Abstract
We report a case of Nocardia exalbida (N. exalbida)-induced pneumonia in a 70-year old Japanese man with lung cancer and radiation pneumonitis. He initially received doripenem (1.5 g/day) for pneumonia treatment, and N. exalbida was identified by a clone library analysis of bronchoalveolar lavage fluid obtained from the pneumonia lesion. The doripenem dosage was therefore increased to 3.0 g/day with adjunctive trimethoprim/sulfamethoxazole, and his pneumonia improved. N. exalbida is susceptible to antibiotics; thus, in nocardiosis, N. exalbida infection might be associated with a good response to treatment, although its clinical findings are non-specific and similar to those of other Nocardia infections.
Keywords: nocardia, pulmonary nocardiosis, lung cancer, radiation pneumonitis
Introduction
Nocardia is an aerobic gram-positive rod bacterium that belongs to the Actinomycetes genus and which is primarily distributed in the soil (1-3). Human infection is predominantly caused via direct inoculation of the skin or inhalation (1-3). Nocardiosis occurs in various organs, including the brain, lungs, skin, and eyes. The lung is the most commonly infected organ (1-9). Immunosuppressed hosts are particularly susceptible to nocardiosis, which can occasionally be severe (10), and the incidence of nocardiosis has been increasing according to an increase in the number of elderly and immunocompromised patients (1,11,12). In addition, the prognosis of pulmonary nocardiosis often depends on the underlying disease, and the 1-year survival rate of pulmonary nocardiosis patients treated with immunosuppressants is approximately 40% (8).
Ninety-two Nocardia species have been reported thus far, and fifty-four have been recognized as clinically significant bacteria (10,11). Clinically, the identification of the Nocardia species in nocardiosis is highly important because the drug susceptibility differs among the species (1,4,8). N. exalbida was first reported in Japan in 2006 (9). To date, only a few cases of N. exalbida infection have been reported (4,5,13-17), and the clinical characteristics of N. exalbida infection have not been fully elucidated.
We herein report a case of pulmonary nocardiosis caused by N. exalbida in a patient with lung cancer and radiation pneumonitis, and review the reported cases of N. exalbida infection.
Case Report
A 70-year old Japanese man was diagnosed with right hilar squamous cell carcinoma (SCC) (cT3N3M0, stageIIIB) in February 2011. He was an ex-smoker (39 pack-years). A single administration of systemic chemotherapy with cisplatin and vinorelbine and concurrent radiotherapy (total radiation dose: 50 Gy) to the right hilum and mediastinum were performed, resulting in a decrease in the tumor size. Radiation pneumonitis occurred in April, and treatment with prednisolone [PSL (50 mg/day)] was initiated. The radiation pneumonitis gradually improved, and the dose of PSL was reduced to 35 mg/day in May and was continued without prophylactic treatment with trimethoprim/sulfamethoxazole (TMP-SMZ).
In June, he suddenly experienced a high-grade fever (38.0°C), and his chest radiograph revealed infiltration in the right middle lung field with elevated C-reactive protein (CRP) levels (15.3 mg/dL). Bacterial pneumonia was suspected, and the oral administration of levofloxacin [LVFX (500 mg/day)] was started, resulting in a worsening of the chest radiography findings. He was eventually admitted to our hospital in July, due to a persistent high-grade fever (38.0°C) and blood-stained sputum.
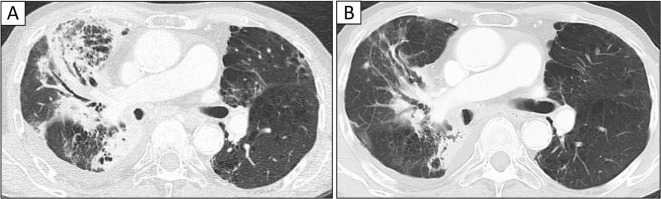
Upon admission, he exhibited a poor general condition and strong breathlessness. A physical examination revealed the following findings: height, 159.1 cm; body weight, 49.5 kg; body temperature, 38.2ºC; heart rate, 130 bpm; blood pressure, 104/60 mmHg; and oxygen saturation, 85% in room air. Chest auscultation demonstrated audible coarse crackles in the right upper lung field, and pitting edema was observed in both lower legs. The laboratory findings on admission (Table 1) demonstrated an elevated peripheral blood white blood cell count (16,900 U/μL) and CRP level (13.5 mg/dL), and an interferon-gamma releasing assay for M. tuberculosis (QuantiFeronⓇ) was inconclusive. The patient’s serum was positive for Aspergillus antigen, but his ß-D glucan titer was within the normal range, and his serum was negative for Cryptococcus neoformans antigen. Increased serum levels of CYFRA21-1 (10.0 ng/dL) and the SCC antigen (7.0 ng/mL) were observed. The serum Krebs von den Lungen (KL)-6 level was within the normal limit (350 U/mL). A chest radiograph on admission revealed novel infiltrative shadows in the right upper and middle lung fields, and chest computed tomography on admission showed infiltrative shadows with air bronchograms in the right middle lobe as well as opacities in the hilum of the right lung that were associated with lung cancer and which narrowed the airway (Fig. 1, 2).
Table 1.
The Results of the Peripheral Blood Analysis on Admission.
| <Blood cell counts> | <Blood chemistry> | ||||||||||||
| WBC | 16,900 | /μL | TP | 5.2 | g/dL | CYFRA21-1 | 10.0 | ng/mL | |||||
| Neutrophils | 93.7 | % | Alb | 2.3 | g/dL | SCC | 7.0 | ng/mL | |||||
| Lymphocytes | 3.3 | % | T-bil | 0.5 | mg/dL | QFT (QuantiFeron®) | indeterminant | ||||||
| Eosinophils | 1.0 | % | AST | 68 | IU/L | measurements A | <0.10 | IU/mL | |||||
| Monocytes | 2.8 | /μL | ALT | 53 | IU/L | measurements M | <0.50 | IU/mL | |||||
| Basophils | 0.1 | g/dL | LDH | 260 | IU/L | β-D glucan | <6.0 | pq/mL | |||||
| RBC | 300×104 | /μL | ALP | 429 | IU/L | Aspergillus antigen | 2.9 | ||||||
| Hb | 10.4 | g/dL | γ-GTP | 230 | IU/L | Cryptococcus neoformans | (-) | ||||||
| Ht | 30.9 | % | BUN | 17 | mg/dL | ||||||||
| Platelets | 18.7×104 | /μL | Cre | 0.54 | mg/dL | <Blood gas analysis (O2 3 L/min)> | |||||||
| pH | 7.538 | ||||||||||||
| <Serology> | PaO2 | 86.0 | mmHg | ||||||||||
| CRP | 13.5 | mg/dL | PaCO2 | 41.5 | mmHg | ||||||||
| KL-6 | 350 | U/mL | HCO3- | 34.5 | mmol/L | ||||||||
WBC: white blood cell, RBC: red blood cell, Hb: haemoglobin, Ht: haematocrit, TP: total protein, Alb: albumin, T-bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: gamma-glutamyl transferase, BUN: blood urea nitrogen, CRP: c-reactive protein, KL-6: Krebs von den Lungen-6, CYFRA21-1: cytokeratin-19 fragments, SCC: squamous cell carcinoma, O2: oxygen, PaO2: partial pressure of arterial oxygen, PaCO2: partial pressure of arterial carbon dioxide, HCO3-: bicarbonate ion
Figure 1.
A chest X-ray obtained on admission showed new infiltration in the right upper and middle lung fields (A). The chest X-ray obtained on day 4 showed worsening infiltration of the right lung field with right pleural effusion (B). After increasing the dose of DRPM from 1.5 g/day to 3.0 g/day with adjunctive TMP-SMZ treatment (day 14), the pulmonary infiltration and right pleural effusion on chest X-ray improved (C).
Figure 2.
Chest computed tomography (CT) on admission (A). Chest CT on admission demonstrated consolidation with air bronchogram in the right middle lobe (A). After antibiotic treatment (day 14), most sites of pulmonary infiltration improved (B).
Bronchoscopy was performed on admission to investigate the source of the airway bleeding, and the adhesion of blood to the trachea, right main bronchus, and right upper leaf branch was revealed, but no active bleeding was observed. The examination of a bronchoalveolar lavage fluid (BALF) specimen obtained from a pneumonia lesion in the right B5 revealed the presence of numerous filamentous gram-positive bacteria (Fig. 3). In addition, similar bacteria were observed in sputum smears and cultures obtained on admission. A clone library analysis targeting the 16S rRNA gene was performed using the BALF in order to evaluate the bacterial flora according to the methods of our previous reports (18-21). Briefly, approximately 600 bp of part of the 16S rRNA gene extracted from DNA samples from the BALF specimen were amplified via a polymerase chain reaction (PCR) using a universal primer, the PCR products were cloned, and the clone library was constructed (18-21). The sequences of the 16S rRNA gene of 96 randomly selected clones from the clone library were determined, and a homology search comparing the sequences with the recorded reference strains was then performed using the basic local alignment search tool algorithm (21). As a result, approximately 65.3% (49/75 clones) of the bacterial clones in his BALF sample were identified as N. exalbida (accession number, NR 11,732.1) (Table 2). The patient was then suspected of having pneumonia caused by N. exalbida. Sputum and BALF cultures were positive for Nocardia species on day 9; however, the Nocardia species was not identifiable. The bacterial strain obtained by culturing was identified as N. exalbida by a PCR targeting the 16S rRNA gene (1,500 bp). According to these findings, the patient was diagnosed with pulmonary nocardiosis caused by N. exalbida.
Figure 3.

The observation of Gram-stained bronchial lavage fluid by light microscopy (×1,000).
Table 2.
The Results of Clone Library Analysis Targeting the 16S Ribosomal RNA Gene Using Bronchoalveolar Lavage Fluid.
| Species | % | |
|---|---|---|
| Nocardia exalbida | 65.3 | |
| Propionibacterium acnes | 1.3 | |
| Prevotella veroralis | 2.7 | |
| Gemella haemolysans | 1.3 | |
| Gemella sanguinis | 2.7 | |
| Staphylococcus epidermidis | 1.3 | |
| Granulicatella adiacens | 2.7 | |
| Veillonella dispar | 1.3 | |
| Fusobacterium canifelinum | 1.3 | |
| Leptotrichia shahii | 1.3 | |
| Brevundimonas vesicularis | 2.7 | |
| Curvibacter delicatus | 4.0 | |
| Campylobacter mucosalis | 4.0 | |
| Shigella flexneri | 5.3 | |
| Actinetobacter junii | 1.3 | |
| unclassified | 1.3 |
Doripenem (DRPM) (1.5 g/day) was initiated after admission, with the continuation of PSL (35 mg/day) for radiation pneumonitis. However, recurrent blood-stained sputum and an elevated serum CRP level were observed on day 3, and his hypoxemia worsened and a chest radiograph showed worsening infiltrative shadows of the right lung field and increased right pleural effusion on day 4, Thus, the dose of DRPM was increased from 1.5 g/day to 3.0 g/day on day 4. The blood-stained sputum, hypoxemia, serum levels of CRP, and radiological findings subsequently improved. Adjunctive treatment with TMP-SMZ (3,600 mg/720 mg) was administered in addition to DRPM (3.0 g/day) after the identification of N. exalbida. This led to a gradual improvement in his laboratory and chest radiography findings (Fig. 1, 2, 4).
Figure 4.
The clinical course of the present patient. DRPM: doripenem, SMX/TMP: sulfamethoxazole/trimethoprim, PSL: prednisolone
The sudden progression of hypoxemia was observed on day 18, and chest radiography revealed right middle lobe atelectasis, due to airway narrowing in association with progressive lung cancer. With the deterioration of his respiratory function and general condition, intravenous continuous administration of morphine hydrochloride was started from day 20 to provide relief from pain and dyspnea. He died on day 22.
Discussion
N. exalbida was first isolated from two Japanese immunocompromised patients with a cutaneous lesion and lung abscess in 2006 (13). To date, nine cases of nocardiosis caused by N. exalbida have been reported, including our patient (Table 3). The lung is generally the most common site of infection in nocardiosis, with lung infections accounting for approximately 40-70% of nocardiosis cases (2,22-25). Four of the nine reported cases of N. exalbida infection involved the lung (44.4%) (4-13), and ocular lesions were also reported in three of the cases (33.3%) (15-17). Nocardiosis tends to develop in patients with underlying diseases such as diabetes, malignancy, or chronic obstructive pulmonary disease, and immunosuppressed patients. Comorbid diseases are reported in approximately 60-90% in nocardiosis patients (2,22,23,25). Seven (77.7%) of the nine cases of N. exalbida infections had comorbid diseases. There are no characteristic clinical symptoms or radiological findings that can be used to distinguish N. exalbida infection from other nocardiosis infections, although the symptoms and radiological findings are generally nonspecific in nocardiosis (1-3,8,26,27). It is therefore difficult to differentiate N. exalbida infection and other nocardiosis infections based on the clinical background, symptoms, and radiographic findings.
Table 3.
The Reported Cases of Nocardia exalbida.
| Reference | Age(y) / Sex | Presentation | Comorbidity | Antibiotics used for outcome | Outcome |
|---|---|---|---|---|---|
| 4 | 47 / M | Pneumonia | HIV, Hepatitis B, Type-II diabetes | IPM+AMK for 17days → GRNX for 6 months |
improved |
| 13 | 43 / unknown | Pulmonary abscess | Immunocompromised patient (details unknown) | unknown | unknown |
| 14 | 68 / M | Pneumonia | HIV | TMP/SMX for 12 months | improved |
| 5 | 63 / M | Brain abscess | Follicular lymphoma | MEPM+TMP-SMZ for 2 months → TMP |
improved |
| 15 | 38 / F | Keratitis | none | TMP-SMZ for 10 days Topical therapy: TOB+CP+SZ+ colistin sodium methanesulfonate | improved |
| 16 | 56 / M | Endophthalmitis | none | TMP-SMZ for 6 months | improved |
| 17 | 57 / M | Blebitis | Open-angle glaucoma | TMP-SMZ+Topical therapy: sulfonamide+AMK for 6 months | improved |
| 13 | 60 / unknown | Pemphigus vulgaris | Immunocompromised patient (details unknown) | unknown | unknown |
| Present case | 70 / M | Pneumonia | Lung cancer, Radiation pneumonia (oral steroids) | DRPM+TMP-SMZ | improved |
HIV: human immunodeficiency virus, IPM: imipenem, AMK: amikacin, GRNX: garenoxacin, MINO: minocycline, TMP-SMZ: trimethoprim/sulfamethoxazole, MEPM: meropenem, TOB: tobramycin, CP: chloramphenicol, SZ: sulfisoxazole., DRPM: doripenem
The early diagnosis and treatment of pulmonary nocardiosis are highly important because the mortality rate of such patients is approximately 40%, and a higher mortality rate of approximately 60% has been reported in cases with dissemination (27). However, the diagnosis of pulmonary nocardiosis is often delayed due to its nonspecific clinical symptoms and radiological findings, and the absence of specific methods for the serological diagnosis of nocardiosis. A definitive diagnosis of nocardiosis is only made by the separation and identification of Nocardia species using the culture method (3,26). However, culturing of Nocardia species is difficult and requires several days to several weeks (1,3,6), and the rate of successful sputum culture ranges from 10 to 70% (28). Gram staining of Nocardia species reveals a characteristic structure of delicate, beaded, branching filaments; thus, gram staining is useful when nocardiosis is suspected (1,3).
The identification of the Nocardia species is important in nocardiosis because they show susceptibility to different antibiotics (1,4,7,13,29). For example, N. farcinica, a common species in nocardiosis, is resistant to most of the antibiotics that are normally used for the treatment of nocardial infections (13,30,31). The identification of the Nocardia species can be performed according to a combination of biochemical tests, growth characteristics, and the antimicrobial susceptibility patterns of cultured bacteria (2,3,26). However, the relatively low rate of successful sputum culture of Nocardia species (28), the relatively long time required to identify the cultured bacteria, and the number of Nocardia species may make it more difficult to a achieve completely accurate identification of the Nocardia species. Thus, a bacterial 16S rRNA sequence analysis has recently become the gold standard for the identification of Nocardia species (4-7,26,32), and all nine cases of N. exalbida infection, including our patient, were identified by 16S rRNA sequencing. In addition, we evaluated the bacterial flora of the BALF sample by a clone library analysis targeting the 16S rRNA gene according to previous reports (18-21); 65.3% of the bacterial clones were found to be N. exalbida, and the patient was determined to have pulmonary infection with N. exalbida (Table 2). 16S rRNA sequencing to identify the bacterial species is generally performed after obtaining a cultured bacterial strain, and is therefore time-consuming. However, the clone library analysis we used takes only a few days after specimen collection without estimating the bacterial species, and we believe that an earlier definitive diagnosis of nocardiosis can help to facilitate appropriate treatment.
TMP-SMZ is widely used as the mainstay in the treatment of nocardiosis (4,5,27). Carbapenems such as imipenem/cilastatin sodium (IPM/CS) and meropenem (MEPM), amikacin, minocycline, and linezolid are also used (4,5,11). In the present patient, the combination of TMP-SMZ and DRPM was administered after the diagnosis of N. exalbida infection. Although our patient ultimately died due to the progression of lung cancer, the clinical response to antibiotic therapy was good (Fig. 4). Susceptibility to antibiotics greatly affects the prognosis for nocardiosis (12,32), and as summarized in Table 4, among the Nocardia species, N. exalbida is susceptible to antibiotics. All seven reported cases of N. exalbida infection noted that the prognosis improved after treatment, and that the identification of N. exalbida might be associated with a good response to treatment in nocardiosis. DRPM is effective for the treatment of pulmonary nocardiosis as well as IPM/CS and MEPM. In our patient, DRPM (1.5 g/day) was ineffective, but the increased dose of 3.0 g/day was effective. Carbapenem antibiotics require ≥50% time above minimum inhibitory concentration (% TAM) to achieve the maximum bactericidal effect, and the plasma concentration of MEPM clearly increased after increasing the dose of MEPM from 1.5 g/day to 3.0 g/day (33). Thus, an increase in the antibiotic dose can be effective when the clinical response to a medium dose of carbapenem is poor.
Table 4.
Antimicrobial Susceptibility to Nocardia exalbida.
| Reference | Presentation | CTX | CTRX | MEPM | IPM/CS | MINO | GRNX | AMK | SMZ/TMP | LZD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | Pneumonia | 4 (S) |
1 (-) |
0.5 (S) |
1 (S) |
2 (S) |
≤1 (S) |
≤4.75/0.25 (S) |
0.5 (S) |
|||||||||||
| 13 | Pulmonary abscess | 16 (-) |
2/4 (-) |
|||||||||||||||||
| 14 | Pneumonia | <0.5 (-) |
4.75/0.25 (-) |
|||||||||||||||||
| 5 | Brain abscess | 0.12 (S) |
0.5 (-) |
<0/13 (S) |
0.25 (S) |
0.12 (S) |
||||||||||||||
| 13 | Pemphigus vulgaris | >16 (-) |
2/4 (-) |
|||||||||||||||||
| Present case | Pneumonia | 1 (-) |
1 (-) |
0.5 (-) |
<0.12 (-) |
2 (-) |
The upper row presents the minimal inhibitory concentration (MIC), μg/mL.
The lower row shows drug susceptibility. S: sensitive, I: intermediate, R: resistant, (-): not described
CTX: cefotaxime, CTRX: ceftriaxone, MEPM: meropenem, IPM/CS: imipenem/cilastatin sodium, MINO: minocycline, GRNX: garenoxacin, AMK: amikacin, TMP-SMZ: trimethoprim/sulfamethoxazole, LZD: linezolid
Combination treatment with TMP-SMZ and DRPM was effective in our case. The synergistic effect of combination therapy with IPM/CS and TMP-SMZ on N. asteroides infection was reported in vitro (34), and the synergistic effect of a combination of DRPM and TMP-SMZ might have been obtained in our case. In addition, it is reported that TMP-SMZ monotherapy resulted in a high mortality rate in nocardiosis. Thus, physicians should consider combination therapy with amikacin, imipenem, or a third-generation cephalosporin, in addition to TMP-SMZ in severe cases (5,28,35).
In conclusion, we reported a case of pulmonary nocardiosis caused by N. exalbida in a patient with lung cancer and radiation pneumonitis treated with corticosteroids. N. exalbida is extremely rare among the Nocardia species, but the response to proper treatment seems to be favorable. The further accumulation of the clinical characteristics in each Nocardia species is expected to facilitate their early diagnosis and appropriate treatment.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Lerner PI. Nocardiaosis. Clin Infect Dis 22: 891-905, 1996. [DOI] [PubMed] [Google Scholar]
- 2. Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev 7: 213-264, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tania CS, David HM, Jonathan RI, Sharon CC. Nocardia species. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th ed. Gerald LM, John EB, Raphael D, Eds. Churchill Livingstone, Philadelphia, 2015: 2853-2863. [Google Scholar]
- 4. Imai K, Koibuchi T, Kikuchi T, et al. . Pulmonary nocardiosis caused by nocardia exalbida complicating pneumocystis pneumonia in an HIV-infected patient. J Infect Chemother 17: 547-51, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Ono M, Kobayashi Y, Shibata T, et al. . Nocardia exalbida brain abscess in a patient with follicular lymphoma. Int J Hematol 88: 95-100, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Curry WA. Human nocardiosis: a clinical review with selected case reports. Arch Intern Med 140: 818-826, 1980. [DOI] [PubMed] [Google Scholar]
- 7. Boiron P, Provost F, Chevrier G, Dupont B. Review of nocardial infections in France 1987 to 1990. Eur J Clin Microbiol Infect Dis 11: 709-714, 1992. [DOI] [PubMed] [Google Scholar]
- 8. Takiguchi Y, Ishizaki S, Kobayashi T, et al. . Pulmonary nocardiosis: a clinical analysis of 30 cases. Intern Med 56: 1485-1490, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev 7: 213-264, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conville PS, Brown-Elliott BA, Smith T, Zelazny AM. The complexities of nocardia taxonomy and identification. J Clin Microbiol 56: e01419-17, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog 114: 369-384, 2018. [DOI] [PubMed] [Google Scholar]
- 12. Minero MV, Marín M, Cercenado E, Rabadán PM, Bouza E, Muñoz P. Nocardiosis at the turn of the century. Medicine (Baltimore) 88: 250-261, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Iida S, Kageyama A, Yazawa K, et al. . Nocardia exalbida sp. nov., isolated from Japanese patients with nocardiosis. Int J Syst Evol Microbiol 56: 1193-1196, 2006. [DOI] [PubMed] [Google Scholar]
- 14. Kiyasu K, Koganemaru H, Kurihara Y, Hitomi S. Pulmonary infection due to Nocardia exalbida complicated with pneumococcal pneumonia. JMM Case Reports 2: 2015(Epub ahead of print). [Google Scholar]
- 15. Mizota A, Haki K, Shiina C, et al. . The first case of keratitis caused by Nocardia exalbida. Int Ophthalmol 27: 333-336, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Milman T, Trubnik V, Shah M, McCormick SA, Finger PT. Isolated Nocardia exalbida endogenous endophthalmitis. Ocul Immunol Inflamm 19: 237-239, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Ifantides C, Batlle OR, Mushatt D, Ayyala RS. Nocardia exalbida blebitis: a case report. J Glaucoma 24: e19-e21, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Kawanami T, Fukuda K, Yatera K, Kido M, Mukae H, Taniguchi H. A higher significance of anaerobes: the clone library analysis of bacterial pleurisy. Chest 139: 600-608, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Yamasaki K, Kawanami T, Yatera K, et al. . Significance of anaerobes and oral bacteria in community-acquired pneumonia. PLoS One 8: e63103, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noguchi S, Mukae H, Kawanami T, et al. . Bacteriological assessment of healthcare-associated pneumonia using a clone library analysis. PLoS One 10: e0124697, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamasaki K, Yatera K, Kato K, et al. . Successful additional corticosteroid treatment in a patient with mycoplasma pneumoniae pneumonia in whom a monobacterial infection was confirmed by a molecular method using bronchoalveolar lavage fluid. Intern Med 55: 703-707, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Mazzaferri F, Cordioli M, Segato E, et al. . Nocardia infection over 5 years (2011-2015) in an Italian tertiary care hospital. New Microbiol 41: 136-140, 2018. [PubMed] [Google Scholar]
- 23. Minero MV, Marín M, Cercenado E, Rabadán PM, Bouza E. Nocardiosis at the turn of the century. Muñoz P. Medicine (Baltimore) 88: 250-261, 2009. [DOI] [PubMed] [Google Scholar]
- 24. Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection 38: 89-97, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Mootsikapun P, Intarapoka B, Liawnoraset W. Nocardiosis in Srinagarind Hospital, Thailand: review of 70 cases from 1996-2001. Int J Infect Dis 9: 154-158, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Kurahara Y, Tachibana K, Tsuyuguchi K, Akira M, Suzuki K, Hayashi S. Pulmonary nocardiosis: a clinical analysis of 59 cases. Respir Investig 52: 160-166, 2014. [DOI] [PubMed] [Google Scholar]
- 27. Martínez Tomás R, Menéndez Villanueva R, Reyes Calzada S, et al. . Pulmonary nocardiosis: risk factors and outcomes. Respirology 12: 394-400, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Li S, Song Xy, Zhao Yy, et al. . Clinical analysis of pulmonary nocardiosis in patients with autoimmune disease. Medicine (Baltimore) 94: e1561, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gordon RE, Barnerr DA, Handerhan JE, Pang CHN. Nocardia autotrophica, and the nocardin stain. Int J Syst Bacteriol 24: 54-63, 1974. [Google Scholar]
- 30. Ishikawa J, Yamashita A, Mikami Y, et al. . The complete genomic sequence of Nocardia farcinica IFM 10152. Proc Natl Acad Sci USA 101: 14925-14930, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kageyama A, Poonwan N, Yazawa K, Mikami Y, Nishimura K. Nocardia asiatica sp. nov., isolated from patients with nocardiosis in Japan and clinical specimens from Thailand. Int J Syst Evol Microbiol 54: 125-130, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Mazzaferri F, Cordioli M, Segato E, et al. . Nocardia infection over 5 years (2011-2015) in an Italian tertiary care hospital. New Microbiol 41: 136-140, 2018. [PubMed] [Google Scholar]
- 33. Kawanami T, Mukae H, Noguchi S, et al. . Efficacy and safety of meropenem (3 g daily) in Japanese patients with refractory respiratory infections. J Infect Chemother 20: 768-773, 2014. [DOI] [PubMed] [Google Scholar]
- 34. Gombert ME, Aulicino TM. Synergism of imipenem and amikacin in combination with other antibiotics against Nocardia asteroides. Antimicrob Agents Chemother 24: 810-811, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smego RA Jr, Moeller MB, Gallis HA. Trimethoprim-sulfamethoxazole therapy for Nocardia infections. Arch Intern Med 143: 711-718, 1983. [PubMed] [Google Scholar]