Abstract
Objective
Salt loading induces renal damage independently of blood pressure (BP) elevation via reactive oxygen species and sympathetic activity. Melatonin, a hormone that regulates the circadian rhythm, has multiple functions, including anti-oxidant effects and the inhibition of sympathetic activity. We have shown that impaired melatonin secretion is associated with renal damage in chronic kidney disease (CKD) patients. However, the associations between salt loading, melatonin secretion, and urinary albumin and protein have not been clarified.
Methods
We recruited 32 CKD patients, conducted 24-hour ambulatory BP monitoring and collected daytime and nighttime urine while the patients were consuming a standard salt (10 g/day) or low salt (6 g/day) diet. The excretion levels of albumin, protein and 6-sulfatoxymelatonin (aMT6s), a metabolite of melatonin, in daytime and nighttime urine were investigated in patients consuming standard salt and low salt diets.
Results
The urinary aMT6s levels in daytime and nighttime of the patients consuming standard salt and low salt diets did not differ to a statistically significant extent. However, the urinary aMT6s levels in patients consuming a standard salt diet-but not patients consuming a low salt diet-were significantly and negatively correlated with the daytime and nighttime urinary albumin and protein levels. Contrarily, no significant correlations were found between the urinary aMT6s levels and the BP levels, renal function, and plasma angiotensin II levels in patients consuming either a standard salt or low salt diet. A multiple regression analysis adjusted for age, sex, and body mass index revealed that the urinary albumin and protein levels were significantly and negatively associated with the urinary aMT6s levels in patients consuming a standard salt diet, but not in patients consuming a low salt diet.
Conclusion
Salt loading aggravates the relationship between melatonin secretion and albuminuria or proteinuria.
Keywords: salt loading, renal damage, melatonin, chronic kidney disease, urinary 6-sulfatoxymelatonin
Introduction
Chronic kidney disease (CKD) is a risk factor for cardiovascular disease (CVD) and end-stage renal failure (1,2). More than 13 million people suffer from CKD in Japan, and this number is expected to increase in the future. Thus, there is an urgent need to establish an effective therapy for this disease. However, there are few strategies for suppressing the occurrence and development of CKD.
Salt loading causes blood pressure (BP) elevation due to an increase in body fluid, which is proportional to the amount of sodium in the body, and BP levels were found to decrease according to the degree of salt restriction (3). Moreover, salt restriction is known to reduce urinary protein excretion and to suppress the progression of renal damage (4,5). Furthermore, recent studies have found that salt loading induces renal damage independently of the increase of body fluids (6-9).
Melatonin is a hormone produced by the pineal gland, and plays a pivotal role in regulating the circadian rhythm of several biological systems. It has been clarified that melatonin not only regulates the biological circadian clock, but also serves a variety of biological functions (10-12). However, the relationships among salt loading, melatonin secretion, and the urinary albumin and protein levels remain to be clarified. Thus, the present study was performed with the aim of clarifying these associations in CKD patients.
Materials and Methods
Subjects
The present study was approved by the ethics committee of Hamamatsu University School of Medicine and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients. We recruited 32 CKD patients who had been admitted to our hospital to undergo renal biopsy between February 2013 and March 2016. Although patients on antihypertensive medication were included in this study, no changes to their antihypertensive regimen were permitted during the study.
Study protocols
Blood samples were drawn at admission to evaluate the patients' basal characteristics (hemoglobin A1c, total cholesterol, low-density lipoprotein cholesterol, and uric acid). Either a standard salt diet (10 g/day of salt) or low salt diet (6 g/day of salt) was provided after admission. Examinations were performed as described below after the patient had consumed the salt diet for a certain period. Subsequently, some patients moved from the standard salt diet to the low salt diet and some patients moved from the low salt diet to the standard diet, and the examinations were repeated after the patient had consumed the salt diet for a certain period. This time (3.88±1.56 days for the standard salt diet and 4.44±1.19 days for the low salt diet) was necessary to maintain a stable body fluid balance. The patients were randomly allocated to receive the standard or low salt diet. The patients' vital signs, such as their height and body weight, were measured and ambulatory BP monitoring (ABPM) was conducted at 30-min intervals for 24 hours using an automatic device (TM-2431; A and D, Tokyo, Japan). Daytime (6:00 AM to 9:00 PM) and nighttime (9:00 PM to 6:00 AM) urine collection was conducted on the day of the experiment. The daytime and nighttime for 24-hour ABPM were divided based on the sleep and wake times that were recorded in patients' behavior records. Blood samples were drawn at 9:00 PM and 6:00 AM on the following day, after patients rested in the supine position for a minimum of 15 minutes. The blood samples taken at 9:00 PM and 6:00 AM were considered to be the daytime and nighttime samples, respectively, as previously described (13,14).
Clinical data
The daytime BP was calculated as the average of the readings taken during waking hours; the nighttime BP was the average of the remaining values. The serum creatinine (sCr), urinary creatinine, albumin, protein, sodium, and potassium concentrations were measured in the hospital's clinical laboratory. The levels of urinary 6-sulfatoxymelatonin (U-aMT6s), the main melatonin metabolite, were measured using an enzyme-linked immunoassay (ELISA) (Bühlmann Laboratories AG, Schönenbuch, Switzerland) (15). The U-aMT6s excretion per hour (U-aMT6s/h) during daytime and nighttime was calculated as a surrogate marker of the rate of melatonin secretion, as previously described (13,16,17). The plasma renin activity (PRA) and plasma angiotensin II (Ang II) levels were assessed by a radioimmunoassay (SRL, Tokyo, Japan). The estimated glomerular filtration rate (eGFR) was calculated using the Japanese eGFR equation (18). The daytime and nighttime urinary albumin/creatinine (U-Alb/Cr) and protein/creatinine (U-Pro/Cr) ratios were calculated.
Statistical analyses
The results were expressed as the mean±standard deviation. Because the PRA, U-aMT6s/h, U-Alb/Cr, and U-Pro/Cr excretion levels were not normally distributed, these results were reported as the median with the 25% and 75% quartiles using the Mann-Whitney test. In addition, they were logarithmically transformed prior to the statistical analysis (13,14). The significance of the differences between gender, as well as the standard and low salt diets in both daytime and nighttime, was determined using Student's t-test for paired samples. The correlations between U-aMT6s/h levels and other parameters [body weight, body mass index (BMI), BP, sCr, eGFR, PRA, plasma Ang II, U-Alb/Cr, and U-Pro/Cr] were evaluated using Pearson's product-moment correlation coefficient for both daytime and nighttime. Multivariate regression analyses were performed for daytime and nighttime using independent predictors of the U-aMT6s/h. P values of <0.05 were considered to indicate statistical significance. All statistical analyses were performed using the IBMⓇSPSSⓇ software program (version 23, IBM, Armonk, USA).
Results
Patient characteristics
This study included 32 patients (male, n=12; female, n=20; mean age, 50.3±15.8 years) who had undergone renal biopsy to determine the patient's definitive diagnosis, recognize the degree of renal damage, and determine the treatment strategy. The histological diagnoses of CKD were as follows: IgA nephropathy (n=19), focal segmental glomerulosclerosis (n=3), membranous nephropathy (n=2), and other diagnoses (n=8). One patient had impaired glucose tolerance treated with a dipeptidyl peptidase-4 inhibitor, 6 patients had hyperlipidemia treated with statins or fibrates, and 2 patients had hypertension treated with calcium channel blockers or beta blockers. One patient had hypertension treated with a calcium channel blocker and hyperuricemia treated with febuxostat and one patient had hypertension treated with a calcium channel blocker and hyperlipidemia treated with a statin. Treatment was effective in all patients. No patient received diuretics. Although the height and body weight of the male patients was significantly higher and heavier in comparison to the female patients, the male and female patients showed similar BMI values. The systolic and diastolic BP values of the male patients were significantly higher in comparison to the female patients. In addition, the levels of uric acid in male patients were significantly higher than those in female patients. The patients' baseline characteristics are presented in Table 1.
Table 1.
Patient Characteristics.
| Total | Male | Female | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Number of patients | 32 | 12 | 20 | |||||
| Age, year | 50.3±15.7 | 49.8±16.0 | 50.6±15.9 | 0.89 | ||||
| Causes of CKD | 0.30 | |||||||
| IgA nephropathy | 19 | 6 | 13 | |||||
| Focal segmental glomerulosclerosis | 3 | 1 | 2 | |||||
| Membranus nephropathy | 2 | 0 | 2 | |||||
| Others | 8 | 5 | 3 | |||||
| Comorbidity | ||||||||
| Impaired glucose tolerance | 1 | 1 | 0 | 0.19 | ||||
| Hypertension | 4 | 3 | 1 | 0.098 | ||||
| Hyperlipidemia | 7 | 3 | 4 | 0.74 | ||||
| Hyperuricemia | 1 | 1 | 0 | 0.19 | ||||
| Height (cm) | 161.7±9.9 | 172.3±6.2 | 155.4±5.1 | <0.01 | ||||
| Body weight (kg) | 57.9±10.2 | 67.3±6.7 | 52.3±7.4 | <0.01 | ||||
| Body mass index (kg/m2) | 22.1±2.9 | 22.8±3.1 | 21.6±2.7 | 0.28 | ||||
| Systolic BP (mmHg) | 118.7±15.7 | 128.4±16.4 | 112.8±12.1 | <0.01 | ||||
| Diastolic BP (mmHg) | 72.1±10.3 | 77.8±12.4 | 68.8±7.1 | 0.014 | ||||
| HbA1c (%) | 5.64±0.48 | 5.78±0.59 | 5.55±0.38 | 0.21 | ||||
| T-cho (mg/dL) | 202.6±39.2 | 190.2±34.7 | 210.1±40.6 | 0.17 | ||||
| LDL-cho (mg/dL) | 116.1±33.0 | 108.1±31.5 | 120.3±34.0 | 0.38 | ||||
| Uric acid (mg/dL) | 5.92±1.60 | 7.47±0.70 | 4.99±1.20 | <0.01 |
CKD: chronic kidney disease, BP: blood pressure, HbA1C: hemoglobin A1C, T-cho: Total cholestreol, LDL-cho: low-density lipoprotein cholestreol
Comparison of specific clinical parameters of the patients consuming standard salt and low salt diets
The clinical parameters of patients consuming standard and low salt diets were compared. The urinary daily sodium excretion was 128.0±32.5 mEq/day in patients consuming the standard salt diet and 89.7±22.8 mEq/day in patients consuming the low salt diet; the difference was statistically significant (p<0.01). The patients consuming standard salt and low salt diets showed similar body weight and BMI values (body weight; standard salt diet; 57.9±10.3 kg, low salt diet; 57.8±10.4 kg, p=0.35 and BMI; standard salt diet; 22.0±2.95 kg/m2, low salt diet; 21.0±4.7 kg/m2, p=0.34, respectively).
Comparison of the specific clinical parameters during daytime and nighttime between patients consuming standard salt and low salt diets
As melatonin is mainly produced by the pineal gland during nighttime (Log U-aMT6s/h; standard salt diet: daytime 4.39±0.28 ng/h, nighttime 5.20±0.42 ng/h, p<0.01, low salt diet: daytime 4.46±0.35 ng/h, nighttime 5.24±0.40 ng/h, p<0.01), we compared specific clinical parameters during daytime and nighttime between patients consuming standard salt and low salt diets (Table 2).
Table 2.
Comparison of Some Clinical Parameters between the Standard and Low Salt Diets in Daytime (A) or Nighttime (B).
| A Daytime | |||
|---|---|---|---|
| Standard salt diet | Low salt diet | p value | |
| Systolic BP (mmHg) | 120.9±15.7 | 119.9±15.1 | 0.30 |
| Diastolic BP (mmHg) | 73.1±8.7 | 73.1±8.7 | 0.96 |
| sCr (mg/dL) | 0.93±0.30 | 0.98±0.33 | 0.027 |
| eGFR (mL/min/1.73 m2) | 60.8±18.2 | 58.1±18.6 | 0.057 |
| PRA (ng/mL/h) | 0.80 [0.40-1.30] | 1.05 [0.63-2.08] | <0.01 |
| Log PRA (ng/mL/h) | -0.087±0.31 | 0.083±0.36 | <0.01 |
| Ang II (pg/mL) | 8.9±3.1 | 11.0±3.8 | <0.01 |
| U-Alb/Cr (mg/gCr) | 382.5 [220.3-800.8] | 327.0 [150.8-828.8] | 0.79 |
| Log U-Alb/Cr (mg/gCr) | 2.57±0.34 | 2.54±0.39 | 0.56 |
| U-Pro/Cr (mg/gCr) | 756.1 [445.9-1,425.8] | 682.3 [344.5-1,301.7] | 0.61 |
| Log U-Pro/Cr (mg/gCr) | 2.87±0.27 | 2.84±0.32 | 0.41 |
| U-aMT6s/h (ng/h) | 23,147.1 [18,464.4-36,015.0] | 29,295.6 [22,098.4-53,048.2] | 0.38 |
| Log U-aMT6s/h (ng/h) | 4.39±0.28 | 4.46±0.35 | 0.21 |
| Urinary sodium excretion (mEq) | 82.8±24.0 | 54.0±18.0 | <0.01 |
| Urinary pottasium excretion (mEq) | 22.3±6.5 | 20.0±7.6 | 0.074 |
| Urinary volume (mL) | 1,008.9±372.1 | 1,005.0±490.2 | 0.96 |
| Standard salt diet | Low salt diet | p value | |
|---|---|---|---|
| Systolic BP (mmHg) | 112.8±14.6 | 111.7±12.8 | 0.36 |
| Diastolic BP (mmHg) | 67.0±8.1 | 66.8±9.1 | 0.74 |
| sCr (mg/dL) | 0.90±0.30 | 0.94±0.32 | <0.01 |
| eGFR (mL/min/1.73 m2) | 64.1±19.3 | 61.4±18.8 | <0.01 |
| PRA (ng/mL/h) | 0.60 [0.40-1.08] | 1.00 [0.70-1.78] | <0.01 |
| Log PRA (ng/mL/h) | -0.22±0.33 | 0.0020±0.37 | <0.01 |
| Ang II (pg/mL) | 8.9±3.4 | 10.1±3.8 | 0.082 |
| U-Alb/Cr (mg/gCr) | 230.0 [110.8-352.0] | 197.5 [88.9-475.0] | 0.78 |
| Log U-Alb/Cr (mg/gCr) | 2.35±0.34 | 2.35±0.43 | 0.87 |
| U-Pro/Cr (mg/gCr) | 522.9 [286.5-678.9] | 484.8 [229.2-843.3] | 0.74 |
| Log U-Pro/Cr (mg/gCr) | 2.65±0.27 | 2.64±0.32 | 0.69 |
| U-aMT6s/h (ng/h) | 14,0426.3 [90,295.6-33,9728.0] | 155,539.8 [90,707.8-347,552.3] | 0.39 |
| Log U-aMT6s/h (ng/h) | 5.20±0.42 | 5.24±0.40 | 0.34 |
| Urinary sodium excretion (mEq) | 45.2±17.2 | 35.7±20.6 | 0.021 |
| Urinary pottasium excretion (mEq) | 9.5±4.2 | 11.6±9.6 | 0.14 |
| Urinary volume (mL) | 562.1±271.5 | 596.8±339.4 | 0.48 |
PRA, U-aMT6s/h, U-Alb/Cr, and U-Pro/Cr excretion levels did not show a normal distribution, they were shown as median along with 25% and 75% quartiles in parenthesis. BP: blood pressure, sCr: serum creatinine, eGFR: estimated glomerular filtration rate, PRA: plasma renin activity, Ang II: angiotensin II, U-Alb: urinary albumin, U-Pro: urinary protein, U-aMT6s: urinary 6-sulfatoxymelatonin
The levels of urinary sodium excretion in patients consuming the standard salt diet were significantly increased in comparison to those in patients consuming the low salt diet in both daytime and nighttime. However, no significant differences in the urinary potassium excretion levels or urinary volume were found between the standard and low salt diets in either daytime or nighttime.
The daytime and nighttime Cr and PRA levels of patients consuming the standard salt diet were significantly decreased in comparison to those in patients consuming the low salt diet. Similar results were found for the plasma Ang II levels. In addition, the nighttime eGFR of patients consuming the standard salt diet was significantly increased in comparison to that of patients consuming the low salt diet and a similar tendency was found in daytime.
On the other hand, daytime and nighttime U-aMT6s/h levels of patients consuming the standard salt diet tended to decrease in comparison to those of patients consuming the low salt diet. Contrarily, the daytime and nighttime BP, U-Alb/Cr, and U-Pro/Cr levels of patients consuming the standard salt diet tended to increase in comparison to those of patients consuming the low salt diet.
Relationships between U-aMT6s/h and specific daytime and nighttime parameters of patients consuming standard salt and low salt diets
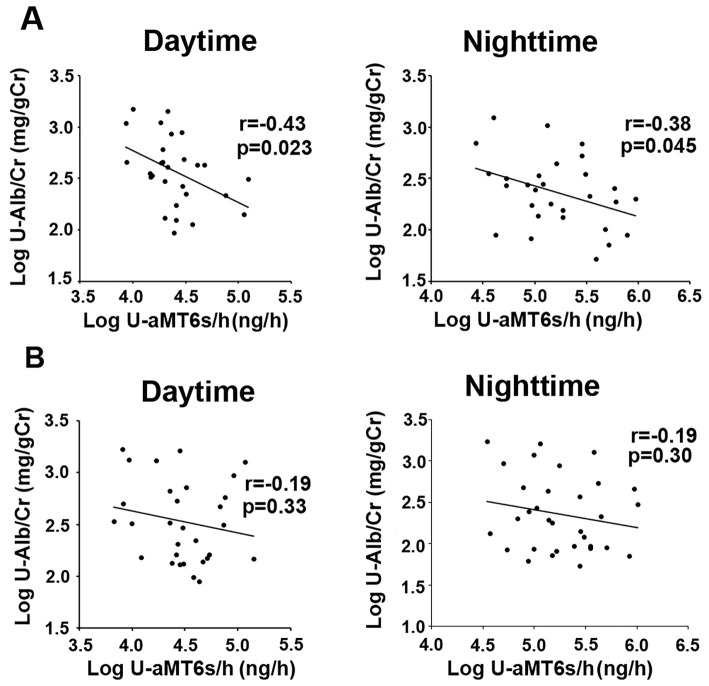
We investigated the relationships between U-aMT6s/h and specific daytime and nighttime parameters in patients consuming standard salt and low salt diets. The urinary aMT6s excretion levels of patients consuming the standard salt diet were significantly and negatively correlated with albumin excretion levels during daytime and nighttime (Fig. 1A). On the other hand, no significant association was detected between the urinary aMT6s excretion level and the urinary albumin excretion level of patients consuming a low salt diet (Fig. 1B). In addition, the urinary aMT6s excretion level of patients consuming a standard salt diet was significantly and negatively correlated with the urinary protein excretion level during both daytime and nighttime. On the other hand, no significant correlations were found between the urinary aMT6s excretion levels and BP, renal function, PRA, or plasma AngII levels during daytime or nighttime in patients consuming standard salt or low salt diets (Table 3).
Figure 1.
The relationship between daytime and nighttime urinary melatonin excretion (U-aMT6s/h) and urinary albumin excretion (U-Alb/Cr) in patients consuming a standard salt diet (A) and those consuming a low salt diet (B). U-aMT6s/h levels in patients consuming a standard salt diet were significantly and negatively correlated with the U-Alb/Cr levels in daytime (r=-0.43, p=0.023) and nighttime (r=-0.38, p=0.045) (A). On the other hand, no significant associations were detected between the U-aMT6s/h levels and U-Alb/Cr levels in daytime (r=-0.19, p=0.33) or nighttime (r=-0.19, p=0.30) in patients consuming a low salt diet (B).
Table 3.
Relationships between Urinary Melatonin Excretion Levels and Some Parameters in Daytime or Nighttime during the Standard or Low Salt Diet.
| Standard salt diet | Low salt diet | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Daytime | Nighttime | Daytime | Nighttime | ||||||||
| r | p | r | p | r | p | r | p | ||||
| Body weight (kg) | -0.038 | 0.85 | -0.12 | 0.54 | 0.18 | 0.35 | -0.067 | 0.72 | |||
| Body mass index (kg/m2) | -0.038 | 0.85 | -0.18 | 0.33 | 0.19 | 0.33 | 0.25 | 0.18 | |||
| Daytime systolic BP (mmHg) | -0.006 | 0.98 | 0.11 | 0.57 | |||||||
| Nighttime systolic BP (mmHg) | -0.32 | 0.092 | -0.14 | 0.44 | |||||||
| Daytime diastolic BP (mmHg) | 0.012 | 0.95 | 0.053 | 0.78 | |||||||
| Nighttime diastolic BP (mmHg) | -0.18 | 0.35 | -0.050 | 0.79 | |||||||
| Daytime sCr (mg/dL) | -0.17 | 0.38 | 0.26 | 0.16 | |||||||
| Nighttime sCr (mg/dL) | -0.16 | 0.40 | -0.081 | 0.66 | |||||||
| Daytime eGFR (mL/min/1.73 m2) | 0.26 | 0.18 | 0.057 | 0.76 | |||||||
| Nighttime eGFR (mL/min/1.73 m2) | 0.34 | 0.063 | 0.11 | 0.57 | |||||||
| Daytime Log PRA (ng/mL/h) | -0.21 | 0.30 | 0.14 | 0.45 | |||||||
| Nighttime Log PRA (ng/mL/h) | -0.12 | 0.53 | 0.037 | 0.84 | |||||||
| Daytime Ang II (pg/mL) | 0.31 | 0.11 | 0.26 | 0.16 | |||||||
| Nighttime Ang II (pg/mL) | 0.25 | 0.18 | -0.11 | 0.56 | |||||||
| Daytime Log U-Pro/Cr (mg/gCr) | -0.47 | 0.012 | -0.19 | 0.33 | |||||||
| Nighttime Log U-Pro/Cr (mg/gCr) | -0.37 | 0.044 | -0.096 | 0.62 | |||||||
BP: blood pressure, sCr: serum creatinine, eGFR: estimated glomerular filtration rate, PRA: plasma renin activity, Ang II: angiotensin II, U-Pro: urinary protein
Multiple regression analyses of the factors associated with the daytime and nighttime urinary aMT6s excretion levels in patients consuming standard salt and low salt diets
Multiple regression analyses were performed to investigate the relationships between the U-aMT6s/h level and the levels of urinary albumin and urinary protein. Analyses adjusted for age, gender and BMI revealed that the nighttime urinary albumin level and the daytime protein level were significantly and negatively associated with the urinary aMT6s levels in patients consuming a standard diet. Similar tendencies were found between U-aMT6s/h and the daytime urinary albumin level and nighttime protein level. On the other hand, no significant associations were observed between the urinary aMT6s level and other daytime and nighttime parameters in patients consuming a low salt diet (Table 4).
Table 4.
Multiple Regression Analyses for Urinary Melatonin Excretion Levels in Daytime or Nighttime during the Standard (A) or Low Salt Diet (B).
| A: Standard salt diet | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Daytime | Nighttime | ||||||||||
| R=0.55, p=0.072 | R=0.58, p=0.042 | R=0.57, p=0.041 | R=0.53, p=0.076 | ||||||||
| β | p | β | p | β | p | β | p | ||||
| Age year | -0.37 | 0.069 | -0.35 | 0.080 | -0.41 | 0.035 | -0.32 | 0.093 | |||
| Gender | 0.060 | 0.75 | 0.11 | 0.54 | 0.033 | 0.85 | 0.15 | 0.39 | |||
| Body mass index (kg/m2) | 0.088 | 0.67 | 0.038 | 0.85 | -0.048 | 0.81 | -0.046 | 0.81 | |||
| Daytime Log U-Alb/Cr (mg/gCr) | -0.44 | 0.020 | |||||||||
| Nighttime Log U-Alb/Cr (mg/gCr) | -0.43 | 0.018 | |||||||||
| Daytime Log U-Pro/Cr (mg/gCr) | -0.49 | 0.010 | |||||||||
| Nighttime Log U-Pro/Cr (mg/gCr) | -0.36 | 0.045 | |||||||||
| B: Low salt diet | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Daytime | Nighttime | ||||||||||
| R=0.33, p=0.61 | R=0.33, p=0.61 | R=0.50, p=0.11 | R=0.44, p=0.28 | ||||||||
| β | p | β | p | β | p | β | p | ||||
| Age year | -0.096 | 0.65 | -0.096 | 0.65 | -0.30 | 0.11 | -0.27 | 0.19 | |||
| Gender | -0.12 | 0.55 | -0.10 | 0.61 | 0.25 | 0.16 | 0.24 | 0.22 | |||
| Body mass index (kg/m2) | 0.18 | 0.41 | 0.18 | 0.39 | 0.21 | 0.27 | 0.21 | 0.31 | |||
| Daytime Log U-Alb/Cr (mg/gCr) | -0.20 | 0.32 | |||||||||
| Nighttime Log U-Alb/Cr (mg/gCr) | -0.22 | 0.23 | |||||||||
| Daytime Log U-Pro/Cr (mg/gCr) | -0.21 | 0.31 | |||||||||
| Nighttime Log U-Pro/Cr (mg/gCr) | -0.19 | 0.34 | |||||||||
U-Alb: urinary albumin, U-Pro: urinary protein
Discussion
This study demonstrated that the level of urinary aMT6s, a surrogate marker of melatonin secretion, was significantly and negatively associated with the urinary albumin and protein levels in patients consuming a standard salt diet, but not a low salt diet, and that the relationships in patients consuming a standard salt diet were maintained after adjustment for age, gender and BMI.
It is well-known that salt loading causes BP elevation according to the increase in the amount of sodium in the body, and that hypertension is a major risk factor for CVD, stroke, and end-stage renal disease (3-5,19,20). However, volume-independent mechanisms through which salt loading causes BP elevation have recently been identified. Dornas et al. reported that salt-dependent hypertension was accompanied by a decrease in renal superoxide dismutase activity, and that the metabolic changes and the hypertensive effects were reversed by a superoxide scavenger, Tempol, which potentially reduced ROS production (6). These data indicate that salt causes renal damage due to ROS activation.
Moreover, Zhang et al. used Sprague-Dawley rats that received a high-salt diet and bilateral hypothalamic paraventricular nucleus (PVN) microinjection with an analog of endogenous catalase (polyethylene glycol catalase), a catalase inhibitor (aminotriazole), or vehicle. They showed that high salt diet-fed rats had significantly increased renal sympathetic nerve activity, plasma norepinephrine, and NOX-2 and NOX-4 (subunits of NADPH oxidase) levels, and that the microinjection of polyethylene glycol catalase into the bilateral PVN restored the balance of neurotransmitters, while the microinjection of aminotriazole into the PVN augmented these changes in hypertensive rats. According to their findings, hydrogen peroxide component of ROS in the PVN regulating renal sympathetic nerve activity are partly due to modulate neurotransmitters within the PVN in salt-induced hypertension (8). Similarly, Ando et al. clarified that the overproduction of ROS in the brain increases the BP through central sympathoexcitation in salt-induced hypertension (9).
Melatonin not only regulates the biological rhythm, but also plays some pathophysiological roles (10-13,21-23). Muller et al. reported that mental stress increased skin sympathetic nerve activity (SSNA) and that SSNA was attenuated by melatonin ingestion in 12 healthy subjects (21). Ray et al. examined orthostatic stress in 12 healthy subjects and clarified that orthostatic stress augments muscle sympathetic nerve activity and that melatonin attenuates the muscle sympathetic nerve response to sympathoexcitatory stimuli (22). These data show that melatonin inhibits sympathetic activity induced by some stress.
The renal protective effect of anti-ROS activity is among the most studied mechanisms of melatonin. We previously made a 5/6 nephrectomized rat model of progressive CKD, divided the nephrectomized rats into untreated and melatonin-treated rats and compared them with control rats. We found that the untreated nephrectomized rats exhibited significantly higher oxidative stress activity, lower antioxidant activity, and increased markers of interstitial fibrosis in comparison to control rats, and that treatment with melatonin significantly ameliorated the abnormalities (23). In addition, when we investigated the relationships between melatonin secretion and some clinical parameters in 53 CKD patients, we found that the nocturnal melatonin concentrations decreased according to the progression of the CKD stage, and that impaired nighttime melatonin secretion was associated with nighttime urinary albumin excretion, a surrogate marker of renal damage in CKD patients (13). These data indicate that melatonin has renoprotective effects that are mediated by anti-ROS activity.
As described above, it is possible that salt loading increases the urinary albumin and protein levels by causing a decrease in melatonin secretion due to the activation of sympathetic activity and augmented ROS activity. Our schematic illustration of our hypothesized mechanism is shown in Fig. 2.
Figure 2.
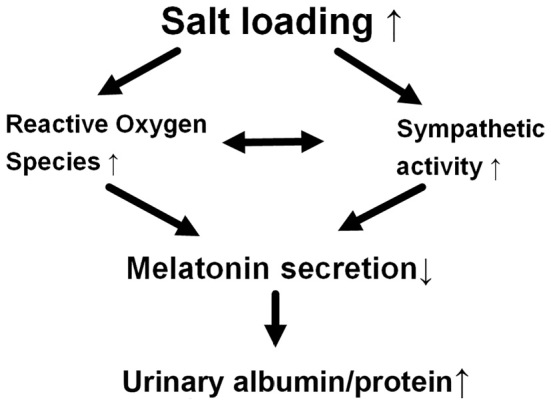
A schematic illustration of the hypothesized mechanism through which salt loading causes an increase in the albumin and protein levels of chronic kidney disease patients. This schematic illustration shows our hypothesized mechanism underlying the increase in urinary albumin and protein levels by salt loading through the decreased secretion of melatonin in patients with chronic kidney disease.
The present study was associated with some limitations. First, the sample size of our single center cohort was relatively small. However, the relationship between melatonin secretion and urinary albumin and protein levels in a standard salt diet was consistent, even after adjustment for age, gender and BMI. Second, no significant differences were found in the daytime or nighttime urinary aMT6s, BP, urinary albumin and protein excretion levels between the patients consuming standard salt diets and those consuming low salt diets. In addition, no significant correlations were found between the melatonin secretion levels and the renal function in patients consuming a standard salt or low salt diet. These findings may be related to the small differences between the standard salt diet (10 g/day) and low salt diet (6 g/day). Moreover, when the salt intake was calculated using urinary daily sodium excretion, the sodium intake was 7.53±1.91 g/day in patients consuming the standard salt diet and 5.28±1.34 g/day in patients consuming the low salt diet. Almost all patients consumed the full standard salt or low salt diet. However, pickled ume and Japanese pickle, which included large amounts of salt, were served, and some patients did not eat them. Moreover, the amount of daily salt was calculated under the assumption that all seasoning, including sauce, soy sauce, and dressing, was used. However, some patients did not use all of the seasonings due to their individual tastes. In addition, because some seasonings remained in the containers, it was impossible for the patient to consume all of the seasoning. This may be why the sodium intake levels, especially in the standard salt diet, were lower than expected. Thus, the fact that the difference in the salt intake of the standard salt and low salt diets was 2.25 g/day, not 4 g/day, may have been why no significant differences were found in some of the daytime and nighttime clinical parameters, including the urinary aMT6s level, between the patients consuming standard salt diets and those consuming low salt diets, and why no significant correlations were found between the melatonin secretion levels and the renal function in patients consuming a standard salt or low salt diet. Actually, our previous clinical study that recruited chronic kidney disease patients with a various degrees of renal function showed a significant positive relationship between melatonin secretion and the renal function (13). The present study also showed a non-significant association between the nighttime melatonin secretion and the nighttime eGFR (r=0.34, p=0.063, Table 3). Heavier salt loading is necessary to show the relationship between melatonin secretion and salt intake more clearly. However, heavier salt loading was not ethically permitted because of its association with renal damage, and we therefore considered it difficult to continue this study. However, even under these conditions, we could identify significant and negative relationships between the urinary aMT6s excretion level and the urinary albumin and protein excretion levels in patients consuming a standard salt diet.
Conclusion
Although the levels of melatonin secretion and urinary albumin and protein excretion were not significantly different between the standard salt and low salt diets in the present study, salt loading may increase urinary the albumin and protein levels via a decrease in melatonin secretion.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported by grants from the Salt Science Research Foundation (Awarded to Naro Ohashi, No. 1630).
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296-1305, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 42: 677-684, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Sacks FM, Svetkey LP, Vollmer WM, et al. ; DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 344: 3-10, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Swift PA, Markandu ND, Sagnella GA, He FJ, MacGregor GA. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: a randomized control trial. Hypertension 46: 308-312, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Slagman MC, Waanders F, Hemmelder MH, et al. ; Holland Nephrology Study Group Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ 26: d4366, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dornas WC, Cardoso LM, Silva M, et al. . Oxidative stress causes hypertension and activation of nuclear factor-κB after high-fructose and salt treatments. Sci Rep 7: 46051, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim YH, Hwang JH, Noh JR, et al. . Prevention of salt-induced renal injury by activation of NAD(P)H:quinone oxidoreductase 1, associated with NADPH oxidase. Free Radic Biol Med 52: 880-888, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Zhang M, Qin DN, Suo YP, et al. . Endogenous hydrogen peroxide in the hypothalamic paraventricular nucleus regulates neurohormonal excitation in high salt-induced hypertension. Toxicol Lett 15: 206-215, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Ando K. Increased salt sensitivity in obese hypertension: role of the sympathetic nervous system. Curr Hypertens Rev 2014(Epub ahead of print). [PubMed] [Google Scholar]
- 10. Russcher M, Koch B, Nagtegaal E, van der Putten K, ter Wee P, Gaillard C. The role of melatonin treatment in chronic kidney disease. Front Biosci 17: 2644-2656, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Kalra S, Agrawal S, Sahay M. The reno-pineal axis: a novel role for melatonin. Indian J Endocrinol Metab 16: 192-194, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simko F, Reiter RJ, Pechanova O, Paulis L. Experimental models of melatonin-deficient hypertension. Front Biosci 18: 616-625, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Ishigaki S, Ohashi N, Isobe S, et al. . Impaired endogenous nighttime melatonin secretion relates to intrarenal renin-angiotensin system activation and renal damage in patients with chronic kidney disease. Clin Exp Nephrol 20: 878-884, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Isobe S, Ohashi N, Fujikura T, et al. . Disturbed circadian rhythm of the intrarenal renin-angiotensin system: relevant to nocturnal hypertension and renal damage. Clin Exp Nephrol 19: 231-239, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Garfinkel D, Laudon M, Nof D, Zisapel N. Improvement of sleep quality in elderly people by controlled-release melatonin. Lancet 346: 541-544, 1995. [DOI] [PubMed] [Google Scholar]
- 16. Ritzenthaler T, Nighoghossian N, Berthiller J, et al. . Nocturnal urine melatonin and 6-sulphatoxymelatonin excretion at the acute stage of ischaemic stroke. J Pineal Res 46: 349-352, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Deacon SJ, Arendt J. Phase-shifts in melatonin, 6-sulphatoxymelatonin and alertness rhythms after treatment with moderately bright light at night. Clin Endocrinol 40: 413-420, 1994. [DOI] [PubMed] [Google Scholar]
- 18. Matsuo S, Imai E, Horio M, et al. . Revised equation for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992, 2009. [DOI] [PubMed] [Google Scholar]
- 19. Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 165: 923-928, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Bakris GL, Williams M, Dworkin L, et al. ; National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group Preserving renal function in adults with hypertension and diabetes: a consensus approach. Am J Kidney Dis 36: 646-661, 2000. [DOI] [PubMed] [Google Scholar]
- 21. Muller MD, Sauder CL, Ray CA. Melatonin attenuates the skin sympathetic nerve response to mental stress. Am J Physiol Heart Circ Physiol 305: H1382-H1386, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ray CA. Melatonin attenuates the sympathetic nerve responses to orthostatic stress in humans. J Physiol 15: 1043-1048, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishigaki S, Ohashi N, Matsuyama T, et al. . Melatonin ameliorates intrarenal renin-angiotensin system in a 5/6 nephrectomy rat model. Clin Exp Nephrol 22: 539-549, 2018. [DOI] [PubMed] [Google Scholar]