Abstract
Although multiple myeloma (MM) had been an incurable hematological malignancy with a poor prognosis, recent advances in novel anti-neoplastic agents, including carfilzomib (a proteasome inhibitor), have improved the prognosis. We herein report two cases of congestive heart failure in patients treated with carfilzomib. Although there are some reports on the cardiotoxicity of carfilzomib, to our knowledge, this is the first report on the cardiac involvement of carfilzomib in Japanese MM patients. We review the critical points from our two cases, with the aim of avoiding carfilzomib-associated heart failure in MM patients.
Keywords: heart failure, carfilzomib, multiple myeloma, cardiotoxity
Introduction
Multiple myeloma (MM) is a plasma cell neoplasm with symptoms such as bone fracture, hypercalcemia, renal insufficiency, and anemia. Although MM is an incurable hematological malignancy with a poor prognosis, recent advancements in novel anti-neoplastic agents have resulted in an improved prognosis, increasing the median overall survival in MM patients to 6.1 years in 2006-2010 from 4.6 years in 2001-2005 (1).
Carfilzomib (CFZ) is a selective and irreversible proteasome inhibitor, used for the treatment of patients with relapsed or refractory MM (RRMM). In a phase III trial in patients with RRMM, CFZ resulted in a better prognosis in RRMM patients in comparison to bortezomib treatment (median progression-free survival: CFZ, 18.7 months; bortezomib, 9.4 months). The major adverse effects associated with CFZ were diarrhea (42.3%), fatigue (32.9%), cough (28.8%), and pyrexia (28.6%), and there were limited reports (6.4%) of heart failure (2). We herein report two cases of MM in Japanese individuals who developed new-onset heart failure after the administration of CFZ.
Case Reports
Case 1
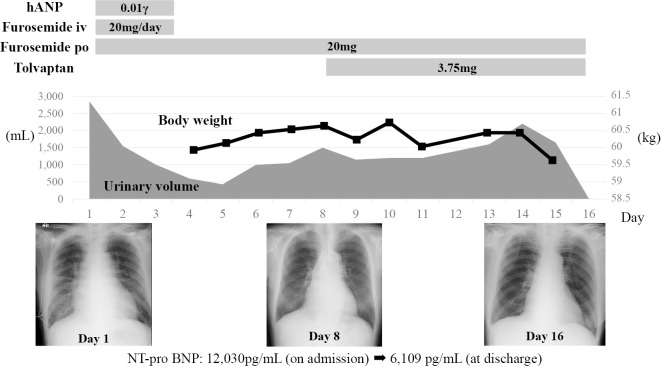
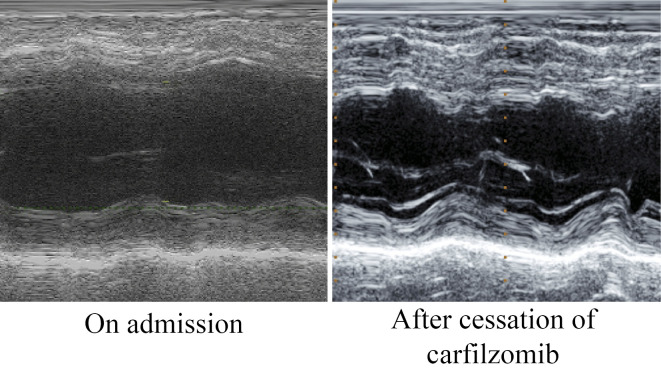
The patient was a 76-year-old man who had history of hypertension and who had been diagnosed with MM 12-years previously. At age of 65, he underwent peripheral blood stem cell transplantation (PBSCT) after 3 courses of vincristine-doxorubicin (adriamycin)-dexamethasone (VAD) therapy (total amount of doxorubicin: 216 mg). However, his disease relapsed, and he finally received more than 20 chemotherapy cycles. He was then treated with a new KRd regimen (CFZ, lenalidomide and dexamethasone). The patient presented an impaired renal function (creatinine clearance, 27 mL/min), hypoalbuminemia (albumin, 3.2 g/dL), increased urine M protein level (806 mg/day) and his serum calcium level was within the normal range (total calcium, 8.2 mg/dL). Twelve units of red blood cells (RBCs) had been transfused in three months before the initiation of KRd therapy due to anemia (RBC, 276×104/μL; hemoglobin, 8.6 g/dL; ferritin, 1,272 ng/mL). At 3 months after the initiation KRd therapy, he visited the emergency room of our hospital complaining of dyspnea and orthopnea. His blood pressure was 210/106 mmHg, his pulse rate was 143 bpm, and oxygen saturation was 96% (with 10 L of oxygen inhalation). He presented with bilateral coarse crackles in his chest and pretibial pitting edema. Chest radiography revealed cardiomegaly, congestion of the pulmonary arteries, and bilateral pleural effusion. Echocardiography revealed diffuse left ventricular wall hypokinesis, a reduced left ventricle ejection fraction [LVEF, 39% (by Teichholtz)], dilatation of the left ventricular end diastolic diameter (LVDd, 64.9 mm) and a normal left ventricular wall thickness (interventricular septal thickness, 11.0 mm; posterior wall thickness, 9 mm). In addition, moderate mitral valve regurgitation (MR) was observed. The patient's serum N-terminal pro brain natriuretic peptide (NT-proBNP) level was 12,030 pg/mL (normal <125 pg/mL). Based on these findings, he was diagnosed with congestive heart failure and treatment was initiated. Although the patient had undergone the VAD therapy and RBC transfusion, we did not consider the cumulative amounts to be problematic. We therefore considered the possibility that the patient's cardiac manifestations were associated with CFZ and discontinued the KRd therapy and initiated treatment with diuretics. Within 16 days, the symptoms of heart failure disappeared completely, and improvement was observed on chest radiography [cardiothoracic ratio (CTR), 58% to 45.3%] and echocardiography [LVEF, 39% to 56% (by Teichholtz); LVDd, 64.9 mm to 50.4 mm; MR, moderate to trivial] findings (Fig. 1, 2) and in the NT-proBNP levels (12,030 pg/mL to 6,109 pg/mL at discharge and 1,250 pg/mL at 3 months after discharge). Hence, we concluded that the patient's cardiac manifestations were associated with the administration of CFZ.
Figure 1.
The clinical course of Case 1. A 76-year-old man, who was treated with KRd therapy for 3 months, visited the emergency room of our hospital complaining of dyspnea and orthopnea. He was diagnosed with congestive heart failure and treatment for heart failure was initiated.
Figure 2.
The change in the echocardiography findings of Case 1. Representative M-mode echocardiographic images of Case 1. Echocardiography showed significant improvement in the cardiac function after the cessation of CFZ.
Case 2
The patient was a 75-year-old woman who had been diagnosed with MM 6 years previously, and who had received over 20 chemotherapy cycles. She has not received either VAD therapy or undergone PBSCT. KRd therapy was initiated due to refractory MM.
She had no history of heart disease and echocardiography showed a normal LV function [LVEF, 58% (by Teichholtz)]. At KRd treatment, she showed a preserved renal function (creatinine clearance, 64 mL/min), hypoalbuminemia (albumin, 3.1 g/dL) and normal serum calcium level (total calcium, 8.2 mg/dL). Sixteen units of RBCs had been transfused in the 16 months before the initiation of KRd therapy due to anemia (RBC, 256×104/μL; Hb, 8.4 g/dL; and ferritin, 676 ng/mL).
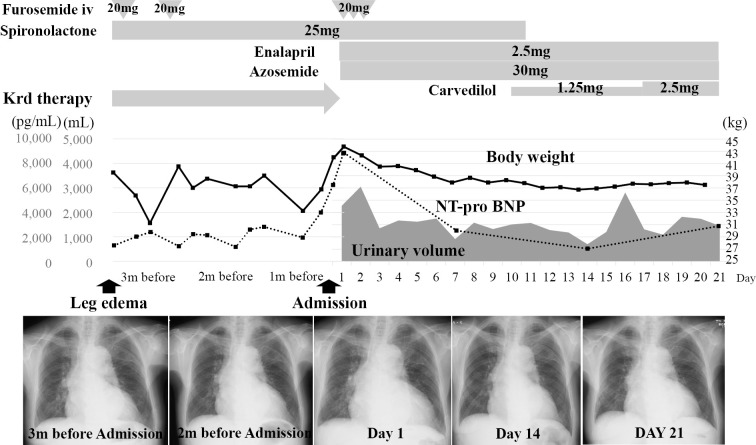
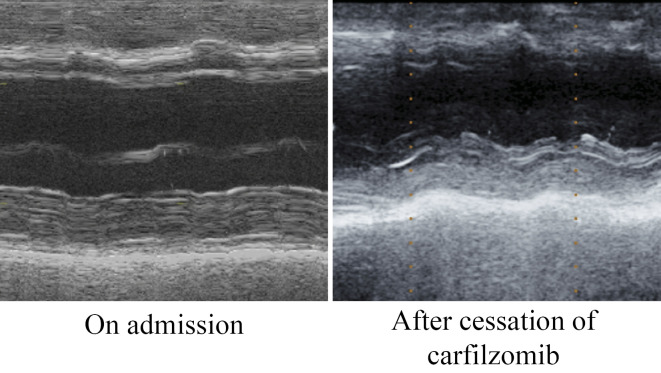
At 5 months after the initiation of KRd therapy, she presented with lower limb edema. Although a cardiotoxic effect of CFZ was suspected, KRd therapy was continued, along with the administration of diuretics, as KRd therapy was found to effective (M protein improved from 787 mg/day to 110 mg/day) in the treatment of MM. One month later, she complained of dyspnea on exertion and presented with exacerbated lower limb edema and coarse crackles in the bilateral chest. Chest radiography showed cardiomegaly, congestion of the pulmonary arteries and bilateral pleural effusion. The patient's NT-proBNP level increased to 8,812 pg/mL, and echocardiography revealed diffuse left ventricular wall hypokinesis, a reduced left ventricle ejection fraction [LVEF, 35% (by Teichholtz)], dilatation of the left ventricular end diastolic diameter (LVDd, 57.2 mm) and a normal left ventricular wall thickness (interventricular septal thickness, 9.7 mm; posterior wall thickness, 10.9 mm). In addition, moderate MR due to tethering was observed. Neither coronary angiography nor cardiac biopsy revealed any specific findings. Since Congo-red staining of a cardiac biopsy specimen was negative and echocardiography revealed no specific findings, we ruled out the possibility of cardiac amyloidosis. Thus, we considered the possibility that the patient's cardiac manifestations were associated with the administration of CFZ and discontinued the KRd therapy, while intensifying the diuretic treatment. Although her heart failure-related symptoms disappeared within 10 days and the left ventricular dilatation and MR were restored (LVDd, 57.2 mm to 46.1 mm; MR, moderate to trivial), the cardiac function was not restored [LVEF, 35% to 36% (by Teichholtz)] after the cessation of CFZ (Fig. 3, 4). Thus, we considered that the prolonged administration of CFZ after the onset of the patient's cardiac manifestations disturbed the recovery from cardiac injury in this case.
Figure 3.
The clinical course of Case 2. A 75-year-old woman, who was treated with KRd therapy due to relapsed MM, presented with symptoms related to heart failure at 5 months after the initiation of KRd therapy. As we considered the possibility that the patient’s cardiac manifestations were associated with CFZ, KRd therapy was discontinued with the intensification of diuretic treatment.
Figure 4.
Changes in the echocardiography findings in Case 2. Representative M-mode echocardiographic images of Case 2. Although the patient’s symptoms of heart failure disappeared within 10 days after cessation of CFZ treatment, the cardiac function, as measured by echocardiography, was not restored.
Discussion
CFZ is a selective proteasome inhibitor that leads tumor cells to apoptosis due to an increase in protein misfolding. It is approved for use, in combination with lenalidomide and dexamethasone in patients with RRMM. Despite the preferable effects of CFZ in the treatment of RRMM, CFZ has been reported to have significant cardiotoxic effects because to maintain functional protein homeostasis, cardiac cells require the highly efficient degradation of terminally misfolded proteins, proteasome inhibition by CFZ can promote cardiac dysfunction through the increase in misfolded-proteins (3,4). Post-marketing surveillance suggested that the incidence of heart failure was approximately 7% in the patients who were treated with CFZ (5), and Chari et al. reported that the cardiotoxic effects of CFZ predominantly occurred within 2 cycles of CFZ-treatment in patients who with a history of cardiovascular disease (6).
Because of multiple comorbidities, including age-related cardiovascular risk factors, chronic anemia, amyloidosis, arteriovenous shunt with bony lesions, hyperviscosity, and possible prior anthracycline exposure, MM patients are at high risk of heart failure. In addition, the excessive body fluid volume caused by hydration before and after the administration of anti-cancer drugs (as an adjunct therapy) may increase the risk of heart failure in MM patients (5,6). Thus, physicians should pay attention to the risk of cardiotoxicity in MM patients before initiating CFZ treatment.
We reported two cases of Japanese patients who experienced cardiotoxic effects in association with the administration of CFZ, and who showed different courses of improvement in their cardiac function after the cessation of CFZ treatment. There have been few reports on factors predicting cardiotoxicity in patients treated with CFZ. Uncontrollable hypertension and the concomitant use of immunomodulators have been reported to be associated with the cardiotoxicity of CFZ (7,8). In addition, although it remains controversial, the elevation of serum BNP (NT-proBNP) after CFZ therapy may help to predict the development of heart failure (9,10). Similarly, although it is currently unclear, cardiac Troponins (I and T) may be biomarkers that predict cardiotoxicity in CFZ-treated patients (11). To the best of our knowledge, no reports have investigated the recovery from CFZ-induced cardiotoxicity after the cessation of CFZ. However, our cases may simply suggest that the improvement in the cardiac function is dependent on the duration of CFZ exposure, as evidenced in Case 2, in which CFZ treatment was continued even after the onset of the cardiac manifestations and no significant improvement was observed. As written clearly in the drug information, the cessation of CFZ is recommended, even when it is deemed to be effective in patients with relapsed MM.
Conclusion
We reported 2 cases in which patients developed cardiac symptoms in association with the administration of CFZ. Before the introduction of CFZ, physicians should pay attention to potential cardiac risk factors in patients with MM. In addition, the prompt cessation of CFZ is recommended when cardiac symptoms appear.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 28: 1122-1128, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stewart AK. Carfilzomib for the treatment of patients with relapsed and/or refractory multiple myeloma. Future Oncol 11: 2121-2136, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Grandin EW, Ky B, Cornell RF, Carver J, Lenihan DJ. Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. J Card Fail 21: 138-144, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Herrmann J, Wohlert C, Saguner AM, et al. Primary proteasome inhibition results in cardiac dysfunction. Eur J Heart Fail 15: 614-623, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siegel D, Martin T, Nooka A, et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica 98: 1753-1761, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chari A, Hajje D. Case series discussion of cardiac and vascular events following carfilzomib treatment: possible mechanism, screening, and monitoring. BMC Cancer 14: 915, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah C, Bishnoi R, Jain A, et al. Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leuk Lymphoma 59: 2257-2569, 2018. [DOI] [PubMed] [Google Scholar]
- 8. Milan A, Bruno G, Maffei I, et al. Arterial hypertension and multiple myeloma: physiopathology and cardiovascular risk and 'practical' indications in patients receiving carfilzomib. Curr Hypertens Rev, 2018(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 9. Atrash S, Tullos A, Panozzo S, et al. Cardiac complications in relapsed and refractory multiple myeloma patients treated with carfilzomib. Blood Cancer J 5: e272, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenthal A, Luthi J, Belohlavek M, et al. Carfilzomib and the cardiorenal system in myeloma: an endothelial effect? Blood Cancer J 6: e384, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 35: 893-911, 2017. [DOI] [PubMed] [Google Scholar]