Abstract
A 35-year-old man with refractory Crohn's disease showed a loss of response to infliximab after requiring treatment with infliximab at 10 mg/kg together with steroid to maintain remission. His symptoms recurred, and colonoscopy showed extensive active ulcers in the colon. Adrenomedullin therapy was started in addition to the conventional infliximab therapy. A few days after, his symptoms went into remission. Endoscopy at 2 and 7 weeks revealed significant mucosal remission without steroid therapy. Adrenomedullin promoted mucosal healing and led to the re-induction of remission in Crohn's disease in a patient with a loss of response to infliximab.
Keywords: adrenomedullin, Crohn's disease, inflammatory bowel disease, infliximab, mucosal healing, loss of response
Introduction
Adrenomedullin (AM) is a biologically active peptide that comprises 52 amino acids and was first isolated from human pheochromocytoma tissue (1). It is expressed ubiquitously in all body tissues and has a potent vasodilating effect. Its expression is enhanced by tissue ischemia or hypoxia, and it is involved in blood and lymph vessel hemostasis. AM is known to exert a wide range of physiologic effects (2), including antiinflammation, mucosal restoration, and organ protection. We have attempted translational research on inflammatory bowel disease (IBD) treatment, with s focus on these physiological actions (3-5). Based on the results of this basic research, we recently conducted a preliminary clinical trial on ulcerative colitis (UC) patients who were unresponsive to conventional treatments and showed that AM had a superior treatment effect for active UC and promoted mucosal healing of the colon (6). However, the therapeutic effect of AM in patients with Crohn's disease (CD) is still unknown.
In this report, we treated a CD patient with AM, making this, to our knowledge, the first report of its use as a treatment for CD.
Case Report
A 35-year-old man was first diagnosed with colonic CD at 19 years of age and developed steroid dependency. He did not have perianal fistula or abscess. At 27 years of age, bloody diarrhea with deep ulcers in the sigmoid colon led to the initiation of infliximab (IFX) at 5 mg/kg, every 8 weeks, which induced remission. However, he developed a loss of response to IFX one year later. The IFX administration period was therefore shortened, and subsequently, the IFX dose was increased to 10 mg/kg every 8 weeks. Nevertheless, since remission was not maintained, combination steroid therapy became necessary from the fourth week of IFX administration. Because he refused immunosuppressive therapy, IFX was administered as monotherapy.
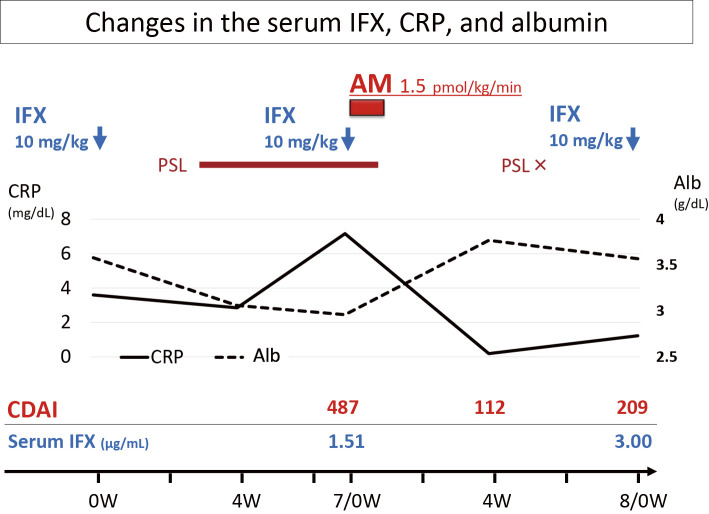
In March 2016, at 35 years of age, he developed diarrhea, bloody stool, abdominal pain, and a deteriorating general condition; at this time the Crohn's Disease Activity Index (CDAI) score increased to 487. Elevated serum C-reactive protein (CRP) and hypoalbuminemia were observed, and the serum IFX trough levels decreased to 1.5 μg/mL. On endoscopy, active longitudinal ulcers were extensively seen in the transverse and sigmoid colon (Fig. 1a and b). The addition of immunomodulators (IM) or switching from IFX to adalimumab was therefore considered. However, the patient instead chose to participate in our clinical study on AM therapy for patients with CD (UMIN000021421).
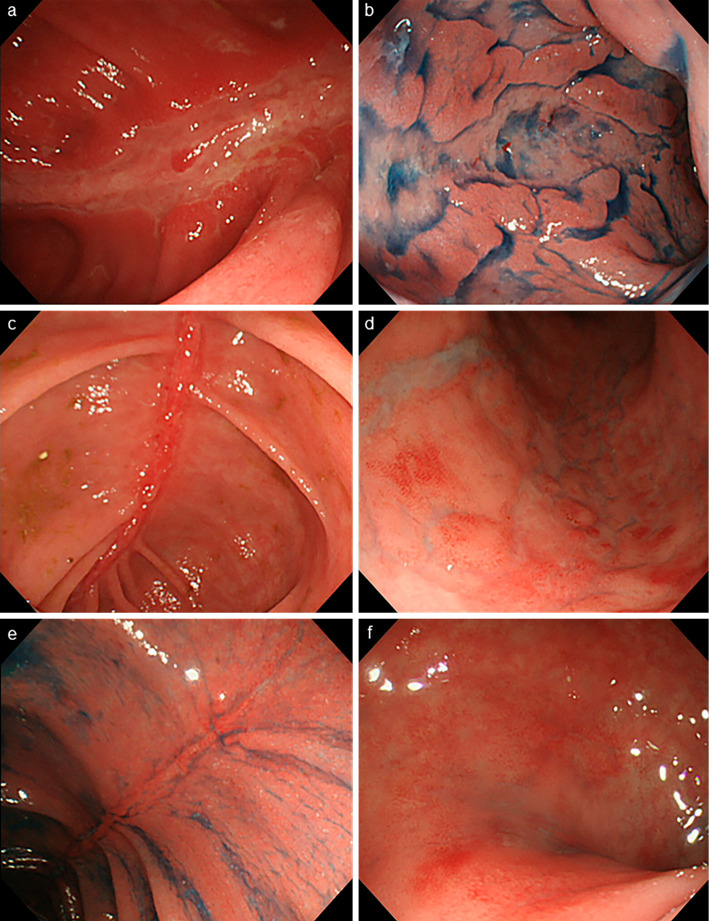
Figure 1.
Colonoscopy findings. Extensive longitudinal ulcers are observed in the transverse colon (a) and sigmoid colon (b) before AM therapy. Two weeks after AM therapy, significant mucosal regeneration is observed at the base of the ulcers in the transverse colon (c), and there is resolution of the ulcers in the sigmoid colon (d). Seven weeks after treatment with AM, the mucosa of the transverse colon (e) and sigmoid colon (f) remain in remission without steroid therapy. AM: adrenomedullin
Continuous infusion of AM at 1.5 pmol/kg/min for 8 h/day on days 1-7 in combination with IFX at 10 mg/kg on day 1 was used. The patient tolerated this treatment well without adverse events, such as reduced blood pressure or headache, during AM administration. Improvement in bloody stools, diarrhea, and abdominal pain was seen following the administration of IFX and AM. Colonoscopy two weeks later revealed that the transverse and sigmoid colon ulcers were healing and were covered with regenerating epithelium (Fig. 1c and d).
Four weeks after the administration of IFX with AM, he remained free from abdominal symptoms, and remission was maintained without the use of steroids. The serum CRP level became negative, and his hypoalbuminemia improved (Fig. 2). Mucosal healing was maintained, as seen during endoscopic evaluation at week 7 (Fig. 1e and f), suggesting that AM potentiated the effect of IFX. The serum IFX trough levels increased to 3.0 μg/mL, which was twice the level before the addition of AM. His condition was maintained using IFX monotherapy for over one year after the administration of AM.
Figure 2.
Clinical course of the patient. Remarkable improvement of the CDAI and an increase in the IFX trough level are seen after AM administration. PSL was discontinued after AM administration. Alb: albumin, AM: adrenomedullin, CDAI: Crohn’s disease activity index, CRP: C-reactive protein, IFX: infliximab, PSL: prednisolone, W: weeks
Discussion
In Japan today, 40-60% of CD patients are reportedly given biologics, which have come to play a central role in the treatment of CD. However, there are patients who experience a loss of effect during biologic maintenance therapy, which is generally dealt with by increasing the dose or switching to other biologics. Despite this, a fair number of patients do not obtain sustained relief and face difficulties in treatment.
In an experimental animal model, AM has been shown to be effective in improving enteritis, the mechanisms for which have been reported to be suppression of inflammatory cytokines (4,7), maintenance of epithelial intracellular junctions (4), improved microvascular flow (8), and facilitation of mucosal healing, as seen on wound healing assays (9). Our patient, who was unresponsive to treatment and had a loss of response to IFX, showed improvement of abdominal symptoms and significant mucosal healing after the addition of AM therapy, such that the use of steroids was unnecessary.
Along with mucosal healing, the patient's markedly reduced serum IFX trough level increased to nearly twice the lowest level. The factors reported to affect serum IFX trough levels include leakage from mucosal lesions into feces (10) and the appearance of antibodies to IFX (ATI) (11). In recent years, reports on patients who have lost their response to IFX showed that the serum IFX trough levels increased again with the additional administration of IM, the mechanism for which was presumed to be suppression of ATI production by IM (12,13). However, it seems unlikely that AM would inhibit ATI production. In the present patient, the serum CRP level decreased while that of serum albumin increased with the induction of remission, suggesting that IFX consumption may have been decreased by the inhibition of CD activity and that leakage of IFX to the intestine may have decreased with mucosal healing.
AM has been reported to enhance tumor progression and metastasis in various organs, including the gastrointestinal tract (14). IBD patients are prone to develop gastrointestinal tumors due to chronic inflammation. Although AM is not a carcinogenic substance, it does promote tumor growth; therefore, careful endoscopic surveillance for tumors is crucial. In addition, we excluded patients with a history of tumor, even if it had been cured. Nevertheless, we presumed that the suppression of inflammation and an improved degree of mucosal healing due to the administration of AM could help prevent tumorigenesis in the future (15).
AM was able to revive the response to IFX or prevent the negative cycle of loss of response to IFX by increasing the serum IFX level and maintaining mucosal healing. AM is a promising novel therapeutic agent for both UC and CD. The effective period of AM remains unclear at present, so we are currently investigating the long-term prognosis with AM in an ongoing clinical trial on CD. If a sufficiently long effective period is detected, e.g. six months or longer, AM may be used as an agent for remission maintenance.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This research was supported by the Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development (AMED, Grant Number JP17ek0109089) and by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number JP16K09316, Japan).
References
- 1. Kitamura K, kangawa K, Kawamoto M, et al. . Adrenomedullin a novel hypotensive peptide isolated from human pheochromacytoma. Biochem Biophys Res Commun 192: 553-560, 1993. [DOI] [PubMed] [Google Scholar]
- 2. Cheung BM, Tang F. Adrenomedullin: exciting new horizons. Recent Pat Endocr Metab Immune Drug Discov 6: 4-17, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Ashizuka S, Ishikawa N, Kato J, et al. . Effect of adrenomedullin administration on acetic acid-induced colitis in rats. Peptides 26: 2610-2615, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Ashizuka S, Inagaki-Ohara K, Kuwasako K, et al. . Adrenomedullin treatment reduces intestinal inflammation and maintains epithelial barrier function in mice administered dextran sulphate sodium. Microbiol Immunol 53: 573-581, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Ashizuka S, Inatsu H, Inagaki-Ohara K, et al. . Adrenomedullin as a potential therapeutic agent for inflammatory bowel disease. Curr Protein Pept Sci 14: 246-255, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Ashizuka S, Inatsu H, Kita T, et al. . Adrenomedullin therapy in patients with refractory ulcerative colitis: a case series. Dig Dis Sci 61: 872-880, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzales-Rey E, Fernandez-Martin A, Chorny A, Delgado M. Therapeutic effect of urocortin and adrenomedullin in a murine model of Crohn's disease. Gut 55: 824-832, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Talero E, Alvarez de, Sotomayor M, et al. . Vascular contribution of adrenomedullin to microcirculatory improvement in experimental colitis. Eur J Pharmacol 670: 601-607, 2011. [DOI] [PubMed] [Google Scholar]
- 9. Hayashi Y, Narumi K, Tsuji S, et al. . Impact of adrenomedullin on dextran sulfate sodium-induced inflammatory colitis in mice: insights from in vitro and in vivo experimental studies. Int J Colorectal Dis 26: 1453-1462, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Brandse JF, van den, Brink GR, Wildenberg ME, et al. . Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 149: 350-355, 2015. [DOI] [PubMed] [Google Scholar]
- 11. Vande Casteele N, Khanna R, Levesque BG, et al. . The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn's disease. Gut 64: 1539-1545, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ben-Horin S, Waterman M, Kopylov U, et al. . Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 11: 444-447, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Strik AS, van den Brink GR, Ponsioen C, et al. . Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther 45: 1128-1134, 2017. [DOI] [PubMed] [Google Scholar]
- 14. Nouguerède E, Berenguer C, Garcia S, et al. . Expression of adrenomedullin in human colorectal tumors and its role in cell growth and invasion in vitro and in xenograft growth in vivo. Cancer Med 2: 196-207, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Ochoa-Callejero L, García-Sanmartín J, Martínez-Herrero S, et al. . Small molecules related to adrenomedullin reduce tumor burden in a mouse model of colitis-associated colon cancer. Sci Rep 7: 17488, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]