Abstract
Objective
Sound hypersensitivity is highly comorbid with migraine headaches. To elucidate the pathogenic mechanism of migraine attacks, we must first identify the types of everyday environmental sounds they perceive as unpleasant and clarify the acoustic properties of such sounds. This study aimed to clarify the true nature of “noise,” i.e. everyday sounds perceived as unpleasant by migraineurs, by evaluating their subjective comfort/discomfort in response to several sounds commonly heard in everyday life.
Methods
Participants were presented with 20 environmental sounds they would likely hear daily. Subjects rated the pleasantness/unpleasantness of each stimulus using a nine-step scale.
Patients
We recruited 50 adults with migraine headaches (46 women, 4 men) and 50 healthy controls (35 women, 15 men).
Results
Migraineurs provided statistically significantly lower (more unpleasant) ratings to ambulance sirens, police car sirens, and railroad crossing bells than did controls. Our analysis also investigated the acoustic characteristics associated with higher rating gaps between the two groups. Greater divergence in ratings for the same stimulus was associated with less power (smaller amplitude envelope) and slower temporal variation in signals in the 400-Hz band.
Conclusion
We identified specific signal components associated with different subjective (un)pleasantness scores between migraineurs and healthy adults, which may lead to the elucidation of the pathogenic mechanism underlying migraine attacks triggered by sound.
Keywords: migraine, noise, frequency components, sound hypersensitivity
Introduction
Migraines are a condition characterized by pulsing, throbbing headaches, usually affecting one half of the head, occurring spontaneously during events termed “attacks.” They are the world's most common neurological disorder, and even in Japan, where the prevalence is relatively low by global standards, migraine affects as many as 8.4% of people (1). Hypersensitivity to lights, smells, and sounds is known to accompany the attacks (2-15). Sound hypersensitivity reportedly affects 70-80% of migraine patients (8) and can range from a condition termed hyperacusis (increased sensitivity to certain frequencies and volume ranges) to phonophobia (an anxiety disorder considered an extreme form of hyperacusis). Furthermore, white noise has been used in experimental settings to trigger migraine attacks (14,15). This association suggests that understanding the true nature of sound hypersensitivity is an essential part of explaining the pathogenic mechanism underlying migraine attacks.
Noise has often been conceptualized in questionnaire-based studies and other investigational research as sounds heard in everyday life that migraine patients prefer to avoid (9) or that trigger attacks (10-13). Ashkenazi et al. empirically determined sound aversion thresholds (more than 90 dB), the intensity at which listeners experienced hearing discomfort in response to artificial, tone-burst (pure-tone) stimuli, as a noise level (16,17).
However, what constitutes environmental noise for migraine patients remains unexamined. In addition, very rarely do we encounter tone-burst stimuli exceeding 90 dB in daily life. To identify environmental noise that trigger hearing discomfort in migraineurs, experiments involving sounds frequently heard in daily life should be conducted. Our research team attempted to determine which sounds migraineurs perceive as comfortable versus uncomfortable using a wide variety of sounds encountered in daily life. However, our sample size was small, and we did not identify which types of sounds were associated with different comfort/discomfort ratings, nor did we look at the acoustic properties of sounds associated with high discomfort scores (18).
The goal of this study was to shed light on what really constitutes unpleasant ‘noise’ in the daily lives of migraine patients. Specifically, we aimed to assess how pleasant or unpleasant they regarded a variety of sounds ubiquitous in everyday settings. After identifying which sounds they regarded as unpleasant, we aimed to clarify the acoustic characteristics of these sounds. Our goal is to improve the understanding of the pathogenic mechanism underlying migraine attacks.
Materials and Methods
Participants
This study was approved by the respective ethics committees of Utsunomiya University and Dokkyo Medical University. Informed consent was obtained from all participants.
Migraines were diagnosed using a medical interview comprising 19 questions based on the International Classification of Headache Disorders 3 beta version (19). We selected 50 individuals diagnosed with migraines (46 women, 4 men; age: 24.5±5.8 years; without aura, n=34; with aura, n=16) to participate in the experiment. Migraines are reportedly more common in women than in men in several countries (20-23), which is consistent with our female-biased ratio. During interviews, we identified sound hypersensitivity in 58% (29/50) of migraineurs, affecting those with and without auras. Fifty healthy adults with no personal history of migraines or chronic pain and no family history of migraines were also enrolled as a control group (35 women, 15 men; age: 32.1±3.6 years).
Sound stimuli
We selected 20 types of environmental sounds likely to be heard daily as auditory stimuli for presentation. Table presents the stimulus descriptions and names. Stimuli were prepared by extracting the 15 seconds that best captured the representative characteristics of the sound in question from a relevant track. These sounds can be grouped into four categories based on Shafer's proposed concept of ‘soundscapes’ (24): animal sounds, natural sounds, emotional sounds, and excessive noise/sirens (Fig. 2). The experiments took place in relatively quiet settings, such as conference rooms at Dokkyo Medical University, Utsunomiya University, and the Institute of Technologists. All sounds were stored in a digital audio player (Kana RS GH-KANARS-8GK; Green House, Tokyo, Japan) and presented to subjects using headphones (MDR-CD900ST; Sony, Tokyo, Japan) via a headphone amplifier (HA400; Behringer, Willich, Germany). The intensity of two types of sound stimuli for each environmental sound were 61±5.8 dB and 71±5.8 dB in simple measurement. These sound pressure levels (SPLs) were chosen to model the relatively loud volumes of some of the sounds likely heard in daily life.
Table.
Sound Stimuli Presented to Participants.
| Stimulus No. | Name | Description | ||
|---|---|---|---|---|
| 1 | Cat | A cat mewing | ||
| 2 | Sparrows | 2–3 sparrows chirping | ||
| 3 | Frogs | Frogs croaking | ||
| 4 | Evening cicadas | Evening cicadas singing | ||
| 5 | Children | Children chatting while at play | ||
| 6 | Dogs | Dogs barking | ||
| 7 | Rain | Falling rain, somewhat heavy | ||
| 8 | Babbling stream | Running water, as down a stream | ||
| 9 | Waves | Waves crashing | ||
| 10 | Church bells | Church bells, continuously peeling in a cavernous, resonant church | ||
| 11 | Wind chimes | Glass wind chimes tinkling | ||
| 12 | Fireworks | Fireworks being launched | ||
| 13 | Destruction | Explosive destruction, as of a building | ||
| 14 | Automobile | Automobile engine turning on, car starting to move | ||
| 15 | Car horn | A car horn | ||
| 16 | Crossing bell | Warning bells seen at railroad crossings in Japan | ||
| 17 | Construction work | Stakes being continuously pounded into the ground, as at a building site | ||
| 18 | Ambulance | An ambulance siren | ||
| 19 | Pachinko parlor | Ambient noise inside a pachinko parlor | ||
| 20 | Police car | Warning siren of a Japanese police car |
Figure 1.
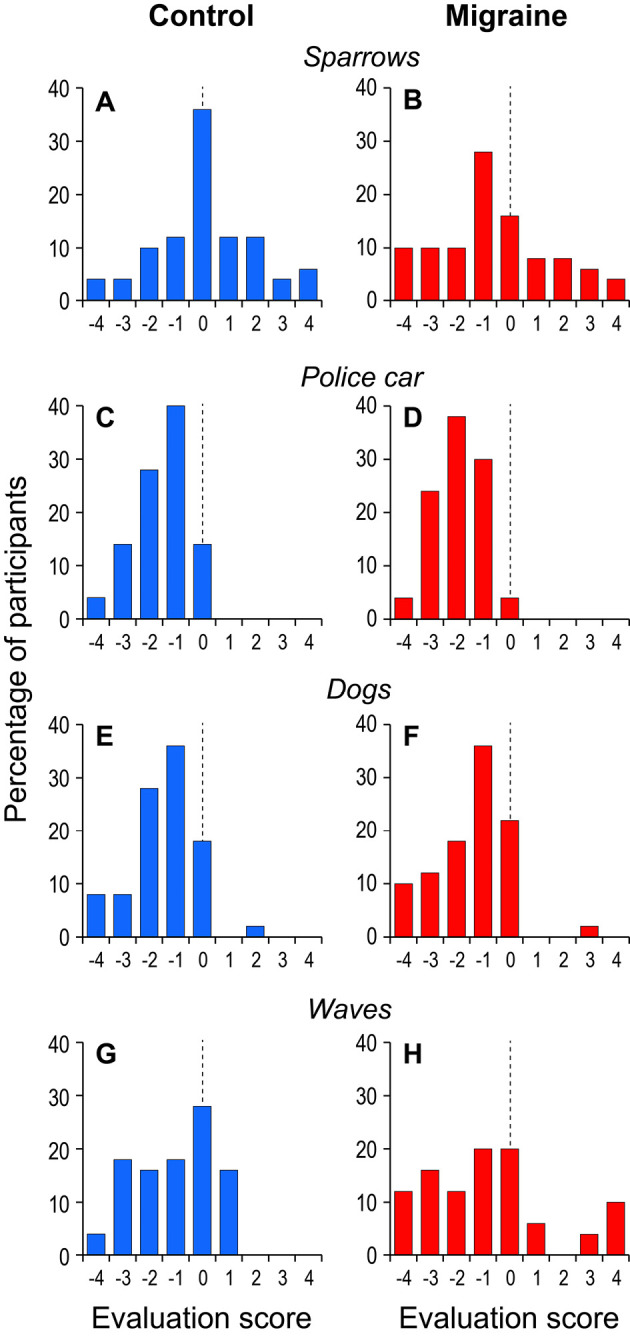
Representative histograms of ratings of different sounds provided by migraineurs (right side) and healthy controls (left side). A, B: Sparrows, C, D: Police car, E, F: Dogs, G, H: Waves
Figure 2.
Ratings for all sound stimuli for migraineurs and controls. The bold horizontal line in the box indicates the median of the data. The upper and lower ends of the box show the 25th and 75th percentile points of the data, respectively. Whiskers (or vertical lines) represent the 10th and 90th percentile points of the data.
Experimental procedure
Participants first listened to an explanation of the experiment. Subsequently, they donned the headphones, closed their eyes, and were presented with a single sound stimulus. Participants then opened their eyes and were allowed 15 seconds to rate how pleasant or unpleasant they perceived the sound to be. We employed a nine-step rating scale as follows: “extremely uncomfortable (-4),” “very uncomfortable (-3),” “uncomfortable (-2),” “slightly uncomfortable (-1),” “neither comfortable nor uncomfortable (0),” “slightly comfortable (1),” “comfortable (2),” “very comfortable (3),” “extremely comfortable (4).” Participants marked the score that best described their feelings toward the sound presented in each trial during the evaluation step. Each participant completed 40 trials. To mitigate fatigue, the session was divided into two 20-trial halves, with a 5-minute rest period in between. Stimuli were presented in three randomized orders, with each pattern used for one third of the study population.
SPL correction and data analyses
Values on the nine-step rating scale were used as the raw data for analyses. Increasing the presentation intensity would heighten the discomfort experienced in response to the same sound. Therefore, it is necessary to precisely calibrate the SPL of presented stimuli when comparing a variety of different sounds. To calibrate the SPL of each sound stimulus, we created a sound-hearing environment using a dummy head (25) and performed measurements also considering the frequency-transfer function of a representative middle-ear model (26). Finally, linear prediction was used to estimate the ratings of sounds presented at 70 dB based on the 2 corrected SPLs and the ratings of each stimulus.
Results
Distribution of evaluation scores for participants with and without migraine
Fig. 1 shows some representative histograms of ratings for selected sounds for the control and migraine groups. For sparrow calls, the most common rating was 0 in the control group, but a lower value of -1 was observed in the migraine group. Similarly, the most common rating for police car in the migraine group (-2) was lower than that in the control group (-1). These trends indicate that a greater percentage of migraine patients than of healthy controls were distressed by these sounds.
In contrast, both groups frequently rated dogs as -1. Migraineurs responded differently than controls to waves, most often rating them -1 or 0 (control mode: 0). Therefore, the histogram distributions may have different modes but similar profiles depending on the sound presented.
Fig. 2 shows the distributions of rating scores in the form of box-and-whisker plots for each of the 20 sounds for the control and migraine groups. Our analysis first focused on differences in ratings attributable to sound type rather than to cohort. Participants provided a median rating of -1 or lower to all sounds considered to be Excessive noise/sirens, suggesting that stimuli in this category caused greater discomfort than did sounds in the other categories. Conversely, Emotional sounds tended to be rated more highly than sounds in other categories, with the 75th percentile reaching 2 in some cases.
Next, we focused on which specific sounds were associated with differences in ratings between the control and migraine groups. Migraine patients provided a lower median rating than controls to approximately half of the sound types in Fig. 2 (i.e. cat calls, sparrow calls, evening cicada calls, wind chimes, and fireworks). In addition, there appeared to be no relationship between the differences in the median ratings of migraineurs versus controls or in how individuals evaluated the sound itself across both groups. For example, a rating gap was apparent for both wind chimes, which was rated highly (comfortable) by both groups, and ambulance, which was rated poorly (uncomfortable) by both groups.
We conducted a two-sample Mann-Whitney U test for each sound stimulus to clarify which were associated with a real ‘rating gap’ between the two groups. Statistically significant differences were observed for sparrow calls, evening cicada calls, car horns, crossing bells, ambulances, and police cars (Fig. 2: p<0.01-0.05).
Rating gaps between migraineurs and controls
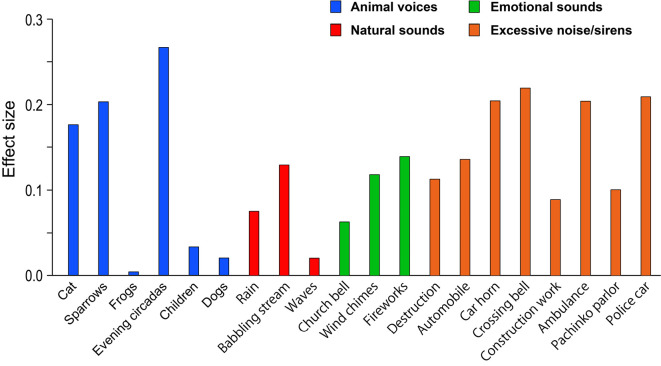
Effect sizes were calculated based on the Mann-Whitney U tests described above as an indicator of how much migraine patients' ratings for each sound type differed from the controls' ratings (Fig. 3). A large effect size indicates a greater ‘rating gap’ between controls and migraineurs (27-29).
Figure 3.
Effect sizes of rating gaps between migraineurs and controls for all sound stimuli. The vertical axis indicates the effect size (r) of the difference in ratings between the two cohorts as calculated with the Mann-Whitney U test.
While the greatest value was quite large (r=0.27), major variation among sound types was apparent. Some of the sounds with the highest effect sizes included sparrow and evening cicada calls among Animal sounds, and car horns, crossing bells, ambulances, and police cars among Excessive noise/sirens. In contrast, frog and dog calls in Animal sounds and waves in Natural sounds had small effect sizes. Taken together, these findings demonstrate how migraineurs and healthy adults can provide divergent ratings for the same sound depending on the type of sound being evaluated.
Spectral composition and its relationship with effect size
We next focused on frequency bands containing significant spectral information associated with larger rating gaps between migraineurs and controls. Knowing that a signal's power is equivalent to the root-mean-square (RMS) (30) of its amplitude envelope, we calculated the RMS for several of these partial signals and divided by the RMS of the entire signal. This yielded a quantity we termed the “signal RMS ratio” with a maximum value of 1. The extraction bandwidth for each partial signal was a constant one-third octave to account for the characteristics of the human auditory system (31). After these calculations, we investigated the relationship between the signal RMS ratio at each bandwidth and the effect size for all sound stimuli.
Fig. 4A shows a plot of the signal RMS ratio for a spectral band of interest (center frequency: 400 Hz) versus the effect size. Each data point represents a single sound stimulus. The signal RMS ratio was negatively correlated with the effect size (Pearson's product-moment correlation: r=-0.55, p=0.01), denoting that the rating gap between cohorts thus shrank even more when the proportion of the 400-Hz component in the entire signal had increased. Fig. 4B shows a plot similar to that in 4A, but the center frequency is different at 4,000 Hz, which suggests that this relationship is positive. However, there is no obvious association in the results if we exclude the two data points where the signal RMS ratio exceeds 0.6. Pearson's product-moment correlation analysis failed to identify any statistically significant association between the signal RMS ratio and effect size (r=0.36, p=0.12).
Figure 4.
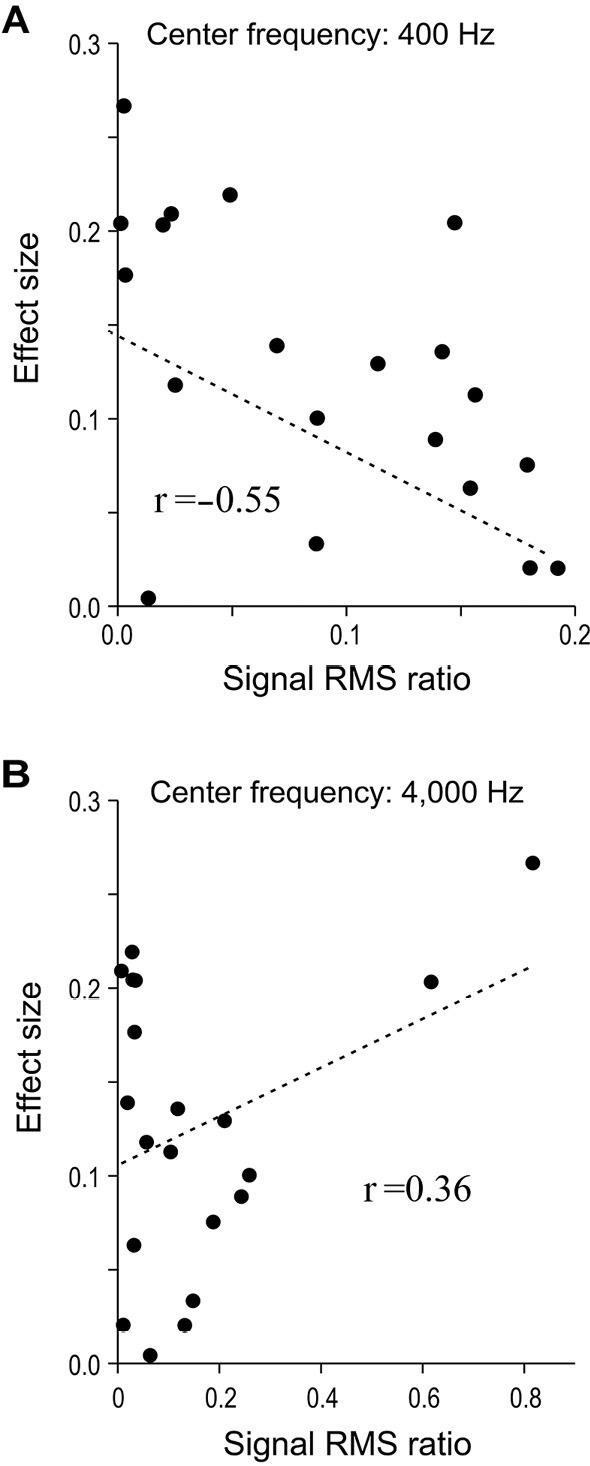
Relative contribution of designated frequency bands to overall signal versus effect size for all sound stimuli. The horizontal axis indicates the “signal root-mean-square (RMS) ratio”: the strength of the relative contribution of sound in a frequency band centered at the designated frequency to the raw signal. The vertical axis indicates the “effect size”: the magnitude of the rating gap between migraineurs and controls. A and B show this relationship for extracted partial signals with central frequencies of 400 and 4,000 Hz, respectively. One data point corresponds to a single sound type (n=20 per graph).
Fig. 5A shows the Pearson's product-moment correlation coefficients for various bandwidths of interest for all sound stimuli. The graph confirms our observations from Fig. 4 that the association between the two variables is large and negative at approximately 400 Hz and large and positive at approximately 4,000 Hz.
Figure 5.
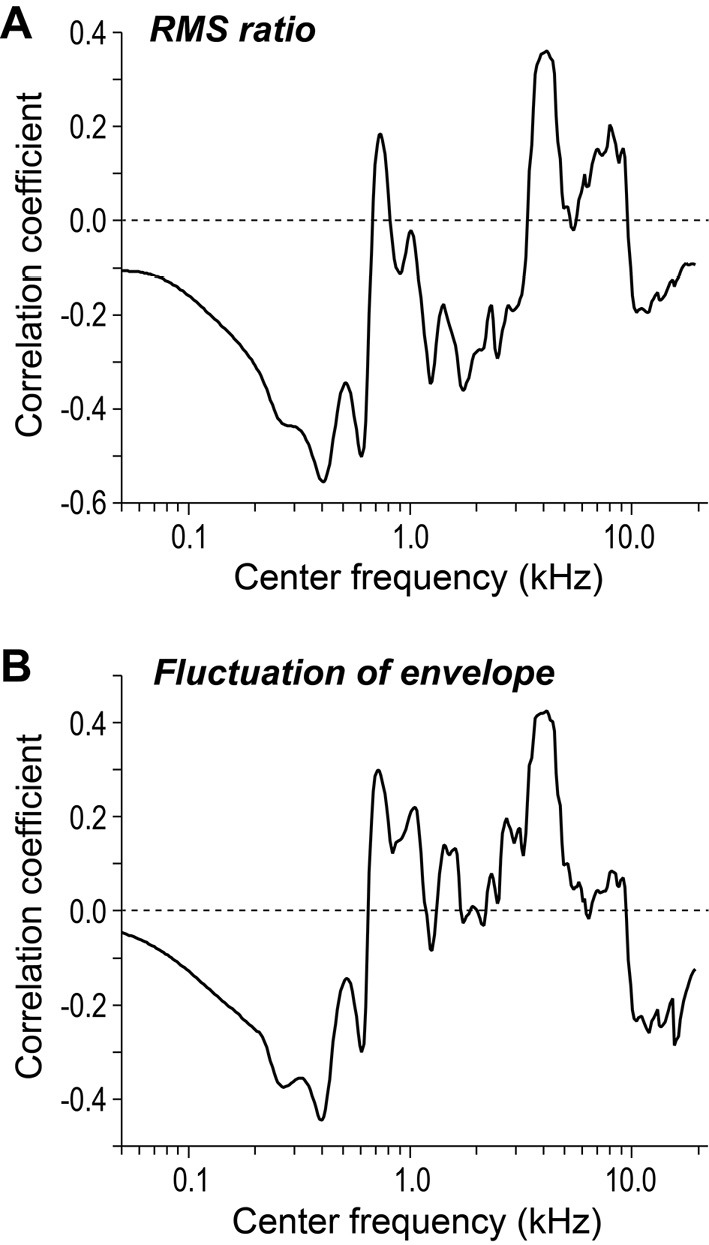
The association of the effect size with two spectral characteristics across all frequency bands for all sound stimuli. The figures show the strength of the correlation of the effect size with the signal root-mean-square ratio (RMS) (A: Fig. 4) and temporal variation (B) at various frequencies.
Auditory perception of a waveform is reportedly influenced by slow temporal variation in different spectral bands of its amplitude envelope (32). We therefore decided to calculate the temporal variation in the amplitude envelopes for a range of partial signals (defined by the center frequency) and to examine its association with the effect size. Partial signals (all bandwidths=one-third octave) were also processed through a bandpass filter to exclude all spectral components over 16 Hz (32). Temporal variation was conceptualized as the standard deviation of the amplitude envelope. Fig. 5B shows the relationship between the effect size and temporal variation in the amplitude envelopes for various frequency bands. It was apparent that, similar to Fig. 5A, the correlation was strongest (in terms of absolute magnitude) at approximately 400 and 4,000 Hz.
Discussion
Migraine patients versus healthy adults
In the present study, we compared the ratings of pleasantness for 20 sound stimuli among adult migraine patients and healthy controls. Two patterns shared by both groups were relatively low (unpleasant) ratings for sounds in the Excessive noise/sirens category and relatively high (pleasant) scores for sounds in the Emotional sounds category. This makes intuitive sense, as a typical person would generally perceive car horns and construction work as unpleasant, but not waves and church bells.
We observed statistically significant differences in ratings between migraineurs and controls for six sounds: sparrow calls, evening cicada calls, car horns, crossing bells, ambulances, and police cars. We calculated the effect sizes for each sound to evaluate the relative importance of the rating gap between the two groups. In addition, we observed differences in effect size among the different sound stimuli, suggesting that the groups rated these sounds differently depending on the sound type, even when controlling for volume. Our study showed this trend using everyday sounds for the first time.
Spectral composition and its association with rating gaps (effect size)
We found a tendency for migraineurs to rate sounds lower than controls when their signal component at approximately 400 Hz was smaller in amplitude and less variable over time. In fact, the 400-Hz band was very weak for cat calls, evening cicada calls, ambulances, and police cars, all sounds with large effect sizes between migraineurs and controls. Sparrow calls and crossing bells also had relatively high effect sizes. While the signals for these sounds are not exactly weak in the 400-Hz band, the corresponding envelope exhibits almost no slow variation over time.
We believe our discovery of the importance of certain frequency bandwidths and temporal properties in the auditory perception of migraine patients is a significant one for the field. In the study by Ashkenazi et al., tone-burst stimuli at frequencies lower than 1,000-Hz were not tested (16). Our study offers novel findings by using everyday sounds, including stimuli with significant low-frequency components.
Next, by focusing on sound categories, we found that different sounds in the same category had very different effect sizes. For example, cat calls had a larger effect size than dog calls, even though both sounds were animal vocalizations (Fig. 3). Almost none of the cat signals lay in the 400-Hz spectrum. In contrast, for dogs, barking was lower in pitch than the mewing of a cat, and accordingly, its partial signal at approximately 400 Hz was quite sizable. In addition, we noted major temporal variation in the amplitude of the signal in that range. Furthermore, despite observing some major trends in comfort/discomfort level by sound category (Fig. 2), we were unable to deduce any meaningful relationship between the sound category and the rating gap between the two groups. In short, this ‘rating gap’ between migraine patients and healthy adults appears to be strongly dependent on a given noise's spectral properties rather than its category or type.
Noises that may trigger migraine attacks and future steps to elucidate the pathogenic mechanism of migraines
Studies have long shown that noises in everyday living environments can trigger attacks in migraine patients (9-13). Experiments involving sound presentation have often utilized white noise as the noise stimulus, an artificial signal consisting of many component frequencies of equal strength across a broad spectrum (14,15). However, these past investigations and experiments have not elucidated the ‘true nature’ of triggering noises, in terms of their specific constituent sounds and acoustic properties.
Our data showed major differences between the ratings of migraineurs and healthy adults for the ambulance, police car, and crossing bell stimuli, with nearly all migraine patients rating them as unpleasant (Fig. 2). The acoustic properties of these sounds included a lower amplitude and a lack of temporal variation for a signal in the 400-Hz band. Ambulances, police cars, and crossing bells are sounds often heard in everyday life and are more likely than artificial white noise to correspond to the “noise” envisioned in previous papers. The ear is known to be highly sensitive to sounds higher than 400 Hz (33). Thus, the prominence of these 400-Hz spectral components in the sounds perceived as unpleasant by migraine patients in this study may have some connection with such inherent properties of human hearing. To speculate, it is possible that sounds with a weak 400-Hz component are perceived as more unpleasant because their high-frequency component (e.g. 1-4 kHz) contributes more to the signal and therefore appears ‘louder’ in comparison. To elucidate the pathogenic mechanism underlying migraine attacks, we wish to continue in this line of inquiry and shed more light on the relationship between the perceptual characteristics of human hearing and migraine attacks.
In this study, we attempted to identify the types of sounds perceived as less pleasant (more unpleasant) by migraine patients than by healthy adults by measuring the comfort/discomfort they experience in response to a variety of sounds ubiquitous in everyday life. Migraineurs experienced high discomfort in response to the sirens of ambulances and police cars and the warning bells of a railroad crossing, sounds frequently heard in daily life. Furthermore, their ratings for these noises diverged greatly from those of healthy adults. This discovery sheds light on the concept of ‘noise’ as an everyday phenomenon that may trigger migraine attacks, showing that the sounds above are highly likely to be part of the environmental noise experienced by migraine patients, something that has never been explicitly shown before.
Our data also showed that the rating gap for many sounds between migraineurs and controls became pronounced for sounds with a 400-Hz component characterized by low and/or slowly varying amplitude. Our findings will allow future research to develop practical counter-measures to improve the quality of life of migraine patients and eliminate the triggers for migraine attacks, such as earplugs to alleviate discomfort in migraineurs and sound-processing equipment to reduce the likelihood and severity of attacks.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by JSPS KAKENHI Grant Number JP17K19995.
Acknowledgement
The authors are indebted to the individuals at Dokkyo Medical University and Utsunomiya University for their cooperation with this study and for serving as study participants.
References
- 1. Sakai F, Igarashi H. Prevalence of migraine in Japan: a nationwide survey. Cephalalgia 17: 15-22, 1997. [DOI] [PubMed] [Google Scholar]
- 2. Noseda R, Kainz V, Jakubowski M, et al. . A neural mechanism for exacerbation of headache by light. Nat Neurosci 13: 239-245, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tatsumoto M, Eda T, Ishikawa T, Hirata K. Light of intrinsically photosensitive retinal ganglion cell (ipRGC) causing migraine headache exacerbation. Cephalalgia 33: 2, 2013. [Google Scholar]
- 4. Saisu A, Tatsumoto M, Hoshiyama E, Aiba S, Hirata K. Evaluation of olfaction in patients with migraine using an odour stick identification test. Cephalalgia 31: 1023-1028, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache 37: 492-495, 1997. [DOI] [PubMed] [Google Scholar]
- 6. Woodhouse A, Drummond PD. Mechanisms of increased sensitivity to noise and light in migraine headache. Cephalalgia 13: 417-421, 1993. [DOI] [PubMed] [Google Scholar]
- 7. Schulte LH, Jürgens TP, May A. Photo-, osmo- and phonophobia in the premonitory phase of migraine: mistaking symptoms for triggers? J Headache Pain 16: 14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vingen JV, Pareja JA, Støren O, et al. . Phonophobia in migraine. Cephalalgia 18: 243-249, 1998. [DOI] [PubMed] [Google Scholar]
- 9. Scharff L, Turk DC, Marcus DA. Triggers of headache episodes and coping responses of headache diagnostic groups. Headache 35: 397-403, 1995. [DOI] [PubMed] [Google Scholar]
- 10. Fraga MDB, Pinho RS, Andreoni S, et al. . Trigger factors mainly from the environmental type are reported by adolescents with migraine. Arq Neuropsiquiatr 71: 290-293, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Chakravarty A, Mukherjee A, Roy D. Trigger factors in childhood migraine: a clinic-based study from eastern India. J Headache Pain 10: 375-380, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neut D, Fily A, Cuvellier JC, Vallée L. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain 13: 61-65, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Solotareff L, Cuvellier JC, Alain D, Vallée L, Tich SNT. Trigger factors in childhood migraine: a prospective clinic-based study from north of France. J Child Neurol 32: 754-758, 2017. [DOI] [PubMed] [Google Scholar]
- 14. Martin PR, Todd J, Reece J. Effects of noise and a stressor on head pain. Headache 45: 1353-1364, 2005. [DOI] [PubMed] [Google Scholar]
- 15. Martin PR, Reece J, Forsyth M. Noise as a trigger for headaches: relationship between exposure and sensitivity. Headache 46: 962-972, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Ashkenazi A, Mushtaq A, Yang I, Oshinsky ML. Ictal and interictal phonophobia in migraine-a quantitative controlled study. Cephalalgia 29: 1042-1048, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ashkenazi A, Yang I, Mushtaq A, Oshinsky ML. Is phonophobia associated with cutaneous allodynia in migraine? J Neurol Neurosurg Psychiatry 81: 1256-1260, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishikawa T, Komatsuzaki T, Mitsui T, Tatsumoto M, Hasegawa H, Hirata K. Exploration of comfortable sound space for migraine patients - investigation of phonophobia to household noises. Int J Affect Eng 14: 1-8, 2015. [Google Scholar]
- 19. Headache Classification Committee of the International Headache Society (IHS).. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33: 629-808, 2013. [DOI] [PubMed] [Google Scholar]
- 20. Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population-a prevalence study. J Clin Epidemiol 44: 1147-1157, 1991. [DOI] [PubMed] [Google Scholar]
- 21. Henry P, Michel P, Brochet B, Dartigues JF, Tison S, Salamon R. A nationwide survey of migraine in France: prevalence and clinical features in adults. GRIM. Cephalalgia 12: 229-237, 1992. [DOI] [PubMed] [Google Scholar]
- 22. Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 267: 64-69, 1992. [PubMed] [Google Scholar]
- 23. Honkasalo ML, Kaprio J, Heikkilä K, Sillanpaa M, Koskenvuo M. A population-based survey of headache and migraine in 22,809 adults. Headache 33: 403-412, 1993. [DOI] [PubMed] [Google Scholar]
- 24. Schafer RM. The Tuning of the World. Arcana, Ontario, 1977: 1-276. [Google Scholar]
- 25.Comparative Hearing: Mammals. In: Springer Handbook of Auditory Research Series. Fay RR, Popper AN, Eds. Springer-Verlag, New York, 1994: 185. [Google Scholar]
- 26. Zwislocki J. Analysis of the middle-ear function. Part I: input impedance. J Acoust Soc Am 34: 1514-1523, 1962. [Google Scholar]
- 27. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum, Hillsdale, MI, 1988. [Google Scholar]
- 28. Field A. Discovering Statistics Using SPSS. 2nd ed. Sage Publications, London, 2005. [Google Scholar]
- 29. Tabachnick BG, Fidell LS. Using Multivariate Statistics (5th internationaled.). Pearson/Allyn & Bacon, Boston, 2006. [Google Scholar]
- 30. Moore BCJ. Hearing, Handbook of Perception and Cognition. 2nd ed. Academic Press, San Diego, 1995: 1-40. [Google Scholar]
- 31. Plomp R. The Intelligent Ear. Erlbaum, Mahwah, 2002: 13-18. [Google Scholar]
- 32. Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science 270: 303-305, 1995. [DOI] [PubMed] [Google Scholar]
- 33. Moore BCJ. An Introduction to the Psychology of Hearing. 6th ed. Emerald Group, Bingley, West Yorkshire, 2013: 133-167. [Google Scholar]