Abstract
Sperm velocity is a key determinant of competitive fertilization success in many species. Selection is therefore expected to favour the evolution of faster sperm when the level of sperm competition is high. However, several aspects can determine the direction and strength of selection acting on this key performance trait, including ecological factors that influence both sperm competition and the strength of selection acting on correlated traits that may constrain evolutionary responses in sperm velocity. Here, we determine how a key ecological variable, the level of predation, shapes sperm swimming speed across 18 Trinidadian populations of guppies (Poecilia reticulata). We use performance analysis, a statistical tool akin to the familiar methods of multivariate selection analyses, to determine how the level of predation influences sperm velocity (modelled as a performance trait) when accounting for correlated pre- and postcopulatory traits that are also impacted by predation. We show that predation affects the combination of pre- and postcopulatory traits that ultimately predict sperm performance. Overall, we report evidence for disruptive relationships between sperm performance and combinations of ornaments and sperm morphology, but the specific combinations of traits that predict sperm velocity depended on the level of predation. These analyses underscore the complex nonlinear interrelationships among pre- and postcopulatory traits and the importance of considering ecological factors that may ultimately change the way in which multiple traits interact to determine a trait's performance value. As such, our results are likely to be broadly applicable across systems where selection is influenced by ecological conditions.
Keywords: multivariate selection analyses, postcopulatory sexual selection, trade-off, ejaculate quality
1. Introduction
Sexual selection represents a powerful selective force shaping reproductive traits. Importantly, an increasing body of work reveals that the strength and form of sexual selection are not immutable and can vary according to ecological variables [1–3]. Ever since Darwin [4], evolutionary biologists have stressed the importance of considering ecological variation in determining how sexual selection acts on reproductive traits that function in the competition for access to mates (i.e. precopulatory sexual selection). For instance, elevated levels of predation can favour male–male competition (promoting weapon-like traits), constrain opportunities for female mate choice (selecting against ornaments) and/or promote sneak matings behaviours over courting tactics [5,6]. Ecological variables also have the potential to influence how sexual selection influences reproductive traits that promote fitness after mating (i.e. postcopulatory sexual selection). For example, ecologically driven variance in territory quality, food availability and predation pressure on population densities, sex ratios and mating systems [1,7] all have the potential to alter rates of female multiple mating, and therefore how often and strongly sperm competition occurs [8]. However, surprisingly few studies have considered how variation in ecological factors influences sexual selection operating after mating (i.e. postcopulatory sexual selection [3]).
Postcopulatory sexual selection imposes a significant evolutionary force on ejaculates (reviewed in [8,9]). In particular, numerous studies have revealed that sperm velocity is a reliable predictor of fertilization success in competitive and non-competitive matings [10]. Consequently, selection imposed through sperm competition is commonly hypothesized to favour the evolution of faster sperm [9,10]. However, the factors that influence sperm velocity are complex [11]. Sperm velocity can be influenced by other components of the ejaculate, including sperm morphology and seminal fluid composition, which may constrain evolutionary responses in sperm swimming speed [12]. Furthermore, sperm traits commonly exhibit strong phenotypic or genetic covariance with traits targeted by precopulatory sexual selection (e.g. sexual ornamentation), many of which are sensitive to prevailing ecological or environmental conditions [3,13]. Thus, ecological variables that impact the expression of precopulatory traits may also influence ejaculate traits [13]. Consequently, to understand how selection targets sperm swimming velocity, we need to account for the complex interrelationship among pre- and postcopulatory sexual traits, all of which may be modified by ecological factors.
The guppy Poecilia reticulata provides an ideal model system for testing how variation in a key ecological variable—predation risk—influences sperm traits among natural populations [14]. Guppies are small livebearing fish that inhabit rivers and streams that are bisected by waterfalls that limit the migration of guppies and impede the movement of predatory fish species. Typically, upstream populations are characterized by low levels of predation (imposed only by small Cyprinodontidae) and downstream populations are characterized by high levels of predation (mainly from Characidae and Ciclidae, [14]). This difference in predation risk between up- and downstream populations generates predictable differences in the strength of natural and sexual selection between predation regimes, with well-known consequences for male ornamentation, female mate choice, male–male competition, antipredator behaviour, mating behaviour, population demography, life-history evolution and, possibly, the level of multiple mating by females (reviewed in [15–17]). In general, where predation is limited (upstream), males are more coloured and tend to rely more on courtship displays to obtain matings. In these upstream populations, relative to high-predation (downstream) sites, the level of multiple mating by females is marginally lower and precopulatory sexual selection is stronger [15,16]. By contrast, in high-predation (downstream) populations, natural selection has a stronger impact on trait evolution [15,16], and males tend to be less coloured and rely more on forced copulations (termed gonopodial thrusts) to achieve matings [14,15]. While multiple paternity, and thus sperm competition, is generally high in both type of populations, broods exhibit relatively higher levels of multiple paternity in high-predation populations, possibly reflecting higher levels of sperm competition [18].
In guppy populations subject to high-predation males have lower sperm production (as estimated from stripped ejaculate size) but produce faster-swimming sperm [19], compared to their low-predation counterparts [20]. In guppies, no linear relationships between sperm morphology and fitness have been described [21,22]. Sperm velocity instead plays an important role in sperm competition [22,23], predicting male reproductive fitness [24]. Sperm velocity exhibits complex relationships with a range of sexual and somatic traits in guppies. For example, sperm velocity is positively phenotypically correlated with orange coloration [25,26] but negatively genetically correlated with both courtship rate and the area of iridescent coloration [27]. Importantly, these relationships between sperm velocity and male sexual and somatic traits are likely to be influenced by predation, which, as described above, can shape selection on coloration, sexual behaviour (and consequently the level of sperm competition) and life-history traits [15,16]. Accordingly, we should expect complex relationships between the level of predation and patterns of (pre- and postcopulatory) trait variation. Yet despite this prediction, only one study on guppies has considered multiple natural populations for testing the effect of predation on postcopulatory traits [19], and we know nothing about how ecological factors shape interrelationships between pre- and postcopulatory sexual traits.
In this study, we examine how predation influences patterns of trait variation that ultimately determine sperm ‘performance’ across multiple guppy populations. We overcome the approach of studying only linear relationship between sperm velocity and other sexually selected traits by also considering nonlinear relationships between sperm velocity (which can be seen as a performance parameter, i.e. the ability to perform key tasks or functions [28]) and other phenotypic traits [29]. Despite the widespread use of this method for describing performance [29], this approach has never been employed to describe sperm performance. Here, we adopted part of a classical analytical framework [29] to evaluate how predation regime shapes variation in sperm performance while accounting for phenotypic covariance with other sexually selected traits [29,30]. Our analyses generate sperm performance surfaces [31] for low- and high-predation populations in order to understand how trait combinations interact to produce high (sperm) performance phenotypes and how predation intensity shapes these patterns. We predict that, given the relative differences in the strength of natural and sexual selection, as well as the relative costs and benefits of male traits in the different environments, the shape of performance surfaces will have different features in populations subject to low- and high-predation regimes.
2. Material and methods
Males used in this study are a subsample of those used for the analysis described by Grueber and colleagues [32] and include all populations that were sampled in both high- (downstream populations) and low- (upstream populations) predation sites (electronic supplementary material). The methods used for fish collection and the characterization of male traits are the same as those described previously [32] and are therefore only briefly summarized here.
(a). Populations sampling
Male guppies were collected in Trinidad's Northern Range Mountains in May–June 2011 from nine rivers spanning the Caroni, Oropouche and Northern drainages (electronic supplementary material and [32] for details). We sampled 60 males from each of nine rivers (total n = 540 males), where for each river, 30 males came from a low-predation site (upstream populations) and 30 were taken from a high-predation site (downstream populations). Fish were then returned to the laboratory at the University of the West Indies, in St Augustine, and kept in tanks containing conditioned freshwater. Three to four days after collection, morphological measurements of males and analyses of ejaculate traits were performed.
(b). Male size and ornamentation
For morphological measurements, each male was euthanized in iced water and photographed on his left side under standard lighting on a white background using a digital camera (Nikon D70s with Nikon 105 mm macro lens; Nikon Corporation, Tokyo, Japan). Each image included a measurement scale. We subsequently used the software ImageJ (https://imagej.nih.gov/ij/) to measure each individual's body size (lateral fish area, caudal fin included, in mm2; hereafter body area) and ornamentation. Briefly, the area (mm2) of the fish covered by carotenoid and pteridine pigments (orange/red and yellow, hereafter orange), structural colours (from white to green, hereafter iridescence) and melanic (black) spots was measured using the polygon selection tool in ImageJ. We measured size and ornamentation for a total of 539 males (photograph was missing for one male).
(c). Sperm assays
Ejaculates were obtained using standard procedures [32] and subsequently used for measuring sperm velocity and sperm morphology (electronic supplementary material). Computer-assisted sperm analysis (CASA) was performed using a CEROS Sperm Tracker (Hamilton Thorne Research, Beverly, MA, USA) to quantify sperm velocity for 480 males (i.e. the reduced sample size reflects cases where ejaculates could not be extracted, there were technical difficulties in obtaining velocity data, or the number of motile sperm cells was less than 10). Sperm velocity was estimated with 70.6 ± 1.3 (mean ± s.e.) sperm per male. To estimate sperm morphology, we photographed preserved sperm cells under a 400× magnification microscope (Leica DM750) and then used ImageJ to measure sperm head, midpiece and flagellum length of 264 males.
(d). Statistical analysis
We compared male traits (size, orange, iridescent, black, sperm velocity and the sperm length measures) between low- and high-predation populations using a series of linear mixed-effects models fit by REML, using the lmer function in lme4 R package (see electronic supplementary material for details).
We used multiple regression analyses to explore the relationship between sperm velocity and the various morphological, ornamental and sperm traits [29–31]. In these analyses, we treated sperm velocity (measured using the average path velocity, VAP; which is highly correlated with the other measures of sperm velocity obtained using CASA: all p-values <0.001) as an estimate of ‘performance’ (dependent variable) and included sperm morphology (measured as the length of the sperm head, midpiece and flagellum), male ornamentation (measured as size of orange, iridescent, and black spots) and size (male body area) as predictor variables. Importantly, each of these performance predictors covaries with sperm velocity [25–27,33,34]. Our modelling is based on full quadratic multiple regression [35], where the performance estimate (sperm velocity) is the dependent variable, and traits, quadratic transformed traits and cross-trait combinations were fitted as predictors. Sperm velocity was standardized to a mean of one (i.e. divided by observed population mean) whereas the predictor variables were standardized to a mean of zero and s.d. of one (i.e. subtracted the observed population mean and divided for the observed population s.d. [35]). The regressions were used to obtain the average slope (βp) and curvature (γp) coefficients of the performance surface (hereafter ‘performance gradients’), and returned an estimate of linear and nonlinear (quadratic and correlational) relationships between traits and performance [29]. We estimated βp separately with a linear regression and then estimated γp with a full quadratic regression [36]. For the performance analysis, we used only males for which we obtained measures of all traits and performed the analysis on low- and high-predation populations separately (n = 240 males; n = 122 high-predation males and n = 118 low-predation males).
We first tested if performance gradients within low and high-predation populations differed from the same gradients obtained when removing predation regime grouping. To do this, and to avoid an overparametrized model where predation would have been a fixed factor together with all two-way interactions with covariates (i.e. the regression predictors), we compared linear βp and quadratic γp coefficients obtained with full regressions performed within predation regime (i.e. for low- and high-predation populations separately) with a distribution of coefficients obtained with regressions performed on 10 000 simulated populations. Simulated populations consisted of n = 120 individuals (i.e. similar sample size as the observed populations) randomly chosen (without replacement, i.e. shuffled) from the original dataset (all measured males, n = 240). Simulated populations thus varied in predation depending on the number of individuals randomly chosen from each regime. Observed coefficients that were greater or smaller than the 95% CI obtained with the simulated population were considered to be affected by predation regime. With a similar procedure, we tested if the absolute difference observed for each gradient between low- and high-predation populations was greater than the 95 upper percentile of a distribution of 10 000 differences obtained by regressions performed in simulated populations (see results and electronic supplementary material, tables S5 and S6, and figures S1 and S2).
As we found that the observed performance gradients of distinct traits in low- and high-predation populations were different from the null distribution (see results), we performed nonlinear analyses in low- and high-predation populations separately by performing canonical analyses (see electronic supplementary material for a detailed description). This approach reveals nonlinear relationships on axes that represent combinations of multiple traits, thus simplifying interpretation of correlational coefficients [37]. These axes are represented by eigenvectors with associated loadings of the original traits. Each vector eigenvalue (λp) represents the curvature of the performance surface along this vector, whereas theta (θp) represents the steepness of the vector. The significance of the curvature on these vectors was tested statistically using a permutation approach [38]. Finally, we visualized the performance surface with non-parametric thin-plate splines [39] using the Tps function in the fields package of R. This method avoids the quadratic assumptions in the construction of the surface based on the major eigenvectors [37,40]. As eigenvectors (surface axes) are loaded by different predictors (traits) based on predation regime (see results), our analyses generate performance surfaces of individuals of low and high predations populations separately and restrict our comparison of low- and high-predation performance surfaces to a qualitative level. All analyses described were performed in R (version 3.4.3) and Excel (Microsoft Corporation).
3. Results
(a). Differences in male traits between low- and high-predation populations
Males from low-predation populations (upstream populations) tended to be larger, with more orange, black and iridescent (albeit a statistical trend) coloration than males from high-predation populations (downstream populations; electronic supplementary material, table S3), which is consistent with previous research [15]. Sperm velocity (VAP) did not differ between high- and low-predation populations (electronic supplementary material, table S3). Flagellum length was greater in low-predation populations than in high-predation populations, whereas sperm head and midpiece length did not differ between predation regimes (electronic supplementary material, table S3).
(b). Predation regime and performance gradients
Four observed nonlinear performance gradients in low- and high-predation populations, involving six traits, differed from the simulated null distribution (electronic supplementary material, table S5 and figure S1). Specifically, two performance gradients in low-predation populations (iridescent × sperm head γp and midpiece × flagellum γp) and two in high-predation populations (black γp and orange × black γp) significantly differed from the corresponding simulated values. Linear performance gradients did not differ significantly from the simulated linear gradients (electronic supplementary material, table S5 and figure S1). Ten differences in nonlinear performance gradients between low- and high-predation populations, involving all the traits considered, differed from the simulated null distribution (electronic supplementary material, table S6 and figure S2), with differences in size γp and coloration (orange γp and orange × black γp) being the most pronounced, and suggesting that performance surfaces of low- and high-predation populations have different features. Given these results, we performed nonlinear analyses and generated sperm performance surfaces for low- and high-predation populations separately.
(c). Performance analysis in low- and high-predation populations
We found no significant linear performance gradients (βp coefficients) between sperm velocity and the measured traits in low-predation populations. However, correlational performance gradients (γp coefficients) between orange area and black area and between sperm head length and iridescent area were positive and significant (electronic supplementary material, table S4). This suggests that males producing faster sperm had either large carotenoid and black spots or small carotenoid and black spots and either large iridescent spot and long sperm head or small iridescent spot and short sperm head.
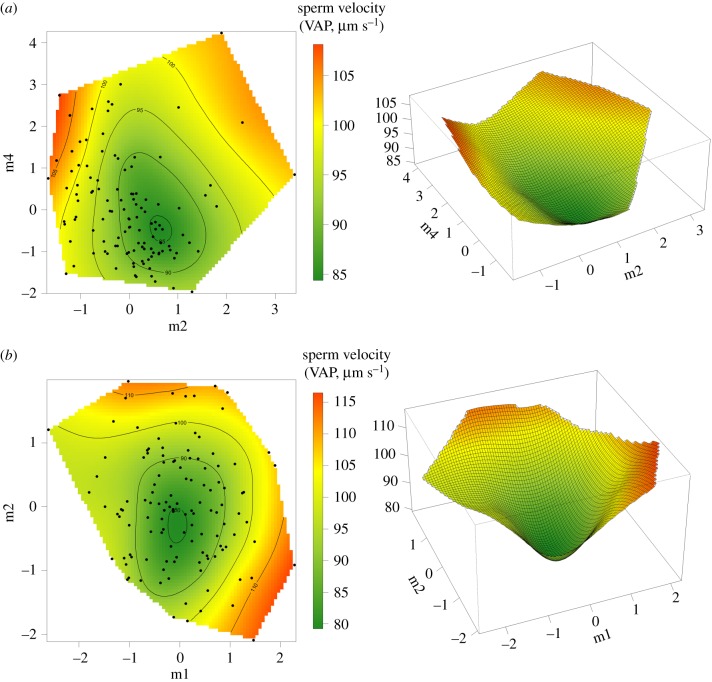
After canonical analysis, we detected two major axes (m2 and m4) where the curvature was significantly different from zero (table 1a). The eigenvector m2 was mainly positively loaded by body size and sperm head length, whereas m4 was mainly positively loaded by the area of orange and black spots and by flagellum length (table 1a). The performance surface based on these two vectors shows two sperm performance peaks close to positive m4 and extreme (positive and negative) m2 values (figure 1a). Overall, in low-predation populations, males with faster sperm have either small or large body size and small or large sperm head but tend to be more coloured and to have a longer flagellum.
Table 1.
Matrix of eigenvalues λp representing the nonlinear analysis of the relationship between sperm velocity (VAP) and body size (i.e. body area, mm2), ornamentation (orange, black and iridescent coloration, mm2) and sperm morphology (sperm head, midpiece and flagellum lengths, µm) traits in (a) low-predation and (b) high-predation populations. Linear Θp obtained with a full regression are also reported. Significant nonlinear vectors and trait loadings greater than 0.5 are presented in bold. In parenthesis are p-values.
| trait loadings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| size | ornamentation |
sperm morphology |
||||||||
| eigenvectors |
Θp (p)a | λp (p)a | body area | orange | black | iridescent | head | midpiece | flagellum | |
| (a) low predation (n = 118) | m1 | −0.058 (0.198) | 0.229 (0.673) | 0.550 | −0.187 | −0.178 | −0.540 | −0.518 | −0.266 | −0.010 |
| m2 | −0.030 (0.454) | 0.213 (0.035) | −0.575 | 0.314 | 0.067 | −0.037 | −0.595 | −0.205 | −0.411 | |
| m3 | −0.037 (0.255) | 0.057 (0.420) | −0.392 | −0.048 | −0.597 | −0.198 | −0.190 | 0.377 | 0.519 | |
| m4 | −0.016 (0.603) | 0.035 (0.043) | 0.131 | 0.579 | 0.541 | −0.193 | −0.162 | 0.189 | 0.505 | |
| m5 | −0.014 (0.617) | −0.040 (0.356) | −0.190 | −0.493 | 0.448 | −0.409 | −0.027 | 0.545 | −0.234 | |
| m6 | −0.011 (0.668) | −0.052 (0.665) | −0.380 | −0.071 | 0.113 | −0.529 | 0.428 | −0.576 | 0.209 | |
| m7 | −0.001 (0.984) | −0.187 (0.570) | 0.128 | 0.529 | −0.316 | −0.428 | 0.363 | 0.286 | −0.455 | |
| (b) high predation (n = 122) | m1 | 0.026 (0.428) | 0.235 (0.038) | −0.273 | 0.675 | −0.610 | 0.295 | 0.022 | −0.094 | 0.031 |
| m2 | −0.006 (0.839) | 0.195 (0.016) | −0.211 | −0.383 | 0.035 | 0.607 | 0.265 | −0.210 | 0.571 | |
| m3 | 0.016 (0.655) | 0.120 (0.104) | 0.465 | −0.014 | −0.317 | −0.155 | 0.723 | 0.343 | 0.137 | |
| m4 | 0.007 (0.733) | 0.004 (0.720) | 0.604 | −0.011 | 0.016 | 0.612 | −0.073 | −0.185 | −0.470 | |
| m5 | 0.011 (0.692) | −0.027 (0.706) | 0.336 | 0.083 | −0.096 | −0.364 | 0.000 | −0.815 | 0.273 | |
| m6 | 0.001 (0.978) | −0.056 (0.521) | 0.330 | 0.538 | 0.484 | 0.115 | −0.181 | 0.252 | 0.508 | |
| m7 | −0.038 (0.263) | −0.092 (0.802) | −0.281 | 0.318 | 0.531 | 0.018 | 0.607 | −0.260 | −0.319 | |
Figure 1.
Two-dimensional contour plots and three-dimensional surfaces showing the relationship between sperm velocity (VAP, µm s−1) and significant vectors in the rotated γp matrix (table 1). (a) In low-predation populations, both vectors m2 and m4 are characterized by a positive eigenvalue (concave surface). (b) High-predation populations, where m1 and m2 are all characterized by a positive eigenvalue (concave surface). (Online version in colour.)
As in low-predation populations, we found no significant βp coefficients between sperm velocity and the measured traits in high-predation populations (electronic supplementary material, table S4). However, we detected a significant positive quadratic γp coefficient for carotenoid coloration, together with a negative correlational coefficient between orange and black coloration. A significant negative correlational performance gradient was also present between sperm head and sperm flagellum length. After canonical analysis, we obtained two significant vectors (m1 and m2), both with significant positive λp eigenvalues in high-predation populations (table 1b). The eigenvector m1 was primarily loaded by the area of orange (positive loading) and black (negative loading) spots whereas the m2 vector was primarily and positively loaded by iridescent spot size and flagellum length (table 1b). The surface built on these vectors revealed two distinct peaks at extreme m2 values and high and average m1 values (figure 1b). Thus, in high-predation populations, males with faster swimming sperm tend to have either a short flagellum, low iridescence and black coloration, and high orange coloration, or a long flagellum and high iridescent coloration with intermediate orange and large black coloration.
In both low and high-predation populations, a dip in the surfaces (representing the lowest sperm velocity) was present close to the average value of the vectors (corresponding to average traits values). This suggests a common disruptive relationship between sperm performance and other traits. Accordingly, positive eigenvectors are slightly more numerous, have bigger absolute values and are the only significant vectors in both low- and high-predation populations. Differences, however, are present as the contribution of traits to the main vectors varies between predation regimes, in the strength, sign and combination.
4. Discussion
Our results reveal that the predation regime can shape sperm performance surfaces in guppies and underscore the importance of considering ecological and environmental factors when evaluating how phenotypic traits interact to influence performance and possibly fitness. Specifically, observed quadratic and correlational, but not linear, performance gradients differed from those generated by simulated populations, where predation effect was removed. Low- and high-predation populations, however, did not share the same differences, suggesting that predation can generate different sperm performance surfaces. Moreover, when directly compared, gradients in low- and high-predation populations are broadly more different than expected by chance. Within the predation regime, low- and high-predation populations differ in how precopulatory (colour, body size) and postcopulatory (sperm morphology) traits interact to determine sperm performance. Despite these differences, there was a general trend for disruptive relationships in sperm performance surfaces. Males with intermediate trait values in both low- and high-predation populations exhibited slower-swimming sperm.
Theories suggest that the extent of sexual ornamentation displayed by males can reflect underlying variation in ejaculate quality, such that males in good condition advertise either their fertility (fertility-linked sperm hypothesis [42]) or genetic quality (good sperm hypothesis [43]). Accordingly, we found that the area of orange pigmentation was associated with peak sperm velocity in both low- and high-predation populations, which is consistent with previous findings [25] and explains why colourful male guppies have been shown to be successful sperm competitors [44]. A possible explanation for this finding, which is consistent with evidence from other taxa [45,46], is that the size of the male's orange spots may be an indicator of genetic quality [47], and good-quality males may be able to both allocate more carotenoids (which are costly to obtain) to sexual secondary characters and produce better-quality sperm [25,26]. However, the way in which orange coloration combined with other sexually selected traits to predict sperm performance was complex and differed between predation regimes, possibly as a consequence of underlying differences in patterns of genetic covariance between populations [48]. Furthermore, it is possible that the level of predation determines the level of investment in costly sexual coloration and courtship by males [16], which in turn may expose energetic trade-offs among traits [49] that differ between low- and high-predation populations. The complexity of patterns revealed by our study suggests that no single mechanism (e.g. signalling functions, trade-offs, changes in genetic architecture, etc.) can explain the differences in performance surfaces between populations. Instead, differences in predation pressure may trigger a number of mechanistic processes that ultimately determine how sexually selected and reproductive traits combine to influence sperm performance. Interestingly, a greater disruptive relationship (i.e. positive eigenvalue) for ornamentation is found in high-predation populations, where the effect of natural selection against ornaments is stronger. This suggests that where predation is higher males successful in postcopulatory competition may be either relying on ornaments or on sneaky copulation, whereas in low-predation populations, this dichotomy is less pronounced.
The way in which sperm length predicted sperm velocity in association with precopulatory traits also differed between predation regimes. It is often assumed that sperm velocity will exhibit a linear positive relationship with sperm length [12,50]. While we found that in low-predation populations flagellum length was associated with sperm velocity, this relationship was moderated by the phenotypic covariance between sperm velocity and other traits. Specifically, in low-predation populations, males with longer flagella had faster-swimming sperm when they had more orange and black coloration and extreme (both long and short) sperm head sizes. By contrast, in high-predation populations, we observed a stronger positive nonlinear (disruptive) relationship between flagellum length and sperm velocity, also moderated by correlational patterns of selection on sexual ornamentation. This latter finding is at odds with the longstanding assumption of a linear relationship between sperm length and velocity. Moreover, this result was unexpected given that stabilizing selection is likely to act on sperm morphology in guppies [51]. Our results suggest that complex nonlinear relationships between sperm form and function may be more common than currently appreciated and may explain the lack of a clear linear correlation between size and velocity in different taxa [11].
Regardless of the mechanisms underlying the complex relationships between sperm velocity and other traits, our results depict a complex scenario where the relationships between sperm performance and precopulatory (size and ornamentation) and postcopulatory traits (sperm components size) are mainly nonlinear. Moreover, only by moving beyond the analysis of univariate linear relationships was it possible to detect the numerous and interdependent relationships among sexually selected traits. Recently, Tuni et al. [52] demonstrated in southern field crickets (Gryllus bimaculatus) that the multiple mechanisms that jointly shape phenotypic associations (trade-offs or positive correlations) between pre- and postcopulatory traits can be revealed by taking into account the hierarchical structure of trait correlations (i.e. at the phenotypic, genotypic and environmental level). We suggest that not only the direction (positive or negative) of these correlations but also the shape (linear or nonlinear) can similarly vary due to multiple interacting biological mechanisms and that only a multivariate approach can reveal such patterns (see [53] for other merits in multivariate analysis). It is important to stress that the present analyses do not allow us to infer causation for the various performance–trait relationships. Nevertheless, such analyses do enable us to develop a predictive experimental framework for testing among various putative causal factors. Furthermore, the approaches used here have the potential to be applied to other systems in order to detect and describe complex interactions between traits, with or without clear a priori expectations.
In conclusion, our results demonstrate that variance in predation risk can affect how pre- and postcopulatory traits interact to determine sperm performance in wild guppy populations. Interestingly, males with average precopulatory and postcopulatory traits tended to exhibit the poorest sperm performance, suggesting that postcopulatory sexual selection acts disruptively on precopulatory traits and sperm morphology simultaneously. As a consequence, the combination of pre- and postcopulatory selection for sexual traits may not be directional, as is sometimes assumed [54]. By comparing sperm performance surfaces between low- and high-predation populations, our findings reveal that pre- and postcopulatory sexual selection can vary in their relative strength and direction within a species and that ecological factors can be important determinants of such patterns. We anticipate that future studies that employ similar performance analyses will advance our understanding of the complex nature of trait relationships observed in other species.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Kharran Deonarinesingh, Rajindra Mahibir and Indar Ramnarine for logistical support with the original fish collections in Trinidad. We also thank Jessica Abbott, Michael Jennions, Facundo X. Palacio and two anonymous reviewers for helpful comments on previous drafts of the paper.
Ethics
Collections were approved by the Director of Fisheries, Fisheries Division, Ministry of Food Production (Trinidad and Tobago), and all animal procedures were approved by UWA's Animal Ethics Committee (permit number RA/3/100/513).
Data accessibility
The full dataset has been uploaded as electronic supplementary material.
Authors' contributions
All authors carried out fieldwork. A.D. and J.L.F. performed statistical analyses. All authors helped in drafting and writing the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Funding for this project was provided by the Australian Research Council Discovery Projects grants (DP110102789 and DP1096253) and a Knut and Alice Wallenberg Academy Fellowship to J.L.F., and by the Wenner-Gren Foundation and the MIUR (PRIN 2008 no. 2008Z8ACTN) to A.D.
References
- 1.Emlen ST, Oring LW. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223. ( 10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 2.Miller CW, Svensson EI. 2014. Sexual selection in complex environments. Annu. Rev. Entomol. 59, 427–445. ( 10.1146/annurev-ento-011613-162044) [DOI] [PubMed] [Google Scholar]
- 3.Evans JP, Garcia-Gonzalez F. 2016. The total opportunity for sexual selection and the integration of pre- and post-mating episodes of sexual selection in a complex world. J. Evol. Biol. 29, 2338–2361. ( 10.1111/jeb.12960) [DOI] [PubMed] [Google Scholar]
- 4.Darwin CR. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 5.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. Revue Canadienne De Zoologie 68, 619–640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 6.Magnhagen C. 1991. Predation risk as a cost of reproduction. Trends Ecol. Evol. 6, 183–186. ( 10.1016/0169-5347(91)90210-O). [DOI] [PubMed] [Google Scholar]
- 7.Davies NB, Lundberg A. 1984. Food distribution and a variable mating system in the dunnock, Prunella modularis. J. Anim. Ecol. 53, 895–912. ( 10.2307/4666) [DOI] [Google Scholar]
- 8.Birkhead TR, Møller AP. 1998. Sperm competition and sexual selection. San Diego, CA: Academic Press. [Google Scholar]
- 9.Birkhead TR, Hosken DJ, Pitnick S. 2009. Sperm biology: an evolutionary perspective. Burlington, MA: Academic Press. [Google Scholar]
- 10.Simmons LW, Fitzpatrick JL. 2012. Sperm wars and the evolution of male fertility. Reproduction 144, 519–534. ( 10.1530/rep-12-0285) [DOI] [PubMed] [Google Scholar]
- 11.Simpson JL, Humphries S, Evans JP, Simmons LW, Fitzpatrick JL. 2014. Relationships between sperm length and speed differ among three internally and three externally fertilizing species. Evolution 68, 92–104. ( 10.1111/Evo.12199) [DOI] [PubMed] [Google Scholar]
- 12.Pizzari T, Parker GA. 2009. Sperm competition and sperm phenotype. In Sperm biology: an evolutionary perspective (eds Birkhead TR, Hosken DJ, Pitnick S), pp. 207–245. Burlington, MA: Academic Press. [Google Scholar]
- 13.Simmons LW, Lüpold S, Fitzpatrick JL. 2017. Evolutionary trade-off between secondary sexual traits and ejaculates. Trends Ecol. Evol. 32, 964–976. ( 10.1016/j.tree.2017.09.011) [DOI] [PubMed] [Google Scholar]
- 14.Magurran AE. 2005. Evolutionary ecology: the Trinidadian guppy. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Houde AE. 1997. Sex, color, and mate choice in guppies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 16.Endler JA. 1995. Multiple-trait coevolution and environmental gradients in guppies. Trends Ecol. Evol. 10, 22–29. ( 10.1016/s0169-5347(00)88956-9) [DOI] [PubMed] [Google Scholar]
- 17.Travis J, Reznick D, Bassar RD, Lopez-Sepulcre A, Ferriere R, Coulson T. 2014. Do eco-evo feedbacks help us understand nature? Answers from studies of the Trinidadian Guppy. Adv. Ecol. Res. 50, 1–40. ( 10.1016/B978-0-12-801374-8.00001-3) [DOI] [Google Scholar]
- 18.Neff BD, Pitcher TE, Ramnarine IW. 2008. Inter-population variation in multiple paternity and reproductive skew in the guppy. Mol. Ecol. 17, 2975–2984. ( 10.1111/j.1365-294X.2008.03816.x) [DOI] [PubMed] [Google Scholar]
- 19.Elgee KE, Evans JP, Ramnarine IW, Rush SA, Pitcher TE. 2010. Geographic variation in sperm traits reflects predation risk and natural rates of multiple paternity in the guppy. J. Evol. Biol. 23, 1331–1338. ( 10.1111/j.1420-9101.2010.01996.x) [DOI] [PubMed] [Google Scholar]
- 20.Evans JP, Magurran AE. 1999. Geographic variation in sperm production by Trinidadian guppies. Proc. R. Soc. B 266, 2083–2087. [Google Scholar]
- 21.Gasparini C, Marino IA.M., Boschetto C, Pilastro A. 2010. Effect of male age on sperm traits and sperm competition success in the guppy (Poecilia reticulata). J. Evol. Biol. 23, 124–135. ( 10.1111/j.1420-9101.2009.01889.x) [DOI] [PubMed] [Google Scholar]
- 22.Boschetto C, Gasparini C, Pilastro A. 2011. Sperm number and velocity affect sperm competition success in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 65, 813–821. ( 10.1007/s00265-010-1085-y) [DOI] [Google Scholar]
- 23.Devigili A, Di Nisio A, Grapputo A, Pilastro A.. 2016. Directional postcopulatory sexual selection is associated with female sperm storage in Trinidadian guppies. Evolution 70, 1829–1843. ( 10.1111/evo.12989) [DOI] [PubMed] [Google Scholar]
- 24.Devigili A, Evans JP, Di Nisio A, Pilastro A.. 2015. Multivariate selection drives concordant patterns of pre- and postcopulatory sexual selection in a livebearing fish. Nat. Commun. 6, 8291 ( 10.1038/ncomms9291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locatello L, Rasotto MB, Evans JP, Pilastro A. 2006. Colourful male guppies produce faster and more viable sperm. J. Evol. Biol. 19, 1595–1602. ( 10.1111/j.1420-9101.2006.01117.x) [DOI] [PubMed] [Google Scholar]
- 26.Pitcher TE, Rodd FH, Rowe L. 2007. Sexual colouration and sperm traits in guppies. J. Fish Biol. 70, 165–177. ( 10.1111/j.1095-8649.2006.01292.x) [DOI] [Google Scholar]
- 27.Evans JP. 2010. Quantitative genetic evidence that males trade attractiveness for ejaculate quality in guppies. Proc. R. Soc. B 277, 3195–3201. ( 10.1098/rspb.2010.0826). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kingsolver JG, Huey RB. 2003. Introduction: the evolution of morphology, performance, and fitness. Integr. Comp. Biol. 43, 361–366. ( 10.1093/Icb/43.3.361) [DOI] [PubMed] [Google Scholar]
- 29.Arnold SJ. 2003. Performance surfaces and adaptive landscapes. Integr. Comp. Biol. 43, 367–375. ( 10.1093/Icb/43.3.367) [DOI] [PubMed] [Google Scholar]
- 30.Arnold SJ. 1983. Morphology, performance and fitness. Am. Zool. 23, 347–361. [Google Scholar]
- 31.Arnold SJ, Bennett AF. 1988. Behavioural variation in natural populations. V. Morphological correlates of locomotion in the garter snake (Thamnophis radix). Biol. J. Linnean Soc. 34, 175–190. ( 10.1111/j.1095-8312.1988.tb01955.x) [DOI] [Google Scholar]
- 32.Grueber CE, Fitzpatrick JL, Devigili A, Gasparini C, Ramnarine IW, Evans JP. 2017. Population demography and heterozygosity-fitness correlations in natural guppy populations: an examination using sexually selected fitness traits. Mol. Ecol. 26, 4631–4643. ( 10.1111/mec.14243) [DOI] [PubMed] [Google Scholar]
- 33.Devigili A, Doldan-Martelli V, Pilastro A. 2015. Exploring simultaneous allocation to mating effort, sperm production, and body growth in male guppies. Behav. Ecol. 26, 1203–1211. ( 10.1093/beheco/arv067) [DOI] [Google Scholar]
- 34.Skinner AM.J, Watt PJ. 2006. Phenotypic correlates of spermatozoon quality in the guppy, Poecilia reticulata. Behav. Ecol. 18, 47–52. ( 10.1093/beheco/arl049) [DOI] [Google Scholar]
- 35.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.1111/j.1558-5646.1983.tb00236.x) [DOI] [PubMed] [Google Scholar]
- 36.Chenoweth SF, Hunt J, Rundle HD. 2012. Analyzing and comparing the geometry of individual fitness surfaces. In The adaptive landscape in evolutionary biology (eds Svensson EI, Calsbeek R), pp. 126–149. New York, NY: Oxford University Press. [Google Scholar]
- 37.Blows MW, Brooks R. 2003. Measuring nonlinear selection. Am. Nat. 162, 815–820. ( 10.1086/378905). [DOI] [PubMed] [Google Scholar]
- 38.Reynolds RJ, Childers DK, Pajewski NM. 2010. The distribution and hypothesis testing of eigenvalues from the canonical analysis of the gamma matrix of quadratic and correlational selection gradients. Evolution 64, 1076–1085. ( 10.1111/j.1558-5646.2009.00874.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green PJ, Silverman BW. 1994. Nonparametric regression and generalised linear models. London, UK: Chapman and Hall. [Google Scholar]
- 40.Schluter D. 1988. Estimating the form of natural selection on a quantitative trait. Evolution 42, 849–861. ( 10.1111/j.1558-(5646.1988.tb02507.x) [DOI] [PubMed] [Google Scholar]
- 41.Bisgaard S, Ankenman B. 1996. Standard errors for the eigenvalues in second-order response surface models. Technometrics 38, 238–246. ( 10.1080/00401706.1996.10484503) [DOI] [Google Scholar]
- 42.Sheldon BC. 1994. Male phenotype, fertility, and the pursuit of extra-pair copulations by female birds. Proc. R. Soc. Lond. B 257, 25–30. ( 10.1098/rspb.1994.0089) [DOI] [Google Scholar]
- 43.Yasui Y. 1997. A ‘good-sperm’ model can explain the evolution of costly multiple mating by females. Am. Nat. 149, 573–584. ( 10.1086/286006) [DOI] [Google Scholar]
- 44.Evans JP, Zane L, Francescato S, Pilastro A. 2003. Directional postcopulatory sexual selection revealed by artificial insemination. Nature 421, 360–363. ( 10.1038/nature01367) [DOI] [PubMed] [Google Scholar]
- 45.Peters A, Denk AG, Delhey K, Kempenaers B. 2004. Carotenoid-based bill colour as an indicator of immunocompetence and sperm performance in male mallards. J. Evol. Biol. 17, 1111–1120. [DOI] [PubMed] [Google Scholar]
- 46.Malo AF, Roldan ER.S., Garde J, Soler AJ, Gomendio M. 2005. Antlers honestly advertise sperm production and quality. Proc. R. Soc. Lond. B 272, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans JP, Kelley JL, Bisazza A, Finazzo E, Pilastro A. 2004. Sire attractiveness influences offspring performance in guppies. Proc. R. Soc. Lond. B 271, 2035–2042. ( 10.1098/rspb.2004.2815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood CW, Brodie ED. 2015. Environmental effects on the structure of the G-matrix. Evolution 69, 2927–2940. ( 10.1111/evo.12795) [DOI] [PubMed] [Google Scholar]
- 49.Stearns SC. 1989. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268. [Google Scholar]
- 50.Humphries S, Evans JP, Simmons LW. 2008. Sperm competition: linking form to function. BMC Evol. Biol. 8, 319 ( 10.1186/1471-2148-8-31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gasparini C, Devigili A, Dosselli R, Pilastro A. 2013. Pattern of inbreeding depression, condition dependence, and additive genetic variance in Trinidadian guppy ejaculate traits. Ecol. Evol. 3, 4940–4953. ( 10.1002/ece3.870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tuni C, Han CS, Dingemanse NJ. 2018. Multiple biological mechanisms result in correlations between pre- and post-mating traits that differ among versus within individuals and genotypes. P. R. Soc. B 285, 20180951 ( 10.1098/rspb.2018.0951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walsh B, Blows MW. 2009. Abundant genetic variation plus strong selection = multivariate genetic constraints: a geometric view of adaptation. Ann. Rev. Ecol. Evol. Syst. 40, 41–59. ( 10.1146/annurev.ecolsys.110308.120232) [DOI] [Google Scholar]
- 54.Radwan J. 2008. Maintenance of genetic variation in sexual ornaments: a review of the mechanisms. Genetica 134, 113–127. ( 10.1007/s10709-007-9203-0) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full dataset has been uploaded as electronic supplementary material.