Abstract
Sexually selected weapons often function as honest signals of fighting ability. If poor-quality individuals produce high-quality weapons, then receivers should focus on other, more reliable signals. Cost is one way to maintain signal integrity. The costs of weapons tend to increase with relative weapon size, and thereby restrict large weapons to high-quality individuals who can produce and maintain them. Weapon cost, however, appears to be unpredictably variable both within and across taxa, and the mechanisms underlying this variation remain unclear. We suggest variation in weapon cost may result from variation in weapon composition—specifically, differences in the amount of muscle mass directly associated with the weapon. We test this idea by measuring the metabolic cost of sexually selected weapons in seven arthropod species and relating these measures to weapon muscle mass. We show that individuals with relatively large weapon muscles have disproportionately high resting metabolic rates and provide evidence that this trend is driven by weapon muscle mass. Overall, our results suggest that variation in weapon cost can be partially explained by variation in weapon morphology and that the integrity of weapon signals may be maintained by increased metabolic cost in species with relatively high weapon muscle mass.
Keywords: animal weapons, sexual selection, cost, metabolic rate
1. Introduction
Sexually selected weapons are some of the most exaggerated and diverse structures in the animal world. They grow out of proportion with body size and other non-sexually selected structures [1–6], and when viewed across clades, they exhibit astounding diversity (e.g. [7–9]).
These weapons typically function as tools for intrasexual competition [9–15]. Animals use these structures to compete with same-sex rivals over direct access to mates [16–20], or over resources required by their mates [21–26]. Evidence also suggests that weapons function as intra- and intersexual signals. Weapon size often scales positively with overall body size [1–6,27], and overall body size typically reflects the genetic and environmental variation underlying individual fitness (hereafter referred to as ‘quality’ [28–32] (but see [33–35]). Through this connection, relative weapon size provides an effective signal of an opponent's resource-holding potential [25,33–36], and members of the opposite sex can use this metric to assess potential mates [25,37,38].
Honesty is essential to the function and persistence of sexual signals, and weapons are no exception [39–46]. If poor-quality animals can cheat and produce high-quality signals (i.e. large weapons), receivers should shift focus to other, more reliable indicators of quality. Cost is one way to maintain signal honesty within a population, particularly when costs are steepest for poor-quality males [39,41–44,46,47]. Weapon cost tends to increase as structures increase in relative size [48–50]. Thus, large structures are both more conspicuous and more difficult to fake, which helps explain why sexual selection so often favours increases in weapon size [39–46].
The cost of sexually selected weapons has been examined from many perspectives and across a variety of taxa [48–57]. From this work, it is clear that the type (developmental, locomotor, metabolic, etc.) and magnitude of these costs is highly variable [58–60]. Some species, for example, experience developmental costs as they invest resources in weapons at the expense of other traits [48,51,57]. Others endure heightened energy expenditure during locomotion, which results from the large weapons they carry (e.g. while running [49,52,54] and flying [50,52]). To date, the source of this variation remains unclear. However, we suggest that some types of weapon cost may be dependent on weapon composition—specifically, the amount of muscle associated with the weapon—and that identifying species with especially costly weapon morphology could help explain observed variation in weapon cost.
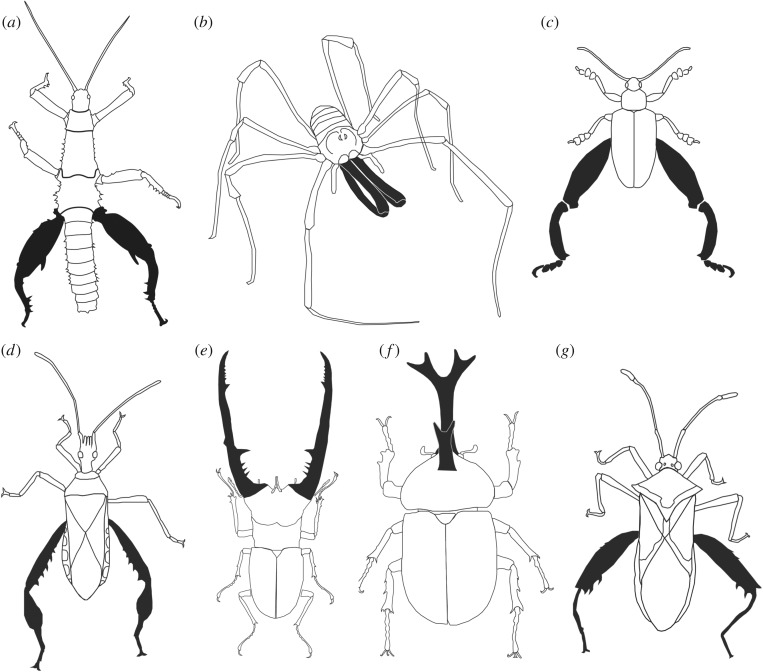
Here, we focus on the muscle content of sexually selected weapons and its relation to metabolic maintenance costs. Increased metabolism is an especially relevant measure of weapon cost, since variation in metabolic rate directly affects an animal's finite resource pool and impacts all other body functions. We predict that variation in weapon muscle mass, which is metabolically expensive to maintain [49,50,54,61,62], will help explain species differences in the metabolic costs associated sexually selected weapons. Using measures of resting metabolic rate (RMR) and weapon muscle mass, we report the metabolic maintenance cost of sexually selected weapons in seven arthropod species (figure 1): thorny devil stick insects (Phasmatodea: Phasmatidae: Eurycantha calcarata), New Zealand long-legged harvestmen (Arachnida: Opiliones: Neopilionidae: Forsteropsalis pureora), frog-legged beetles (Coleoptera: Chrysomelidae: Sagra femorata), leaf-footed cactus bugs (Hemiptera: Coreidae: Narnia femorata), Indonesian stag beetles (Coleoptera: Lucanidae: Cyclommatus metallifer), Japanese rhinoceros beetles (Coleoptera: Scarabaeidae: Trypoxylus dichotomus) and heliconia bugs (Hemiptera: Coreidae: Leptoscelis tricolor). We show that, both within and across species, individuals with large weapon muscles relative to their body size have substantially higher RMRs than individuals with relatively small muscles, and we provide evidence that these trends are driven by muscle mass. We discuss our results in the context of honest signalling and costly weapons, and show that the observed variation in weapon cost is probably associated with variation in the morphology of the weapons studied.
Figure 1.
Study species with weapons defined by shaded area: (a) thorny devil stick insect, (b) New Zealand long-legged harvestman, (c) frog-legged beetle, (d) leaf-footed cactus bug, (e) Indonesian stag beetle, (f) Japanese rhinoceros beetle and (g) heliconia bug.
2. Material and methods
(a). Study species
Species were chosen based on the presence of sexually selected weapons and ease of collection/sourcing through commercial breeders (electronic supplementary material, table S1). Thorny devil stick insects [63,64], frog-legged beetles [20,65], leaf-footed cactus bugs [26,66] and heliconia bugs [67] all have enlarged hindleg weapons. These hindleg weapons are used to squeeze rivals and either (a) dislodge them from high value territories [66,67] or (b) remove them from receptive females mid-copula [20,65]. Indonesian stag beetles have enlarged mandibles used to dislodge rivals from potential nesting sites [68,69]. Japanese rhinoceros beetles have a twice-bifurcated head horn and a smaller, bifurcated thoracic horn, which are used to pry rivals from feeding sites frequented by potential mates [23,70]. New Zealand long-legged harvestmen have enlarged chelicera used to grapple opponents during contests over reproductive territories and/or females [71–74] (figure 1).
(b). Measurement of resting metabolic rate
Flow-through respirometry was used to measure carbon dioxide (CO2) emission at rest (estimate of RMR) for stick insects (n = 19), frog-legged beetles (n = 27), leaf-footed cactus bugs (n = 39), stag beetles (n = 13), rhinoceros beetles (n = 16) and heliconia bugs (n = 22). The entire system was calibrated using pure N2 and 2000 ppm CO2.
CO2 emission rate was measured for 1 h using a two-cell infrared analyser (Licor LI-7000, Licor, NE, USA) in differential mode. Dry, CO2-free air was directed through the reference cell, which measured the fractional CO2 concentration, then through a glass chamber containing the focal animal (2.2 l for stick insects; 14 ml for frog-legged beetles, leaf-looted cactus bugs, stag beetles and heliconia bugs; 500 ml for rhinoceros beetles) and into the measurement cell, which measured fractional CO2 concentration of excurrent air. All gas circulated in 3 mm inner diameter plastic tubing. Air flow was controlled by a mass-flow controller (Unit instruments, CA, USA; 0–500 cm3 min−1), connected to controlling electronics (MFC-4, Sable Systems International, NV, USA). Flow rates were selected based on the body size and CO2 production of each species to balance detectability and temporal resolution and were as follows: stick insects = 2 l min−1, frog-legged beetles, stag beetles, rhinoceros beetles = 500 ml min−1, leaf-footed cactus bugs, heliconia bugs = 250 ml min−1 [75]. Temperature was monitored using T-type thermocouples connected to a thermocouple meter (TC-1000, Sable Systems International). Activity was monitored visually or, when possible, using an activity detector (AD-1, Sable Systems International) and periods of activity were removed from the analysis. Before and after each trial, baseline CO2 in the system was measured for 2 min with the experimental chamber empty. These measures were used to correct for baseline drift by constructing a linear model between CO2 levels at the beginning and end of the trial and subtracting it from each CO2 measurement.
Data were collected for frog-legged beetles using Laboratory Chart (v. 7.2, ADInstruments, AUS) receiving signals from an AD converter (PowerLab 8sp, ADInstruments). For all other species, data were collected using ExpeData (v. 1.1.9, Sable Systems International) receiving signals from an AD converter (UI2, Sable Systems International). AD converters received analogue signals from the two-cell infrared analyser, thermocouple meter and activity detector. The traces collected showed relative concentration of CO2 (ppm) according to time (sampling frequency: 1 Hz). Raw measures were converted to molar rates of CO2 production using known flow rate and the ideal gas law (equation (2.1)),
| 2.1 |
where ṀCO2 = rate of CO2 production, P, pressure (1 ATM), FR, flow rate, Fe, excurrent CO2 concentration, Fi, incurrent CO2 concentration, R = gas constant (0.08206 1 atm K−1 mol−1) and T = temperature. A continuous period of at least 20 min during which the animal was completely inactive was isolated, and mean ṀCO2 production during this time was collected as an estimate of RMR. The first 10 min of each trial were excluded to avoid effects of handling.
Flow-through respirometry was not available for harvestmen. Instead, Warburg manometers were constructed [76]. Each animal (n = 27) was placed in a 60 ml syringe containing soda lime (Ca(OH)2 and NaOH) soaked cotton wool. The syringe was then sealed to a graduated 1 ml syringe containing an ink filled water bubble. As the animal respired, CO2 reacted with the soda lime and formed solid CaCO3, which decreased gas volume and pressure within the syringe. This change in volume/pressure was measured via movement of the water bubble and collected as a measure of O2 consumption and used as an estimate of RMR. Control manometers were run concurrently to correct for the baseline CO2 and changes in atmospheric pressure. Measurements were collected over 20 min trials. Prior to each trial, animals were completely inactive for five minutes. Warburg manometers provide a high level of precision for measuring small organisms despite the lack the control provided by flow-through respirometry [75]. Still, to account for this lack of control, pressure and temperature fluctuation in the system was controlled for using a control manometer and CO2 build up in the system was prevented by limiting trials to 20 min. We believe these actions allowed for reliable comparisons between data collected using Warburg manometers and flow-through respirometry. We recognize, however, the limitations associated with making direct comparisons using different methodologies and urge the reader to consider these limitations when interpreting our results.
(c). Morphological measures and muscle digestion/dissection
All morphological measures were collected after measuring RMR (electronic supplementary material, table S2). For frog-legged beetles, stag beetles, rhinoceros beetles and harvestmen, weapon and body size were measured using digital callipers. For stick insects, leaf-footed cactus bugs and heliconia bugs, weapon and body size were measured from photographs using ImageJ v. 1.50i software (NIH, USA). Body mass of stick insects, harvestmen, leaf-footed bugs, rhinoceros beetles and heliconia bugs was measured directly using digital scales. Body mass of frog legged beetles was estimated using a linear model between body mass and body size PC1 (electronic supplementary material, table S2), which was constructed from a sample of frog-legged beetles collected from the same population at an earlier date. This appeared to be a reliable estimate of body mass for the frog-legged beetles measured here, but it should be noted that this method could have masked differences in individual quality encoded in deviations from the average relationship between body mass and overall size. Direct measures of body mass were unavailable for stag beetles. Instead, mean body mass for stag beetles was sourced from [52] and used as an estimate of mean stag beetle body mass.
Weapon muscle mass was measured using potassium hydroxide (KOH) digestion for frog-legged beetles, leaf-footed cactus bugs, stag beetles, rhinoceros beetles and heliconia bugs [77–79]. Weapon muscle mass was measured in harvestmen using papain digestion. Weapons and associated muscle (electronic supplementary material, table S2) were dissected, dried at 70°C (50°C for harvestmen) and weighed. After initial weighing, weapons were submerged in 10% KOH (18.5 U.ml−1 papain in 100 mM TRIS–HCl pH 7 buffer for harvestmen) and incubated at 70°C (room temperature for harvestmen) to digest soft tissue, primarily muscle (frog-legged beetles, stag beetles, rhinoceros beetles = 12 h; leaf-footed cactus bugs, heliconia bugs = 8 h; long-legged harvestmen = 72 h). After digestion, weapons were rinsed with water and dried at 70°C (50°C for harvestmen). Once dry, weapons were weighed a second time. The difference between first and second weighing was taken as an estimate of dry muscle mass. For rhinoceros beetles, wet muscle mass was collected in place of dry muscle mass (using the protocol described above, except for drying steps), which likely overestimated weapon muscle mass compared to other species in this study. For stick insects, hind femurs were dissected, dried at 70°C for 24 h and weighed. Muscles were then manually dissected (due to their large size) and the hindleg was weighed a second time. The difference between first and second weighing was taken as an estimate of dry muscle mass. For species with paired weapons (stick insects, harvestmen, frog-legged beetles, leaf-footed bugs and heliconia bugs), weapon muscle mass was measured from a single weapon and multiplied by two to calculate total weapon muscle mass.
(d). Statistical analyses
All statistical analyses were performed in R v. 3.5.0 (R Core Development Team, 2018). For analyses of RMR, principal component analyses (PCA; R package FactoMineR [80]) were constructed separately for each species and used to estimate weapon and body size in an effort to capture the effects of overall body size on RMR (variables included in PCA summarized in electronic supplementary material, table S2). For all other analyses, a single, log-transformed linear measure of weapon and body size was used (electronic supplementary material, table S2, bold). This allowed for direct and transparent assessment of scaling relationships against isometry [81].
All data were log-transformed prior to analysis of scaling relationships. Two analyses were performed in each species to assess the relationship between RMR, weapon size and body size. First, RMR was regressed on body size and weapon size in the same model using ordinary least-squares (OLS) regression [82]. Second, residual RMR was regressed on residual weapon size using OLS regression to determine whether males with large relative weapon sizes (i.e. high residual weapon sizes) have high RMRs for their body size (i.e. high residual RMR). Residuals for the latter analyses were collected from separate OLS regressions of RMR on body size and weapon size on body size. Both analyses allowed us to assess the effect of weapon size on RMR while controlling for the effects of overall body size.
OLS regression was also used to assess the relationship between weapon size and body size and muscle mass and body size for all species. Relative weapon muscle mass was calculated first as muscle mass divided by linear measures of body size (relative muscle mass A) and second as muscle mass divided by body mass (relative muscle mass B). Measuring relative weapon muscle mass two ways provided two measures of relative weapon muscle mass for most animals, as well as a reliable measure of weapon muscle mass for stag beetles, where body mass was unavailable. Log mean relative weapon muscle mass was compared across species using means and 95% confidence intervals. For stag beetles, mean weapon muscle mass/mean body mass was used in place of mean relative weapon muscle mass (see above). RMR was then regressed on log relative muscle mass A and B in stick insects, frog-legged beetles, stag beetles (species with a significant relationship between residual RMR and residual weapon size) to determine the role weapon muscle mass plays in the observed trends (note: relative muscle mass B was not regressed on RMR in stag beetles).
3. Results
(a). Principal component analyses
In stick insects, harvestmen, frog-legged beetles, cactus bugs, stag beetles and rhinoceros beetles, principal component 1 (PC1) explained the majority of variation in every PCA and was used as the measure of weapon and/or body size (electronic supplementary material, table S2).
(b). Scaling relationships
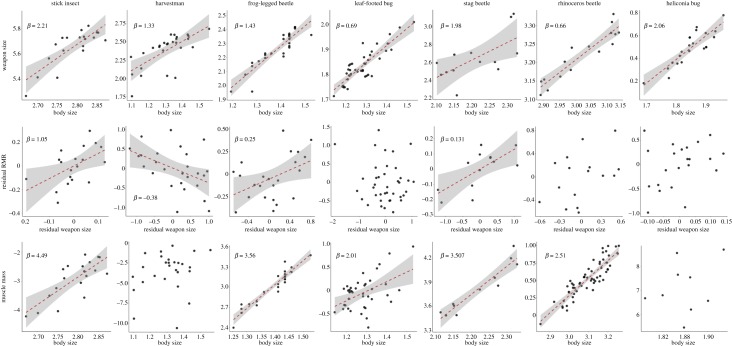
Weapon size increased hyperallometrically (β > 1 for linear measurements) with body size in harvestmen (β = 1.33 ± 0.28, F1,25 = 22.28, p < 0.0001), frog-legged beetles (β = 1.43 ± 0.14, F1,25 = 108.8, p < 0.0001), stag beetles (β = 1.975 ± 0.69, F1,11 = 8.083, p = 0.016) and heliconia bugs (β = 2.064 ± 0.26, F1,20 = 60.81, p < 0.0001). Weapon size increased isometrically with body size in stick insects (β = 2.206 ± 0.38, F1,17 = 32.83, p < 0.0001; isometry for area measurements, β = 2). Weapon size increased hypoallometrically with body size in cactus bugs (β = 0.691 ± 0.06, F1,37 = 113.2, p < 0.0001) and rhinoceros beetles (β = 0.662 ± 0.07, F1,14 = 92, p < 0.0001; figure 2).
Figure 2.
Scaling relationship between log weapon and log body size (top), log residual resting metabolic rate and log residual weapon size (middle), and log muscle mass and log body size (bottom) for all species. Red dashed lines represent OLS regression. Shaded areas represent 95% confidence intervals around OLS regressions. Slopes (β) omitted for non-significant scaling relationships. (Online version in colour.)
RMR increased with weapon size in stick insects (β = 1.263, F2,15 = 10.8, p < 0.001), frog-legged beetles (β = 0.259, F3,19 = 14.65, p < 0.001) and stag beetles (β = 0.133, F3,9 = 15.03, p < 0.001), but there was no significant effect of body size on RMR and no significant interaction between weapon size and body size in these models (electronic supplementary material, table S3). There were no significant relationships between RMR and weapon size or body size in rhinoceros beetles, heliconia bugs, leaf-footed cactus bugs or harvestmen (electronic supplementary material, table S3). Residual RMR increased with residual weapon size in stick insects (β = 1.05, F1,17 = 6.439 p = 0.021), frog-legged beetles (β = 0.254, F1,21 = 6.519, p = 0.019) and stag beetles (β = 0.131, F1,11 = 10.37, p = 0.008), suggesting males with large relative weapon sizes experience higher RMRs than predicted by their body size (figure 2). Residual RMR decreased with residual weapon size in harvestmen (β = −0.375, F1,24 = 6.561, p = 0.017; figure 2). There was no significant relationship between residual RMR and residual weapon size in cactus bugs, rhinoceros beetles or heliconia bugs.
Muscle mass increased hyperallometrically (β > 3 for volumetric measurements [75]) with body size in stick insects (β = 4.849, F1,17 = 27.29, p < 0.001), frog-legged beetles (β = 3.559, F1,22 = 207.5, p < 0.0001) and stag beetles (β = 3.507, F1,9 = 81.82, p < 0.0001; figure 2). Muscle mass increased hypoallometrically with body size in cactus bugs (β = 2.055, F1,37 = 11.86, p < 0.01) and rhinoceros beetles (β = 2.505, F1,55 = 204, p < 0.0001; figure 2). In harvestmen and heliconia bugs, there was no significant relationship between muscle mass and body size.
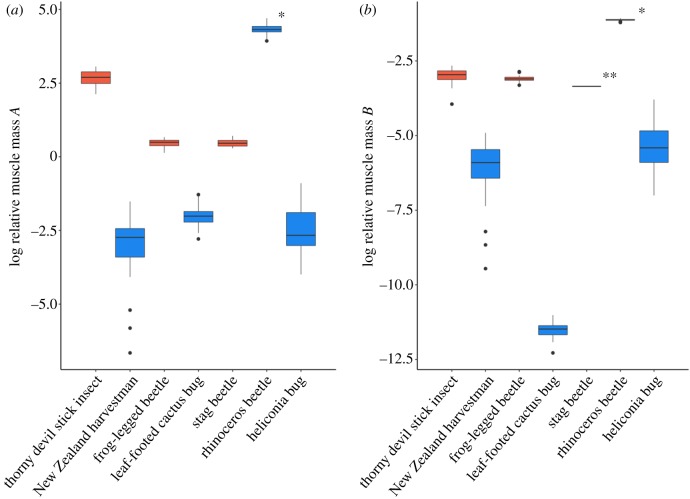
Log relative weapon muscle mass for each species is summarized in figure 3 and electronic supplementary material, table S4. In stick insects, frog-legged beetles and stag beetles, log RMR increased with log relative weapon muscle mass A (stick insects: β = 0.61, F1,17 = 28.55, p < 0.0001; frog-legged beetles: β = 1.367, F1,21 = 30.31, p < 0.0001; stag beetles: β = 1.99, F1,9 = 13.76, p < 0.01) and log relative muscle mass B (stick insects: β = 0.996 F1,17 = 29.65, p < 0.0001; frog-legged beetles: β = 1.988, F1,25 = 12.67, p < 0.01; stag beetles: NA; electronic supplementary material, figure S1), suggesting the observed trends in RMR are indeed driven by high relative weapon muscle mass.
Figure 3.
Boxplot of relative weapon muscle mass for all species. (a) relative muscle mass A = muscle mass/body size. (b) Relative muscle mass B = muscle mass/body mass. Shades of red indicate significant relationships between residual RMR and residual weapon size. Shades of blue indicate no significant relationship between residual RMR and weapon size. Error bars represent 95% confidence intervals around mean relative muscle mass. *Wet muscle mass was measured for rhinoceros beetles in place of dry muscle mass. **Relative muscle mass B calculated for stag beetles as mean muscle mass/mean body mass.
4. Discussion
We suggest that much of the variation observed in costly sexually selected weapons stems from variation in the types of weapons studied and their associated musculature. Here, we surveyed RMR as a metric of metabolic maintenance cost in seven arthropod species (figure 1) and related these measures to weapon muscle mass. In stick insects, frog-legged beetles and stag beetles, three species with high relative muscle mass (figure 3; electronic supplementary material, table S4) and steep scaling relationships between muscle mass and body size, there was a significant positive relationship between RMR and weapon size after controlling for body size (electronic supplementary material, table S3), and residual RMR increased with residual weapon size (figure 2). This suggests that males with large relative weapon sizes have unusually high RMRs, independent of their typically large body sizes. In addition, these species all had significant, positive scaling relationships between RMR and relative muscle mass (electronic supplementary material, figure S1), further suggesting these trends in RMR are driven by weapon muscle mass. By contrast, other species surveyed here showed shallow or non-significant scaling relationships between muscle mass and body size (figure 2), had low relative weapon muscle mass (figure 3), and either a negative or nonsignificant relationship between RMR and weapon size and/or body size (electronic supplementary material, figure S3; figure 2).
Our results suggest that when animals have high weapon-associated muscle mass that scales steeply with body size, they experience relatively high metabolic maintenance costs. These types of heavily muscled weapons should be especially prevalent in animals where performance depends on muscle content (rather than speed or strength-amplifying levers), and when hyperallometric scaling of weapon muscle mass is required to overcome mechanical disadvantage in large weapons [83–85]. Indeed, this is the case for both frog-legged beetles [83] and stag beetles [84], and in both systems residual RMR increased with residual weapon size (figure 1). (Note: there is no published work to date on weapon force production in thorny devil stick insects.)
By extension, we suggest inconsistency in weapon literature regarding cost may, in part, result from interspecific variation in the way weapon strength is generated and the associated variation in weapon muscle mass. Leaf-footed cactus bugs, for example, show no perceptible strength [83] or metabolic cost associated with hindleg weapons and have relatively small muscles that scale hypoallometrically with body size (figures 2 and 3). The heliconia bugs measured here also showed no metabolic cost, and large-weaponed individuals did not have relatively large weapon muscles (figures 2 and 3). Recent work by Somjee et al. [86] does suggest weapon muscle contributes to RMR in this species, but RMR in that study scaled shallowly across weapon sizes, suggesting large-weaponed males experience low metabolic cost relative to their weapon size. In long-legged harvestmen, where relative weapon muscle mass is low and there is no significant relationship between weapon muscle mass and body size, residual RMR decreased as residual weapon size increased (figures 2 and 3). This pattern may result from biological variation in the way chelicera function across body/weapon sizes or may be an artefact of the way weapon/muscle size was measured. For example, weapon-associated muscle in the body, which was not measured here, may be important for weapon function in long-legged harvestmen. Additionally, physiologically relevant variation in harvestmen weapon morphology may not be fully captured by linear measurements of weapon size [71]. Finally, Japanese rhinoceros beetles show no locomotor, immune, developmental or metabolic cost associated with their large, hollow horns, and the prothoracic muscles regulating weapon movement scaled hypoallometrically with body size (figure 2) [58–60]. Rhinoceros beetles did have relatively massive prothoracic muscles compared with weapon muscle mass in other species (figure 3), but this was probably an overestimation since wet muscle mass was measured in place of dry muscle mass. Overall, these trends could result from the difficulty associated with detecting patterns in data composed of small measurements with low variation. It is more likely, however, that weapon strength in each of these species is generated and maintained through modifications of the weapon lever system, rather than hyperallometric increases in weapon muscle (e.g. [69,85]), and that the resulting low muscle mass explains why costs have never been observed.
It should be noted, however, that weapon honesty is still expected in species where metabolic cost was not observed. These weapons still function as signals and should reliably display individual quality. Rather than metabolic costs driven by large muscles, species with small muscles may experience production costs resulting from differential resource allocation during weapon development [48,51,57] or locomotor costs, not from heavy, muscular weapons, but from bulky, otherwise lightweight structures [53,87]. Honesty may also be maintained through heightened condition-dependent development [28,32,47,88–96]. Sexually selected weapons are famously sensitive to developmental nutrition [95], the abiotic environment [97,98], parasite load [99] and stress [100]. When weapon growth is sensitive to these factors, only individuals of the highest quality can produce large weapons. Along with costs, condition dependence can effectively restrict the biggest weapons to the highest quality individuals, ensuring that weapon size persists as a reliable signal through time.
Overall, we suggest that much of the controversy surrounding the presence/absence of cost in weapon systems can be resolved, in part, by recognizing that both the type and magnitude of cost may be dependent on the composition of the weapon studied. Notably, we suspect some of the variation in weapon cost is driven by variation in the ways weapon force is generated and the associated variation in weapon muscle mass. The work presented here clearly suggests a relationship between weapon use, muscle content and cost across arthropod species. However, our interpretation of these results may have been limited by relatively small sample sizes, lack of phylogenetic control, and variation in the methodology used throughout. We therefore encourage those exploring the cost of sexually selected weapons to focus on large groups of species where robust phylogenetic methods are available [101], to use large samples sizes and consistent methodology when possible, and to direct their study using both a priori knowledge of the biomechanical mode of action of the structure and the behavioural ecology of the focal species.
Supplementary Material
Acknowledgements
We thank the NSF, JSPS and RSNZ for funding, Art Woods (U. of Montana) for use of laboratory space, respirometry equipment and assistance in data analysis, Teruyuki Niimi (National Institute for Basic Biology, Okazaki Japan) for use of laboratory space, Christine Miller for assistance in early planning of the project, Christine Miller and Pablo Allen for supplying Narnia femorata, Hiroki Gotoh for supplying Cylcomattus metallifer, Masako Katsuki and Yasu Okada for assistance in collecting Sagra femorata, and Lizanne Gomes, Caitlin Selleck and Nikolas Willmott for assistance in field and data collection of Forsteropsalis pureora.
Data accessibility
Data supporting this article are available through Harvard Dataverse.
Authors' contributions
Conceptualization: D.M.O.; methodology: D.M.O., R.P.B., A.J.H.; formal analysis: D.M.O., R.P.B., U.S., M.D., E.M., E.C.P., S.S., A.J.H.; investigation: D.M.O., R.P.B., U.S., M.D., E.M., E.C.P., S.S; resources: D.M.O., R.P.B., U.S., M.D., E.M., E.C.P., A.J.H., G.I.H., C.J. P., D.J.E.; data curation: D.M.O., R.P.B., U.S., M.D., A.J.H., E.C.P., E.M.; writing—original draft: D.M.O.; writing—reviewing and editing: D.M.O., R.P.B., U.S., M.D., E.M., E.C.P., S.S, A.J.H., G.I.H., C.J.P., D.J.E.; supervision: D.M.O., A.J.H., G.I.H., C.J.P. and D.J.E.; project administration: D.M.O; funding acquisition; D.M.O, U.S., M.D., E.M., E.C.P., G.I.H., D.J.E.
Competing interests
We have no competing interests to report.
Funding
D.M.O. NSF/JSPS—award no. 1515085. D.J.E. NSF—IOS-1456133. G.I.H. RSNZ—Marsden fund no. 15-UOA-241.
References
- 1.Stern DL, Emlen DJ. 1999. The developmental basis for allometry in insects. Development 126, 1091–1101. [DOI] [PubMed] [Google Scholar]
- 2.Emlen DJ, Allen CE. 2003. Genotype to phenotype: physiological control of trait size and scaling in insects. Integr. Comp. Biol. 43, 617–634. ( 10.1093/icb/43.5.617) [DOI] [PubMed] [Google Scholar]
- 3.Bonduriansky R, Day T. 2003. The evolution of static allometry in sexually selected traits. Evolution 57, 2450–2458. ( 10.1111/j.0014-3820.2003.tb01490.x) [DOI] [PubMed] [Google Scholar]
- 4.Kodric-Brown A, Sibly RM, Brown JH. 2006. The allometry of ornaments and weapons. Proc. Natl Acad. Sci. USA 103, 8733–8738. ( 10.1073/pnas.0602994103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shingleton AW, Frankino WA. 2013. New perspectives on the evolution of exaggerated traits. BioEssays News Rev. Mol. Cell. Dev. Biol. 35, 100–107. ( 10.1002/bies.201200139) [DOI] [PubMed] [Google Scholar]
- 6.Fromhage L, Kokko H. 2014. Sexually selected traits evolve positive allometry when some matings occur irrespective of the trait. Evolution 68, 1332–1338. ( 10.1111/evo.12349) [DOI] [PubMed] [Google Scholar]
- 7.Eberhard WG. 1980. Horned beetles. Sci. Am. 242, 166–183. ( 10.1038/scientificamerican0380-166) [DOI] [Google Scholar]
- 8.Geist V. 1966. The evolution of horn-like organs. Behaviour 27, 175–214. ( 10.1163/156853966X00155) [DOI] [Google Scholar]
- 9.Emlen DJ. 2008. The evolution of animal weapons. Annu. Rev. Ecol. Evol. Syst. 39, 387–413. ( 10.1146/annurev.ecolsys.39.110707.173502) [DOI] [Google Scholar]
- 10.Rico-Guevara A, Hurme KJ. 2018. Intrasexually selected weapons. Biol. Rev. 94, 60–101. ( 10.1111/brv.12436) [DOI] [PubMed] [Google Scholar]
- 11.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 12.Hardy IC, Briffa M. 2013. Animal contests. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Emlen DJ. 2014. Animal weapons: the evolution of battle. New York, NY: Henry Holt and Company. [Google Scholar]
- 14.Shuker D, Simmons L, others. 2014. The evolution of insect mating systems. Oxford: Oxford University Press. [Google Scholar]
- 15.McCullough EL, Miller CW, Emlen DJ.. 2016. Why sexually selected weapons are not ornaments. Trends Ecol. Evol. 31, 742–751. ( 10.1016/j.tree.2016.07.004) [DOI] [PubMed] [Google Scholar]
- 16.Lundrigan B. 1996. Morphology of horns and fighting behavior in the family Bovidae. J. Mammal. 77, 462–475. ( 10.2307/1382822) [DOI] [Google Scholar]
- 17.Simmons LW, Ridsdill-Smith TJ. 2011. Ecology and evolution of dung beetles. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 18.Okada K, Miyatake T. 2009. Genetic correlations between weapons, body shape and fighting behaviour in the horned beetle Gnatocerus cornutus. Anim. Behav. 77, 1057–1065. ( 10.1016/j.anbehav.2009.01.008) [DOI] [Google Scholar]
- 19.Painting CJ, Holwell GI. 2014. Observations on the ecology and behaviour of the New Zealand giraffe weevil (Lasiorhynchus barbicornis). N. Z. J. Zool. 41, 147–153. ( 10.1080/03014223.2013.854816) [DOI] [Google Scholar]
- 20.O'Brien D M, Katsuki M, Emlen DJ. 2017. Patterns of selection in the frog legged beetle (Sagra femorata). Evolution 71, 2584–2598. ( 10.1111/evo.13336) [DOI] [PubMed] [Google Scholar]
- 21.Zeh DW, Zeh JA, Tavakilian G. 1992. Sexual selection and sexual dimorphism in the harlequin beetle Acrocinus longimanus. Biotropica 24, 86–96. ( 10.2307/2388476) [DOI] [Google Scholar]
- 22.Kelly CD. 2006. Fighting for harems: assessment strategies during male–male contests in the sexually dimorphic Wellington tree weta. Anim. Behav. 72, 27–736. ( 10.1016/j.anbehav.2006.02.007) [DOI] [Google Scholar]
- 23.Hongo Y. 2007. Evolution of male dimorphic allometry in a population of the Japanese horned beetle Trypoxylus dichotomus septentrionalis. Behav. Ecol. Sociobiol. 62, 245–253. ( 10.1007/s00265-007-0459-2) [DOI] [Google Scholar]
- 24.Judge KA, Bonanno VL. 2008. Male weaponry in a fighting cricket. PLoS ONE 3, e3980 ( 10.1371/journal.pone.0003980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennenmoser S, Christy JH. 2013. The design of a beautiful weapon: compensation for opposing sexual selection on a trait with two functions. Evolution 67, 1181–1188. ( 10.1111/evo.12018) [DOI] [PubMed] [Google Scholar]
- 26.Addesso KM, Short KA, Moore AJ, Miller CW. 2014. Context-dependent female mate preferences in leaf-footed cactus bugs. Behaviour 151, 479–492. ( 10.1163/1568539X-00003137) [DOI] [Google Scholar]
- 27.O'Brien DM, Allen CE, Van Kleeck MJ, Hone D, Knell R, Knapp A, Christiansen S, Emlen DJ. 2018. On the evolution of extreme structures: static scaling and the function of sexually selected signals. Anim. Behav. 144, 95–108. ( 10.1016/j.anbehav.2018.08.005) [DOI] [Google Scholar]
- 28.Bonduriansky R. 2006. The evolution of condition-dependent sexual dimorphism. Am. Nat. 169, 9–19. [DOI] [PubMed] [Google Scholar]
- 29.Peig J, Green AJ. 2010. The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct. Ecol. 24, 1323–1332. ( 10.1111/j.1365-2435.2010.01751.x) [DOI] [Google Scholar]
- 30.Bolger T, Connolly PL. 1989. The selection of suitable indices for the measurement and analysis of fish condition. J. Fish Biol. 34, 171–182. ( 10.1111/j.1095-8649.1989.tb03300.x) [DOI] [Google Scholar]
- 31.Cuervo JJ, Møller AP. 2001. Components of phenotypic variation in avian ornamental and non-ornamental feathers. Evol. Ecol. 15, 53–72. ( 10.1023/A:1011913804309) [DOI] [Google Scholar]
- 32.Cotton S, Fowler K, Pomiankowski A. 2004. Condition dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae). Evolution 58, 1038–1046. ( 10.1111/j.0014-3820.2004.tb00437.x) [DOI] [PubMed] [Google Scholar]
- 33.Clutton-Brock TH, Albon SD, Gibson RM, Guinness FE. 1979. The logical stag: adaptive aspects of fighting in red deer (Cervus elaphus L.). Anim. Behav. 27(Part 1), 211–225. ( 10.1016/0003-3472(79)90141-6) [DOI] [Google Scholar]
- 34.Barrette C, Vandal D. 1990. Sparring, relative antler size, and assessment in male caribou. Behav. Ecol. Sociobiol. 26, 383–387. ( 10.1007/BF00170894) [DOI] [Google Scholar]
- 35.Panhuis TM, Wilkinson GS. 1999. Exaggerated male eye span influences contest outcome in stalk-eyed flies (Diopsidae). Behav. Ecol. Sociobiol. 46, 221–227. ( 10.1007/s002650050613) [DOI] [Google Scholar]
- 36.Painting CJ, Holwell GI. 2014. Exaggerated rostra as weapons and the competitive assessment strategy of male giraffe weevils. Behav. Ecol. 25, 1223–1232. ( 10.1093/beheco/aru119) [DOI] [Google Scholar]
- 37.Wilkinson GS, Reillo PR. 1994. Female choice response to artificial selection on an exaggerated male trait in a stalk-eyed fly. Proc. R. Soc. Lond. B 255, 1–6. ( 10.1098/rspb.1994.0001) [DOI] [Google Scholar]
- 38.Vanpé C, Gaillard J-M, Kjellander P, Liberg O, Delorme D, Hewison AJ. 2010. Assessing the intensity of sexual selection on male body mass and antler length in roe deer Capreolus capreolus: is bigger better in a weakly dimorphic species? Oikos 119, 1484–1492. ( 10.1111/j.1600-0706.2010.18312.x) [DOI] [Google Scholar]
- 39.Biernaskie JM, Grafen A, Perry JC. 2014. The evolution of index signals to avoid the cost of dishonesty. Proc. R. Soc. B 281, 20140876 ( 10.1098/rspb.2014.0876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zahavi A. 1975. Mate selection—a selection for a handicap. J. Theor. Biol. 53, 205–214. ( 10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 41.Pomiankowski A. 1987. Sexual selection: the handicap principle does work—sometimes. Proc. R. Soc. Lond. B 231, 123–145. ( 10.1098/rspb.1987.0038) [DOI] [Google Scholar]
- 42.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546. ( 10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 43.Iwasa Y, Pomiankowski A, Nee S. 1991. The evolution of costly mate preferences. II. The 'handicap' principle. Evolution 45, 1431–1442. ( 10.1111/j.1558-5646.1991.tb02646.x) [DOI] [PubMed] [Google Scholar]
- 44.Johnstone RA. 1995. Sexual seletoin, honest advertisement and the handicap principle: reviewing the evidence. Biol. Rev. 70, 1–65. ( 10.1111/j.1469-185X.1995.tb01439.x) [DOI] [PubMed] [Google Scholar]
- 45.Eshel I, Volovik I, Sansone E. 2000. On Fisher-Zahavi's handicapped sexy son. Evol. Ecol. Res. 2, 509–523. [Google Scholar]
- 46.Számadó S. 2011. The cost of honesty and the fallacy of the handicap principle. Anim. Behav. 81, 3–10. ( 10.1016/j.anbehav.2010.08.022) [DOI] [Google Scholar]
- 47.Rowe L, Houle D. 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263, 1415–1421. ( 10.1098/rspb.1996.0207) [DOI] [Google Scholar]
- 48.Emlen DJ. 2001. Costs and the diversification of exaggerated animal structures. Science 291, 1534–1536. ( 10.1126/science.1056607) [DOI] [PubMed] [Google Scholar]
- 49.Allen BJ, Levinton JS. 2007. Costs of bearing a sexually selected ornamental weapon in a fiddler crab. Funct. Ecol. 21, 154–161. ( 10.1111/j.1365-2435.2006.01219.x) [DOI] [Google Scholar]
- 50.Goyens J, Van Wassenbergh S, Dirckx J, Aerts P.. 2015. Cost of flight and the evolution of stag beetle weaponry. J. R. Soc. Interface 12, 20150222 ( 10.1098/rsif.2015.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baxter BJ, Andrews RN, Barrell GK.. 1999. Bone turnover associated with antler growth in red deer (Cervus elaphus). Anat. Rec. 256, 14–19. () [DOI] [PubMed] [Google Scholar]
- 52.Goyens J, Dirckx J, Aerts P. 2015. Costly sexual dimorphism in Cyclommatus metallifer stag beetles. Funct. Ecol. 29, 35–43. ( 10.1111/1365-2435.12294) [DOI] [PubMed] [Google Scholar]
- 53.Moczek AP, Emlen DJ. 2000. Male horn dimorphism in the scarab beetle, Onthophagus taurus: do alternative reproductive tactics favour alternative phenotypes? Anim. Behav. 59, 459–466. ( 10.1006/anbe.1999.1342) [DOI] [PubMed] [Google Scholar]
- 54.Bywater CL, White CR, Wilson RS. 2014. Metabolic incentives for dishonest signals of strength in the fiddler crab Uca vomeris. J. Exp. Biol. 217, 2848–2850. ( 10.1242/jeb.099390) [DOI] [PubMed] [Google Scholar]
- 55.Picard K, Festa-Bianchet M, Thomas D. 1996. The cost of horniness: heat loss may counter sexual selection for large horns in temperate bovids. Écoscience 3, 280–284. ( 10.1080/11956860.1996.11682343) [DOI] [Google Scholar]
- 56.Caravello HE, Cameron GN. 1987. The effects of sexual selection on the foraging behaviour of the Gulf Coast fiddler crab, Uca panacea. Anim. Behav. 35, 1864–1874. ( 10.1016/S0003-3472(87)80079-9) [DOI] [Google Scholar]
- 57.Simmons LW, Emlen DJ. 2006. Evolutionary trade-off between weapons and testes. Proc. Natl Acad. Sci. USA 103, 16 346–16 351. ( 10.1073/pnas.0603474103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCullough EL, Emlen DJ. 2013. Evaluating the costs of a sexually selected weapon: big horns at a small price. Anim. Behav. 86, 977–985. ( 10.1016/j.anbehav.2013.08.017) [DOI] [Google Scholar]
- 59.McCullough EL, Weingarden PR, Emlen DJ. 2012. Costs of elaborate weapons in a rhinoceros beetle: how difficult is it to fly with a big horn? Behav. Ecol. 23, 1042–1048. ( 10.1093/beheco/ars069) [DOI] [Google Scholar]
- 60.McCullough EL, Tobalske BW. 2013. Elaborate horns in a giant rhinoceros beetle incur negligible aerodynamic costs. Proc. R. Soc. B 280, 20130197 ( 10.1098/rspb.2013.0197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beltman JGM, Van Der Vliet MR, Sargeant AJ, De Haan A.. 2004. Metabolic cost of lengthening, isometric and shortening contractions in maximally stimulated rat skeletal muscle. Acta Physiol. Scand. 182, 179–187. ( 10.1111/j.1365-201X.2004.01338.x) [DOI] [PubMed] [Google Scholar]
- 62.Hortobágyi T, Finch A, Solnik S, Rider P, DeVita P. 2011. Association between muscle activation and metabolic cost of walking in young and old adults. J. Gerontol. Ser. A 66A, 541–547. ( 10.1093/gerona/glr008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bedford GO. 1976. Defensive behaviour of the New Guinea stick insect Eurycantha (Phasmatodea: Phasmatidae: Eurycanthinae). Proc. Linnean Soc. NSW 100, 218–222. [Google Scholar]
- 64.Hsiung C-C. 1987. Aspects of the biology of the Melanesian stick-insect Eurycantha calcarata Lucas (Cheleutoptera: Phasmatidae). J. Nat. Hist. 21, 1241–1258. ( 10.1080/00222938700770761) [DOI] [Google Scholar]
- 65.Katsuki M, Yokoi T, Funakoshi K, Oota N. 2014. Enlarged hind legs and sexual behavior with male-male intercation in Sagra femorata (Coleoptera: Chrysomelidae). Entomol. News 124, 211–220. ( 10.3157/021.124.0306) [DOI] [Google Scholar]
- 66.Procter DS, Moore AJ, Miller CW. 2012. The form of sexual selection arising from male–male competition depends on the presence of females in the social environment. J. Evol. Biol. 25, 803–812. ( 10.1111/j.1420-9101.2012.02485.x) [DOI] [PubMed] [Google Scholar]
- 67.Miller CW. 2007. Maternal effects and sexual selection in the heliconia bug, Leptoscelis tricolor (Hemiptera: Coreidae). PhD dissertation, University of Montana, Missoula, MT.
- 68.Gotoh H, Fukaya K, Miura T. 2012. Heritability of male mandible length in the stag beetle Cyclommatus metallifer. Entomol. Sci. 15, 430–433. ( 10.1111/j.1479-8298.2012.00527.x) [DOI] [Google Scholar]
- 69.Goyens J, Dirckx J, Aerts P. 2015. Stag beetle battle behavior and its associated anatomical adaptations. J. Insect Behav. 28, 227–244. ( 10.1007/s10905-015-9495-3) [DOI] [Google Scholar]
- 70.Hongo Y. 2003. Appraising behaviour during male-male interaction in the Japanese horned beetle Trypoxylus dichotomus septentrionalis (Kono). Behaviour 140, 501–517. ( 10.1163/156853903322127959) [DOI] [Google Scholar]
- 71.Painting CJ, Probert AF, Townsend DJ, Holwell GI. 2015. Multiple exaggerated weapon morphs: a novel form of male polymorphism in harvestmen. Sci. Rep. 5, 16368 ( 10.1038/srep16368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buzatto BA, Macías-Ordóñez R, Machado G. 2013. Macroecology of harvestman mating systems. In Sexual selection (eds Macedo RH, Machado G, pp. 115–162. London, UK: Academic Press. [Google Scholar]
- 73.Taylor CK. 2004. New Zealand harvestmen of the subfamily Megalopsalidinae (Opiliones: Monoscutidae)—the genus Pantopsalis. Tuhinga 15, 53–76. [Google Scholar]
- 74.Buzatto BA, Machado G. 2014. Male dimorphism and alternative reproductive tactics in harvestmen (Arachnida: Opiliones). Behav. Processes 109, 2–13. ( 10.1016/j.beproc.2014.06.008) [DOI] [PubMed] [Google Scholar]
- 75.Lighton JR. 2008. Measuring metabolic rates: a manual for scientists: a manual for scientists. Oxford, UK: Oxford University Press. [Google Scholar]
- 76.Warburg O. 1956. On the origin of cancer cells. Science 123, 309–314. ( 10.1126/science.123.3191.309) [DOI] [PubMed] [Google Scholar]
- 77.Holwell GI. 2008. Geographic variation in genital morphology of Ciulfina praying mantids. J. Zool. 276, 108–114. ( 10.1111/j.1469-7998.2008.00475.x) [DOI] [Google Scholar]
- 78.Holwell GI, Winnick C, Tregenza T, Herberstein ME. 2010. Genital shape correlates with sperm transfer success in the praying mantis Ciulfina klassi (Insecta: Mantodea). Behav. Ecol. Sociobiol. 64, 617–625. ( 10.1007/s00265-009-0879-2) [DOI] [Google Scholar]
- 79.Holwell GI, Ginn SG, Herberstein ME. 2007. Three new species of Ciulfina Giglio-Tos (Mantodea: Liturgusidae) from north-eastern Australia. Zootaxa 1583, 23–35. ( 10.11646/zootaxa.1583.1.3) [DOI] [Google Scholar]
- 80.Le S, Josse J, Husson F.. 2008. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25, 1–18. ( 10.18637/jss.v025.i01) [DOI] [Google Scholar]
- 81.Corruccini RS. 1983. Principal components for allometric analysis. Am. J. Phys. Anthropol. 60, 451–453. ( 10.1002/ajpa.1330600406) [DOI] [PubMed] [Google Scholar]
- 82.Kilmer JT, Rodríguez RL. 2016. Ordinary least squares regression is indicated for studies of allometry. J. Evol. Biol. 30, 4–12. ( 10.1111/jeb.12986) [DOI] [PubMed] [Google Scholar]
- 83.O'Brien DM, Boisseau RP. 2018. Overcoming mechanical adversity in extreme hindleg weapons. PLoS ONE 13, e0206997 ( 10.1371/journal.pone.0206997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mills MR, Nemri RS, Carlson EA, Wilde W, Gotoh H, Lavine LC, Swanson BO. 2016. Functional mechanics of beetle mandibles: honest signaling in a sexually selected system. J. Exp. Zool. Part Ecol. Genet. Physiol. 325, 3–12. ( 10.1002/jez.1961) [DOI] [PubMed] [Google Scholar]
- 85.Vogel S. 2013. Comparative biomechanics: life's physical world. Princeton, NJ: Princeton University Press. [Google Scholar]
- 86.Somjee U, Woods HA, Duell M, Miller CW. 2018. The hidden cost of sexually selected traits: the metabolic expense of maintaining a sexually selected weapon. Proc. R. Soc. B 285, 20181685 ( 10.1098/rspb.2018.1685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Swallow JG, Wilkinson GS, Marden JH. 2000. Aerial performance of stalk-eyed flies that differ in eye span. J. Comp. Physiol. B 170, 481–487. ( 10.1007/s003600000124) [DOI] [PubMed] [Google Scholar]
- 88.Andersson M. 1986. Evolution of condition-dependent sex ornaments and mating preferences: sexual selection based on viability differences. Evolution 40, 804–816. ( 10.1111/j.1558-5646.1986.tb00540.x) [DOI] [PubMed] [Google Scholar]
- 89.David P, Hingle A, Greig D, Rutherford A, Pomiankowski A, Fowler K. 1998. Male sexual ornament size but not asymmetry reflects condition in stalk-eyed flies. Proc. R. Soc. Lond. B 265, 2211–2216. ( 10.1098/rspb.1998.0561) [DOI] [Google Scholar]
- 90.Gosden TP, Chenoweth SF. 2011. On the evolution of heightened condition dependence of male sexual displays. J. Evol. Biol. 24, 685–692. ( 10.1111/j.1420-9101.2010.02205.x) [DOI] [PubMed] [Google Scholar]
- 91.Johns A, Gotoh H, McCullough EL, Emlen DJ, Lavine LC. 2014. Heightened condition-dependent growth of sexually selected weapons in the rhinoceros beetle, Trypoxylus dichotomus (Coleoptera: Scarabaeidae). Integr. Comp. Biol. 54, 614–621. ( 10.1093/icb/icu041) [DOI] [PubMed] [Google Scholar]
- 92.Zeh DW, Zeh JA. 1988. Condition-dependent sex ornaments and field tests of sexual-selection theory. Am. Nat. 132, 454–459. ( 10.1086/284863) [DOI] [Google Scholar]
- 93.Wilkinson GS, Taper M. 1999. Evolution of genetic variation for condition-dependent traits in stalk-eyed flies. Proc. R. Soc. Lond. B 266, 1685–1690. ( 10.1098/rspb.1999.0832) [DOI] [Google Scholar]
- 94.Suttie JM, Fennessy PF, Corson ID, Laas FJ, Crosbie SF, Butler JH, Gluckman PD. 1989. Pulsatile growth hormone, insulin-like growth factors and antler development in red deer (Cervus elaphus scoticus) stags. J. Endocrinol. 121, 351–360. ( 10.1677/joe.0.1210351) [DOI] [PubMed] [Google Scholar]
- 95.Emlen DJ, Warren IA, Johns A, Dworkin I, Lavine LC. 2012. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science 337, 860–864. ( 10.1126/science.1224286) [DOI] [PubMed] [Google Scholar]
- 96.Gotoh H, Miyakawa H, Ishikawa A, Ishikawa Y, Sugime Y, Emlen DJ, Lavine LC, Miura T. 2014. Developmental link between sex and nutrition; doublesex regulates sex-specific mandible growth via juvenile hormone signaling in stag beetles. PLoS Genet. 10, e1004098 ( 10.1371/journal.pgen.1004098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Emlen DJ. 1994. Environmental control of horn length dimorphism in the beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Proc. R. Soc. Lond. B 256, 131–136. ( 10.1098/rspb.1994.0060) [DOI] [Google Scholar]
- 98.Gillespie SR, Scarlett Tudor M, Moore AJ, Miller CW. 2014. Sexual selection is influenced by both developmental and adult environments. Evolution 68, 3421–3432. ( 10.1111/evo.12526) [DOI] [PubMed] [Google Scholar]
- 99.Folstad I, Arneberg P, Karter AJ. 1996. Antlers and parasites. Oecologia 105, 556–558. ( 10.1007/BF00330020) [DOI] [PubMed] [Google Scholar]
- 100.Topiński P. 1975. Abnormal antler cycles in deer as a result of stress inducing factors. Acta Theriol. (Warsz.) 20, 267–279. ( 10.4098/AT.arch.75-23) [DOI] [Google Scholar]
- 101.Blomberg SP, Garland T, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. ( 10.1111/j.0014-3820.2003.tb00285.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this article are available through Harvard Dataverse.