Abstract
A growing body of research demonstrates the impacts of invasive alien plants on native animals, but few studies consider thermal effects as a driver of the responses of native organisms. As invasive alien plants establish and alter the composition and arrangement of plant communities, the thermal landscapes available to ectotherms also change. Our study reviews the research undertaken to date on the thermal effects of alien plant invasions on native reptiles, amphibians, insects and arachnids. The 37 studies published between 1970 and early 2019 portray an overall detrimental effect of invasive plants on thermal landscapes, ectothermic individuals' performance and species abundance, diversity and composition. With a case study of a lizard species, we illustrate the use of thermal ecology tools in plant invasion research and test the generality of alien plant effects: changes in thermoregulation behaviour in invaded landscapes varied depending on the level of invasion and lizard traits. Together, the literature review and case study show that thermal effects of alien plants on ectotherms can be substantial albeit context-dependent. Further research should cover multiple combinations of native/invasive plant growth forms, invasion stages and ectotherm traits. More attention is also needed to test causality along the chain of effects from thermal landscapes to individuals, populations and communities.
Keywords: arthropod, climate change, habitat modification, herpetofauna, individual-based model, temperature tolerance
1. Introduction
The numbers of established alien species show no sign of saturation [1], posing a major threat to biodiversity globally [2]. Evidence has been accumulating of the impacts of alien plants on biodiversity at the organism, population, species, community and ecosystem levels [3–5], and their repercussions for human well-being [6]. For resident animals, research has addressed mostly the impacts at the community level. One of the most comprehensive reviews of invasive plant impacts [4] reported the results of 147 case studies of impacts on animal abundance and species richness and diversity. Establishing causality requires an understanding of the underlying organismal mechanisms and the way in which the effects on organisms cascade across levels of organization, but the mechanisms associated with changing animal abundance or richness in invaded landscapes are seldom tested [7,8]. The same review of invasive plant impacts [4] included only 46 case studies documenting species-level effects on the survival, fecundity and activity of resident animals.
Animal responses to alien plants are associated with disrupted herbivory or predator–prey interactions, altered reproductive success or modified habitat structure and associated niche availability [7,9]. Habitat structure changes are particularly important for ectothermic animals that use behavioural thermoregulation to optimize performance. Vegetation influences the levels of solar radiation and wind that reach the soil [10], mediating the thermal quality of available micro-sites [9,11]. At the landscape scale, vegetation modulates the spatial arrangement of micro-climates. Land cover changes modifying the vegetation thus inevitably alter the thermal landscapes. For example, fragmented or deforested habitats can become warmer [12–14], whereas temperatures tend to decrease with conversion to silviculture [15]. Mounting evidence for habitat modification-mediated changes in thermal landscapes has led to calls for the integration of the thermal ecology and habitat fragmentation fields of research [12]. Recent work has examined the importance of the thermal tolerance of resident animals in determining their responses to habitat fragmentation, land conversion or deforestation [16,17]. By contrast, studies of habitat modification caused by plant invasions have thus far given insufficient attention to thermal landscape change as a mechanism driving ectotherm responses.
Similarly to habitat fragmentation and conversion, the introduction of invasive alien plants alters the composition and arrangement of plant communities [3,4,7]. Alien plant invasions often result in a reduction in the cover, abundance and diversity of resident biota [3,4,18]. Such changes may result in landscapes with more homogeneous vegetation, although the level of change depends on the type and number of native and alien plants and the degree of similarity between the two communities [18,19]. For example, in South African Fynbos shrublands, invasion by Acacia saligna trees led to an increase in the height of the vegetation community and a reduction in understory shrubs [20]. In another example, alien moss spreading in coastal dune scrubland resulted in reduced layer cover and vegetation height, simpler micro-habitat structure and more shading [21]. Vegetation changes such as these affect the thermal heterogeneity available to ectotherms, with implications for thermoregulation.
To explore the extent to which thermal effects of alien plants have been addressed so far, we review the plant invasion literature focused on thermal landscape changes and consequences for reptiles, amphibians, insects and arachnids. These are suitable taxonomic groups for this question, as their energetic and activity budgets are strongly coupled to the thermal landscapes they inhabit. First, we summarize published evidence of changes in thermal landscapes, organisms’ responses and population and community dynamics. We identify gaps in the integration of thermal ecology into plant invasion impacts research and highlight useful tools to pursue this integration. Second, to illustrate the use of these tools and test the generality of impacts, we present a case study of lizard thermoregulation in invaded landscapes. We compare the thermal landscapes along a gradient of alien plant invasion and the responses of lizards with different motility levels.
2. The chain of effects of alien plants from thermal landscapes to ectotherm communities
By altering the structural complexity of habitats, invasive alien plants initiate a chain of effects on thermal landscapes and resident ectotherms. Where optimal micro-climates become less abundant or accessible, thermoregulation costs for ectotherms can increase [22], imposing constraints on activity and affecting performance. Effects at the individual level trickle down to demographic changes [23], species range shifts [24] and community re-structuring [25]. Our literature review covered the different stages in this chain of effects: changes in thermal landscapes, responses of ectothermic organisms and changes in ectotherm populations or communities. We searched the ISI Web of Knowledge for articles published since 1970, using a combination of search terms for non-native plants, thermal effects and native reptiles, amphibians, insects or arachnids (electronic supplementary material, table S1). Studies were considered relevant when they presented quantitative comparisons between native and invaded habitats of both (i) the thermal landscape stage and (ii) at least one of the native ectotherm response stages. We considered comparisons between sites of native vegetation with and without the presence of alien plants, thus excluding plantations and other anthropogenic landscapes as invaded sites but including restored habitats as native sites. When studies performed multiple comparisons for different native ectotherms, invasive or native plant species, invasion levels, sites or experimental venues, we recorded separate entries for each comparison (see detailed methods in the electronic supplementary material, S1).
We found 37 relevant studies published mostly from 2007 (see the electronic supplementary material, S2 and figure S1), covering a total of 169 comparisons. The studies were biased towards reptiles and insects (35% and 29%, respectively), with half of all comparisons across taxonomic groups covering insects. Lizards and frogs received the most attention among reptiles and amphibians, respectively, and Lepidoptera and Araneae were the best represented orders among insects and arachnids, respectively (electronic supplementary material, figure S2). Among single-alien species invasions, the Pinus radiata, Olea europaea and Acacia longifolia trees and the Arundo donax and Microstegium vimineum grasses had the largest numbers of comparisons (electronic supplementary material, figure S3). There were more studies in the Northern Hemisphere (57%), particularly in North America (electronic supplementary material, figure S4), and in woodland or riparian habitats. In each comparison, one or several variables were used to describe a particular stage of the chain of effects (electronic supplementary material, table S2). For example, mean, maximum and minimum temperatures were some of the variables applied to the thermal landscape change stage.
(a). Changes in thermal landscapes
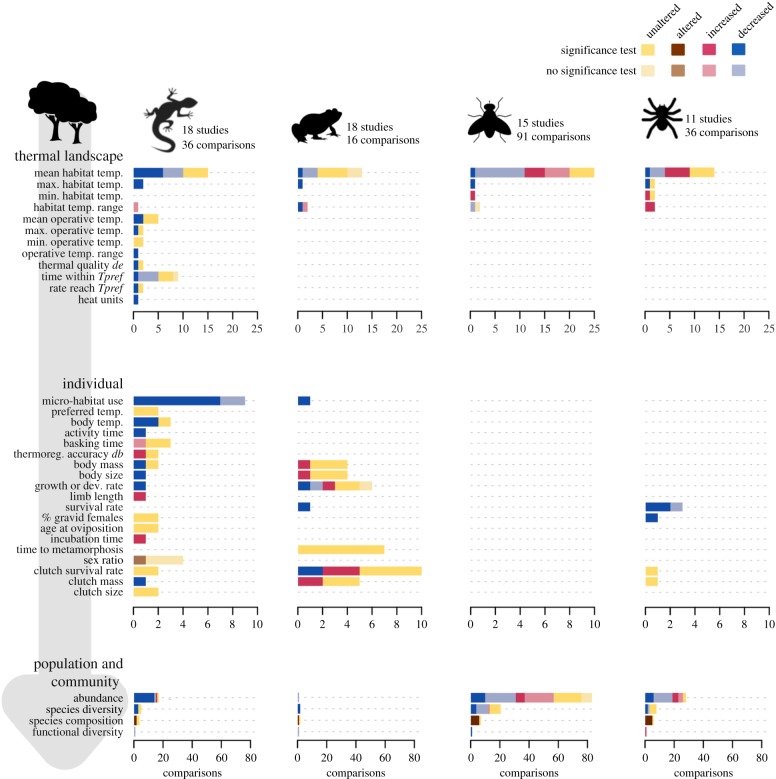
In most comparisons for herpetofauna, invaded habitats had cooler or similar mean temperatures relative to native habitats, but there was more variation in results for insects and arachnids (figure 1). Differences in methodologies used to measure temperature, including the temporal and spatial sampling intensity as well as the type of temperature measured, probably contributed to these inconsistent trends. In particular, reptile and amphibian studies measured mostly substrate and water temperature, respectively, whereas most insect and arachnid studies measured soil temperature. In addition, comparisons involving invasive plants of larger size than the dominant native plants were most frequent in studies with reptiles. Changes in thermal quality caused by plant invasions are expected to be largest when the alien plants present novel growth forms or structural features relative to the dominant native vegetation [9,26]. For example, larger size and faster-growing alien trees can cause reductions in light and temperature in shrubland habitats [27,28], and those with extended phenology can lengthen thermal effects beyond the growing season of native vegetation [29].
Figure 1.
Numbers of comparisons reporting effects (variable altered, decreased or increased relative to native areas) or no effects (variable unaltered) of plant invasions on reptiles, amphibians, insects and arachnids, with indication of statistical significance testing. The results are presented for each stage of the chain of effects of plant invasions on native ectotherms, from thermal landscapes to individual responses and population and community dynamics. Note the different scales on the ‘comparisons’ axis for each stage. Thermal landscape comparisons in studies covering several taxonomic groups were entered separately for each group when these comparisons were not specific to a given native species. Tpref, preferred temperature; see the electronic supplementary material, table S2 for a list of variables including the ones reported here.
Only three studies with reptiles [29–31] measured operative environmental temperatures, which integrate the influence of local features and the body's physical properties to obtain a more accurate estimate of body temperature at equilibrium with the environment [32]. These studies deployed physical replicas of the study organism fitted with temperature sensors. Use of physical replicas or biophysical model simulations [33,34] to estimate operative temperature is growing in climate change biology and has potential in plant invasion biology.
Most studies reported mean habitat temperatures and might thus have overlooked biologically relevant aspects of the thermal landscape, such as extreme temperatures and the relative availability of suitable and unsuitable micro-climates [35]. Whereas a focus on averages has traditionally dominated the climate change biology research [35], recent years have seen increased attention devoted to temperature variance and extremes (e.g. [36]). In our review, only 15 comparisons measured thermal ranges or extremes (figure 1). Describing the thermal heterogeneity available to ectotherms requires sufficient sampling in space and time [37], but a large number of studies relied on measurements at one single location and/or one single point in time. For small organisms in particular, such as invertebrates, thermal imagery tools have proven useful to characterize the thermal landscape at scales as small as a single bush or plant leaf [38]. Information on temperature distributions across habitats and time also allows more complete assessments relative to the organism's thermal preferences. Studies doing so generally found that optimal temperatures in invaded landscapes were available for shorter periods and were reached later in the day ([27,29–31,39,40]; figure 1).
Vegetation changes affect micro-climate accessibility as well as composition. Landscapes where preferred temperatures are more dispersed across an ectothermic animal's home range provide better opportunities for thermoregulation, with consequences for organism performance [22,41,42]. Many invasive plants can inhibit native vegetation growth and form dense stands [43], leading to homogeneous or clumped spatial patterns. Many of the plant invasion studies reviewed here applied landscape metrics to alien vegetation but not to available temperatures. Local-scale, fine-resolution studies of species' vulnerability to climate change have used thermal imagery [44] or spatially explicit individual-based process models [41,42] to explore the role of the spatial configuration of thermal landscapes in animal behavioural thermoregulation. The same tools can be applied in plant invasion research (see the case study below).
(b). Ectothermic individuals’ responses
Using radio-tracking, field observations and experimental trials, several studies in this review showed that invaded areas were actively avoided by individuals, including females selecting incubation sites (e.g. [27,29,45]). The responses of ectothermic individuals using invaded areas were also assessed. One possible response to modified thermal landscapes is to shift thermoregulatory set-points to match micro-climate availability, in line with, for example, seasonal acclimation effects [46,47]. The only study testing this hypothesis, using laboratory thermal gradients, found no difference in the thermal preferences of juvenile lizards reared under different levels of plant invasion ([48]; figure 1). A more common response of individuals to thermal habitat changes is to adjust the micro-habitat use and activity patterns to avoid effects on body temperatures and thermoregulation accuracy. For example, some individuals of garden skinks climbed invasive vines when ground-level basking sites became scarce owing to increased shading [48], whereas lizards in another comparison restricted activity to open micro-habitats similar to those they inhabit in natural areas [40]. In two independent comparisons, lizards in invaded landscapes were active for shorter periods or spent more time basking ([28,48]; figure 1). These studies measured body temperatures in the field or in the laboratory, but assessments can also rely on individual-based models that simulate the body temperature of virtual individuals moving through landscapes with known operative temperature distributions [41,42]. Likewise, the use of bio-loggers with accelerometers holds great promise for assessing both activity patterns and energy expenditure rates [49].
Thermoregulatory behaviour adjustments involve costs, such as reduced time for feeding or mating, increased energy expenditure associated with movement, lower rates of digestive processes and increased exposure to predators [50]. In landscapes with lower habitat quality, the costs of thermoregulation might exceed the benefits [50,51] and, over continued periods, lead to reduced growth rates and reproductive output [52]. In line with these expectations, lizards, toads and ticks in five studies had decreased body size, body mass, growth rate or survival rates in invaded areas [48,53–56]. Negative effects on reproduction in invaded areas included smaller clutch mass for garden skinks, reduced metamorphosis rate for toads and decreased proportion of egg-laying females for cattle ticks [48,54,57]. The remaining studies showed no changes in body condition, growth or reproductive traits, with two studies reporting positive effects for frogs in the larval stage ([58,59]; figure 1). Decreased incubation temperatures for turtles in invaded areas led to longer incubation times [60], but no significant changes in sex ratio were found for turtles or terrapins ([61,62]; figure 1). This variation in results probably reflects, in part, differences in ectothermic species' traits. Larger impacts are expected, for example, for amphibians and reptiles that are oviparous and less plastic in their choice of oviposition sites, lack parental transport of eggs, have larval young and/or froglets, and are short-lived and mature early [9].
For both reptiles and amphibians, about 75% of the studies examined individual responses. Reptiles were the only native group for which thermoregulatory behaviour was directly tested. Sub-optimal thermal environments played an important role in micro-habitat selection by reptiles, either in isolation or in combination with food availability or predation rates [27,39]. Mechanistic studies for amphibians covered mostly reproduction and larval development traits, and non-thermal mechanisms were linked to changes in water chemistry and predation (e.g. [54,56,58,59]). The complex life cycle of most amphibians and their susceptibility to water loss make them vulnerable to habitat changes in both aquatic and terrestrial systems. The presence of alien plants in freshwater systems can alter the quality of breeding, oviposition and refuge micro-habitats for amphibians [54,63,64]. More work is needed to understand how both hydric and thermal tolerances of amphibians mediate their response to plant invasions [17], and how responses differ across life-stages. In contrast to herpetofauna, insects and arachnids were poorly represented in mechanistic studies (figure 1). Where tested, arachnid responses to alien plants were predicted by temperature as well as by vegetation height, humidity and light penetration, through thermal or other mechanisms [55,57]. The low thermal inertia of insects and arachnids warrants more mechanistic tests of their responses to invasive plant-induced changes in thermal landscapes.
(c). Ectotherm population and community dynamics
Overall, the effects of alien plants on populations and communities were detrimental but with some variation, echoing the general trend portrayed in previous reviews [3–5,8,9]. Reptile and amphibian species abundance in invaded sites significantly decreased in 14 of the 18 comparisons addressing the population stage of the chain of effects (figure 1). However, for insects and arachnids, the contrast between the number of significant decreases and increases was smaller and, for insects, a larger fraction of comparisons showed no change in invaded areas (figure 1). Variation in results for insects and arachnids probably stems from the wide range of taxonomic levels that were used across studies to measure abundance, from class down to species level. By contrast, herpetofauna abundances were measured mainly at the species level and included fewer taxonomic entities.
Across taxonomic groups, species diversity in invaded areas decreased or remained unaltered (figure 1). Unaltered species diversity was found, for example, in invaded habitats that retained some of the structural complexity of undisturbed areas [65] or that maintained high-species richness despite altered composition [66]. Where measured, community composition and functional diversity were affected (figure 1), highlighting the trait-specific sensitivity of native ectotherms to invasive plants. Small-bodied species and those with specialized habitat, diet or micro-climate requirement are expected to be more sensitive to disturbances such as plant invasions [9,67,68]. The studies that quantified or discussed functional changes supported some of these expectations. For example, fossorial, ground-dependent and vegetation-dwelling reptiles and amphibians, as well as small-bodied and specialist herbivorous insects or arachnids, were particularly affected by invasive plants [66,69,70]. Species’ thermal affiliation, such as cold- or warm-specialization, was also noted as a likely cause of altered composition of amphibian and leaf-litter invertebrate assemblages in invaded areas [63,70]. The use of community thermal indices would allow the capture of community changes in temperature affiliation specifically (e.g. [71]).
Establishing causality between observed changes in thermal landscapes and in animal communities requires investigation of possible underlying mechanisms of response at the individual level [7,8,72]. However, only three of the 20 studies reporting changes at the population or community levels in invaded areas also tested for changes in individuals' behaviour, physiology or reproduction [45,53,57]. For the majority of the studies collated here, the conclusions about the thermal effects of plants on native animal populations and communities relied on correlations between temperature and species abundance or richness or on speculation based on existing theory.
3. An illustration of thermal ecology tool use in plant invasion research
The studies reviewed here showed the importance of considering temperature as a mechanism driving responses of ectotherms to plant invasions. However, they also revealed variation in results (figure 1). Rather than a systematic cooling or warming of habitats with negative consequences for native ectothermic animals, the effects of alien plants are likely to be context-dependent. First, general patterns can be obscured by differences in sampling methods, experimental venues and statistical techniques among studies. Second, the direction and magnitude of effects can be influenced by factors such as the plant invasion history, the age, cover and density of alien plants, the degree of contrast between native and alien plant growth forms, the ecosystem type and climatic conditions, the spatial and temporal scales of analysis, and the life stage and traits of the native animal species [3–5,73,74]. We applied key thermal ecology tools (electronic supplementary material, table S2) in a case study to explore two such context-dependencies: the level of plant invasion and native ectotherm species traits.
Our case study focused on renosterveld shrublands [75] in the western Cape Province of South Africa and assessed the effects of invasive Acacia saligna trees (Fabaceae family, commonly known as Port Jackson wattle) on the thermoregulation of an exemplar species, the Cape skink, Trachylepis capensis [76]. To characterize the thermal landscape of each of our three sites, we deployed 36 operative environmental temperature models [32] over a gridded area with a grid size of approximately 20 cm. The models were simplified copper replicas of the organism and were programmed to log data every 2 min for 48 h. The aim was to capture small-scale spatial thermal heterogeneity relative to the organism body size and home range, as well as thermal fluctuations throughout the animal's daily active period. However, achieving this fine resolution in three sites simultaneously would require a large number of operative temperature models. We thus opted for sampling a relatively small area in each site at fine resolution and, in a post-sampling data processing step, replicating it to obtain a larger squared area of 3 m side (see detailed methods in the electronic supplementary material, S3). We are assuming that each sampled area is representative of the larger landscape, an assumption that is supported by reports of similar levels of thermal heterogeneity across the micro-, local and landscape levels [77].
To simulate the thermoregulatory behaviour of individuals across time, we built a spatially explicit individual-based model [41,42]. Every 2 min, a virtual individual in a given location sampled the nearby landscape to select the grid cell with the operative temperature that would yield the most optimal body temperature (taken as Tpref ±s.d.; see the electronic supplementary material, S3). For comparison, a thermoconforming individual moved randomly irrespective of the resulting body temperature. The time-series of operative temperature as well as body temperature and selected cells were used to calculate several variables describing the thermal landscape and the lizard's response in native and invaded habitats (electronic supplementary material, table S2).
(a). The invasion history context
Time since introduction influences the level of impact of alien plants on native communities [73]. As the invasion progresses in time, ecological and evolutionary processes change the functional distinctiveness and abundance of alien plants, attenuating or amplifying the impacts on native communities [78]. Study approaches covering multiple invasion stages are thus preferred [72], but most studies in our review compared a native to an invaded site with no reference to the time since introduction or the level of invasion. In our case study, we therefore considered two invaded areas with different levels of invasion, reflected in the age and density of alien plants. Our comparison was among areas of (i) native vegetation (native), (ii) sparse invasion by young A. saligna (mildly invaded), and (iii) dense invasion by mature A. saligna (‘highly invaded’; electronic supplementary material, figure S5).
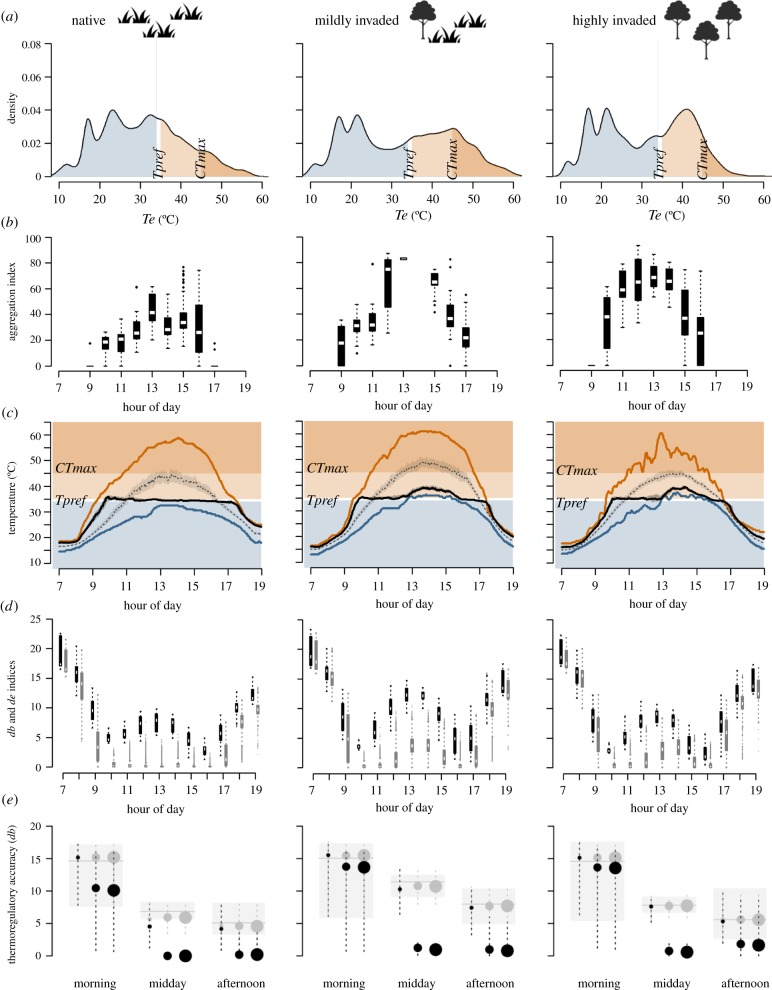
In our literature review, the presence of alien trees in habitats dominated by native plants with smaller growth forms often led to cooler temperatures relative to the native areas (figure 1). By contrast, in our case study only the highly invaded area had lower average and maximum operative temperature than the native area throughout most of the day (electronic supplementary material, figure S6 and table S3). Although A. saligna trees increase shade in shrubland habitats and can thus provide cooler micro-sites, their ability to suppress native ground vegetation can create landscapes with exposed ground [20] where maximum temperatures are amplified. This warming effect dominated in the mildly invaded habitat, where younger trees were sparsely distributed.
The three sites along the invasion gradient also differed in availability and accessibility of T. capensis' preferred temperatures. The native landscape had higher thermal quality according to Hertz's de index [79], reflecting a distribution of temperatures centred around the lizard's mean preferred temperature (Tpref) of 34.5°C ([80]; figure 2a and electronic supplementary material, table S3). Optimal temperatures in this landscape were available for longer periods and across more micro-sites (electronic supplementary material, figure S7). They were also more easily accessed by skinks, as indicated by the higher levels of spatial dispersion of optimal temperatures for longer periods of the day (figure 2b and electronic supplementary material, table S3). By contrast, optimal temperatures in invaded areas were more clumped in space, especially under the denser canopy of older alien A. saligna trees, and less available, particularly in the mildly invaded area (figure 2a,b).
Figure 2.
Operative temperature (Te) and simulated body temperature (Tb) of Trachylepis capensis in native, mildly invaded and highly invaded habitats. (a) Te composition: measurements below (blue), within (white) and above (light orange) the preferred temperature (mean Tpref ± s.d.) of T. capensis, and above its critical thermal maximum (CTmax, dark orange). (b) Te spatial configuration: median (white notch) and interquantile range (black boxes) of the aggregation index computed for the class of Te values within T. capensis' mean Tpref ± s.d. every 2 min within each hour, with the whiskers extending 1.5 times the interquartile range from the nearer quartile (dotted lines). Higher values correspond to clumped thermal landscapes. (c) Te and simulated Tb across the day: the solid and dotted black lines represent Tb for thermoregulating and thermoconforming individuals, respectively, with grey shades reflecting the interquantile ranges of 100 iterations. The blue and orange lines indicate the minimum and maximum available Te across space, respectively. (d) Thermal quality and thermoregulation accuracy for thermoregulating individuals: de and db indices (black and grey boxplots, respectively) for all measurements within each hour and, for db, all model iterations with random start points. The boxplots show the interquartile range (solid boxes), median (white horizontal lines) and whiskers as in (b) above. Lower de and db values indicate higher quality and accuracy, respectively. (e) Thermoregulation accuracy for individuals with different motility levels: the median (solid circles) and interquartile range (vertical dotted lines) of db are shown for thermoregulating (black) and thermoconforming (grey) individuals with low, intermediate and high motility (small, mid-size and large circles, respectively). The grey rectangles show the interquartile range of de over a given period, with the horizontal line indicating the median.
(b). The importance of native ectotherm species’ traits
Whether a thermoregulating ectotherm is affected in altered thermal landscapes depends partly on its response-mediating traits [81]. For example, thermal performance curve parameters such as optimal temperature and thermal breadth [82,83], and their plasticity [47], can mediate the responses of ectotherms in disturbed habitats [9,16,17]. The life-history and morphological traits of ectotherms can also influence their responses to alien plants [9], as discussed in the literature review. In turn, the movement and dispersal of ectotherms can mediate the responses of individuals to changes in the accessibility of suitable micro-habitats [84,85]. In environments with small-scale thermal heterogeneity, where optimal temperatures are dispersed in space, individuals can easily find suitable micro-sites [51] irrespective of their motility level or home range size. By contrast, coarser-scale heterogeneity might require longer movements, carrying higher thermoregulation costs [51] and inaccuracy [42], particularly for less motile species. Alien plant invasions alter the spatial scale of thermal heterogeneity, and thus their effects on lizard thermoregulation probably depend on the individuals' level of motility [9]. We tested this hypothesis in our case study by considering lizards with low, intermediate and high levels of motility, corresponding to sit-and-wait, intermediate and wide-foraging strategies.
Lizards with intermediate motility maintained optimal body temperatures for longer periods of time in the native area (figure 2c and electronic supplementary material, table S4), where thermal quality was higher and optimal temperatures were more dispersed in space (figure 2b). In invaded landscapes, however, individuals had to move longer distances to find suitable micro-sites (electronic supplementary material, table S4). This contrast was more pronounced in the hottest part of the day when thermal quality was lower in all habitats (figure 2d). Differences in individuals’ motility interacted with the spatial configuration of the thermal landscape, producing variation in thermoregulatory accuracy (figure 2e; see figure 2b for the comparison of spatial configurations). In clumped landscapes, such as in invaded habitats at midday, only the most motile lizards were able to explore sufficient variation in available operative temperatures and thus thermoregulate accurately. By contrast, in landscapes with large spatial dispersion of optimal temperatures, such as the native area at midday, even the less motile thermoregulating individuals achieved higher accuracy than thermoconforming ones.
4. Concluding remarks
The thermal ecology field offers useful tools and approaches to measure operative and body temperatures [32], estimate species' thermal performance curve parameters [82,83] and model thermal landscapes and thermoregulation behaviour [33,41,42]. Our literature review highlighted the need for such tools in plant invasion impacts research, and our case study illustrated the application of some of these tools to test hypotheses about ectotherms’ mechanisms of response. Teasing apart alternative mechanisms and assessing cascading effects along organizational levels requires a combination of tools including field or laboratory trials and model simulations. Ideally, studies should test not only thermal but also non-thermal mechanisms, such as changes in environmental resource quality and biotic interactions, and consider the potential role of distinct mechanisms in different life-stages of the native organism.
Although it is tempting to seek broad generalities from studies on alien plant impacts on resident animals, invasion science over the last decades has demonstrated the importance of context-dependencies such as native/alien plant combinations, invasion stages and ectotherm functional groups [72,73]. Our literature review highlighted variation in results that is probably linked to such context-dependencies, and our case study alone uncovered the interaction between invasion level and ectotherm motility. A growing body of research is needed to investigate possible interactions in a wide range of ecosystem types. As resident animal communities experience the synergistic effects of climate and biological invasions [86], research should also cover interactions with possible future climate change scenarios.
Supplementary Material
Acknowledgements
We thank Jacques van der Merwe from the City of Cape Town for granting us access to Joostenbergkloof Reserve for temperature measurements, and A.C. Hougaard for field assistance. Our manuscript was greatly improved following feedback from Sean Tomlinson and an anonymous reviewer.
Data accessibility
The dataset for the literature review and the operative temperature dataset and individual-based model code for the case study are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.3j46s79 [87].
Authors' contributions
Both authors conceptualized and developed the research. R.A.G. carried out the literature review, field data collection and model simulations. Both authors interpreted the results. R.A.G drafted the manuscript with input from S.C.-T.
Competing interests
The authors declare that they have no competing interests.
Funding
R.A.G. received support from a post-doctoral fellowship from the Centre for Invasion Biology and from a L'Oréal-UNESCO For Women in Science Sub-Saharan fellowship. S.C.-T. is supported by the Centre for Invasion Biology, Stellenbosch University and the Incentive Funding for Rated Researchers from the South African National Research Foundation.
References
- 1.Seebens H, et al. 2017. No saturation in the accumulation of alien species worldwide. Nat. Commun. 8, 14435 ( 10.1038/ncomms14435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stohlgren TJ, et al. 2011. Widespread plant species: natives versus aliens in our changing world. Biol. Invasions 13, 1931–1944. ( 10.1007/s10530-011-0024-9) [DOI] [Google Scholar]
- 3.Vilà M, et al. 2011. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 14, 702–708. ( 10.1111/j.1461-0248.2011.01628.x) [DOI] [PubMed] [Google Scholar]
- 4.Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M. 2012. A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species' traits and environment. Glob. Change Biol. 18, 1725–1737. ( 10.1111/j.1365-2486.2011.02636.x) [DOI] [Google Scholar]
- 5.Schirmel J, Bundschuh M, Entling MH, Kowarik I, Buchholz S. 2016. Impacts of invasive plants on resident animals across ecosystems, taxa, and feeding types: a global assessment. Glob. Change Biol. 22, 594–603. ( 10.1111/gcb.13093) [DOI] [PubMed] [Google Scholar]
- 6.Pejchar L, Mooney HA. 2009. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 24, 497–504. ( 10.1016/j.tree.2009.03.016) [DOI] [PubMed] [Google Scholar]
- 7.Levine JM, Vilà M, Antonio CMD, Dukes JS, Grigulis K, Lavorel S. 2003. Mechanisms underlying the impacts of exotic plant invasions. Proc. R. Soc. Lond. B 270, 775–781. ( 10.1098/rspb.2003.2327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clusella-Trullas S, Garcia RA. 2017. Impacts of invasive plants on animal diversity in South Africa: a synthesis. Bothalia 47, a2166 ( 10.4102/abc.v47i2.2166) [DOI] [Google Scholar]
- 9.Martin LJ, Murray BR. 2011. A predictive framework and review of the ecological impacts of exotic plant invasions on reptiles and amphibians. Biol. Rev. 86, 407–419. ( 10.1111/j.1469-185X.2010.00152.x) [DOI] [PubMed] [Google Scholar]
- 10.Hardwick SR, Toumi R, Pfeifer M, Turner EC, Nilus R, Ewers RM. 2015. The relationship between leaf area index and microclimate in tropical forest and oil palm plantation: forest disturbance drives changes in microclimate. Agric. For. Meteorol. 201, 187–195. ( 10.1016/j.agrformet.2014.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suggitt AJ, Gillingham PK, Hill JK, Huntley B, Kunin WE, Roy DB, Thomas CD. 2011. Habitat microclimates drive fine-scale variation in extreme temperatures. Oikos 120, 1–8. ( 10.1111/j.1600-0706.2010.18270.x) [DOI] [Google Scholar]
- 12.Tuff KT, Tuff T, Davies KF. 2016. A framework for integrating thermal biology into fragmentation research. Ecol. Lett. 19, 361–374. ( 10.1111/ele.12579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewers RM, Banks-Leite C. 2013. Fragmentation impairs the microclimate buffering effect of tropical forests. PLoS ONE 8, 1–7. ( 10.1371/journal.pone.0058093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skinner WR. 1999. Regional climatic warming and associated twentieth century land-cover changes in north-western North America. Clim. Res. 12, 39–52. ( 10.3354/cr012039) [DOI] [Google Scholar]
- 15.Tomlinson S, Webber BL, Bradshaw SD, Dixon KW, Renton M. 2018. Incorporating biophysical ecology into high-resolution restoration targets: insect pollinator habitat suitability models. Restor. Ecol. 26, 338–347. ( 10.1111/rec.12561) [DOI] [Google Scholar]
- 16.Frishkoff LO, Hadly EA, Daily GC. 2015. Thermal niche predicts tolerance to habitat conversion in tropical amphibians and reptiles. Glob. Change Biol. 21, 3901–3916. ( 10.1111/gcb.13016) [DOI] [PubMed] [Google Scholar]
- 17.Nowakowski AJ, et al. 2018. Thermal biology mediates responses of amphibians and reptiles to habitat modification. Ecol. Lett. 21, 345–355. ( 10.1111/ele.12901) [DOI] [PubMed] [Google Scholar]
- 18.Hejda M, Pyšek P, Jarošík V. 2009. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 97, 393–403. ( 10.1111/j.1365-2745.2009.01480.x) [DOI] [Google Scholar]
- 19.Olden JD, Poff NL. 2003. Toward a mechanistic understanding and prediction of biotic homogenization. Am. Nat. 162, 442–460. ( 10.1086/378212) [DOI] [PubMed] [Google Scholar]
- 20.van Wilgen BW, Richardson DM.. 1985. The effects of alien shrub invasions on vegetation structure and fire behaviour in south African Fynbos shrublands: a simulation study. J. Appl. Ecol. 22, 955–966. ( 10.2307/2403243) [DOI] [Google Scholar]
- 21.Pehle A, Schirmel J. 2015. Moss invasion in a dune ecosystem influences ground-dwelling arthropod community structure and reduces soil biological activity. Biol. Invasions 17, 3467–3477. ( 10.1007/s10530-015-0971-7) [DOI] [Google Scholar]
- 22.Basson CH, Levy O, Angilletta MJ, Clusella-Trullas S. 2017. Lizards paid a greater opportunity cost to thermoregulate in a less heterogeneous environment. Funct. Ecol. 31, 856–865. ( 10.1111/1365-2435.12795) [DOI] [Google Scholar]
- 23.Buckley LB. 2008. Linking traits to energetics and population dynamics to predict lizard ranges in changing environments. Am. Nat. 171, E1–E19. ( 10.1086/523949) [DOI] [PubMed] [Google Scholar]
- 24.Calosi P, Bilton DT, Spicer JI, Votier SC, Atfield A. 2010. What determines a species’ geographical range? Thermal biology and latitudinal range size relationships in European diving beetles (Coleoptera: Dytiscidae). J. Anim. Ecol. 79, 194–204. ( 10.1111/j.1365-2656.2009.01611.x) [DOI] [PubMed] [Google Scholar]
- 25.Barnagaud J-Y, Barbaro L, Hampe A, Jiguet F, Archaux F. 2013. Species' thermal preferences affect forest bird communities along landscape and local scale habitat gradients. Ecography 36, 1218–1226. ( 10.1111/j.1600-0587.2012.00227.x) [DOI] [Google Scholar]
- 26.Steidl RJ, Litt AR, Matter WJ. 2013. Effects of plant invasions on wildlife in desert grasslands. Wildl. Soc. Bull. 37, 527–536. ( 10.1002/wsb.308) [DOI] [Google Scholar]
- 27.Valentine LE, Roberts B, Schwarzkopf L. 2007. Mechanisms driving avoidance of non-native plants by lizards. J. Appl. Ecol. 44, 228–237. ( 10.1111/j.1365-2664.2006.01244.x) [DOI] [Google Scholar]
- 28.Block C, Stellatelli OA, García GO, Vega LE, Isacch JP. 2013. Factors affecting the thermal behavior of the sand lizard Liolaemus wiegmannii in natural and modified grasslands of temperate coastal dunes from Argentina. J. Therm. Biol. 38, 560–569. ( 10.1016/j.jtherbio.2013.09.009) [DOI] [Google Scholar]
- 29.Carter ET, Eads BC, Ravesi MJ, Kingsbury BA. 2015. Exotic invasive plants alter thermal regimes: implications for management using a case study of a native ectotherm. Funct. Ecol. 29, 683–693. ( 10.1111/1365-2435.12374) [DOI] [Google Scholar]
- 30.Schreuder E, Clusella-Trullas S. 2017. Exotic trees modify the thermal landscape and food resources for lizard communities. Oecologia 182, 1213–1225. ( 10.1007/s00442-016-3726-y) [DOI] [PubMed] [Google Scholar]
- 31.Carter ET, Ravesi MJ, Eads BC, Kingsbury BA. 2017. Invasive plant management creates ecological traps for snakes. Biol. Invasions 19, 443–453. ( 10.1007/s10530-016-1289-9) [DOI] [Google Scholar]
- 32.Bakken GS. 1992. Measurement and application of operative and standard operative temperatures in ecology. Am. Zool. 32, 194–216. ( 10.1093/icb/32.2.194) [DOI] [Google Scholar]
- 33.Kearney MR, Shine R, Porter WP. 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840. ( 10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gates DM. 1980. Biophysical ecology. New York, NY: Springer. [Google Scholar]
- 35.Sheldon KS, Dillon ME. 2016. Beyond the mean: biological impacts of cryptic temperature change. Integr. Comp. Biol. 56, 110 ( 10.1093/icb/icw005) [DOI] [PubMed] [Google Scholar]
- 36.Benedetti-Cecchi L, Bertocci I, Vaselli S, Maggi E. 2006. Temporal variance reverses the impact of high mean intensity of stress in climate change experiments. Ecology 87, 2489–2499. ( 10.1890/0012-9658(2006)87[2489:TVRTIO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 37.Garcia RA, Allen JL, Susana C-T. 2019. Rethinking the scale and formulation of indices assessing organism vulnerability to warmer habitats. Ecography 42, 1024–1036. ( 10.1111/ecog.04226) [DOI] [Google Scholar]
- 38.Pincebourde S, Suppo C. 2016. The vulnerability of tropical ectotherms to warming is modulated by the microclimatic heterogeneity. Integr. Comp. Biol. 56, 85–97. ( 10.1093/icb/icw014) [DOI] [PubMed] [Google Scholar]
- 39.Hacking J, Abom R, Schwarzkopf L. 2014. Why do lizards avoid weeds? Biol. Invasions 16, 935–947. ( 10.1007/s10530-013-0551-7) [DOI] [Google Scholar]
- 40.Stellatelli OA, Vega LE, Block C, Cruz FB. 2013. Effects on the thermoregulatory efficiency of two native lizards as a consequence of the habitat modification by the introduction of the exotic tree Acacia longifolia. J. Therm. Biol. 38, 135–142. ( 10.1016/j.jtherbio.2012.12.005) [DOI] [Google Scholar]
- 41.Vickers M, Schwarzkopf L. 2016. A random walk in the park: an individual-based null model for behavioral thermoregulation. Am. Nat. 187, 481–490. ( 10.1086/685433) [DOI] [PubMed] [Google Scholar]
- 42.Sears MW, Angilletta MJ. 2015. Costs and benefits of thermoregulation revisited: both the heterogeneity and spatial structure of temperature drive energetic costs. Am. Nat. 185, E94–E102. ( 10.1086/680008) [DOI] [PubMed] [Google Scholar]
- 43.Richardson DM, Pyšek P, Rejmánek M, Barbour MG, Panetta FD, West CJ. 2000. Naturalization and invasion of alien plants: concepts and definitions. Divers. Distrib. 6, 93–107. ( 10.1046/j.1472-4642.2000.00083.x) [DOI] [Google Scholar]
- 44.Faye E, Rebaudo F, Yánez-Cajo D, Cauvy-Fraunié S, Dangles O. 2016. A toolbox for studying thermal heterogeneity across spatial scales: from unmanned aerial vehicle imagery to landscape metrics. Methods Ecol. Evol. 7, 437–446. ( 10.1111/2041-210X.12488) [DOI] [Google Scholar]
- 45.Stellatelli OA, Vega LE, Block C, Cruz FB. 2013. Effects of tree invasion on the habitat use of sand lizards. Herpetologica 69, 455–465. ( 10.1655/HERPETOLOGICA-D-12-00033) [DOI] [Google Scholar]
- 46.Basson CH, Clusella-Trullas S. 2015. The behavior-physiology nexus: behavioral and physiological compensation are relied on to different extents between seasons. Physiol. Biochem. Zool. 88, 384–394. ( 10.1086/682010) [DOI] [PubMed] [Google Scholar]
- 47.Clusella-Trullas S, Chown S. 2014. Lizard thermal trait variation at multiple scales: a review. J. Comp. Physiol. B 184, 5–21. ( 10.1007/s00360-013-0776-x) [DOI] [PubMed] [Google Scholar]
- 48.Downes S, Hoefer A-M. 2007. An experimental study of the effects of weed invasion on lizard phenotypes. Oecologia 153, 775–785. ( 10.1007/s00442-007-0775-2) [DOI] [PubMed] [Google Scholar]
- 49.Kays R, Crofoot MC, Jetz W, Wikelski M. 2015. Terrestrial animal tracking as an eye on life and planet. Science 348, aaa2478 ( 10.1126/science.aaa2478) [DOI] [PubMed] [Google Scholar]
- 50.Huey RB, Slatkin M. 1976. Cost and benefits of lizard thermoregulation. Q. Rev. Biol. 51, 363–384. ( 10.1086/409470) [DOI] [PubMed] [Google Scholar]
- 51.Sartorius SS, do Amaral JPS, Durtsche RD, Deen CM, Lutterschmidt WI. 2002. Thermoregulatory accuracy, precision, and effectiveness in two sand-dwelling lizards under mild environmental conditions. Can. J. Zool. 80, 1966–1976. ( 10.1139/z02-191) [DOI] [Google Scholar]
- 52.Adolph SC, Porter WP. 1993. Temperature, activity, and lizard life histories. Am. Nat. 142, 273–295. ( 10.1086/285538) [DOI] [PubMed] [Google Scholar]
- 53.Stellatelli OA, Block C, Vega LE, Cruz FB. 2014. Responses of two sympatric sand lizards to exotic forestations in the coastal dunes of Argentina: some implications for conservation. Wildl. Res. 41, 480–489. ( 10.1071/WR14078) [DOI] [Google Scholar]
- 54.Brown C, Blossey B, Maerz J, Joule S. 2006. Invasive plant and experimental venue affect tadpole performance. Biol. Invasions 8, 327–338. ( 10.1007/s10530-004-8244-x) [DOI] [Google Scholar]
- 55.Civitello DJ, Flory SL, Clay K. 2008. Exotic grass invasion reduces survival of Amblyomma americanum and Dermacentor variabilis ticks (Acari: Ixodidae). J. Med. Entomol. 45, 867–872. ( 10.1093/jmedent/45.5.867) [DOI] [PubMed] [Google Scholar]
- 56.DeVore JL, Maerz JC. 2014. Grass invasion increases top-down pressure on an amphibian via structurally mediated effects on an intraguild predator. Ecology 95, 1724–1730. ( 10.1890/13-1715.1) [DOI] [PubMed] [Google Scholar]
- 57.Racelis AE, Davey RB, Goolsby JA, de León AAP, Varner K, Duhaime R.. 2012. Facilitative ecological interactions between invasive species: Arundo donax stands as favorable habitat for cattle ticks (Acari: Ixodidae) along the U.S.–Mexico border. J. Med. Entomol. 49, 410–417. ( 10.1603/ME11104) [DOI] [PubMed] [Google Scholar]
- 58.Earl JE, Castello PO, Cohagen KE, Semlitsch RD. 2014. Effects of subsidy quality on reciprocal subsidies: how leaf litter species changes frog biomass export. Oecologia 175, 209–218. ( 10.1007/s00442-013-2870-x) [DOI] [PubMed] [Google Scholar]
- 59.Rogalski MA, Skelly DK. 2012. Positive effects of nonnative invasive Phragmites australis on larval bullfrogs. PLoS ONE 7, 1–8. ( 10.1371/journal.pone.0044420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolton RM, Brooks RJ. 2010. Impact of the seasonal invasion of Phragmites australis (common reed) on turtle reproductive success. Chelonian Conserv. Biol. 9, 238–243. ( 10.2744/CCB-0793.1) [DOI] [Google Scholar]
- 61.Schmid JL, Addison DS, Donnelly MA, Shirley MA, Wibbels T. 2008. The effect of Australian pine (Casuarina equisetifolia) removal on loggerhead sea turtle (Caretta caretta) incubation temperatures on Keewaydin Island, Florida. J. Coast. Res. Special Issue 10055, 214–220. ( 10.2112/SI55-001.1) [DOI] [Google Scholar]
- 62.Cook CE, McCluskey AM, Chambers RM. 2018. Impacts of invasive Phragmites australis on diamondback terrapin nesting in Chesapeake Bay. Estuaries Coasts 41, 966–973. ( 10.1007/s12237-017-0325-z) [DOI] [Google Scholar]
- 63.Watling JI, Hickman CR, Orrock JL. 2011. Invasive shrub alters native forest amphibian communities. Biol. Conserv. 144, 2597–2601. ( 10.1016/j.biocon.2011.07.005) [DOI] [Google Scholar]
- 64.Nunes AL, Fill JM, Davies SJ, Louw M, Rebelo AD, Thorp CJ, Vimercati G, Measey J. 2019. A global meta-analysis of the ecological impacts of alien species on native amphibians. Proc. R. Soc. B 286, 20182528 ( 10.1098/rspb.2018.2528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Somaweera R, Wijayathilaka N, Bowatte G, Meegaskumbura M. 2015. Conservation in a changing landscape: habitat occupancy of the critically endangered Tennent's leaf-nosed lizard (Ceratophora tennentii) in Sri Lanka. J. Nat. Hist. 49, 31–32. ( 10.1080/00222933.2015.1006280) [DOI] [Google Scholar]
- 66.Trimble MJ, van Aarde RJ.. 2014. Amphibian and reptile communities and functional groups over a land-use gradient in a coastal tropical forest landscape of high richness and endemicity. Anim. Conserv. 17, 441–453. ( 10.1111/acv.12111) [DOI] [Google Scholar]
- 67.Litt AR, Cord EE, Fulbright TE, Schuster GL. 2014. Effects of invasive plants on arthropods. Conserv. Biol. 28, 1532–1549. ( 10.1111/cobi.12350) [DOI] [PubMed] [Google Scholar]
- 68.Wong MKL, Guénard B, Lewis OT. 2019. Trait-based ecology of terrestrial arthropods. Biol. Rev. 94, 999–1022. ( 10.1111/brv.12488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schirmel J, Buchholz S. 2013. Invasive moss alters patterns in life-history traits and functional diversity of spiders and carabids. Biol. Invasions 15, 1089–1100. ( 10.1007/s10530-012-0352-4) [DOI] [Google Scholar]
- 70.Nguyen KQ, Cuneo P, Cunningham SA, Krix DW, Leigh A, Murray BR. 2016. Ecological effects of increasing time since invasion by the exotic African olive (Olea europaea ssp. cuspidata) on leaf-litter invertebrate assemblages. Biol. Invasions 18, 1689–1699. ( 10.1007/s10530-016-1111-8) [DOI] [Google Scholar]
- 71.Devictor V, et al. 2012. Differences in the climatic debts of birds and butterflies at a continental scale. Nat. Clim. Chang. 2, 121–124. ( 10.1038/nclimate1347) [DOI] [Google Scholar]
- 72.Kueffer C, Pyšek P, Richardson DM. 2013. Integrative invasion science: model systems, multi-site studies, focused meta-analysis and invasion syndromes. New Phytol. 200, 615–633. ( 10.1111/nph.12415) [DOI] [PubMed] [Google Scholar]
- 73.Kumschick S, et al. 2015. Ecological impacts of alien species: quantification, scope, caveats, and recommendations. Bioscience 65, 55–63. ( 10.1093/biosci/biu193) [DOI] [Google Scholar]
- 74.Gaertner M, Breeyen AD, Hui C, Richardson DM. 2009. Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: a meta-analysis. Prog. Phys. Geogr. Earth Environ. 33, 319–338. ( 10.1177/0309133309341607) [DOI] [Google Scholar]
- 75.Mucina L, Rutherford MC, Powrie LW, van Niekerk A, van der Merwe JH. 2014. Vegetation field atlas of continental South Africa, Lesotho and Swaziland. Strelitzia 33. Pretoria, South Africa: South African National Biodiversity Institute. [Google Scholar]
- 76.Gray JE. 1831. A synopsis of the species of Class Reptilia. In The animal kingdom arranged in conformity with its organisation by the Baron Cuvier with additional descriptions of all the species hither named, and of many before noticed (eds Griffith E, Pidgeon E), p. 481 + 110 pp London, UK: V Whittaker, Treacher and Co. [Google Scholar]
- 77.Pincebourde S, Murdock CC, Vickers M, Sears MW. 2016. Fine-scale microclimatic variation can shape the responses of organisms to global change in both natural and urban environments. Integr. Comp. Biol. 56, 45–61. ( 10.1093/icb/icw016) [DOI] [PubMed] [Google Scholar]
- 78.Dostál P, Müllerová J, Pyšek P, Pergl J, Klinerová T. 2013. The impact of an invasive plant changes over time. Ecol. Lett. 16, 1277–1284. ( 10.1111/ele.12166) [DOI] [PubMed] [Google Scholar]
- 79.Hertz PE, Huey RB, Stevenson RD. 1993. Evaluating temperature regulation by field-active ectotherms: the fallacy of the inappropriate question. Am. Nat. 142, 796–818. ( 10.1086/285573) [DOI] [PubMed] [Google Scholar]
- 80.Withers PC. 1981. Physiological correlates of limblessness and fossoriality in scincid lizards. Copeia 1981, 197–204. ( 10.2307/1444055) [DOI] [Google Scholar]
- 81.Isaac NJB, Cowlishaw G. 2004. How species respond to multiple extinction threats. Proc. R. Soc. Lond. B 271, 1135–1141. ( 10.1098/rspb.2004.2724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomlinson S. 2019. The mathematics of thermal sub-optimality: nonlinear regression characterization of thermal performance of reptile metabolic rates. J. Therm. Biol. 81, 49–58. ( 10.1016/j.jtherbio.2019.02.008) [DOI] [PubMed] [Google Scholar]
- 83.Angilletta MJ. 2006. Estimating and comparing thermal performance curves. J. Therm. Biol. 31, 541–545. ( 10.1016/j.jtherbio.2006.06.002) [DOI] [Google Scholar]
- 84.Fletcher RJ, Reichert BE, Holmes K. 2018. The negative effects of habitat fragmentation operate at the scale of dispersal. Ecology 99, 2176–2186. ( 10.1002/ecy.2467) [DOI] [PubMed] [Google Scholar]
- 85.Lenda M, Witek M, Skórka P, Moroń D, Woyciechowski M. 2013. Invasive alien plants affect grassland ant communities, colony size and foraging behaviour. Biol. Invasions 15, 2403–2414. ( 10.1007/s10530-013-0461-8) [DOI] [Google Scholar]
- 86.Mitchell AB, Litt AR. 2016. Nonnative plant shifts functional groups of arthropods following drought. Biol. Invasions 18, 1351–1361. ( 10.1007/s10530-016-1072-y) [DOI] [Google Scholar]
- 87.Garcia R, Clusella-Trullas S. 2019. Data from: Thermal landscape change as a driver of ectotherm responses to plant invasions Dryad Digital Repository. ( 10.5061/dryad.3j46s79) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Garcia R, Clusella-Trullas S. 2019. Data from: Thermal landscape change as a driver of ectotherm responses to plant invasions Dryad Digital Repository. ( 10.5061/dryad.3j46s79) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The dataset for the literature review and the operative temperature dataset and individual-based model code for the case study are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.3j46s79 [87].