Abstract
Artificial light at night (ALAN) is an increasing phenomenon associated with worldwide urbanization. In birds, broad-spectrum white ALAN can have disruptive effects on activity patterns, metabolism, stress response and immune function. There has been growing research on whether the use of alternative light spectra can reduce these negative effects, but surprisingly, there has been no study to determine which light spectrum birds prefer. To test such a preference, we gave urban and forest great tits (Parus major) the choice where to roost using pairwise combinations of darkness, white light or green dim light at night (1.5 lux). Birds preferred to sleep under artificial light instead of darkness, and green was preferred over white light. In a subsequent experiment, we investigated the consequence of sleeping under a particular light condition, and measured birds' daily activity levels, daily energy expenditure (DEE), oxalic acid as a biomarker for sleep debt and cognitive abilities. White light affected activity patterns more than green light. Moreover, there was an origin-dependent response to spectral composition: in urban birds, the total daily activity and night activity did not differ between white and green light, while forest birds were more active under white than green light. We also found that individuals who slept under white and green light had higher DEE. However, there were no differences in oxalic acid levels or cognitive abilities between light treatments. Thus, we argue that in naive birds that had never encountered light at night, white light might disrupt circadian rhythms more than green light. However, it is possible that the negative effects of ALAN on sleep and cognition might be observed only under intensities higher than 1.5 lux. These results suggest that reducing the intensity of light pollution as well as tuning the spectrum towards long wavelengths may considerably reduce its impact.
Keywords: artificial light at night, light pollution, Parus major, sleep, oxalic acid, urbanization
1. Introduction
Light pollution refers to the diminishing of darkness during night-time, caused by light from anthropogenic sources. Artificial light at night (ALAN) can threaten ecosystem dynamics through alterations in the biological timing of a wide range of species, with far-reaching consequences [1,2]. For instance, ALAN can lead to lethal consequences due to attraction to light sources, such as for hatching sea turtles [3] and migrating birds [4]. Night-time illumination can also have more subtle effects through changes in physiological processes and behaviour due to disruption of natural circadian rhythms and sleep, which in turn may affect the individual's health and ultimately fitness [1].
Birds use light cues for synchronizing their biological rhythms [5] and ALAN can alter their photoperiodic perception [6–9]. Consequently, ALAN can affect the timing of reproductive physiology and behaviour [8,10,11], timing of dawn singing [10,12,13] and sleep behaviour [14] of free-living passerine songbird species. Experimental studies on captive songbirds have confirmed work in the wild [7,15]. Blackbirds increase locomotor activity at night when roosting under light compared with darkness [15]. Similarly, great tits advance activity, delay activity offset, and move a higher proportion of their daily activity into the night when exposed to ALAN [7].
Although there is increasing evidence that ALAN alters biological rhythms, the consequences of such alteration are not always fully understood. ALAN can decrease melatonin production at night [16], increase blood inflammatory markers [17] and increase susceptibility to pathogens [18]. However, the increase in nocturnal activity due to ALAN could have a major impact also on energy consumption and sleep [19,20]. Energy is a crucial and limited resource for animals, and there is a trade-off between investment decisions on behavioural and/or physiological processes and these trade-offs are often associated with fitness [21]. A measurement of energy metabolism is daily energy expenditure (DEE). While DEE is mostly affected by body mass [22], it can also be influenced by environmental factors such as human disturbance, temperature and food availability [21,22]. In the context of light pollution, higher activity at night due to ALAN could potentially increase the energy expenditure of diurnal animals, with carry-over consequences on other physiological systems as well as fitness. However, in a recent field study on great tits (Parus major), we showed that a lower DEE was related to breeding in territories illuminated with white and green lights compared with dark areas [16]. This decrease in DEE could be explained by other ecological factors, such as the increase in food availability (insects) in artificially illuminated areas [16]. Furthermore, in forest areas, birds can avoid artificial lighting by choosing a distant nesting location [17], thereby possibly evading the negative effects of nocturnal light. As such, the direct effects of artificial light on DEE are yet unknown.
Other potential ecological costs of ALAN might arise through loss of sleep, as shown in humans [18]. Indeed, in birds previous studies suggested that ALAN is associated with nocturnal restlessness (i.e. activity bouts that are clearly distinguishable from sleep behaviour). For instance, female great tits exposed to ALAN for two nights in nest boxes slept less and had shorter sleep bouts compared with birds who roosted under darkness [14]. However, such short-term manipulation appeared to be transient as birds showed regular sleep behaviour when the exposure to light at night was stopped. Moreover, it is unclear whether such nocturnal restlessness really represents sleep disruption. Recently, reduced plasma levels of oxalic acid have been established as a biomarker of sleep disruption in humans and rodents [23]. This opens the possibility to measure sleep disruption in non-model organisms in the field. A recent study in great tits showed that a higher nocturnal activity due to ALAN was associated with a decrease in oxalic acid, thereby suggesting a negative effect of ALAN on sleep [24]. Sleep is a key state for the consolidation of memory, and thereby affects information use [25]. Information processing, retention and use is a part of cognition and important for behavioural decision-making processes, and cognitive abilities allow animals to detect danger, remember food resources and nesting sites based on environmental cues [25]. Studies with great tits show that they are able to memorize locations of cached food by observing other bird species and steal resources, indicating the importance of cognition on fitness [26]. In birds, cognition is affected by sleep, and thus cognitive abilities may be altered by sleep disruption due to ALAN [19]. In a recent study, birds kept under constant daylight showed a disruption in their activity patterns and a deterioration in their cognitive performance [27]. However, the effects of dim rather than constant bright ALAN on cognition remain unknown.
Although ALAN is increasingly associated with negative ecological effects, it is also necessary in human society for economic and safety reasons. Currently, there is an increase in the use of broad-spectrum light emitting diode (LED) lamps due to their cost-effectiveness [28]. As LED lights can easily be adjusted to different light spectra, this may offer the possibility of using a different light spectrum to decrease the ecological negative impact of light pollution. In birds, broad-spectrum white LED light seems to have major impacts, such as altering immune response [24], advancing reproductive activities [29] and increasing corticosterone levels [30] compared with control birds not exposed to ALAN. Experiments with blue tits (Cyanistes caeruleus) have shown that, at lower intensities, green light is less disruptive (compared with white and red light) on activity patterns [31].
While the effects of different light colours are yet to be fully appreciated, it is also unclear whether animals would prefer any type of light spectra when selecting for a roosting location, everything else being equal. Animals generally make behavioural decisions that maximizes their fitness, and therefore should choose for environments that satisfy their requirements the most [32]. On the one hand, animals might benefit from roosting in lit areas because they could forage at night, but on the other hand, they could suffer from increased predation risk and sleep disruption. These trade-offs may be modulated by light intensity and spectra. There has been some research in the poultry sector about the preference of chickens for artificial light of different colours, which showed that these birds seem to prefer light with high colour temperature (spectra) [33]. However, these studies were not carried out in the context of nocturnal lighting. Furthermore, even closely related species can show behavioural differences with regards to ALAN [34], and thus it is difficult to make generalizations. To date, there has not been any research into whether wild bird species prefer to roost in dark versus lit areas, and into whether a specific spectrum of ALAN would be preferred.
The aim of this study is to test the preference of birds for roosting in darkness versus different light spectra, and understand the physiological, behavioural and cognitive consequences of different spectra of ALAN. In a laboratory setting, we exposed male great tits to green light, white light (at similar intensities of 1.5 lux) or darkness. This light intensity is comparable to what wild birds are exposed to in light polluted areas [11,17] and in captive studies has been shown to have moderate effects on activity patterns of great tits [7]. We chose to use green light because there is considerable interest to find light colours that minimize the effects of light pollution on wildlife, and green has been suggested to be a potential option as it is also suitable for outdoor lighting [35]. We used birds both from urban and rural areas to assess whether urban birds respond differently to night light compared with forest birds, as previous research suggested that prolonged exposure to ALAN might alter sensitivity to light [11]. In a first experiment, we tested the preference of birds by giving them the choice of where to roost between pairwise combinations of darkness, green light or white light. In a second experiment, we forced birds to roost under a specific night light and measured daily activity patterns, DEE, plasma concentrations of oxalic acid, sleep behaviour and cognitive abilities. Our hypothesis is that birds prefer to roost under darkness than any light colour. White light, and to a lesser extent green light, will increase night time activity and cause sleep debt, thereby increasing the DEE and negatively affecting cognitive ability. We also expect that night light will have less disruptive effects on the physiology of urban birds as they might have developed tolerance to the presence of ALAN.
2. Methods
(a). Birds and housing
We studied 35 (17 forest and 18 urban) male great tits. The birds were caught in the wild (see electronic supplementary material, figure S1, for a map of catching locations) and transported to the Netherlands Institute of Ecology (NIOO-KNAW) Wageningen, the Netherlands. Birds were housed in individual cages (90 × 50 × 40 m), initially spread over three adjacent rooms. Each cage had two light sources, one for day and one for night. The front of each cage was covered with a wooden board to exclude any external light from the outside and neighbouring cages. Birds were provided with food and water ad libitum. Over the course of the first experiment (experiment 1), which was from 9 October until 28 October (21 days), birds had a constant photoperiod of 10.15 h light : 13.45 h dark, and for the second experiment (experiment 2), which was from 6 November until 17 December (42 days) birds had a constant photoperiod of 8.15 h light : 15.45 h dark. This was the average of natural daytime and night-time hours throughout the dates that the experiments were carried out.
(b). General experimental set-up
In both experiments, we used a within-individual design such that all birds were exposed to every treatment. Each experiment consisted of three treatment periods and the order of treatments was randomized across birds and rooms. During the daytime, birds were exposed to full florescent spectral light at ±1000 lux (Activa 172, Philips), and at night they were exposed to LED lamps with either green light, white light or darkness (no light). While both green and white lights emit full spectrum light, green lamps have an increased blue and reduced red emission (for spectra see supplement to [31]). Green and white light intensities during night time were set at 1.5 lux, measured at perch level. To ensure that light intensities were the same in all cages we tested lights at perch level with a lux meter before the start of the experiments. Between the two experiments, birds had 7 days of recovery period. During this recovery period, birds were exposed to dark nights. In both experiments, night lights had a 15 min overlap with daylights both in the morning and in the evening.
(c). Experiment 1: choice experiment
(i). Experimental set-up
Each bird was placed into a combined cage made up of two adjacent individual cages that were connected through a 7 cm diameter hole. Birds were allowed to move freely between the sub-cages (electronic supplementary material, figure S1-A). Individuals were assigned randomly to a treatment group and to one of 12 blocks of cages divided over three rooms. Each block contained all three treatments and both origins. During the daytime, the conditions of the sub-cages were the same but at night time, the light in the two sub-cages was different. Birds were exposed to one of three treatments: white light–green light (WG), darkness–green light (DG) or darkness–white light (DW). Treatments lasted five nights followed by 2 days of recovery (electronic supplementary material, figure S1-B). After the second night of the five-night treatment, the placement of the light in the sub-cages was switched around to account for the possibility of the bird choosing one sub-cage over the other regardless of the presence or absence of light.
(ii). Light preference
Camera traps were used to record the sub-cage chosen by the birds. The cameras were set to take a picture at 1 min intervals as well as to take a picture based on motion detection. While an actual camera was placed in one sub-cage to record preference, a dummy camera was placed in the adjacent sub-cage. The dummy was used to correct for the possibility of the camera to act as a novel object and thereby affect cage choice independent of light. For every night, in each sub-cage, the choice of the bird was recorded as a binary response of either yes (bird in cage) or no (bird not present in cage). Birds perched everywhere in the cage during their sleep, including perches, the ground, on top or behind feeders and even on the hole dividing the two sub-cages. in the case of the latter event, given that the head of the bird was in one cage and the tail in the other, the position of the head was considered the bird's choice, as bird photoreceptors are located in the head (eye, pineal gland and hypothalamus) [5].
(d). Experiment 2: forced light exposure
(i). Experimental set-up
In experiment 2, all birds were placed into separate individual cages. The treatments to which the birds were exposed at night were: white light, green light or darkness. Individuals were randomly placed into a treatment group and to one of six blocks divided over two rooms. Each block contained all three treatments and both origins. Every treatment lasted two weeks (14 days), where birds received 11 nights of treatment, followed by three nights of recovery (electronic supplementary material, figure 1b). Two birds (one forest and one urban) died during the recovery week between the two experiments due to unknown causes (thus n = 33).
Figure 1.
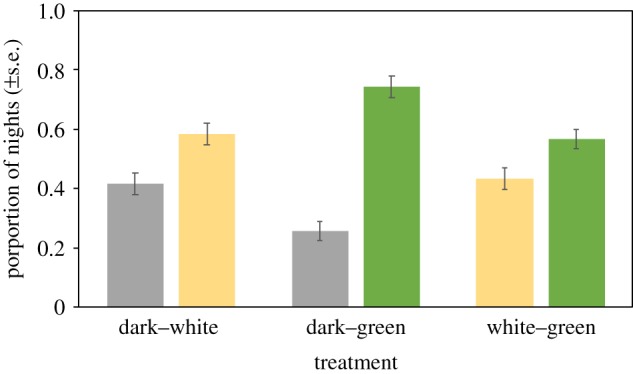
Proportion of nights spent under different light conditions in pairwise light treatments. Bars and errors bars represent means ± s.e.m. (Online version in colour.)
(ii). Activity measurements
Daily activity patterns of each individual bird were measured continuously following the same method described in de Jong et al. [7]. We focused on onset of activity, offset of activity, total activity and nocturnal activity. For a more detailed explanation of how the measurements were obtained please see the electronic supplementary material.
(iii). Nocturnal restlessness
We used camera traps for the assessment of slight movements of birds during sleep. Cameras were set to take pictures on motion detection as well as at 1 min intervals. We looked at the time frames between latest activity offset and earliest activity onset to observe sleep behaviour. In every treatment, for each bird, one night was selected. We aimed to select the same night (the seventh night after the treatment started) as an observatory unit for each treatment period of two weeks. If that was not possible because the bird was not clearly visible on that night, then we selected the closest available night. The light treatment did not affect the likelihood of a bird being clearly visible in the camera frame (p > 0.1). To assess sleep behaviour, we went through the night recordings frame by frame. If the bird was in the same sleep position between two subsequent frames, and with the head tucked beneath the shoulder, it was recorded as ‘no movement’ (0), and whenever the bird moved its head or changed sleeping position over the period of time frame, it was recorded as ‘restlessness’ (1). This distinction was based on previous papers that assessed sleep behaviour in great tits. We recognize that sometimes birds might sleep also with the head outside of the feather, especially during REM-related sleep [36], but unfortunately, we did not have any mean to distinguish such events without having corresponding EEG recordings.
(iv). Daily energy expenditure
DEE of birds was measured in a subsample of 11 birds with the doubly labelled water (DLW) technique through the collection of breath samples, which has been validated in previous studies [16,37]. All 11 birds were measured in each treatment period and thus we obtained a total of 33 DEE measurements. The order of treatments and origins were randomized. A detailed explanation of this procedure can be found in the electronic supplementary material.
(v). Oxalic acid
Before the start of experiment 2 and at the end of each 12 day treatment, we took a blood sample (in total four per bird) to measure plasma concentrations of oxalic acid. Details of sampling and laboratory assays are described in [24] and in the electronic supplementary material.
(vi). Cognitive abilities
Cognition was measured with a subsample of 22 birds (11 urban and 11 forest) in experiment 2, through learning and memory tasks adapted from the dimensional shift paradigm by Titulaer et al. [38]. A dimensional shift paradigm examines learning ability through behavioural responses to environmental cues. Overall, we tested six tasks, four learning and two memory tasks (see electronic supplementary material, table S3 for a schematic). A detailed explanation of these procedures can be found in the electronic supplementary material.
(e). Statistical analysis
All data were analysed with SPSS statistics (v. 24, IBM SPSS), with a significance level of α = 0.05. We used generalized linear mixed-effect models with logistic regression for binary responses (light preference, cognition and movement), and for all other response variables, we used linear mixed-effects models (LMMs). Assumptions for using linear models were met. Individuals nested within blocks were added into all models as random effects to account for the repeated measurements of birds and location of cages. If an interaction term was significant, we performed post hoc tests with Bonferroni correction. Model selection was done by step-wise deletion of non-significant terms, starting with the interaction term.
In experiment 1, we separated and analysed data per treatment (WG, DW, DG). Night lights in sub-cage (green, white and dark), origin and position of cage (left/right) were added into the models as fixed effects with interaction of night light × origin. In experiment 2, we ran four separate models to analyse activity patterns. In these models, the following four response variables were used: activity onset, activity offset, nocturnal activity and total activity. The three-way interaction of treatment, origin and treatment day (i.e. the days of treatment—night lights—in each treatment period) was initially fitted into all models. In the analysis of DEE, change in oxalic acid and sleep restlessness, we included origin, treatment and their interaction as fixed effects. For cognition, we ran a model with the interaction of type × treatment × origin. Type was defined as the sort of task (memory/learning) birds had to complete in the cognition test. If the interaction was significant we separated data by type.
3. Results
(a). Experiment 1: choice experiment
In all three treatments, night light had a major effect on the choice of birds, and green light was the predominant preference (figure 1; electronic supplementary material, table S1), regardless of the origin of the birds. Birds generally chose to roost under light at night compared with darkness, both in DW (p = 0.002) and DG treatments (p < 0.001). In WG, birds chose to roost under green light (p = 0.014) in comparison with white light. Light position (left/right) only had a minor effect in the WG treatment (p = 0.041), where birds preferred to roost in the cage on the right (estimate = 0.55, s.e. = 0.04) over left.
(b). Experiment 2: forced light exposure
(i). Effects of ALAN on activity traits
In experiment 2, activity patterns were disrupted by ALAN compared with darkness, and more so for birds roosting under white light, especially for forest birds (electronic supplementary material, figure S2; table 1). The interaction between origin and treatment had an effect on all variables except activity offset (table 1). The greatest changes were observed in the activity onset of birds. White light had the most severe effect (urban: estimate = −148 min, s.e. = 8.5; forest: estimate = −158 min, s.e. = 8.8), advancing onset almost by three hours. Birds roosting under green light also started their day earlier, but to a lesser extent compared with white light (urban: estimate = −123 min, s.e. = 8.5; forest: estimate = −117 min, s.e. = 8.7). Moreover, there was a significant treatment × origin effect: while urban birds responded more strongly than forest birds to green light, the reverse was true for white light (table 1; electronic supplementary material, figure S2). The effect of ALAN on activity offset was weaker and did not depend on origin (electronic supplementary material, figure S2-B). It was highest for birds roosting under green light (estimate: 31 min, s.e. = 3.8), followed by white light (estimate = 20 min, s.e. = 3.9) and then dark, where offset was close to lights off (estimate = 4 min, s.e. = 3.9). Nocturnal activity was higher in birds exposed to ALAN compared with birds under darkness (DW, DG: p < 0.001, electronic supplementary material, figure S2-C). For forest birds, there was a significant difference in nocturnal activity between light spectra (p < 0.001), because birds were more active under white light (estimate = 118 min, s.e. = 8.4) compared with green light (estimate = 86 min, s.e. = 8.4). However, the difference was not significant (p = 0.08) for urban birds. Similarly, total activity was higher under ALAN compared with darkness (DW, DG p < 0.001; electronic supplementary material, figure S2-D). While forest birds had a higher total activity (p < 0.001) under white light (estimate = 481 min, s.e. = 18.0) compared with green light (estimate = 442 min, s.e. = 18.0), urban birds showed no difference in total activity with regards to light spectra (p = 0.07).
Table 1.
Results of the LMMs on the four activity response variables in experiment 2. Model outputs for significant terms are given. The numerator degrees of freedom (ndf), denominator degrees of freedom (ddf), F-statistic (F) and p-value (p) are given for each term.
| response | explanatory | ndf, ddf | F | p |
|---|---|---|---|---|
| activity onset | origin × treatment | 2, 882.1 | 3.2 | 0.041 |
| treatment × treatment day | 20, 878.8 | 4.3 | <0.001 | |
| activity offset | treatment × treatment day | 20, 883.6 | 1.8 | 0.014 |
| nocturnal activity | origin × treatment | 2, 970.9 | 5.6 | 0.004 |
| total activity | origin × treatment | 2, 969.2 | 18.1 | <0.001 |
| treatment day | 10, 968.2 | 8.9 | <0.001 |
There was also a major effect of treatment day on the activity patterns of birds excluding nocturnal activity (table 1). Activity onset advanced over time under both treatments, but while there were similarities in the first treatment days, onset declined more so under white light than under green light (electronic supplementary material, figure S2-A). Conversely, to onset, activity offset was delayed more in the first few treatment days under ALAN, and the offset times became closer to dark nights over time (electronic supplementary material, figure S2-B). During the relative night, birds did not change activity levels over time (electronic supplementary material, figure S2-C). The total activity measured in a 24 h period increased in the first couple of treatment days and then plateaued over time (electronic supplementary material, figure S2-D).
(ii). Effects of ALAN on nocturnal restlessness, oxalic acid, cognition and DEE
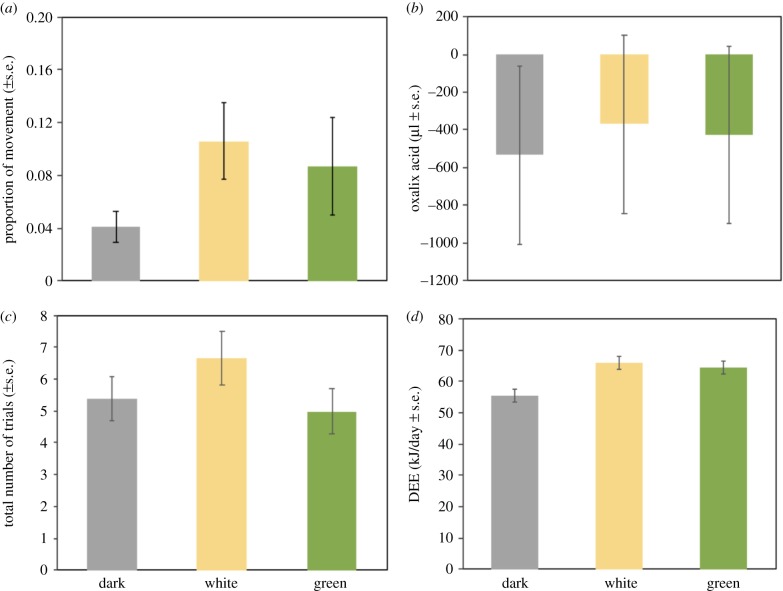
ALAN affected the proportion of movements at night displayed by birds. Specifically, white light (estimate = 0.11, s.e. = 0.03) induced more movement compared with darkness (estimate = 0.04, s.e. = 0.01, p = 0.02), whereas no difference was found between green light (estimate = 0.09, s.e. = 0.04) and other treatments (figure 2a; electronic supplementary material, table S2).). However, ALAN had no effect on the change in levels of blood oxalate (figure 2b; electronic supplementary material, table S2). Similarly, cognition was not affected by ALAN (figure 2c; electronic supplementary material, table S2). Only the type of task had an effect on the total number of trials for task completion (p < 0.001), because in memory tasks birds were quicker (estimate = 3.3, s.e. = 0.46), compared with learning tasks (estimate = 6.6, s.e. = 0.58), independent of treatment or origin (p > 0.1 in both cases). DEE was significantly affected by treatment (p = 0.002). Post-hoc tests revealed that birds that were exposed to ALAN, regardless of their origin and the light spectrum, had a higher DEE compared with the dark control group (DW, p = 0.003; DG, p = 0.011, WG, p = 0.87) (figure 2d; electronic supplementary material, table S2).
Figure 2.
Response of great tits to the presence of artificial light at night and different light spectra. (a) Proportion of minutes at night that birds spent without head tucked under feathers. (b) Oxalic acid calculated as the change in levels between baseline measurement and each treatment period. (c) Total number of trials until task completion during the cognition tests. (d) Daily energy expenditure of birds. Bars and error bars represent raw data as means ± s.e.m. (Online version in colour.)
4. Discussion
(a). Great tits prefer to sleep under green ALAN
ALAN can have detrimental effects on birds, such as altering susceptibility to infection [39], increasing stress [30] and inhibiting body mass gain [14]. Moreover, previous studies on free-living great tits and European blackbirds showed that birds might avoid illuminated areas at night [11,17], possibly in an attempt to evade the adverse effects of nocturnal light. Therefore, we hypothesized that birds would prefer to roost in darkness. Contrary to our expectations, in the choice experiment, birds had a clear preference for roosting under light instead of darkness. In particular, they chose to sleep under green light more often when the alternative choice was white light or darkness, and white light was also (slightly) preferred over darkness.
While we do not know the exact mechanism behind the choice of roosting under light versus darkness, we suggest that when light intensity is dimmed enough, birds prefer to roost under light to extend their days and possibly increase foraging time and extra-pair mate attraction (assuming that such preference does not vary seasonally, since these experiments were run in autumn). Indeed, in birds extension of activity into the night under ALAN has been associated with increased extra-pair paternity gain [10] and food intake [40]. The benefits of increase foraging at night might be particularly beneficial in winter, when the energetic costs of thermoregulation during cold nights might impose strong selection on the ability of birds to acquire sufficient food to survive the night. However, we also stress that our birds were held in captivity with constant warm temperature and ad libitum food. Future captive studies could deprive birds of food at night to test whether birds would still extend their activity into the night under ALAN.
(b). The physiological and behavioural consequences of sleeping under ALAN
The preference for birds to sleep under artificial light raises the question of whether ALAN exposure has any real negative effect on health, cognition and ultimately fitness. In the follow-up of the choice experiment, the forced light exposure experiment, both light treatments had a strong effect on activity patterns, but white light more so compared with green light. In particular, the largest differences were seen in the activity onset of birds, where birds started their day around 30 min earlier under white light compared with green light. Conversely, birds under dark nights confined their activity during the daylight hours. A similar experiment with blue tits showed the same pattern, with white light having a more severe effect on nocturnal activity compared with green light [31]. Interestingly, many of these effects of activity patterns plateaued or even reversed after a few days of exposure (electronic supplementary material, figure S2), possibly suggesting habituation to light. Future studies should directly test this hypothesis.
Parallel to these strong changes in activity patterns, birds under green and white light showed elevated levels of DEE. Our findings support the idea that an increase in locomotor activity could lead to higher levels of DEE. The increase in DEE under ALAN found in our experiment contradicts what was found in a recent study by Welbers et al. [16], where a lower DEE was observed under experimental green and white lights installed in forest areas. However, this study was conducted on free-living birds, and it was suggested that the decrease in DEE might be related to other ecological factors, such as the attraction of insects to light and thus increase in food availability in illuminated areas. Such factors were missing in our laboratory experiment, which might explain the discrepancy in the data from the field. Field data might be more biologically relevant. However, great tits are more often exposed to light at night in urban areas, where availability of preferred insect preys is usually scarce [41,42]. Indeed, the only previous study which measured DEE in urban and rural great tits found energy expenditure to be higher in urban individuals [43].
Despite the effects of ALAN on nocturnal activity and energy expenditure, no clear impact on sleep disruption was found, as measured by the plasma levels of oxalic acid. There could be several reasons for this. One potential explanation is that oxalic acid is not a valid biomarker for sleep debt in birds, as studies so far are contradictory. In fact, in a previous experimental study in the field, birds living in forest areas that were artificially illuminated did show more nocturnal restlessness and a reduction in oxalic acid over time [24]. A more recent study showed that levels of oxalic acid increased in great tit nestlings exposed to ALAN in their nest boxes [44]. However, in this study it was also noted that sleep patterns differed between developing birds and adults, which may reflect age-specific differences in sleep loss in response to ALAN and thus, changes in levels oxalic acid. An alternative explanation is that our experimental treatment of 1.5 lux of ALAN was not sufficiently strong to cause sleep disruption and ultimately alter cognitive responses. As mentioned in the introduction, an intensity of light of 1.5 lux is within the range of what wild birds can be exposed to in light polluted areas. However, artificial light levels measured underneath street lamps can be as high as 20 lux, and on average between 5 and 10 lux [3,11]. Thus, 1.5 lux might simply represent a level of light that birds can tolerate without suffering sleep disruption and an associated reduction in oxalic acid level.
We hypothesized that ALAN would have an effect on cognition as cognitive abilities in birds, like learning and memory, can be affected by sleep quality [45], and nocturnal illumination can lead to restlessness [14,24]. Contrary to our expectations, we did not find any effects of ALAN on cognition, which might be due to several reasons. First, birds in our experiment might not have experienced sleep disruption (see above). Previous studies showed that very high levels of light at night can have detrimental effects on cognition of birds. For instance, birds exposed to constant daylight for the whole 24 h showed a decrease in neuronal activity in brain regions associated with cognition and a decline in cognitive functions [46]. However, our experimental manipulation was closer to a natural situation compared with these previous studies, as essentially birds were still exposed to LD cycles with only dim light at night. Thus, as mentioned above, the birds in our experiment might not have experienced the same degree of circadian sleep disruption under dim light at night and consequently cognitive responses were not altered.
(c). Urban and forest great tits respond differently to ALAN
Urban and forest birds respond differently to ALAN in many features of their activity patterns. ALAN, and in particular white light, was consistently more disruptive on the activity patterns of forest birds compared with urban conspecifics. In the forced light exposure experiment, while the amount of nocturnal activity and total activity was similar for both urban and forest birds under green light, it was higher for forest birds under white light. Thus, our results suggest that forest birds are more sensitive to nocturnal lighting, and in particular to white light, than urban birds. It has been proposed that prolonged exposure to anthropogenic factors, including ALAN, should lead to acclimation or even adaptation, resulting in habitat-specific differences in behaviour and physiology between populations inhabiting urban and forest areas [47,48]. However, such differences might depend on the specific biological function considered, and also on the species. Indeed, when exposed to ALAN, blackbirds from city areas showed a stronger reproductive response [11], but no difference in daily activity pattern [15], compared with forest conspecifics. In a common-garden experiment, urban blackbirds also showed lower responsiveness of the stress axis compared with forest conspecifics [48]. The increase in night activity and total activity for forest birds under white light compared with green light, and the lack of differences between the two light treatments for urban birds, supports the idea that white lights could possibly have stronger effects on activity patterns of naive animals. However, in our experiment origin was assigned to birds depending on the location in which the birds were caught. We had no knowledge on the previous experiences of the birds, and as such we cannot directly relate our outcomes to previous light exposure.
(d). Light spectra matter
The different spectra we used clearly had an effect on light preference and activity patterns. Birds preferred to sleep under green light, and white light had the strongest effects on activity levels. We deployed green and white lights with the same measured illuminance (i.e. lux levels). However, lux is a unit of measure that is calibrated to the photo-sensitivity of the human eye. We used these human-based light measurements in lux because these will be the real currency when city councils install new lights, regardless of the action spectra of wild animals. We recognized that the avian action spectrum is different from that of humans [5]. However, the spectral characteristics of the visual system are a limited predictor of how intensely birds perceive light. For instance, a study by Prayitno & Phillips [49] showed that the difference in perceived colour-dependent light intensity (in a discrimination test) can be difficult to predict from the known spectral sensitivity of the eye. Moreover, the circadian system of birds is complex and relies upon the action of several types of photoreceptors located in different areas, including the retina, the pineal gland and the hypothalamus [5]. Our understanding of which set of photoreceptors may be more affected by dim ALAN, and how in turn they might affect circadian behaviour and physiology, is currently scarce. This limits our ability to understand the mechanisms by which light pollution affects the circadian system of birds and other animals. Future studies should look at filling this gap.
5. Conclusion
We provide the first and only evidence that a wild bird species prefers to roost under light instead of darkness when given the choice in the laboratory. We proposed the idea that birds may actively select to roost under light at night when this is sufficiently dim not to disrupt their sleep, as this offers the opportunity for increased foraging at night, which has been shown in other species [40,50]. From our camera recordings, we could detect nocturnal foraging in some birds, although this was difficult to quantify. Moreover, birds clearly preferred to roost under green light and light levels of 1.5 lux did not likely result in sleep disruption and cognitive impairment. Thus, negative behavioural and physiological effects of ALAN might be observed only under intensities higher than 1.5 lux. These results suggest that reducing the intensity of light pollution as well as tuning the spectrum towards long wavelengths may considerably reduce its impact. Such simple, clear guidelines should be taken into considerations when installing new artificial illumination.
Supplementary Material
Acknowledgements
We wish to thank all the people who helped us catching birds in the field: Robin Heinen, Martijn van der Sluijs and Peter Alblas. We are also grateful to Coretta Jongeling, Ruben de Wit, Franca Kropman and Marylou Aaldering for providing outstanding care to all the birds in this experiment. Jeroen Laurens and Gilles Wijlhuizen provided technical assistance for the installation of the cages, the light system and the activity recorders. Kees van Oers helped designing the cognitive tests and lent us the necessary equipment.
Ethics
The study was approved by an ethical committee (DEC-KNAW protocol NIOO 14.05, addendum 2 to M.E.V.).
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.dr8277c [51].
Authors' contributions
Z.N.U., K.S., M.E.V. and D.M.D. conceived and designed the study. D.M.D. captured the birds. Z.N.U. and D.M.D. set-up the experimental facilities. Z.N.U. performed the experiments and analysed all data, except for the camera recordings of nocturnal behaviour which were analysed by T.K. P.M. conducted the laboratory analysis of oxalic acid. Z.N.U. wrote the paper with help from M.E.V. and D.M.D. All authors agreed on the final version of the manuscript.
Competing interests
We declare we have no competing interests.
References
- 1.Dominoni D, Borniger J, Nelson R. 2016. Light at night, clocks and health: from humans to wild organisms. Biol. Lett. 12, 20160015 ( 10.1098/rsbl.2016.0015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders D, Gaston KJ. 2018. How ecological communities respond to artificial light at night. J. Exp. Zool. Part A Ecol. Integr. Physiol. 329, 394–400. ( 10.1002/jez.2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longcore T, Rich C. 2004. Ecological light pollution. Front. Ecol. Environ. 2, 191–198. ( 10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2) [DOI] [Google Scholar]
- 4.Van Doren BM, Horton KG, Dokter AM, Klinck H, Elbin SB, Farnsworth A.. 2017. High-intensity urban light installation dramatically alters nocturnal bird migration. Proc. Natl Acad. Sci. USA 114, 11 175–11 180. ( 10.1073/PNAS.1708574114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassone VM. 2014. Avian circadian organization: a chorus of clocks. Front. Neuroendocrinol. 35, 76–88. ( 10.1016/j.yfrne.2013.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominoni DM, Partecke J. 2015. Does light pollution alter daylength? A test using light-loggers on free-ranging European blackbirds (Turdus merula). Phil. Trans. R. Soc. B 370, 20140118 ( 10.1098/rstb.2014.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong M, Jeninga L, Ouyang JQ, van Oers K, Spoelstra K, Visser ME.. 2016. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol. Behav. 155, 172–179. ( 10.1016/j.physbeh.2015.12.012) [DOI] [PubMed] [Google Scholar]
- 8.Dominoni D, de Jong M, Bellingham M, O'Shaughnessy P, van Oers K, Robinson J, Smith B, Visser ME, Helm B. 2018. Dose-response effects of light at night on the reproductive physiology of great tits (Parus major): Integrating morphological analyses with candidate gene expression. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 1–15. ( 10.1002/jez.2214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar J, Malik S, Bhardwaj SK, Rani S. 2018. Bright light at night alters the perception of daylength in Indian weaver bird (Ploceus philippinus). J. Exp. Zool. Part A Ecol. Integr. Physiol. 329, 488–496. ( 10.1002/jez.2201) [DOI] [PubMed] [Google Scholar]
- 10.Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. ( 10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 11.Dominoni D, Quetting M, Partecke J.. 2013. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B 280, 20123017 ( 10.1098/rspb.2012.3017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordt A, Klenke R. 2013. Sleepless in town: drivers of the temporal shift in dawn song in urban European blackbirds. PLoS ONE 8, 1–10. ( 10.1371/journal.pone.0071476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Silva A, Samplonius J, Schlicht E, Valcu M, Kempenaers B.. 2014. Artificial night lighting rather than traffic noise affects the daily timing of dawn and dusk singing in common European songbirds. Behav. Ecol. 25, 1037–1047. ( 10.1093/beheco/aru103) [DOI] [Google Scholar]
- 14.Raap T, Pinxten R, Eens M. 2015. Light pollution disrupts sleep in free-living animals. Sci. Rep. 5, 13557 ( 10.1038/srep13557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominoni D, Goymann W, Helm B, Partecke J. 2013. Urban-like night illumination reduces melatonin release in European blackbirds (Turdus merula): implications of city life for biological time-keeping of songbirds. Front. Zool. 10, 60 ( 10.1186/1742-9994-10-60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welbers AAMH, van Dis NE, Kolvoort AM, Ouyang J, Visser ME, Spoelstra K, Dominoni DM.. 2017. Artificial light at night reduces daily energy expenditure in breeding great tits (Parus major). Front. Ecol. Evol. 5, 1–10. ( 10.3389/fevo.2017.00055) [DOI] [Google Scholar]
- 17.de Jong M, Ouyang JQ, van Grunsven RHA, Visser ME, Spoelstra K.. 2016. Do wild great tits avoid exposure to light at night? PLoS ONE 11, e0157357 ( 10.1371/journal.pone.0157357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho YM, Ryu SH, Lee BR, Kim KH, Lee E, Choi J. 2015. Effects of artificial light at night on human health: a literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 32, 1294–1310. ( 10.3109/07420528.2015.1073158) [DOI] [PubMed] [Google Scholar]
- 19.Aulsebrook AE, Jones TM, Mulder RA, Lesku JA. 2018. Impacts of artificial light at night on sleep: a review and prospectus. J. Exp. Zool. Part A Ecol. Integr. Physiol. 329, 409–418. ( 10.1002/jez.2189 [DOI] [PubMed] [Google Scholar]
- 20.Ouyang JQ, Davies S, Dominoni D. 2018. Hormonally mediated effects of artificial light at night on behavior and fitness: linking endocrine mechanisms with function. J. Exp. Biol. 221, jeb156893 ( 10.1242/jeb.156893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Careau V, Killen SS, Metcalfe NB. 2015. Adding fuel to the ‘fire of life’: energy budgets across levels of variation in ectotherms and endotherms. In Integrative organismal biology (eds Martin LB, Woods HA, Ghalambor C), pp. 219–233. Chichester, UK: John Wiley. [Google Scholar]
- 22.Nagy KA. 2005. Field metabolic rate and body size. J. Exp. Biol. 208, 1621–1625. ( 10.1242/jeb.01553) [DOI] [PubMed] [Google Scholar]
- 23.Weljie AM, Meerlo P, Goel N, Sengupta A, Kayser MS, Abel T, Birnbaum MJ, Dinges DF, Sehgal A.. 2015. Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt. Proc. Natl Acad. Sci. USA 112, 2569–2574. ( 10.1073/pnas.1417432112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang JQ, de Jong M, van Grunsven RHA, Matson KD, Haussmann MF, Meerlo P, Visser ME, Spoelstra K.. 2017. Restless roosts: light pollution affects behavior, sleep and physiology in a free-living songbird. Glob. Chang. Biol. 23, 4987–4994. ( 10.1111/gcb.13756) [DOI] [PubMed] [Google Scholar]
- 25.Vorster AP, Born J. 2015. Sleep and memory in mammals, birds and invertebrates. Neurosci. Biobehav. Rev. 50, 103–119. ( 10.1016/j.neubiorev.2014.09.020) [DOI] [PubMed] [Google Scholar]
- 26.Brodin A, Urhan AU. 2014. Interspecific observational memory in a non-caching Parus species, the great tit Parus major. Behav. Ecol. Sociobiol. 68, 649–656. ( 10.1007/s00265-013-1679-2) [DOI] [Google Scholar]
- 27.Jha NA, Kumar V. 2017. Effect of no-night light environment on behaviour, learning performance and personality in zebra finches. Anim. Behav. 132, 29–47. ( 10.1016/j.anbehav.2017.07.017) [DOI] [Google Scholar]
- 28.Kyba CCM, et al. 2017. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 3, e1701528 ( 10.1126/sciadv.1701528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Jong M, Ouyang JQ, Da Silva A, van Grunsven RHA, Kempenaers B, Visser ME, Spoelstra K.. 2015. Effects of nocturnal illumination on life-history decisions and fitness in two wild songbird species. Phil. Trans. R. Soc. B 370, 20140128 ( 10.1098/rstb.2014.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouyang JQ, de Jong M, Hau M, Visser ME, van Grunsven RH, Spoelstra K.. 2015. Stressful colours: corticosterone concentrations in a free-living songbird vary with the spectral composition of experimental illumination. Biol. Lett. 11, 20150517 ( 10.1098/rsbl.2015.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Jong M, Caro SP, Gienapp P, Spoelstra K, Visser ME.. 2017. Early birds by light at night: effects of light color and intensity on daily activity patterns in blue tits. J. Biol. Rhythms 32, 323–333. ( 10.1177/0748730417719168) [DOI] [PubMed] [Google Scholar]
- 32.Dawkins MS. 1990. From an animal's point of view: motivation, fitness, and animal welfare. Behav. Brain Sci. 13, 1–9. ( 10.1017/S0140525X00077104) [DOI] [Google Scholar]
- 33.Riber AB. 2015. Effects of color of light on preferences, performance, and welfare in broilers. Poult. Sci. 94, 1767–1775. ( 10.3382/ps/pev174) [DOI] [PubMed] [Google Scholar]
- 34.Sun J, Raap T, Pinxten R, Eens M. 2017. Artificial light at night affects sleep behaviour differently in two closely related songbird species. Environ. Pollut. 231, 882–889. ( 10.1016/j.envpol.2017.08.098) [DOI] [PubMed] [Google Scholar]
- 35.Gaston KJ, Davies TW, Bennie J, Hopkins J. 2012. REVIEW: reducing the ecological consequences of night-time light pollution: options and developments. J. Appl. Ecol. 49, 1256–1266. ( 10.1111/j.1365-2664.2012.02212.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szymczak JT, Helb HW, Kaiser W. 1993. Electrophysiological and behavioral correlates of sleep in the blackbird (Turdus merula). Physiol. Behav. 53, 1201–1210. ( 10.1016/0031-9384(93)90380-x) [DOI] [PubMed] [Google Scholar]
- 37.Mitchell GW, Guglielmo CG, Hobson KA. 2015. Measurement of whole-body CO2 production in birds using real-time laser-derived measurements of hydrogen (δ2H) and oxygen (δ18O) isotope concentrations in water vapor from breath. Physiol. Biochem. Zool. 88, 599–606. ( 10.1086/683013) [DOI] [PubMed] [Google Scholar]
- 38.Titulaer M, van Oers K, Naguib M.. 2012. Personality affects learning performance in difficult tasks in a sex-dependent way. Anim. Behav. 83, 723–730. ( 10.1016/j.anbehav.2011.12.020) [DOI] [Google Scholar]
- 39.Kernbach ME, Miller JM, Hall RJ, Unnasch TR, Burkett-Cadena ND, Martin LB. 2018. Light pollution increases West Nile virus competence in a ubiquitous passerine reservoir species. bioRxiv, 269209 ( 10.1101/269209) [DOI]
- 40.Russ A, Rüger A, Klenke R. 2015. Seize the night: European blackbirds (Turdus merula) extend their foraging activity under artificial illumination. J. Ornithol. 156, 123–131. ( 10.1007/s10336-014-1105-1) [DOI] [Google Scholar]
- 41.Pollock CJ, Capilla-Lasheras P, McGill RAR, Helm B, Dominoni DM. 2017. Integrated behavioural and stable isotope data reveal altered diet linked to low breeding success in urban-dwelling blue tits (Cyanistes caeruleus). Sci. Rep. 7, 5014 ( 10.1038/s41598-017-04575-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seress G, Hammer T, Bókony V, Vincze E, Preiszner B, Pipoly I, Sinkovics C, Evans KL, Liker A. 2018. Impact of urbanization on abundance and phenology of caterpillars and consequences for breeding in an insectivorous bird. Ecol. Appl. 28, 1143–1156. ( 10.1002/eap.1730) [DOI] [PubMed] [Google Scholar]
- 43.Hinsley SA, Hill RA, Bellamy PE, Harrison NM, Speakman JR, Wilson AK, Ferns PN. 2008. Effects of structural and functional habitat gaps on breeding woodland birds: working harder for less. Landsc. Ecol. 23, 615–626. ( 10.1007/s10980-008-9225-8) [DOI] [Google Scholar]
- 44.Raap T, Pinxten R, Eens M. 2018. Artificial light at night causes an unexpected increase in oxalate in developing male songbirds. Conserv. Physiol. 6, coy005 ( 10.1093/conphys/coy005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rattenborg NC, Martinez-Gonzalez D, Lesku JA. 2009. Avian sleep homeostasis: convergent evolution of complex brains, cognition and sleep functions in mammals and birds. Neurosci. Biobehav. Rev. 33, 253–270. ( 10.1016/j.neubiorev.2008.08.010) [DOI] [PubMed] [Google Scholar]
- 46.Taufique SKKT, Kumar V. 2016. Differential activation and tyrosine hydroxylase distribution in the hippocampal, pallial and midbrain brain regions in response to cognitive performance in Indian house crows exposed to abrupt light environment. Behav. Brain Res. 314, 21–29. ( 10.1016/j.bbr.2016.07.046) [DOI] [PubMed] [Google Scholar]
- 47.Bonier F, Martin PR, Wingfield JC. 2007. Urban birds have broader environmental tolerance. Biol. Lett. 3, 670–673. ( 10.1098/rsbl.2007.0349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Partecke J, Schwabl I, Gwinner E. 2006. Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology 87, 1945–1952. ( 10.1890/0012-9658(2006)87[1945:SATCUA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 49.Prayitno DS, Phillips CJC. 1997. Equating the perceived intensity of coloured lights to hens. Br. Poult. Sci. 38, 136–141. ( 10.1080/00071669708417958) [DOI] [PubMed] [Google Scholar]
- 50.Stracey CM, Wynn B, Robinson SK. 2014. Light pollution allows the northern mockingbird (Mimus polyglottos) to feed nestlings after dark. Wilson J. Ornithol. 126, 366–369. ( 10.1676/13-107.1) [DOI] [Google Scholar]
- 51.Ulgezen ZN, Käpyla T, Meerlo P, Spoelstra K, Visser ME, Dominoni DM. 2019. Data from: The preference and costs of sleeping under light at night in forest and urban great tits. Dryad Digital Repository. ( 10.5061/dryad.dr8277c) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ulgezen ZN, Käpyla T, Meerlo P, Spoelstra K, Visser ME, Dominoni DM. 2019. Data from: The preference and costs of sleeping under light at night in forest and urban great tits. Dryad Digital Repository. ( 10.5061/dryad.dr8277c) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.dr8277c [51].