Abstract
Background
Switzerland's demographic trends show, as elsewhere on the planet, increasing numbers of older and very old adults. This suggests that its healthcare system will suffer serious repercussions, including in the use of care and especially the use of emergency services. Significant numbers of older adults will be at risk of developing multiple chronic conditions including one or more geriatric syndromes, such as frailty and delirium. Few studies to date have documented associations between frailty and delirium.
Aim
To explore the relationships between frailty and delirium in older adult patients consulting (n = 114) at an emergency department (ED) in Switzerland.
Method
A cross-sectional study was conducted in a peripheral hospital ED in the French-speaking part of Switzerland. Frailty was assessed using the Tilburg Frailty Indicator (TFI). Delirium was assessed using the Confusion Assessment Method (CAM). Participants' cognitive states were assessed using the 6-item Cognitive Impairment Test (6CIT) and the Informant Questionnaire on Cognitive Decline in the Elderly (IQ-CODE), completed by the participant's most significant informal caregiver.
Results
The mean participant age was 77.6 years (SD = 7.7); the majority of the subjects were women (54%). The participants took an average of 4.7 different medications a day (SD = 3.2, median = 4). More than half (62%) of the participants were frail; 2 and 14% presented signs and symptoms of delirium and subsyndromal delirium, respectively. A weak but significant association between scores for frailty and delirium (p < 0.05) was demonstrated, and clinical observation confirmed this. A 4-h follow-up measurement of delirium in the ED revealed no significant or clinical difference.
Conclusion
Although the literature describes strong associations between frailty and delirium in surgical units and community care settings, the present study only demonstrated a weak-to-moderate association between frailty and delirium in our ED.
Key Words: Older adults, Delirium, Frailty, Nursing care, Preventative nursing, Screening, Emergency care
Introduction
The number of persons aged 65 years or older has grown significantly throughout Switzerland and in the canton of Valais [1, 2]. According to the canton's Health Observatory, the number of persons aged 65 years or older quadrupled between 1950 and 2015 [3]. This demographic transition is having far-reaching but predictable repercussions on the healthcare system, including greater recourse to the canton's emergency departments (ED), which are the 24-h entry points to Switzerland and Valais' healthcare systems [4]. However, the inevitable aging process and the consequences of increasing frailty do not affect all older adults equally [5, 6]. Reactions to (endogenous and exogenous) psychological, physical, and environmental stressors can tip some older adults towards a functional decline accelerated by the geriatric syndromes of frailty and delirium [7, 8, 9].
Frailty is a condition characterized by a person's increased vulnerability resulting from the decay of their physiological and cognitive reserves [10, 11, 12, 13]. It evolves silently, causing a declining ability to resist stressors and thus increasing the risk of undesirable events such as falls, bedsores, repeated hospitalization and ED admissions, loss of autonomy, and premature death [14, 15, 16, 17]. Both Santangelo et al. [18] and Clegg et al. [19] documented that frailty was associated with adverse effects, with the development and progression of numerous chronic illnesses, and with post-surgical complications [20].
Delirium is characterized by fluctuating disturbances in arousal, a sudden and rapid onset, trouble concentrating, and cognition secondary to an acute medical condition; it is common and affects 8–17% of older patients attending ED and 51% of patients in postacute care [21]. This syndrome has some important common consequences compared with frailty, such as a loss of executive function leading to falls, increased rehospitalizations, institutionalizations, and premature death [22, 23, 24, 25, 26, 27]. Delirium in the ED among older patients has negative consequences and is an independent predictor of prolonged hospitalizations and early death [28, 29]. Older adults visiting ED often experience delirium, but this is rarely recognized [30].
The rapid detection of geriatric syndromes in the ED, such as frailty and delirium, is related to awareness and risks of an increased length of stay, more adverse effects, increased admissions and readmissions, and increased mortality [21]. Frailty and delirium would appear to be two clinically distinct geriatric syndromes; however, their simultaneous onset has often been documented in the scientific literature [20, 24, 25]. Few studies have explored the associations between frailty and delirium on admission to Swiss ED [31, 32, 33, 34, 35]. Highlighting an association between frailty and delirium could lead to a better understanding of both these geriatric syndromes and their interdependence, to prevent and detect them more consequently in the ED [33, 35]. Nevertheless, few authors have sought to evidence any relationship between frailty and the initiation of other geriatric syndromes in the ED [20, 21].
We hypothesized that frailty underlies and predisposes older adults to delirium [22]. It is important to establish whether frailty and subsequent delirium are associated in order to develop further strategies and interventions [8, 36].
ED health care professionals carry out rapid, triage type, clinical evaluations and general, clinical, geriatric evaluations of older patients [37, 38]. A more profound understanding of the relationship between frailty and delirium could increase the detection of any acute changes in patients' mental state. Healthcare providers such as nurses and physicians could play a major role in prevention and the implementation of strategies to reduce the risk factors for frailty and delirium [40, 41]. An ED is both a hub and an entry point to the healthcare system – one via which some older patients return home and others are hospitalized [39]. We can conclude that rapid or early detection of frailty by ED healthcare professionals would help to orient older adults towards appropriate departments or wards and to adapt patients' management all along their clinical and care trajectories, whether in the hospital or at home. The objective is to identify and minimize the risk factors leading to adverse effects, complications, avoidable rehospitalizations, and early deaths [28, 29, 40].
The present study's main objective was to explore the presence of frailty and delirium among older adults admitted to an ED. Its secondary objective was to analyze associations between frailty and delirium among those older adults during the 4 h following their admission to an ED. Finally, this study explored the relationships between frailty, delirium, and the sociodemographic characteristics of older adult patients consulting at the ED.
The study's theoretical framework was based on the Neuman Systems Model developed by Neuman and Fawcett [42]. The emphasis is put on the healthcare professional's role in preserving and maintaining health using primary, secondary, and tertiary preventative interventions.
Materials and Methods
Study Design and Setting
This cross-sectional study was conducted in the ED of a peripheral hospital in the French-speaking region of Switzerland. About 50,000 yearly admissions in the ED of the regional hospital, i.e., almost one third, are community-dwelling older adults aged 65 or older (n = 15,000).
Recruitment
All older adults, both male and female, who consulted at the ED during the recruitment period were invited to participate in this study. The study inclusion criteria were: (1) age 65 years or older, (2) capacity for discernment or a legal representative's agreement to the patient's participation, (3) ability to speak and understand French, and (4) medical assessment confirming discernment of the patient's ability to participate. Each patient underwent an evaluation of their capacity for discernment carried out by a specialist physician, and a family caregiver was asked to agree to participation when patients were incapable of discernment. Oral and written consent were thus obtained from either the patient or their legal representative.
Data Collection
Data collection took place between October 2016 and February 2017 on Mondays and Tuesdays between 7:00 a.m. and 7:00 p.m. The entire multidisciplinary team of the ED was informed about the study's objectives and how data would be collected. Two master's degree study nurses underwent a training course on delirium and how to use the CAM with a recognized expert in the mental and physical evaluation of older adults [43]. At the first encounter with potential participating patients, the study nurses presented the study and distributed information sheets and informed consent forms. Patients were given an hour to make up their minds. If the patient was incapable of discernment, then their legal representative was asked for consent. During the training period, interrater agreement between each of the two study nurses and the expert was acceptable, with a κ of 0.80 [44].
Measurement
Primary Outcomes
Assessment of Frailty. Frailty was evaluated using the Tilburg Frailty Indicator (TFI) of Gobbens et al. [45]. Although there is as yet no psychometrically validated French version of the TFI, the tool was translated using the scientific method described by Le May et al. [46] and an ulterior psychometric validation is planned. The TFI is divided into 2 parts. Part A contains 10 items which identify the determinants of frailty, such as age and sex. Part B contains 15 questions to evaluate the physical, psychological, and social domains of frailty, and its final score ranges between 0 and 15. Each questionnaire item is represented by a variable: 11 items are dichotomized (yes/no) and 4 are categorical (yes/no/perhaps). Each question results in a score of 1 or 0. A total score superior or equal to 5 suggests that the person is frail. The greater the score, the more the degree of frailty is considered to be significant. Internal coherence demonstrated a satisfactory Cronbach's α coefficient of 0.73. Construct validity between the different domains revealed significant Pearson's correlation coefficients (p ≤ 0.05). These were r = 0.42 between the physical and psychological domains, r = 0.19 between the physical and social domains, and r = 0.18 between the psychological and social domains. Convergent validity was judged to be good. Divergent validity was also tested and considered good. The TFI demonstrated good temporal-fidelity stability, with a frailty score of 0.79; after 1 year and 2 weeks, scores were 0.90 [45].
Assessment of Delirium. The signs and symptoms of delirium were evaluated using the French version of the Confusion Assessment Method (CAM), as developed by Inouye et al.[47] and Laplante et al. [48]. The CAM is made up of 9 items, and items 1, 2, and 3 and/or 4 must be present for the result to be positive. The psychometric values of the CAM have been documented as being excellent, with 94% sensitivity, 89% specificity, and an interrater reliability of 0.70 and 1.00 (Cohen's κ). In the present study, the interrater reliability was 0.89, which is considered excellent [44].
Despite the absence of a clear definition and a validated tool, this study took incomplete delirium signs and symptoms into consideration (also named subsyndromal delirium; SSD). SSD has been documented as an acute confusion syndrome and shares characteristic core domain symptoms with full-blown delirium which distinguish each of them from the nondelirium groups, although the severity was intermediate in the subsyndromal group. Milder disturbances of delirium core domain symptoms are highly suggestive of SSD [49]. Based on the criteria of Meagher et al. [50] for the nondelirium cases to delineate the SSD group, the following criteria were included: (1) absence of full-blown delirium, (2) acute or subacute onset, (3) at least one symptom documented using the CAM in a dimensional approach (disturbed attention was the most significant), and (4) evidence of other cognitive and/or neuropsychiatric disturbances which could not be better accounted for by another neuropsychiatric condition.
Secondary Outcomes
Cognitive State. The patients' cognitive state was evaluated using the Informant Questionnaire on Cognitive Decline in the Elderly (IQ-CODE) of Jorm and Jacomb [51] and the 6-Item Cognitive Impairment Test (6CIT) developed by Katzman et al.[52]. The 6CIT evaluates the patient's orientation in time and space, memory, and concentration, with a maximum total score of 28. A score from 0–7 is considered “normal,” one of 8–9 is considered to indicate a “slight cognitive deficit,” and one ≥10 is considered to represent a “significant cognitive deficit.” For overall data, the 6CIT shows 90% sensitivity and 100% specificity; for light dementia it demonstrates 78% sensitivity and 100% specificity [52].
The IQ-CODE is aimed a participants' close family caregivers, and it allows us to differentiate existing, long-term neurocognitive disorders from recent ones. The questionnaire included 16 questions using a 5-point Likert type scale ranging from 1 (much better) to 5 (much worse). The mean score for the 16 items (or the mean of the number of items answered) is calculated. The threshold score for suspected dementia is 3.36 [51]. The IQ-CODE demonstrates a high reliability, with a Cronbach αfrom 0.93 to 0.97 over 3 days and of 0.75 over a year; it shows 79% sensitivity and 82% specificity.
Participant Characteristics
Participants' sociodemographic data were collected using a questionnaire and included sex, age, marital status, level of education, income data (revenue from the Valais cantonal pension), and the number of medications kept at home (including contingency treatments). A pretest was carried out on a sample of 5 people with the same characteristics as the population aimed for.
Statistical Analysis
For treating descriptive data, proportion testing was carried out for categorical variables. For numerical variables, we carried out measures of dispersion and central tendency. Measures of correlation were used to answer our main study questions examining the degrees of association between participants' scores for frailty and delirium, their health status, and their sociodemographic data: the point biserial correlation coefficient (rpb) was used for dichotomous variables and Kendall's τ was used for polytomous variables. Data were stored on and statistical analyses were carried out using IBM-SPSS version 22.0 [53]. p = 0.05 was considered statistically significant. Less than 5% of the data was missing and this was not treated.
Results
Participants
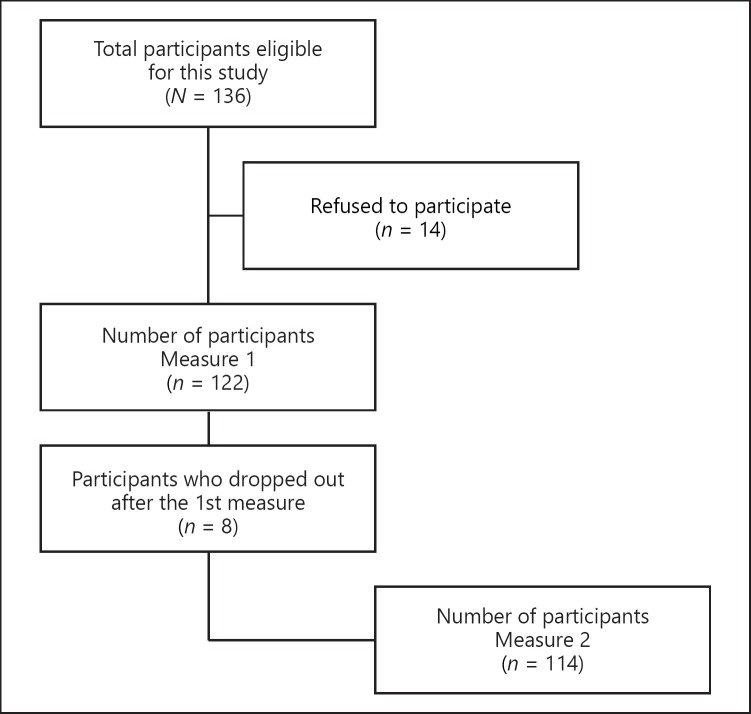
A total of 136 older patients who arrived at the ED during the recruitment days between 7:00 a.m. and 7:00 p.m. were eligible for this study; 14 refused to participate for various reasons such as fatigue, pain, and disinterest. The sample at the baseline measurement included 122 participants. At the second measurement, 4 h later, 8 participants had left the ED, reducing our sample to 114 patients analyzed (84% response rate) (Fig. 1).
Fig. 1.
Flow chart of participant recruitment in the ED.
Sociodemographic Data
Participants were mainly women aged over 77 years (SD = 7.7), and most were married. Most participants were Swiss (88%), 45% had been educated to apprenticeship level, and 81% received a Valais cantonal pension of over CHF 2,500 per month (Table 1).
Table 1.
Participants' sociodemographic characteristics in the ED
| Characteristic | Value |
|---|---|
| Sex | |
| Female | 65 (54) |
| Male | 56 (46) |
| Marital status | |
| Married | 68 (56) |
| Widowed | 39 (32) |
| Divorced | 13 (11) |
| Single | 1 (1) |
| Nationality | |
| Swiss | 106 (88) |
| Other | 15 (12) |
| Level of education | |
| Obligatory schooling | 20 (16) |
| Secondary school | 27 (22) |
| Apprenticeship | 55 (45) |
| Further education or university | 19 (16) |
| Monthly household incomea | |
| Below CHF 1,500 | 0 (0) |
| Between CHF 1,500 and 2,500 | 23 (19) |
| Above CHF 2,500 | 97 (81) |
The total number of patients was 122. Values are presented as numbers (%).
Pension revenue from canton Valais.
Health Status
The mean 6CIT score was 4.6 (median = 2). The participants were taking an average of 4.7 medications a day (SD = 3.2). Only 9 participants were diagnosed with a prior major neurocognitive disorder, including clinical manifestations of memory problems (mean = 4.6), as evaluated by their legal representatives using the IQ-CODE (Table 2).
Table 2.
Participants' health statuses at ED admission
| Variable | Value |
|---|---|
| 6CIT scale (M1) | |
| Mean±SD | 4.6±6.8 |
| Median (IQR) | 2 (8.2) |
| Range | 0–28 |
| IQ-CODE (n = 9) | |
| Mean±SD | 4.6±4.3 |
| Median (IQR) | 4.8 (4.4) |
| Range | 4.2–5 |
| Medications taken,n | |
| Mean±SD | 4.7±3.2 |
| Median (IQR) | 5.0 (6.0) |
| Range | 0–16 |
The total number of patients is 122.
Frailty
Our findings showed an overall mean TFI score of 5.3, which is above the indicator's threshold nonfrail/frail score of 5. With regard to these TFI findings, nearly two thirds (62%) of the participants were considered frail on their arrival at the ED (Table 3).
Table 3.
TFI scores in the ED
| TFI | Value |
|---|---|
| Score (0–15) | |
| Mean±SD | 5.3±2.5 |
| Median (IQR) | 5 (7.0) |
| Range | 0–13 |
| Frailty (%) | |
| Not frail | 46 (38) |
| Frail | 75 (62) |
Delirium
Table 4 presents the evolution in the signs and symptoms of full-blown and SSD as measured using the CAM algorithm for full-blown delirium and the dimensional approach for SSD at ED admission (baseline) and 4 hours after ED admission. Two participants presented with full-blown delirium (2%) at admission, and 15 (12%) showed clinical features of SSD. The figures for full-blown delirium hardly changed between the baseline measurements (M1) and measurements taken 4 h later (M2). However, presentation with SSD increased to 18 participants (16%). No significant differences were found between the measurements of M1 and M2 (d.f. = 114), with a mean difference of −0.08 (SD = 0.05; 95% CI −0.18 to −0.02; p = 0.9).
Table 4.
Evolution of delirium and SSD
| No delirium | SSD | Full-blown delirium | |
|---|---|---|---|
| Upon admission to the ED (n = 122) | 105 (86) | 15 (12) | 2 (2) |
| 4 h after admission (n = 114) | 94 (82) | 18 (16) | 2 (2) |
Values are presented as numbers (%).
Associations between Sociodemographic Data, Health Status, Frailty, and Delirium
Due to the low number of patients identified as CAM+ (i.e., with full-blown delirium), patients with SSD (1–3 symptoms) were grouped together with them. With regard to sociodemographic data and the TFI score, there was a weak, nonsignificant, negative association with sex (–0.17; p = 0.06) and marital status (–0.10; p = 0.3) and a weak, nonsignificant, positive association with age (0.16; p = 0.07) and monthly pension revenue (0.05; p = 0.6). Nationality showed a weak but significant positive association (0.25; p ≤ 0.01) with the TFI score and no significant association with the participants' level of education (–0.13; p = 0.08). A weak, nonsignificant, positive association was observed between the baseline (0.13; p = 0.17) and 4-h (0.08; p = 0.3) evaluations of cognitive deficiency. A mean significant positive association (0.44; p ≤ 0.01) was found between the TFI score and polypharmacy. The TFI score also presented a weak but significant positive association between the signs and symptoms of delirium at admission (0.23; p ≤ 0.01) and 4 h later (0.20; p = 0.03) (Table 5).
Table 5.
Associations between sociodemographic data, health status, frailty, and delirium
| Variable | TFI score |
|
|---|---|---|
| Pearson'srpb | p value | |
| CAM – (n = 121) | 0.23 | <0.01* |
| CAM – M2 (n = 114) | 0.20 | 0.03* |
| Cognitive deficit (6CIT) M1 (n = 121) | 0.13 | 0.17 |
| Cognitive deficit (6CIT) M2 (n = 114) | 0.08 | 0.3 |
| Medicationa (n = 121) | 0.44 | <0.01* |
| Sex (n = 121) | −0.17 | 0.06 |
| Age (n = 121) (65–80 vs. >80 years) | 0.16 | 0.07 |
| Nationality (n = 121) | 0.25 | <0.01* |
| Monthly pension income (n = 120) | 0.05 | 0.6 |
| Marital status (n = 121) (single vs. couple) | −0.10 | 0.3 |
| Level of education (n = 121) | −0.13 | 0.08b |
p ≤ 0.05.
Polypharmacy (>5 medications/day).
Kendall's τ.
Discussion
The sociodemographic data describing the population recruited to the present study corroborate those in other research concerning geriatric syndromes in ED [54, 55, 56]. Women made up 54% of the present study's participants, which is similar to the percentage described by Salvi et al. [57], and the mean age and the distribution of marital status were also similar. A high percentage of study participants had completed apprenticeships, showing levels of education similar to those in the study by Guessous et al. [58]. Although most participants' monthly income from the Valais cantonal pension scheme was above CHF 2,500, this was significantly less than the figure found by Guessous et al. [58], whose study was carried out in another Swiss canton, which probably explains the discrepancy. Associations between sociodemographic data and frailty scores showed no significant associations linked to participants' age or sex, contradicting the studies by Fried et al. [59], Rockwood et al. [60], and Guessous et al. [58]. Indeed, these results were surprising because the literature suggests that age and female sex are substantial risk factors for the development of frailty [61, 62, 63]. This may be explained by the higher mean ages in these studies, but cultural influences on the particular prevention strategies and healthcare systems chosen by different regions and countries cannot be ignored either. In opposition to the findings of van Assen et al. [64], the present study found no differences between mean frailty scores and the level of education. Household income was not significantly associated with scores for frailty, and this finding was also at odds with the studies by Fried et al. [59], van Assen et al. [64], and Guessous et al. [58]. One hypothesis is that these results are considerably influenced by national differences in healthcare systems and the financial situation of the retired older adults. Nationality was only weakly associated with frailty scores, but the studies by Guessous et al. [58] and Santos-Eggimann et al. [65] do not corroborate our results. The present study only examined nationality – not ethnic origin or country of birth – and this may have influenced our results. Furthermore, the instruments used were not the same, and they measured physical frailty only. Study participants took an average of 4.7 medications a day, whereas the study by Herr et al. [66] found a mean of 6.1 and associated polypharmacy with the frailty score. Considering these results, we could hypothesize that the rate of frailty was influenced by polypharmacy and comorbidities. However, a more detailed analysis of the types of medication and comorbidities would contribute to a better understanding of the links between polypharmacy, medication categories, comorbidities, and frailty scores. Our population's mean TFI score was higher than that of the designer's original study carried out on a general population sample in 2010 [45]. Two thirds of our participants showed signs of frailty, which is close to the prevalence recorded in an ED by Salvi et al. [57]. Nevertheless, the prevalence in the general population of older adults is lower, as in the studies by van Assen et al. [64] and Santos-Eggimann et al. [65]. These studies used different measurement instruments, however, and thus other classifications in other settings. The hypothesis is that the population consulting at an ED presents with more health difficulties than the population in general. It would have been interesting to analyze why patients consulted at the ED and to compare those data with levels of frailty. No significant associations were found between the two measurements for cognitive deficiency and the frailty score, and these findings do not correspond to those in the studies by Verloo et al. [8] and Leung et al. [67]. However, the reasons for hospitalizations and the study settings are quite heterogeneous and hard to compare [8, 67]. On admission to the ED, 12% of the participants had developed an SSD and 2% were suffering from full-blown delirium. Four hours later, these levels were, respectively, 16 and 2%, demonstrating the fluctuating component of delirium, as previously described by Inouye et al. [68] and Meagher and Trzepacz [69]. However, these rates were lower than those in the study of Marcantonio et al. [72], and other observational studies [70, 71], using the same measurement instrument, found a quiet higher delirium rate for their sample population [72]. Their study, however, was carried out in acute hospital units, which is a significant risk factor for delirium. We could hypothesize that the reasons for admission and participants' pathologies were confounding factors in our study. The present study demonstrated that frailty was weakly but significantly associated with the signs and symptoms of delirium. This is in opposition to the study by Verloo et al. [8], in which 9 out of 10 participants with delirium were frail and the association was strong. Leung et al. [67] and Jung et al. [73] also revealed a strong association, with patients who were frail before surgery at a greater risk of developing delirium after surgery. It is important to note, however, that these studies used different measurement instruments and that their settings and populations were dissimilar. Indeed, the population in an ED is often very heterogeneous, and the reasons for their consultations and the health conditions involved are numerous.
ED face the significant challenge of providing comprehensive, thoughtful evaluations of older patients presenting with delirium combined with frailty. One issue is that dementia and mild cognitive impairment are common in geriatric ED patients and their signs often go undetected [30]. Routine cognitive screening by ED nurses provides a formal assessment of the patient's mental status before their evaluation using another instrument, but the routine comprehensive geriatric assessment also provides a baseline for future ED visits. Since frailty is supposed to be a marker for vulnerability, it seems congruent that frail patients are at risk of higher rates of delirium and SSD. The cause of delirium is often multifactorial, including an acute medical illness overlaying a baseline cognitive dysfunction, medication effects and interactions, and decompensating comorbidities. The appropriate evaluation and management of frailty and delirium are critical to a positive outcome.
Frailty and delirium share several commonalities but also have specific differences. Both should be considered multifactorial health conditions as they are characterized by multiple risk factors and their causation is not necessarily specific to a given organ system failure. In particular, both are predictive of several negative health-related outcomes, most of which could be prevented by applying adapted and personalized interventions. Bellelli et al.[20] showed that frailty and delirium differ in many respects. Frailty is a long-term condition of decline that disturbs an individual's homeostasis and their capacities across multiple physiological systems; it is usually considered the endpoint in the progression of chronic diseases during the aging process [20]. On the contrary, delirium is an acute condition that occurs in response to a stressor; it may resolve relatively rapidly, though it can sometimes persist for weeks, months, or even years [23]. SSD is a condition in which one or more symptoms of delirium do not progress to full-blown delirium. SSD has been explained as an “incomplete delirium syndrome” [74]. Based upon our findings in the ED, we suggest that SSD should be considered to encompass both categorical and dimensional components, as recent studies have stated [49, 75]. Applying these criteria could allow the identification of SSD in ED patients, appropriate follow-up of the prognosis, and exploration of how SSD is temporally connected to full-blown delirium. SSD could be a stage of an evolving or resolving illness, but we do not understand to what extent it may be a distinct disorder whereby ED patients can experience it without progressing to full-blown delirium [76]. Frailty may thus represent the physiological condition for the development of delirium, and delirium may represent the clinical manifestation of the underlying frailty of an older adult in acute decompensation [20]. From a clinical perspective, cognitive frailty describes the heterogeneous cognitive conditions characterized by the simultaneous presence of both physical frailty and cognitive impairment [77]. Similar to frailty, delirium cannot be regarded as an isolated mental disorder, and there is some evidence that it affects motor function as well [26]. The impaired mobility occurring during delirium may be the sign of a complex system being close to failure, as has been demonstrated in many acute care units for older hospitalized inpatients [78, 79, 80]. Frailty reflects the life-long accumulation of physical and cognitive deficits, thus defining the greater or lesser extent of an individual's vulnerability [81]. Indeed, the appearance of delirium might be the condition which finally provides clinical evidence of a previously overlooked frailty syndrome [20].
This study contains certain methodological weaknesses, and its findings should be treated carefully. Firstly, in addition to the relatively small sample group, data were collected from the unplanned (urgent) admissions to the ED of a single-site, secondary care hospital serving a population of 350,000 people – results should be generalized with caution. Secondly, despite a standardized protocol and training procedure, more than one rater performed the psychiatric evaluations, possibly introducing interrater variability. Thirdly, patients were only assessed for delirium at admission (immediately upon their arrival) and 4 h later. Any patients developing delirium after this time were not included in the analysis, possibly underestimating the onset of delirium. Finally, using the TFI imposed some methodological limitations on this study, as the most widely used screening tool for assessing frailty is that proposed by Fried et al. [59], which requires the fulfilment of at least 3 of the 5 following criteria: weight loss, exhaustion, weak grip strength, slow walking speed, and low physical activity. However, exhausted and severely ill older adults admitted to an ED are often unable to complete these performance-based tests, and thus many of the present study's participants could not have been assessed using such physical testing. The TFI, however, is a user-friendly questionnaire based on a multidimensional approach to frailty, assessing the physical, psychological, and social aspects of human functioning. A systematic review by Sutton et al.[82] concluded that out of 38 frailty assessment instruments the TFI provided the most extensive examination of frailty's psychometric properties. The authors concluded that the TFI was a robust, reliable, and valid screening instrument for frailty and that it was easy and quick to administer. The psychometric validation of the French version of the TFI is ongoing.
Conclusion
This descriptive correlational study's goals were to identify the participants state of frailty, to transversally and temporally evaluate the signs and symptoms of delirium, and to find associations between those geriatric syndromes among older adults consulting at an ED. This study revealed a weak but statistically significant association between frailty and delirium in an ED setting. In view of Switzerland's and the world's inevitable demographic transition, the role of the interdisciplinary teams staffing ED will become ever more important. Indeed, systematic screening for these two geriatric syndromes among persons at risk would help to optimize coordination between different professional groups and thus to put in place preventive interventions and actions to reduce rates of complications. Our findings are consistent with the growing body of literature that supports the existence of a long-lasting functional relationship between frailty and delirium. Further research is needed to determine whether interventions aimed at delirium and/or frailty could also help to reduce longer-term functional decline.
Statement of Ethics
This study's protocol was approved by the Human Research Ethics Committee of the canton Vaud (CER-VD-2016-01505) and by the particular peripheral hospital's care management team.
Disclosure Statement
The authors have no conflict of interests to declare.
Funding Sources
None.
Author Contributions
H.V. is the guarantor, and all of the authors contributed to drafting of the original research, the development of the selection criteria, data collection, and analysis.
Acknowledgement
The authors acknowledge all the involved older adults, legal representatives, and ED health care providers for their participation in this study.
References
- 1.OFS OFdS . Neuchatel, 2017, 2018. Les scenarios de l'évolution de la population de la Suisse 2015 - 2045. [Google Scholar]
- 2.OECD OfECaD . OECD. Paris: Organization for Economic Co-operation and Development; 2017. OECD Health Data 2017. [Google Scholar]
- 3.Wahlen R, Favre F, Gloor V, Clausen F, Konzelmann I, Fornerod L. Sion, 2015, 2018. La santé de la population valaisanne: 5ème rapport [Internet]. Observatoire Valaisan de la santé; 2015. [Google Scholar]
- 4.Sanchez B, Hirzel A, Bingisser R, Ciurea A, Exadaktylos A, Lehmann B, Matter H, Meier K, Osterwalder J, Sieber R, Yersin B, Camargo C, Hugli O. State of Emergency Medicine in Switzerland: a national profile of emergency departments in 2006. 2013 doi: 10.1186/1865-1380-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuh D, New Dynamics of Ageing (NDA) Preparatory Network A life course approach to healthy aging, frailty, and capability. J Gerontol A Biol Sci Med Sci. 2007 Jul;62((7)):717–21. doi: 10.1093/gerona/62.7.717. [DOI] [PubMed] [Google Scholar]
- 6.Arcand M, Hébert R. Edisem- Maloine; 2008. Précis pratique de gériatrie. [Google Scholar]
- 7.Quinlan N, Marcantonio ER, Inouye SK, Gill TM, Kamholz B, Rudolph JL. Vulnerability: the crossroads of frailty and delirium. J Am Geriatr Soc. 2011 Nov;59(Suppl 2):S262–8. doi: 10.1111/j.1532-5415.2011.03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verloo H, Goulet C, Morin D, von Gunten A. Association between frailty and delirium in older adult patients discharged from hospital. Clin Interv Aging. 2016 Jan;11:55–63. doi: 10.2147/CIA.S100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verloo H, Goulet C, Morin D, von Gunten A. Delirium among Home-Dwelling Elderly after a Recent Hospitalization: An Urgent Need for Effective Nursing Interventions. Dement Geriatr Cogn Disord Extra. 2012 Jan;2((1)):187–9. doi: 10.1159/000338229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. Toward a conceptual definition of frail community dwelling older people. Nurs Outlook. 2010 Mar-Apr;58((2)):76–86. doi: 10.1016/j.outlook.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Gordon AL, Masud T, Gladman JR. Now that we have a definition for physical frailty, what shape should frailty medicine take? Age Ageing. 2014 Jan;43((1)):8–9. doi: 10.1093/ageing/aft161. [DOI] [PubMed] [Google Scholar]
- 12.Kelaiditi E, Cesari M, Canevelli M, van Kan GA, Ousset PJ, Gillette-Guyonnet S, et al. IANA/IAGG Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013 Sep;17((9)):726–34. doi: 10.1007/s12603-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 13.Aubertin-Leheudre M, Woods AJ, Anton S, Cohen R, Pahor M. Frailty Clinical Phenotype: A Physical and Cognitive Point of View. 2014 doi: 10.1159/000382061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima G. Frailty as a Predictor of Future Falls Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc. 2015 Dec;16((12)):1027–33. doi: 10.1016/j.jamda.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Cardon-Verbecq C, Loustau M, Guitard E, Bonduelle M, Delahaye E, Koskas P, et al. Predicting falls with the cognitive timed up-and-go dual task in frail older patients. Ann Phys Rehabil Med. 2016 doi: 10.1016/j.rehab.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Andela RM, Dijkstra A, Slaets JP, Sanderman R. Prevalence of frailty on clinical wards: description and implications. Int J Nurs Pract. 2010 Feb;16((1)):14–9. doi: 10.1111/j.1440-172X.2009.01807.x. [DOI] [PubMed] [Google Scholar]
- 17.Eeles E, Low Choy N. Frailty and Mobility. 2015 doi: 10.1159/000381200. [DOI] [PubMed] [Google Scholar]
- 18.Santangelo A, Testai M, Maugeri D. Delirium is marker of frailty? Study in a population over 90-year old recovered in a sicilian RSA. Eur Psychiatry. 2010;•••:25. [Google Scholar]
- 19.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013 Mar;381((9868)):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellelli G, Moresco R, Panina-Bordignon P, Arosio B, Gelfi C, Morandi A, et al. Is Delirium the Cognitive Harbinger of Frailty in Older Adults? A Review about the Existing Evidence. Front Med (Lausanne) 2017 Nov;4:188. doi: 10.3389/fmed.2017.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puig Campmany M, Ris Romeu J, Blázquez Andión M, Benito Vales S. Development of a comprehensive, multidisciplinary program of care for frailty in an emergency department. Eur Geriatr Med. 2018 doi: 10.1007/s41999-018-0151-2. [DOI] [PubMed] [Google Scholar]
- 22.Bunce D, Batterham PJ, Mackinnon AJ. Long-term Associations Between Physical Frailty and Performance in Specific Cognitive Domains. The Journals of Gerontology: Series B. 2018 doi: 10.1093/geronb/gbx177. gbx177-gbx177. [DOI] [PubMed] [Google Scholar]
- 23.von Gunten A, Baumgartner M, Georgescu D, Hafner M, Hasemann W, Kressig RW, et al. Etat confusionnel aigu de la personne âgée. Swiss Medical Forum. 2018;18:277–284. [Google Scholar]
- 24.Dani M, Owen LH, Jackson TA, Rockwood K, Sampson EL, Davis D. Delirium, Frailty, and Mortality: Interactions in a Prospective Study of Hospitalized Older People. J Gerontol A Biol Sci Med Sci. 2018 Mar;73((3)):415–8. doi: 10.1093/gerona/glx214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eeles EM, White SV, O'Mahony SM, Bayer AJ, Hubbard RE. The impact of frailty and delirium on mortality in older inpatients. Age Ageing. 2012 May;41((3)):412–6. doi: 10.1093/ageing/afs021. [DOI] [PubMed] [Google Scholar]
- 26.Babine RL, Hyrkäs KE, Hallen S, Wierman HR, Bachand DA, Chapman JL, et al. Falls and delirium in an acute care setting: A retrospective chart review before and after an organisation-wide interprofessional education. J Clin Nurs. 2018 Apr;27((7-8)):e1429–41. doi: 10.1111/jocn.14259. [DOI] [PubMed] [Google Scholar]
- 27.Eeles EM, Hubbard RE, White SV, O'Mahony MS, Savva GM, Bayer AJ. Hospital use, institutionalisation and mortality associated with delirium. Age Ageing. 2010 Jul;39((4)):470–5. doi: 10.1093/ageing/afq052. [DOI] [PubMed] [Google Scholar]
- 28.Han JH, Eden S, Shintani A, Morandi A, Schnelle J, Dittus RS, Storrow AB, Ely EW. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2011;18:451–457. doi: 10.1111/j.1553-2712.2011.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han JH, Shintani A, Eden S, Morandi A, Solberg LM, Schnelle J, et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010 Sep;56((3)):244–252.e1. doi: 10.1016/j.annemergmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaMantia MA, Messina FC, Hobgood CD, Miller DK. Screening for delirium in the emergency department: a systematic review. Ann Emerg Med. 2014 May;63((5)):551–560.e2. doi: 10.1016/j.annemergmed.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Eeles EM, White SV, O'Mahony SM, Bayer AJ, Hubbard RE. The impact of frailty and delirium on mortality in older inpatients. Age Ageing. 2012 May;41((3)):412–6. doi: 10.1093/ageing/afs021. [DOI] [PubMed] [Google Scholar]
- 32.Fallon A, Dyer A, Nabeel S, Coughlan T, Collins D, O'Neill D, Kennelly DS. Frailty in older patients attending an emergency department. 2015 [Google Scholar]
- 33.Émond M, Boucher V, Carmichael PH, Voyer P, Pelletier M, Gouin É, et al. Incidence of delirium in the Canadian emergency department and its consequences on hospital length of stay: a prospective observational multicentre cohort study. BMJ Open. 2018 Mar;8((3)):e018190. doi: 10.1136/bmjopen-2017-018190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brousseau AA, Dent E, Hubbard R, Melady D, Émond M, Mercier É, et al. Multinational Emergency Department Study Identification of older adults with frailty in the Emergency Department using a frailty index: results from a multinational study. Age Ageing. 2018 Mar;47((2)):242–8. doi: 10.1093/ageing/afx168. [DOI] [PubMed] [Google Scholar]
- 35.Cirbus J, MacLullich AM, Noel C, Ely EW, Chandrasekhar R, Han JH. Delirium etiology subtypes and their effect on six-month function and cognition in older emergency department patients. Int Psychogeriatr. 2018;•••:1–10. doi: 10.1017/S1041610218000777. [DOI] [PubMed] [Google Scholar]
- 36.Verloo H, Goulet C, Morin D, von Gunten A. Effect Estimation of an Innovative Nursing Intervention to Improve Delirium among Home-Dwelling Older Adults: A Randomized Controlled Pilot Trial. Dement Geriatr Cogn Disord Extra. 2015 Apr;5((1)):176–90. doi: 10.1159/000375444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrohknia N, Castrén M, Ehrenberg A, Lind L, Oredsson S, Jonsson H, et al. Emergency department triage scales and their components: a systematic review of the scientific evidence. Scand J Trauma Resusc Emerg Med. 2011 Jun;19((1)):42. doi: 10.1186/1757-7241-19-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mélot C. To score or not to score during triage in the emergency department? Intensive Care Med. 2015 Jun;41((6)):1135–7. doi: 10.1007/s00134-015-3814-1. [DOI] [PubMed] [Google Scholar]
- 39.Han JH, Vasilevskis EE, Chandrasekhar R, Liu X, Schnelle JF, Dittus RS, Ely EW. Delirium in the Emergency Department and Its Extension into Hospitalization (DELINEATE) Study: Effect on 6-month Function and Cognition. J Am Geriatr Soc. 2017 doi: 10.1111/jgs.14824. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han JH, Wilson A, Ely EW. Delirium in the older emergency department patient: a quiet epidemic. Emerg Med Clin North Am. 2010 Aug;28((3)):611–31. doi: 10.1016/j.emc.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy M, Enander RA, Tadiri SP, Wolfe RE, Shapiro NI, Marcantonio ER. Delirium risk prediction, healthcare use and mortality of elderly adults in the emergency department. J Am Geriatr Soc. 2014 Mar;62((3)):462–9. doi: 10.1111/jgs.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuman B, Fawcett J. ed 5th. New-York: Pearson Education; 2011. The Neuman Systems Model. [Google Scholar]
- 43.Robertsson B. Assessment scales in delirium. Dement Geriatr Cogn Disord. 1999 Sep-Oct;10((5)):368–79. doi: 10.1159/000017173. [DOI] [PubMed] [Google Scholar]
- 44.Cohen J. ed 2nd Edition. Hillsdale, New York: Lawrence ERdbaum Associates; 1988. Statistical power and analysis for the behavioral sciences. [Google Scholar]
- 45.Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc. 2010 Jun;11((5)):344–55. doi: 10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Le May L, Loiselle CG, Gélinas C, Lampron A, Bouchard E, Goulet C. Critères de sélection et d'adaptation d'un questionnaire pour la recherche clinique. Douleur Analg. 2008;21((2)):114–29. [Google Scholar]
- 47.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990 Dec;113((12)):941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 48.Laplante J, Cole M, McCusker J, Singh S, Ouimet MA. [Confusion Assessment Method. Validation of a French-language version] Perspect Infirm. 2005 Sep-Oct;3((1)):12–4. [PubMed] [Google Scholar]
- 49.Sepulveda E, Leonard M, Franco JG, Adamis D, McCarthy G, Dunne C, Trzepacz PT, Gaviria AM, de Pablo J, Vilella E, Meagher DJ. Subsyndromal delirium compared with delirium, dementia, and subjects without delirium or dementia in elderly general hospital admissions and nursing home residents. Alzheimer's & dementia (Amsterdam, Netherlands) 2016;7:1–10. doi: 10.1016/j.dadm.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meagher D, O'Regan N, Ryan D, Connolly W, Boland E, O'Caoimhe R, et al. Frequency of delirium and subsyndromal delirium in an adult acute hospital population. Br J Psychiatry. 2014 Dec;205((6)):478–85. doi: 10.1192/bjp.bp.113.139865. [DOI] [PubMed] [Google Scholar]
- 51.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989 Nov;19((4)):1015–22. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 52.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983 Jun;140((6)):734–9. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 53.SPSS I . Somer (NY): IBM Corporation; 2011. Statistical Package for Social Sciences 22.0 - IBM. [Google Scholar]
- 54.McCusker J, Bellavance F, Cardin S, Trépanier S, Identification of Seniors at Risk (ISAR) Steering Committee Screening for geriatric problems in the emergency department: reliability and validity. Acad Emerg Med. 1998 Sep;5((9)):883–93. doi: 10.1111/j.1553-2712.1998.tb02818.x. [DOI] [PubMed] [Google Scholar]
- 55.McCabe JJ, Kennelly SP. Acute care of older patients in the emergency department: strategies to improve patient outcomes. Open Access Emerg Med. 2015 Sep;7:45–54. doi: 10.2147/OAEM.S69974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Costa AP, Hirdes JP, Heckman GA, Dey AB, Jonsson PV, Lakhan P, et al. Geriatric syndromes predict postdischarge outcomes among older emergency department patients: findings from the interRAI Multinational Emergency Department Study. Acad Emerg Med. 2014 Apr;21((4)):422–33. doi: 10.1111/acem.12353. [DOI] [PubMed] [Google Scholar]
- 57.Salvi F, Morichi V, Grilli A, Lancioni L, Spazzafumo L, Polonara S, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR) J Nutr Health Aging. 2012 Apr;16((4)):313–8. doi: 10.1007/s12603-011-0155-9. [DOI] [PubMed] [Google Scholar]
- 58.Guessous I, Luthi JC, Bowling CB, Theler JM, Paccaud F, Gaspoz JM, et al. Prevalence of frailty indicators and association with socioeconomic status in middle-aged and older adults in a swiss region with universal health insurance coverage: a population-based cross-sectional study. J Aging Res. 2014;2014:198603. doi: 10.1155/2014/198603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56((3)):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 60.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005 Aug;173((5)):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner G, Clegg A, British Geriatrics Society. Age UK. Royal College of General Practioners Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing. 2014 Nov;43((6)):744–7. doi: 10.1093/ageing/afu138. [DOI] [PubMed] [Google Scholar]
- 62.Panza F, Solfrizzi V, Barulli MR, Santamato A, Seripa D, Pilotto A, et al. Cognitive Frailty: A Systematic Review of Epidemiological and Neurobiological Evidence of an Age-Related Clinical Condition. Rejuvenation Res. 2015 Oct;18((5)):389–412. doi: 10.1089/rej.2014.1637. [DOI] [PubMed] [Google Scholar]
- 63.Summerbell J, Wynne H, Hankey CR, Williams FM. The effect of age and frailty upon blood esterase activities and their response to dietary supplementation. Br J Clin Pharmacol. 1993 Nov;36((5)):399–404. doi: 10.1111/j.1365-2125.1993.tb00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Assen MA, Pallast E, Fakiri FE, Gobbens RJ. Measuring frailty in Dutch community-dwelling older people: Reference values of the Tilburg Frailty Indicator (TFI) Arch Gerontol Geriatr. 2016 Nov-Dec;67:120–9. doi: 10.1016/j.archger.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Santos-Eggimann B, Cuénoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009 Jun;64((6)):675–81. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herr M, Robine JM, Pinot J, Arvieu JJ, Ankri J. Polypharmacy and frailty: prevalence, relationship, and impact on mortality in a French sample of 2350 old people. Pharmacoepidemiol Drug Saf. 2015 Jun;24((6)):637–46. doi: 10.1002/pds.3772. [DOI] [PubMed] [Google Scholar]
- 67.Leung JM, Tsai TL, Sands LP. Brief report: preoperative frailty in older surgical patients is associated with early postoperative delirium. Anesth Analg. 2011 May;112((5)):1199–201. doi: 10.1213/ANE.0b013e31820c7c06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;•••:383. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meagher D, Trzepacz PT. Phenomenological distinctions needed in DSM-V: delirium, subsyndromal delirium, and dementias. J Neuropsychiatry Clin Neurosci. 2007;19((4)):468–70. doi: 10.1176/jnp.2007.19.4.468. [DOI] [PubMed] [Google Scholar]
- 70.Bucht G, Gustafson Y, Sandberg O. Epidemiology of delirium. Dement Geriatr Cogn Disord. 1999 Sep-Oct;10((5)):315–8. doi: 10.1159/000017161. [DOI] [PubMed] [Google Scholar]
- 71.Johnson J. Identifying and recognizing delirium. Dement Geriatr Cogn Disord. 1999 Sep-Oct;10((5)):353–8. doi: 10.1159/000017170. [DOI] [PubMed] [Google Scholar]
- 72.Marcantonio ER, Kiely DK, Simon SE, John Orav E, Jones RN, Murphy KM, et al. Outcomes of older people admitted to postacute facilities with delirium. J Am Geriatr Soc. 2005 Jun;53((6)):963–9. doi: 10.1111/j.1532-5415.2005.53305.x. [DOI] [PubMed] [Google Scholar]
- 73.Jung P, Pereira MA, Hiebert B, Song X, Rockwood K, Tangri N, et al. The impact of frailty on postoperative delirium in cardiac surgery patients. J Thorac Cardiovasc Surg. 2015 Mar;149((3)):869–75.e1. doi: 10.1016/j.jtcvs.2014.10.118. [DOI] [PubMed] [Google Scholar]
- 74.Cole M. Subsyndromal delirium in old age: conceptual and methodological issues. International psychogeriatrics / IPA. 2013;25:863–866. doi: 10.1017/S1041610213000434. [DOI] [PubMed] [Google Scholar]
- 75.Velilla NM, Bouzon CA, Contin KC, Beroiz BI, Herrero AC, Renedo JA. Different functional outcomes in patients with delirium and subsyndromal delirium one month after hospital discharge. Dement Geriatr Cogn Disord. 2012;34((5-6)):332–6. doi: 10.1159/000345609. [DOI] [PubMed] [Google Scholar]
- 76.Cole MG, McCusker J, Voyer P, Monette J, Champoux N, Ciampi A, Belzile E, Vu M. Core symptoms not meeting criteria for delirium are associated with cognitive and functional impairment and mood and behavior problems in older long-term care residents. International psychogeriatrics / IPA. 2014;26:1181–1189. doi: 10.1017/S1041610214000313. [DOI] [PubMed] [Google Scholar]
- 77.Ruan Q, Yu Z, Chen M, Bao Z, Li J, He W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res Rev. 2015 Mar;20:1–10. doi: 10.1016/j.arr.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Church S, Robinson TN, Angles EM, Tran ZV, Wallace JI. Postoperative falls in the acute hospital setting: characteristics, risk factors, and outcomes in males. Am J Surg. 2011 Feb;201((2)):197–202. doi: 10.1016/j.amjsurg.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 79.Dean E. Reducing falls among older people in hospital. Nurs Older People. 2012 Jun;24((5)):16–9. doi: 10.7748/nop2012.06.24.5.16.c9114. [DOI] [PubMed] [Google Scholar]
- 80.Ferguson A, Uldall K, Dunn J, Blackmore CC, Williams B. Effectiveness of a Multifaceted Delirium Screening, Prevention, and Treatment Initiative on the Rate of Delirium Falls in the Acute Care Setting. J Nurs Care Qual. 2017 doi: 10.1097/NCQ.0000000000000297. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 81.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011 Feb;27((1)):17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 82.Sutton JL, Gould RL, Daley S, Coulson MC, Ward EV, Butler AM, et al. Psychometric properties of multicomponent tools designed to assess frailty in older adults: A systematic review. BMC Geriatr. 2016 Feb;16((1)):55. doi: 10.1186/s12877-016-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]