Abstract
Objective
The statin family of cholesterol-lowering drugs has been shown to induce tumor-specific apoptosis by inhibiting the rate-limiting enzyme of the mevalonate (MVA) pathway, HMG-CoA reductase (HMGCR). Accumulating evidence suggests that statin use may delay prostate cancer (PCa) progression in a subset of patients; however, the determinants of statin drug sensitivity in PCa remain unclear. Our goal was to identify molecular features of statin-sensitive PCa and opportunities to potentiate statin-induced PCa cell death.
Methods
Deregulation of HMGCR expression in PCa was evaluated by immunohistochemistry. The response of PCa cell lines to fluvastatin-mediated HMGCR inhibition was assessed using cell viability and apoptosis assays. Activation of the sterol-regulated feedback loop of the MVA pathway, which was hypothesized to modulate statin sensitivity in PCa, was also evaluated. Inhibition of this statin-induced feedback loop was performed using RNA interference or small molecule inhibitors. The achievable levels of fluvastatin in mouse prostate tissue were measured using liquid chromatography–mass spectrometry.
Results
High HMGCR expression in PCa was associated with poor prognosis; however, not all PCa cell lines underwent apoptosis in response to treatment with physiologically-achievable concentrations of fluvastatin. Rather, most cell lines initiated a feedback response mediated by sterol regulatory element-binding protein 2 (SREBP2), which led to the further upregulation of HMGCR and other lipid metabolism genes. Overcoming this feedback mechanism by knocking down or inhibiting SREBP2 potentiated fluvastatin-induced PCa cell death. Notably, we demonstrated that this feedback loop is pharmacologically-actionable, as the drug dipyridamole can be used to block fluvastatin-induced SREBP activation and augment apoptosis in statin-insensitive PCa cells.
Conclusion
Our study implicates statin-induced SREBP2 activation as a PCa vulnerability that can be exploited for therapeutic purposes using clinically-approved agents.
Keywords: Statins, Dipyridamole, Prostate cancer, Mevalonate pathway, Tumor metabolism, Drug repurposing
Highlights
-
•
High HMGCR protein expression in prostate cancer is associated with poor prognosis.
-
•
Statin-mediated HMGCR inhibition induces apoptosis in a subset of prostate cancer cells.
-
•
Statin-induced SREBP2 activation modulates statin sensitivity in prostate cancer.
-
•
Inhibiting SREBP2 sensitizes prostate cancer cells to statin-induced apoptosis.
-
•
Combined statin and dipyridamole therapy significantly delays prostate tumor growth.
Abbreviations
- 25-HC
25-hydroxycholesterol
- BCR
biochemical relapse
- FPP
farnesyl pyrophosphate
- GGPP
geranylgeranyl pyrophosphate
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- HMGCR
HMG-CoA reductase
- HMGCS1
HMG-CoA synthase 1
- IC50
half-maximal inhibitory concentration
- INSIG1
insulin-induced gene 1
- MVA
mevalonate
- PCa
prostate cancer
- PDX
patient-derived xenograft
- PSA
prostate-specific antigen
- RP
radical prostatectomy
- SCD
stearoyl-CoA desaturase
- SREBP
sterol regulatory element-binding protein
- TMA
tissue microarray
1. Introduction
Prostate cancer (PCa) is the most commonly diagnosed malignancy in men and the third-leading cause of cancer-related male mortality in developed countries [1]. Given the long natural history of PCa, many patients suffer from disease-related morbidity and compromised quality of life, due in part to side-effects of radical therapies such as androgen deprivation [2]. An estimated 20–50% of PCa patients relapse after frontline treatment and inevitably progress to more advanced, lethal forms of the disease [3]. Hence, there is an unmet need for safe and effective therapies to treat PCa and delay disease progression.
Statins are clinically-approved agents that are commonly prescribed for the management of high cholesterol, but more recently have been shown to possess anti-cancer properties [4], [5]. A number of retrospective studies have reported an association between statin medication use and reduced PCa risk, particularly more advanced and lethal forms of the disease [6], [7], [8], [9]. In addition to chemoprevention, statin use has been associated with improved patient outcome following radical therapy [10], [11], [12]; however, studies are conflicting as to the extent to which statin use at the time of frontline therapy improves patient outcome [13]. This suggests that a subset of PCa patients may benefit from the addition of statins to their treatment regimen, and, in other patients, additional targeted agents may be required to improve patient responses to statin therapy.
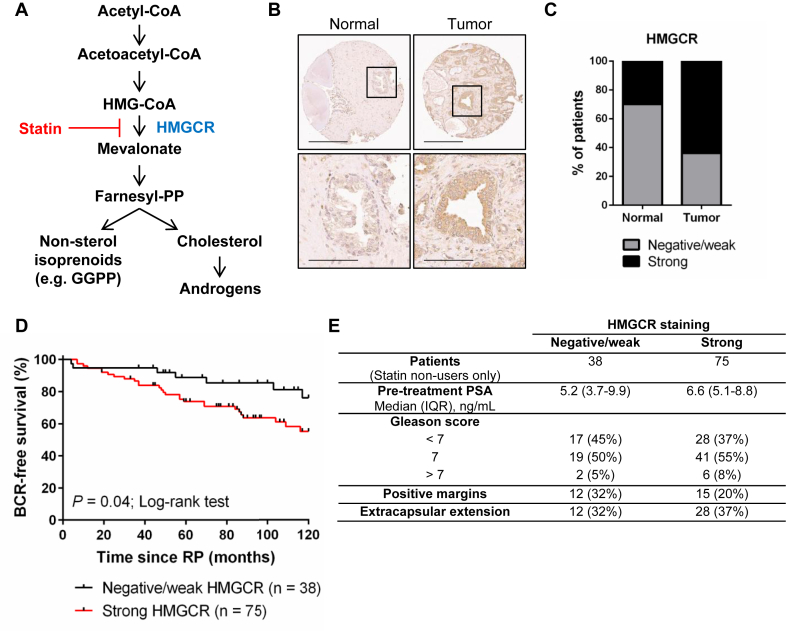
Statins are specific inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (HMGCR), the rate-limiting enzyme of the mevalonate (MVA) pathway. The MVA pathway is an integral metabolic pathway that converts acetyl-CoA to sterols and other isoprenoids that are important for cell growth and survival [14] (Figure 1A). The enzymes of the MVA pathway are transcriptionally regulated by sterol regulatory element-binding protein 2 (SREBP2), which is activated in response to intracellular sterol depletion. Aberrant SREBP2 expression and activity has been associated with PCa progression [15], [16], [17], suggesting that prostate tumors may be particularly dependent on cholesterol and other isoprenoid metabolites, and therefore vulnerable to HMGCR inhibition by statins.
Figure 1.
Expression of the metabolic enzyme HMGCR is elevated in primary PCa tissues and is associated with poor prognosis. (A) Schematic representation of the MVA pathway. Statins inhibit the rate-limiting enzyme of the pathway, HMGCR. GGPP = geranylgeranyl pyrophosphate. (B) Representative images of a patient-matched normal and malignant prostate tissue pair stained for HMGCR expression. Scale bars = 300 μm (top row) and 100 μm (bottom row). (C) Prostate tumor tissues stained more intensely for HMGCR expression compared to adjacent normal prostate tissue controls. N = 149 matched normal and tumor tissues. p < 0.0001 (McNemar's test). (D) HMGCR expression in prostate tumors was associated with early biochemical relapse (BCR) in patients who were statin non-users. Hazard Ratio (95% confidence interval) = 0.43 (0.24–0.97); p = 0.04 (Log-rank test). (E) HMGCR expression among statin non-users by clinical and pathological features. IQR = interquartile range.
Statins have been shown to induce cancer cell-specific apoptosis in a number of different cancer cell types via the direct inhibition of HMGCR [5]; however, heterogeneous responses to statin exposure have been reported [18], [19], [20], [21]. A number of mechanisms have been proposed to explain why some cancer cells are more sensitive to statins than others. For example, in breast cancer, high basal expression of sterol biosynthesis genes has been associated with resistance to atorvastatin [22]. Moreover, we previously demonstrated in multiple myeloma that lovastatin sensitivity was inversely associated with the ability of cells to induce the expression of MVA metabolism genes in response to statin exposure [23]. In PCa, however, the determinants of statin sensitivity remain to be defined.
Before statins can be repurposed for the treatment of PCa, data from prospective clinical trials are necessary. While such data in PCa have been limited thus far [24], statins have been evaluated in clinical trials in a number of other cancer types. In line with the epidemiological data, mixed efficacies have been reported by these studies and the need for patient stratification and/or combination therapies has been proposed [25], [26], [27]. Hence, a better understanding of the mechanisms of MVA pathway deregulation and statin sensitivity in PCa will be crucial for the successful design of future clinical trials. Here, we provide evidence that deregulation of the MVA pathway at the level of HMGCR expression is associated with poor prognosis in PCa patients; however, inhibition of HMGCR activity in vitro was insufficient to induce apoptosis in the majority of PCa cell lines evaluated. Sensitivity to fluvastatin was inversely associated with SREBP2 activation following statin treatment. Importantly, inhibition of SREBP2 with the clinically-approved agent dipyridamole potentiated fluvastatin-induced apoptosis in PCa cells that were relatively insensitive to fluvastatin as a single agent. Taken together, these findings provide strong rationale for the combined inhibition of HMGCR and SREBP2 to induce PCa cell death and warrant the clinical evaluation of fluvastatin and dipyridamole for the treatment of PCa.
2. Materials and methods
2.1. Tissue microarrays and immunohistochemistry
Tissue microarrays (TMAs) comprised of radical prostatectomy (RP) tissue samples from 149 PCa patients treated at the Princess Margaret Cancer Centre between 2003 and 2013 were obtained with Research Ethics Board approval. Each patient was represented by 3 malignant and 2 benign cores. TMAs were probed with a monoclonal antibody against HMGCR (A9, prepared in-house) by the Pathology Research Program (PRP) Laboratory (University Health Network, Toronto, Canada), and staining was scored by practicing PCa pathologists. Clinical and pathological data, including statin use information, were obtained through a comprehensive chart review. As validation, an independent TMA (US Biomax, PR807b) was stained and scored. To evaluate apoptosis in our xenograft experiments, excised tumor tissues were fixed in 10% buffered formalin for at least 24 h, paraffin-embedded, sectioned and stained with an anti-TUNEL antibody (prepared in-house) by the PRP Laboratory. TUNEL positivity was quantified using Aperio ImageScope v11.2.0.780 software.
2.2. Cell culture and compounds
LNCaP (CRL-1740) and PC-3 (CRL-1435) cells were purchased from the American Type Culture Collection (ATCC) and maintained in RPMI 1640 and Ham's F-12K medium, respectively. DU145 cells were a kind gift of Dr. Robert Bristow (University Health Network) and were maintained in alpha-Modified Eagle's Medium (α-MEM). VCaP cells were a kind gift of Dr. Mathieu Lupien (University Health Network) and maintained in Dulbecco's Modified Eagle's Medium (DMEM). All cell lines were supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin and 100 μg/mL streptomycin. Cell lines were routinely confirmed to be mycoplasma-free using the MycoAlert Mycoplasma Detection Kit (Lonza), and their authenticity was verified by short tandem repeat (STR) profiling. Fluvastatin (US Biological) was dissolved in ethanol, dipyridamole (Sigma) was dissolved in DMSO, mevalonate (Sigma) was dissolved in water, 25-hydroxycholesterol (Sigma) was dissolved in ethanol and doxycycline hyclate (Sigma) was dissolved in water.
2.3. Cell viability assays
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were performed as previously described [23]. Briefly, PCa cells were seeded at 2,000–15,000 cells/well in 96-well plates overnight, then treated in triplicate with 0–400 μM fluvastatin for 72 h.
2.4. RNA interference
Two independent short hairpin RNAs (shRNAs) against SREBF2 were designed using The RNAi Consortium (TRC) Genetic Perturbation Platform (portals.broadinstitute.org/gpp/public) and cloned into the doxycycline-inducible pLKO shRNA lentiviral system (shRNA sequences in Supplementary Table 1). HEK-293Tv cells were co-transfected with the shRNA constructs, pMD2.G and psPAX2 via calcium phosphate transfection. Viral supernatants were harvested 48 h post-transfection. LNCaP cells were transduced with the lentiviral supernatants in the presence of 8 μg/mL polybrene, after which they were selected in 1 μg/mL puromycin.
2.5. Cell death assays
Cells were seeded at 0.5–1 × 106 cells/plate and treated the next day as indicated. After 72 h, cells were fixed in 70% ethanol for >24 h, stained with propidium iodide and analyzed by flow cytometry for DNA fragmentation (% pre-G1 population) as a measure of cell death, as previously described [23].
2.6. Xenograft experiments
All animal experiments were carried out in accordance with the regulations of the Canadian Council on Animal Care. 7–9 week-old male NOD/SCID (non-obese diabetic/severe combined immunodeficiency) mice were injected with 5 million LNCaP cells subcutaneously in the flank in a 1:1 mixture with Matrigel (Corning). When tumor volumes reached 200 mm3, mice were randomized and treated with one of the following: 50 mg/kg/day fluvastatin (resuspended in phosphate-buffered saline (PBS), administered orally), 120 mg/kg/day dipyridamole (5 mg/mL dipyridamole in 50 mg/mL polyethylene glycol 600 and 2 mg/mL tartaric acid, administered intraperitoneally), the combination of fluvastatin and dipyridamole or vehicle controls. For the patient-derived xenograft (PDX) experiment, 5 × 5 mm pieces of LTL-484 [28] were surgically implanted subcutaneously in the flank of NOD/SCID mice. When tumor volumes reached 200 mm3, mice were randomized and treated with vehicle controls or fluvastatin and dipyridamole, as described above.
2.7. Fluvastatin quantification
Fluvastatin concentrations were quantified by high-performance liquid chromatography (HPLC)-tandem mass spectrometry (MS/MS) with atorvastatin used as the internal standard. Flash-frozen mouse prostate and liver tissues (up to 250 mg) were homogenized in liquid nitrogen and resuspended in 500 μL of water. Serum and tissue samples were subjected to methyl tert-butyl ether extraction, after which the supernatants were separated, dried at room temperature and reconstituted in 250 μL of methanol:water (1:1). 10 μL of sample was injected into a Shimadzu CBM-20A system coupled with a triple quadrupole mass spectrometer (API 3200, Applied Biosystems/MDS SCIEX). Chromatographic separation was achieved using a Phenomenex HyperClone BDS C18 column (50 × 2.0 mm, 5 μm). The binary mobile phase consisted of 5 mM ammonium acetate in water (mobile phase A) and 5 mM ammonium acetate in acetonitrile (mobile phase B), and was delivered at a flow rate of 0.5 mL/min. The following gradient schedule was used: 10%–100% B (0.0–1.0 min), 100% B (1.0–3.0 min), 100%–10% B (3.0–3.2 min) and 10% B (3.2–6.0 min). Data collection, peak integration and processing were performed using Analyst version 1.4.2 software (Applied Biosystems/MDS SCIEX).
2.8. Immunoblotting
Whole cell lysates were prepared by washing cells twice with cold PBS and lysing cells in RIPA buffer (50 mM Tris–HCl pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, 0.1% SDS, 1 mM EDTA, protease inhibitors) on ice for 30 min. Lysates were cleared by centrifugation and protein concentrations were determined using the Pierce 660 nm Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of protein were diluted in Laemmli sample buffer, boiled for 5 min and resolved by SDS-polyacrylamide gel electrophoresis. The resolved proteins were then transferred onto nitrocellulose membranes. Membranes were blocked for 1 h in 5% milk in PBS/0.1% Tween-20 (PBS-T) at room temperature, and then probed with the following primary antibodies in 5% milk/PBS-T overnight at 4 °C: SREBP2 (1:250; BD Biosciences, 557037), SREBP1 (1:250; Santa Cruz, sc-13551), α-Tubulin (1:3000; Calbiochem, CP06), Actin (1:3000; Sigma, A2066), PARP (1:1000; Cell Signaling Technology, #9542). Primary antibodies were detected using IRDye-conjugated secondary antibodies and the Odyssey Classic Imaging System (LI-COR Biosciences). Densitometric analysis was performed using ImageJ 1.47v software.
2.9. Quantitative RT-PCR
Total RNA was isolated from subconfluent cells using TRIzol Reagent (Invitrogen). cDNA was synthesized from 500 ng RNA using SuperScript III (Invitrogen). Quantitative reverse transcription PCR (qRT-PCR) was performed using the ABI Prism 7900HT sequence detection system and TaqMan probes (Applied Biosystems) for the following genes: HMGCR (Hs00168352), HMGCS1 (Hs00266810), INSIG1 (Hs01650979), SCD (Hs01682761) and RPL13A (Hs01578913).
3. Results
3.1. Expression of the metabolic enzyme HMGCR is elevated in primary PCa tissues and is associated with poor prognosis
Given the promising epidemiological data that support an association between statin use and improved PCa patient outcome, we first evaluated whether expression of the enzymatic target of statins, HMGCR, is deregulated in primary PCa tissues. We performed immunohistochemistry (IHC) for HMGCR using a validated antibody [29] (Supplementary Fig. 1) on tissue microarrays (TMAs) comprised of matched normal and malignant prostate tissues from 149 PCa patients who underwent a radical prostatectomy (RP) (Figure 1, Supplementary Fig. 2). Staining intensity was scored as either “negative/weak” or “strong” by PCa pathologists. A greater proportion of PCa tissues scored as having high HMGCR expression compared to normal prostate tissues, suggesting that HMGCR expression is deregulated in PCa (Figure 1B–C). This observation was validated by staining an independent TMA comprised of 30 benign (normal and hyperplasia) prostate and 45 PCa tissue samples (Supplementary Fig. 3).
We next evaluated whether high HMGCR protein expression was associated with biochemical relapse (BCR)-free survival in this cohort of patients. When considering all 149 patients, no significant association was observed between HMGCR expression and BCR-free survival (Supplementary Fig. 2A). However, 36 patients (24%) were documented statin users. Given that statin-mediated HMGCR inhibition has been reported to activate a feedback response that ultimately results in the upregulation of MVA pathway enzyme expression, including HMGCR [23], [30], there was the potential that statin use was a confounding variable. Interestingly, when considering only statin non-users, a statistically significant association was observed between HMGCR expression and BCR-free survival, where patients with high HMGCR expression relapsed earlier than patients with lower HMGCR expression (Figure 1D). When comparing HMGCR expression to other clinical and pathological features such as pre-treatment prostate-specific antigen (PSA) levels, Gleason score and extracapsular extension, no association was observed, even when accounting for statin use (Figure 1E, Supplementary Fig. 2B).
3.2. Sensitivity to HMGCR inhibition is inversely associated with fluvastatin-induced SREBP2 activation in PCa cell lines
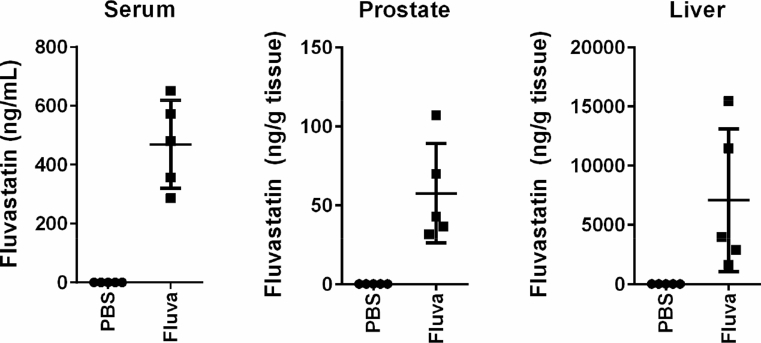
To evaluate the effects of HMGCR inhibition on PCa viability, we treated PCa cell lines with increasing doses of fluvastatin in vitro. We chose to evaluate fluvastatin because we previously demonstrated that fluvastatin does not interact with P-glycoprotein, a major drug efflux pump associated with drug resistance, at clinically-achievable concentrations [31]. Fluvastatin also offers a lower potential for drug–drug interactions compared to many of the other statins, as it is not metabolized by the cytochrome P450 3A4 (CYP3A4) complex, and therefore foods or the many drugs that can modulate CYP3A4 function will not affect fluvastatin activity [32]. Importantly, while hydrophilic statins (e.g. pravastatin, rosuvastatin) exhibit high hepatoselectivity, lipophilic statins have been measured in extrahepatic tissues such as the brain [33]. It is therefore hypothesized that lipophilic statins, such as fluvastatin, can reach tumors in distant organs, including the prostate. Indeed, we were able to measure fluvastatin in the prostate of NOD/SCID mice after oral delivery, albeit at a concentration approximately 10-fold less than what was measured in the serum (Figure 2).
Figure 2.
Fluvastatin can be measured in the mouse prostate. Male NOD/SCID mice were treated with PBS or 50 mg/kg/day fluvastatin by oral gavage for 4 consecutive days. 2 h after the last treatment, serum samples were collected, the mice were euthanized and prostate and liver tissues were harvested. Fluvastatin concentrations were quantified by HPLC-MS/MS. Error bars represent the mean ± SD, n = 5 mice per group.
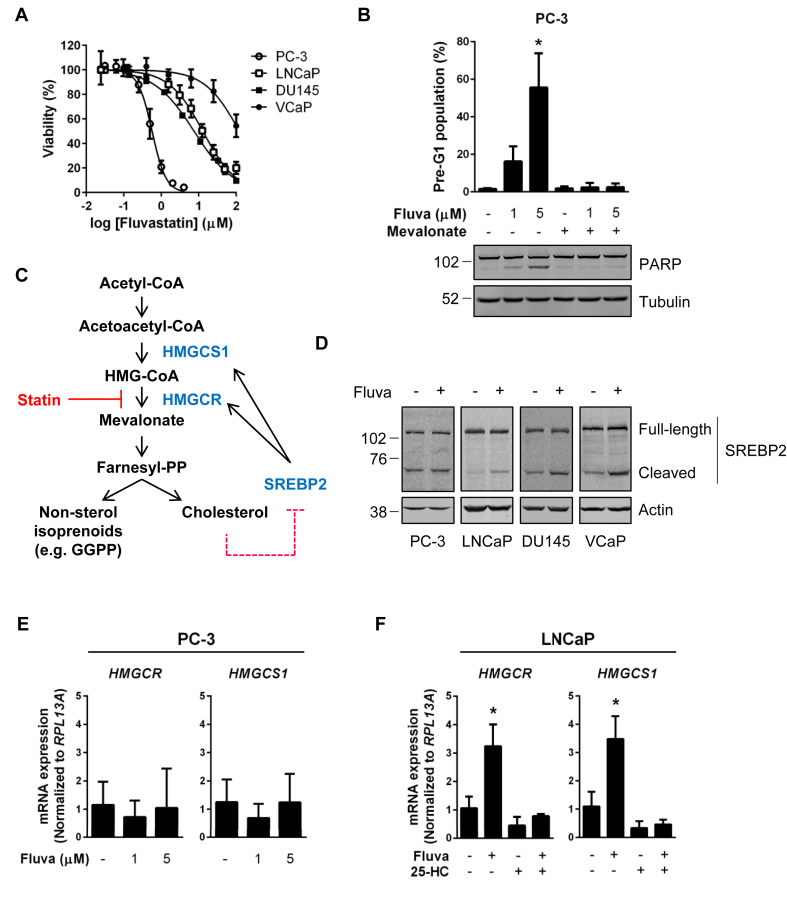
Intriguingly, a range of fluvastatin sensitivities was observed among the four PCa cell lines evaluated (Figure 3A). Fluvastatin exhibited cytotoxic effects in PC-3 cells at low micromolar concentrations similar to those measurable in the mouse prostate, whereas LNCaP, DU145 and VCaP cells were less sensitive to fluvastatin exposure (Figure 3A). Treatment of statin-sensitive PC-3 cells with fluvastatin resulted in cell death, as evidenced by increased DNA fragmentation and PARP cleavage, which was fully rescued by the addition of MVA (Figure 3B). This supports that the apoptotic response in PC-3 cells is due to direct HMGCR inhibition.
Figure 3.
Sensitivity to HMGCR inhibition is inversely associated with fluvastatin-induced SREBP2 activation in PCa cell lines. (A) PCa cell lines were treated with a range of fluvastatin doses for 72 h, and cell viability was determined using an MTT assay. Error bars represent the mean ± SD, n = 3–5. (B) PC-3 cells were treated with fluvastatin ±200 μM MVA for 72 h, fixed in ethanol and assayed for DNA fragmentation (% pre-G1 population) as a marker of cell death by propidium iodide staining. Error bars represent the mean + SD, n = 3, *p < 0.05 (one-way ANOVA with Tukey's multiple comparisons test). Protein was also isolated from PC-3 cells after 72 h of treatment and immunoblotting was performed to assay for PARP cleavage. (C) Schematic representation of the MVA pathway and its sterol-regulated feedback loop. Depletion of cholesterol following statin-mediated inhibition of HMGCR results in the cleavage and activation of SREBP2 and upregulation of the MVA pathway enzymes HMGCR and HMGCS1. (D) PCa cell lines were treated with 10 μM fluvastatin for 8 h. Protein was then isolated and lysates were analyzed for statin-induced SREBP2 activation by immunoblotting. Both the full-length (inactive) and cleaved forms of SREBP2 were detected. (E) PC-3 cells were treated with 1 or 5 μM fluvastatin for 16 h, and RNA was isolated to assay for HMGCR and HMGCS1 expression by qRT-PCR. mRNA expression data are normalized to RPL13A expression. Error bars represent the mean + SD, n = 3. (F) LNCaP cells were treated with 5 μM fluvastatin ±1 μM 25-hydroxycholesterol (25-HC) for 16 h, and RNA was isolated to assay for HMGCR and HMGCS1 expression by qRT-PCR. mRNA expression data are normalized to RPL13A expression. Error bars represent the mean + SD, n = 3, *p < 0.05 (one-way ANOVA with Bonferroni's multiple comparisons test, where each group was compared to the solvent control group).
Inhibition of HMGCR activity results in the depletion of intracellular sterol levels, which in turn results in the activation of SREBP2 [30], [34]. SREBP2 resides in the endoplasmic reticulum (ER) in its precursor, full-length form. In response to sterol depletion, SREBP2 is escorted to the Golgi apparatus, where it is cleaved. Cleavage of SREBP2 liberates the N-terminal transcription factor, which then translocates to the nucleus to activate the transcription of sterol metabolism genes, including those that encode enzymes of the MVA pathway (Figure 3C). Most normal and cancer cells demonstrate robust SREBP2 activation in response to sterol depletion; however, impairment of this sterol-regulated feedback response has been documented in a subset of cancer cells [23], [35], [36]. We next evaluated SREBP2 activation in PCa cell lines in response to fluvastatin treatment. Intriguingly, while increased SREBP2 cleavage was evident after fluvastatin treatment in LNCaP, DU145 and VCaP cells, no fluvastatin-induced SREBP2 cleavage was observed in statin-sensitive PC-3 cells (Figure 3D). In line with this observation, treatment of PC-3 cells with fluvastatin failed to induce the expression of the SREBP2 target genes HMGCR and HMG-CoA synthase 1 (HMGCS1) after 16 h of treatment (Figure 3E). In contrast, treatment of LNCaP cells with fluvastatin resulted in the upregulation of both HMGCR and HMGCS1 mRNA expression (Figure 3F). This response was completely abrogated by the addition of 25-hydroxycholesterol (25-HC), supporting that this restorative feedback mechanism is sterol-regulated in LNCaP cells (Figure 3F).
3.3. Inhibition of the sterol-regulated feedback loop of the MVA pathway potentiates fluvastatin-induced cell death in PCa cell lines
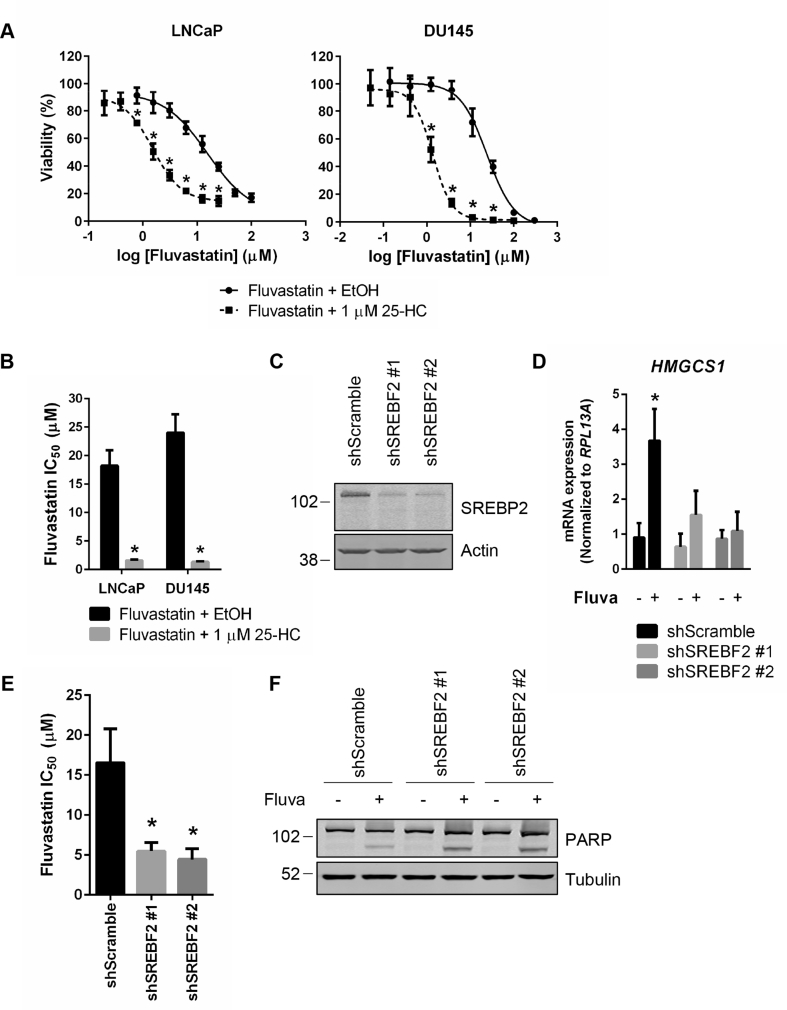
Given that fluvastatin sensitivity seemed to be inversely associated with the ability of PCa cells to activate SREBP2 and upregulate the expression of sterol metabolism genes in response to fluvastatin treatment, we next evaluated whether inhibition of the sterol-regulated feedback loop of the MVA pathway potentiated the cytotoxic effects of fluvastatin. Given that the addition of 25-HC prevented the upregulation of HMGCR and HMGCS1 mRNA expression in response to fluvastatin treatment (Figure 3F), we tested whether 25-HC could sensitize PCa cells to fluvastatin. Treatment of LNCaP and DU145 cells with a sub-toxic concentration of 25-HC significantly decreased the IC50 value of fluvastatin, suggesting that inhibition of SREBP2 activation can potentiate the cytotoxic effects of fluvastatin (Figure 4A–B). As a complementary approach, we knocked down SREBP2 in LNCaP cells using two independent doxycycline-inducible shRNAs (Figure 4C). SREBP2 knockdown abrogated fluvastatin-induced HMGCS1 expression and significantly decreased the IC50 value of fluvastatin (Figure 4D–E). Moreover, treatment of LNCaP cells with fluvastatin in the presence of SREBP2 knockdown resulted in increased apoptosis, as evidenced by increased PARP cleavage (Figure 4F). Collectively, these data suggest that inhibiting the sterol-regulated feedback loop of the MVA pathway is a viable approach to potentiate statin-induced PCa cell death.
Figure 4.
Inhibition of the sterol-regulated feedback loop of the MVA pathway potentiates fluvastatin-induced cell death in PCa cell lines. (A) LNCaP and DU145 cells were treated with a range of fluvastatin doses ± a sub-toxic dose (1 μM) of 25-hydroxycholesterol (25-HC) for 72 h, and cell viability was determined using an MTT assay. Error bars represent the mean ± SD, n = 3, *p < 0.05 (Student t test, unpaired, two-tailed). (B) Fluvastatin IC50 values for LNCaP and DU145 cells treated with fluvastatin alone or in combination with 1 μM 25-HC. Error bars represent the mean + SD, n = 3, *p < 0.05 (Student t test, unpaired, two-tailed). (C) LNCaP cells expressing inducible shRNAs against SREBF2 were induced for 72 h with 1 μg/mL doxycycline and protein was isolated to assay for SREBP2 expression by immunoblotting. (D) LNCaP shScramble and shSREBF2 cells were treated with 1 μg/mL doxycycline for 56 h and then EtOH or 10 μM fluvastatin for an additional 16 h in the presence of 1 μg/mL doxycycline. RNA was isolated to assay for HMGCS1 expression by qRT-PCR. mRNA expression data are normalized to RPL13A expression. Error bars represent the mean + SD, n = 3, *p < 0.05 (Student t test, unpaired, two-tailed). (E) LNCaP shScramble and shSREBF2 cells were treated with a range of fluvastatin doses in the presence of 1 μg/mL doxycycline for 72 h, and cell viability was determined using an MTT assay. The IC50 values are plotted. Error bars represent the mean + SD, n = 3, *p < 0.05 (one-way ANOVA with Bonferroni's multiple comparisons test, where each group was compared to the shScramble control). (F) LNCaP shScramble and shSREBF2 cells were treated with EtOH or 10 μM fluvastatin for 72 h in the presence of 1 μg/mL doxycycline. Protein was then isolated to assay for PARP cleavage by immunoblotting.
3.4. Dipyridamole inhibits fluvastatin-induced SREBP activation and potentiates fluvastatin-induced apoptosis in PCa cell lines
There is significant interest in targeting the SREBP family of transcription factors in PCa, as reactivation of lipogenesis has been shown to promote disease progression [17]. In addition to SREBP2, the master transcriptional regulator of fatty acid metabolism (SREBP1) has also been implicated as a viable therapeutic target in PCa [37]. Small molecule inhibitors, such as fatostatin, have been identified to inhibit both SREBP1 and SREBP2 and exhibit anti-cancer activity in vivo [37]; however, fatostatin has yet to be evaluated in clinical trials. More recently, our lab identified that the drug dipyridamole, which is currently approved as an anti-platelet agent, can also inhibit statin-induced SREBP2 activation [38]. Given that inhibiting SREBP2 potentiated statin-induced cell death in PCa cells (Figure 4), dipyridamole could potentially offer an immediately-available option to increase the therapeutic window of statins as anti-PCa agents.
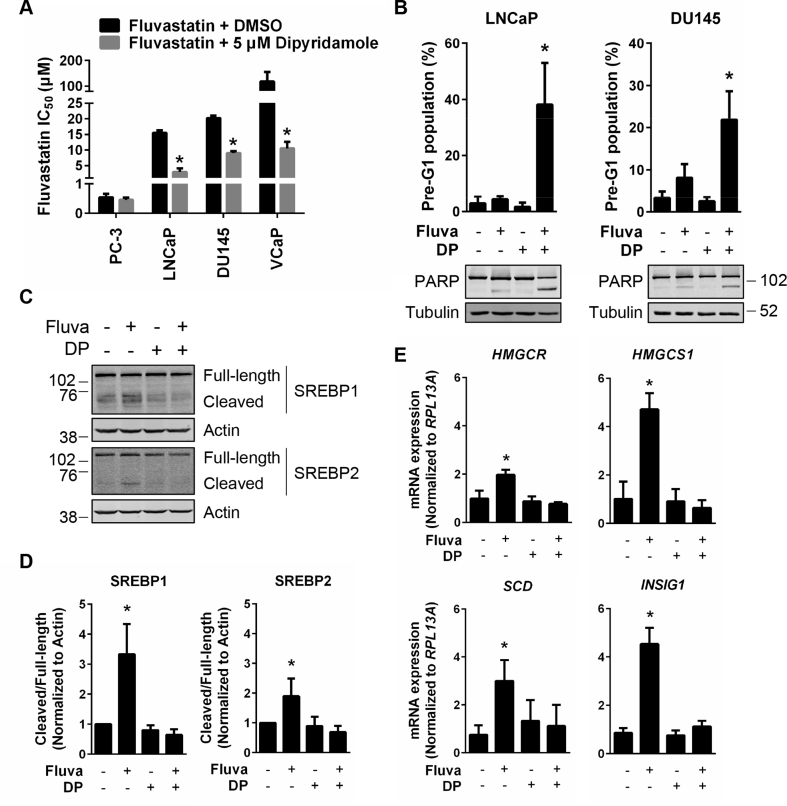
To evaluate whether dipyridamole treatment could sensitize PCa cells to fluvastatin, we treated PC-3, LNCaP, DU145 and VCaP cells with fluvastatin alone or in combination with a sub-lethal, physiologically-achievable dose of dipyridamole [38]. Treatment of LNCaP, DU145 and VCaP cells with dipyridamole significantly lowered the IC50 value of fluvastatin in these cell lines (Figure 5A). Furthermore, combining fluvastatin and dipyridamole, at doses that had a minimal effect when used as single agents, resulted in significantly increased apoptosis in both LNCaP and DU145 cells (Figure 5B). Cell death in response to the fluvastatin and dipyridamole combination was fully rescued by the addition of MVA (Supplementary Fig. 4).
Figure 5.
Dipyridamole inhibits fluvastatin-induced SREBP activation and potentiates fluvastatin-induced apoptosis in PCa cell lines. (A) PCa cell lines were treated with a range of fluvastatin doses ± a sub-lethal dose (5 μM) of dipyridamole for 72 h, and cell viability was determined using an MTT assay. The IC50 values are plotted. Error bars represent the mean + SD, n = 3–5, *p < 0.05 (Student t test, unpaired, two-tailed). (B) LNCaP and DU145 cells were treated with solvent controls, 10 μM fluvastatin, 5 μM dipyridamole (DP) or the combination for 72 h, fixed in ethanol and assayed for DNA fragmentation (% pre-G1 population) as a marker of cell death by propidium iodide staining. Error bars represent the mean + SD, n = 3, *p < 0.05 (one-way ANOVA with Tukey's multiple comparisons test). Protein was also isolated from cells after 72 h of treatment and immunoblotting was performed to assay for PARP cleavage. (C) LNCaP cells were treated with 10 μM fluvastatin ±5 μM DP for 8 h, and protein was isolated to assay for SREBP1 and SREBP2 expression and cleavage (activation) by immunoblotting. (D) SREBP1 and SREBP2 cleavage (cleaved/full-length) was quantified by densitometry and normalized to Actin expression. Error bars represent the mean + SD, n = 3, *p < 0.05 (one-way ANOVA with Bonferroni's multiple comparisons test, where each group was compared to the solvent controls group). (E) LNCaP cells were treated with 10 μM fluvastatin ±5 μM DP for 16 h, and RNA was isolated to assay for HMGCR, HMGCS1, INSIG1 and SCD expression by qRT-PCR. mRNA expression data are normalized to RPL13A expression. Error bars represent the mean + SD, n = 3, *p < 0.05 (one-way ANOVA with Bonferroni's multiple comparisons test, where each group was compared to the solvent controls group).
Interestingly, the fluvastatin IC50 value of PC-3 cells remained unaffected by dipyridamole co-treatment, which is consistent with the observation that PC-3 cells failed to upregulate sterol metabolism gene expression in response to fluvastatin (Figure 3, Figure 5, Supplementary Fig. 5).
Treatment of less statin-sensitive PCa cell lines with dipyridamole abrogated fluvastatin-induced cleavage and activation of SREBP2 and upregulation of lipid metabolism gene expression (Figure 5C–E, Supplementary Fig. 6). Given that activation of SREBP1 is post-translationally regulated by the same mechanism as SREBP2, we evaluated whether dipyridamole could also inhibit SREBP1. Indeed, fluvastatin-induced cleavage of SREBP1 was also inhibited by dipyridamole, an effect that was previously undocumented for this clinically-approved agent (Figure 5C–D).
3.5. The combination of fluvastatin and dipyridamole delays prostate tumor growth
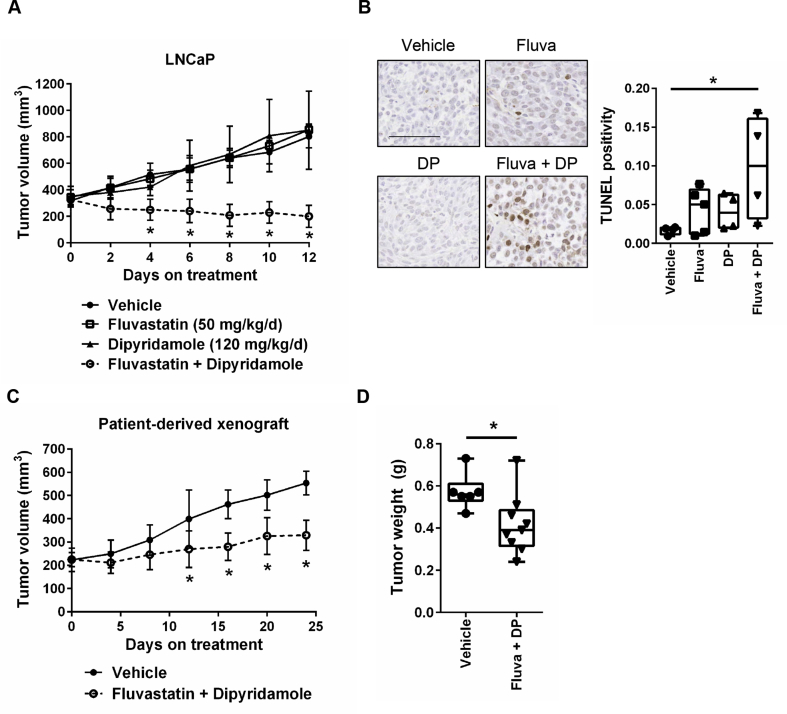
Given that both fluvastatin and dipyridamole are clinically-approved and poised for repurposing, we evaluated whether the fluvastatin-dipyridamole combination was effective at delaying tumor growth in vivo. We treated NOD/SCID mice harboring established LNCaP xenografts orally with fluvastatin and/or intraperitoneally (i.p.) with dipyridamole. The combination of fluvastatin and dipyridamole significantly decreased tumor volumes, while each agent alone had no effect compared to vehicle-treated mice (Figure 6A). After 12 days of treatment, LNCaP tumors from mice treated with both fluvastatin and dipyridamole had increased TUNEL staining compared to vehicle-treated mice, suggesting that the drug combination induced apoptosis in vivo (Figure 6B). We further evaluated the drug combination in a clinically-relevant patient-derived xenograft (PDX) model of androgen-sensitive PCa. Consistent with the LNCaP xenograft results, the fluvastatin-dipyridamole drug combination significantly decreased tumor volumes and final tumor weights in the PDX model (Figure 6C–D).
Figure 6.
The combination of fluvastatin and dipyridamole delays prostate tumor growth. (A) Male NOD/SCID mice were injected subcutaneously with 5 million LNCaP cells. Once tumors reached a volume of 200 mm3, the mice were randomized to receive 50 mg/kg/day fluvastatin (oral), 120 mg/kg/day dipyridamole (i.p. injection), the combination or vehicle controls. The drug combination resulted in significantly reduced tumor volumes. Error bars represent the mean ± SD, n = 4–5 mice per treatment group, *p < 0.05 (one-way ANOVA with Tukey's multiple comparisons test). (B) After 12 days of treatment, tumors were excised, fixed and assayed for TUNEL staining by IHC. TUNEL-positive cells were quantified and representative images are shown (scale bar = 100 μm). Box plot with whiskers representing minimum and maximum values, n = 4–5 mice per treatment group, *p < 0.05 (one-way ANOVA with Tukey's multiple comparisons test). (C) Male NOD-SCID mice were engrafted subcutaneously with LTL-484 patient-derived xenograft tissue. Once tumors reached a volume of 200 mm3, the mice were randomized to receive fluvastatin and dipyridamole (as above) or vehicle controls. The drug combination resulted in significantly reduced tumor volumes. Error bars represent the mean ± SD, n = 6–9 mice per treatment group, *p < 0.05 (Student t test, unpaired, two-tailed). (D) After 24 days of treatment, the mice were euthanized, and the tumors were excised and weighed. Tumors from the mice treated with the drug combination weighed significantly less than those from the mice treated with the vehicle controls. Box plot with whiskers representing minimum and maximum values, n = 6–9 mice per treatment group, *p < 0.05 (Student t test, unpaired, two-tailed).
4. Discussion
Increasing evidence supports that statins possess anti-cancer properties; however, in PCa, a broad range of sensitivity to fluvastatin was observed (Figure 3A). In order to advance statins as anti-cancer agents for the treatment of PCa, it is crucial to understand what features distinguish the subset of prostate tumors that are responsive to statins and/or identify effective statin-drug combinations to increase their therapeutic potential.
Sensitivity to fluvastatin was inversely associated with the ability to induce the expression of SREBP2 target genes following fluvastatin exposure. When HMGCR activity is inhibited by statins, intracellular pools of sterols and other non-sterol isoprenoids are depleted [5]. The depletion of sterols typically leads to the cleavage and activation of the SREBP transcription factors, which subsequently activate the transcription of MVA pathway genes, thus restoring MVA pathway activity [30], [34]. In this study, we demonstrated that relatively statin-insensitive PCa cell lines cleave SREBP2 and activate this restorative feedback loop in response to fluvastatin treatment. Inhibiting this sterol-regulated feedback loop with dipyridamole potentiated fluvastatin-induced apoptosis in these cell lines (Figure 5). This was also achieved by knockdown of SREBP2 (Figure 4C–F), which is consistent with the results of our recent genome-wide shRNA dropout screen that identified SREBP2 knockdown as a potentiator of statin-induced tumor cell death [39]. This suggests that statin-induced SREBP2 activation is a tumor vulnerability across multiple cancer types.
Intriguingly, while we demonstrated that dipyridamole can inhibit both SREBP1 and SREBP2 activation in response to statin treatment (Figure 5C–D), knockdown of SREBP2 alone was sufficient to phenocopy the effects of dipyridamole and potentiate statin-induced apoptosis in LNCaP cells (Figure 4C–F). While SREBP1 primarily regulates the expression of genes involved in fatty acid metabolism, it shares a subset of target genes with SREBP2 [40], suggesting some functional redundancies between these transcription factors. Indeed, it has previously been reported that SREBP1 can compensate for reduced SREBP2 expression in a tissue-specific manner [41]. Hence, it is possible that inhibition of SREBP2 alone in some tumors may be insufficient to potentiate statin-induced apoptosis due to compensation by SREBP1, and therefore an inhibitor against both SREBP1 and SREBP2, such as dipyridamole, would have broader utility and greater anti-cancer efficacy in combination with a statin.
In contrast to the other PCa cell lines that were evaluated, the statin-sensitive PC-3 cell line failed to induce SREBP2 target gene expression in response to fluvastatin treatment (Figure 3D–E). In line with this observation, co-treatment with dipyridamole did not potentiate cell death in this cell line (Figure 5A, Supplementary Fig. 5). Impaired feedback regulation of sterol metabolism has been documented in a number of different cancer types [23], [35], [36]; however, the mechanisms of deregulation in these cancer cells remain to be elucidated. In the context of PC-3 cells, the failure to upregulate HMGCR and HMGCS1 expression in response to fluvastatin was not due to a lack of SREBP2 expression (Figure 3D). Rather, these cells expressed high levels of both full-length and cleaved SREBP2, suggesting that additional post-translational or epigenetic mechanisms may be contributing to their inability to mount this feedback response. Further investigation into the mechanisms of impaired feedback regulation of the MVA pathway in cancer is warranted, as this could potentially reveal predictive biomarkers of statin sensitivity.
We also demonstrated that, in addition to impaired feedback regulation, the MVA pathway was deregulated at the level of HMGCR protein expression in primary PCa tissues. Analysis of matched normal and malignant prostate tissues from PCa patients revealed that a greater proportion of prostate tumors express high levels of HMGCR compared to normal prostate controls (Figure 1B, C, Supplementary Fig. 3). Moreover, high HMGCR expression in prostate tumors was significantly associated with earlier BCR (Figure 1D), which is consistent with evidence that HMGCR overexpression and MVA pathway activation contribute to tumorigenesis [29]. Deregulation of the MVA pathway at the level of HMGCR expression in PCa suggests that prostate tumors may be particularly sensitive to statin-induced apoptosis; however, data in the literature are conflicting as to whether or not HMGCR expression alone can accurately predict statin sensitivity [20], [22], [23], [42]. One possible explanation for these conflicting data is the poor specificity of many commercially-available HMGCR antibodies [22], [29]. In this study, we used a monoclonal antibody that we previously showed can detect human HMGCR at the predicted 97 kDa molecular weight by immunoblotting, and which reliably detects the upregulation of HMGCR expression following statin treatment in cell lines [29] and in the mouse liver (Supplementary Fig. 1). Further comprehensive studies using validated HMGCR reagents are needed to accurately evaluate the utility of HMGCR expression as a predictive biomarker of statin sensitivity.
Interestingly, the association between HMGCR expression and BCR was only observed among statin non-users. While statins decrease serum cholesterol by primarily inhibiting cholesterol biosynthesis in the liver, it has been suggested that statin use may result in the compensatory activation of the MVA pathway in extrahepatic tissues [43], [44]. In line with this hypothesis, data from a window-of-opportunity clinical trial in breast cancer revealed that women treated with high cholesterol-lowering doses of atorvastatin had higher HMGCR expression in their tumor tissue after two weeks of treatment [42]. In our cohort of PCa patients, no significant difference in HMGCR expression was observed between statin users and non-users; however, the dose, type and duration of statin use at the time of RP varied among patients. As a result, HMGCR expression at the time of RP is a poor marker of statin-regulated MVA pathway activity in this cohort of patients. Analysis of tissue samples pre- and post-statin treatment from recently completed window-of-opportunity clinical trials in PCa (e.g. Murtola et al. [24] or NCT01992042) will be useful in determining how statin use affects intratumoral expression of MVA pathway genes and in identifying potential biomarkers of statin sensitivity.
A number of mechanisms have been proposed to explain why PCa cells are dependent on MVA metabolism [44], [45]. Cholesterol serves as the precursor for steroid hormones, including androgens, which are important for PCa cell survival and disease progression [46]. Cholesterol is also an important component of lipid rafts, which facilitate cell signaling events initiated at the cell membrane that are important for cell survival [47]. However, with the exception of a few studies [47], attempts to rescue the anti-cancer effects of statins with sterols have failed in a variety of different cancer cell types, including PCa [48], [49], [50]. Rather, in these studies, addition of the non-sterol isoprenoid geranylgeranyl pyrophosphate (GGPP), and sometimes farnesyl pyrophosphate (FPP), more consistently rescued statin-induced cell death. Our observations, as well as those of others [51], that inhibition of the sterol-regulated feedback loop of the MVA pathway potentiates statin-induced apoptosis offers a possible explanation for these results. Rather than compensate for the inhibition of HMGCR, increasing sterol levels retains SREBP2 in its inactive form at the ER, thus abrogating the ability of cells to compensate for MVA pathway inhibition, which in turn potentiates cell death. This indicates that both the sterol and non-sterol isoprenoid branches of the MVA pathway are important for PCa cell survival. While depletion of non-sterol isoprenoid pools results in cancer cell death, the depletion of sterols triggers a homeostatic feedback response through which the cell attempts to compensate for the depletion of these crucial MVA-derived metabolites.
The result that statin-induced cell death can be rescued by exogenous mevalonate suggests that, in part, statins act by directly inhibiting HMGCR in tumor cells. Achieving the same effect clinically would require sufficient statin drug accumulation in the peripheral circulation and prostate tissue. For our studies, we evaluated fluvastatin, a lipophilic statin that can achieve high maximum serum concentrations [52], which we hypothesized could reach the prostate. To the best of our knowledge, we report here for the first time that fluvastatin can be measured in the mouse prostate (Figure 2); however, assuming that these concentrations are similar to those achievable in the human prostate, fluvastatin monotherapy is unlikely to be effective at killing PCa cells in every patient. Two potential strategies to increase the anti-cancer effects of statins in the prostate are: i) dose-escalation and ii) statin-drug combination therapy. In our xenograft studies, fluvastatin was administered at 2–3× the typical cholesterol-lowering dose. Evidence from a number of phase I clinical studies has demonstrated that statins are well-tolerated at these higher doses [53], [54]. We, and others, have also demonstrated that the cytotoxic effects of statins are both dose- and time-dependent [19], [20], [49], and therefore longer treatment durations at lower doses may be equally as effective, especially for a disease with a long natural history such as PCa. Prospective dose-finding studies are necessary to evaluate how best to prescribe statins in the context of PCa. In addition to dose-escalation, statins can be combined with other agents that potentiate their anti-cancer effects. Here, we provided evidence to support that inhibiting the sterol-regulated feedback loop of the MVA pathway with dipyridamole can potentiate fluvastatin-induced apoptosis, both in vitro and in vivo. Furthermore, co-treatment with dipyridamole significantly reduces the dose of fluvastatin required to induce PCa cell death.
5. Conclusion
Inhibiting the MVA pathway at the level of HMGCR with physiologically-achievable concentrations of fluvastatin is only effective at killing a minority of PCa cells. In other PCa cells, where SREBP2 is activated following fluvastatin treatment, fluvastatin-induced cell death is dampened. In these cells, inhibiting both the MVA pathway and SREBP2 is necessary to induce apoptosis, which can be achieved by combining fluvastatin with a second clinically-approved agent, dipyridamole. Our study provides strong pre-clinical rationale to warrant further clinical evaluation of these immediately-available and well-tolerated drugs for the treatment of PCa.
Author contributions
JL, PJM and LZP conceived and designed the study. JL, PJM, RY and JEV performed experiments. JL analyzed and interpreted the experimental data. MM, JMS and THV scored the TMAs. RY and EXC performed the HPLC-MS/MS analysis. YW provided the PDX model. DTSW, RJH and NEF provided clinical data and expertise. JL and LZP wrote the manuscript. All authors read and approved the manuscript. LZP supervised the study.
Acknowledgments
We thank all members of the Penn lab for helpful discussions. We especially thank Dr. Carolyn Goard and Melanie Peralta for their assistance with purifying and validating the HMGCR antibody for IHC. We also thank Drs. Robert Bristow and Mathieu Lupien for reagents, and Dr. Miran Kenk, Wenjiang Zhang and Maria Monroy for their technical support. This work was supported by funding from the Canada Research Chairs program (L.Z. Penn), Canadian Institutes of Health Research (FRN: 142263; L.Z. Penn), CIHR Doctoral Research Awards (J. Longo and R. Yu) and Ontario Student Opportunity Trust Fund (J.E. van Leeuwen). This work was also supported by the Princess Margaret Cancer Foundation Hold'em for Life Prostate Cancer Research Fund.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.04.003.
Conflicts of interest
None declared.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA. A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Sanda M.G., Dunn R.L., Michalski J., Sandler H.M., Northouse L., Hembroff L. Quality of life and satisfaction with outcome among prostate-cancer survivors. The New England Journal of Medicine. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 3.Paller C.J., Antonarakis E.S. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clinical Advances in Hematology and Oncology. 2013;11(1):14–23. [PMC free article] [PubMed] [Google Scholar]
- 4.Wong W.W.L., Dimitroulakos J., Minden M.D., Penn L.Z. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16(4):508–519. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 5.Clendening J.W., Penn L.Z. Targeting tumor cell metabolism with statins. Oncogene. 2012;31(48):4967–4978. doi: 10.1038/onc.2012.6. [DOI] [PubMed] [Google Scholar]
- 6.Platz E.A., Leitzmann M.F., Visvanathan K., Rimm E.B., Stampfer M.J., Willett W.C. Statin drugs and risk of advanced prostate cancer. Journal of the National Cancer Institute. 2006;98(24):1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs E.J., Rodriguez C., Bain E.B., Wang Y., Thun M.J., Calle E.E. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiology, Biomarkers & Prevention. 2007;16(11):2213–2217. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 8.Flick E.D., Habel L.A., Chan K.A., Van Den Eeden S.K., Quinn V.P., Haque R. Statin use and risk of prostate cancer in the California men's health study cohort. Cancer Epidemiology, Biomarkers & Prevention. 2007;16(11):2218–2225. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- 9.Murtola T.J., Tammela T.L.J., Lahtela J., Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiology, Biomarkers & Prevention. 2007;16(11):2226–2232. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 10.Gutt R., Tonlaar N., Kunnavakkam R., Karrison T., Weichselbaum R.R., Liauw S.L. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. Journal of Clinical Oncology. 2010;28(16):2653–2659. doi: 10.1200/JCO.2009.27.3003. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton R.J., Banez L.L., Aronson W.J., Terris M.K., Platz E.A., Kane C.J. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Cancer. 2010;116(14):3389–3398. doi: 10.1002/cncr.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harshman L.C., Wang X., Nakabayashi M., Xie W., Valenca L., Werner L. Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncology. 2015;1(4):495–504. doi: 10.1001/jamaoncol.2015.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park H.S., Schoenfeld J.D., Mailhot R.B., Shive M., Hartman R.I., Ogembo R. Statins and prostate cancer recurrence following radical prostatectomy or radiotherapy: a systematic review and meta-analysis. Annals of Oncology. 2013;24(6):1427–1434. doi: 10.1093/annonc/mdt077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullen P.J., Yu R., Longo J., Archer M.C., Penn L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nature Reviews Cancer. 2016;16(11):718–731. doi: 10.1038/nrc.2016.76. [DOI] [PubMed] [Google Scholar]
- 15.Ettinger S.L., Sobel R., Whitmore T.G., Akbari M., Bradley D.R., Gleave M.E. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Research. 2004;64(6):2212–2221. doi: 10.1158/0008-5472.can-2148-2. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Wu J.B., Li Q., Shigemura K., Chung L.W.K., Huang W.-C. SREBP-2 promotes stem cell-like properties and metastasis by transcriptional activation of c-Myc in prostate cancer. Oncotarget. 2016;7(11):12869–12884. doi: 10.18632/oncotarget.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M., Zhang J., Sampieri K., Clohessy J.G., Mendez L., Gonzalez-Billalabeitia E. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nature Genetics. 2018;50(2):206–218. doi: 10.1038/s41588-017-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong W.W.-L., Clendening J.W., Martirosyan A., Boutros P.C., Bros C., Khosravi F. Determinants of sensitivity to lovastatin-induced apoptosis in multiple myeloma. Molecular Cancer Therapeutics. 2007;6(6):1886–1897. doi: 10.1158/1535-7163.MCT-06-0745. [DOI] [PubMed] [Google Scholar]
- 19.Martirosyan A., Clendening J.W., Goard C.A., Penn L.Z. Lovastatin induces apoptosis of ovarian cancer cells and synergizes with doxorubicin: potential therapeutic relevance. BMC Cancer. 2010;10:103. doi: 10.1186/1471-2407-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goard C.A., Chan-Seng-Yue M., Mullen P.J., Quiroga A.D., Wasylishen A.R., Clendening J.W. Identifying molecular features that distinguish fluvastatin-sensitive breast tumor cells. Breast Cancer Research and Treatment. 2014;143(2):301–312. doi: 10.1007/s10549-013-2800-y. [DOI] [PubMed] [Google Scholar]
- 21.Yu R., Longo J., Van Leeuwen J.E., Mullen P.J., Ba-Alawi W., Haibe-Kains B. Statin-induced cancer cell death can be mechanistically uncoupled from prenylation of RAS family proteins. Cancer Research. 2018;78(5):1347–1357. doi: 10.1158/0008-5472.CAN-17-1231. [DOI] [PubMed] [Google Scholar]
- 22.Kimbung S., Lettiero B., Feldt M., Bosch A., Borgquist S. High expression of cholesterol biosynthesis genes is associated with resistance to statin treatment and inferior survival in breast cancer. Oncotarget. 2016;7(37):59640–59651. doi: 10.18632/oncotarget.10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clendening J.W., Pandyra A., Li Z., Boutros P.C., Martirosyan A., Lehner R. Exploiting the mevalonate pathway to distinguish statin-sensitive multiple myeloma. Blood. 2010;115(23):4787–4797. doi: 10.1182/blood-2009-07-230508. [DOI] [PubMed] [Google Scholar]
- 24.Murtola T.J., Syvälä H., Tolonen T., Helminen M., Riikonen J., Koskimäki J. Atorvastatin versus placebo for prostate cancer before radical prostatectomy – a randomized, double-blind, placebo-controlled clinical trial. European Urology. 2018;74(6):697–701. doi: 10.1016/j.eururo.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 25.Knox J.J., Siu L.L., Chen E., Dimitroulakos J., Kamel-Reid S., Moore M.J. A Phase I trial of prolonged administration of lovastatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or of the cervix. European Journal of Cancer. 2005;41(4):523–530. doi: 10.1016/j.ejca.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Garwood E.R., Kumar A.S., Baehner F.L., Moore D.H., Au A., Hylton N. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Research and Treatment. 2010;119(1):137–144. doi: 10.1007/s10549-009-0507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seckl M.J., Ottensmeier C.H., Cullen M., Schmid P., Ngai Y., Muthukumar D. Multicenter, phase III, randomized, double-blind, placebo-controlled trial of pravastatin added to first-line standard chemotherapy in small-cell lung cancer (LUNGSTAR) Journal of Clinical Oncology. 2017;35(14):1506–1514. doi: 10.1200/JCO.2016.69.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hao J., Ci X., Xue H., Wu R., Dong X., Choi S.Y.C. Patient-derived hormone-naive prostate cancer xenograft models reveal growth factor receptor bound protein 10 as an androgen receptor-repressed gene driving the development of castration-resistant prostate cancer. European Urology. 2018;73(6):949–960. doi: 10.1016/j.eururo.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Clendening J.W., Pandyra A., Boutros P.C., El Ghamrasni S., Khosravi F., Trentin G.A. Dysregulation of the mevalonate pathway promotes transformation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(34):15051–15056. doi: 10.1073/pnas.0910258107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein J.L., Brown M.S. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 31.Goard C.A., Mather R.G., Vinepal B., Clendening J.W., Martirosyan A., Boutros P.C. Differential interactions between statins and P-glycoprotein: implications for exploiting statins as anticancer agents. International Journal of Cancer. 2010;127(12):2939–2948. doi: 10.1002/ijc.25295. [DOI] [PubMed] [Google Scholar]
- 32.Kapur N.K., Musunuru K. Clinical efficacy and safety of statins in managing cardiovascular risk. Vascular Health and Risk Management. 2008;4(2):341–353. doi: 10.2147/vhrm.s1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C., Lin J., Smolarek T., Tremaine L. P-glycoprotein has differential effects on the disposition of statin acid and lactone forms in mdr1a/b knockout and wild-type mice. Drug Metabolism and Disposition. 2007;35(10):1725–1729. doi: 10.1124/dmd.107.015677. [DOI] [PubMed] [Google Scholar]
- 34.Radhakrishnan A., Goldstein J.L., McDonald J.G., Brown M.S. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metabolism. 2008;8(6):512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatidis L., Gruber A., Vitols S. Decreased feedback regulation of low density lipoprotein receptor activity by sterols in leukemic cells from patients with acute myelogenous leukemia. Journal of Lipid Research. 1997;38(12):2436–2445. [PubMed] [Google Scholar]
- 36.Chen Y., Hughes-Fulford M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. International Journal of Cancer. 2001;91(1):41–45. doi: 10.1002/1097-0215(20010101)91:1<41::aid-ijc1009>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Chen Y., Hu P., Huang W. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Molecular Cancer Therapeutics. 2014;13(4):855–866. doi: 10.1158/1535-7163.MCT-13-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandyra A., Mullen P.J., Kalkat M., Yu R., Pong J.T., Li Z. Immediate utility of two approved agents to target both the metabolic mevalonate pathway and its restorative feedback loop. Cancer Research. 2014;74(17):4772–4782. doi: 10.1158/0008-5472.CAN-14-0130. [DOI] [PubMed] [Google Scholar]
- 39.Pandyra A.A., Mullen P.J., Goard C.A., Ericson E., Sharma P., Kalkat M. Genome-wide RNAi analysis reveals that simultaneous inhibition of specific mevalonate pathway genes potentiates tumor cell death. Oncotarget. 2015;6(29):26909–26921. doi: 10.18632/oncotarget.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. The Journal of Clinical Investigation. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vergnes L., Chin R.G., de Aguiar Vallim T., Fong L.G., Osborne T.F., Young S.G. SREBP-2-deficient and hypomorphic mice reveal roles for SREBP-2 in embryonic development and SREBP-1c expression. Journal of Lipid Research. 2016;57(3):410–421. doi: 10.1194/jlr.M064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjarnadottir O., Romero Q., Bendahl P.O., Jirström K., Rydén L., Loman N. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Research and Treatment. 2013;138(2):499–508. doi: 10.1007/s10549-013-2473-6. [DOI] [PubMed] [Google Scholar]
- 43.Duncan R.E., El-Sohemy A., Archer M.C. Statins and the risk of cancer. Journal of the American Medical Association. 2006;295(23):2720. doi: 10.1001/jama.295.23.2720-a. [DOI] [PubMed] [Google Scholar]
- 44.Brown A.J. Cholesterol, statins and cancer. Clinical and Experimental Pharmacology and Physiology. 2007;34(3):135–141. doi: 10.1111/j.1440-1681.2007.04565.x. [DOI] [PubMed] [Google Scholar]
- 45.Alfaqih M.A., Allott E.H., Hamilton R.J., Freeman M.R., Freedland S.J. The current evidence on statin use and prostate cancer prevention: are we there yet? Nature Reviews Urology. 2017;14(2):107–119. doi: 10.1038/nrurol.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locke J.A., Guns E.S., Lubik A.A., Adomat H.H., Hendy S.C., Wood C.A. Androgen Levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Research. 2008;68(15):6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 47.Zhuang L., Kim J., Adam R.M., Solomon K.R., Freeman M.R. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. The Journal of Clinical Investigation. 2005;115(4):959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia Z., Tan M.M., Wong W.W., Dimitroulakos J., Minden M.D., Penn L.Z. Blocking protein geranylgeranylation is essential for lovastatin-induced apoptosis of human acute myeloid leukemia cells. Leukemia. 2001;15(9):1398–1407. doi: 10.1038/sj.leu.2402196. [DOI] [PubMed] [Google Scholar]
- 49.Jiang P., Mukthavaram R., Chao Y., Nomura N., Bharati I.S., Fogal V. In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells. British Journal of Cancer. 2014;111(8):1562–1571. doi: 10.1038/bjc.2014.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown M., Hart C., Tawadros T., Ramani V., Sangar V., Lau M. The differential effects of statins on the metastatic behaviour of prostate cancer. British Journal of Cancer. 2012;106(10):1689–1696. doi: 10.1038/bjc.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casella C., Miller D.H., Lynch K., Brodsky A.S. Oxysterols synergize with statins by inhibiting SREBP-2 in ovarian cancer cells. Gynecologic Oncology. 2014;135(2):333–341. doi: 10.1016/j.ygyno.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mason R.P., Walter M.F., Day C.A., Jacob R.F. Intermolecular differences of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors contribute to distinct pharmacologic and pleiotropic actions. The American Journal of Cardiology. 2005;96(5A):11F–23F. doi: 10.1016/j.amjcard.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Thibault A., Samid D., Tompkins A.C., Figg W.D., Cooper M.R., Hohl R.J. Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clinical Cancer Research. 1996;2(3):483–491. [PubMed] [Google Scholar]
- 54.Kornblau S.M., Banker D.E., Stirewalt D., Shen D., Lemker E., Verstovsek S. Blockade of adaptive defensive changes in cholesterol uptake and synthesis in AML by the addition of pravastatin to idarubicin + high-dose Ara-C: a phase 1 study. Blood. 2007;109(7):2999–3006. doi: 10.1182/blood-2006-08-044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.