ABSTRACT
Background
In the absence of dose-response data, Dietary Reference Values for vitamin D in nonpregnant adults are extended to pregnancy.
Objective
The aim was to estimate vitamin D intake needed to maintain maternal 25-hydroxyvitamin D [25(OH)D] in late gestation at a concentration sufficient to prevent newborn 25(OH)D <25–30 nmol/L, a threshold indicative of increased risk of nutritional rickets.
Design
We conducted a 3-arm, dose-response, double-blind, randomized placebo-controlled trial in Cork, Ireland (51.9oN). A total of 144 white-skinned pregnant women were assigned to receive 0, 10 (400 IU), or 20 (800 IU) µg vitamin D3/d from ≤18 wk of gestation. Vitamin D metabolites at 14, 24, and 36 wk of gestation and in cord sera, including 25(OH)D3, 3-epi-25(OH)D3, 24,25(OH)2D3, and 25(OH)D2 were quantified by liquid chromatography–tandem mass spectrometry. A curvilinear regression model predicted the total vitamin D intake (from diet and antenatal supplements plus treatment dose) that maintained maternal 25(OH)D in late gestation at a concentration sufficient to maintain cord 25(OH)D at ≥25–30 nmol/L.
Results
Mean ± SD baseline 25(OH)D was 54.9 ± 10.7 nmol/L. Total vitamin D intakes at the study endpoint (36 wk of gestation) were 12.1 ± 8.0, 21.9 ± 5.3, and 33.7 ± 5.1 µg/d in the placebo and 10-µg and 20-µg vitamin D3 groups, respectively; and 25(OH)D was 24.3 ± 5.8 and 29.2 ± 5.6 nmol/L higher in the 10- and 20-µg groups, respectively, compared with placebo (P < 0.001). For maternal 25(OH)D concentrations ≥50 nmol/L, 95% of cord sera were ≥30 nmol/L and 99% were >25 nmol/L. The estimated vitamin D intake required to maintain serum 25(OH)D at ≥50 nmol/L in 97.5% of women was 28.9 µg/d.
Conclusions
Thirty micrograms of vitamin D per day safely maintained serum 25(OH)D concentrations at ≥50 nmol/L in almost all white-skinned women during pregnancy at a northern latitude, which kept 25(OH)D at >25 nmol/L in 99% and ≥30 nmol/L in 95% of umbilical cord sera. This trial was registered at www.clinicaltrials.gov as NCT02506439.
Keywords: 25-hydroxyvitamin D, dietary requirements, dose-response, ODIN, neonatal, pregnancy, randomized controlled trial, vitamin D, vitamin D requirements
INTRODUCTION
There is currently insufficient evidence from randomized controlled trials (RCTs) to determine whether 25-hydroxyvitamin D [25(OH)D] targets specific for perinatal outcomes are required (1–4). Thus, Dietary Reference Values for vitamin D, which are the recommended intakes to meet 25(OH)D targets of between 25 and 50 nmol/L for bone health outcomes, have been extended to pregnancy and lactation (summarized in Table 1) (5). Although setting Dietary Reference Values is an iterative process, reliant on the evidence basis at that time, the current recommendations for vitamin D during pregnancy suffer from a lack of evidence across a number of criteria. Not only are they not specific for perinatal health outcomes, they do not consider fetal and neonatal requirements specifically and rely on an assumption that pregnancy does not increase the metabolic demand for vitamin D.
TABLE 1.
Summary of the current dietary recommendations for vitamin D in pregnant women1
| 25(OH)D threshold, nmol/L | Vitamin D, µg/d | |||||
|---|---|---|---|---|---|---|
| Agency, year (ref) | Region | Deficiency | Population average | Individual target | EAR | RI |
| IOM (2011) (1) | United States/Canada | <30 | 40 | ≥50 | 10 | 15 |
| NORDEN (2012) (2) | Nordic | <30 | — | ≥50 | 7.5 | 10 |
| SACN (2016) (3) | United Kingdom | <25 | — | ≥25 | — | 10 |
| EFSA (2016) (4) | EU | — | — | ≥50 | — | 15 (AI) |
1Table modified and reproduced from reference 5. AI, Adequate Intake; EAR, Estimated Average Requirement; EFSA, European Food Safety Authority; IOM, Institute of Medicine; NORDEN, Nordic Council of Ministers; ref, reference; RI, Recommended (individual) Intake; SACN, Scientific Advisory Committee on Nutrition; 25(OH)D, 25-hydroxyvitamin D.
Endemic vitamin D deficiency has been reported among pregnant women and newborns worldwide, with wide variations between and within regions. In the systematic review by Saraf et al. (6), the global prevalence of 25(OH)D concentrations <50 nmol/L was 54% among pregnant women and 75% among newborns, whereas 18% of pregnant women and 29% of newborns had concentrations <25 nmol/L, a threshold identified as indicative of an increased risk of nutritional rickets (1–4). Acknowledging that neonatal requirements for 25(OH)D are unknown and the application of adult references to newborns may be questionable (7), it seems prudent nonetheless to ensure maintenance of newborn circulating 25(OH)D above a minimum of 25–30 nmol/L, consistent with the prevention of nutritional rickets (8, 9). Because cord 25(OH)D concentrations are usually ∼60–80% of maternal values at delivery (10, 11), maintenance of maternal 25(OH)D >25–30 nmol/L will not ensure newborn protection at the same threshold. In the absence of sufficient trial-based data to set maternal requirements for 25(OH)D on the basis of perinatal outcomes, we propose in the interim that dietary recommendations for vitamin D during pregnancy should be established with the aim of maintaining maternal 25(OH)D in late gestation at sufficient concentrations to ensure that newborn 25(OH)D concentrations are ≥25–30 nmol/L. Researchers in New Zealand and Canada have proposed that maternal 25(OH)D should be maintained at 50 nmol/L (12, 13). To our knowledge, this is the first placebo-controlled, dose-response, randomized trial that was designed specifically to estimate the maternal vitamin D intake needed to maintain serum 25(OH)D in late gestation at a concentration sufficient to keep umbilical cord 25(OH)D ≥25–30 nmol/L, a threshold indicative of increased risk of nutritional rickets.
METHODS
This trial was conducted as part of the European Commission–funded collaborative project ODIN (Food-based solutions for optimal vitamin D nutrition and health throughout life; www.odin-vitd.eu). This study was a 3-arm, parallel, dose-response, double-blind, randomized trial of vitamin D3 (cholecalciferol) compared with placebo.
Participants
A total of 144 healthy, pregnant women were recruited to the trial. Recruitment began in November 2014 and continued until April 2016. The primary center for recruitment was the Cork University Maternity Hospital, Cork, Ireland, where women attending early pregnancy and ultrasound clinics were approached by a member of the study team and informed about the trial. Social media and advertisements in local General Practitioners surgeries and pharmacies provided another avenue for recruitment. Women were considered eligible for participation if they were white-skinned adults ≥18 y of age, with a gravidae of ≤18 wk of gestation, in good general health, and not identified as having a high-risk pregnancy. Exclusion criteria were as follows: current smoker, vegan, diagnosed hypertension before commencement of the study, diagnosed medical disorder including type 1 or 2 diabetes, chronic kidney disease or gastrointestinal disease, consumption of medications known to interfere with vitamin D metabolism (e.g., corticosteroids), and consumption of supplemental vitamin D [>10 µg/d (>400 IU)] or calcium (>650 mg/d) before randomization. Women were permitted to continue with self-administration of antenatal supplements containing ≤10 µg vitamin D/d but were excluded from the study if personal supplement use exceeded this dose.
Sample size
The power calculation for this study was based on similar dose-response studies of vitamin D nutritional requirements designed by our research group, whereby 31 participants/arm are adequate to detect a 10-nmol/L difference in 25(OH)D concentrations and provide 90% power to show a dose-response relation with a slope of 1.5 and α = 0.05 (14–17). Because this was a pregnancy study, with 3 assessment points at baseline and the second and third trimesters, it was implemented throughout the year, unlike our previous trials in other population subgroups, which were conducted during winter. Therefore, we increased the sample size to 48/arm (144 in total) to enable a season-specific analysis and to account for a potentially higher drop-out rate in late gestation than we would usually see (typically, ≤15%).
Ethics
Ethical approval was obtained from the Clinical Research Ethics Committee of the Cork Teaching Hospitals [ECM4(o)04/02/14] and written informed consent was provided by all women before commencing the trial. The study protocol followed the guidelines laid down in the Declaration of Helsinki, and the trial was registered at the US NIH Clinical Trials Registry (www.clinicaltrials.gov; ID: NCT02506439).
Randomization
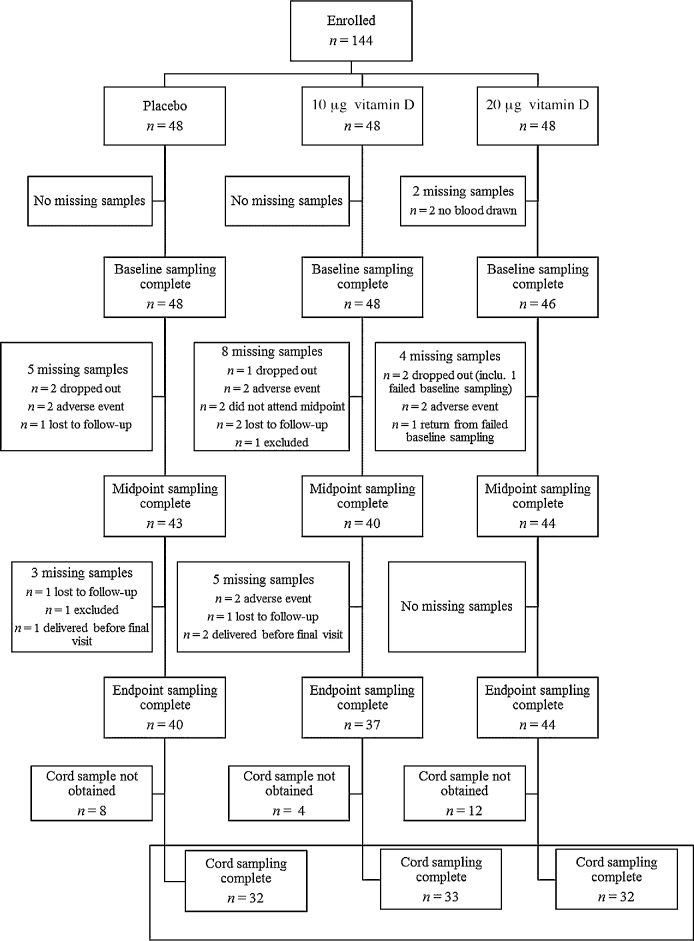
Given that this was a single-sex sample, within the reproductive age range, women were randomly assigned to receive 10 µg (400 IU) or 20 µg (800 IU) vitamin D3/d or a matching placebo in a 1:1:1 ratio (Figure 1). A senior scientist, who was not involved in the implementation or analysis of the study, randomly assigned group codes to a computer-generated list of random numbers, which were assigned to consecutive participant identification numbers. Participants were allocated an identification number in order of attendance of the baseline visit. The treatment allocation was blinded to participants and investigators throughout the study period.
FIGURE 1.
CONSORT flow diagram of participant enrollment, random assignment, and biochemical analysis throughout the study by treatment group, where n is based on the total number of samples available for each visit. Any participant who did not provide a blood sample at baseline was included in the descriptive and biochemical analysis at later time points but excluded from the dose-response analysis, whereas women missing a midpoint sample only were included in both the dose-response analysis and the analysis at endpoint, if a blood sample was collected at this time point. The numbers of women who provided both a baseline and ≥1 follow-up sample (midpoint or endpoint) were 43, 42, and 43 for the placebo and 10- and 20-µg groups, respectively, which left a final number of 128 for the dose-response analysis. CONSORT, Consolidated Standards of Reporting Trials; inclu., including.
Intervention
Placebo and Minisun vitamin D3 tablets were provided by OY Verman Ab. All tablets (placebo and 10- and 20-µg vitamin D3) were identical in appearance and taste and free from sugar, lactose, yeast, gluten, and gelatin. Tablets were packaged and coded into identical, white, plastic containers in a food sensory facility at our center, ensuring concealment of the treatment to the study team and participants. Independent analysis of the vitamin D content of the study tablets was conducted by liquid chromatography–tandem mass spectrometry (LC-MS/MS) at the National Food Institute at the Technical University of Denmark. All of the analyses were based on a sample of 5 tablets and were performed in triplicate. The content of the placebo and the 10- and 20-µg labeled tablets corresponded to a value of <0.02, 10.9, and 22.4 µg vitamin D3, respectively. The dose range of vitamin D was chosen on the basis of the distribution of intakes and 25(OH)D concentrations observed in our previous dose-response vitamin D trials (14, 15). We estimated that the mean intake in the 20 µg group would be ∼25 µg/d (1000 IU), accounting for the average contribution of the diet to vitamin D intakes in Ireland (18). In the current study, given that women were allowed to continue with their own supplements as long as these did not provide >10 µg/d, we estimated that the range of total intakes in the sample from the base diet, fortified foods, personal antenatal supplements, and assigned trial supplements would lie between 1 and ∼50 µg/d. We aimed to include a supplemental dose that would ensure a total vitamin D intake that would not exceed the accepted Tolerable Upper Intake Level of 100 µg/d (1). All of the participants received a container of 90 tablets at both the baseline and midpoint visits, corresponding to 1 tablet/d for up to 14 wk. Compliance was monitored by a tablet count at each visit, and noncompliance was defined a priori as tablet consumption <80%.
Data collection
All of the study visits took place at the Human Nutrition Studies Unit at the Cork Center for Vitamin D and Nutrition Research, University College Cork, Cork, Ireland. The RCT was conducted in compliance with Good Clinical Practice. Participants were seen 3 times throughout their pregnancy: at baseline (14 wk of gestation; range: 8–18 wk), midgestation (24 wk of gestation; range: 20–26 wk), and late gestation (36 wk of gestation; range: 34–38 wk). Gestational age was established by date of last menstrual period and confirmed by fetal ultrasound at the participants’ first ultrasound visit. At baseline, interviewer-led assessments collected information on general health, lifestyle, and sociodemographic characteristics. Habitual calcium and vitamin D intakes were estimated by using a validated interviewer-administered quantitative food-frequency questionnaire for vitamin D and calcium (19) at baseline, and antenatal supplement use was re-assessed at the second and third study visit to account for any changes in supplemental vitamin D or calcium intake. Anthropometric measurements (height and weight) were taken at baseline with the use of standard scales (Leicester height measure; CMS Weighing Equipment Ltd.; digital weighing scales; Seca Ltd.), and body weight measurements were repeated at the second and third visits. Weight was measured in kilograms, without shoes or heavy clothing, to the nearest 2 decimal places. A venous nonfasting blood sample (30 mL) was collected at each visit, because it would be inappropriate to request a pregnant woman to fast. Blood samples were taken by a research nurse and processed to serum within 3 h. A venous umbilical cord blood sample was collected at delivery, refrigerated immediately, and processed to serum at University College Cork. All of the blood samples were stored at –80°C until further analysis.
Laboratory analysis
Serum 25(OH)D concentrations
Circulating serum 25-hydroxyvitamin D3 [25(OH)D3], 3-epimer of 25-hydroxyvitamin D3 [3-epi-25(OH)D3], 24,25-dihydroxyvitamin D3 [24,25(OH)2D3], and 25-hydroxyvitamin D2 [25(OH)D2] were analyzed at the Cork Center for Vitamin D and Nutrition Research using a CDC-certified LC-MS/MS method, which has been described in detail elsewhere (20). Briefly, the instrument used was a Waters Acquity UPLC system coupled to an Acquity Triple Quadrupole (TQD) mass spectrometer detector (Waters, Santry, Dublin 9, Ireland). Concentrations of 25(OH)D3 and 25(OH)D2 were quantified separately and summed to generate total 25(OH)D. Chromatographic separation and quantification of 3-epi-25(OH)D3 were also achieved. Four levels of serum-based National Institute of Standards and Technology (NIST)–certified quality-assurance material (SRM 972) were used for method validation, whereas quality-control materials assayed in parallel to all samples were purchased from Chromsystems. NIST calibrators (SRM 2972) were used throughout the analysis. The intra-assay CVs for 25(OH)D3, 3-epi-25(OH)D3, 24,25(OH)2D3, and 25(OH)D2 were 2.5%, 12.7%, 9.3%, and 8.5% and the interassay CVs were 6.4%, 10.6%, 7.2%, and 3.5%, respectively. The limits of detection (LoDs) for 25(OH)D3, 3-epi-25(OH)D3, 24,25(OH)2D3, and 25(OH)D2 were 0.31, 0.12, 0.65, and 0.17 nmol/L and the limits of quantification were 2.52, 1.35, 1.09, and 2.19 nmol/L, respectively. Mean biases were −1.5%, −4.9%, 12.0%, and −1.7%, respectively. Our method does not measure the C-3 epimer of 25(OH)D2, but given the low mean ± SD concentrations of 25(OH)D2 found in sera from pregnant women (3.7 ± 2.7 nmol/L) (20) and umbilical cords (2.2 ± 1.9 nmol/L) (9), extremely low concentrations of 3-epi-25(OH)D2 would be expected.
The quality and accuracy of the vitamin D metabolite analysis in our laboratory are monitored on an ongoing basis by participation in the Vitamin D External Quality Assessment Scheme (DEQAS; Charing Cross Hospital, London, United Kingdom). We have been certified by the CDC's Vitamin D Standardization Certification Program, which has reported accuracy and bias for total 25(OH)D, 25(OH)D3, 3-epi-25(OH)D3, and 25(OH)D2 since 2013. For transparency, and to promote international comparison, we have reported data across a range of currently applied international 25(OH)D thresholds (1–4, 21).
Serum calcium concentrations
As a safety measure, serum calcium is preferable to urinary calcium in studies involving pregnant women because physiologic hypercalciuria can occur as a result of normal pregnancy (1). Serum calcium and albumin were quantified at our laboratory by colorimetric and immunoturbidimetric assays, respectively, with the use of the Randox Monaco Automated Clinical Chemistry Analyser (Randox Laboratories Ltd., Co.). Serum calcium was corrected for albumin as follows: corrected calcium (mmol/L) = measured total calcium (mmol/L) + 0.02 × [40 − serum albumin (g/L)], where 40 represents the average albumin concentration in grams per liter (22). Batch analysis allowed for continuous sampling throughout the trial to ensure that all participants remained below the predefined safety threshold (hypercalcemia defined as serum calcium ≥2.63 mmol/L) (1). A normal reference range of 2.02–2.60 mmol/L is in agreement with that provided by the manufacturer of the equipment used in this laboratory (Randox Monaco, Randox Laboratories Ltd., Co.). The mean interassay CV for the analysis was 3%.
Serum parathyroid hormone concentrations
Serum intact parathyroid hormone (iPTH) was analyzed at baseline and endpoint at our laboratory with the use of an ELISA (MD Biosciences, Inc.) on the automated Dynex DS2 ELISA processing platform (Dynex Technologies). This 2-site assay is designed to measure biologically iPTH 1–84 and utilizes 2 purified goat polyclonal antibodies, each specific to a distinct region on the parathyroid hormone (PTH) molecule. A biotinylated antibody binds to midregion and C-terminal PTH 39–84. The detection antibody, a horseradish peroxidase–conjugated antibody, binds N-terminal PTH 1–34. The mean intra- and interassay CVs for this analysis were <3%.
Statistical analysis
Statistical analysis was conducted with the use of SPSS for Windows version 23.0 (released 2015; IBM Corp., Armonk, NY, USA). Distribution of the data for all variables at baseline was tested for normality with the use of histograms and formal tests (Kolmogorov-Smirnov and Shapiro-Wilk). Data are presented as means ± SDs, medians (IQRs), and frequencies with percentages, where appropriate. Where data could not be normalized after transformation, the alternative nonparametric tests were used. A Mann-Whitney U test assessed the difference in participant characteristics between women who completed the trial and women who had either withdrawn or for whom data were missing at any stage. Pre- and postintervention 25(OH)D status and iPTH concentrations within each group were compared by using paired t tests, as were differences between maternal and umbilical cord vitamin D metabolites. For continuous data, a between-group ANOVA with post hoc Tukey's test was used to assess the differences in participant characteristics, biochemical measures, and dietary intakes between the 3 arms of the trial and a mixed within-between-subject ANOVA assessed changes in biochemical measures between the groups over time. For comparison between categorical variables, including the proportion of women achieving serum 25(OH)D concentration thresholds of ≥25, 30, 40, 50, and 75 nmol/L, chi-square tests were used. Bivariate correlations between variables were examined by using Pearson or Spearman correlations, where appropriate. Season of sampling was dichotomized into winter (November–May) and summer (June–October). Associations between maternal and umbilical cord vitamin D metabolites, vitamin D intakes, and iPTH were described by using a linear trendline or power best-fit curve, where appropriate. P < 0.05 was considered significant.
Mathematical modeling of distribution of serum 25(OH)D in relation to total vitamin D intake
Total vitamin D intake was assessed as habitual dietary intake plus that derived from personal antenatal supplementation, if used, plus the intervention dose from the assigned treatment, on the basis of the analytically verified vitamin D content of the tablets. The relation between total vitamin D intake and postintervention serum 25(OH)D was described by using the curvilinear regression model y = b2 + b0 × [1 − exp(–x/b1)], as detailed previously (16, 17). We conducted an intention-to-treat analysis; given that the primary outcome was the dose-relation between total vitamin D intake and achieved 25(OH)D postsupplementation, all participants who had endpoint data were included, regardless of compliance. We carried forward data from their own midpoint assessment for 7 participants who were not available at endpoint, according to best practice. Ninety-five percent prediction intervals of the required vitamin D intake were calculated to assess the probable range of intake in the target population. Moreover, the required vitamin D intakes to maintain 97.5% of the pregnant women above serum 25(OH)D thresholds of 25, 30, and 50 nmol/L were estimated from the model by inverse regression applied to the lower limits of the prediction intervals. In addition, 95% CIs for these vitamin D intakes were obtained with the use of percentiles from a nonparametric bootstrap procedure based on 1000 replications. Curvilinear regression models were fitted to all data and to data stratified by season (summer and winter). Analyses were carried out by using R version 3.2.2 (23).
RESULTS
Recruitment, retention, and adverse events
Our target sample of 144 women completed the baseline visit. Serum specimens were available for all but 2 women at baseline, due to the inability to draw blood. A total of 121 women completed every visit, corresponding to an overall retention rate of 84%. The numbers of women who provided both a baseline and ≥1 follow-up sample (midpoint or endpoint) were 43, 42, and 43 for the placebo and 10- and 20-µg groups, respectively, which left a final number of 128 for the dose-response analysis. Of the 23 participants who did not provide an endpoint sample, 8 were due to a pregnancy-associated adverse event, 5 withdrew for personal reasons, 5 were lost to follow-up, 2 began consuming vitamin D supplements containing >10 µg/d and were excluded, and 3 delivered their infants before the final visit was conducted. Overall, 9 adverse events were reported: 1 sexually transmitted disorder, 1 case of gastrointestinal upset unrelated to the intervention, 1 suspected irritated uterus, 1 miscarriage, 1 chorionic hematoma, 1 symphysis pubic dysfunction, 1 case of severe hypotension, 1 case of pregnancy-induced hypertension, and 1 diagnosed case of preeclampsia. The number of adverse events and withdrawals from the trial did not differ by intervention group (P > 0.05). There were no differences in the subject characteristics between women with and without a full data set. Compliance with the intervention was high and was similar between each of the intervention arms: 79%, 87%, and 90% of participants in the placebo and 10- and 20-µg/d groups were compliant at the midpoint visit (P = 0.376) and 67%, 83%, and 76% were compliant at endpoint (P = 0.310), respectively. A detailed description of participation retention and progression throughout the study by treatment group is provided in Figure 1.
Baseline characteristics
Participant characteristics are shown in Table 2. Maternal age at baseline ranged from 21 to 41 y. Women in the placebo group were, on average, 3 y younger than women allocated to the highest vitamin D3-dose group (P = 0.004). Overall, 70% of participants had a university degree. Thirty-three percent of women were first-time mothers and 5 women were having a twin pregnancy (3 in the placebo group and 2 in the 20-μg group). Total vitamin D and calcium intakes did not differ between the groups before the intervention (P > 0.05). BMI at first visit was not associated with baseline 25(OH)D status, nor did BMI influence the change in maternal 25(OH)D after the intervention (P > 0.05 for both). More than two-thirds (69%) of women were taking a vitamin D–containing supplement, from which half obtained 10 µg/d in addition to their dietary intake. Doses ranged from 0.7 to 10 µg/d and almost all products (97%) contained vitamin D3. Thus, mean total vitamin D intakes, including habitual diet plus supplemental antenatal vitamin D plus the assigned treatment dose, were 12.1 ± 8.0, 21.9 ± 5.3, and 33.7 ± 5.1 µg/d in the placebo group and 10- and 20-µg groups, respectively. The range of total vitamin D intakes was 1.7–53.4 µg/d (68–2136 IU).
TABLE 2.
Baseline characteristics of the 144 participants by treatment group1
| Vitamin D3/d | ||||
|---|---|---|---|---|
| Placebo (n = 48) | 10 μg (n = 48) | 20 μg (n = 48) | P | |
| Age, y | 32 ± 4a | 34 ± 4a,b | 35 ± 3b | 0.007 |
| Gestational age, wk | 14 ± 2 | 14 ± 2 | 14 ± 2 | 0.87 |
| First-time mother, n (%) | 20 (42) | 13 (27) | 14 (29) | 0.26 |
| University education, n (%) | 31 (65) | 36 (75) | 34 (71) | 0.53 |
| BMI at 14 wk of gestation, kg/m2 | 25.7 ± 4.3 | 26.8 ± 5.1 | 24.5 ± 3.1 | 0.095 |
| Season enrolled, n (%) | ||||
| Winter (November–April) | 39 (81) | 39 (81) | 32 (67) | 0.15 |
| Summer (May–October) | 9 (19) | 9 (19) | 16 (33) | |
| Vitamin D intake,2 μg/d | 10.6 ± 5.2 | 10.5 ± 5.4 | 11.4 ± 5.0 | 0.43 |
| Calcium intake,2 mg/d | 1143 ± 440 | 1188 ± 571 | 1216 ± 443 | 0.76 |
| Vitamin D supplements (yes), n (%) | 33 (69) | 31 (65) | 35 (73) | 0.68 |
| Calcium supplements (yes), n (%) | 9 (19) | 13 (27) | 12 (25) | 0.61 |
1Values are means ± SDs unless otherwise indicated. P values are for differences between groups by ANOVA. Means in the same row without a common superscript letter differ, P < 0.05 (Tukey's test).
2Includes intake from diet and antenatal supplements.
Total serum 25(OH)D, iPTH, and albumin-adjusted calcium concentrations were similar between the treatment groups at baseline (Table 3). Baseline 25(OH)D was normally distributed, as confirmed by both Kolmogorov-Smirnov and Shapiro-Wilk tests. With a mean ± SD concentration of 54.9 ± 22.6 nmol/L, 13% had a 25(OH)D concentration <30 nmol/L and 44% had concentrations <50 nmol/L. As expected, the prevalence of women with low vitamin D status was higher in winter (November–May; 15% <30 nmol/L and 56% <50 nmol/L) than in summer (June–October; 3% <30 nmol/L and 7% <50 nmol/L). Although only 3% of mothers took a vitamin D2–containing supplement (dose range: 2.5–10 µg/d), 25(OH)D2 was detectable (i.e., concentrations more than the LoD) in >98% of maternal sera, with a sample median concentration of 2.3 nmol/L, ranging from less than the LoD to 22.0 nmol/L across all time points.
TABLE 3.
Maternal and cord vitamin D metabolites and serum calcium and maternal iPTH throughout pregnancy, by treatment group1
| Treatment group2 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (14 wk mean gestational age) (n = 142) | Midpoint (24 wk mean gestational age) (n = 127) | Endpoint (36 wk mean gestational age) (n = 121) | Umbilical cord (n = 97) | |||||||||||||
| Placebo | 10 μg/d | 20 μg/d | P | Placebo | 10 μg/d | 20 μg/d | P | Placebo | 10 μg/d | 20 μg/d | P | Placebo | 10 μg/d | 20 μg/d | P | |
| Total 25(OH)D, nmol/L | 57.2 ± 24.5 | 49.6 ± 19.6 | 58.0 ± 22.9 | 0.13 | 63.6 ± 25.0a | 81.9 ± 27.8b | 92.8 ± 22.1b | <0.001 | 71.4 ± 24.3a | 96.0 ± 29.2b | 100.6 ± 23.3b | <0.001 | 39.2 ± 16.3a | 44.1 ± 14.6a,b | 50.5 ± 15.1b | 0.016 |
| 25(OH)D3, nmol/L | 54.8 ± 24.4 | 46.9 ± 19.7 | 54.9 ± 23.2 | 0.14 | 60.9 ± 25.1a | 79.1 ± 27.2b | 90.0 ± 21.9b | <0.001 | 68.4 ± 24.2a | 92.9 ± 28.9b | 97.6 ± 23.1b | <0.001 | 37.7 ± 16.2a | 42.4 ± 14.1a,b | 48.1 ± 14.1b | 0.023 |
| 3-Epi-25(OH)D3, nmol/L | 2.0 ± 1.2 | 1.6 ± 0.8 | 1.9 ± 1.3 | 0.30 | 2.6 ± 1.5a | 3.2 ± 1.6a | 4.1 ± 1.8b | <0.001 | 3.4 ± 1.5a | 4.5 ± 2.0b | 4.8 ± 1.7b | 0.001 | 3.8 ± 2.4 | 4.1 ± 1.8 | 4.6 ± 1.5 | 0.27 |
| 24,25(OH)2D3, nmol/L | 3.2 ± 2.0 | 2.7 ± 1.9 | 3.3 ± 2.1 | 0.35 | 3.9 ± 2.3a | 5.3 ± 2.6b | 6.2 ± 2.4b | <0.001 | 4.6 ± 2.5a | 7.3 ± 3.4b | 7.6 ± 2.5b | <0.001 | 2.9 ± 1.7a | 3.8 ± 2.1a,b | 4.0 ± 1.4b | 0.026 |
| Serum calcium,3 mmol/L | 2.21 ± 0.1 | 2.23 ± 0.1 | 2.24 ± 0.1 | 0.53 | 2.31 ± 0.1 | 2.31 ± 0.1 | 2.33 ± 0.1 | 0.609 | 2.31 ± 0.1a,b | 2.26 ± 0.2a | 2.36 ± 0.1b | 0.019 | 2.67 ± 0.2 | 2.61 ± 0.5 | 2.72 ± 0.2 | 0.40 |
| iPTH,4 pg/mL | 11.1 ± 10.7 | 11.2 ± 8.2 | 11.7 ± 8.8 | 0.95 | — | — | — | 13.6 ± 9.4 | 12.5 ± 11.8 | 11.9 ± 8.4 | 0.74 | — | — | — | ||
1Values are means ± SDs. P values are for differences between groups by ANOVA. Means in the same row without a common superscript letter differ, P < 0.05 (Tukey's test). P < 0.05 for change in 25(OH)D, 25(OH)D3, 3-epi-25(OH)D3, 24,25(OH)2D3, and serum calcium from baseline and P > 0.05 for iPTH. iPTH, intact parathyroid hormone; 3-epi-25(OH)D3, 3-epi-25-hydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D3, 25-hydroxyvitamin D3.
2Represents total vitamin D intakes (including habitual diet plus supplemental vitamin D plus the treatment dose) of 12.1 ± 8.0, 21.9 ± 5.3, and 33.7 ± 5.1 µg/d in placebo and 10- and 20-µg groups, respectively.
3Serum albumin-corrected calcium values.
4Serum iPTH was not measured at midpoint.
Effect of vitamin D3 supplementation on maternal serum total 25(OH)D, iPTH, and calcium
To show the impact of the intervention on maternal serum total 25(OH)D status, mean 25(OH)D concentrations at each study visit are shown in Table 3 and the prevalence of women with 25(OH)D concentrations below a range of thresholds is described in Table 4. Serum 25(OH)D increased in all groups (Figure 2), corresponding to mean increases of 5.9 ± 17.3, 33.0 ± 21.7, and 33.4 ± 23.5 nmol/L from baseline to midpoint in the placebo and 10- and 20-µg groups, respectively (P < 0.05 in all cases), and of 10.0 ± 24.0, 12.2 ± 16.9, and 7.8 ± 11.0 nmol/L from midpoint to endpoint in the placebo and 10- and 20-µg groups, respectively (P < 0.05 in all cases). The range of 25(OH)D concentrations postintervention was 14.7–150.7 nmol/L. The increase in 25(OH)D concentrations in the placebo group reflects seasonal variability in vitamin D status, because 81% of women in this group were recruited in winter and delivered during summer. Mean maternal serum total 25(OH)D concentrations at 36 wk of gestation were 24.3 ± 5.8 nmol/L and 29.2 ± 5.6 nmol/L higher in the 10- and 20-µg groups, respectively, compared with in the placebo group (P < 0.001) (Table 3).
TABLE 4.
Prevalence of maternal 25(OH)D concentrations at baseline and endpoint of the intervention study and umbilical cord 25(OH)D concentrations <25, 30, 40, 50, and 75 nmol/L by intervention group1
| Treatment group2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (14 wk mean gestational age) | Endpoint (36 wk mean gestational age) | Umbilical cord | ||||||||||
| (n = 142) | (n = 121) | (n = 97) | ||||||||||
| 25(OH)D | Placebo | 10 μg/d | 20 μg/d | P | Placebo | 10 μg/d | 20 μg/d | P | Placebo | 10 μg/d | 20 μg/d | P |
| <25 nmol/L | 10 | 10 | 4 | 0.48 | 8a | 0b | 0b | 0.044 | 19 | 6 | 3 | 0.07 |
| <30 nmol/L | 13 | 17 | 9 | 0.51 | 8 | 3 | 0 | 0.15 | 31a | 15a,b | 3b | 0.010 |
| <40 nmol/L | 27 | 35 | 24 | 0.44 | 13 | 5 | 2 | 0.16 | 56a | 49a | 22b | 0.014 |
| <50 nmol/L | 40 | 52 | 41 | 0.41 | 23a | 5a,b | 2b | 0.004 | 72 | 67 | 56 | 0.41 |
| <75 nmol/L | 75 | 81 | 74 | 0.66 | 45a | 32a,b | 16b | 0.015 | 97 | 100 | 94 | 0.35 |
1Values are percentages. P values are for differences in the proportion of women achieving serum 25(OH)D cutoffs between groups by chi-square test. Means in the same row without a common superscript letter differ, P < 0.05. 25(OH)D, 25-hydroxyvitamin D.
2Represents total vitamin D intakes (including habitual diet plus supplemental vitamin D plus the treatment dose) of 12.1 ± 8.0, 21.9 ± 5.3, and 33.7 ± 5.1 µg/d in the placebo and 10- and 20-µg groups, respectively.
FIGURE 2.
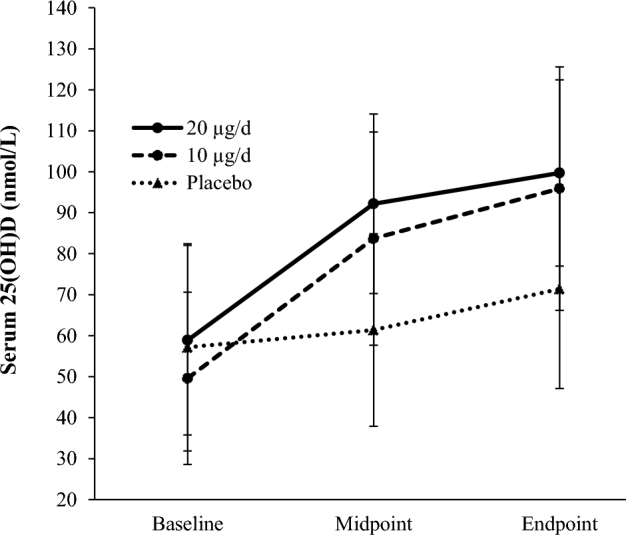
Mean maternal serum 25(OH)D concentrations achieved at each time point by intervention group. n = 40, 35, and 43 for the placebo and 10- and 20-µg groups, respectively, in which the analysis includes women who provided a blood sample at all 3 time points. Total mean ± SD vitamin D intakes (including habitual diet and supplementation plus treatment dose) were 12.1 ± 8.0, 21.9 ± 5.3, and 33.7 ± 5.1 µg/d in the placebo and 10- and 20-µg groups, respectively. Mean gestational age = 14, 24, and 36 wk at baseline, midpoint, and endpoint, respectively. Differences in mean serum 25(OH)D within groups from baseline to endpoint were all significantly different (P < 0.01 in all cases, paired-samples t test for each group). 25(OH)D, 25-hydroxyvitamin D.
On completion of the intervention (mean of 36 wk of gestation), no participant in the 10- or 20-µg groups had a serum 25(OH)D concentration <25 nmol/L (Table 4). One woman who received 10 µg vitamin D had a 25(OH)D <30 nmol/L and 2 had concentrations <50 nmol/L. One participant in the 20-µg group had a concentration <50 nmol/L. In the placebo group, 8% had a postintervention 25(OH)D concentration <30 nmol/L and 23% had concentrations <50 nmol/L. Postintervention, 68% and 84% had concentrations >75 nmol/L in the 10- and 20-µg groups, respectively. In total, 9% (n = 11) had a 25(OH)D concentration >125 nmol/L at endpoint, a threshold designated as cautionary (pending further data) by the Institute of Medicine in 2011 (1); none were in the placebo group, 5 were taking 10 µg and 6 were taking 20 µg, with average total intakes of 21.9 and 33.7 µg/d, respectively. There were no significant differences in serum calcium between treatment groups at any of the time points (Table 3) and there were no cases of hypercalcemia throughout the intervention study. There were no significant differences in serum iPTH between treatment groups at baseline or endpoint (midpoint not measured) (Table 3), and no significant changes from baseline to endpoint (P > 0.05). There was a negative correlation between serum 25(OH)D concentrations and iPTH at both baseline (rho = −0.337, P < 0.001; n = 142) and endpoint (rho = −0.289, P = 0.006; n = 121).
Dose-response of total vitamin D intake and maternal 25(OH)D
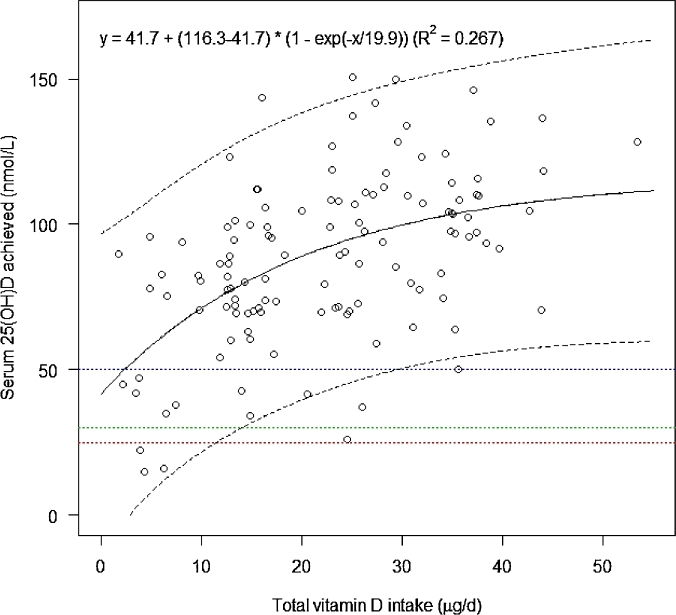
Figure 3 shows the nonlinear relation between total vitamin D intake and postintervention total 25(OH)D concentrations. The estimated year-round vitamin D intakes required to maintain serum 25(OH)D concentrations ≥25, 30, and 50 nmol/L in 97.5% of gravidae were 11.3, 13.8, and 28.9 µg/d, respectively (Table 5). Stratification by winter and summer delivered higher estimates for winter, at 16.2, 18.3, and 30.8 µg vitamin D/d to meet the ≥25-, 30-, and 50-nmol/L thresholds, respectively, compared with summer, at 5.8, 8.4, and 23.5 µg/d, respectively (Table 5).
FIGURE 3.
The relation between achieved serum 25(OH)D concentrations and total vitamin D intake in pregnant women living at 51.9°N, assessed by using a curvilinear regression model. The mean response is indicated by the central line, and the outer lines are its 95% prediction intervals; n = 128. Horizontal lines represent serum 25(OH)D thresholds of 25, 30, and 50 nmol/L, respectively. 25(OH)D, 25-hydroxyvitamin D.
TABLE 5.
Estimated vitamin D intakes to maintain serum 25(OH)D above selected thresholds in 97.5% of pregnant women at 51.9oN, year-round, during winter and summer1
| Vitamin D intakes, µg/d | |||
|---|---|---|---|
| 25(OH)D | Year-round | Winter | Summer |
| >25 nmol/L | 11.3 (6.8, 15.0) | 16.2 (9.9, 21.9) | 5.8 (1.1, 10.6) |
| >30 nmol/L | 13.8 (8.8, 18.1) | 18.3 (11.7, 24.3) | 8.4 (2.0, 13.1) |
| >50 nmol/L | 28.9 (20.6, 41.1) | 30.8 (19.5, 40.7) | 23.5 (14.0, 30.6) |
1Values are the vitamin D intakes (95% CIs) that will maintain serum 25(OH)D >25, 30 and 50 nmol/L in 97.5% of white-skinned pregnant women. Results are based on a nonlinear regression model of serum 25(OH)D concentration as a function of vitamin D intake (y = b2 + b0 × [1 − exp(–x/b1)]), n = 128; 95% CIs for the lower prediction limits were obtained with the use of bias-corrected bootstrap procedures based on 1000 replications. Winter season of delivery was defined as November–May; summer season of delivery was defined as June–October. 25(OH)D, 25-hydroxyvitamin D.
Umbilical cord 25(OH)D
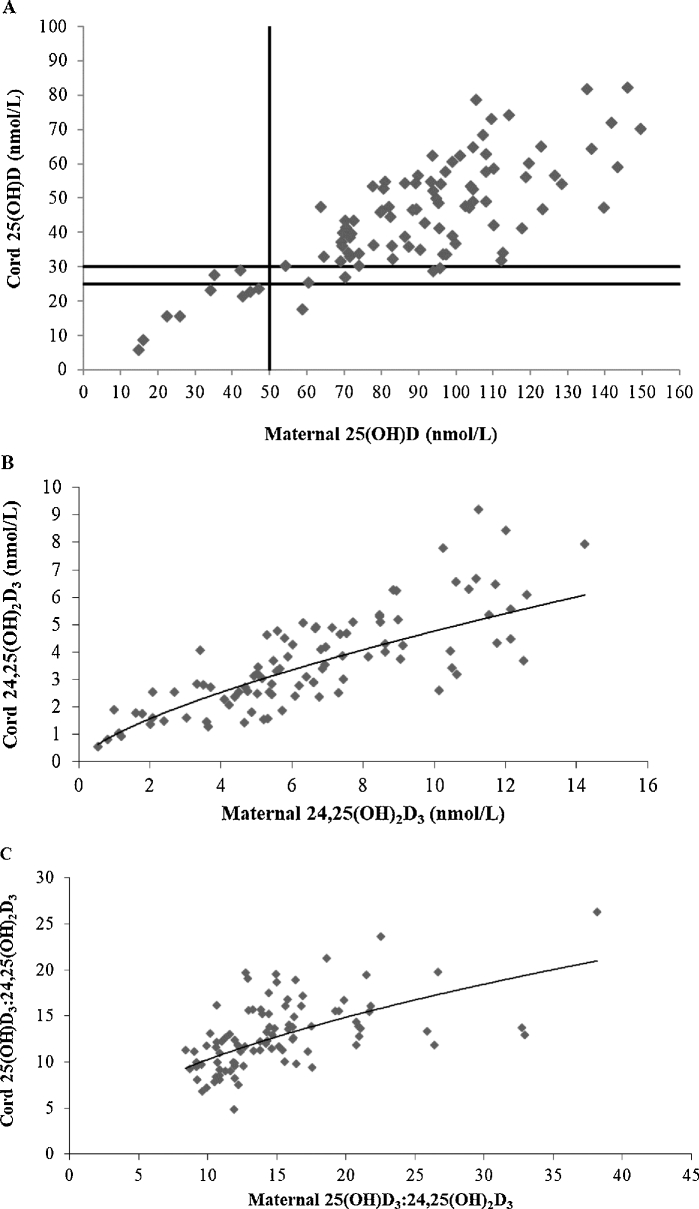
Mean gestational age at delivery was 39 wk (range: 36–42 wk). In total, 97 umbilical cord blood samples were collected, with 96 matching maternal samples. Maternal and cord blood 25(OH)D concentrations were highly correlated (r = 0.789, P < 0.001; n = 96, Figure 4A), with mean cord 25(OH)D concentrations reflecting an average of 52% of maternal values (range: 28–79%). Infants born to mothers in the placebo group had significantly lower 25(OH)D concentrations than those born to mothers in the 20-µg group (mean ± SEM difference: 11.3 ± 3.83 nmol/L; P = 0.011), with no significant difference in mean cord values between group receiving 10 µg and the other 2 treatment arms (Table 3). Thirty-one percent of newborns born to mothers in the placebo group had 25(OH)D concentrations <30 nmol/L compared with 3% of those receiving 20 µg (P = 0.008) (Table 4). Cord 25(OH)D concentrations did not fall below 25 and 30 nmol/L when maternal 25(OH)D concentrations at 36 wk of gestation were ≥44 and 55.4 nmol/L, respectively (Figure 4A). When maternal 25(OH)D concentration was ≥50 nmol/L, cord concentrations were ≥25 nmol/L in all but 1 and ≥30 nmol/L in 95% of newborns.
FIGURE 4.
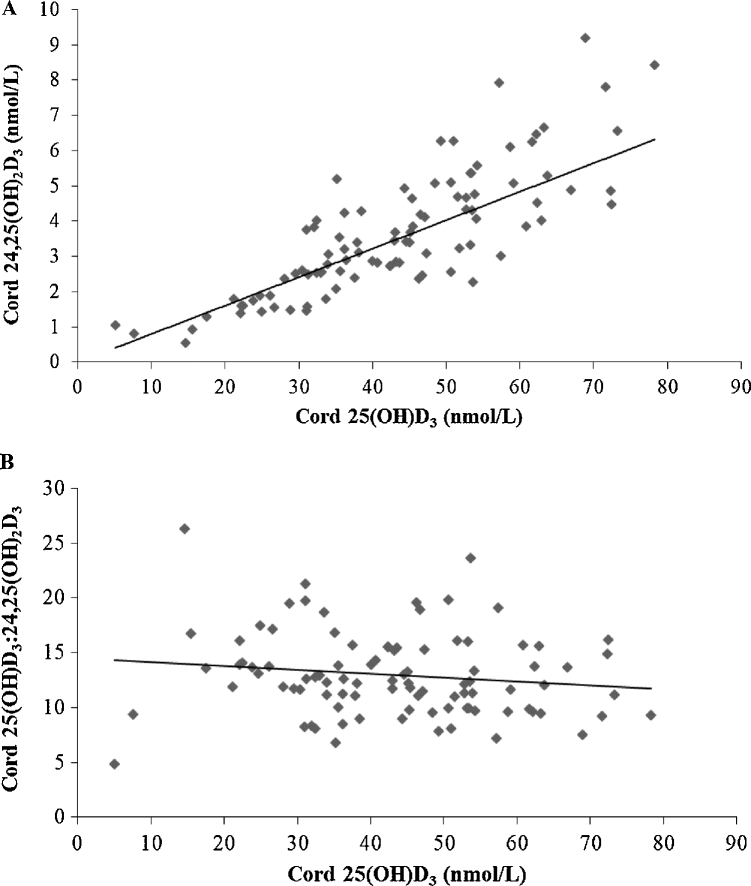
Association of maternal 25(OH)D at 36 wk of gestation and umbilical cord 25(OH)D concentrations (linear trendline: y = 0.4391x + 5.6922; R² = 0.62; n = 96; r = 0.79, P < 0.001) (A). The vertical line represents maternal 25(OH)D at 50 nmol/L, and the horizontal lines represent cord 25(OH)D at 25 and 30 nmol/L. When maternal 25(OH)D concentration was ≥50 nmol/L, cord concentrations were ≥25 nmol/L in all but 1 and ≥30 nmol/L in 95% of newborns. Association of maternal 24,25(OH)2D3 at 36 wk of gestation and umbilical cord 24,25(OH)2D3 (power fit trendline: y = 0.9715x0.6905; R² = 0.68; n = 95; r = 0.80, P < 0.001) (B). Association of the ratio of maternal 25(OH)D3 to 24,25(OH)2D3 at 36 wk of gestation and the ratio of umbilical cord 25(OH)D3 to 24,25(OH)2D3 (power fit trendline: y = 2.9938x0.5346; R² = 0.33; n = 95; r = 0.55, P < 0.001) (C). 25(OH)D, 25-hydroxyvitamin D; 25(OH)D3, 25-hydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3.
Other serum vitamin D metabolites
The effects of the vitamin D3 intervention on maternal and cord serum 3-epi-25(OH)D3 and 24,25(OH)2D3 concentrations at each study visit are shown in Table 3. Serum 3-epi-25(OH)D3 was detectable in all maternal samples, with the exception of 2 mothers at baseline who had a 25(OH)D3 concentration <12 nmol/L. 3-Epi-25(OH)D3 increased in line with treatment dose and showed a dose-dependent difference (P < 0.001) at midpoint, with no difference between the 10- and 20-µg groups at endpoint. The median (IQR) molar ratio of 25(OH)D3 to 3-epi-25(OH)D3 was 30.0 (12.8), 25.4 (9.6), and 21.2 (25.0) at baseline, midpoint, and endpoint, respectively, indicating an increase in the relative expression of 3-epi-25(OH)D3 as pregnancy progressed (P < 0.001), which did not differ by intervention dose (P = 0.93). 3-Epi-25(OH)D3 was detectable in 100% of cord sera; the median (IQR) molar ratio of 25(OH)D3 to 3-epi-25(OH)D3 was lower than in pregnancy at 10.8 (3.1) (P < 0.001), and the correlation between maternal and cord 3-epi-25(OH)D3 was 0.8 (P < 0.001).
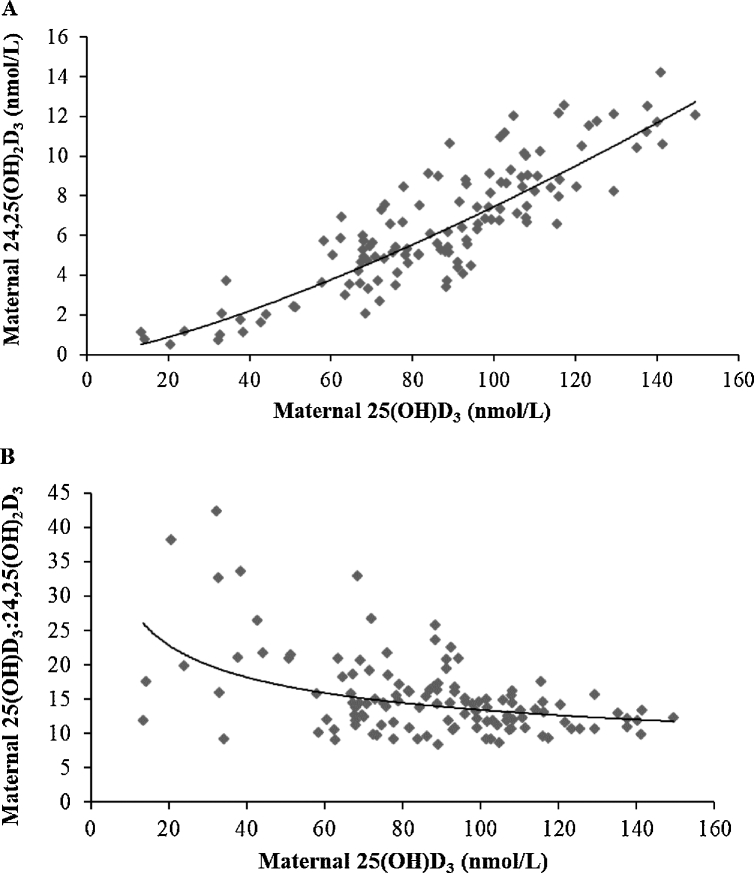
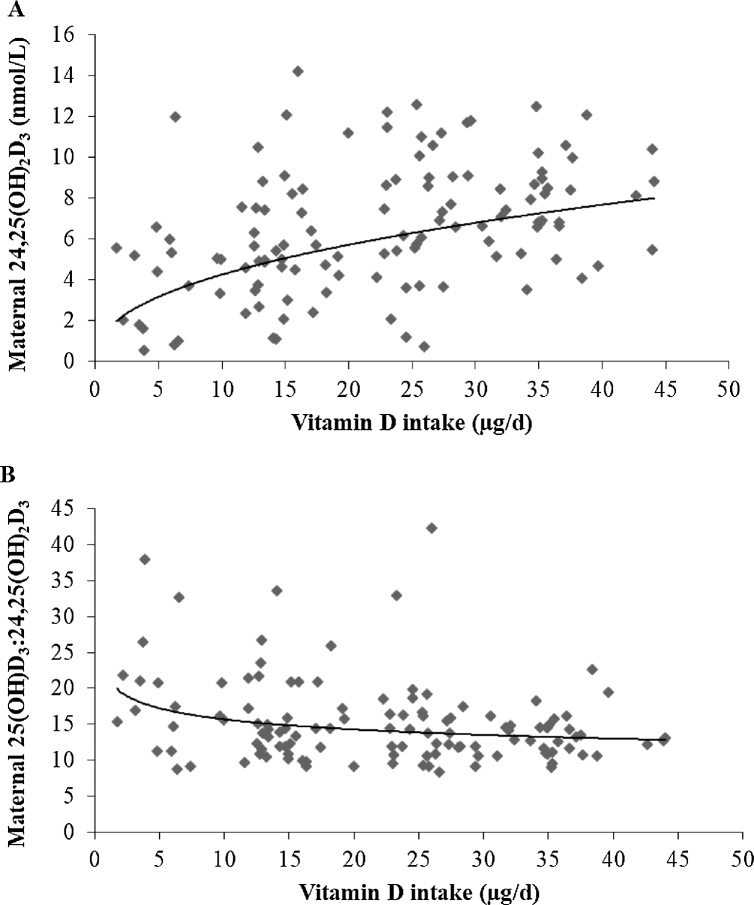
Maternal 24,25(OH)2D3 concentrations increased with increasing 25(OH)D3, as shown in Figure 5A. As 25(OH)D3 increased, the ratio of 25(OH)D3 to 24,25(OH)2D3 decreased. At 36 wk of gestation, the association between the maternal ratio of 25(OH)D3 to 24,25(OH)2D3 and 25(OH)D3 was r = −0.479 (P < 0.001), indicating ratios of ∼20 and ∼18 at serum 25(OH)D3 concentrations of 30 and 50 nmol/L, respectively (Figure 5B). Similarly, associations of cord 24,25(OH)2D3 and 25(OH)D3 and the cord ratio of 25(OH)D3 to 24,25(OH)2D3 and 25(OH)D3 are shown in Figure 6. There was a strong correlation between maternal and cord ratios of 25(OH)D3 to 24,25(OH)2D3 (r = 0.553, P < 0.001; n = 95), with a lower mean ± SD molar ratio of 25(OH)D3 to 24,25(OH)2D3 in cord (12.9 ± 3.8) than in maternal (15.1 ± 5.4) sera sampled in late gestation (P < 0.001) (Figure 4B, C). Finally, associations between 24,25(OH)2D3 and the ratio of 25(OH)D3 to 24,25(OH)2D3 and vitamin D intake are shown in Figure 7.
FIGURE 5.
Association of maternal 25(OH)D3 and 24,25(OH)2D3 concentrations at 36 wk of gestation (power fit trendline: y = 0.0162x1.3309; R² = 0.80; n = 121; r = 0.86, P < 0.001) (A); association of maternal 25(OH)D3 concentrations and the ratio of 25(OH)D3 to 24,25(OH)2D3 at 36 wk of gestation (power fit trendline: y = 61.575x−0.331; R² = 0.20; n = 121; r = –0.48, P < 0.001) (B). 25(OH)D3, 25-hydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3.
FIGURE 6.
Association of umbilical cord 25(OH)D3 and 24,25(OH)2D3 concentrations (power fit trendline: y = 0.0794x1.0036; R² = 0.71; n = 95; r = 0.82, P < 0.001) (A); association of the ratio of umbilical cord 25(OH)D3 concentrations and the ratio of 25(OH)D3 to 24,25(OH)2D3 (linear trendline: y = –0.0353x + 14.473; R² = 0.02; n = 95; r = –0.14, P = 0.173) (B). 25(OH)D3, 25-hydroxycholecalciferol; 24,25(OH)2D3, 24,25-dihydroxycholecalciferol.
FIGURE 7.
Association of maternal vitamin D intake and 24,25(OH)2D3 concentrations (power fit trendline: y = 1.604x0.4247; R² = 0.20; n = 121; r = 0.401, P < 0.001) (A); association of maternal vitamin D intake and the ratio of 25(OH)D3 to 24,25(OH)2D3 at 36 wk of gestation (power fit trendline: y = 21.431x−0.135; R² = 0.08; n = 121; r = −0.26, P = 0.004) (B). 25(OH)D3, 25-hydroxycholecalciferol; 24,25(OH)2D3, 24,25-dihydroxycholecalciferol.
DISCUSSION
Despite growing evidence to support a role for vitamin D in perinatal and infant health, pregnancy-specific 25(OH)D targets are not available thus far, which presents a risk to maternal and fetal health. On the basis of current knowledge, reflected in the reports from international agencies (1–4) and consensus groups (8, 21), the prevention of newborn vitamin D deficiency, in the context of protecting the fetal and newborn skeleton, is an appropriate foundation for specifying meaningful maternal 25(OH)D targets and the vitamin D intakes to meet them. In this dose-response, double-blind, placebo-controlled RCT conducted from 14 wk of gestation to late pregnancy, we estimated that a maternal vitamin D intake of ∼30 µg/d (1200 IU) would maintain 25(OH)D concentrations ≥50 nmol/L in 97.5% of women in late pregnancy, which would prevent 95–99% of umbilical cord sera falling below the minimum 25(OH)D thresholds associated with the prevention of adverse consequences for bone health in newborns, at 25–30 nmol/L.
Until now, the question of protecting newborn infants from very low vitamin D status has not been specifically addressed and maternal vitamin D requirements have been considered in isolation from the fetal and neonatal requirement. Our study shows that the 25(OH)D thresholds of 25 and 30 nmol/L proposed by the United Kingdom (3) and the Institute of Medicine (1) as indicative of an increased risk of vitamin D deficiency, on the basis of bone health outcomes, would be achieved by 97.5% of mothers with a total vitamin D intake of 11 and 14 µg/d, respectively. Because our recruitment strategy covered the calendar year, stratification of the dose-response analysis by winter and summer season of delivery was possible. There was a wide variation in season-specific intake estimates at the lower 25(OH)D thresholds, from 18 to 8 μg/d to meet the 30-nmol/L threshold and from 16 to 6 μg/d to meet the 25-nmol/L threshold, in winter and summer, respectively. This variability highlights not only the seasonal changes in the contribution of UV-B availability to vitamin D status at a northern latitude (24) but also the vulnerability of women with little UV-B exposure and low vitamin D intakes to vitamin D deficiency during winter. Because gestation occurs over a 40-wk period, vitamin D recommendations for pregnancy based on year-round estimates are likely to be most appropriate.
The current intakes required to meet particular 25(OH)D targets in pregnancy extend the findings of our recent individual participant data–level meta-analysis involving 882 healthy children and adults from 7 winter-based RCTs, in which we reported that intakes of 10, 13, and 26 µg vitamin D/d would maintain winter serum 25(OH)D concentrations of >25, 30, and 50 nmol/L, respectively (25). At each 25(OH)D threshold, current vitamin D estimates for pregnant women are similar, albeit slightly higher than the individual participant data analysis.
The current data, which show protection of newborn vitamin D status at minimum thresholds of 25–30 nmol/L with maternal 25(OH)D concentrations ≥50 nmol/L, confirm suggestions from 2 trials in Canada (13) and New Zealand (12), which concurred that although 10 µg vitamin D/d may prevent maternal 25(OH)D falling below 30 nmol/L, a minimum of 25 µg/d might be needed to maintain newborn 25(OH)D at similar concentrations. The UK-based Maternal Vitamin D Osteoporosis Study (MAVIDOS) showed that supplementation with 25 µg/d was sufficient to prevent the seasonal decline in 25(OH)D status among women delivering in winter and maintained 83% at concentrations ≥50 nmol/L (26). Both Hollis et al. (27) and Dawodu et al. (28) reported higher dosing regimens of ≤100 µg/d in 2 distinct population groups in the United States and United Arab Emirates, respectively, noting large disparities in the range of 25(OH)D obtained, whereas the loading dose of 1750 µg followed by 875 µg/wk (125 µg/d) used by Roth et al. (29) ensured that all women in the Bangladesh trial reached 50 nmol/L. It is important to acknowledge that the wide range of 25(OH)D concentrations achieved in intervention trials to date results from variations in study design, latitude, vitamin D status, and nutritional status of the sample populations before intervention. A selection of vitamin D intervention studies in pregnancy is summarized in Supplemental Table 1 to enable a thorough comparison of doses and 25(OH)D concentrations achieved.
Calcium intakes are not presented in most studies of vitamin D in pregnancy, which adds to the variability between trial data. Disruptions in calcium homeostasis during pregnancy arise from inadequate dietary calcium or secondary hyperparathyroidism resulting from low vitamin D status. In the current study, serum iPTH was unchanged from the beginning to the end of the intervention period, irrespective of the treatment dose. Dietary recommendations for vitamin D are established with the assumption that dietary calcium intakes are adequate (1–4). In our sample, 22% had calcium intakes below the current US Estimated Average Requirement of 800 mg (1). Adolescents and ethnic minorities, who may have a higher prevalence of low calcium intakes, should be considered separately, because this may affect the vitamin D–25(OH)D dose-response relation in these groups.
Due to a lack of certainty surrounding the threshold at which the risk of hypercalcemia increases and some evidence for a reverse J-shaped relation between high 25(OH)D concentrations and adverse outcomes, caution has been urged with regard to vitamin D dosing, particularly in pregnancy (1). In the current study, no participant exceeded the Tolerable Upper Intake Level of 100 μg/d (1, 4), which was set on the basis of avoiding the adverse effect of hypercalcemia. Although a small proportion of women (9%; n = 11) achieved a serum 25(OH)D concentration above the cautionary threshold of 125 nmol/L (1), with total intakes of 21–34 μg/d (1), we did not detect any hypercalcemia. Doses of ≤100 µg/d have been shown to be safe in pregnancy, with no indication of hypercalcemia or hypercalciuria even at 25(OH)D concentrations >240 nmol/L (27, 30). Our dosing strategy plus careful assessment of background vitamin D intakes were conservative and are a strength of the study, because this minimized risk while achieving the target 25(OH)D concentrations, albeit in a highly compliant sample.
In agreement with our recent report (9), we confirmed that 3-epi-25(OH)D3 was present in almost all maternal and all infant cord sera, and, to our knowledge, these are the first data showing an increase in the relative expression of 3-epi-25(OH)D3 as pregnancy progresses, independent of supplemental vitamin D intake. We have also presented the first dose-response data of 24,25(OH)2D3 in a pregnancy trial and observed strong correlations between maternal 24,25(OH)2D3 and 25(OH)D3 and between maternal 24,25(OH)2D3 and total vitamin D intake. Compared with nonpregnant adults (31), the ratio of 25(OH)D3 to 24,25(OH)2D3 in pregnant women was slightly higher. At 25(OH)D concentrations of 30 and 50 nmol/L, the ratios of 25(OH)D3 to 24,25(OH)2D3 were ∼20 and 18 in pregnant women compared with ∼18 and 15 in nonpregnant adults, suggesting lower 24-hydroxylase [CYP24A1] activity in late pregnancy. Hanson et al. (32), in an observational study in 131 maternal-cord dyads, reported identical ratios of 25(OH)D3 to 24,25(OH)2D3 in mothers and cords, at 18.5, alongside highly correlated maternal and cord metabolite concentrations (r = 0.78, 0.90, and 0.89 for 25(OH)D3, 24,25(OH)2D3, and 3-epi-25(OH)D3, respectively). With similar cord and higher average postintervention maternal 25(OH)D concentrations, our metabolite correlations were in the same range (r = 0.8), and there was a strong correlation between maternal and cord ratios of 25(OH)D3 to 24,25(OH)2D3, but we observed a lower molar ratio of 25(OH)D3 to 24,25(OH)2D3 in cord blood, at 13, compared with maternal sera, at 15. This may be partly attributable to the wider distribution of our intervention data compared with the observational study.
This trial has a number of strengths, including CDC-accredited analysis of 25(OH)D and metabolites and the use of a method traceable to the NIST higher-order reference measurement procedure. Our vitamin D intake assessment was validated for the measurement of habitual vitamin D intake in females of different ages and has been implemented in almost 1000 trial participants to date (19). Both compliance with the intervention and overall retention rate were high, and we detected no hypercalcemia or intervention-related adverse events. Because our sample was confined to white women, our findings are not generalizable to all women, and we recommend that the protocol be repeated in ethnicity-specific studies, because the food-frequency questionnaire would need to be tailored and the dose-response relation may vary.
In conclusion, we report evidence for an increased dietary requirement for vitamin D among white pregnant women than is currently recommended. We have shown that a total vitamin D intake of 30 µg/d is sufficient to maintain serum 25(OH)D concentrations ≥50 nmol/L in 97.5% of gravidae at high latitude and will prevent 95–99% of umbilical cord 25(OH)D concentrations falling to <25–30 nmol/L, a clinically relevant and achievable target. The question of whether pregnancy-specific thresholds based on perinatal outcomes are required is still outstanding.
Supplementary Material
Online Supporting Material.
Acknowledgements
The authors’ responsibilities were as follows—MEK: designed the study and is the principal investigator and guarantor and has responsibility for the final content; KMO, KH, and AH: conducted clinical follow-up and sample analysis; AH: performed dietary assessment and analysis; GLJH: performed LC-MS/MS analysis; LCK: is a consultant obstetrician at Cork University Maternity Hospital, Ireland, and was responsible for clinical governance; MEK and KDC: are grant holders; KMO, MEK, KDC, and CR: performed the statistical analysis; KMO and MEK: wrote the manuscript; and all authors: read, contributed to, and approved the final manuscript. The authors had no conflicts of interest to report.
Notes
Supported by funding to MEK and KDC from the European Commission under grant agreement 613977 for the ODIN Integrated Project (Food-based solutions for optimal vitamin D nutrition and health throughout the life cycle; http://www.odin-vitd.eu/).
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- IOM
Institute of Medicine
- iPTH
intact parathyroid hormone
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- LoD
limit of detection
- NIST
National Institute of Standards and Technology
- PTH
parathyroid hormone
- RCT
randomized controlled trial
- 25(OH)D
25-hydroxyvitamin D
- 25(OH)D2
25-hydroxyvitamin D2
- 25(OH)D3
25-hydroxyvitamin D3
- 24,25(OH)2D3
24,25-dihydroxyvitamin D3
- 3-epi-25(OH)D3
3-epimer of 25-hydroxyvitamin D3
REFERENCES
- 1. Institute of Medicine Dietary Reference Intakes for calcium and vitamin D. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 2. Nordic Council of Ministers Nordic Nutrition Recommendations 2012: integrating nutrition and physical activity. Copenhagen (Denmark): Nordic Council of Ministers; 2012. [Google Scholar]
- 3. Scientific Advisory Committee on Nutrition Vitamin D and health. London: The Stationary Office; 2016. [Google Scholar]
- 4. European Food Safety Authority Scientific opinion on Dietary Reference Values for vitamin D. Parma (Italy): European Food Safety Authority; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiely ME, Hemmingway A, O’ Callaghan KM. Vitamin D in pregnancy—current perspectives and future directions. Ther Adv Musculoskelet Dis 2017;9:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saraf R, Morton SM, Camargo CA Jr., Grant CC. Global summary of maternal and newborn vitamin D status—a systematic review. Matern Child Nutr 2016;12:647–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brannon PM, Picciano MF. Vitamin D in pregnancy and lactation in humans. Annu Rev Nutr 2011;31:89–115. [DOI] [PubMed] [Google Scholar]
- 8. Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O et al.. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab 2016;101:394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiely ME, O'Donovan SM, Kenny LC, Hourihane JOB, Irvine AD, Murray DM. Vitamin D metabolite concentrations in umbilical cord blood serum and associations with clinical characteristics in a large prospective mother-infant cohort in Ireland. J Steroid Biochem Mol Biol 2016;167:162–8. [DOI] [PubMed] [Google Scholar]
- 10. Hollis BW, Pittard WB. Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab 1984;59:652–7. [DOI] [PubMed] [Google Scholar]
- 11. Við Streym S, Kristine Moller U, Rejnmark L, Heickendorff L, Mosekilde L, Vestergaard P. Maternal and infant vitamin D status during the first 9 months of infant life—a cohort study. Eur J Clin Nutr 2013;67:1022–8. [DOI] [PubMed] [Google Scholar]
- 12. Grant CC, Stewart AW, Scragg R, Milne T, Rowden J, Ekeroma A, Wall C, Mitchell EA, Crengle S, Trenholme A et al.. Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics 2014;133:e143–53. [DOI] [PubMed] [Google Scholar]
- 13. March KM, Chen NN, Karakochuk CD, Shand AW, Innis SM, Von Dadelszen P, Barr SI, Lyon MR, Whiting SJ, Weiler HA et al.. Maternal vitamin D3 supplementation at 50 mug/d protects against low serum 25-hydroxyvitamin D in infants at 8 wk of age: a randomized controlled trial of 3 doses of vitamin D beginning in gestation and continued in lactation. Am J Clin Nutr 2015;102:402–10. [DOI] [PubMed] [Google Scholar]
- 14. Cashman KD, Hill TR, Lucey AJ, Taylor N, Seamans KM, Muldowney S, Fitzgerald AP, Flynn A, Barnes MS, Horigan G et al.. Estimation of the dietary requirement for vitamin D in healthy adults. Am J Clin Nutr 2008;88:1535–42. [DOI] [PubMed] [Google Scholar]
- 15. Cashman KD, Wallace JM, Horigan G, Hill TR, Barnes MS, Lucey AJ, Bonham MP, Taylor N, Duffy EM, Seamans K et al.. Estimation of the dietary requirement for vitamin D in free-living adults ≥ 64 y of age. Am J Clin Nutr 2009;89:1366–74. [DOI] [PubMed] [Google Scholar]
- 16. Mortensen C, Damsgaard CT, Hauger H, Ritz C, Lanham-New SA, Smith TJ, Hennessy Á, Dowling K, Cashman KD, Kiely M et al.. Estimation of the dietary requirement for vitamin D in white children aged 4–8 y: a randomized, controlled, dose-response trial. Am J Clin Nutr 2016;104:1310–7. [DOI] [PubMed] [Google Scholar]
- 17. Smith TJ, Tripkovic L, Damsgaard CT, Mølgaard C, Ritz C, Wilson-Barnes SL, Dowling KG, Hennessy Á, Cashman KD, Kiely M et al.. Estimation of the dietary requirement for vitamin D in adolescents aged 14–18 y: a dose-response, double-blind, randomized placebo-controlled trial. Am J Clin Nutr 2016;104:1301–9. [DOI] [PubMed] [Google Scholar]
- 18. Black LJ, Walton J, Flynn A, Cashman KD, Kiely M. Small increments in vitamin D intake by Irish adults over a decade show that strategic initiatives to fortify the food supply are needed. J Nutr 2015;145:969–76. [DOI] [PubMed] [Google Scholar]
- 19. Kiely M, Collins A, Lucey AJ, Andersen R, Cashman KD, Hennessy A. Development, validation and implementation of a quantitative food frequency questionnaire to assess habitual vitamin D intake. J Hum Nutr Diet 2016;29:495–504. [DOI] [PubMed] [Google Scholar]
- 20. Kiely ME, Zhang JY, Kinsella M, Khashan AS, Kenny LC. Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am J Clin Nutr 2016;104:354–61. [DOI] [PubMed] [Google Scholar]
- 21. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 22. Goltzman D. Approach to hypercalcemia [updated 2016 Aug 8]. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000[cited 2017 Jul 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279129/. [Google Scholar]
- 23. R Development Core Team. A language and environment for statistical computing [Internet]. [cited 2017 Apr30]. Available from: http://www.r-proj.org. [Google Scholar]
- 24. O'Neill CM, Kazantzidis A, Ryan MJ, Barber N, Sempos CT, Durazo-Arvizu RA, Jorde R, Grimnes G, Eiriksdottir G, Gudnason V et al.. Seasonal changes in vitamin D-effective UVB availability in Europe and associations with population serum 25-hydroxyvitamin D. Nutrients 2016;8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cashman KD, Ritz C, Kiely ME; ODIN Collaborators. Improved dietary guidelines for vitamin D: application of individual participant data (IPD)-level meta-regression analyses. Nutrients 2017;9:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, Fraser R, Gandhi SV, Carr A, D'Angelo S et al.. Maternal Gestational Vitamin D Supplementation and Offspring Bone Health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol 2016;4:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011;26:2341–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, Hollis BW. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J Clin Endocrinol Metab 2013;98:2337–46. [DOI] [PubMed] [Google Scholar]
- 29. Roth DE, Al Mahmud A, Raqib R, Akhtar E, Black RE, Baqui AH. Pharmacokinetics of high-dose weekly oral vitamin D3 supplementation during the third trimester of pregnancy in Dhaka, Bangladesh. Nutrients 2013;5:788–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wagner CL, McNeil R, Hamilton SA, Winkler J, Rodriguez Cook C, Warner G, Bivens B, Davis DJ, Smith PG, Murphy M et al.. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol 2013;208:137.e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cashman KD, Hayes A, Galvin K, Merkel J, Jones G, Kaufmann M, Hoofnagle AN, Carter GD, Durazo-Arvizu RA, Sempos CT. Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: a double-edged sword? Clin Chem 2015;61:636–45. [DOI] [PubMed] [Google Scholar]
- 32. Hanson C, Anderson-Berry A, Lyden E, Kaufmann M, Wu A, Elliott E, Lee JI, Jones G. Dynamics of vitamin D metabolism in maternal-fetal dyads. J Pediatr Gastroenterol Nutr. 2016;62:486–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supporting Material.