Abstract
Background
The field of neuromodulation is continually evolving, with the past decade showing significant advancement in the therapeutic efficacy of neuromodulation procedures. The continued evolution of neuromodulation technology brings with it the promise of addressing the needs of both patients and physicians, as current technology improves and clinical applications expand.
Design
This review highlights the current state of the art of neuromodulation for treating chronic pain, describes key areas of development including stimulation patterns and neural targets, expanding indications and applications, feedback-controlled systems, noninvasive approaches, and biomarkers for neuromodulation and technology miniaturization.
Results and Conclusions
The field of neuromodulation is undergoing a renaissance of technology development with potential for profoundly improving the care of chronic pain patients. New and emerging targets like the dorsal root ganglion, as well as high-frequency and patterned stimulation methodologies such as burst stimulation, are paving the way for better clinical outcomes. As we look forward to the future, neural sensing, novel target-specific stimulation patterns, and approaches combining neuromodulation therapies are likely to significantly impact how neuromodulation is used. Moreover, select biomarkers may influence and guide the use of neuromodulation and help objectively demonstrate efficacy and outcomes.
Keywords: Technology, Stimulation Patterns, Closed-Loop, Miniaturization, Noninvasive, Optogenetics
Eras in Spinal Cord Stimulation
The year 2017 marked the 50th anniversary since the first reported use of dorsal column electrical stimulation to treat pain [1,2]. Originally based on the proposed concept of the gate control theory of pain [3], spinal cord stimulation has advanced quite rapidly in recent years [4–7]. Mechanisms of action beyond gate control theory, such as the direct modulation of wide–dynamic range neurons, the modulation of activity in cortical and subcortical brain regions, and activation of descending inhibitory pathways, have been proposed [8].
The first spinal cord stimulator (SCS) was made commercially available in 1968. This technology used a radiofrequency receiver coupled with dorsal column–stimulating electrodes and an external power supply [9]. Surgical complications and practical considerations gave way to the first fully implantable spinal cord stimulation system in 1981 [10]. The first rechargeable implantable pulse generator (IPG) was introduced to the market in 2004 [11]. Innovation in the field after Food and Drug Administration commercial approval focused on decreasing the size of the IPG and improving the stability and maneuverability of leads [12,13]. The evolution of leads, including multicolumn and multicontact leads, led to a significant decrease in surgical revision rates [14].
An initial trend showed a tendency to favor rechargeable systems due to smaller IPG size and longer lifespan [15]. The rationale behind this was mainly focused on cost-effectiveness and decreased potential for complications, with fewer IPG replacements needed over time [16]. However, recent studies have consistently shown higher rates of explanation for a rechargeable system when compared with a primary cell IPG [17,18]. Also, it is not entirely clear that smaller rechargeable devices are what patients prefer, as opposed to having to endure the burden of recharging the device, although there may be a size-to-charging burden ratio that would favor a smaller profile [19]. Research on efficacy, at times lacking both in quantity and quality, has historically focused on a limited number of clinical applications such as postlaminectomy syndrome and complex regional pain syndrome [20,21]. In the past several years, high-quality level 1 evidence has been generated in the field of neuromodulation for pain treatment [4,5,7]. With emerging technology involving novel targets and pulse trains, a trend toward paresthesia-free stimulation modalities has been observed. In its present form, neuromodulation is used as a generally safe and reversible therapy that is used to treat chronic pain, decrease the need for systemic medications, including opioids, and more efficiently utilize health care resources [13,22,23].
Device Programming and Electrical Fields
Traditional SCS programming has involved selecting various combinations of lead cathodes (-) and anodes (+) in order to shape an electrical field within the dorsal columns with the goal of creating overlapping paresthesias in painful areas. Manipulation of stimulation parameters (apart from contact selection), including frequency, amplitude, and pulse width, can impact the strength, intensity, and perception of associated paresthesias from the device. Creative arrays or configurations make it possible to preferentially target specific fibers within the dorsal column [24]. However, conventional tonic paresthesia-based stimulation has limitations. Postural changes affecting the distance of the spinal cord from the leads can result in changes in the perceived strength of the stimulation [25]. Additionally, paresthesias may be felt outside of the painful region and can be uncomfortable for the patient [26]. For example, a patient requiring paresthesia coverage solely for low back pain may experience intense paresthesia in the legs in an effort to capture a therapeutic effect on the low back. Moreover, given the anatomical distribution of the axons in the dorsal columns at the midthoracic area, it can be challenging to effectively modulate fiber physiology innervating various axial structures with lower tonic stimulation frequencies [27]; it should be acknowledged, however, that recent improvements in technology make capturing the low back much more feasible than in past decades. Although SCS has been effectively utilized with traditional paresthesia-based programming, it is clearly not without limitations. Despite these shortcomings, traditional stimulation has yielded relief for many patients with moderate efficacy; even given the demonstrated effectiveness of some novel pulse trains, some patients prefer paresthetic tonic stimulation [28,29].
A New Era—SCS Patterns
With the increasing study of spinal cord stimulation physiology, the concept of altering stimulation parameters to subsequently modulate the pathophysiology in chronic pain has become the focus of therapy development [8,30,31]. For decades, it has been known that the nervous system uses “codes” to encapsulate and transmit information. Patterns of stimulation can evoke very different responses in multiple regions of the central nervous system, and thus provide an opportunity to better address the complex neurophysiology in chronic pain. Moreover, clinical research on the contemporary use of newer stimulation patterns has focused on generating high-level evidence to support therapeutic outcomes.
kHz Frequency SCS
In contrast to traditional tonic stimulation, which typically is programmed between 40 and 100 Hz, higher frequencies of SCS (HF-SCS) have also been tested up to 10 kHz [7,32,33]. One of the results of HF-SCS stimulation is the absence of sustained action potential generation in the dorsal columns, despite current amplitudes being at or above threshold [34]. This, combined with lower thresholds and pulse amplitudes, effectively reduces the likelihood of a patient feeling paresthesia [31]. In contrast to traditional SCS lead placement, which is based on intraoperative paresthesia mapping, leads for HF-SCS are anatomically placed to span the T9-10 interspace [35]. Multiple published studies, including prospective, open-label studies and RCTs, have shown that HF stimulation can provide significant pain relief for the treatment of back and leg pain [7,32,33,35,36]. The SENZA RCT study, utilizing a stimulation frequency of 10 kHz, found superior pain relief when compared with tonic, low-frequency (LF) stimulation. The control group utilizing tonic-LF stimulation demonstrated typical outcomes for this therapy modality in low back pain patients with a component of leg pain. Patient-reported pain relief in this study was maintained out to two years [37].
The relation between stimulation frequency and patient-reported outcomes was recently studied by Al-Kaisy and colleagues [32]. This sham-controlled RCT found that higher frequencies (5,882 kHz) showed a larger decrease in patient-reported pain when compared with lower kHz frequencies (1,200 and 3,030 Hz). Interestingly, the sham control group demonstrated comparable relief to the lower kHz HF-SCS groups. Another sham-controlled study using 5,000-kHz stimulation in tonic-LF responders showed no difference vs sham in responder rate using the Patient Global Impression of Change and visual analog scale (VAS) score reductions [33].
As with all sham-controlled trials in neuromodulation, there are inherent difficulties in running these types of studies. They often lead to more questions about results and study designs, as well as the utility of the treatment under investigation. In 2013, a European study reported significant reductions in VAS using HF-SCS in 74% of patients at six months [35]. Al-Kaisy et al. reported results from 198 patients with chronic intractable back and leg pain enrolled in a prospective, randomized study, comparing HF SCS with tonic stimulation. Patients experienced significant reductions in VAS for not only leg pain but also traditionally difficult-to-treat back pain that were durable out to 24 months [36]. Reductions in scores (indicating improvement) on the Oswestry Disability Index (ODI) and reduced opioid usage were also reported. Investigators reported a responder rate of >80% for both back and leg pain in the HF-SCS group, compared with responder rates in the traditional SCS group of 43.8% and 55.5% for back and leg pain, respectively. The superiority of 10-kHz stimulation over traditional SCS was seen through 12 months [7].
HF stimulation has also been reported to improve axial low back pain in patients without prior back surgery [38]. In contrast to these results, two other published studies, including an RCT, did not support these results [39,40]. Both the Russo et al. and De Andres et al. studies found lower effectiveness (<50% pain relief) in the HF-SCS groups when compared with the aforementioned pivotal RCT. Recently, Thomson and colleagues published results from the PROCO study [41], demonstrating equivalent pain relief with SCS at 1-, 4-, 7-, and 10-kHz SCS. These latter findings suggest that lower frequencies within the kHz range might be able to produce similar clinical results at 10 kHz with considerable energy savings.
The mechanisms of action of HF-SCS have not been clearly defined. Several “working hypotheses” have been proposed via animal and computational models that include wide–dynamic range (WDR) neuron modulation, dorsal horn fiber recruitment, and local depolarization blockade [34,42,43]. However, some modeling studies have suggested that local blockade does not occur at clinical amplitudes [44]. Human studies have also shown a lack of efferent motor activity with kilohertz frequency SCS, consistent with the abbreviated DC fiber activation patterns seen in animals [34,45]. Thus, although the clinical findings are promising, the underlying mechanism(s) of action using 10-kHz stimulation remain to be elucidated. A unique observational case series examining intraoperative electromyography (EMG) with HF-SCS stimulation showed no observable EMG responses with 10,000-Hz stimulation. This contrasted with other observed waveforms, including traditional tonic and burst SCS, which displayed various signal-generating thresholds, distal to proximal muscle activation, and a phenomenon of hyperexcitability, suggesting different underlying mechanisms of action [45].
Burst SCS
Burst is another novel stimulation pattern that utilizes a specific pulse train [46–48]. De Ridder et al. first described burst stimulation (five 1-ms pulses with a 1-ms interpulse interval at 500 Hz, applied at a 40-Hz frequency with passive regeneration of the charge balance at the end of the pulse burst) using cortical stimulation for the treatment of tinnitus [49,50]. Neuronal bursting is a well-studied phenomenon that helps code presynaptic information and responses that have a multitude of postsynaptic effects including temporal summation, short- and long-term potentiation, and information filtering. Classically defined burst effects have been described from the spinal cord up to thalamocortical relay centers [51–53]. Multiple elements of the stimulation pattern are central components of the burst signal. These include basic parameters such as pulse width and amplitude, but also aspects of the pulse burst itself such as the interburst timing and burst signature. All of these components affect the dimension and dynamics of the electrical field and impact the function of the target tissues being directly influenced by the field. Moreover, these effects will also indirectly impact structures downstream from the targeted neural tissue. The importance of the specific burst signature and parameters has been studied using computational models, as well as in animals and humans [30,45,54,55]. What may seem like subtle differences in signature (e.g., passive vs active recharge) can have a cascading effect on primary and secondary neural elements. Moreover, specific timing elements are also important, which is not surprising as neuronal channel kinetics and synapses are highly sensitive to spike timing. Thus, slight differences in evoked ensemble responses in the DC from variations in a burst pattern can have meaningful neurophysiologic ramifications.
Burst SCS has been clinically evaluated in multiple prospective studies, including sham-controlled and comparative RCTs [4,56–60]. The SUNBURST study demonstrated the statistical superiority of pain ratings with burst stimulation relative to pain ratings with tonic stimulation, and the majority of study subjects preferred burst stimulation over tonic due to better pain relief and lack of paresthesias [4]. Clinically, burst stimulation has been studied in the treatment of diabetic neuropathy, complex regional pain syndrome, and failed back surgery syndrome, with the predominant research being conducted on low back and leg pain. Burst SCS has also been shown to recover analgesia in patients who have failed, or shown accommodation to, tonic SCS [61]. Both electroencephalography (EEG) and positron emission tomography–computed tomography imaging studies have shown that burst SCS demonstrates differences in cortical and subcortical cerebral activation patterns, suggesting that this waveform may have the ability to directly and differentially modulate the brain regions involved in the emotional and affective aspects of the pain experience [46,62]. Mechanistically, burst SCS has been shown to involve different neurochemical pathways in the dorsal horn of the spinal cord and also have a pronounced effect on WDR neurons within the spinothalamic tract [63,64]. Similarly, burst SCS does not seem to alter firing rates of neurons in the dorsal column nuclei, suggesting that there is an enhanced ability to modulate pain processing in the dorsal horn without activating the neuronal fibers, which would create the sensation of paresthesia [64]. This difference in the ability to selectively modulate different somatic nerve processing is a possible mechanism for the resulting paresthesia-free analgesia.
High–Pulse Width SCS Programming
Another stimulation method that is being explored comprises a higher-charge delivery (or density) over a given period of time. Typically, this approach has been programmed at subparesthetic levels. Increasing pulse width and frequency increases the charge delivered per second [65]. It is hypothesized to provide an increased ability to modulate neural structures without activating fibers to the extent that it generates paresthesias. A critical element of this paradigm would be the frequency component; otherwise the increased pulse width would simply follow a strength–duration curve [66,67]. Provenzano et al. reported a retrospective review on patients using high-density stimulation that showed no significant difference when compared with conventional paresthesia-based stimulation, thus bringing into question the clinical utility of the technique [68]. However, alterations in programmed electrical parameters continue to be an area of exploration [69].
Random/Stochastic Stimulation Patterns
Several disorders of the central nervous system (CNS) are characterized by abnormal neuronal synchrony. Random stimulation was developed to selectively counteract abnormal neuronal synchrony by eliciting desynchronization [70–73]. Random stimulation patterns could evoke antikindling or correct alterations of abnormal synaptic connectivity and synchrony. Zeitler et al. demonstrated that a random slowly varying sequence (SVS) stimulation pattern significantly improved the antikindling effect of random stimulation and tremor suppression [74]. Further support for this concept has been published by Manos et al., demonstrating that there is an optimal frequency and intensity of random acoustic patterns in the treatment of tinnitus. These findings suggest that random stimulation pattern usage may be co-dependent upon other stimulation parameters [75]. Adamchic et al. also demonstrated that the type of stimulation is important for acute and long-term effects in acoustic neuromodulation for durable improvement in tinnitus symptomatology [76]. Patient-reported tinnitus improvement was correlated with EEG recordings, demonstrating an objective, electrophysiologic biomarker for improvement. The application of random patterns is not limited to the auditory cortex, but also subcortical networks involved in movement such as the basal ganglia [70,77].
Stochastic approaches have also been suggested for peripheral afferent sensory fibers in the treatment of tremor [78]. In a slightly different paradigm, Dideriksen et al. demonstrated the suppression of pathologic tremor through electrical stimulation of afferent fibers. In this case, either surface or intramuscular EMG recordings were used to detect tremor and time stimulation. Different stimulation timing (in-phase or out-of-phase with tremor) and parameters were trialed, and the data suggest the need for patient-specific stimulation protocols for tremor suppression [79]. This concept is not foreign to neuromodulation for the treatment of pain, as physicians must personalize treatment by choosing the right device and stimulation settings for each patient. Although randomized stimulation patterns have shown promise for longer residual effects on neurologic function [77], it is unknown how different systems and underlying conditions may respond to this type of stimulation.
Therapy Tolerance and Habituation
Loss of therapeutic effect is the most common reason for SCS therapy failure over time [80]. This has been demonstrated in both the United States and Europe through the examination of explant data [17,18,81]. Although the loss of efficacy could be due to a variety of reasons, habituation or tolerance to neurostimulation has been one of the greatest challenges to long-term efficacy in neuromodulation for chronic pain [26]. As new technologies are being developed to improve efficacy and therapy response rates, physiologic adaptation to therapeutic stimulation remains a challenge for the entire field. Future devices may utilize multiple stimulation strategies or a more “random” pattern of stimulation (see Random/StochasticStimulation Patterns) in order to help mitigate CNS adaptation over time [82]. Other sources of inadequate analgesia include disease alteration and progression and alterations in patient psychological status. Habituation of neurostimulation for chronic pain is poorly understood, but there are common physiologic examples of the human body adapting to a new “set point” in a disease-based maladaptive homeostatic change. In hypertension, cerebral autonomic regulation adjusts a set point over time as part of the pathophysiologic state [83]. Of course, tolerance and adaptations to centrally acting pharmaceuticals are also well-studied phenomena, with a myriad of underlying mechanisms, including but not limited to neurochemical receptor endocytosis, activation of intracellular second messenger systems, and transcriptional control of cellular function [84]. It is reasonable to assume that a similar principle may govern the body’s adaptation to neurostimulation over time. In fact, as an external stimulus acting through multiple electrophysiological and neurochemical mechanisms, it would be surprising to not see some form of adaptation with neural stimulation. However, because different stimulation patterns and signatures likely evoke different cellular mechanisms of analgesia, it is possible that patients who fail one form of stimulation may achieve analgesia with another. This phenomenon is supported in clinical reports of patients who have failed tonic stimulation and subsequently experienced improvements when switched to burst SCS [61,85]. We have yet to see how newer waveforms and pulse trains will perform over time as a salvage solution to short- and long-term SCS failures. Future research is warranted to investigate this possibility.
Feedback Control of Neurostimulation
Virtually all neural systems in the body utilize feedback from the environment or the body habitus to maintain homeostasis. Feedback in the skeletal motor, autonomic, and sensory systems provides a much finer fidelity and accuracy of controlling physiologic function. A feedback-based system, or closed-loop control, is based upon the classic homeostatic mechanism whereby a biosignal is detected and measured, and the resulting signal is then used to adjust physiologic function. For example, blood pressure is partly controlled via baroreceptors in the carotid sinus. The baroreceptors detect a change in pressure, then rapidly adjust arterial pressure through autonomic control of heart rate, stroke volume, and total peripheral resistance. There are multiple examples of medical device-based approaches that utilize biomarker feedback, including deep brain stimulation for Parkinson’s disease [86–88] and epilepsy [89,90], cochlear stimulation for hearing, and spinal cord stimulation for chronic pain [6,91,92].
Conventional neuromodulation is based on an open-loop paradigm, in which stimulation parameters are statically set by a clinician and changes in stimulation intensity (pulse amplitude) are made by the patient. This strategy poses several challenges with SCS, especially as the most common anatomic location for stimulation is the midthoracic spine, corresponding to back and leg pain targets [93]. There are a number reasons why conventional SCS has traditionally had limited success in treating axial back pain. The low back is not particularly well represented in the spinal cord as compared with other areas of the body, such as the hands or feet, because the back is not used for fine touch or sensation. There are comparatively fewer sensory fibers corresponding to the low back, and these fibers tend to be deeper and also more lateral in the spinal cord [93]. The dorsal cerebrospinal fluid (dCSF) layer is thickest around the midthoracic spine, such that stimulation in this area requires more energy to cross that fluid barrier; in fact, only 10–20% of the current from spinal cord–stimulating electrodes provides stimulation of the dorsal columns. To complicate matters further, the dCSF thickness changes positionally (i.e., thicker when patients are prone and thinner when patients are supine) [94]. This creates a clinical and technical dilemma—different amounts of energy are needed to pass current across the fluid layer and can lead to supratherapeutic or subtherapeutic stimulation. There are a number of physiologic parameters that further influence the energy requirements and SCS experience, including dynamic factors such as heartbeat and respiration that continuously vary. On one hand, this can lead to unwanted or unpleasant stimulation, and on the other hand, it may lead to understimulation and lack of efficacy.
The first commercially available closed-loop SCS system involved detection of body position, rather than a neural signal [92]. This system used a gravitational signal to help control intensity of neurostimulation, which fluctuates with bodily position (supine vs upright). The system uses a three-axis accelerometer, which is calibrated at a baseline visit to recognize different body positions and programmed with patient-specific stimulation parameters for each position to avoid over- or understimulation. A prospective, multicenter, open-label randomized crossover study of 79 patients comparing automatic position-adaptive stimulation with conventional manual programming found a 41% reduction in number of daily patient-directed programming button pushes (18.2 vs 30.7 per day) [92]. Not surprisingly, using body position as a feedback loop for neurophysiologic parameters does not account for all variables involved in maintaining stimulation in the therapeutic window but served as an incremental improvement over conventional stimulation.
Toward developing a feedback-controlled system in SCS, Parker et al. reported measurements of evoked compound action potentials (ECAPs) from the spinal cords of sheep as well as patients undergoing stimulation for pain relief [91,95]. This represents an objective electrophysiologic biomarker in response to neurostimulation of the dorsal columns. Furthermore, it has been demonstrated that the amplitude of sheep A-beta fiber potentials during SCS exhibits dependence on electrode location, highlighting the potential for optimization of A-beta recruitment, pain relief, and power consumption in SCS devices [95]. From these observations, the idea of a closed-loop system with feedback to adjust “dose” of stimulation input to achieve constant neural recruitment and to avoid both overstimulation and inadequate pain relief was initially tested. In a recently published study, Russo and colleagues demonstrated the clinical effectiveness of a closed-loop SCS system utilizing low-frequency tonic stimulation and an improvement in neuromodulating a well-established target, the dorsal columns [6]. Pain was significantly reduced in the legs and, to a greater degree, in the back. This suggests that there is good recruitment of back-specific dorsal column fibers, although the exact mechanisms are yet to be elucidated. Furthermore, a significant advantage of a closed-loop feedback-based SCS system may be its prevention or limitation of stimulation amplitudes reaching the high levels required to recruit A-beta nociceptors. To extend the preliminary findings above, a multicenter, double-blind, randomized controlled trial in the United States has just been completed, and results are forthcoming (ClinicalTrials.gov ID #NCT02924129). A salient factor in closed-loop SCS may be that its sensing is dependent on activation of fibers that generate perceptible paresthesias; the applicability of closed-loop feedback technology to paresthesia-free SCS waveforms, then, remains to be seen.
System Miniaturization
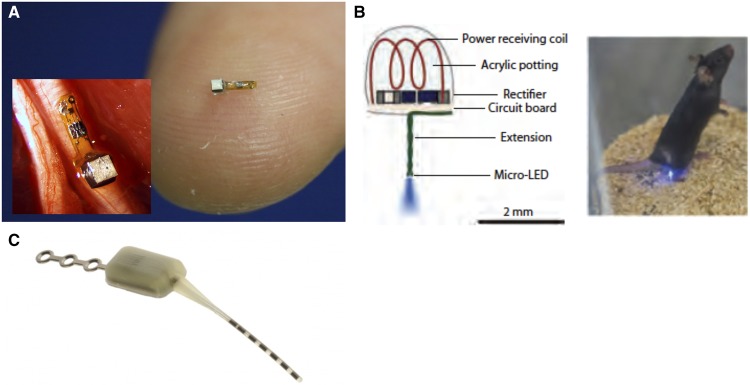
Modern neurostimulators consist of leads and an implanted pulse generator, which have evolved from radiofrequency-powered devices to internal battery systems that are both primary cell and rechargeable. Some contemporary technology is now shifting back to externally powered devices for peripheral nerve stimulation and for other neurostimulation applications or patients in whom internal batteries would be anatomically challenging or unwanted or perceived as unsightly by patients. Implantable nerve stimulators have been the first-generation miniature wireless stimulators to reach patients for peripheral applications such as chronic hemiplegic shoulder pain and central applications such as sphenopalatine ganglion stimulation for cluster headache (Figure 1) [99–103]. These externally powered devices have been developed with a wide range of delivery options, from percutaneous ultrasound-guided options to open implants. In peripheral stimulation, there are significant advantages to externally powered devices, and the versatility afforded by externalizing certain components has significant implications in miniaturization. The data supporting the clinical efficacy of these devices are beyond the scope of this paper, but the concepts of versatility and upgradeability embodied by their existence are paramount to the advanced concepts described below, including injectable neurostimulators, microelectrode arrays, and optogenetics.
Figure 1.
Forward looking approaches to neuromodulation. A) A “neural dust” miniaturization of systems that can be externally powered for various recording and, potentially, stimulating applications [96]. B) An optogenetic approach to modulate specific cells in the central nervous system to induce analgesia. Image used with permission from Montgomery et al. [97]. C) All-in-one small stimulator designed specifically for sphenopalatine ganglion stimulation [98].
Despite their relatively compact sizes, the above neurostimulation systems are still orders of magnitude larger than cells. There have been significant advances in materials that allow for size scales of devices to approach the dimensions of cells, which may improve the selectivity of stimulation [104]. Microelectrodes with arrays of varying length have been created to target different fascicles, perhaps ideal for peripheral nerve stimulation applications. Current microelectrode arrays (0.5–5 mm in length and 15–50 µm wide) are capable of neural recording and stimulation, but they have been observed in vivo to trigger a limiting foreign body response in brain tissue that is similar to neurotrauma and neurodegeneration [104]. This response can be mitigated by decreasing the size and enhancing the flexibility of the implant; however, this presents challenges for device fabrication, device delivery, ability to handle appropriate stimulation current levels, and long-term stability. Furthermore, there are challenges with accurate positioning and tracking of the devices over time in addition to programming.
Conceptually, an injectable neurostimulator requires most, if not all, of the components of its full-sized progenitors to be contained in a package small enough to be delivered percutaneously. This includes two or more electrodes, a telemetry module, sensors, a power supply, a microprocessor, a data transceiver, and power management/recharging capabilities [105]. Recently, a working ultramicro neural sensor was developed [106,107] with a microelectronics platform that utilizes ultrasound wavelengths to both power and communicate with the device (Figure 1). The entire footprint of the unit was designed for nerve recordings to fit onto a single nerve. The so-called “neural dust” embodies the rapidly decreasing scale of neurorecording and neurostimulation platforms. As these become nanoscale, the potential for injectable distribution becomes more of a reality, as does the discrete targeting of specific cell groups.
Optogenetics
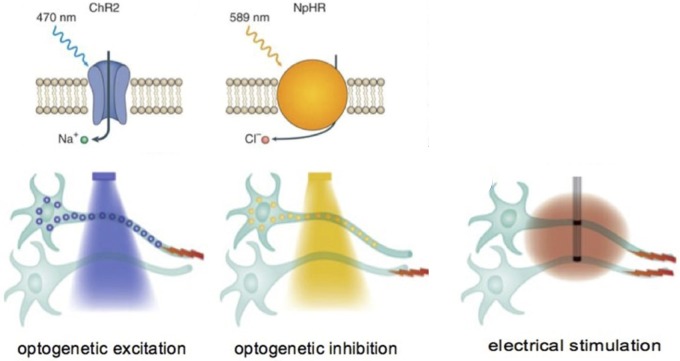
All the neuromodulation techniques that have been described thus far involve the use of electricity for neural manipulation. In 2005, Ed Boyden and Karl Deisseroth reported a technique by which neurons not previously sensitive to light could be genetically manipulated to express light-sensitive ion channels and, thus, become depolarized by photons [108]. The field of optogenetics has exploded with the utility of this technique, and it is currently being utilized for both basic discovery within systems neuroscience and as a part of therapy development efforts. Conceptually, virally mediated transfection of genes coding for light-sensitive proteins (opsins, similar to those in the eye) conjugated to specific ion channels is achieved in neurons via promotor-driven expression. Thus, very targeted expression can be achieved within a specific neuronal phenotype, even if those cells are interspersed heterogeneously within a group of other cells. The capacity to specifically generate very targeted expression results in the ability to use light to modulate activity in a very select population of neurons (Figure 2). This approach has been utilized to demonstrate the feasibility of selectively manipulating neurons controlling nociception and pain within the peripheral and central nervous system [97,109–113]. Pain can be both induced or inhibited based upon the cells that are targeted and the specific channel-rhodopsins that are expressed. Although it is still early, the approach of using light to specifically inhibit or activate neurons provides a powerful tool to control neural function and induce analgesia. Beyond the design and construction of viral vectors that can selectively induce the expression of light-sensitive channels and convey optical sensitivity to neurons, there is also a significant effort to miniaturize light sources on the device side of this therapeutic approach in order to make the application anatomically flexible and battery-free. Overall, this technique still has developmental hurdles to overcome, and it is yet to be seen if the ability to target specific neurons in discrete anatomical regions will translate into effective pain management in humans.
Figure 2.
Optogenetic approaches to modulating neural function. Light-sensitive ion channels (channelrhodopsins) can be expressed in neurons of a specific phenotype that, in turn, allow cell membranes to become depolarized (excited) or hyperpolarized (inhibited) by applying light to the cell. Specific wavelengths of light (blue, yellow, etc.) can be used to excite or inhibit cells based upon the type of channelrhodopsin expressed in the neuron [175].
Neural Targets
Dorsal Root Ganglion
The dorsal root ganglion has emerged as an important neuromodulation target [5,114–116]. The combination of special anatomy and physiology of the primary sensory neuron at the level of the ganglion demonstrates the multiple aspects to stimulation that need to be tailored and accounted for when designing a stimulation therapy. There are several papers that address the clinical and mechanistic aspects of DRG stimulation in this special edition. As such, this neural target will not be fully addressed here.
Vagal Nerve Stimulation—Immune Function and Headache
Cytokine production by the immune system contributes to both health and disease. The nervous system, via an inflammatory reflex of the vagus nerve, can inhibit cytokine release and thereby prevent tissue injury and death. The efferent neural signaling pathway is termed the cholinergic anti-inflammatory pathway. Stimulation of the vagus nerve prevents the damaging effects of cytokine release in animal models of sepsis, endotoxemia, ischemia/reperfusion injury, hemorrhagic shock, arthritis, and other inflammatory syndromes [117].
Using this information, and the fact that it previously was unknown whether directly stimulating the inflammatory reflex in humans inhibits tumor necrosis factor (TNF)–α production, Koopman et al. demonstrated that peripheral blood production of TNF-α, interleukin (IL)-1β, and IL-6 was inhibited in epilepsy patients with an implantable left cervical vagus nerve–stimulating device [118]. Thereafter, it was shown that vagus nerve stimulation in rheumatoid arthritis patients significantly inhibited TNF-α production for up to three months and disease severity improved significantly, establishing that the inflammatory reflex modulates TNF-α production and modulating the vagus nerve reduces inflammation in humans. These findings suggest the possibility of using mechanism-based neurostimulation devices in the treatment of rheumatoid arthritis (RA) and potentially other autoimmune and auto-inflammatory diseases such as inflammatory bowel disease. In fact, there is currently a US pilot trial (Safety and Efficacy of Vagus Nerve Stimulator in Patients with Rheumatoid Arthritis) evaluating vagus nerve stimulation (VNS) in the treatment of RA (ClinicalTrials.gov NCT03437473). Mechanistically, VNS activates the cholinergic anti-inflammatory pathway that ameliorates cytokine-mediated disease and is dependent on an intact splenic nerve [119]. Therefore, the splenic nerve may be another target for neuromodulation in the treatment of autoimmune inflammatory diseases underlying chronic pain conditions.
The vagus nerve is also a target for the treatment of headache disorders. The idea of stimulating the vagus nerve to treat headache first came from the unanticipated observation that patients being treated with a vagus nerve stimulator for epilepsy had reduced severity and incidence of their coexistent migraines while using the stimulator [120]. Furthermore, noninvasive vagus nerve stimulation (nVNS) at the carotid artery can be used for prophylactic and abortive treatment of cluster and migraine headaches with reduced cortical spreading depression and demonstrated progression in efficacy over time [121].
Speculations on the pathophysiological mechanisms that mediate the effect of nVNS in migraine mainly originate from animal studies. The main mechanistic hypothesis is that the afferent anatomical connection between the vagus nerve and the trigeminal nucleus caudalis [122] and the nociceptive inputs from the dura mater terminating in the nucleus tractus solitarius [123] are interrupted. Neurophysiological evidence showing a reduction in glutamate levels and neuronal firing in the spinal trigeminal nucleus secondary to continuous vagus stimulation [124] could justify vagal ascending antinociceptive effects. This seems to be further confirmed by studies in rats, which have demonstrated that vagus nerve stimulation can reduce pain [125] and allodynia [126] in the trigeminal area.
SPG Stimulation
The sphenopalatine ganglion (SPG) is another neuromodulatory target for the treatment of headaches. The SPG has been targeted with injections of local anesthetic [127] and has been subject to neurolysis/radiofrequency ablation [128] to abort and reduce migraine and cluster headaches. Recent sham-controlled RCTs have shown significant ability to provide pain relief and reduce the number of headache days when utilizing neurostimulation of the SPG [100,129]. These results have been maintained for up to two years [129,130]. Tepper et al. performed a pilot study to evaluate the effectiveness of SPG stimulation in the treatment of migraine headaches. Migraine attacks were induced in 11 patients with refractory headache. They were then treated with electrical stimulation through a needle introduced into the sphenopalatine fossa via an infrazygomatic approach. The treatment was effective in less than half the patients, with two reporting a completely aborted attack and three with significant pain relief. These results might have been caused by suboptimal lead placement, inadequate lead design for the pterygopalatine fossa, and inclusion of medication overuse in a subgroup of patients [131]. Given the role that the SPG plays in autonomic regulation within the head (parasympathetic outflow to the periorbital, nasal, meningeal, and cerebral blood vessels) [132–134], the SPG is an intriguing target that may benefit from advances in neuromodulation, including application of different waveforms.
Trigeminal System Stimulation
It is reasonable to assume that neuromodulation for the treatment of trigeminal neuralgia and intractable facial pain might provide good relief, as neuroablative procedures of the trigeminal nucleus caudalis and spinal trigeminal tract have resulted in pain relief [135–139]. Despite this assumption, the body of evidence in the literature for Trigemino-cervical complex (TCC) region stimulation for facial pain is relatively small [136].
Upper cervical SCS is one approach that has been attempted to modulate the TCC. A retrospective case series found that 75% of implanted patients continued to derive benefit for up to 10 years after implantation [140]. Velasquez et al. conducted a retrospective, consecutive, single-center series of 12 patients with trigeminal neuropathy treated with upper cervical spinal cord stimulation [141]. The average coverage in the pain zone was 72%, and the median baseline, trial, and postoperative numeric rating scale (NRS) scores were 7, 3, and 3, respectively. The mean reduction in the numeric rating scale from baseline was 4 points, resulting in an average 57.1% pain reduction. The long-term treatment failure rate was 25%. These results are encouraging and point to another approach to modulating the trigeminal system in the treatment of cranial pain conditions. Recently, noninvasive methods have been utilized to stimulate the supraorbital nerve, a branch of V1, that eventually feeds into the trigeminal system. In an open label study, Chou et al. demonstrated the safety and efficacy of an external device to stimulate the supraorbital nerve to treat acute migraine headaches [142]. Similar findings were also observed in a blinded, pilot RCT [143]. Subsequent research has demonstrated alterations in brain metabolism, with this type of noninvasive neuromodulation providing further support of the physiologic mechanisms underlying the approach [144].
Neuromodulation for Joint Pain and Preventing Articular Disease Progression
Pulsed radiofrequency (PRF) to address pain has been applied to multiple anatomies, including intraspinal and extraspinal articular structures, the shoulder, and the knee [145–147]. Several studies have shown that PRF of the suprascapular nerve may relieve shoulder pain and can improve mobility of the shoulder joint [102,146,148]. Intra-articular PRF has resulted in significant and durable pain relief in a majority of patients with joint pain [147]. Although the exact mechanism is unclear, it may be related to the exposure of immune cells to low-strength RF fields, stimulating an anti-inflammatory effect.
With the knowledge of pulsed radiofrequency of neural structures (including those innervating joints), it seems reasonable to apply current and future implantable continuous neuromodulation devices to treat joint pain with intra-articular implantation. Apart from providing palliative treatment effects such as pain relief, it is also possible that stimulation may help treat underlying inflammatory mechanisms in joints [149]. With miniaturization or the advent of thin film-like devices, intra-articular implantation may be possible. Joints are generally anatomies with a high degree of movement. Thus it is important to consider whether the joint itself, or the nerve branches innervating the structure, should be the location of the neuromodulation.
Occipital Nerve Stimulation
The greater and lesser occipital nerves send afferent projections through the C2-3 dorsal roots, which then make synaptic connections in the upper cervical spinal cord, including cells in the TCC [150,151]. Stimulation of this afferent pathway, in turn, may lead to an ability to modulate TCC function and downstream trigeminal dysfunction in head and facial pain. Occipital nerve stimulation (ONS) has been extensively used to treat various headaches, including cervicogenic, migraine, cluster, and occipital neuralgia, since the initial report by Weiner and Reed [152]. A recent systematic review with meta-analysis showed that there is favorable but low-quality evidence to support use of tonic ONS for decreasing the intensity and frequency of headache pain associated with intractable primary headaches [153]. Another systematic review makes a level 3 recommendation for the use of tonic ONS as a treatment option for medically refractory occipital nerve pain [154]. Puledda and Goadsby reviewed the current evidence and status for neuromodulation devices for the acute and preventive treatment of migraine. They concluded that more studies with appropriate blinding strategies are needed to confirm the results of new treatments [155]. Multiple attempts have been made to demonstrate the efficacy of occipital nerve stimulation through sham-controlled studies, yet these have been met with failure to meet the primary end point [156,157]. Likely reasons for these failures include poor patient selection and inclusion, primary outcome measures, method of sham control, and variability in the outcomes that may have been better controlled. Interestingly, one study examined the effects of a burst stimulation pattern during ONS in healthy volunteers and found that there were different patterns of cerebral activation, suggesting that, like SCS, burst may provide a differential clinical outcome [158]. In general, however, further work needs to be completed on novel stimulation paradigms in ONS.
Application of Novel Waveforms to Peripheral Targets
As previously discussed, newer stimulation patterns of the dorsal column have led to improved pain relief and function over classic lower-frequency tonic stimulation. Physiologically, a particular structure may require a specific stimulation pattern and stimulation timing that are different from another target. For example, as opposed to spinal cord stimulation, VNS may be used for short sessions periodically throughout the day (although the optimal treatment regime has not yet been identified). Thus, because of the neural target and the desired effect, a specific, and minimal, stimulation paradigm can be employed. Applying ideal target-specific stimulation patterns may improve outcomes in areas where current neuromodulation has shown less than impressive results. Another example of this comes from Kent et al., who applied computational modeling to elucidate the mechanisms of action of DRG stimulation for low- and high-frequency waveforms. Afferent signals measured in nociceptive neurons could be suppressed during delivery of 40-Hz LFS, as well as 1-kHz and 10-kHz HFS, suggesting that high-frequency DRG stimulation may not provide any more relief in chronic pain conditions [159]. On the other hand, high-frequency stimulation of peripheral nerves causes a complete conduction block [160] and relieves postamputation pain [161]. Applying different waveforms or stimulation patterns may improve outcomes in painful conditions underserved by present neurostimulation targeting. Revisiting these targets with new patterns may improve outcomes. Thus, as new neural targets are identified, it will be incumbent to understand not only the best physical embodiment of the neuromodulation device for the intended target, but also the most effective stimulation pattern and the timing of the pattern to other temporally important physiological biomarkers (e.g., hormonal fluctuations, immunological rhythms, patterned neural events).
Biomarkers and Neuromodulation for Pain
Chronic neuropathic pain treatment has had limited success, due in part to a relatively poor understanding of the mechanisms underlying the development and maintenance of the condition. Additionally, the effectiveness of neuropathic pain management regimens and procedures can be difficult to determine, due to the subjective nature of pain perception and a lack of standardized assessment tools. Study in the fields of neuroimaging, genomics, and molecular biology is helping identify unique biomarkers and signatures that could lead to improved objectivity and standardization in measuring nociceptive correlates. Surprisingly, relatively little research has been conducted on the impact of SCS on various objective biomarkers in the form of easily obtainable and measurable physiologic responses. Most recently, there have been studies beginning to examine the impact of SCS on various protein levels in cerebrospinal fluid [162–165], circulating and regional inflammatory intermediates [166,167], and gene transcripts [168,169]. Regional changes in cerebral activation patterns have also been examined [46,170–172], as well as electrophysiological analyses in humans [45,91,95,173,174]. Some of this work has been used to examine neuromonitoring for safety during implantation. Beyond the large body of mechanistic work that is outside the scope of this review, it is unclear how biomarkers might play a role in spinal cord therapy stimulation. Some possibilities include markers to help determine stimulation candidates or predict therapy success, provide an objective measure of a physiologic component or proxy of pain or an indicator to help determine optimal therapy delivery. As this aspect of neuromodulation continues to develop, it will be interesting to note how these types of biomarkers may play an increasing role in the therapy.
Conclusions
Technological advancements in microelectronics, feedback-based system design, biomimetic stimulation patterns, and the interplay between genomics and device-based therapies are transforming how neuromodulation is being conceived. Miniaturization and noninvasive therapies are providing the templates for increasingly smaller devices or noninvasive therapies that do not require surgery. As our understanding of how the interplay between neural targets and stimulation patterns continues to develop, the landscape of the therapeutic arsenal that physicians have at their disposal will most certainly continue to grow.
As technology and approaches to neuromodulation advance, it is clear that systems need to be more flexible in their ability to produce multiple programmable outputs in order to not only tailor treatment to specific diagnoses and conditions, but also individual patients. The ability to shift stimulation output from one pattern to the next could also potentially help avoid therapeutic fatigue, which is the primary reason for device explants. To most effectively do this, we echo comments from De Ridder in a call for software-driven systems, as opposed to hardware being the limiting factor [82]. Having hardware platforms capable of delivering multiple evidence-based therapeutic modalities will be a benefit to patients. In concert with this, various biomarkers may become an increasingly important component of the treatment algorithm to help understand how to individualize neuromodulation therapy. This includes understanding why therapies are failing over time. As such, the need for continued high-quality clinical evidence will also be required to establish the right foundation for this growing field. This will also include, as appropriate and possible, the use of sham controls in clinical studies. Lastly, we continue the call to better understand the physiologic underpinnings of these therapies through basic research to help understand how to continually improve upon existing technologies and drive discovery in areas not yet explored. To this end, it remains to be seen if neuromodulation therapies can positively prevent or impact underlying disease mechanisms and move from a palliative treatment paradigm to a disease prevention model.
Acknowledgment
The authors wish to thank Jeff Kramer, PhD, an independent scientific consultant, for his intellectual contributions and assistance in preparing the manuscript.
Funding sources: This manuscript was prepared with financial support from Abbott.
Conflicts of interest: Dr. Fishman is a consultant for Abbott, Biotronik, Cornerloc, Medtronic, and Nevro; funded research for Abbott, Biotronik, Medtronic, Nuvectra, Stimgenics; majority stock holder of Celéri Health, Inc. and a minority stock holder of Thermaquil. Dr. Antony is a consultant and principle investigator for Abbott; is an advisory board member for Boston Scientific. Dr. Esposito is a consultant for Abbott and Flowonix. Dr. Deer is a minority stock holder in Axonics, Bioness, Nalu, Saluda, Spinethera, Vertos, Vertiflex, Cornerloc; is a consultant for Abbott, Saluda, Flowonix, Vertos, and Vertiflex; has received funded research from Abbott, Mainstay, Vertiflex, Vertos, Saluda. Dr. Levy is a minority stock holder in Bioness, Nalu, Saluda, Vertos; a consultant for Abbott, Mainstay, Nalu, Nuvectra, Saluda, Vertos.
Supplement sponsorship: This article appears as part of the supplement “Neuromodulation of the Spine and Nervous System” sponsored by Abbott.
References
- 1. Shealy CN, Mortimer JT, Reswick JB.. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth Analg 1967;464:489–91. [PubMed] [Google Scholar]
- 2. Shealy CN, Taslitz N, Mortimer JT, et al. Electrical inhibition of pain: Experimental evaluation. Anesth Analg 1967;463:299–305. [PubMed] [Google Scholar]
- 3. Melzack R, Wall PD.. Pain mechanisms: A new theory. Science 1965;1503699:971–9. [DOI] [PubMed] [Google Scholar]
- 4. Deer T, Slavin KV, Amirdelfan K, et al. Success Using Neuromodulation with BURST (SUNBURST) study: Results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation 2018;211:56–66. [DOI] [PubMed] [Google Scholar]
- 5. Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: A randomized comparative trial. Pain 2017;1584:669–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russo M, Cousins MJ, Brooker C, et al. Effective relief of pain and associated symptoms with closed-loop spinal cord stimulation system: Preliminary results of the Avalon Study. Neuromodulation 2018;211:38–47. [DOI] [PubMed] [Google Scholar]
- 7. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: The SENZA-RCT randomized controlled trial. Anesthesiology 2015;1234:851–60. [DOI] [PubMed] [Google Scholar]
- 8. Linderoth B, Foreman RD.. Conventional and novel spinal stimulation algorithms: Hypothetical mechanisms of action and comments on outcomes. Neuromodulation 2017;206:525–33. [DOI] [PubMed] [Google Scholar]
- 9. Kumar K, Rizvi S.. Historical and present state of neuromodulation in chronic pain. Curr Pain Headache Rep 2014;181:387.. [DOI] [PubMed] [Google Scholar]
- 10. Waltz JM. Computerized percutaneous multi-level spinal cord stimulation in motor disorders. Appl Neurophysiol 1982;45(1–2):73–92. [DOI] [PubMed] [Google Scholar]
- 11. Oakley JC, Krames ES, Prager JP, et al. A new spinal cord stimulation system effectively relieves chronic, intractable pain: A multicenter prospective clinical study. Neuromodulation 2007;103:262–78. [DOI] [PubMed] [Google Scholar]
- 12. Deer TR, Krames E, Mekhail N, et al. The appropriate use of neurostimulation: New and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014;176:599–615; discussion 615. [DOI] [PubMed] [Google Scholar]
- 13. Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014;176:515–50; discussion 550. [DOI] [PubMed] [Google Scholar]
- 14. Kumar K, Hunter G, Demeria D.. Spinal cord stimulation in treatment of chronic benign pain: Challenges in treatment planning and present status, a 22-year experience. Neurosurgery 2006;583:481–96; discussion 481–96. [DOI] [PubMed] [Google Scholar]
- 15. Hornberger J, Kumar K, Verhulst E, et al. Rechargeable spinal cord stimulation versus non-rechargeable system for patients with failed back surgery syndrome: A cost-consequences analysis. Clin J Pain 2008;243:244–52. [DOI] [PubMed] [Google Scholar]
- 16. Kumar K, Rizvi S.. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med 2013;1411:1631–49. [DOI] [PubMed] [Google Scholar]
- 17. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation 2017;206:543–52. [DOI] [PubMed] [Google Scholar]
- 18. Van Buyten J-P, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: Results of an International Retrospective Chart Review study. Neuromodulation 2017;207:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lam CK, Rosenow JM.. Patient perspectives on the efficacy and ergonomics of rechargeable spinal cord stimulators. Neuromodulation 2010;133:218–23. [DOI] [PubMed] [Google Scholar]
- 20. Kumar K, North R, Taylor R, et al. Spinal cord stimulation vs. conventional medical management: A prospective, randomized, controlled, multicenter study of patients with failed back surgery syndrome (PROCESS study). Neuromodulation 2005;84:213–8. [DOI] [PubMed] [Google Scholar]
- 21. North RB, Kidd DH, Farrokhi F, et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: A randomized, controlled trial. Neurosurgery 2005;561:98–106; discussion 106–7. [DOI] [PubMed] [Google Scholar]
- 22. Song JJ, Popescu A, Bell RL.. Present and potential use of spinal cord stimulation to control chronic pain. Pain Physician 2014;173:235–46. [PubMed] [Google Scholar]
- 23. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007;132(1–2):179–88. [DOI] [PubMed] [Google Scholar]
- 24. Holsheimer J, Wesselink WA.. Effect of anode-cathode configuration on paresthesia coverage in spinal cord stimulation. Neurosurgery 1997;413:654–9; discussion 659–60. [DOI] [PubMed] [Google Scholar]
- 25. Holsheimer J, Barolat G, Struijk JJ, et al. Significance of the spinal cord position in spinal cord stimulation. Acta Neurochir Suppl 1995;64:119–24. [DOI] [PubMed] [Google Scholar]
- 26. Kumar K, Caraway DL, Rizvi S, et al. Current challenges in spinal cord stimulation. Neuromodulation 2014;17(Suppl 1): 22–35. [DOI] [PubMed] [Google Scholar]
- 27. Holsheimer J, Buitenweg JR.. Review: Bioelectrical mechanisms in spinal cord stimulation. Neuromodulation 2015;183:161–70; discussion 170. [DOI] [PubMed] [Google Scholar]
- 28. Taylor RS, Desai MJ, Rigoard P, et al. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: A systematic review and meta-regression analysis. Pain Pract 2014;146:489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kemler MA, Barendse GA, van Kleef M, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med 2000;3439:618–24. [DOI] [PubMed] [Google Scholar]
- 30. Chakravarthy K, Kent AR, Raza A, Xing F, Kinfe TM.. Burst spinal cord stimulation: Review of preclinical studies and comments on clinical outcomes. Neuromodulation 2018;215:431–9. [DOI] [PubMed] [Google Scholar]
- 31. Chakravarthy K, Richter H, Christo PJ, et al. Spinal cord stimulation for treating chronic pain: Reviewing preclinical and clinical data on paresthesia-free high-frequency therapy. Neuromodulation 2018;211:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Kaisy A, Palmisani S, Pang D, et al. Prospective, randomized, sham-control, double blind, crossover trial of subthreshold spinal cord stimulation at various kilohertz frequencies in subjects suffering from failed back surgery syndrome (SCS Frequency Study). Neuromodulation 2018;215:457–65. [DOI] [PubMed] [Google Scholar]
- 33. Perruchoud C, Eldabe S, Batterham AM, et al. Analgesic efficacy of high-frequency spinal cord stimulation: A randomized double-blind placebo-controlled study. Neuromodulation 2013;164:363–9; discussion 369. [DOI] [PubMed] [Google Scholar]
- 34. Crosby ND, Janik JJ, Grill WM.. Modulation of activity and conduction in single dorsal column axons by kilohertz-frequency spinal cord stimulation. J Neurophysiol 2017;1171:136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Buyten J-P, Al-Kaisy A, Smet I, et al. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: Results of a prospective multicenter European clinical study. Neuromodulation 2013;161:59–65; discussion 65–6. [DOI] [PubMed] [Google Scholar]
- 36. Al-Kaisy A, Van Buyten J-P, Smet I, et al. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med 2014;153:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery 2016;795:667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Al-Kaisy A, Palmisani S, Smith TE, et al. 10 kHz high-frequency spinal cord stimulation for chronic axial low back pain in patients with no history of spinal surgery: A preliminary, prospective, open label and proof-of-concept study. Neuromodulation 2017;201:63–70. [DOI] [PubMed] [Google Scholar]
- 39. Russo M, Verrills P, Mitchell B, Salmon J, Barnard A, Santarelli D.. High frequency spinal cord stimulation at 10 kHz for the treatment of chronic pain: 6-month Australian clinical experience. Pain Physician 2016;194:267–80. [PubMed] [Google Scholar]
- 40. De Andres J, Monsalve-Dolz V, Fabregat-Cid G, et al. Prospective, randomized blind effect-on-outcome study of conventional vs high-frequency spinal cord stimulation in patients with pain and disability due to failed back surgery syndrome. Pain Med 2017;1812:2401–21. [DOI] [PubMed] [Google Scholar]
- 41. Thomson SJ, Tavakkolizadeh M, Love-Jones S, et al. Effects of rate on analgesia in kilohertz frequency spinal cord stimulation: Results of the PROCO randomized controlled trial. Neuromodulation 2018;211:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arle JE, Mei L, Carlson KW, et al. High-frequency stimulation of dorsal column axons: Potential underlying mechanism of paresthesia-free neuropathic pain relief. Neuromodulation 2016;194:385–97. [DOI] [PubMed] [Google Scholar]
- 43. Li S, Farber JP, Linderoth B, Chen J, Foreman RD.. Spinal cord stimulation with “conventional clinical” and higher frequencies on activity and responses of spinal neurons to noxious stimuli: An animal study. Neuromodulation 2018;215:440–7. [DOI] [PubMed] [Google Scholar]
- 44. Lempka SF, McIntyre CC, Kilgore KL, et al. Computational analysis of kilohertz frequency spinal cord stimulation for chronic pain management. Anesthesiology 2015;1226:1362–76. [DOI] [PubMed] [Google Scholar]
- 45. Falowski SM. An observational case series of spinal cord stimulation waveforms visualized on intraoperative neuromonitoring. Neuromodulation 2019;222:219–28. [DOI] [PubMed] [Google Scholar]
- 46. De Ridder D, Vanneste S.. Burst and tonic spinal cord stimulation: Different and common brain mechanisms. Neuromodulation 2016;191:47–59. [DOI] [PubMed] [Google Scholar]
- 47. De Ridder D, Vanneste S, Plazier M, et al. Mimicking the brain: Evaluation of St Jude Medical's Prodigy Chronic Pain system with burst technology. Expert Rev Med Devices 2015;122:143–50. [DOI] [PubMed] [Google Scholar]
- 48. De Ridder D, Vanneste S, Plazier M, et al. Burst spinal cord stimulation: Toward paresthesia-free pain suppression. Neurosurgery 2010;665:986–90. [DOI] [PubMed] [Google Scholar]
- 49. De Ridder D, Vanneste S, van der Loo E, et al. Burst stimulation of the auditory cortex: A new form of neurostimulation for noise-like tinnitus suppression. J Neurosurg 2010;1126:1289–94. [DOI] [PubMed] [Google Scholar]
- 50. De Ridder D, et al. Theta, alpha and beta burst transcranial magnetic stimulation: Brain modulation in tinnitus. Int J Med Sci 2007;45:237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Remy S, Spruston N.. Dendritic spikes induce single-burst long-term potentiation. Proc Natl Acad Sci U S A 2007;10443:17192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Swadlow HA, Gusev AG.. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat Neurosci 2001;44:402–8. [DOI] [PubMed] [Google Scholar]
- 53. Sherman SM. Tonic and burst firing: Dual modes of thalamocortical relay. Trends Neurosci 2001;242:122–6. [DOI] [PubMed] [Google Scholar]
- 54. Kent AR, Venkatesan L, Kramer J, Min X. Dorsal column fiber response is dependent on the temporal pattern of burst spinal cord stimulation. Paper presented at: 20th Annual North American Neuromodulation Society Meeting (NANS); Jan 19–22, 2017; Las Vegas, NV.
- 55. Crosby ND, Goodman Keiser MD, Smith JR, et al. Stimulation parameters define the effectiveness of burst spinal cord stimulation in a rat model of neuropathic pain. Neuromodulation 2015;181:1–8; discussion 8. [DOI] [PubMed] [Google Scholar]
- 56. Schu S, Slotty PJ, Bara G, et al. A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation 2014;175:443–50. [DOI] [PubMed] [Google Scholar]
- 57. De Ridder D, Plazier M, Kamerling N, et al. Burst spinal cord stimulation for limb and back pain. World Neurosurg 2013;805:642–9 e1. [DOI] [PubMed] [Google Scholar]
- 58. de Vos CC, Bom MJ, Vanneste S, et al. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation 2014;172:152–9. [DOI] [PubMed] [Google Scholar]
- 59. Muhammad S, Roeske S, Chaudhry SR, et al. Burst or high-frequency (10 kHz) spinal cord stimulation in failed back surgery syndrome patients with predominant back pain: One year comparative data. Neuromodulation 2017;207:661–7. [DOI] [PubMed] [Google Scholar]
- 60. Kinfe TM, Pintea B, Link C, et al. High frequency (10 kHz) or burst spinal cord stimulation in failed back surgery syndrome patients with predominant back pain: Preliminary data from a prospective observational study. Neuromodulation 2016;193:268–75. [DOI] [PubMed] [Google Scholar]
- 61. De Ridder D, Lenders MWPM, De Vos CC, et al. A 2-center comparative study on tonic versus burst spinal cord stimulation: Amount of responders and amount of pain suppression . Clin J Pain 2015;315:433–7. [DOI] [PubMed] [Google Scholar]
- 62. Yearwood T, Falowski S, Venkatesan L, Vanneste S. Comparison of neural activity in chronic pain patients during tonic and burst spinal cord stimulation: A SUNBURST substudy. Paper presented at: NANS-NIC; June 25–29, 2016; Baltimore, MD.
- 63. Crosby ND, Weisshaar CL, Smith JR, et al. Burst and tonic spinal cord stimulation differentially activate GABAergic mechanisms to attenuate pain in a rat model of cervical radiculopathy. IEEE Trans Biomed Eng 2015;626:1604–13. [DOI] [PubMed] [Google Scholar]
- 64. Tang R, Martinez M, Goodman-Keiser M, et al. Comparison of burst and tonic spinal cord stimulation on spinal neural processing in an animal model. Neuromodulation 2014;172:143–51. [DOI] [PubMed] [Google Scholar]
- 65. Sweet J, Badjatiya A, Tan D, et al. Paresthesia-free high-density spinal cord stimulation for postlaminectomy syndrome in a prescreened population: A prospective case series. Neuromodulation 2016;193:260–7. [DOI] [PubMed] [Google Scholar]
- 66. Lee D, Hershey B, Bradley K, Yearwood T.. Predicted effects of pulse width programming in spinal cord stimulation: A mathematical modeling study. Med Biol Eng Comput 2011;497:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yearwood TL, Hershey B, Bradley K, et al. Pulse width programming in spinal cord stimulation: A clinical study. Pain Physician 2010;134:321–35. [PubMed] [Google Scholar]
- 68. Provenzano DA, Rebman J, Kuhel C, et al. The efficacy of high-density spinal cord stimulation among trial, implant, and conversion patients: A retrospective case series. Neuromodulation 2017;207:654–60. [DOI] [PubMed] [Google Scholar]
- 69. Miller JP, Eldabe S, Buchser E, et al. Parameters of spinal cord stimulation and their role in electrical charge delivery: A review. Neuromodulation 2016;194:373–84. [DOI] [PubMed] [Google Scholar]
- 70. Tass PA, Qin L, Hauptmann C, et al. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann Neurol 2012;725:816–20. [DOI] [PubMed] [Google Scholar]
- 71. Tass PA, Silchenko AN, Hauptmann C, Barnikol UB, Speckmann EJ.. Long-lasting desynchronization in rat hippocampal slice induced by coordinated reset stimulation. Phys Rev E Stat Nonlin Soft Matter Phys 2009;80(1 Pt 1):011902.. [DOI] [PubMed] [Google Scholar]
- 72. Tass PA, Hauptmann C.. Therapeutic modulation of synaptic connectivity with desynchronizing brain stimulation. Int J Psychophysiol 2007;641:53–61. [DOI] [PubMed] [Google Scholar]
- 73. Tass PA. A model of desynchronizing deep brain stimulation with a demand-controlled coordinated reset of neural subpopulations. Biol Cybern 2003;892:81–8. [DOI] [PubMed] [Google Scholar]
- 74. Zeitler M, Tass PA.. Augmented brain function by coordinated reset stimulation with slowly varying sequences. Front Syst Neurosci 2015;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Manos T, Zeitler M, Tass PA.. How stimulation frequency and intensity impact on the long-lasting effects of coordinated reset stimulation. PLoS Comput Biol 2018;145:e1006113.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Adamchic I, Toth T, Hauptmann C, et al. Acute effects and after-effects of acoustic coordinated reset neuromodulation in patients with chronic subjective tinnitus. Neuroimage Clin 2017;15:541–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang J, Nebeck S, Muralidharan A, et al. Coordinated reset deep brain stimulation of subthalamic nucleus produces long-lasting, dose-dependent motor improvements in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine non-human primate model of Parkinsonism. Brain Stimul 2016;94:609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tass PA. Vibrotactile coordinated reset stimulation for the treatment of neurological diseases: Concepts and device specifications. Cureus 2017;98:e1535.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dideriksen JL, Laine CM, Dosen S, et al. Electrical stimulation of afferent pathways for the suppression of pathological tremor. Front Neurosci 2017;11:178.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hayek SM, Veizi E, Hanes M.. Treatment-limiting complications of percutaneous spinal cord stimulator implants: A review of eight years of experience from an academic center database. Neuromodulation 2015;187:603–8; discussion 608–9. [DOI] [PubMed] [Google Scholar]
- 81. Dupre DA, Tomycz N, Whiting D, Oh M.. Spinal cord stimulator explantation: Motives for removal of surgically placed paddle systems. Pain Pract 2018;184:500–4. [DOI] [PubMed] [Google Scholar]
- 82. De Ridder D, Vanneste S.. Visions on the future of medical devices in spinal cord stimulation: What medical device is needed? Expert Rev Med Devices 2016;133:233–42. [DOI] [PubMed] [Google Scholar]
- 83. Rowell LB. Human Cardiovascular Control. Oxford, New York: Oxford University Press; 1993. [Google Scholar]
- 84. Argoff CE, ed. Pain Management Secrets. 3rd ed Philadelphia, PA: Mosby Elsevier; 2009. [Google Scholar]
- 85. Pope JE, Falowski S, Deer TR.. Advanced waveforms and frequency with spinal cord stimulation: Burst and high-frequency energy delivery. Expert Rev Med Devices 2015;124:431–7. [DOI] [PubMed] [Google Scholar]
- 86. Khanna P, Swann NC, de Hemptinne C, et al. Neurofeedback control in Parkinsonian patients using electrocorticography signals accessed wirelessly with a chronic, fully implanted device. IEEE Trans Neural Syst Rehabil Eng 2017;2510:1715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. de Hemptinne C, Ryapolova-Webb ES, Air EL, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A 2013;11012:4780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Arlotti M, Rossi L, Rosa M, et al. An external portable device for adaptive deep brain stimulation (aDBS) clinical research in advanced Parkinson's disease. Med Eng Phys 2016;385:498–505. [DOI] [PubMed] [Google Scholar]
- 89. Geller EB, Skarpaas TL, Gross RE, et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia 2017;586:994–1004. [DOI] [PubMed] [Google Scholar]
- 90. Jobst BC, Kapur R, Barkley GL, et al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia 2017;586:1005–14. [DOI] [PubMed] [Google Scholar]
- 91. Parker JL, Karantonis DM, Single PS, et al. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain 2012;1533:593–601. [DOI] [PubMed] [Google Scholar]
- 92. Schultz DM, Webster L, Kosek P, et al. Sensor-driven position-adaptive spinal cord stimulation for chronic pain. Pain Physician 2012;151:1–12. [PubMed] [Google Scholar]
- 93. Deer T, Pope J, Hayek S, et al. Neurostimulation for the treatment of axial back pain: A review of mechanisms, techniques, outcomes, and future advances. Neuromodulation 2014;17(Suppl 2): 52–68. [DOI] [PubMed] [Google Scholar]
- 94. Holsheimef J, Barolat G.. Spinal geometry and paresthesia coverage in spinal cord stimulation. Neuromodulation 1998;13:129–36. [DOI] [PubMed] [Google Scholar]
- 95. Parker JL, Karantonis DM, Single PS, et al. Electrically evoked compound action potentials recorded from the sheep spinal cord. Neuromodulation 2013;164:295–303; discussion 303. [DOI] [PubMed] [Google Scholar]
- 96. Sanders R. (Image credits to Roxanne Makasdjian, Stephen McNally, and Ryan Neely). Sprinkling of neural dust opens door to electroceuticals. Berkeley News; August. 3, 2016. [Google Scholar]
- 97. Montgomery KL, Yeh AJ, Ho JS, et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat Methods 2015;1210:969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Autonomic Technologies, Inc. News media kit. 2015. Available at: http://www.ati-spg.com/us/en/news/media-kit/ (accessed January 2019).
- 99. Deer T, Pope J, Benyamin R, et al. Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation 2016;191:91–100. [DOI] [PubMed] [Google Scholar]
- 100. Schoenen J, Jensen RH, Lantéri-Minet M, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: A randomized, sham-controlled study. Cephalalgia 2013;3310:816–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sokal P, Harat M, Zieliński P, et al. Tibial nerve stimulation with a miniature, wireless stimulator in chronic peripheral neuropathic pain. J Pain Res 2017;10:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gofeld M, Agur A.. Peripheral nerve stimulation for chronic shoulder pain: A proof of Concept Anatomy Study. Neuromodulation 2018;213:284–9. [DOI] [PubMed] [Google Scholar]
- 103. Wilson RD, Bennett ME, Nguyen VQC, et al. Fully implantable peripheral nerve stimulation for hemiplegic shoulder pain: A multi-site case series with two-year follow-up. Neuromodulation 2018;213:290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pancrazio JJ, Deku F, Ghazavi A, et al. Thinking small: Progress on microscale neurostimulation technology. Neuromodulation 2017;208:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li X, Serdijn WA, Zheng W, et al. The injectable neurostimulator: An emerging therapeutic device. Trends Biotechnol 2015;337:388–94. [DOI] [PubMed] [Google Scholar]
- 106. Neely RM, Piech DK, Santacruz SR, et al. Recent advances in neural dust: Towards a neural interface platform. Curr Opin Neurobiol 2018;50:64–71. [DOI] [PubMed] [Google Scholar]
- 107. Seo D, Neely RM, Shen K, et al. Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron 2016;913:529–39. [DOI] [PubMed] [Google Scholar]
- 108. Boyden ES, Zhang F, Bamberg E, et al. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005;89:1263–8. [DOI] [PubMed] [Google Scholar]
- 109. Mondello SE, Sunshine MD, Fischedick AE, et al. Optogenetic surface stimulation of the rat cervical spinal cord. J Neurophysiol 2018;1202:795–811. [DOI] [PubMed] [Google Scholar]
- 110. Daou I, Beaudry H, Ase AR, Wieskopf JS, Ribeiro-da-Silva A, Mogil JS, Séguéla P.. Optogenetic silencing of Nav1.8-positive afferents alleviates inflammatory and neuropathic pain. eNeuro 2016;31:ENEURO.0140-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Li B, Yang X-y, Qian F-P, et al. A novel analgesic approach to optogenetically and specifically inhibit pain transmission using TRPV1 promoter. Brain Res 2015;1609:12–20. [DOI] [PubMed] [Google Scholar]
- 112. Gu L, Uhelski ML, Anand S, et al. Pain inhibition by optogenetic activation of specific anterior cingulate cortical neurons. PLoS One 2015;102:e0117746.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Iyer SM, Montgomery KL, Towne C, et al. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol 2014;323:274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Liem L, van Dongen E, Huygen FJ, et al. The dorsal root ganglion as a therapeutic target for chronic pain. Reg Anesth Pain Med 2016;414:511–9. [DOI] [PubMed] [Google Scholar]
- 115. Vuka I, Vučić K, Repić T, et al. Electrical stimulation of dorsal root ganglion in the context of pain: A systematic review of in vitro and in vivo animal model studies. Neuromodulation 2018;213:213–24. [DOI] [PubMed] [Google Scholar]
- 116. Forget P, Boyer T, Steyaert A, Masquelier E, Deumens R, Le Polain de Waroux B.. Clinical evidence for dorsal root ganglion stimulation in the treatment of chronic neuropathic pain. A review. Acta Anaesthesiol Belg 2015;662:37–41. [PubMed] [Google Scholar]
- 117. Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007;1172:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Koopman FA, Chavan SS, Miljko S, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A 2016;11329:8284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rosas-Ballina M, Ochani M, Parrish WR, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A 2008;10531:11008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sadler RM, Purdy RA, Rahey S.. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia 2002;226:482–4. [DOI] [PubMed] [Google Scholar]
- 121. Yuan H, Silberstein SD.. Vagus nerve stimulation and headache. Headache 2017;57(Suppl 1):29–33. [DOI] [PubMed] [Google Scholar]
- 122. Ruggiero DA, Underwood MD, Mann JJ, et al. The human nucleus of the solitary tract: Visceral pathways revealed with an “in vitro” postmortem tracing method. J Auton Nerv Syst 2000;79(2–3):181–90. [DOI] [PubMed] [Google Scholar]
- 123. Kaube H, Hoskin KL, Goadsby PJ.. Activation of the trigeminovascular system by mechanical distension of the superior sagittal sinus in the cat. Cephalalgia 1992;123:133–6. [DOI] [PubMed] [Google Scholar]
- 124. Lyubashina OA, Sokolov AY, Panteleev SS.. Vagal afferent modulation of spinal trigeminal neuronal responses to dural electrical stimulation in rats. Neuroscience 2012;222:29–37. [DOI] [PubMed] [Google Scholar]
- 125. Ren K, Randich A, Gebhart GF.. Vagal afferent modulation of spinal nociceptive transmission in the rat. J Neurophysiol 1989;622:401–15. [DOI] [PubMed] [Google Scholar]
- 126. Oshinsky ML, Murphy AL, Hekierski H, et al. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain 2014;1555:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Maizels M, Geiger AM.. Intranasal lidocaine for migraine: A randomized trial and open-label follow-up. Headache 1999;398:543–51. [DOI] [PubMed] [Google Scholar]
- 128. Bayer E, Racz GB, Miles D, et al. Sphenopalatine ganglion pulsed radiofrequency treatment in 30 patients suffering from chronic face and head pain. Pain Pract 2005;53:223–7. [DOI] [PubMed] [Google Scholar]
- 129. Jurgens TP, Barloese M, May A, et al. Long-term effectiveness of sphenopalatine ganglion stimulation for cluster headache. Cephalalgia 2017;375:423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Barloese MC, Jürgens TP, May A, et al. Cluster headache attack remission with sphenopalatine ganglion stimulation: Experiences in chronic cluster headache patients through 24 months. J Headache Pain 2016;171:67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tepper SJ, Rezai A, Narouze S, et al. Acute treatment of intractable migraine with sphenopalatine ganglion electrical stimulation. Headache 2009;497:983–9. [DOI] [PubMed] [Google Scholar]
- 132. Tepper SJ, Caparso A.. Sphenopalatine ganglion (SPG): Stimulation mechanism, safety, and efficacy. Headache 2017;57(Suppl 1):14–28. [DOI] [PubMed] [Google Scholar]
- 133. Puledda F, Goadsby PJ.. Current approaches to neuromodulation in primary headaches: Focus on vagal nerve and sphenopalatine ganglion stimulation. Curr Pain Headache Rep 2016;207:47.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Khan S, Schoenen J, Ashina M.. Sphenopalatine ganglion neuromodulation in migraine: What is the rationale? Cephalalgia 2014;345:382–91. [DOI] [PubMed] [Google Scholar]
- 135. Sjoqvist O. The conduction of pain in the fifth nerve and its bearing on the treatment of trigeminal neuralgia. Yale J Biol Med 1939;116:593–600. [PMC free article] [PubMed] [Google Scholar]
- 136. Deer TR, Mekhail N, Petersen E, et al. The appropriate use of neurostimulation: Stimulation of the intracranial and extracranial space and head for chronic pain. Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014;176:551–70; discussion 570. [DOI] [PubMed] [Google Scholar]
- 137. Green MW. Long-term follow-up of chronic cluster headache treated surgically with trigeminal tractotomy. Headache 2003;435:479–81. [DOI] [PubMed] [Google Scholar]
- 138. Bullard DE, Nashold BS Jr.. The caudalis DREZ for facial pain. Stereotact Funct Neurosurg 1997;68(1–4 Pt 1):168–74. [DOI] [PubMed] [Google Scholar]
- 139. Bernard EJ, Nashold BS, Caputi F Jr, et al. Nucleus caudalis DREZ lesions for facial pain. Br J Neurosurg 1987;11:81–91. [DOI] [PubMed] [Google Scholar]
- 140. Tomycz ND, Deibert CP, Moossy JJ.. Cervicomedullary junction spinal cord stimulation for head and facial pain. Headache 2011;513:418–25. [DOI] [PubMed] [Google Scholar]
- 141. Velasquez C, Tambirajoo K, Franceschini P, Eldridge PR, Farah JO.. Upper cervical spinal cord stimulation as an alternative treatment in trigeminal neuropathy. World Neurosurg 2018;114:e641–6. [DOI] [PubMed] [Google Scholar]
- 142. Chou DE, Gross GJ, Casadei CH, et al. External trigeminal nerve stimulation for the acute treatment of migraine: Open-label trial on safety and efficacy. Neuromodulation 2017;207:678–83. [DOI] [PubMed] [Google Scholar]
- 143. Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: A randomized controlled trial. Neurology 2013;808:697–704. [DOI] [PubMed] [Google Scholar]
- 144. Russo A, Tessitore A, Esposito F, et al. Functional changes of the perigenual part of the anterior cingulate cortex after external trigeminal neurostimulation in migraine patients. Front Neurol 2017;8:282.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Facchini G, Spinnato P, Guglielmi G, et al. A comprehensive review of pulsed radiofrequency in the treatment of pain associated with different spinal conditions. Br J Radiol 2017;901073:20150406.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Vanneste T, Van Lantschoot A, Van Boxem K, et al. Pulsed radiofrequency in chronic pain. Curr Opin Anaesthesiol 2017;305:577–82. [DOI] [PubMed] [Google Scholar]
- 147. Schianchi PM, Sluijter ME, Balogh SE.. The treatment of joint pain with intra-articular pulsed radiofrequency. Anesth Pain Med 2013;32:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gofeld M, Restrepo-Garces CE, Theodore BR, et al. Pulsed radiofrequency of suprascapular nerve for chronic shoulder pain: A randomized double-blind active placebo-controlled study. Pain Pract 2013;132:96–103. [DOI] [PubMed] [Google Scholar]
- 149. Pan B, Zhang Z, Chao D, et al. Dorsal root ganglion field stimulation prevents inflammation and joint damage in a rat model of rheumatoid arthritis. Neuromodulation 2018;213:247–53. [DOI] [PubMed] [Google Scholar]
- 150. Leone M, Cecchini AP.. Central and peripheral neural targets for neurostimulation of chronic headaches. Curr Pain Headache Rep 2017;213:16.. [DOI] [PubMed] [Google Scholar]
- 151. Mammis A, Agarwal N, Mogilner AY.. Occipital nerve stimulation. Adv Tech Stand Neurosurg 2015;42:23–32. [DOI] [PubMed] [Google Scholar]
- 152. Weiner RL, Reed KL.. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation 1999;23:217–21. [DOI] [PubMed] [Google Scholar]
- 153. Cadalso R, Daugherty J, Holmes C Jr, et al. Efficacy of electrical stimulation of the occipital nerve in intractable primary headache disorders: A systematic review with meta-analyses. J Oral Facial Pain Headache 2018;321:40–52. [DOI] [PubMed] [Google Scholar]
- 154. Sweet JA, Mitchell LS, Narouze S, et al. Occipital nerve stimulation for the treatment of patients with medically refractory occipital neuralgia: Congress of neurological surgeons systematic review and evidence-based guideline. Neurosurgery 2015;773:332–41. [DOI] [PubMed] [Google Scholar]
- 155. Puledda F, Goadsby PJ.. An update on non-pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache 2017;574:685–91. [DOI] [PubMed] [Google Scholar]
- 156. Silberstein SD, Dodick DW, Saper J, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: Results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 2012;3216:1165–79. [DOI] [PubMed] [Google Scholar]
- 157. Saper JR, Dodick DW, Silberstein SD, et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia 2011;313:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kovacs S, Peeters R, De Ridder D, et al. Central effects of occipital nerve electrical stimulation studied by functional magnetic resonance imaging. Neuromodulation 2011;141:46–55; discussion 56–7. [DOI] [PubMed] [Google Scholar]
- 159. Kent AR, Kramer J, Min X. Comparison of low- and high-frequency dorsal root ganglion stimulation: A computational modeling analysis. Paper presented at: International Neuromodulation Society 13th World Congress; May 27– June 1, 2017; Edinburgh, UK.
- 160. Ackermann DM, Foldes EL, Bhadra N, Kilgore KL.. Conduction block of peripheral nerve using high frequency alternating currents delivered through an intrafascicular electrode. Muscle Nerve 2010;411:117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Soin A, Shah NS, Fang ZP.. High-frequency electrical nerve block for postamputation pain: A pilot study. Neuromodulation 2015;183:197–206. [DOI] [PubMed] [Google Scholar]
- 162. Lind A-L, Wetterhall M, Sjödin M, et al. Proteomic analysis of cerebrospinal fluid gives insight into the pain relief of spinal cord stimulation. Scand J Pain 2017;44:257–8. [Google Scholar]
- 163. Lind A, Emami P, Sjödin M, et al. New players in the mechanism of spinal cord stimulation for neuropathic pain. Scand J Pain 2017;81:52. [Google Scholar]
- 164. Lind A-L, Emami Khoonsari P, Sjödin M, et al. Spinal cord stimulation alters protein levels in the cerebrospinal fluid of neuropathic pain patients: A proteomic mass spectrometric analysis. Neuromodulation 2016;196:549–62. [DOI] [PubMed] [Google Scholar]
- 165. Korvela M, Lind A-L, Wetterhall M, et al. Quantification of 10 elements in human cerebrospinal fluid from chronic pain patients with and without spinal cord stimulation. J Trace Elem Med Biol 2016;37:1–7. [DOI] [PubMed] [Google Scholar]
- 166. Muhammad S, Chaudhry SR, Yearwood TL, et al. Changes of metabolic disorders associated peripheral cytokine/adipokine traffic in non-obese chronic back patients responsive to burst spinal cord stimulation. Neuromodulation 2018;211:31–7. [DOI] [PubMed] [Google Scholar]
- 167. Kinfe TM, Muhammad S, Link C, et al. Burst spinal cord stimulation increases peripheral antineuroinflammatory interleukin 10 levels in failed back surgery syndrome patients with predominant back pain. Neuromodulation 2017;204:322–30. [DOI] [PubMed] [Google Scholar]
- 168. Tilley DM, Cedeño DL, Kelley CA, et al. Spinal cord stimulation modulates gene expression in the spinal cord of an animal model of peripheral nerve injury. Reg Anesth Pain Med 2016;416:750–6. [DOI] [PubMed] [Google Scholar]
- 169. Vallejo R, Tilley DM, Cedeño DL, et al. Genomics of the effect of spinal cord stimulation on an animal model of neuropathic pain. Neuromodulation 2016;196:576–86. [DOI] [PubMed] [Google Scholar]
- 170. Deogaonkar M, Sharma M, Oluigbo C, et al. Spinal cord stimulation (SCS) and functional magnetic resonance imaging (fMRI): Modulation of cortical connectivity with therapeutic SCS. Neuromodulation 2016;192:142–53. [DOI] [PubMed] [Google Scholar]
- 171. Bentley LD, Duarte RV, Furlong PL, et al. Brain activity modifications following spinal cord stimulation for chronic neuropathic pain: A systematic review. Eur J Pain 2016;204:499–511. [DOI] [PubMed] [Google Scholar]
- 172. Moens M, Sunaert S, Mariën P, et al. Spinal cord stimulation modulates cerebral function: An fMRI study. Neuroradiology 2012;5412:1399–407. [DOI] [PubMed] [Google Scholar]
- 173. Muncie LM, Ellens NR, Tolod-Kemp E, Feler CA, Winestone JS.. Intraoperative electrophysiological monitoring for C1-2 spinal cord stimulation. J Neurosurg Spine 2017;262:183–9. [DOI] [PubMed] [Google Scholar]
- 174. Sindou MP, Mertens P, Bendavid U, et al. Predictive value of somatosensory evoked potentials for long-lasting pain relief after spinal cord stimulation: Practical use for patient selection. Neurosurgery 2003;526:1374–83; discussion 1383-4. [DOI] [PubMed] [Google Scholar]
- 175. ektalks. Optogenetics—controlling neurons with light—a way to understand brain circuit organisation? Available at: http://ektalks.blogspot.com/2016/02/optogenetics-controlling-neurons-with.html (accessed January 2019).