Abstract
Objective
Obesity has increased to pandemic levels and enhanced understanding of adipose regulation is required for new treatment strategies. Although bone morphogenetic proteins (BMPs) influence adipogenesis, the effect of BMP antagonists such as Noggin is largely unknown. The aim of the study was to define the role of Noggin, an extracellular BMP inhibitor, in adipogenesis.
Methods
We generated adipose-derived progenitor cells and a mouse model with adipocyte-specific Noggin deletion using the AdiponectinCre transgenic mouse, and determined the adipose phenotype of Noggin-deficiency.
Results
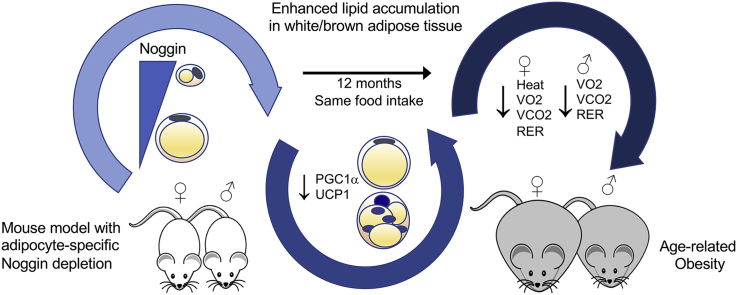
Our studies showed that Noggin is expressed in progenitor cells but declines in adipocytes, possibly allowing for lipid accumulation. Correspondingly, adipocyte-specific Noggin deletion in vivo promoted age-related obesity in both genders with no change in food intake. Although the loss of Noggin caused white adipose tissue hypertrophy, and whitening and impaired function in brown adipose tissue in both genders, there were clear gender differences with the females being most affected. The females had suppressed expression of brown adipose markers and thermogenic genes including peroxisome proliferator activated receptor gamma coactivator 1 alpha (PGC1alpha) and uncoupling protein 1 (UCP1) as well as genes associated with adipogenesis and lipid metabolism. The males, on the other hand, had early changes in a few BAT markers and thermogenic genes, but the main changes were in the genes associated with adipogenesis and lipid metabolism. Further characterization revealed that both genders had reductions in VO2, VCO2, and RER, whereas females also had reduced heat production. Noggin was also reduced in diet-induced obesity in inbred mice consistent with the obesity phenotype of the Noggin-deficient mice.
Conclusions
BMP signaling regulates female and male adipogenesis through different metabolic pathways. Modulation of adipose tissue metabolism by select BMP antagonists may be a strategy for long-term regulation of age-related weight gain and obesity.
Keywords: Noggin, Bone morphogenetic protein, Adipocyte, Adipogenesis, Obesity
Graphical abstract
Highlights
-
•
Expression of Noggin is lower in adipocytes than in adipose progenitor cells, allowing for lipid accumulation.
-
•
Noggin deletion in mice promotes age-related obesity in both genders without change in food intake.
-
•
Loss of Noggin causes hypertrophy of white adipose tissue, and whitening and impaired function in brown adipose tissue.
-
•
Both genders have reductions in VO2, VCO2 and RER, whereas females also have reduced heat production.
-
•
Select BMP antagonists may be essential for long-term regulation of age-related weight gain and obesity.
Although, bone morphogenetic proteins (BMPs) influence fat development, the role of their inhibitors is largely unknown. This study shows that reduction of Noggin causes obesity related to age but not food intake, suggesting that select BMP antagonists may regulate age-related weight gain and obesity.
Abbreviations
- Adipoq
adiponectin
- aP2
adipocyte protein 2
- ASC
adipose stromal cell
- ALK
activin receptor-like kinase
- Cdh5
cadherin 5, VE-cadherin
- BAT
brown adipose tissue
- BMI
body mass index
- BMP
bone morphogenetic protein
- CD
cluster of differentiation
- Cebpa
CCAAT/enhancer-binding protein alpha
- Cox
cytochrome c oxidase
- Cpt1a/Cpt1b
carnitine palmitoyltransferase 1A/1B
- CT
computed tomography
- CV2
crossveinless 2
- DAPI
4′,6-diamidino-2-phenylindole
- DFAT cells
dedifferentiated fat cells
- Dio2
iodothyronine deiodinase 2
- DMEM
Dulbecco's modified Eagle's Medium
- EE
energy expenditure
- Elovl
ELOVL fatty acid elongase
- ERa
estrogen receptor alpha
- Fabp4
fatty acid-binding protein
- FACS
fluorescence-activated cell sorting
- FASN
fatty acid synthase
- 18F-FDG
18F-fludeoxyglucose
- FITC
fluorescein isothiocyanate
- F, Fl
flox
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HF/HS
high-fat/high-sucrose
- HOMA-IR
insulin resistance index
- HMDP
hybrid mouse diversity panel
- HSL
hormone-sensitive lipase
- ICAM
intercellular adhesion molecule
- ID
inhibitor of DNA binding
- IL
interleukin
- MGP
matrix Gla protein
- Nog
noggin
- OCR
oxygen consumption rate
- Oct
octamer-binding transcription factor
- p
phosphorylated, phospho
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- PET
positron emission tomography
- PGC1a
peroxisome proliferator activated receptor gamma coactivator 1 alpha
- PRDM16
PR domain-containing 16
- RER
respiratory exchange ratio
- SCD1
stearoyl-CoA desaturase-1
- Slc27a2
solute carrier family 27 member2
- SMAD
transcription factors with homology to the Caenorhabditis elegans SMA and Drosophila MAD family of genes
- TCF21
transcription factor 21
- TGFβ
transforming growth factor β
- UCP1
uncoupling protein 1
- VCAM-1
vascular cell adhesion protein 1
- VO2
oxygen consumption
- WAT
white adipose tissue
- WB
washing buffer
- Zic1
Zic family member 1
1. Introduction
Approximately two-thirds of the adult American population suffer from overweight or obesity, which is closely linked to adipose-tissue dysfunction and inflammation, type 2 diabetes, insulin resistance and cardiovascular disease [1], [2]. There are at least two types of adipose tissue in mammals [3], [4], [5]: white adipose tissue (WAT), which has a central role in energy storage, hormone production and organ protection, and brown adipose tissue (BAT), which is highly vascularized and dissipates energy in the form of heat. BAT is considered to play an important role in the depletion of excess calories and may have a mitigating effect on cardiovascular disease [6]. Recent studies have shown that WAT can transition into BAT and vice versa, phenomena that are usually referred to as “browning” and “whitening”, respectively [7], [8]. The adipocytes that are responsible for “browning” have been labeled either “beige” or “brite” (brown-in-white) adipocytes [9], [10]. The classical brown precursor cells derive from the dermatomyotome that express Engrailed and Myf5, whereas the beige/brite adipocytes emerge in WAT, which derive from pluripotent mesodermal stem cells [4], [5]. In addition, both types of adipocytes have been traced from progenitor cells in perivascular areas, and even the vascular endothelium [11], [12].
The bone morphogenetic proteins (BMPs) are part of the transforming growth factor β (TGFβ) superfamily, and regulate development and tissue differentiation through their influences on cell proliferation, lineage and migration [13], [14]. The best known functions of the members of the BMP subfamily are related to bone and cartilage formation, but many BMPs are also involved in the development and pathophysiology of other organs, including the cardiovascular system [15], [16], nervous system [17], kidneys [18], lungs [19] and adipose tissue [5], [20]. During development, gradients of BMPs and BMP inhibitors are established and help specify cell differentiation in tissue formation [21]. Analogously, niches with ‘hot spots’ of high local BMP concentrations may exist in various tissues. For example, perivascular niches have been described where endothelial responses to circulating factors are transmitted to adipogenic progenitor cells [22].
BMP2 and BMP4 have been reported to stimulate white adipogenesis, whereas BMP4 and BMP7 are essential in the induction of brown adipogenesis [20], [23], [24], [25], [26]. Roles for BMP4 and BMP7 have also been described in the browning and whitening of adipose tissue [23], [24], [27]. BMP8b may have a role in modulating thermogenesis in BAT [28] and adrenergic-induced remodeling of the adipose neuro-vascular network [29]. It has also been described that the absence of the activin receptor-like kinase (ALK)3 (also referred to as BMPR1A) either in cells from the Myf5+ lineage (brown preadipocytes) or in all adipocytes leads to a deficiency in brown fat [30]. Interestingly, BMP9, which is highly expressed in liver and circulates in plasma, has been shown to regulate enzymes involved in glucose homeostasis and to be a strong inducer of brown adipogenesis [31].
The BMP activity is frequently modulated by extracellular inhibitors such as matrix Gla protein (MGP), Noggin, and Gremlin [32], [33], [34]. However, these are secreted proteins with abilities to bind BMP2, 4, and 7 [32], [33], [34], [35] and could therefore affect adipogenesis. However, the understanding of their roles in adipose tissue remains in large part unclear and contradictory. MGP is highly expressed in differentiating human preadipocytes [36], and is higher in omental than subcutaneous adipose tissue [37]. Noggin, however, was reported to induce adipogenesis in mesenchymal stem cells from mouse bone marrow, and to circulate at higher levels in human subjects with body mass index (BMI) > 27 [38], whereas other investigators found that Noggin reduced cell differentiation in human adipose stromal cells [39]. Gremlin has been reported to inhibit adipocyte differentiation [40], [41], cause preadipocyte resistance to BMP4 and impair regulation of beige and brown adipogenesis [42].
Here we address whether the BMP inhibitor Noggin has the ability of modulating adipose tissue and obesity in vivo through adipocyte-specific gene deletion of the Noggin gene in mouse. Our findings provide evidence that Noggin is a regulator of adipose tissue, and Noggin deficiency promotes age-related whitening of BAT and white adipose hypertrophy associated with obesity.
2. Methods
2.1. Mice
Nogginflox/flox (Nogfl/fl) (Nog tm1.1Rmh/J, stock #016117) [43], Adiponectin (Adipoq)Cre (B6; FVB-Tg(Adipoq-cre)1Evdr/J, stock #010803) [44], and VE-cadherin (Cadherin5, Cdh5)Cre (B6.FVB-Tg(Cdh5-cre)7Mlia/J, stock #006137) [45] were obtained from the Jackson Laboratory. All mice were on C57BL/6J background. Genotypes were confirmed by PCR [46], and experiments were performed with generations F4-F6. Littermates were used as wild type controls. All mice were fed a standard chow diet (Teklad Rodent Diet 8604, Envigo, Placentia, CA). The use of animals and all experimental procedures were reviewed and approved by the University of California Los Angeles (UCLA) Chancellor's Animal Research Committee and conducted in accordance with the animal care guideline set by UCLA. The investigation conformed to the National Research Council, Guide for the Care and Use of Laboratory Animals, Eighth Edition (Washington, DC: The National Academies Press, 2011).
2.2. Preparation of adipose tissue-derived progenitor cells
Dedifferentiated fat (DFAT) cells and adipose stromal cells (ASCs) were prepared from 2 g of subcutaneous WAT or interscapular BAT collected from wild type C57BL/6J mice aged 8–10 weeks, as previously described [47], [48], [49]. The DFAT cells were used after passage of the initial preparation from adipocytes, between passages 1–3, and the experiments where performed in adipogenic medium from the StemPro™ Adipogenesis Differentiation Kit (Gibco/Thermofisher Scientific, Waltham, MA). Recombinant human BMP4 and Noggin, and anti-Noggin antibodies (Cat. No. AF719) were from R&D Systems (Minneapolis, MN). The ASCs were sorted by FACS as outlined below.
2.3. Body fat determination
Whole body fat, fluids and lean muscle mass of isoflurane-anesthetized mice were determined biweekly using a Bruker Optics Minispec NMR analyzer (Billerica, MA) in accordance with the manufacturer's recommendations.
2.4. Indirect calorimetry and respiratory measurements
Energy expenditure, food intake, and ambulatory activity were determined by using the Oxymax/CLAMS Comprehensive Lab Animal Monitoring System (Columbus Instruments, Columbus, OH). Respiratory measurements were made in 20 min intervals after an initial 12 h acclimation period. Energy expenditure (EE) was calculated from oxygen consumption (VO2) and respiratory exchange ratio (RER) using the Lusk equation, EE in cal/min = (3.815 + 1.232 × RER) × VO2 in ml/min [50]. Statistical significance for EE measurements was determined by using 2-way-ANOVA.
2.5. Glucose tolerance test
Mice aged one year were fasted for 6 h prior to the beginning of the test. Baseline measurements of blood glucose were obtained (Optium EZ kit from Abbott, Lake Bluff, IL), and the mice were injected with 2 mg glucose (Millipore Sigma, Billerica, MA) per gram of body weight. Additional blood glucose measurements were taken at 15, 30, 60, and 90 min after the injection.
2.6. Immunofluorescence
Tissues were collected at 2 months and 1 year of age. Subcutaneous fat pads were collected in the inguinal and axillary regions (the latter corresponding to the anterior subcutaneous fat pad in [51]). Visceral fat pads were collected in the gonadal and omental regions, and brown fat was collected in the interscapular region.
Tissue sections were prepared and stained as previously described [52]. Briefly, 4 μm-sections were fixed in 4% paraformaldehyde and processed as described. For immunohistochemistry and immunofluorescence, sections were permeabilized with 0.5% Triton X-100 for 10 min, followed by 3 washes with wash buffer (WB, phosphate-buffered saline (PBS) containing 0.1% Tween-20). Non-specific antibody binding sites were blocked by incubating the sections for 30 min in blocking buffer (1% BSA, 2% goat serum and 0.5% Triton X-100 in PBS). Primary antibodies were diluted in antibody buffer (PBS containing 1% BSA, 0.5% Triton X-100), and sections were incubated for overnight at 4 °C, followed by several washes in WB. Alexa Fluor 488-conjugated (green fluorescence) or Alexa Fluor 594-conjugated (red fluorescence) secondary goat anti-chicken or goat anti-rabbit antibodies (Molecular Probes, Eugene, OR) or Streptavidin (Vector Laboratories, Burlingame, CA, USA) were applied to the sections and incubated for 60 min at room temperature. After several washes in WB and a brief equilibration of the sections in PBS, the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, St. Louis, MO). A DAPI stock solution was diluted to 300 nM in PBS, and 300 μl of the diluted solution was added to the sections, making certain that they were completely covered. The sections were incubated for 1–5 min and rinsed several times in PBS. Staining without primary antibodies served as controls. Slides without primary antibodies were used as negative controls. Images were acquired with an inverted Nikon Eclipse Ti–S microscope (Nikon Corporation, Tokyo, Japan). We used biotinylated isolectin B4 (Vector Laboratories, Burlingame, CA), and specific antibodies for Perilipin (Cat. No. 9349S, Cell Signaling, Danvers, MA), Noggin (Cat. No. AF719, R&D Systems, Minneapolis, MN), and beta-catenin (Cat. No. ab16051, Abcam, Cambridge, MA). The preparations were mounted with Prolong™ Gold Antifade Mountant (Thermo Fisher Scientific, Canoga Park, CA). Image analysis was performed on sections stained with anti-perilipin antibodies using Wolfram Mathematica 10 (Wolfram Research, Champaign, IL). Only cells with clear cell boundaries were included in the analysis (Supplemental Fig. 1).
2.7. RNA analysis
Total RNA was isolated from tissues or cultured cells using the miRNeasy Mini kit (Qiagen, Redwood City, CA) according to the manufacturer's instructions. cDNA was obtained using the High Capacity cDNA Reverse Transcription kit from Applied Biosystems (Foster City, CA). Relative quantitative PCR was performed on a 7500 Fast Real-Time PCR System using TaqMan® Universal PCR Master Mix (both from Applied Biosystems). Primers and probes for mouse genes BMP2, BMP4, BMP7, BMP8b, Gremlin, matrix Gla protein (MGP), crossveinless 2 (CV2), Noggin, cluster of differentiation (CD)36, CD31, CD68, Adiponectin (Adipoq), Uncoupling Protein 1 (UCP1), PR domain-containing 16 (PRDM16), octamer-binding transcription factor (Oct)3/4, transcription factor 21 (TCF21), Cidea, peroxisome proliferator activated receptor gamma coactivator 1 alpha (Pgc1a), cytochrome c oxidase polypeptide 7A1 (Cox7a1), cytochrome c oxidase subunit 8B (Cox8b), iodothyronine deiodinase 2 (Dio2), Zic family member 1 (Zic1), CCAAT/enhancer-binding protein alpha (Cebpa), ELOVL fatty acid elongase 3 (Elovl3), Elovl6, solute carrier family 27 member2 (Slc27a2), carnitine palmitoyltransferase 1A (Cpt1a), Cpt1b, interleukin 6 (IL-6), intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion protein 1 (VCAM-1) were obtained from Applied Biosystems as part of Taqman® Gene Expression Assays. Cycle conditions were: one cycle at 50 °C for 2 min, followed by one cycle at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Threshold cycles of specific cDNAs were compared to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or 18S ribosomal RNA (18S rRNA) as previously described [53].
2.8. Immunoblotting
Immunoblotting was performed as previously described [46], [54]. Equal amounts of tissue protein were used. Blots were incubated with specific antibodies to UCP1 (1:1,000 dilution; Cat. No. ab10983, Cell Signaling Technology) or phospho-SMAD1/5 (Ser463/465) (41D10) (1:1,000 dilution; Cell Signaling Technology, Cat. No. #9516), total SMAD (1:1,000 dilution; Santa Cruz Biotechnology, sc-7153), beta-actin (1:2,000 dilution; Sigma-Aldrich, Cat. No. A2228), hormone-sensitive lipase (HSL) (1:1,000 dilution; Cell Signaling Technology, Cat. No. 4107), phospho-HSL (Ser660) (1:1,000 dilution; Cell Signaling Technology, Cat. No. 4126), fatty acid synthase (FASN) (1:1,000 dilution; Cell Signaling Technology, Cat. No. #3180), and stearoyl-CoA desaturase-1 (SCD1) (0.2 μg/ml; Santa Cruz Biotechnology, Cat. No. sc-14719).
2.9. Fluorescence-activated cell sorting (FACS)
FACS was performed as previously described [55], and used to sort freshly isolated ASCs into CD34−CD31−, CD34+CD31−, CD34+CD31+, and CD34−CD31+ cell fractions using fluorescein isothiocyanate (FITC)-conjugated antibodies against CD34 (1:200; Cat. No. 11-0341-85, eBioscience, San Diego, CA) or phycoerythrin (PE)-conjugated antibodies against mouse CD31 (1:200; Cat. No. 561073, BD Biosciences Pharmingen). Nonspecific fluorochrome- and isotype-matched IgGs (BD Pharmingen) served as controls. Flow cytometer gates were set using unstained cells and the isotype-matched controls. Cells were gated by forward scatter (FSC) versus side scatter (SSC) to eliminate debris. A region was established to define positive PE/AF-488 fluorescence using a PE/AF-488-conjugated isotype-specific control. The number of cells stained positive for a given marker was determined by the percentage of cells present within a gate, which was established such that fewer than 2% of positive events represented nonspecific binding by the PE/AF-488-conjugated isotype-specific control. Minimums of 10,000 events were counted for each analysis. All FACS analyses were performed using a BD LSR II flow cytometer (BD Biosciences). FACS files were exported and analyzed using BD Cellquest software v.3.3.
2.10. Oil Red O staining
For Oil Red O staining, cells that were seeded at 70% confluency and treated as indicated in the text. The staining was performed using standard methods. To stain isolated adipocytes, floating adipocytes were collected in a new tube and AdipoRed (Lonza Biosciences, Walkersville, MD) diluted1:50 was added together with DAPI diluted 1:200 and incubated at room temperature for 20 min with soft shaking. The adipocytes were washed once more with PBS before mounting the preparation.
2.11. Brown fat activity
The experiments were performed in sub-thermoneutral conditions (20 °C) and after cold treatment at 4 °C as previously described [56]. Animals of two months and one year of age received one injection of approximately 70 μ Cu of 18F-Fludeoxyglucose (FDG) via the tail vein. A 1-h interval for uptake was allowed between probe administration and micro-positron emission tomography (micro-PET)/micro-computed tomography (micro-CT) scanning. Image acquisition and reconstruction were performed as previously described [57]. Briefly, mice were placed in a dedicated imaging chamber designed for use for the CT and both PET systems. Data were acquired by using a Genisys 8 PET/CT (Sofie Biosciences, Culver City, CA) for 10 min and a MicroCAT II small-animal CT scanner (Siemens ImTek Inc. Knoxville, TN) for 8 min. Micro-PET images were reconstructed by using statistical maximum a posteriori probability algorithms (MAP) into multiple frames. Micro-CT Images were reconstructed using filtered back-projection without scatter or attenuation correction. We chose a ramp filter with a cutoff frequency of 0.5 and a zoom of 5 to give a voxel size of 0.379 mm3. PET and CT Images were analyzed using Amide Software (version 1.16).
2.12. Respiratory rate measurement
The oxygen consumption rate (OCR) in fat pads was measured with an XF24 Analyzer (Seahorse Bioscience) as described [58]. Briefly, freshly isolated brown and white adipose tissues were minced and rinsed in cold PBS, placed in a Seahorse Islet Capture Microplate (3–5 mg per well), and incubated with 625 μl of unbuffered Dulbecco's Modified Eagle Medium (DMEM) (containing 2.5 mM glucose). OCR was measured before and after the injection of 200 μM etomoxir. The etomoxir-sensitive respiration represents the beta-oxidation. Experiments were triplicated; differences were tested by two-way ANOVA.
2.13. The hybrid mouse diversity panel (HMDP)
HMDP data was analyzed from 95 inbred mouse strains fed a standard chow diet and 110 inbred strains fed a high-fat/high sucrose diet for 8 weeks as previously described [59], [60], [61]. Gene expression comparisons were analyzed in 75 overlapping strains from two HMDP studies using global gene expression in epididymal adipose tissue in male mice. The correlations of gene expression and phenotypic data were calculated with the biweight midcorrelation using R package WGCNA.
2.14. Statistical analysis
Data were analyzed for statistical significance by two-way ANOVA with post hoc Tukey's analysis or multiple t test with Holm–Sidak correction for multiple comparisons using GraphPad Prism® 6.0 software (GraphPad Software, San Diego, CA). Unpaired student's t test was used for single variable comparison between two groups. Data are presented as mean ± SEM. p-values less than 0.05 were considered statistically significant. All experiments were repeated a minimum of three times.
3. Results
3.1. Noggin as a potential regulator of adipose tissue
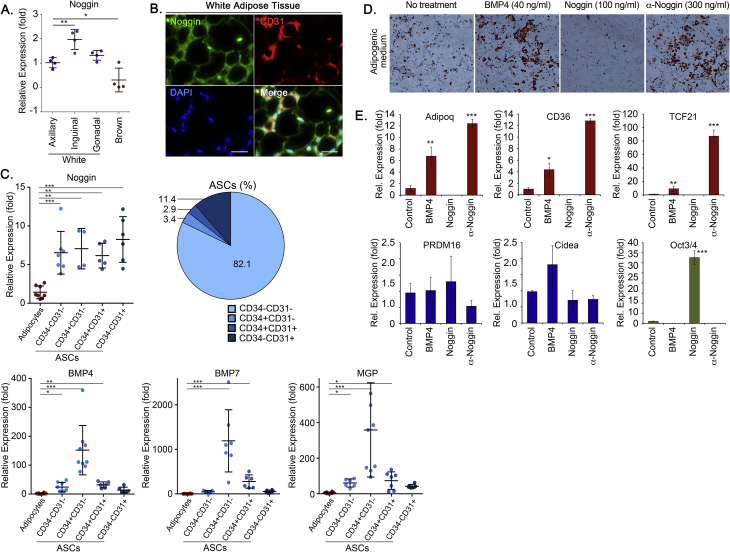
To examine the potential role of BMP inhibition in adipogenic differentiation, we compared the expression of BMP4 and BMP7, which are known to be associated with adipogenesis with that of Noggin, a well-established BMP inhibitor. Noggin was expressed in both WAT and BAT, as determined by qPCR (Figure 1A), and was detected by immunofluorescence in proximity of both adipocytes and CD31+ capillaries (Figure 1B). We then compared Noggin expression to that of BMP4, BMP7 and MGP, another BMP inhibitor, in the adipocytes and the different ASCs fractions. Both adipocytes and ASCs expressed Noggin. The ASCs fraction from subcutaneous WAT was sorted by FACS using antibodies to the vascular markers CD34 and CD31. White adipocytes and four ASCs fractions (82.1% CD34−CD31−, 3.4% CD34+CD31−, 2.9% CD34+CD31+, and 11.4% CD34−CD31+ cells) were analyzed by qPCR. The results revealed that the expression of BMP4, BMP7 and MGP was highest in the CD34+CD31− cells (Figure 1C), suggesting that the BMPs from these cells might trigger or modulate adipogenic differentiation. The expression of Noggin, on the other hand, was similar in all the ASCs fractions and significantly higher than in the adipocytes (Figure 1C). Therefore, we hypothesized that decreases in Noggin allowed for lipid accumulation, a late characteristic of adipogenic differentiation.
Figure 1.
Noggin expression in tissue, and effect of Noggin on adipose-derived progenitor cells (DFAT cells) in vitro. (A) Noggin expression in white and brown fat depots compared to the axillary depot. (B) Immunofluorescence for Noggin (green) in subcutaneous WAT; co-stained with the endothelial marker CD31 (red). DAPI (blue) was used to visualize nuclei (bars, 100 μm). (C) Expression of Noggin, BMP4, BMP7 and MGP in white ASCs fractions obtained by FACS using antibodies to CD31 and CD34, compared to white adipocytes, as determined by qPCR. (D, E) DFAT cells derived from WAT were treated for 12 days with control (vehicle or non-specific IgG), BMP4, Noggin and anti-Noggin antibodies in adipogenic medium. (D) The cells were stained by Oil Red O, and (E) expression of adipogenic markers and the stem cell marker Oct3/4 was determined by qPCR. *<0.05, **<0.01, ***<0.001.
To assess the effect of Noggin on lipid accumulation in adipogenic precursor cells in vitro, we cultured DFAT cells in adipogenic medium and treated the cells with control (non-specific IgG), BMP4 (50 ng/ml), Noggin (100 ng/ml) or neutralizing anti-Noggin antibodies (300 ng/ml) for 12 days. The medium was changed on days 5 and 9. The results showed that BMP4 and the anti-Noggin antibodies promoted lipid accumulation compared to control, as shown by Oil Red O staining, whereas Noggin suppressed the lipid accumulation (Figure 1D). Expression of the adipogenic markers adiponectin, Tcf21 and CD36 were consistent with the Oil Red O staining, whereas the beige/brown markers did not change significantly (Figure 1E). Interestingly, the stem cell marker Oct3/4 was enhanced in the Noggin-treated cells (Figure 1E), supporting that Noggin counteracts differentiation in these cells. Similar results were seen when treating 3T3-L1 cells, an alternative cell line (data not shown).
3.2. Noggin-deficiency promotes obesity and enlargement of lipid droplets in female and male mice
To determine the role of Noggin in adipose tissue, we generated mice with adipocyte-specific Noggin deficiency by crossbreeding Nogfl/fl and AdipoqCre mice to obtain Nogfl/fl;AdipoqCre mice. Noggin expression in the different fat pads from these mice was significantly reduced when compared to their Nogfl/fl littermates, as determined by qPCR and visualized by immunofluorescence (Supplemental Figs. 2A and B). As would be expected, low levels of Noggin continued to co-localize with isolectin B4, a capillary marker, consistent with the adipocyte-specific Noggin deletion. We also generated Nogfl/fl;Cdh5Cre control mice with endothelial cell-specific Noggin deficiency for comparison.
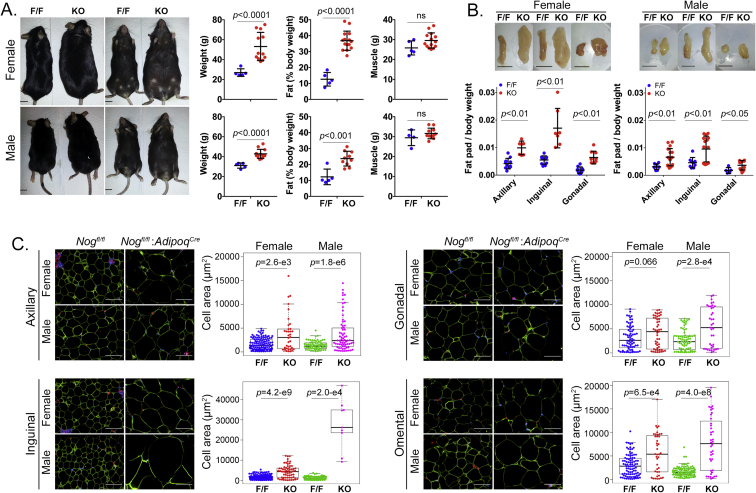
Female and male Nogfl/fl;AdipoqCre mice were allowed an ad libitum chow diet for up to one year, and body weight, body composition, and size of fat pads were monitored. The body weight and percent fat gradually increased and started to show significant differences at 9 and 6–9 months, respectively, compared to Nogfl/fl mice, as assessed by NMR (Supplemental Fig. 3). The differences were highly significant in both genders of the Nogfl/fl;AdipoqCre mice after one year (Figure 3A) when the females had nearly doubled their body weight. The muscle mass did not differ at any time point (Supplemental Fig. 3, Figure 2A). Similarly, the weight of the axillary, inguinal and gonadal fat pads, as compared to body weight, was significantly increased in females after 2 months and in both genders after one year (Supplemental Fig. 4A, Figure 2B). No differences in body weight, body composition or BAT were detected in female or male Nogfl/fl;Cdh5Cre mice with the endothelial-specific Noggin loss, as compared to the Nogfl/fl mice (Supplemental Figs. 5A and B). Together, the results suggested that loss of Noggin in adipocytes promotes weight gain and an increase in adipose tissue with age in both genders.
Figure 3.
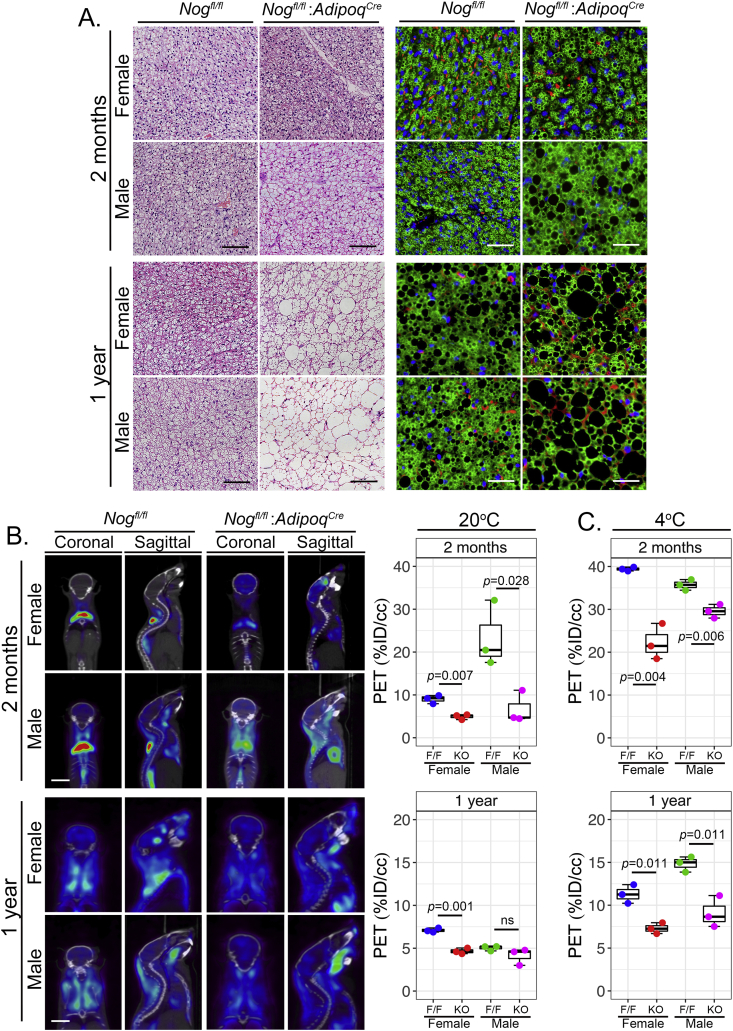
Adipocyte-specific deletion of Noggin impairs BAT function in mice. BAT from female and male mice with adipocyte-specific deletion of Noggin (Nogfl/fl;AdipoqCre or KO) and control mice (Nogfl/fl or F/F) aged 2 months and one year was examined. (A) Sections of BAT were stained by H&E staining (left panels) and immunofluorescence for Perilipin (green) (right panels). Sections were co-stained for CD31 (red) and DAPI (blue) was used to visualize nuclei (bars, 100 μm). (B) 18F-FDG uptake in BAT at 20 °C was visualized by PET (left panels) and subsequently quantified (right panels) (bars, 10 mm). (C) Quantification of 18F-FDG uptake in BAT after cold exposure at 4 °C.
Figure 2.
Adipocyte-specific deletion of Noggin in mice promotes obesity. Female and male mice with adipocyte-specific deletion of Noggin (Nogfl/fl;AdipoqCre or KO) and control mice (Nogfl/fl or F/F) aged one year were analyzed in regards to (A) body weight and percent fat and muscle (bars, 10 mm), (B) fat pad size from the axillary, inguinal and gonadal locations (bars, 10 mm), and (C) adipocyte size as determined by cell area after immunofluorescence for Perilipin (green). Sections were co-stained for CD31 (red) and DAPI (blue) was used to visualize nuclei (bars, 100 μm).
Noggin provides antagonism to several BMPs, including BMP2, 4, and 7 [32] and likely BMP8b based on its close alignment with BMP7 [13]. To determine the effect of Noggin deficiency on white adipocytes, sections from the different fat pads were stained with anti-perilipin antibodies and the area of the sectioned adipocytes was determined using immunofluorescence and image analysis. Subcutaneous (axillary and inguinal) and visceral (gonadal and omental) fat pads were examined in females and males. At 2 months, only the subcutaneous adipocytes in male mice were larger in the Noggin-deficient animals (Supplemental Fig. 4B). After one year, however, adipocytes in subcutaneous fat pads as well as in the omental fat pads were significantly larger in the Noggin-deficient mice of both genders (Figure 2C). The gonadal fat pads also showed a significant enlargement in males, but not in females (Figure 2C). Again, no significant difference was detected in the Nogfl/fl;Cdh5Cre mice with endothelial Noggin loss, as compared to the Nogfl/fl mice at one year of age (Supplemental Fig. 5C). Our results suggest that the reduced Noggin levels allowed for a potential rebalancing of the overall BMP activity that enhanced lipid accumulation with age. However, this effect was influenced by fat location and gender.
3.3. Noggin-deficiency affects BAT structure and functionality
Since Noggin is known to modulate BMP4 as well as BMP7 [32], [33], [34], [62], the latter essential in brown adipogenesis, we expected that brown adipogenesis would also be affected. We examined BAT at 2 months and one year in the Nogfl/fl;AdipoqCre mice and compared to control Nogfl/fl mice by H&E staining and anti-perilipin immunofluorescence. This revealed that brown adipocytes in Noggin-deficient females and males had larger lipid droplets than the respective control animals at both time points (Figure 3A), suggesting that lack of Noggin promotes lipid accumulation also in brown adipocytes. It also suggested that the BAT function was altered as a result of the reduced Noggin expression. Therefore, we assessed the BAT function at 20 °C by PET at 2 months and one year of age. At 2 months, the 18F-FDG uptake in BAT was less in females than in males in both types of mice, and both genders of the Nogfl/fl;AdipoqCre mice exhibited lower 18F-FDG uptake than the Nogfl/fl control mice (Figure 3B). At one year of age, the decrease in BAT activity was more pronounced in females than in males, but the control mice continued to have higher 18F-FDG uptake than the Nogfl/fl;AdipoqCre mice of the same gender (Figure 3B). The alteration in thermogenesis was confirmed by cold exposure experiments at 4 °C, which clearly showed less 18F-FDG uptake in the BAT of both female and male Nogfl/fl;AdipoqCre mice at 2 months and one year of age (Figure 3C). Thus, the results suggested that the reduction in Noggin affected the BAT, and that part of the obesity in the Nogfl/fl;AdipoqCre mice might be due to the impaired BAT function.
3.4. Noggin deficiency affects metabolism and adipogenic differentiation
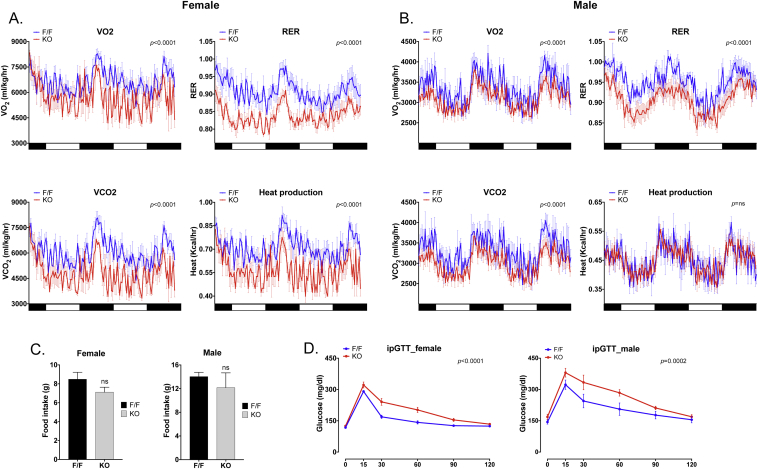
To investigate possible causes of the increase in adipose tissue, we analyzed metabolic differences between Nogfl/fl;AdipoqCre and Nogfl/fl control mice at 2 months of age using metabolic cages. This was prior to detectable differences in weight and body fat. The results showed that the Nogfl/fl;AdipoqCre mice of both genders exhibited significant decreases in VO2, VCO2, and RER (Figure 4A, B). The females also exhibited a decrease in heat production, which was not observed in the males (Figure 4A, B). However, there was no significant difference in the cumulative food intake between the Nogfl/fl;AdipoqCre and Nogfl/fl control mice of the same gender (Figure 4C, D). The food intake was measured from the time the mice were placed in the metabolic cages until the metabolic data collection was complete. In addition, no differences were detected in locomotor or ambulatory activity. On the other hand, there were significant increases in plasma levels of triglycerides and total cholesterol in the Nogfl/fl;AdipoqCre mice (Supplemental Fig. 6A). We also noted an increase in glucose and an increasing trend in insulin levels in the Nogfl/fl;AdipoqCre mice (Supplemental Fig. 6A), suggesting that the mice were developing glucose intolerance. Indeed, both genders showed evidence of glucose intolerance at 2 months of age (Figure 5D), and both genders developed histological signs of fatty liver at one year of age (Supplemental Fig. 7B).
Figure 4.
Adipocyte-specific deletion of Noggin in mice affects heat production, respiratory exchange ratio and oxygen consumption. Female and male mice with adipocyte-specific deletion of Noggin (Nogfl/fl;AdipoqCre or KO) and control mice (Nogfl/fl or F/F) aged 2 months were examined in metabolic cages. (A, B) VO2, VCO2, RER and heat production for female (A) and male (B) mice (n = 4–6). (C) Food intake in female and male mice (n = 4–6). (D) Glucose tolerance test in female and male mice (n = 6–7). p-values are indicated in the respective panels.
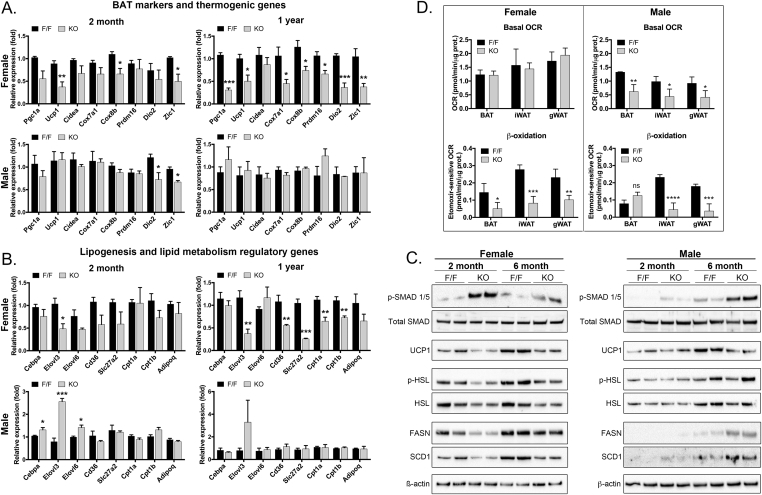
Figure 5.
Adipocyte-specific deletion of Noggin affects expression of markers for adipogenic lineage, mitochondria and β-oxidation. Adipose tissues were collected from female and male mice with adipocyte-specific deletion of Noggin (Nogfl/fl;AdipoqCre or KO) and control mice (Nogfl/fl or F/F) aged 2 months and one year. (A, B) Gene expression was determined by qPCR for (A) BAT markers and thermogenic genes, and (B) lipogenesis and lipid metabolism regulatory genes. n = 3; *<0.05, **<0.01, ***<0.001. (C) Gene expression was determined by immunoblotting for phosphorylated (p)-SMAD1/5 as compared to total SMAD, UCP1, p-HSL and HSL, FASN, and SCD1. Beta-actin is shown as a control. (D) Oxygen consumption rate (OCR) in BAT from female and male Nogfl/fl;AdipoqCre and Nogfl/fl control mice in BAT at 2 months of age; basal OCR in females (top) and after addition of etomoxir (bottom). *<0.05, **<0.01, ***<0.001, ****<0.0001.
To further investigate BAT function, we first confirmed that the Noggin deletion enhanced the canonical SMAD activation in the BAT as expected. Indeed, the activation (phosphorylation) of SMAD1/5, mediators of BMP signaling, was increased in both female and male mice at 2 and 6 months of age, as determined by immunoblotting. Total SMAD, which is shown for comparison, did not change (Figure 5C, top two lanes).
We then investigated the expression by qPCR of BAT markers and thermogenic genes (PGC1a, UCP1, Cidea, Cox7a1, Cox8b, PRDM16, Dio2, and Zic1 [63]), and adipogenic and lipid metabolism regulatory genes (Cebpa, Elovl3, Elovl6, CD36, Slc27a2, Cpt1a, Cpt1b, and adiponectin). The expression was examined in BAT from mice aged 2 months and one year. The results showed that all the BAT markers and thermogenic genes decreased or showed a decreasing trend in female Nogfl/fl;AdipoqCre mice (Figure 5A), whereas only Dio2 and Zic1 showed an early decrease (at 2 months) in male Nogfl/fl;AdipoqCre mice (Figure 5A). The decrease in UCP1 in female Noggin-deficient mice was confirmed by immunoblotting (Figure 5C). Furthermore, the adipogenic and lipid metabolism regulatory genes also decreased in the female Nogfl/fl;AdipoqCre mice, especially after one year (Figure 5B), whereas an early increase of Cebpa, Elovl3 and Elovl6 (at 2 months) was observed in the male Nogfl/fl;AdipoqCre mice (Figure 5B).
In addition, we investigated the expression by immunoblotting of the lipogenic genes FASN and SCD1 and the lipolytic gene HSL. Both FASN and SCD1 as well as HSL and phosphorylated (p)-HSL decreased in the female Nogfl/fl;AdipoqCre mice (Figure 5C). Interestingly, FASN and SCD1 showed a late induction (at 6 months) without a change in HSL or p-HSL in the male Noggin-deficient males.
Thus, there were clear gender differences with the females being the most affected gender. The females showed a suppression in BAT markers and genes associated with thermogenesis, adipogenesis and lipolysis, whereas the males showed early changes in a few of the BAT markers and thermogenic genes, and then a late induction of the lipogenic genes FASN and SCD1. Together, it suggests that the BMP signaling regulates female and male adipogenesis through different metabolic pathways.
Finally we analyzed the oxygen consumption rate (OCR) in BAT from female and male Nogfl/fl;AdipoqCre and Nogfl/fl control mice at 2 months of age, before major weight gains in the Noggin-deficient mice. The analysis showed no difference in the basal OCR in females, whereas it was decreased in the males (Figure 5D). After addition of etomoxir, an inhibitor of Cpt1a, an additional reduction in OCR was seen in the Nogfl/fl;AdipoqCre mice, as compared to the Nogfl/fl control mice (Figure 5D), suggesting an impaired potential for beta-oxidation in Noggin-deficient BAT.
Expression of inflammatory markers, including the macrophage marker CD68, IL-6, and the adhesion molecules ICAM-1 and VCAM-1 were enhanced in BAT at one year of age in females but not in males (Supplemental Fig. 7). No change was detected in the expression of the endothelial marker CD31 (data not shown).
The differential effect of Noggin deletion on the transcriptional landscape may explain the greater obesity in the Nogfl/fl;AdipoqCre mice females, as compared to the males. This suggests that changes in BMP signaling have gender-specific effects, and that the females are more responsive to Noggin depletion and the corresponding increase in BMP activity, especially at an early stage, which is suggested by the strong p-SMAD1/5 activation at 2 months of age (Figure 5C).
3.5. Expression of BMPs and BMP inhibitors in the HMDP
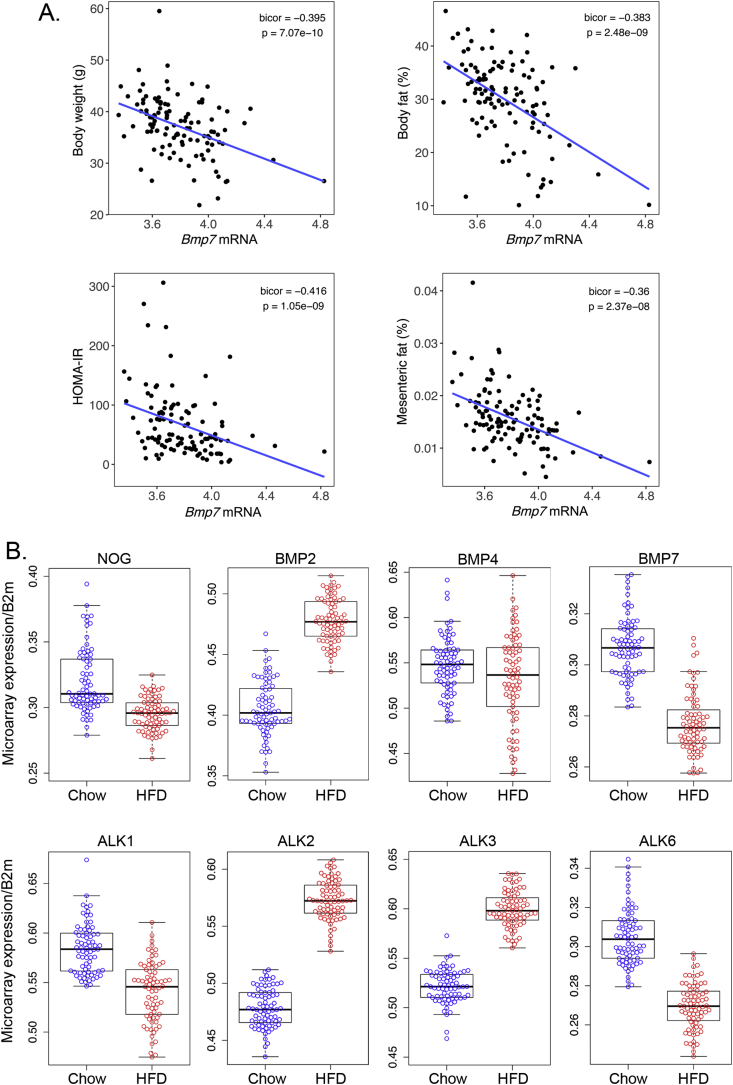
The HMDP is a collection of approximately 100 well-characterized inbred strains of mice that display a substantial diversity of metabolic phenotypes relevant to human obesity. We explored two studies in the HMDP database established by Lusis et al. [59], [60], [61], [64]. First, we found that certain BMPs have relationships with obesity phenotypes, adipose expression of BMP7 in particular correlates negatively with body weight, percent fat, and insulin resistance index (HOMA-IR) (Figure 6A). We further discovered that expression of several BMP signaling components changed significantly in adipose tissue in response to a high-fat/high-sucrose (HF/HS) diet as compared to chow-fed mice by microarray expression. Noggin and BMP7 both decreased and BMP2 increased, whereas BMP4 did not change significantly, although downstream BMP activity might intensify due to the lack of Noggin. In addition, the BMP type 1 receptors ALK2 and ALK3 were induced by the HF/HS diet, whereas the ALK1 and ALK6 receptors were suppressed, allowing for another level of BMP regulation (Figure 6B). Overall, the HMDP results supported that BMP modulation is important in adipose regulation.
Figure 6.
Adipose expression of BMP signaling components respond to high fat/high sucrose feeding in mice in the HMDP database. (A) Relationship between adipose expression of BMP7 and obesity phenotypes. (B) Expression of BMP signaling components responds to HF/HS diet compared to chow, as determined by microarray expression.
4. Discussion
Our results demonstrate that Noggin modulates BMP signaling in adipose tissue in a way that may have long-term protective actions against obesity. The results suggest that Noggin provides such protection by limiting lipid accumulation in individual adipocytes; loss of Noggin causes a “whitening” of BAT and hypertrophy of WAT through regulation of the genes for BAT differentiation and thermogenesis as well as for lipid metabolism regulatory genes. Furthermore, the effect is influenced by age and gender, and was especially pronounced in females. The impaired BAT function was detected early and preceded significant changes in the WAT.
BMP signaling is important in both morphogenesis and cell differentiation, and constellations of BMPs and BMP antagonists often act in time- and space-dependent manners during development [13], [14]. The relationship between the BMPs and their antagonists can be complicated since most of the antagonists inhibit multiple BMPs each [32], [33], [35], and some are induced by the BMP signaling as part of feedback regulation [65]. Therefore, the removal of an inhibitor can sway the entire system rather than make a discrete change in the activity of one BMP. The BMP signaling was increased in the Noggin-deficient adipose tissue, as observed by increased the p-SMAD1/5 phosphorylation. However, we cannot with certainty link this change in SMAD activation to a specific BMP since Noggin antagonizes several BMPs including BMP4 and BMP7 [34], and possibly BMP8b due to its similarity to BMP7.
The results showed that Noggin expression was relatively stable in the sub-fractions of the ASCs, whereas that of BMP4, BMP7 and MGP was strongest in the CD34-positive cells. It is possible that Noggin is part of a temporal regulation, and functions as a barrier to activation of certain BMPs until the cells are ready for lipid accumulation. The in vitro results supported a negative correlation between Noggin and lipid accumulation and adipogenic differentiation, an overall opposite action to that of BMP4, at least in vitro. A decrease in Noggin might also modulate the fat distribution by counteracting BMP2, which stimulates white adipogenesis and is more expressed in visceral than in subcutaneous fat depots [20]. A loss of Noggin would be less likely, however, to be due to enhanced BMP8b activity since this has been reported to increases brown adipose tissue thermogenesis [28].
When comparing our results to the HMDP database, Noggin expression is decreased in diet-induced obesity in mice from the HMDP, which would be consistent with the obesity phenotype seen in Noggin-deficient mice. BMP7 decreased in concert with Noggin, whereas BMP2 increased, suggesting that increased BMP2 activity may be important in handling the incoming lipids. The declining BMP7 would be expected to further enhance the whitening, resulting in an association between low BMP7 levels and increased body weight, body fat, HOMA-IR and mesenteric fat.
Overall, a change in the inhibitor levels may have unexpected effects depending on the underlying BMP balance. Protein characteristics, cellular location, binding to the extracellular matrix, timing and location of expression, and varying affinities for various BMPs or BMP receptors are all aspects that can influence the role of an inhibitor. Indeed, the variety of inhibitors will make them, as a group, the perfect mediators of numerous BMP functions. For example, Noggin is a soluble protein [66] that diffuses in the tissue and contributes to tissue gradients of BMP activity. Gremlin, on the other hand, is able to form large complexes with BMP [67], while MGP is a poorly soluble protein that might immobilize BMPs in the matrix [68]. Both Gremlin and MGP are secreted by preadipocytes [36], [42], but it is unclear how they differ in their BMP inhibitory function during differentiation compared to Noggin.
Our results are consistent with previous reports from Modica et al. [27] that showed that overexpression of BMP4 in brown tissue after intra-brown tissue administration of Bmp4-containing adenoviral vectors induced a shift to white-like phenotype in the brown adipocytes. In contrast, Qian et al. [23] demonstrated that BMP4 overexpression regulated by the fatty acid-binding protein (Fabp4)/adipocyte Protein 2 (aP2) promoter caused induction of oxidative genes and smaller lipid droplets. The use of the aP2-promoter has been reported to result in ectopic expression of BMP4 in multiple cells including macrophages and endothelial cells [69], which might explain the different results, especially when considering that adipocytes have been traced from endothelial cells [11].
It has been reported that the maximum size of adipocytes is around 1.0 μg of lipid per cell [70]. Here it appears that it can be exceeded in Noggin-deficient adipocytes in certain locations. Assuming that the calculated areas represent cross-sections of spherical adipocytes, the estimated size of the largest adipocytes would be in the range of 5–6 μg of lipid, i.e. 5–6-fold larger than the largest adipocyte in obese patients [70].
In our studies, the BMP signaling is affected by gender, and activated p-SMAD1/5 is higher in females at an early age. This effect may derive from gender differences in the upstream regulation of the BMP genes or in the downstream target genes, such as the inhibitor of DNA binding (ID) transcription factors. Indeed, Qian et al. [71] reported that reciprocal interactions between BMP4 and estrogen/ERa signaling could promote obesity and insulin resistance in aging female mice. These interactions could be directly targeted by Noggin through its interaction with BMP4. The findings by Grefhorst et al. [72] might also be applicable in our case. They reported that estrogen increases BMP8b expression in BAT, which might add to the effect on the BAT and the thermogenesis in the female mice. The male mice showed little difference in the thermogenic genes, but did show evidence of enhanced lipogenesis ultimately resulting in a similar phenotype.
On the transcriptional level, the ID1-4 transcription factors have been implicated in the regulation of adipogenic differentiation and development [73]. These are frequently targeted by BMP signaling. Interestingly, overexpression of ID1 in the adipose tissue results in age-associated and diet-induced obesity in mice, which has similarities to the Noggin-deficient phenotype, whereas lack of ID1 enhances the expression of UCP1 and genes associated with beige adipocytes in response to cold exposure [74]. Thus, the ID factors are likely to be important in mediating the BMP effects in adipose tissue. Altogether, the differences that arise from gender-specific differences in the Noggin-deficient mice will require further investigations.
5. Conclusions
Noggin declines in adipose progenitor cells during adipogenesis in vitro allowing for lipid accumulation. In a similar way, adipocyte-specific Noggin depletion in mice promotes age-related obesity with white adipose tissue hypertrophy in subcutaneous and omental fat pads, and whitening, reduced brown adipose function and diminished expression of PGC1alpha and UCP1. The obesity is age-related, independent of food intake, and occurs in both genders, but with reductions in heat production and expression of mitochondria and beta-oxidation markers restricted to female mice. Thus, the BMP antagonists may be essential for long-term regulation of obesity.
Author contributions
A.M.B.M. conceptualization, methodology, validation, formal analysis, investigation, data curation, writing – original draft, writing – review & editing, visualization, M.J. investigation, validation, formal analysis, P.R. investigation, formal analysis, T.S. investigation, formal analysis, Y.G. methodology, formal analysis, J.Y. investigation, formal analysis, L.V. investigation, formal analysis, K.R. investigation, formal analysis, L.Z. conceptualization, investigation, Y.Y. conceptualization, investigation, A.M.F. conceptualization, supervision, project administration, funding acquisition, P.T. conceptualization, supervision, A.J.L. conceptualization, supervision, X.W. methodology, validation, formal analysis, investigation, data curation, writing – review & editing, visualization, K.I.B. conceptualization, formal analysis, writing – original draft, writing – review & editing, visualization, supervision, project administration, funding acquisition
Acknowledgments
Funding for this work was provided in part by NIH/NHLBI: grant number HL30568 (A.M.F., K.I.B., P.T., A.J.L), NIH/NHLBI: grant number HL81397 (K.I.B.), and NIH/NHLBI: grant number HL112839 (K.I.B.), and the American Heart Association: grant number 13SDG17190013 (M.J.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.04.004.
Contributor Information
Xiuju Wu, Email: xiujuwu@mednet.ucla.edu.
Kristina I. Boström, Email: kbostrom@mednet.ucla.edu.
Conflict of interest
There are no financial interests of conflicts of interest to disclose for any of the authors.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.W.H. Organization . vol. 2. 2017. pp. 1–12. (Double-duty actions for nutrition: policy brief WHO/NMH/NHD/17). [Google Scholar]
- 2.Poirier P., Giles T.D., Bray G.A., Hong Y., Stern J.S., Pi-Sunyer F.X., Eckel R.H., A. American Heart, P.A. Obesity Committee of the Council on Nutrition, Metabolism Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Cohen P., Spiegelman B.M. Cell biology of fat storage. Molecular Biology of the Cell. 2016;27:2523–2527. doi: 10.1091/mbc.E15-10-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Q.Q., Lane M.D. Adipogenesis: from stem cell to adipocyte. Annual Review of Biochemistry. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- 5.Kajimura S., Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annual Review of Physiology. 2014;76:225–249. doi: 10.1146/annurev-physiol-021113-170252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiefer F.W. The significance of beige and brown fat in humans. Endocrine Connections. 2017;6:R70–R79. doi: 10.1530/EC-17-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartelt A., Heeren J. Adipose tissue browning and metabolic health. Nature Reviews Endocrinology. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 8.Cinti S. UCP1 protein: the molecular hub of adipose organ plasticity. Biochimie. 2017;134:71–76. doi: 10.1016/j.biochi.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Rosenwald M., Wolfrum C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte. 2014;3:4–9. doi: 10.4161/adip.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishibashi J., Seale P. Medicine. Beige can be slimming. Science. 2010;328:1113–1114. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran K.V., Gealekman O., Frontini A., Zingaretti M.C., Morroni M., Giordano A. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metabolism. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Planat-Benard V., Silvestre J.S., Cousin B., Andre M., Nibbelink M., Tamarat R. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 13.Carreira A.C., Alves G.G., Zambuzzi W.F., Sogayar M.C., Granjeiro J.M. Bone morphogenetic proteins: structure, biological function and therapeutic applications. Archives of Biochemistry and Biophysics. 2014;561:64–73. doi: 10.1016/j.abb.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Wang R.N., Green J., Wang Z., Deng Y., Qiao M., Peabody M. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes and Diseases. 2014;1:87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia de Vinuesa A., Abdelilah-Seyfried S., Knaus P., Zwijsen A., Bailly S. BMP signaling in vascular biology and dysfunction. Cytokine & Growth Factor Reviews. 2016;27:65–79. doi: 10.1016/j.cytogfr.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Morrell N.W., Bloch D.B., Ten Dijke P., Goumans M.J., Hata A., Smith J. Targeting BMP signalling in cardiovascular disease and anaemia. Nature Reviews Cardiology. 2016;13:106–120. doi: 10.1038/nrcardio.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegarty S.V., O'Keeffe G.W., Sullivan A.M. BMP-Smad 1/5/8 signalling in the development of the nervous system. Progress in Neurobiology. 2013;109:28–41. doi: 10.1016/j.pneurobio.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Nishinakamura R., Sakaguchi M. BMP signaling and its modifiers in kidney development. Pediatric Nephrology. 2014;29:681–686. doi: 10.1007/s00467-013-2671-9. [DOI] [PubMed] [Google Scholar]
- 19.Hines E.A., Sun X. Tissue crosstalk in lung development. Journal of Cellular Biochemistry. 2014;115:1469–1477. doi: 10.1002/jcb.24811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guiu-Jurado E., Unthan M., Bohler N., Kern M., Landgraf K., Dietrich A. Bone morphogenetic protein 2 (BMP2) may contribute to partition of energy storage into visceral and subcutaneous fat depots. Obesity. 2016;24:2092–2100. doi: 10.1002/oby.21571. [DOI] [PubMed] [Google Scholar]
- 21.Pera E.M., Acosta H., Gouignard N., Climent M., Arregi I. Active signals, gradient formation and regional specificity in neural induction. Experimental Cell Research. 2014;321:25–31. doi: 10.1016/j.yexcr.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Lin G., Garcia M., Ning H., Banie L., Guo Y.L., Lue T.F. Defining stem and progenitor cells within adipose tissue. Stem Cells and Development. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian S.W., Tang Y., Li X., Liu Y., Zhang Y.Y., Huang H.Y. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E798–E807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tseng Y.H., Kokkotou E., Schulz T.J., Huang T.L., Winnay J.N., Taniguchi C.M. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowers R.R., Lane M.D. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle. 2007;6:385–389. doi: 10.4161/cc.6.4.3804. [DOI] [PubMed] [Google Scholar]
- 26.Xue R., Wan Y., Zhang S., Zhang Q., Ye H., Li Y. Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes. American Journal of Physiology. Endocrinology and Metabolism. 2014;306:E363–E372. doi: 10.1152/ajpendo.00119.2013. [DOI] [PubMed] [Google Scholar]
- 27.Modica S., Straub L.G., Balaz M., Sun W., Varga L., Stefanicka P. Bmp4 promotes a brown to white-like adipocyte shift. Cell Reports. 2016;16:2243–2258. doi: 10.1016/j.celrep.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Whittle A.J., Carobbio S., Martins L., Slawik M., Hondares E., Vazquez M.J. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellegrinelli V., Peirce V.J., Howard L., Virtue S., Turei D., Senzacqua M. Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nature Communications. 2018;9:4974. doi: 10.1038/s41467-018-07453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz T.J., Huang P., Huang T.L., Xue R., McDougall L.E., Townsend K.L. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495:379–383. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo M.M., Kim S., Tseng C.Y., Jeon Y.H., Choe S., Lee D.K. BMP-9 as a potent brown adipogenic inducer with anti-obesity capacity. Biomaterials. 2014;35:3172–3179. doi: 10.1016/j.biomaterials.2013.12.063. [DOI] [PubMed] [Google Scholar]
- 32.Umulis D., O'Connor M.B., Blair S.S. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh D.W., Godson C., Brazil D.P., Martin F. Extracellular BMP-antagonist regulation in development and disease: tied up in knots. Trends in Cell Biology. 2010;20:244–256. doi: 10.1016/j.tcb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Blazquez-Medela A.M., Jumabay M., Bostrom K.I. Beyond the bone: bone morphogenetic protein signaling in adipose tissue. Obesity Reviews. 2019 doi: 10.1111/obr.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao Y., Jumabay M., Wang A., Bostrom K.I. Matrix Gla protein deficiency causes arteriovenous malformations in mice. Journal of Clinical Investigation. 2011;121:2993–3004. doi: 10.1172/JCI57567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutch D.M., Rouault C., Keophiphath M., Lacasa D., Clement K. Using gene expression to predict the secretome of differentiating human preadipocytes. International Journal of Obesity. 2009;33:354–363. doi: 10.1038/ijo.2009.3. [DOI] [PubMed] [Google Scholar]
- 37.van Beek E.A., Bakker A.H., Kruyt P.M., Hofker M.H., Saris W.H., Keijer J. Intra- and interindividual variation in gene expression in human adipose tissue. Pflügers Archiv: European journal of physiology. 2007;453:851–861. doi: 10.1007/s00424-006-0164-4. [DOI] [PubMed] [Google Scholar]
- 38.Sawant A., Chanda D., Isayeva T., Tsuladze G., Garvey W.T., Ponnazhagan S. Noggin is novel inducer of mesenchymal stem cell adipogenesis: implications for bone health and obesity. Journal of Biological Chemistry. 2012;287:12241–12249. doi: 10.1074/jbc.M111.293613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafson B., Smith U. The WNT inhibitor Dickkopf 1 and bone morphogenetic protein 4 rescue adipogenesis in hypertrophic obesity in humans. Diabetes. 2012;61:1217–1224. doi: 10.2337/db11-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Q., Tang S.G., Yuan Z.M. Gremlin 2 inhibits adipocyte differentiation through activation of Wnt/beta-catenin signaling. Molecular Medicine Reports. 2015;12:5891–5896. doi: 10.3892/mmr.2015.4117. [DOI] [PubMed] [Google Scholar]
- 41.Gu C., Xu Y., Zhang S., Guan H., Song S., Wang X. miR-27a attenuates adipogenesis and promotes osteogenesis in steroid-induced rat BMSCs by targeting PPARgamma and GREM1. Scientific Reports. 2016;6:38491. doi: 10.1038/srep38491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gustafson B., Hammarstedt A., Hedjazifar S., Hoffmann J.M., Svensson P.A., Grimsby J. BMP4 and BMP antagonists regulate human white and beige adipogenesis. Diabetes. 2015;64:1670–1681. doi: 10.2337/db14-1127. [DOI] [PubMed] [Google Scholar]
- 43.Stafford D.A., Brunet L.J., Khokha M.K., Economides A.N., Harland R.M. Cooperative activity of noggin and gremlin 1 in axial skeleton development. Development. 2011;138:1005–1014. doi: 10.1242/dev.051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eguchi J., Wang X., Yu S., Kershaw E.E., Chiu P.C., Dushay J. Transcriptional control of adipose lipid handling by IRF4. Cell Metabolism. 2011;13:249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alva J.A., Zovein A.C., Monvoisin A., Murphy T., Salazar A., Harvey N.L. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 46.Yao Y., Jumabay M., Ly A., Radparvar M., Wang A.H., Abdmaulen R. Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood. 2012;119:5037–5047. doi: 10.1182/blood-2011-10-385906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jumabay M., Abdmaulen R., Ly A., Cubberly M.R., Shahmirian L.J., Heydarkhan-Hagvall S. Pluripotent stem cells derived from mouse and human white mature adipocytes. Stem Cells Translational Medicine. 2014;3:161–171. doi: 10.5966/sctm.2013-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jumabay M., Abdmaulen R., Urs S., Heydarkhan-Hagvall S., Chazenbalk G.D., Jordan M.C. Endothelial differentiation in multipotent cells derived from mouse and human white mature adipocytes. Journal of Molecular and Cellular Cardiology. 2012;53:790–800. doi: 10.1016/j.yjmcc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jumabay M., Zhumabai J., Mansurov N., Niklason K.C., Guihard P.J., Cubberly M.R. Combined effects of bone morphogenetic protein 10 and crossveinless-2 on cardiomyocyte differentiation in mouse adipocyte-derived stem cells. Journal of Cellular Physiology. 2018;233:1812–1822. doi: 10.1002/jcp.25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLean J.A., Tobin G. Cambridge University Press; Cambridge Cambridgeshire; New York: 1987. Animal and human calorimetry. [Google Scholar]
- 51.de Jong J.M., Larsson O., Cannon B., Nedergaard J. A stringent validation of mouse adipose tissue identity markers. American Journal of Physiology. Endocrinology and Metabolism. 2015;308:E1085–E1105. doi: 10.1152/ajpendo.00023.2015. [DOI] [PubMed] [Google Scholar]
- 52.Yao Y., Jumabay M., Ly A., Radparvar M., Cubberly M.R., Bostrom K.I. A role for the endothelium in vascular calcification. Circulation Research. 2013;113:495–504. doi: 10.1161/CIRCRESAHA.113.301792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bostrom K., Zebboudj A.F., Yao Y., Lin T.S., Torres A. Matrix GLA protein stimulates VEGF expression through increased transforming growth factor-beta1 activity in endothelial cells. Journal of Biological Chemistry. 2004;279:52904–52913. doi: 10.1074/jbc.M406868200. [DOI] [PubMed] [Google Scholar]
- 54.Yao Y., Bennett B.J., Wang X., Rosenfeld M.E., Giachelli C., Lusis A.J. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circulation Research. 2010;107:485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsumoto T., Kano K., Kondo D., Fukuda N., Iribe Y., Tanaka N. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. Journal of Cellular Physiology. 2008;215:210–222. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 56.Wang X., Minze L.J., Shi Z.Z. Functional imaging of brown fat in mice with 18F-FDG micro-PET/CT. Journal of Visualized Experiments. 2012 doi: 10.3791/4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fueger B.J., Czernin J., Hildebrandt I., Tran C., Halpern B.S., Stout D. Impact of animal handling on the results of 18F-FDG PET studies in mice. Journal of Nuclear Medicine. 2006;47:999–1006. [PubMed] [Google Scholar]
- 58.Martin L.J., Lau E., Singh H., Vergnes L., Tarling E.J., Mehrabian M. ABCC6 localizes to the mitochondria-associated membrane. Circulation Research. 2012;111:516–520. doi: 10.1161/CIRCRESAHA.112.276667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennett B.J., Farber C.R., Orozco L., Kang H.M., Ghazalpour A., Siemers N. A high-resolution association mapping panel for the dissection of complex traits in mice. Genome Research. 2010;20:281–290. doi: 10.1101/gr.099234.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parks B.W., Nam E., Org E., Kostem E., Norheim F., Hui S.T. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell metabolism. 2013;17:141–152. doi: 10.1016/j.cmet.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parks B.W., Sallam T., Mehrabian M., Psychogios N., Hui S.T., Norheim F. Genetic architecture of insulin resistance in the mouse. Cell Metabolism. 2015;21:334–346. doi: 10.1016/j.cmet.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu W., Kim J., Cheng C., Rawlins B.A., Boachie-Adjei O., Crystal R.G. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone. 2006;39:61–71. doi: 10.1016/j.bone.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calderon-Dominguez M., Mir J.F., Fucho R., Weber M., Serra D., Herrero L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte. 2016;5:98–118. doi: 10.1080/21623945.2015.1122857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lusis A.J., Seldin M.M., Allayee H., Bennett B.J., Civelek M., Davis R.C. The hybrid mouse diversity panel: a resource for systems genetics analyses of metabolic and cardiovascular traits. The Journal of Lipid Research. 2016;57:925–942. doi: 10.1194/jlr.R066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao Y., Zebboudj A.F., Shao E., Perez M., Bostrom K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. Journal of Biological Chemistry. 2006;281:33921–33930. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
- 66.Groppe J., Greenwald J., Wiater E., Rodriguez-Leon J., Economides A.N., Kwiatkowski W. Structural basis of BMP signalling inhibition by the cystine knot protein. Noggin Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- 67.Kisonaite M., Wang X., Hyvonen M. Structure of Gremlin-1 and analysis of its interaction with BMP-2. Biochemical Journal. 2016;473:1593–1604. doi: 10.1042/BCJ20160254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Price P.A., Williamson M.K. Primary structure of bovine matrix Gla protein, a new vitamin K-dependent bone protein. Journal of Biological Chemistry. 1985;260:14971–14975. [PubMed] [Google Scholar]
- 69.Jeffery E., Berry R., Church C.D., Yu S., Shook B.A., Horsley V. Characterization of Cre recombinase models for the study of adipose tissue. Adipocyte. 2014;3:206–211. doi: 10.4161/adip.29674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gustafson B., Smith U. Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk. Atherosclerosis. 2015;241:27–35. doi: 10.1016/j.atherosclerosis.2015.04.812. [DOI] [PubMed] [Google Scholar]
- 71.Qian S.W., Liu Y., Wang J., Nie J.C., Wu M.Y., Tang Y. BMP4 cross-talks with estrogen/ERalpha signaling to regulate adiposity and glucose metabolism in females. EBioMedicine. 2016;11:91–100. doi: 10.1016/j.ebiom.2016.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grefhorst A., van den Beukel J.C., van Houten E.L., Steenbergen J., Visser J.A., Themmen A.P. Estrogens increase expression of bone morphogenetic protein 8b in brown adipose tissue of mice. Biology of Sex Differences. 2015;6:7. doi: 10.1186/s13293-015-0025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patil M., Sharma B.K., Satyanarayana A. Id transcriptional regulators in adipogenesis and adipose tissue metabolism. Frontiers in Bioscience. 2014;19:1386–1397. doi: 10.2741/4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patil M., Sharma B.K., Elattar S., Chang J., Kapil S., Yuan J. Id1 promotes obesity by suppressing brown adipose thermogenesis and white adipose. Browning Diabetes. 2017;66:1611–1625. doi: 10.2337/db16-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.