Summary
The immune system has a well‐established contribution to tissue homeostasis and wound healing. However, in many cases immune responses themselves can cause severe tissue damage. Thus, the question arose to which extent cells of the immune system directly contribute to the process of wound healing and to which extent the resolution of excessive immune responses may indirectly contribute to wound healing. FoxP3‐expressing CD4 T‐cells, so‐called regulatory T‐cells (Tregs), have an important contribution in the regulation of immune responses; and, in recent years, it has been suggested that Tregs next to an immune‐regulatory, ‘damage‐limiting’ function may also have an immune‐independent ‘damage‐resolving’ direct role in wound healing. In particular, the release of the epidermal growth factor‐like growth factor Amphiregulin by tissue‐resident Tregs during wound repair suggested such a function. Our recent findings have now revealed that Amphiregulin induces the local release of bio‐active transforming growth factor (TGF) β, a cytokine involved both in immune regulation as well as in the process of wound repair. In light of these findings, we discuss whether, by locally activating TGF β, Treg‐derived Amphiregulin may contribute to both wound repair and immune suppression. Furthermore, we propose that Treg‐derived Amphiregulin in an autocrine way may enable an IL‐33‐mediated survival and expansion of tissue‐resident Tregs upon injury. Furthermore, Treg‐derived Amphiregulin may contribute to a constitutive, low‐level release of bio‐active TGF β within tissues, leading to continuous tissue regeneration and to an immune‐suppressive environment, which may keep inflammation‐prone tissues in an homeostatic state.
Keywords: Amphiregulin, cytokines/cytokine receptors, IL‐33, regulation/suppression, signal transduction, tissue‐resident Tregs
Regulatory T‐cells have an established role in wound healing
Following a breach of tissue homeostasis (be it by infection or tissue injury), a highly complex but also well‐orchestrated process of local inflammation occurs, which guides local wound healing.1, 2 This process can roughly be separated into three distinct, consecutive but overlapping phases. These phases are traditionally divided into a pro‐inflammatory initiation phase, a tissue formation phase, and a resolution and tissue re‐organization phase.1 During each of these phases, different types of leucocytes infiltrate the site of tissue damage and play distinct, clearly defined roles.1 The injury itself induces a pro‐inflammatory initiation phase, during which neutrophils and pro‐inflammatory monocytes are recruited to the site of injury.2 These cells contribute to host defence, and the removal of cell debris and necrotic cells. The pro‐inflammatory initiation phase then transitions into the so‐called tissue formation phase, in which inflammation is already dampened and a differentiation of infiltrating monocytes into alternatively activated macrophages occurs. During this phase, angiogenesis and cell proliferation lead to a closure of the wound. In the following, the so‐called resolution or tissue‐remodelling phase, the immune response is actively suppressed and excessive tissue and cellular matrix growth is reversed.2 This process of wound healing has to be well orchestrated, and disruption of this process, for instance due to the infection of the healing wound, can lead to a failure of wound healing or excessive scar formation.3
The transition from the pro‐inflammatory initiation phase into the tissue formation phase and the resolution phase is critically controlled by immune‐regulatory mechanisms. In particular, FoxP3‐expressing regulatory CD4 T‐cells (Tregs) appear to play a critical role in this transition. Tregs rapidly migrate to and accumulate at sites of inflammation, such as at sites of injury.4, 5, 6 Depletion of Tregs during the different phases of wound healing, for instance by the application of diphtheria toxin (DT) to FoxP3:DTR transgenic mice or by the injection of CD25‐depleting antibodies, consistently led to aggravated inflammation and deteriorated clinical outcomes in a number of different injury model systems in mice.4, 6, 7, 8, 9, 10, 11, 12
For instance, in mouse models of myocardic infarction, the depletion of Tregs resulted in aggravated cardiac inflammation and deteriorated clinical outcome.9, 10 In these experiments, Treg cell depletion was associated with increased neutrophil and monocyte infiltration, and diminished alternatively activated macrophage polarization.9 In contrast, expansion of the Treg compartment, for instance, via activation using super‐agonistic anti‐CD28 monoclonal antibody administration9 or adoptive Treg transfer,10 reduced infarct size and improved tissue remodelling and functional performance of the heart. Similarly, in mouse models of ischaemic‐reperfused kidneys in Rag1 −/− mice, the transfer of Tregs reduced the influx of neutrophils and macrophages, and diminished innate cytokine transcription in the kidney, resulting in diminished renal injury.7 Also in mouse models of muscle injury, such as in the mdx mouse model of Duchenne muscular dystrophy11 or acute muscle injury,4 DTR‐mediated depletion of Tregs in FoxP3:DTR transgenic mice exacerbated muscle injury and the severity of muscle inflammation, which was associated with a prolonged infiltration of pro‐inflammatory monocytes and a bias towards classically activated macrophages. In contrast, expansion of Tregs via the application of IL‐2/anti‐IL‐2 complexes in mdx mice led to decreased myofibre injury and suppressed inflammation in muscles associated with muscle fibre injury.11
Thus, taken together, these findings clearly support the conclusion that Tregs critically contribute to the process of wound healing.
Immune‐mediated roles of regulatory T‐cells during wound healing
One of the underlying mechanisms associated with Treg‐mediated wound healing is assumed to be the suppression of pro‐inflammatory stimuli. Such Treg‐mediated immune suppression appears to be a critical factor allowing for the progression of the wound‐healing process.13 Tregs utilize a number of different mechanisms to locally mediate their immune‐suppressive function.14 These mechanisms include the local activation of bio‐active transforming growth factor (TGF)β, 15, 16 the secretion of the immune‐suppressive cytokine IL‐1017 or the conversion of local adenosine monophosphate into adenosine.18 Accordingly, in mdx mice treated with IL‐2/anti‐IL‐2 complexes, more Tregs and increased IL‐10 concentrations were found in injured muscles.11 Also, in an ischaemic‐reperfused kidney model and an experimental brain ischaemia model in Rag1 −/− mice, the adoptive transfer of wild‐type, but not of IL‐10‐deficient Tregs, was sufficient to ameliorate tissue injury.7, 8 Furthermore, in a model of lipopolysaccharides (LPS)‐induced acute lung injury, the transfer of wild‐type, but not of CD73‐deficient Tregs, ameliorated lung injury in Rag −/− mice; strongly suggesting that the CD73‐mediated adenosine generation and thus induced immune suppression by Tregs contributed to the restoration of tissue homeostasis.19
Thus, the resolution of local inflammation is clearly a key function of Tregs during wound healing (Fig. 1).
Figure 1.
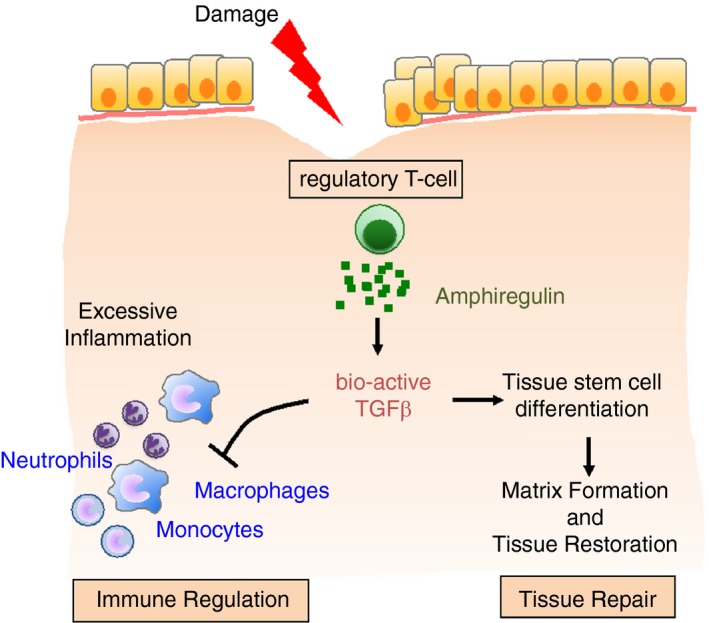
Dual function of regulatory T‐cells during wound healing. The process of wound healing has to be well orchestrated and disruption of this process, for instance due to the infection of the healing wound, can lead to a failure of wound healing or excessive scar formation. Regulatory T‐cells rapidly accumulate at sites of injury and keep local immune responses under control so that no excessive immune responses develop that could cause additional damage. This immune‐regulatory ‘damage‐limiting’ function of regulatory T‐cells is complemented by an immune‐independent ‘damage‐resolving’ direct role in wound healing. In this process, regulatory T‐cells at the site of injury release growth factors, such as the epidermal growth factor (EGF)‐like growth factor Amphiregulin, that directly contribute to the differentiation of cells within the injured tissues, in this way contributing to wound healing and the restoration of tissue homeostasis. TGFβ, transforming growth factor β.
Non‐immune‐mediated roles of regulatory T‐cells during wound healing
In recent years, it has further been suggested that Tregs may also directly contribute to wound healing, independent of their immune‐regulatory function.4, 20 Such a notion was supported by the discovery that in several different tissues, such as the muscle,4 a specific subtype of Tregs, so called tissue‐resident Tregs, 21, 22 expresses the epidermal growth factor (EGF)‐like growth factor Amphiregulin.
Amphiregulin is an EGF‐like growth factor associated with a number of different physiological processes, such as tissue homeostasis, inflammation and immunity.23, 24 With regard to tissue homeostasis, a direct role for Amphiregulin has, for instance, recently been demonstrated for the gingiva.25 The gingiva is a key oral barrier site and Amphiregulin gene‐deficient mice showed at steady‐state a substantially elevated level of oral, periodontal pathology in comparison to wild‐type mice.25 Furthermore, during wound healing, a direct role of Amphiregulin has been suggested. For instance, during infections, the injection of recombinant Amphiregulin (rAREG) has in several different experimental settings demonstrated the amelioration of symptoms, such as during Influenza infection26, 27 or following viral‐bacterial co‐infections.28 In line with these findings, also during wound healing following muscle injury, the injection of rAREG enhanced the restoration of injured muscles, and the presence of rAREG enhanced the differentiation of muscle stem cells in vitro. These findings were further corroborated by findings from the Rudensky group, using a mouse strain with a Treg‐specific deficiency of Amphiregulin (FoxP3:cre x Areg fl/fl).20 These mice showed a substantially more severe form of symptoms following influenza infection than wild‐type mice,20 suggesting a direct role of Treg‐derived Amphiregulin in wound repair.
Other studies further supported the finding that during wound healing Tregs may have, independent of immune modulatory function, a complementary, regenerative role. For instance, in a model of lysolecithin‐mediated demyelination in the spinal cord of mice, Tregs were found to directly promote oligodendrocyte differentiation and myelin production.29 Depletion of Tregs led to a substantially impaired remyelination and oligodendrocyte differentiation, which could be reversed by the adoptive transfer of Tregs. In vitro studies then suggested that Treg‐derived matricellular protein CCN3 – a protein known to induce the expression of TGFβ‐related bone morphogenetic proteins30 – directly promoted oligodendrocyte progenitor cell differentiation and myelination.29 These data suggest that also in vivo Treg‐derived CCN3 may play a similar, wound‐healing supportive role in injured spinal cord tissue.
In addition, in a study using established models of tissue regeneration in zebrafish, the conditional ablation of FoxP3‐expressing zebrafish Tregs hampered organ regeneration.31 Dependent on the injured organ, infiltrating Tregs stimulated the proliferation of tissue precursor cells through the secretion of organ‐specific regenerative factors, such as Ntf3 for the spinal cord, Nrg1 for the heart, and Igf1 for the retina. Moreover, when Foxp3‐deficient zebrafish Tregs infiltrated the injured organs, they failed to express regenerative factors and thus could not contribute to wound healing.31
Combined, these findings strongly suggest that Tregs, in addition to an immune modulatory, ‘damage‐limiting’ function, may also have a direct wound repair, ‘damage‐resolving’ function (Fig. 1).
The role of Amphiregulin in immune regulation and wound healing
The concept of such a double function of Tregs for ‘damage‐limiting’ and ‘damage‐resolving’ during wound healing is rather appealing. Nevertheless, a number of different findings suggest that, on a molecular level, the two functions might not easily be separable.
In mammalians, the concept of a distinction of these two functions is mainly based on the finding that several types of tissue‐resident Tregs express Amphiregulin, and that the injection of rAREG supported the process of wound healing in several different model systems. However, the exact mechanism by which Amphiregulin contributes to wound healing had remained unresolved, while at the same time Amphiregulin had been shown before to enhance the suppressive capacity of Tregs in vitro and in vivo.32, 33, 34, 35, 36
We recently found that one critical function of Amphiregulin is to locally activate latent TGFβ.37 Thus, via this local release of TGFβ, Amphiregulin may contribute to both the local suppression of inflammation as well as to the local differentiation of tissue stem cells, and in this way to the process of wound healing and restoration of tissue homeostasis. In the following, we will discuss how this novel insight may influence our understanding of Treg‐derived Amphiregulin for tissue homeostasis, wound repair and immune suppression.
It has been well established that the receptor of Amphiregulin, the EGF receptor (EGFR), contributes to wound healing.38 So far, it has mainly been assumed that this receptor mediates its function by inducing the proliferation of epithelial cells within the wound;38 and, in line with such an assumption, it has recently indeed been published from the group of Belkaid that a specific function for CD8 T‐cell‐derived Amphiregulin is the induction of keratinocyte expansion within a healing skin wound.39 However, in contrast to high‐affinity EGFR ligands, such as EGF, TGFα or heparin‐binding EGF‐like growth factor (HB‐EGF), which activate the proliferation‐stimulating mitogen‐activated protein kinase (MAPK) signalling pathway,40, 41 the low‐affinity EGFR ligand Amphiregulin preferentially induces the phosphorylation of EGFR‐Y992 and thus the activation of the PLCγ signalling pathway.42, 43 As a consequence, Amphiregulin is a poor mitogen for many epithelial and mesenchymal cell types and it appears highly unlikely that Amphiregulin substantially contributes to wound healing by inducing the proliferation of epithelial cells at the site of wounding. Thus, the underlying mechanism by which Amphiregulin contributes to wound healing has largely remained unresolved.
We recently discovered that Amphiregulin induced the local activation of TGFβ.37 In mouse models of CCl4‐induced acute liver damage and of Nippostrongylus infection‐induced lung damage, we demonstrated that during wound healing Amphiregulin induced the TGFβ‐mediated differentiation of tissue stem cells and in this way critically contributed to the restoration of tissue homeostasis (Fig. 2). TGFβ is secreted in a latent form, and only by releasing it from this latent complex becomes the bio‐active form of TGFβ exposed.44 One way of releasing bio‐active TGFβ is the activation of integrin‐α V‐containing complexes.45 We found that Amphiregulin induced the activation of integrin‐α V‐containing complexes and thus the local activation of TGFβ (Fig. 3). This local activation of TGFβ induced the differentiation of blood vessel‐associated mesenchymal precursor cells, so called pericytes, into collagen‐producing myo‐fibroblasts; a process that critically contributed to the restoration of injured blood vessels and thus wound healing (Fig. 2).
Figure 2.
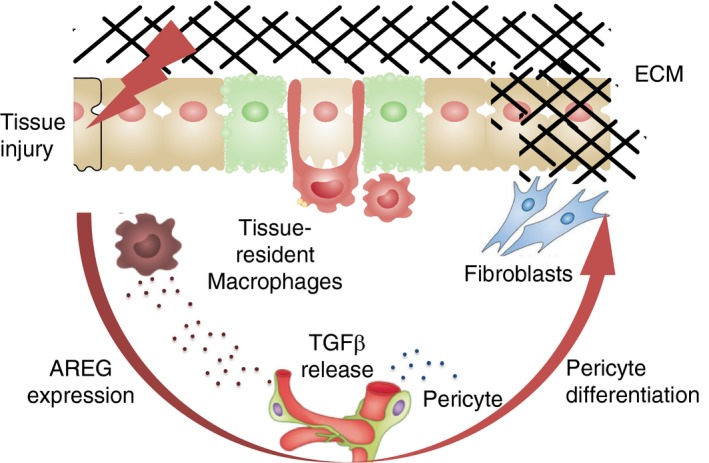
Amphiregulin contributes to wound healing by releasing local, bioactive transforming growth factor (TGF)β. Upon tissue damage, tissue‐resident macrophages sense the breach of tissue homeostasis and release Amphiregulin, which activates the local release of bio‐active TGFβ. This local release of bio‐active TGFβ induces the differentiation of local pericyte populations into myofibroblasts, which produce extracellular matrix components essential for the restoration of blood barrier function and the restoration of tissue homeostasis. AREG, Amphiregulin; ECM, extracellular matrix.
Figure 3.
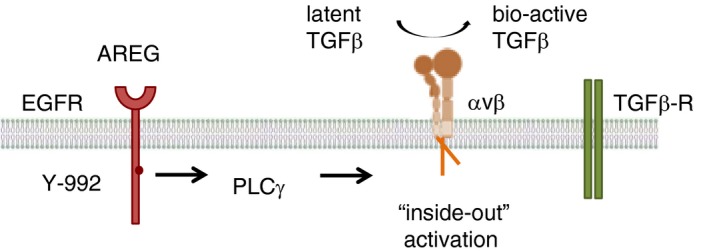
Amphiregulin induces an ‘inside‐out’ activation of integrin complexes that then lead to the local release of bioactive transforming growth factor (TGF)β. Amphiregulin induces a sustained PLCγ‐mediated signal via the epidermal growth factor receptor (EGFR). Such a sustained PLCγ‐mediated signalling induces an intracellular rearrangement of local actin fibres, and the physical separation of complexed, trans‐membrane integrin‐α and integrin‐β subunits, and thus to their extracellular activation. Such an ‘inside‐out’ activation of integrin‐α V‐containing complexes can induce the local release of bio‐active TGFβ from its latent form, and thus to the local activation of the TGFβ signalling pathway. AREG, Amphiregulin.
A number of growth factors and chemokine receptors as well as the T‐cell receptors (TCRs) use such an ‘inside‐out’ activation of integrin complexes to establish immunological synapsis between T‐cells and antigen‐presenting cells,46 or to allow leucocytes to attach themselves to blood vessels in order to cross into inflamed tissues.47 A critical step in this ‘inside‐out’ activation of integrin complexes is the sustained activation of the PLCγ signalling pathway.48 Amphiregulin preferentially induces the PLCγ signalling pathway42, 43 and is thus well situated to induce the activation of integrin‐α V‐containing complexes on target cells, which then results in the release of bio‐active TGFβ from its latent form (Fig. 3). Thus, taken together, our data show that one major function of Amphiregulin is the local induction of TGFβ.
Similar to Amphiregulin, also TGFβ has a double function during wound healing. For one, TGFβ induces the differentiation of tissue‐resident precursor cells; and, for the other, TGFβ is a key mediator of Treg‐mediated immune suppression.15 To achieve TGFβ‐mediated immune suppression, integrin‐α V‐containing complexes on Tregs have to be activated, which then release bio‐active TGFβ.16 A number of different groups have shown in in vitro suppression assays that rAREG enhances the suppressive capacity of Tregs. 32, 34, 35, 36 Also in in vivo settings, Amphiregulin enhances the suppressive capacity of Tregs. 32, 33 For instance, in a T‐cell transfer‐based colitis model, the titrated transfer of wild‐type Tregs into Rag1 −/− mice that had received naïve CD4 T‐cells could in a dose‐dependent manner suppress the induction of colitis, if the recipient Rag1 −/− mice were on a wild‐type background but not if the recipient Rag1 −/− mice had been backcrossed onto an Amphiregulin‐deficient background.32 Furthermore, following the adoptive transfer of antigen‐specific Tregs, these cells could suppress hapten‐induced ear swelling in wild‐type, but not in Amphiregulin gene‐deficient mice. These findings clearly demonstrated that endogenous expression of Amphiregulin is essential to ensure efficient Treg function. In line with this immune‐suppressive function of Amphiregulin, it has been reported that also following ischaemic stroke Treg‐derived Amphiregulin contributes to neurological recovery by suppressing IL‐6 expression within the brain and thus avoiding neurotoxic astrogliosis.6
Although we so far have not yet formally addressed whether Amphiregulin also activates integrin‐α V‐containing complexes on Tregs, our pericyte‐based finding of Amphiregulin‐mediated TGFβ activation suggests that such a mechanism might also be the underlying effect by which Amphiregulin enhances Treg function.32 Nevertheless, in consequence, this TGFβ‐activating role of Amphiregulin also means that in those experimental settings, in which rAREG has been injected into mice, the injected rAREG may have induced the release of bio‐active TGFβ. This release of bio‐active TGFβ may have enhanced the differentiation of tissue precursor cells, such as pericytes37 or muscle satellite cells,4 and thus may have directly contributed to wound healing; or, at the same time, may have enhanced the suppressive capacity of Tregs and thus may also indirectly have contributed to wound healing. Thus, in two ways may have contributed to tissue repair (Fig. 1).
The function of regulatory T‐cell‐derived Amphiregulin
In a similar way, it is possible that Treg‐derived Amphiregulin could induce the local release of bio‐active TGFβ and in this way the differentiation of tissue precursor cells; thus, may directly be contributing to tissue repair. Nevertheless, while Tregs have to be considered the most prominent cell type mediating local immune regulation, Tregs are not the only Amphiregulin‐producing cells within inflamed tissues; but, a wide range of other prominent Amphiregulin‐producing cell types, such as eosinophils, have been shown before to also critically contribute to the process of wound healing.49
Such a redundancy of cell types, which all could be potentially physiological relevant sources of Amphiregulin within injured tissues, raises the question what specific function Treg‐derived Amphiregulin might have. Amphiregulin‐expressing Tregs are typically restricted to tissue‐resident Treg populations and thus suggest an organ‐specific role for Treg‐expressed Amphiregulin.21 These tissue‐resident Treg populations also preferentially express the IL‐33 receptor, T1/ST2.6, 50, 51 The role of T1/ST2 expression on Tregs currently remains unresolved. However, IL‐33 is an important alarmin, released by dying cells, and recent data strongly suggest that IL‐33 signalling in Tregs provides a critical signal for Treg accumulation and maintenance in inflamed tissues.6, 50, 52, 53 However, also how IL‐33 may contribute to such an expansion of Treg populations at the site of inflammation remains largely unknown.
We have shown before that the EGFR forms hetero‐complexes with T1/ST2 on Th2 cells.43 These hetero‐complexes allowed Th2 cells to efficiently activate the MAPK signalling pathway and thus to induce the expression of IL‐13 upon exposure to IL‐3343 (Fig. 4). In CD4 T‐cells, it has been shown that EGFR is one of the most prominently upregulated trans‐membrane receptors upon STAT5 activation.54 We showed that both in Th2 cells as well as in Tregs the EGFR is strongly upregulated upon activation.32, 43 Also, kinome profiling in human Tregs revealed that the EGFR is one of the strongest upregulated kinases upon Treg activation.55 In line with these findings, the group of Rosenblum found induced EGFR expression in Tregs in a model of skin wounding in mice,12 and lineage‐specific deletion of EGFR in Tregs resulted in reduced Treg accumulation within the inflamed skin.12 Thus, in this respect the phenotype of EGFR‐ and T1/ST2‐deficient Tregs resembles each other, with both depicting a deficiency to expand at the site of inflammation.
Figure 4.
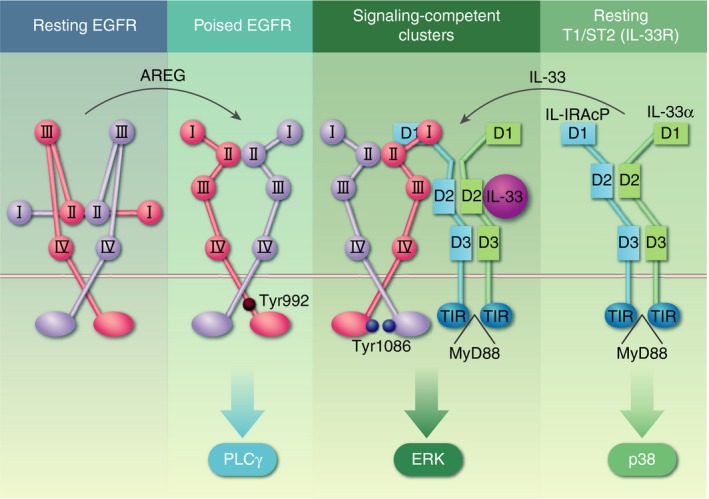
An Amphiregulin‐mediated autocrine activation of the epidermal growth factor receptor (EGFR) facilitates T‐cells to form a hetero‐complex with the IL‐33R (T1/ST2), which enables IL‐33 to activate the mitogen‐activated protein kinase (MAPK) signalling pathway. The low‐affinity EGFR ligand Amphiregulin activates resting EGFR complexes. This places the receptor into a ‘poised state’, which allows it to enter ‘signalling competent’ clusters on the cell surface and to interact with the IL‐33R (T1/ST2). Such clusters of hetero‐complexes between the EGFR and T1/ST2 then enables IL‐33 to mediate a MAPK‐mediated signal via the EGFR, and thus potentially the proliferation of tissue‐resident regulatory T‐cell populations. AREG, Amphiregulin.
These findings provide a scenario in which similar to Th2 cells also on Tregs T1/ST2 and the EGFR could form hetero‐complexes that may allow for IL‐33‐induced activation of the MAPK signalling pathway (Fig. 4). The MAPK signalling pathway is a pivotal signalling pathway for the transduction of mitogenic stimuli.41, 56 Thus, such an IL‐33‐mediated activation of the MAPK signalling pathway might therefore be a possible mechanism that may explain for the observed expansion of tissue‐resident Treg populations upon injection of rIL‐33.50, 52
Such hetero‐complexes between the EGFR and T1/ST2 on tissue‐resident Tregs could further enable these cells to become activated in a TCR‐independent way, as we have demonstrated before for Th2 cells.43 Upon tissue damage, antigen‐specific restimulation of tissue‐resident Treg populations via major histocompatibility complex (MHC)‐II antigen presentation and TCR‐mediated activation might not always be possible. Thus, the recognition of released IL‐33 from damaged tissues may constitute an alternative pathway of activation for tissue‐resident Treg populations. Such an assumption is further supported by a study using a NOD‐based model of autoimmune diabetes. This study demonstrated that Treg populations with a low‐affinity TCR for their cognate antigen preferentially expressed Amphiregulin, preferentially localized to the site of inflammation and functioned there in an antigen‐independent way.57 Thus, taken together, the combined expression of T1/ST2 and EGFR may enable tissue‐resident Treg populations to function in a MHC‐II‐independent way.
In Th2 cells, the induced expression of Amphiregulin, in an autocrine way, enabled the formation of such hetero‐complexes between EGFR and T1/ST2, and only upon expression of Amphiregulin could activated Th2 cells function in a MHC‐II‐independent way.43 Assuming a similar function for Amphiregulin in tissue‐resident Treg populations, then the constitutive expression of Amphiregulin may keep these Tregs in a ‘poised’ state (Fig. 4). In such a ‘poised’ state, tissue‐resident Treg populations would be able to rapidly respond to tissue damage and the exposure to IL‐33.
Such a poised state of tissue‐resident Treg populations could be critical during tissue injury, for instance due to the fact that IL‐33 release from necrotic cells is a very early event during tissue injury in many tissues. Thus, this poised state may allow to already induce the activation and expansion of Treg populations at the site of injury, while the infiltration of Treg populations derived from secondary lymphoid organs into injured tissues may so far not have been initiated yet.5, 6 Thus, the constitutive expression of Amphiregulin by tissue‐resident Tregs may constitute a first line of defence during wounding. However, also under steady‐state conditions, such a ‘poised’ state of tissue‐resident Treg populations could be contributing to tissue homeostasis; for instance in fatty tissues, in which a constant, IL‐33‐dependent but most likely antigen‐independent activation of Treg populations contributes to the control of local and systemic inflammation and metabolism.
Summary and outlook
Taken together, a wide range of different publications in recent years strongly suggested that Tregs play an important role in wound healing, both by suppressing local inflammation and also by directly contributing to the wound‐healing process. The exact mechanism of how Tregs contribute to these processes has remained to be resolved, and further research is necessary to fully resolve the specific function of Treg‐derived Amphiregulin. At this stage, it remains speculative to which extent Treg‐derived Amphiregulin directly contributes to wound healing, and to which extent it contributes to the local release of bioactive TGFβ, which then has both a wound‐healing and immune‐suppressive function at the site of injury.
However, alternatively, Treg‐derived Amphiregulin may also contribute to the survival and expansion of tissue‐resident Tregs upon injury. Because it was also further shown that T1/ST2‐expressing Treg populations preferentially express high levels of integrin‐α V and low levels of IL‐10,51 it is tempting to speculate that this constitutive expression of Amphiregulin by tissue‐resident Tregs may also lead to a constitutive, low‐level release of bio‐active TGFβ, which may contribute to ongoing regeneration of tissues and to a low‐level immune‐suppressive environment in the surrounding of Amphiregulin‐expressing tissue‐resident Treg populations, which might be an evolutionary advantage for instance in inflammation‐prone tissues, such as fatty tissues.
Disclosures
The authors have no competing interests.
References
- 1. Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science 2017; 356:1026–30. [DOI] [PubMed] [Google Scholar]
- 2. Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue‐specific contribution of macrophages to wound healing. Semin Cell Dev Biol 2017; 61:3–11. [DOI] [PubMed] [Google Scholar]
- 3. Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 2005; 15:599–607. [DOI] [PubMed] [Google Scholar]
- 4. Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y et al A special population of regulatory T cells potentiates muscle repair. Cell 2013; 155:1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo CT, Liao W, Dadi S, Toure A, Li MO. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature 2016; 529:532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ito M, Komai K, Mise‐Omata S, Iizuka‐Koga M, Noguchi Y, Kondo T et al Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019; 565:246–50. [DOI] [PubMed] [Google Scholar]
- 7. Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H et al Regulatory T cells suppress innate immunity in kidney ischemia‐reperfusion injury. J Am Soc Nephrol 2009; 20:1744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liesz A, Suri‐Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S et al Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 2009; 15:192–9. [DOI] [PubMed] [Google Scholar]
- 9. Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A et al Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 2014; 115:55–67. [DOI] [PubMed] [Google Scholar]
- 10. Sharir R, Semo J, Shimoni S, Ben‐Mordechai T, Landa‐Rouben N, Maysel‐Auslender S et al Experimental myocardial infarction induces altered regulatory T cell hemostasis, and adoptive transfer attenuates subsequent remodeling. PLoS ONE 2014; 9:e113653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villalta SA, Rosenthal W, Martinez L, Kaur A, Sparwasser T, Tidball JG et al Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med 2014; 6:258ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nosbaum A, Prevel N, Truong HA, Mehta P, Ettinger M, Scharschmidt TC, et al Cutting edge: regulatory T cells facilitate cutaneous wound healing. J Immunol 2016; 196:2010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li J, Tan J, Martino MM, Lui KO. Regulatory T‐cells: potential regulator of tissue repair and regeneration. Front Immunol 2018; 9:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012; 30:531–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor‐beta regulation of immune responses. Annu Rev Immunol 2006; 24:99–146. [DOI] [PubMed] [Google Scholar]
- 16. Worthington JJ, Kelly A, Smedley C, Bauche D, Campbell S, Marie JC et al Integrin alphavbeta8‐mediated TGF‐beta activation by effector regulatory T cells is essential for suppression of T‐cell‐mediated inflammation. Immunity 2015; 42:903–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X et al Regulatory T cell‐derived interleukin‐10 limits inflammation at environmental interfaces. Immunity 2008; 28:546–58. [DOI] [PubMed] [Google Scholar]
- 18. Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′‐adenosine monophosphate to adenosine. J Immunol 2006; 177:6780–6. [DOI] [PubMed] [Google Scholar]
- 19. Ehrentraut H, Clambey ET, McNamee EN, Brodsky KS, Ehrentraut SF, Poth JM et al CD73+ regulatory T cells contribute to adenosine‐mediated resolution of acute lung injury. FASEB J 2013; 27:2207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S et al A distinct function of regulatory T cells in tissue protection. Cell 2015; 162:1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A et al Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009; 15:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Panduro M, Benoist C, Mathis D. Tissue Tregs. Annu Rev Immunol 2016; 34:609–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berasain C, Avila MA. Amphiregulin. Semin Cell Dev Biol 2014; 28:31–41. [DOI] [PubMed] [Google Scholar]
- 24. Zaiss DM, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 2015; 42:216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krishnan S, Prise IE, Wemyss K, Schenck LP, Bridgeman HM, McClure FA et al Amphiregulin‐producing gammadelta T cells are vital for safeguarding oral barrier immune homeostasis. Proc Natl Acad Sci USA 2018; 115:10 738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA et al Innate lymphoid cells promote lung‐tissue homeostasis after infection with influenza virus. Nat Immunol 2011; 12:1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo XJ, Dash P, Crawford JC, Allen EK, Zamora AE, Boyd DF et al Lung gammadelta T cells mediate protective responses during neonatal influenza infection that are associated with type 2 immunity. Immunity 2018; 49:531–44 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jamieson AM, Pasman L, Yu S, Gamradt P, Homer RJ, Decker T et al Role of tissue protection in lethal respiratory viral‐bacterial coinfection. Science 2013; 340:1230–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dombrowski Y, O'Hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P et al Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci 2017; 20:674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tan TW, Huang YL, Chang JT, Lin JJ, Fong YC, Kuo CC et al CCN3 increases BMP‐4 expression and bone mineralization in osteoblasts. J Cell Physiol 2012; 227:2531–41. [DOI] [PubMed] [Google Scholar]
- 31. Hui SP, Sheng DZ, Sugimoto K, Gonzalez‐Rajal A, Nakagawa S, Hesselson D et al Zebrafish regulatory T cells mediate organ‐specific regenerative programs. Dev Cell 2017; 43:659–72 e5. [DOI] [PubMed] [Google Scholar]
- 32. Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Grone A, Sibilia M et al Amphiregulin enhances regulatory T cell‐suppressive function via the epidermal growth factor receptor. Immunity 2013; 38:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meulenbroeks C, van Weelden H, Schwartz C, Voehringer D, Redegeld FAM, Rutten V et al Basophil‐derived amphiregulin is essential for UVB irradiation‐induced immune suppression. J Invest Dermatol 2015; 135:222–8. [DOI] [PubMed] [Google Scholar]
- 34. Dai K, Huang L, Chen J, Yang L, Gong Z. Amphiregulin promotes the immunosuppressive activity of intrahepatic CD4(+) regulatory T cells to impair CD8(+) T‐cell immunity against hepatitis B virus infection. Immunology 2015; 144:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dai K, Huang L, Sun X, Yang L, Gong Z. Hepatic CD206‐positive macrophages express amphiregulin to promote the immunosuppressive activity of regulatory T cells in HBV infection. J Leukoc Biol 2015; 98:1071–80. [DOI] [PubMed] [Google Scholar]
- 36. Wang S, Zhang Y, Wang Y, Ye P, Li J, Li H et al Amphiregulin confers regulatory T cell suppressive function and tumor invasion via the EGFR/GSK‐3beta/Foxp3 Axis. J Biol Chem 2016; 291:21 085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Minutti C, Modak R, Macdonald F, Li F, Smyth D, Dorward D et al A macrophage‐pericyte axis directs tissue restoration via Amphiregulin‐induced TGFβ activation. Immunity 2019; 50:645–54. 10.1016/j.immuni.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schneider MR, Werner S, Paus R, Wolf E. Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol 2008; 173:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Linehan JL, Harrison OJ, Han SJ, Byrd AL, Vujkovic‐Cvijin I, Villarino AV et al Non‐classical immunity controls microbiota impact on skin immunity and tissue repair. Cell 2018; 172:784–96 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shankaran H, Ippolito DL, Chrisler WB, Resat H, Bollinger N, Opresko LK et al Rapid and sustained nuclear‐cytoplasmic ERK oscillations induced by epidermal growth factor. Mol Syst Biol 2009; 5:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Albeck JG, Mills GB, Brugge JS. Frequency‐modulated pulses of ERK activity transmit quantitative proliferation signals. Mol Cell 2013; 49:249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gilmore JL, Scott JA, Bouizar Z, Robling A, Pitfield SE, Riese DJ 2nd et al Amphiregulin‐EGFR signaling regulates PTHrP gene expression in breast cancer cells. Breast Cancer Res Treat 2008; 110:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Minutti CM, Drube S, Blair N, Schwartz C, McCrae JC, McKenzie AN et al Epidermal growth factor receptor expression licenses type‐2 helper T cells to function in a T cell receptor‐independent fashion. Immunity 2017; 47:710–22 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, Noguera I et al TGF‐beta latency: biological significance and mechanisms of activation. Stem Cells 1997; 15:190–7. [DOI] [PubMed] [Google Scholar]
- 45. Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J et al The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999; 96:319–28. [DOI] [PubMed] [Google Scholar]
- 46. Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T‐cell receptor signaling to integrins. Immunol Rev 2007; 218:65–81. [DOI] [PubMed] [Google Scholar]
- 47. Alon R, Shulman Z. Chemokine triggered integrin activation and actin remodeling events guiding lymphocyte migration across vascular barriers. Exp Cell Res 2011; 317:632–41. [DOI] [PubMed] [Google Scholar]
- 48. Lefort CT, Ley K. Neutrophil arrest by LFA‐1 activation. Front Immunol 2012; 3:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM et al Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013; 153:376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA et al The alarmin IL‐33 promotes regulatory T‐cell function in the intestine. Nature 2014; 513:564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Siede J, Frohlich A, Datsi A, Hegazy AN, Varga DV, Holecska V et al IL‐33 receptor‐expressing regulatory T cells are highly activated, Th2 biased and suppress CD4 T cell proliferation through IL‐10 and TGFbeta release. PLoS ONE 2016; 11:e0161507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ et al Poor repair of skeletal muscle in aging mice reflects a defect in local, Interleukin‐33‐dependent accumulation of regulatory T cells. Immunity 2016; 44:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Popovic B, Golemac M, Podlech J, Zeleznjak J, Bilic‐Zulle L, Lukic ML et al IL‐33/ST2 pathway drives regulatory T cell dependent suppression of liver damage upon cytomegalovirus infection. PLoS Pathog 2017; 13:e1006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY et al Priming for T helper type 2 differentiation by interleukin 2‐mediated induction of interleukin 4 receptor alpha‐chain expression. Nat Immunol 2008; 9:1288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tuettenberg A, Hahn SA, Mazur J, Gerhold‐Ay A, Scholma J, Marg I et al Kinome profiling of regulatory T cells: a closer look into a complex intracellular network. PLoS ONE 2016; 11:e0149193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002; 12:9–18. [DOI] [PubMed] [Google Scholar]
- 57. Sprouse ML, Shevchenko I, Scavuzzo MA, Joseph F, Lee T, Blum S et al Cutting edge: low‐affinity TCRs support regulatory T cell function in autoimmunity. J Immunol 2018; 200:909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


