Abstract
Spinal cord ischemia (SCI) is a devastating complication following thoracic endovascular aortic repair (TEVAR). A man with a ruptured thoracic aortic aneurysm (TAA) was transferred to our hospital. Emergency TEVAR, with left subclavian artery (LSA) coverage, was performed for the ruptured TAA. On postoperative day two, the patient had incomplete paralysis in his legs, presumably caused by SCI. We performed LSA revascularization (LSAR) to provide blood supply to the spinal cord; his paralysis improved and almost resolved after surgery. To our knowledge, this is the first report on LSAR’s efficacy for delayed paraplegia due to SCI.
Keywords: delayed paralysis, thoracic endovascular aortic repair, left subclavian artery revascularization
Introduction
Thoracic endovascular aortic repair (TEVAR) is an established treatment for thoracic aortic aneurysm (TAA). Spinal cord ischemia (SCI), an extremely devastating complication, occurs in patients undergoing TEVAR for TAA. However, its causes are multifactorial and not fully understood.
Various approaches to prevent SCI after TEVAR have been reported in the literature. The left subclavian artery (LSA) provides blood supply to the spinal cord via the vertebral artery. Left subclavian artery revascularization (LSAR), following LSA coverage, has proven useful.1) However, a meta-analysis showed a non-significant increase in SCI risk without LSAR1); thus, the need for routine LSAR for LSA coverage is still debatable. Furthermore, the need for LSAR after TEVAR is also unclear and controversial.1) We herein report a case in which LSAR was effective for delayed paraplegia due to SCI.
Case Report
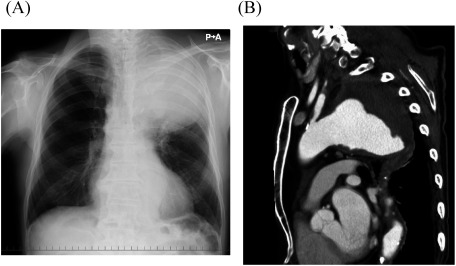
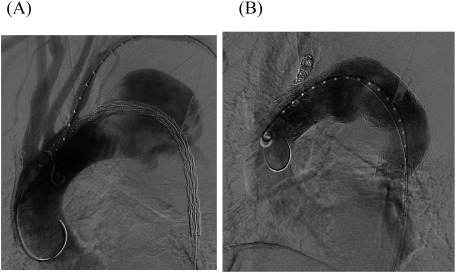
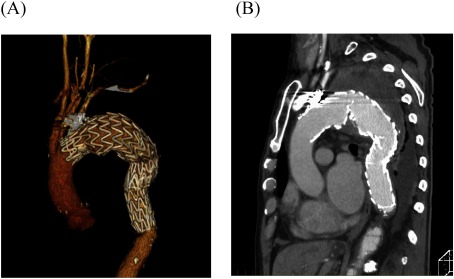
A 77-year-old man with massive hematemesis presented to another hospital’s emergency department. Emergency gastroscopy revealed no bleeding sources in the stomach or duodenum. Chest radiography showed a giant TAA (Fig. 1A). Contrast-enhanced computed tomography (CT) revealed a TAA (112 mm in diameter) close to the LSA (Fig. 1B), pulmonary hemorrhage, and obstructed left internal and external iliac arteries. The aneurysm may have caused hemoptysis from stabbing into the lungs; therefore, he was referred to our hospital for surgery. At the time of arrival, his blood pressure was 112/60 mmHg and his heart rate was 78 beats/min. Laboratory data revealed mild anemia (hemoglobin: 11.2 g/dL). He was diagnosed as having ruptured TAA because of his hemoptysis, and an emergency TEVAR was performed immediately. The descending thoracic aorta was stented with two grafts (GORE TAG®; W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) inserted through the right femoral artery. The proximal stent was 45 mm in diameter and 200 mm long; the distal stent was 45 mm in diameter and 150 mm long. LSA coverage was required to achieve an adequate proximal seal, and the stent graft’s distal site was covered at the level of the ninth thoracic vertebra (Figs. 2A and 2B). Additionally, LSA coil occlusion was performed. The patient was extubated after TEVAR. Neurological deficits were not observed. Postoperatively, the mean blood pressure remained above 90 mmHg in the intensive care unit. On postoperative day two, he showed weakness of the right lower limb muscles with gradual, incomplete paralysis of both lower limbs. At that time, there was no decrease in blood pressure. An emergency CT was performed to determine the cause of paralysis, but the aneurysm was completely shielded (Figs. 3A and 3B) with no obvious cerebral infarction. The neurologist’s opinion was that he had developed muscle weakness in the bilateral lower limbs. Several pyramidal signs appeared, including absent abdominal reflex and bilateral extensor plantar responses. His tendon reflex was diminished and symmetric. Extrapyramidal disorder was noted, and we suspected ischemia of the anterior spinal artery. About 10 h after symptoms appeared, we performed an axillary–axillary bypass to provide blood supply to the spinal cord via the left vertebral artery. Cerebrospinal fluid drainage (CSFD) was not performed because of prior spinal surgery. His postoperative respiratory condition was poor, and he was only extubated on the following day. After extubating, his paralysis improved considerably. He was ambulatory at discharge.
Fig. 1 (A) Chest radiography showing a huge thoracic aortic aneurysm in the upper left lung field. (B) Preoperative, contrast-enhanced computed tomography showing a giant ruptured thoracic aortic aneurysm close to the left subclavian artery.
Fig. 2 (A) Intraoperative angiography showing a thoracic aortic aneurysm close to the left subclavian artery (LSA). The need for LSA coverage is apparent. (B) The proximal stent was 45 mm in diameter and 200 mm long; the distal stent was 45 mm in diameter and 150 mm long. LSA coverage was required to achieve an adequate proximal seal. Additionally, LSA coil occlusion was performed.
Fig. 3 (A, B) Postoperative, contrast-enhanced computed tomography showing the stent graft covering the segment from the left subclavian artery to the level of the ninth thoracic vertebra and completely shielding the aneurysm.
Discussion
TEVAR is increasingly performed for TAA because of low in-hospital mortality and low postoperative morbidity. However, severe complications, such as paraplegia, occasionally occur despite various SCI prevention strategies. Post-TEVAR SCI occurs in up to 10.3% of patients, most of who never return to leading a normal life. Therefore, protective measures to reduce SCI in TEVAR are very important. Currently, there are two hypotheses to explain SCI’s occurrence: inadequate collateral blood supply to the spinal cord and atheroembolism of aortic plaque material in the segmental artery. However, SCI’s precise pathogenesis remains unclear.1) Additionally, multiple post-TEVAR risk factors for SCI occurrence have been identified. Therefore, it was very difficult to completely prevent SCI after TEVER.
Maintaining collateral circulation to the spinal cord to prevent SCI is critical,1,2) although other prevention measures have been reported.3) The LSA provides blood flow to the vertebral artery, which is connected to the anterior spinal artery.4) The 2009 American Society of Vascular Surgery practice guidelines recommended routine, preoperative LSAR for elective TEVAR patients undergoing LSA coverage.5) However, according to a recent meta-analysis, there is no significant difference in the rate of SCI between patients with and without LSAR.1,6) In addition, LSAR itself carries a 10%–12% chance of complications (including brachial plexus, vagus nerve, left recurrent nerve, and thoracic duct injuries; neck hematoma; subclavian dissection; and stroke).1) Some studies have suggested performing LSAR in a limited patient population, such as patients with prior left mammary artery coronary bypass, those with a left side arterio-venous fistula for renal dialysis, and left-hand dominant patients.1,6,7) However, the need for routine LSAR after TEVAR is uncertain.1) Our patient had some risk factors (extensive aortic coverage, LSA coverage, and hypogastric artery obstruction) related to the lack of a collateral blood supply network; in accordance with current practice guidelines, LSAR was essential for maintaining spinal cord viability. Nevertheless, we did not perform LSAR on the same day due to the case’s emergent nature. Immediately after surgery, no neurological deficits were observed, and the patient was kept under observation.
Most SCI cases after TEVAR have a delayed onset (>24 h).8) In our patient, incomplete paralysis was noted on postoperative day two. In delayed SCI, it is also important to maintain adequate blood pressure through collateral circulation of the spinal cord. Estrera et al. proposed a treatment protocol for delayed SCI that included CSFD, oxygen delivery, and patient status.9) They emphasized that delayed SCI could be reduced by preventing complications such as delayed postoperative hypotension and CSFD malfunction. In our patient, blood pressure was strictly controlled, and it was difficult to perform CSFD; therefore, only LSAR was performed. Our patient’s paralysis after TEVAR was also resolved by LSAR. Most previous reports focused on whether to perform prophylactic LSAR during TEVAR.1) Therefore, whether LSAR is necessary for delayed SCI is unclear. However, considering the mechanism of our patient’s recovery, we believe the increased collateral blood flow due to LSAR was important for treating delayed SCI. Although few patients with SCI completely return to baseline function, patients with delayed SCI are more likely to have functional improvement.8) It may have been possible for our patient to recover neurological function without LSAR. However, we believe that LSAR should be considered when LSA coverage has been performed and when there is extensive total aortic coverage and hypogastric artery occlusion.
Several other effective neuroprotective strategies during TEVAR have been developed, including specific anesthetic approaches, maximized oxygen delivery, optimized hemoglobin, mild hypothermia, maintaining cerebrospinal fluid and spinal cord perfusion pressures, neuromonitoring, procedure staging, LSAR, minimally invasive coil embolization, temporary aneurysm sac perfusion, and novel implantation sequences for branched/fenestrated stents.1) A multimodality approach is crucial for reducing the incidence of SCI after TEVAR.10)
Conclusion
We present a novel report on LSAR’s efficacy for delayed paraplegia due to SCI. After emergency TEVAR with LSA coverage was performed for the ruptured TAA, the patient had incomplete paralysis in his legs, presumably caused by SCI. We performed LSAR to provide blood supply to the spinal cord; the patient’s paralysis improved and almost resolved after surgery. LSAR should be considered when LSA coverage has been performed and in cases of extensive total aortic coverage and hypogastric artery occlusion.
Disclosure Statement
None declared.
Author Contributions
Study conception: EN, KN
Data collection: EN, HI
Analysis: all authors
Investigation: all authors
Writing: EN
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Awad H, Ramadan ME, El Sayed HF, et al. Spinal cord injury after thoracic endovascular aortic aneurysm repair. Can J Anaesth 2017; 64: 1218-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Griepp RB, Griepp EB. Spinal cord perfusion and protection during descending thoracic and thoracoabdominal aortic surgery: the collateral network concept. Ann Thorac Surg 2007; 83: S865-9. [DOI] [PubMed] [Google Scholar]
- 3).Acher C, Acher CW, Marks E, et al. Intraoperative neuroprotective interventions prevent spinal cord ischemia and injury in thoracic endovascular aortic repair. J Vasc Surg 2016; 63: 1458-65. [DOI] [PubMed] [Google Scholar]
- 4).Amato ACM, Stolf NAG. Anatomy of spinal blood supply. J Vasc Bras 2015; 14: 248-52. [Google Scholar]
- 5).Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010; 121: e266-369. [DOI] [PubMed] [Google Scholar]
- 6).Dexter D, Maldonado TS. Left subclavian artery coverage during TEVAR: is revascularization necessary? J Cardiovasc Surg (Torino) 2012; 53: 135-41. [PubMed] [Google Scholar]
- 7).Garg K, Maldonado TS. Further consideration for subclavian revascularization with TEVAR. Semin Vasc Surg 2012; 25: 232-7. [DOI] [PubMed] [Google Scholar]
- 8).DeSart K, Scali ST, Feezor RJ, et al. Fate of patients with spinal cord ischemia complicating thoracic endovascular aortic repair. J Vasc Surg 2013; 58: 635-42.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Estrera AL, Sheinbaum R, Miller CC 3rd, et al. Neuromonitor-guided repair of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2010; 140 Suppl: S131-5; discussion, S142-6. [DOI] [PubMed] [Google Scholar]
- 10).Sueda T, Takahashi S. Spinal cord injury as a complication of thoracic endovascular aneurysm repair. Surg Today 2018; 48: 473-7. [DOI] [PubMed] [Google Scholar]