Abstract
Abdominal aortic aneurysm (AAA) is considered to be a potent life-threatening disorder in elderly individuals. Although many patients with a small AAA are detected during routine abdominal screening, there is no effective therapeutic option to prevent the progression or regression of AAA in the clinical setting. Recent advances in molecular biology have led to the identification of several important molecules, including microRNA and transcription factor, in the process of AAA formation. Regulation of these factors using nucleic acid drugs is expected to be a novel therapeutic option for AAA. Nucleic acid drugs can bind to target factors, mRNA, microRNA, and transcription factors in a sequence-specific fashion, resulting in a loss of function of the target molecule at the transcriptional or posttranscriptional level. Of note, inhibition of a transcription factor using a decoy strategy effectively suppresses experimental AAA formation, by regulating the expression of several genes associated with the disease progression. This review focuses on recent advances in molecular therapy of using nucleic acid drugs to treat AAA.
Keywords: abdominal aortic aneurysm, nucleic acid drug, decoy oligodeoxynucleotide, transcription factor
Introduction
Abdominal aortic aneurysm (AAA) is characterized by a permanent dilatation of aorta, associated with weakening of the aortic wall. The prevalence of AAA is approximately 5% in men and 1% in women over 60 years of age.1,2) Although AAA is usually asymptomatic, it gradually expands in many patients, and ruptured AAA has a high mortality.3) Therefore, AAA is considered to be a potent life-threatening disorder in elderly patients. The main purpose of human AAA management is AAA rupture prevention and therapeutic intervention defined by a balance between operative risk and rupture risk. Patients with a large AAA receive elective surgical or endovascular repair to prevent rupture. Although a large number of asymptomatic patients with a small AAA are detected incidentally during routine abdominal screening, survival of patients with a small AAA is not improved by these interventional procedures in contrast to patients with a large AAA.4–7) Therefore, the dimension of a small AAA is monitored using noninvasive imaging methods, and surgical intervention is considered when the aneurysm diameter attains the interventional size. For treating small AAAs, many researchers are seeking a novel therapeutic approach, especially pharmacological therapy. Indeed, the efficacy of several medicines, such as the renin–angiotensin–aldosterone system (RAAS) inhibitors and statin and matrix metalloproteinase (MMP) inhibitors, on AAA formation has been confirmed in experimental studies.8–10) However, there is no evidence for a beneficial effect of these medicines on AAA progression in clinical trials.11) Therefore, the treatment strategy of patients with small AAAs remains an imperative clinical problem.
Recent progress in molecular and cellular biology identifies several important genes and intracellular pathways in the pathogenesis of several disorders, including AAA. These factors are thought to be potent therapeutic targets for treating specific diseases, and gene therapy, including nucleic acid-based therapy, is considered to be an innovative and promising approach to modify the expression of target genes. Indeed, several types of nucleic acid drugs have been investigated for their therapeutic effects on numerous pathologic conditions, such as cancer, inflammatory bowel disease, and atherosclerosis, in experimental studies, and some of these agents have been used in clinical settings.12–14) This review focuses on the potential of gene therapy for the treatment of AAA.
Gene Therapy
Gene therapy is a manipulation of gene expression and/or function to treat both hereditary and acquired diseases. One approach to alter gene expression is administration of a functional exogenous gene (DNA) into cells to restore gene function or to provide a therapeutic mediator. Recently, the candidate gene association studies and genome-wide association studies identified a number of mutated genes associated with AAA formation, and these genes are considered to be a potent therapeutic target.15) In addition, it has been reported that inhibition of experimental AAA development and/or occurrence were achieved via overexpression of therapeutic genes, such as cytochrome P450 epoxygenase 2J2 (CYP2J2), angiotensin converting enzyme 2 (ACE2), and lectin-like domain of thrombomodulin.16–18) CYP2J2, a member of the cytochrome P450 superfamily of enzymes, metabolizes arachidonic acids to epoxyeicosatrienoic acids. Recombinant adeno-associated virus (AAV)-mediated CYP2J2 overexpression increased epoxyeicosatrienoic acids, resulting in the inhibition of angiotensin (Ang) II-induced AAA progression via activation of peroxisome proliferator-activated receptor (PPAR)γ and anti-inflammatory effects in ApoE-deficient mice.16) Similarly, ACE2 is a well-known member of RAAS, and it induces the conversion of Ang I to the Ang 1-9 and Ang II to Ang 1-7. Because these effector molecules mediate anti-inflammatory and anti-Ang II effects, ACE2 gene transfer inhibited Ang II-induced AAA formation in ApoE-deficient mice.17) The therapeutic effects of overexpression of lectin-like domain of thrombomodulin were also investigated in mouse CaCl2- and Ang II-induced AAA models. Thrombomodulin is a co-factor for thrombin and acts as an anti-coagulant factor. One-time intravenous administration of recombinant AAV vectors carrying the lectin-like domain of thrombomodulin inhibited the expression of high-mobility group box 1 (HMGB1) and advanced glycation end product (RAGE), resulting in the prevention of AAA formation through downregulation of inflammatory response and oxidative stress.18) Overexpression of microRNA (miRNA) also induced therapeutic effects on AAA progression. Lentivirus-mediated miRNA-21, -24, or -145 overexpression inhibited AAA expansion or reduced the incidence of AAA in mice.19–21) However, the therapeutic effects of the delivery of a single gene might be limited to treat AAA, because multiple mediators contribute to AAA formation.
Nucleic Acid Medicine
Nucleic acid-based therapy is included in criteria of gene therapy, because of their ability to modify the expression of a specific gene to treat a pathological condition.13,22) Nucleic acid drugs are synthetic single- or double-stranded oligodeoxynucleotides (ODN) that contain a consensus sequence of the target factor. These ODN bind to the target mRNA, miRNA, or transcription factor in a sequence-specific fashion via Watson–Click base pairing, resulting in a loss of function of the target molecule at the transcriptional or posttranscriptional level.22) Typically, antisense ODN, small interfering RNA (siRNA), microRNA, anti-miRNA, aptamer, ribozyme, and decoy ODN are included in the nucleic acid drugs.23) Change in the activity of miRNA and transcription factor via nucleic acid drug can alter the expression of a set of genes associated with disease progression, whereas other technologies inhibit only one target gene. The therapeutic value of nucleic acid drugs has been reported in experimental models of many diseases, including AAA.24) Pathophysiology of AAA is characterized by chronic inflammation and degradation of the aortic wall.1) Therefore, inflammatory and proteolytic factors are considered to be the primary targets for nucleic acid-based therapy to treat AAA.
Antisense ODN
Antisense ODN are short single-stranded DNA or RNA molecules comprising 10–25 nucleotides that can specifically hybridize with a complementary sequence of the target mRNA. After binding of antisense ODN to mRNA, the translation of mRNA is arrested and mRNA is cleaved by ribonuclease H, resulting in the suppression of target protein synthesis.25,26) Therefore, antisense ODN are also useful tools in the study of gene function, because of the specific inhibition of target gene expression without changing the function of other genes. Recently, the effect of antisense ODN against heparin-binding EGF-like growth factor (HB-EGF) on Ang II-induced AAA formation was investigated in low-density lipoprotein-deficient mice. Although the association of hyperlipidemia with AAA development is controversial in humans, systemic administration of HB-EGF specific antisense ODN suppressed AAA formation through antihyperlipidemic effects.27) This study suggests the importance of restoring environmental factors for managing patients with AAA.
Small Interfering RNA and MicroRNA
Both siRNA and miRNA silence the translation of target mRNA using RNA interference system, which is a normal physiological response to control the synthesis of a specific protein in cells. However, aberrant expression of siRNA and miRNA also contributes to initiation and progression of AAA, and several studies reported that miRNA might be available as a biomarker to predispose AAA formation.28) Therefore, a role of these molecules in the process of AAA formation has gained research interest, and modulation of their expression has been investigated in several experimental studies.
siRNA and miRNA are a class of non-coding double-stranded RNA and induce posttranscriptional gene silencing in a sequence-specific fashion. Typically, siRNA is composed of 21–23 nucleotides in the effector phase and has a complete complementary sequence of the target mRNA. After binding to the target mRNA, siRNA degraded mRNA via an RNA-induced silencing complex.26,29) Although siRNA has been used to investigate the function of a gene in in vitro studies, its effects have also been evaluated in experimental models of AAA. Administration of siRNA against resistin-like molecule-beta attenuated the incidence and severity of Ang II-induced mouse AAA via anti-inflammatory effects associated with the inhibition of extracellular signal-regulated kinase 1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK) activation.30) In addition, silencing of hypoxia inducible factor-1 (HIF-1) using lentivirus expressing HIF-1α shRNA also suppressed AAA formation in ApoE-deficient mice.31)
MicroRNA composed of 10–25 nucleotides has an incomplete complementary sequence in the 3′ untranslated regions of mRNA. Because many mRNAs have the binding site against one miRNA, miRNA can hybridize several genes associated with both physiological and pathological conditions. In addition, a different kind of miRNA can bind to the same 3′ untranslated regions of mRNA, and the translation of mRNA is cooperatively regulated by these miRNA.32,33) There are two approaches to regulate miRNA activity using nucleic acid agent. Inhibition of miRNA activity is performed by antagomirs, which are synthetic single-strand RNA including the complementary sequence of target miRNA. An antagomir hybridizes with the target miRNA, resulting in the degradation of miRNA.32,33) In contrast, an increase in miRNA activity is induced by double-strand ODN, pre-miRNA, or miRNA-mimics.34) Several clinical studies have demonstrated the altered miRNA expression in both human AAA wall and serum samples.28,35–39) Regulation of these miRNA via antagomirs or miRNA-mimics induces a potent therapeutic effect on experimental AAA formation. Silencing the expression of miRNA-29b, -155, -181b, and -712 using antagomirs inhibited elastase or Ang II-induced AAA expansion in a mouse model.38–42) A detailed explanation of the role of miRNA in AAA formation and therapeutic value of antagomirs and miRNA-mimics on AAA formation has been provided in previous review articles.36,43,44)
Decoy Strategy
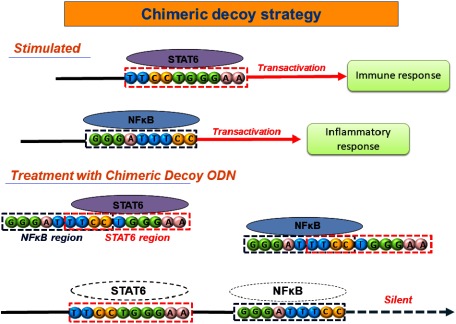
Several intracellular pathways are activated in the process of disease progression in humans. These cascades, including compensatory pathways, converge on the activation of a specific transcription factor network. Activation of transcription factors leads to the transcription of a set of genes associated with a pathologic condition, as well as a physiological phenomenon. Some of these effector molecules have an ability to activate the transcription factors, resulting in the induction of a positive feedback loop that leads to sustaining disease condition. A decoy strategy is available to regulate the activity of endogenous transcription factor (Fig. 1). Decoy ODN are synthetic double-stranded ODN containing the consensus sequence of the target transcription factor (cis-element) binding site. Because decoy ODN can bind to target transcription factors in a sequence-specific fashion, the binding of the transcription factor to the promoter or enhancer region is blocked, resulting in the suppression of gene transcription. In addition, administration of decoy ODN against a negative transcription factor enhances the expression of suppressed genes.45) Therefore, the decoy strategy leads to normalization of the aberrant gene expression profile associated with disease progression. Indeed, in experimental studies, the efficacy of decoy ODN has been reported in several diseases models, such as cancer, inflammatory bowel disease, neointimal hyperplasia, and AAA.46–50) Based on a potent biological effect of decoy ODN, clinical studies using decoy ODN were performed in the field of cancer and restenosis after coronary intervention.51–53)
Fig. 1 Chimeric decoy strategy against NFκB and STAT6.
Chimeric decoy ODN contain consensus sequences of multiple transcription factors in one decoy ODN, resulting in simultaneous inhibition of target transcription factor activation. A part of the consensus sequences of two different transcription factors is overlapped in the structure of ODN. ODN: oligodeoxynucleotide; STAT: signal transducers and activator of transcription
Target of Transcription Factors to Treat AAA
Recent clinical studies have demonstrated the upregulation of several kinds of transcription factors in human AAA walls when compared with non-aneurysmal samples. These transcription factors mainly regulate the expression of pro-inflammatory factors, such as cytokines and adhesion molecules. Among them, nuclear factor-kappa B (NFκB) is thought to play an important role in the process of AAA formation, because it is a key transcription factor in both acute and chronic inflammatory responses. NFκB directly regulates numerous cytokines and proteases, such as Interleukin (IL)-1, IL-6, tumor necrosis factor-α (TNF-α), and MMPs, and TNF-α and IL-1b can also activate NFκB.54–57) NFκB also regulates the expression of adhesion molecules and chemokines, which induce the migration of inflammatory cells.58,59) Because inflammatory cells, including mast cells, are the primary source of inflammatory cytokines and proteases, inhibition of inflammatory cell recruitment indirectly suppresses the excess expression of inflammatory mediators. Indeed, our previous studies demonstrated that treatment with NFκB decoy ODN mediated a potent anti-inflammatory effect in rat and rabbit AAA models.50,60) Furthermore, NFκB inhibited the transcription of elastin and collagen genes, suppressing their synthesis.61,62) Therefore, NFκB is thought to be a main target of the decoy strategy to treat AAA.
Ets regulates the gene expression in response to multiple developmental and mitogenic signals, including cell growth, differentiation, and apoptosis. In addition, it is also known to regulate MMP-1, MMP-2, and MMP-9 transcription.63,64) Several clinical studies have reported the activation of ets-1, -2, and -4 and ELF1 in the human aneurysm wall.60,65,66) Our previous study demonstrated that treatment with ets decoy ODN reduced the size of already-formed experimental AAA in rabbits.60)
The members of KLF family regulate the expression of various genes associated with cellular proliferation, differentiation, and apoptosis, and contribute to the development and homeostasis of several tissues. Previous studies have demonstrated the activation of KLF4 in the human aneurysm wall, and deletion of KLF4 attenuated AAA formation in elastase- and Ang II-induced mouse AAA model.67) In contrast, concentration of KLF15 was reduced in human AAA tissues, and deficiency of KLF15 induced AAA formation and heart failure in mice through activation of p53 and p300 acetyltransferase.68)
Signal transducer and activator of transcription (STAT) regulates the transcription of several genes associated with inflammatory and immune responses. In addition, STAT activation induces cellular differentiation, proliferation, and apoptosis in various cell types. Therefore, regulation of STAT using decoy ODN has been investigated for treating cancer, asthma, and inflammatory bowel disease. A previous study demonstrated the activation of STAT1, 2, 3, and 5 was in human AAA wall compared with non-aneurysm aortic wall samples.69) In an experimental study using ApoE-deficient mice, administration of Ang II induced STAT3 activation in the AAA wall through Toll-like receptor 4 signaling, and pharmacological inhibition of STAT3 reduced the incidence and severity of Ang II-induced AAA formation.70) Similarly, although an increase in IL-17 participates in Ang II-induced AAA formation in mice, IL-6-STAT3 signaling pathway induced the accumulation of Th 17 cells in the AAA wall and inhibition of STAT3 activity suppressed AAA formation.71)
HIF-1 is activated under hypoxic conditions in tissues and regulates several genes responding to this environmental stimulus. The function of these genes is mainly associated with inflammation, angiogenesis, and cell growth. Activation of HIF-1 was also observed in human AAA tissues.72) In addition, silencing of HIF-1 using shRNA reduced AAA diameter in an Ang II-induced ApoE-deficient mouse AAA model via inhibition of upregulation of MMPs and inflammatory and angiogenic factors.31) A similar observation was seen using pharmacological inhibition of HIF-1 in an elastase-induced mouse AAA model.72) In contrast, it has been reported that expression of HIF-1 in myeloid lineage protects AAA formation. In myeloid-specific HIF-1α and ApoE double-knockout mice, deletion of HIF-1 increased aneurysm diameter after infusion of Ang II.73) These findings suggest that the effects of HIF-1 differ among different types of cells in the AAA wall.
Recent studies have also demonstrated that deletion of brain and muscle Arnt-like protein 1 in smooth muscle cells inhibits AAA formation in AAA mice.74) This transcription factor is known to regulate the circadian rhythm. Similarly, Runx1, a transcription factor for hematopoiesis, was also enhanced expression in the human AAA wall.65,75) Therefore, further studies are needed to clarify the transcription factor network associated with AAA formation, which can lead to a new therapeutic approach for AAA (Table 1).
Table 1 Target transcription factor for treating AAA in experimental studies.
| Transcription factor | Deletion/blockade | AAA formation | References |
|---|---|---|---|
| NFκB | Decoy ODN | ↓ | 60 |
| Ets | Decoy ODN | ↓ | 60 |
| KLF family | |||
| KLF4 | KO (SMC) | ↓ | 67 |
| KLF15 | KO | ↑ | 65 |
| STAT3 | STAT3 inhibitor | ↓ | 70, 71 |
| HIF-1α | shRNA | ↓ | 31 |
| KO (myeloid lineage cell) | ↑ | 73 | |
| XBP1 | KO (SMC) | ↑ | 99 |
| BMAL1 | KO (SMC) | ↓ | 74 |
AAA: abdominal aortic aneurysm; BMAL1: brain and muscle Arnt-like protein-1; KO: knock out; SMC: smooth muscle cell; STATS: signal transducer and activator of transcription; ODN: oligodeoxynucleotide; XBP1: X-box binding protein 1
Chimeric Decoy Strategy for Treating AAA
In the promoter region of DNA, there are binding sites for several transcription factors. Therefore, multiple transcription factors can bind to the promoter region of one gene, and are thought to cooperatively regulate target gene expression, whereas the effect of an individual transcription factor on transactivation of target genes differs in disease state, phenotypes, and cell types.76) This phenomenon suggests that the inhibition of multiple transcription factors is necessary to obtain sufficient gene regulation. In addition, combined blockade of multiple transcription factors might affect a number of gene expressions associated with different aspects of disease progression. Therefore, attention of a new therapeutic approaches of decoy strategy are shifting toward inhibition of multiple transcription factors.77–82)
Although it might be possible to administrate several types of decoy ODN against a single transcription factor in cells, transfection efficiency of individual decoy ODN is thought to be significantly low, resulting in insufficient silencing efficiency. Therefore, a chimeric decoy strategy was developed to regulate multiple transcription factors simultaneously. Chimeric decoy ODN contain consensus sequences of multiple transcription factors in one decoy ODN, resulting in simultaneous inhibition of target transcription factor activation.13) Furthermore, a novel chimeric decoy ODN (Fig. 1) was used. Although conventional chimeric decoy ODN contain individual consensus sequences at a separate site in their structure, a part of the consensus sequences of two different transcription factors was overlapped in the structure of ODN. The inhibitory effect of this type of decoy ODN on the activation of two target transcription factors was confirmed in a mouse asthma model.77) This modification results in shortening of the ODN length, leading to an increase in transfection efficiency and a decrease in production cost.
Chimeric decoy ODN typically contain a consensus sequence of two transcription factors, because ODN with a long sequence have low transfection efficiency and may induce conformational changes. Although any transcription factor can be chosen for a chimeric decoy strategy, an appropriate selection of transcription factors against target disease is vital for achieving favorable outcomes (Table 2). We focused on inhibition of NFκB combined with another transcription factor for treating inflammatory diseases. Our previous studies demonstrated that simultaneous inhibition of NFκB and E2F significantly suppresses anastomotic intimal hyperplasia via inhibition of inflammatory response and proliferation of VSMC (vascular smooth muscle cell) in rabbits, because E2F regulates the expression of cell cycle regulated genes.78) In addition, the efficacy of chimeric decoy ODN against NFκB and STAT6 on asthma exacerbation was confirmed in an ovalbumin-induced mouse asthma model.77) For treating AAA, we focused on simultaneous inhibition of NFκB and ets, because these transcription factors synergistically regulate the expression of many inflammatory factors including MMPs. The prevention of AAA progression using chimeric decoy ODN against NFκB and ets was confirmed in elastase-induced rat and rabbit AAA models.50,83) Furthermore, treatment with chimeric decoy ODN induced regression of already-formed AAA in rabbits through the inhibition of inflammatory response and MMPs activation, and upregulation of elastin synthesis in the AAA wall.60) Importantly, the therapeutic effect of simultaneous inhibition of these two transcription factors on disease progression is significantly greater than that of inhibition of single transcription factor, NFκB, or ets.57) Similar observation was achieved in the experimental study of asthma.77) These findings indicate the feasibility of the chimeric decoy strategy for treating several inflammatory diseases via effective regulation of a wide range of aberrant gene expressions.
Table 2 Chimeric decoy strategy in experimental studies.
| Transcription factor | Target disease | Inhibitory effects | References |
|---|---|---|---|
| NFκB/ets | Aneurysm | Inflammation | 50, 60, 87 |
| MMP activity | |||
| NFκB/SP1 | Atherosclerosis | Inflammation | 79 |
| Serum cholesterol level | |||
| Chronic kidney disease | Fibrosis | 80 | |
| NFκB/STAT6 | Bronchial asthma | Inflammation | 77 |
| NFκB/E2F | Intimal hyperplasia | Inflammation | 78 |
| VSMC proliferation | |||
| Smad/SP1 | Chronic kidney disease | Fibrosis | 81 |
| Inflammation | |||
| AP1/Smad | Tissue fibrosis | Fibrosis | 82 |
| Inflammation |
AP1: activator protein 1; MMP: matrix metalloproteinase; SP1: specificity protein 1; STATS: signal transducer and activator of transcription; VSMC: vascular smooth muscle cell
Structural Modification of Decoy ODN and Delivery System
ODN-based therapeutic strategy is expected to treat several diseases, including AAA. However, the clinical use of ODN-based agents is associated with several concerns, such as easy degradation of ODN by endonucleases. Several chemical modifications of ODN, such as locked nucleic acids or morpholino oligomers, are used to increase the stability of antisense ODN or miRNAs.30,84) In contrast, decoy ODN have received structural modification to increase stability and resistance against nucleases. In a ribbon-type (dumbbell-type) decoy ODN, both double-stranded termini of the decoy ODN are linked by a circular structure of nucleic acids, because degradation of decoy ODN by nuclease begins at the site where ODN ends.52,85,86) Indeed, our previous study reported that ribbon-type decoy ODN against NFκB and ets could inhibit AAA progression in an elastase-induced rat AAA model despite systemic administration.87) In addition, our recent study and other studies have demonstrated that sense and antisense strands of decoy ODN were linked with a chemical spacer instead of nucleic acids.52,77) This type of decoy ODN leads to simplification of the synthesis process and a reduction of ODN production cost, in addition to enhanced stability. Furthermore, phosphorothioate modification is also provided to nucleic acids of ribbon-type decoy ODN, resulting in a further increase in their stability and nuclease resistance in vivo.
Other limitation of ODN-based therapy is a lack of an accurate method for ODN administration into the aneurysm wall. In previous experimental studies, we administrated decoy ODN into the aortic wall using a cellulose-based sheet containing decoy ODN, which directly employed outer surface of the aortic wall.50,60) However, administration of decoy ODN in humans should be performed using noninvasive methods, such as systemic administration. Although internalization of ODN into target cells is thought to occur by some form of endocytosis, it is difficult to attach anionic ODN to the positively charged cell membrane. Although structural modifications of decoy ODN has a potential for systemic administration, an application of drug delivery system (DDS) is also an effective approach to deliver ODN into the aneurysm wall.88) Several DDS using nanocarriers, such as nanoparticles, liposomes, and micelles, have been developed. Among them, we used a poly(lactic-co-glycolic acid) (PLGA) nanoparticle-based delivery system, because PLGA is a natural polymer and is thought to be an efficient drug carrier due to its low immunogenicity, high safety, and biocompatibility.89) In this system, decoy ODN are entrapped in the PLGA nano-matrix, resulting in protection against enzymatic degeneration, and the particle surface is positively charged by chitosan coating. In addition, PLGA nanoparticles are thought to escape from endosome via the proton-sponge mechanism.90) Therefore, we consider this delivery system might induce a sufficient dose of decoy ODN into target cells via systemic administration.
Clinical Trial of Nucleic Acid Medicine
There are no clinical trials on treating AAA using nucleic acid drugs to date. However, several clinical trials using antisense or decoy ODN to treat human diseases have been performed. Among them, the second-generation antisense ODN against apolipoprotein B-100 mipomersen was approved by Food and Drug Administration (FDA) for treating patients with homozygous familial hyperlipidemia. In phase 3 trial, this antisense ODN was administrated by weekly subcutaneous injection (200 mg) to patients with familial hyperlipidemia and/or coronary artery disease, and markedly reduced apolipoprotein-containing lipoproteins. However, this drug is known to cause liver and cardiovascular adverse effects.91,92) The use of nucleic acid medicines for human treatment is associated with certain concerns, such as nonspecific effects including nonsequence-specific binding to mRNA or protein.93) In addition, high-dose phosphorothioate ODN bolus injection was reported to have caused kidney damage, elevation of liver enzymes, and hypotension in experimental studies.94,95)
On the contrary, the therapeutic effects of decoy ODN have also been investigated in clinical trials for the prevention of restenosis after vascular intervention and cancer treatment. Treatment with E2F decoy ODN did not prevent graft failure after coronary artery bypass grafting in a phase 3 clinical trial.96) However, the efficacy of NFκB decoy ODN for preventing restenosis after percutaneous coronary intervention (PCI) was demonstrated in a phase I/IIa clinical trial.51) After stent implantation, NFκB decoy ODN (1 mg) was transfected using a Remedy catheter (dual balloon system) into the coronary arterial wall at the site of bare metal stent implantation. Significant restenosis was found in only 1 of the 17 patients at 6 months after treatment, and no significant adverse effect occurred in any patients during this observation period. In addition, 4 years after PCI, treatment with NFκB decoy ODN suppressed neointimal hyperplasia when compared to the site with no decoy ODN transfection in the same artery of one patient.97) A recent clinical trial reported using a balloon catheter containing NFκB decoy ODN for treating arteriovenous fistula (AVF) stenosis.98) Percutaneous transluminal angioplasty via balloon catheter containing NFκB decoy ODN (89–134 µg) encapsulated nanoparticles was safe for clinical use and effective for prolonging the primary patency period, whereas no significant differences between treatment with NFκB decoy ODN and control were observed.
These results suggest that appropriate dose and delivery method of ODN can avoid adverse effects in humans. In addition, chemical and structural modification of ODN for reducing toxicity is important to treat human diseases.
Conclusion
Emerging evidence indicates that treatment with nucleic acid drugs induces a potent therapeutic effect for several diseases including AAA. In addition, recent advances in the modification techniques of ODN have contributed to their increased in vivo stability. Effective delivery systems have also been developed. However, these advances are not adequate to treat human AAA. Further studies to overcome the limitations of ODN-based therapy are needed for use in clinical settings.
Disclosure Statement
Dr Morishita has stocks for AnGes Inc., and is an endowed chair of the Department of Clinical Gene Therapy that is founded by Nippon Boehringer Ingelheim Co., Ltd., Shionogi & Co., Ltd., AnGes, Inc., ROHTO Pharmaceutical Co., Ltd., Grace Labo Co., Ltd., FANCL CORPORATION, EH Inc., OHAYO DAIRY PRODUCTS Co., Ltd., Morishita Jintan Co., Ltd. and WAKASA SEIKATSU Co., Ltd. The other authors report no conflicts.
Author Contributions
Writing: all authors
Final approval of the article: all authors
References
- 1).Miyake T, Morishita R. Pharmacological treatment of abdominal aortic aneurysm. Cardiovasc Res 2009; 83: 436-43. [DOI] [PubMed] [Google Scholar]
- 2).Li X, Zhao G, Zhang J, et al. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population—a meta-analysis. PLoS ONE 2013; 8: e81260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg 2013; 100: 1405-13. [DOI] [PubMed] [Google Scholar]
- 4).Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126: 441-9. [DOI] [PubMed] [Google Scholar]
- 5).Boll AP, Verbeek AL, van de Lisdonk EH, et al. High prevalence of abdominal aortic aneurysm in a primary care screening programme. Br J Surg 1998; 85: 1090-4. [DOI] [PubMed] [Google Scholar]
- 6).The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998; 352: 1649-55. [PubMed] [Google Scholar]
- 7).Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med 2002; 346: 1437-44. [DOI] [PubMed] [Google Scholar]
- 8).Miyake T, Miyake T, Shimizu H, et al. Inhibition of aneurysm progression by direct renin inhibition in a rabbit model. Hypertension 2017; 70: 1201-9. [DOI] [PubMed] [Google Scholar]
- 9).Shiraya S, Miyake T, Aoki M, et al. Inhibition of development of experimental aortic abdominal aneurysm in rat model by atorvastatin through inhibition of macrophage migration. Atherosclerosis 2009; 202: 34-40. [DOI] [PubMed] [Google Scholar]
- 10).Petrinec D, Liao S, Holmes DR, et al. Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: preservation of aortic elastin associated with suppressed production of 92 kD gelatinase. J Vasc Surg 1996; 23: 336-46. [DOI] [PubMed] [Google Scholar]
- 11).Sweeting MJ, Thompson SG, Brown LC, et al. Use of angiotensin converting enzyme inhibitors is associated with increased growth rate of abdominal aortic aneurysms. J Vasc Surg 2010; 52: 1-4. [DOI] [PubMed] [Google Scholar]
- 12).Li J, Wang Y, Zhu Y, et al. Recent advances in delivery of drug-nucleic acid combinations for cancer treatment. J Control Release 2013; 172: 589-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Youn SW, Park KK. Small-nucleic-acid-based therapeutic strategy targeting the transcription factors regulating the vascular inflammation, remodeling and fibrosis in atherosclerosis. Int J Mol Sci 2015; 16: 11804-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res 2018; 46: 1584-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Kim HW, Stansfield BK. Genetic and epigenetic regulation of aortic aneurysms. Biomed Res Int 2017; 2017: 7268521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Cai Z, Zhao G, Yan J, et al. CYP2J2 overexpression increases EETs and protects against angiotensin II-induced abdominal aortic aneurysm in mice. J Lipid Res 2013; 54: 1448-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Hao Q, Dong X, Chen X, et al. Angiotensin-converting enzyme 2 inhibits angiotensin II-induced abdominal aortic aneurysm in mice. Hum Gene Ther 2017; Jan 31. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18).Lai CH, Wang KC, Kuo CH, et al. Recombinant adeno-associated virus vector carrying the thrombomodulin lectin-like domain for the treatment of abdominal aortic aneurysm. Atherosclerosis 2017; 262: 62-70. [DOI] [PubMed] [Google Scholar]
- 19).Maegdefessel L, Azuma J, Toh R, et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med 2012; 4: 122ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Maegdefessel L, Spin JM, Raaz U, et al. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun 2014; 5: 5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Wu J, Wang J, Li X, et al. MicroRNA-145 mediates the formation of angiotensin II-induced murine abdominal aortic aneurysm. Heart Lung Circ 2017; 26: 619-26. [DOI] [PubMed] [Google Scholar]
- 22).Morishita R, Kaneda Y, Ogihara T. Therapeutic potential of oligonucleotide-based therapy in cardiovascular disease. BioDrugs 2003; 17: 383-9. [DOI] [PubMed] [Google Scholar]
- 23).Grassi G, Scaggiante B, Dapas B, et al. Therapeutic potential of nucleic acid-based drugs in coronary hyper-proliferative vascular diseases. Curr Med Chem 2013; 20: 3515-38. [DOI] [PubMed] [Google Scholar]
- 24).Ahmad MZ, Akhter S, Mallik N, et al. Application of decoy oligonucleotides as novel therapeutic strategy: a contemporary overview. Curr Drug Discov Technol 2013; 10: 71-84. [DOI] [PubMed] [Google Scholar]
- 25).Chan JHP, Lim S, Wong WSF. Antisense oligonucleotides: from design to therapeutic application. Clin Exp Pharmacol Physiol 2006; 33: 533-40. [DOI] [PubMed] [Google Scholar]
- 26).Kole R, Krainer AR, Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat Rev Drug Discov 2012; 11: 125-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Kim S, Yang L, Kim S, et al. Targeting hepatic heparin-binding EGF-like growth factor (HB-EGF) induces anti-hyperlipidemia leading to reduction of angiotensin II-induced aneurysm development. PLoS ONE 2017; 12: e0182566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Wanhainen A, Mani K, Vorkapic E, et al. Screening of circulating microRNA biomarkers for prevalence of abdominal aortic aneurysm and aneurysm growth. Atherosclerosis 2017; 256: 82-8. [DOI] [PubMed] [Google Scholar]
- 29).Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov 2004; 3: 318-29. [DOI] [PubMed] [Google Scholar]
- 30).Meng X, Zhang K, Kong J, et al. Deletion of resistin-like molecule-beta attenuates angiotensin II-induced abdominal aortic aneurysm. Oncotarget 2017; 8: 104171-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Yang L, Shen L, Li G, et al. Silencing of hypoxia inducible factor-1α gene attenuated angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice. Atherosclerosis 2016; 252: 40-9. [DOI] [PubMed] [Google Scholar]
- 32).Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281-97. [DOI] [PubMed] [Google Scholar]
- 33).Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010; 11: 597-610. [DOI] [PubMed] [Google Scholar]
- 34).Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov 2013; 12: 847-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Venkatesh P, Phillippi J, Chukkapalli S, et al. Aneurysm-specific miR-221 and miR-146a participates in human thoracic and abdominal aortic aneurysms. Int J Mol Sci 2017; 18: 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Gao P, Si J, Yang B, et al. Upregulation of microRNA-15a contributes to pathogenesis of abdominal aortic aneurysm (AAA) by modulating the expression of cyclin-dependent kinase inhibitor 2B (CDKN2B). Med Sci Monit 2017; 23: 881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Li Y, Maegdefessel L. Non-coding RNA contribution to thoracic and abdominal aortic aneurysm disease development and progression. Front Physiol 2017; 8: 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Zampetaki A, Attia R, Mayr U, et al. Role of miR-195 in aortic aneurysmal disease. Circ Res 2014; 115: 857-66. [DOI] [PubMed] [Google Scholar]
- 39).Maegdefessel L, Azuma J, Toh R, et al. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest 2012; 122: 497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Zhang Z, Liang K, Zou G, et al. Inhibition of miR-155 attenuates abdominal aortic aneurysm in mice by regulating macrophage-mediated inflammation. Biosci Rep 2018; 38: BSR20171432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Di Gregoli K, Mohamad Anuar NN, Bianco R, et al. MicroRNA-181b controls atherosclerosis and aneurysms through regulation of TIMP-3 and elastin. Circ Res 2017; 120: 49-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Kim CW, Kumar S, Son DJ, et al. Prevention of abdominal aortic aneurysm by anti-microRNA-712 or anti-microRNA-205 in angiotensin II-infused mice. Arterioscler Thromb Vasc Biol 2014; 34: 1412-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Maegdefessel L, Dalman RL, Tsao PS. Pathogenesis of abdominal aortic aneurysms: microRNAs, proteases, genetic associations. Annu Rev Med 2014; 65: 49-62. [DOI] [PubMed] [Google Scholar]
- 44).Raffort J, Lareyre F, Clement M, et al. Micro-RNAs in abdominal aortic aneurysms: insights from animal models and relevance to human disease. Cardiovasc Res 2016; 110: 165-77. [DOI] [PubMed] [Google Scholar]
- 45).Morishita R, Higaki J, Tomita N, et al. Application of transcription factor “decoy” strategy as means of gene therapy and study of gene expression in cardiovascular disease. Circ Res 1998; 82: 1023-8. [DOI] [PubMed] [Google Scholar]
- 46).Klein JD, Sano D, Sen M, et al. STAT3 oligonucleotide inhibits tumor angiogenesis in preclinical models of squamous cell carcinoma. PLoS ONE 2014; 9: e81819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Ozaki K, Makino H, Aoki M, et al. Therapeutic effect of ribbon-type nuclear factor-κB decoy oligonucleotides in a rat model of inflammatory bowel disease. Curr Gene Ther 2012; 12: 484-92. [DOI] [PubMed] [Google Scholar]
- 48).Ahn JD, Morishita R, Kaneda Y, et al. Inhibitory effects of novel AP-1 decoy oligodeoxynucleotides on vascular smooth muscle cell proliferation in vitro and neointimal formation in vivo. Circ Res 2002; 90: 1325-32. [DOI] [PubMed] [Google Scholar]
- 49).Yoshimura S, Morishita R, Hayashi K, et al. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element ‘decoy’ of nuclear factor-kB binding site as a novel molecular strategy. Gene Ther 2001; 8: 1635-42. [DOI] [PubMed] [Google Scholar]
- 50).Nakashima H, Aoki M, Miyake T, et al. Inhibition of experimental abdominal aortic aneurysm in the rat by use of decoy oligodeoxynucleotides suppressing activity of nuclear factor κB and ets transcription factors. Circulation 2004; 109: 132-8. [DOI] [PubMed] [Google Scholar]
- 51).Egashira K, Suzuki J, Ito H, et al. Long-term follow up of initial clinical cases with NF-κB decoy oligodeoxynucleotide transfection at the site of coronary stenting. J Gene Med 2008; 10: 805-9. [DOI] [PubMed] [Google Scholar]
- 52).Sen M, Thomas SM, Kim S, et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov 2012; 2: 694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 2006; 43: 742-51.e1. [DOI] [PubMed] [Google Scholar]
- 54).Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 1990; 10: 2327-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Satriano J, Schlondorff D. Activation and attenuation of transcription factor NF-kB in mouse glomerular mesangial cells in response to tumor necrosis factor-alpha, immunoglobulin G, and adenosine 3’:5’-cyclic monophosphate. Evidence for involvement of reactive oxygen species. J Clin Invest 1994; 94: 1629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Takeshita H, Yoshizaki T, Miller WE, et al. Matrix metalloproteinase 9 expression is induced by Epstein–Barr virus latent membrane protein 1 C-terminal activation regions 1 and 2. J Virol 1999; 73: 5548-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Kim H, Koh G. Lipopolysaccharide activates matrix metalloproteinase-2 in endothelial cells through an NF-κB-dependent pathway. Biochem Biophys Res Commun 2000; 269: 401-5. [DOI] [PubMed] [Google Scholar]
- 58).Weber C, Erl W, Pietsch A, et al. Aspirin inhibits nuclear factor-κB mobilization and monocyte adhesion in stimulated human endothelial cells. Circulation 1995; 91: 1914-7. [DOI] [PubMed] [Google Scholar]
- 59).Ledebur HC, Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-κB site and p65 homodimers. J Biol Chem 1995; 270: 933-43. [DOI] [PubMed] [Google Scholar]
- 60).Miyake T, Aoki M, Masaki H, et al. Regression of abdominal aortic aneurysms by simultaneous inhibition of nuclear factor κB and ets in a rabbit model. Circ Res 2007; 101: 1175-84. [DOI] [PubMed] [Google Scholar]
- 61).Kuang PP, Berk JL, Rishikof DC, et al. NF-κB induced by IL-1β inhibits elastin transcription and myofibroblast phenotype. Am J Physiol Cell Physiol 2002; 283: C58-65. [DOI] [PubMed] [Google Scholar]
- 62).Kouba DJ, Chung KY, Nishiyama T, et al. Nuclear factor-κB mediates TNF-α inhibitory effect on alpha 2(I) collagen (COL1A2) gene transcription in human dermal fibroblasts. J Immunol 1999; 162: 4226-34. [PubMed] [Google Scholar]
- 63).Vandenbunder B, Wernert N, Queva C, et al. Does the transcription factor c-ets1 take part in the regulation of angiogenesis and tumor invasion? Folia Biol (Praha) 1994; 40: 301-13. [PubMed] [Google Scholar]
- 64).Gum R, Lengyel E, Juarez J, et al. Stimulation of 92-kDa gelatinase B promoter activity by ras is mitogen-activated protein kinase kinase 1-independent and requires multiple transcription factor binding sites including closely spaced PEA3/ets and AP-1 sequences. J Biol Chem 1996; 271: 10672-80. [DOI] [PubMed] [Google Scholar]
- 65).Pahl MC, Erdman R, Kuivaniemi H, et al. Transcriptional (ChIP-chip) analysis of ELF1, ETS2, RUNX1 and STAT5 in human abdominal aortic aneurysm. Int J Mol Sci 2015; 16: 11229-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Nischan J, Gatalica Z, Curtis M, et al. Binding sites for ETS family of transcription factors dominate the promoter regions of differentially expressed genes in abdominal aortic aneurysms. Circ Cardiovasc Genet 2009; 2: 565-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Salmon M, Johnston WF, Woo A, et al. KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation 2013; 128: S163-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Haldar SM, Lu Y, Jeyaraj D, et al. Klf15 deficiency is a molecular link between heart failure and aortic aneurysm formation. Sci Transl Med 2010; 2: 26ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Liao M, Xu J, Clair AJ, et al. Local and systemic alterations in signal transducers and activators of transcription (STAT) associated with human abdominal aortic aneurysms. J Surg Res 2012; 176: 321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Qin Z, Bagley J, Sukhova G, et al. Angiotensin II-induced TLR4 mediated abdominal aortic aneurysm in apolipoprotein E knockout mice is dependent on STAT3. J Mol Cell Cardiol 2015; 87: 160-70. [DOI] [PubMed] [Google Scholar]
- 71).Ju X, Ijaz T, Sun H, et al. Interleukin-6-signal transducer and activator of transcription-3 signaling mediates aortic dissections induced by angiotensin II via the T-helper lymphocyte 17-interleukin 17 axis in C57BL/6 mice. Arterioscler Thromb Vasc Biol 2013; 33: 1612-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Wang W, Xu B, Xuan H, et al. Hypoxia-inducible factor 1 in clinical and experimental aortic aneurysm disease. J Vasc Surg 2017; Dec 11. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Takahara Y, Tokunou T, Kojima H, et al. Deletion of hypoxia-inducible factor-1α in myeloid lineage exaggerates angiotensin II-induced formation of abdominal aortic aneurysm. Clin Sci (Lond) 2017; 131: 609-20. [DOI] [PubMed] [Google Scholar]
- 74).Lutshumba J, Liu S, Zhong Y, et al. Deletion of BMAL1 in smooth muscle cells protects mice from abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2018; 38: 1063-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Dubis J, Litwin M, Michalowska D, et al. Elevated expression of runt-related transcription factors in human abdominal aortic aneurysm. J Biol Regul Homeost Agents 2016; 30: 497-504. [PubMed] [Google Scholar]
- 76).Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 2013; 12: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Miyake T, Miyake T, Sakaguchi M, et al. Prevention of asthma exacerbation in a mouse model by simultaneous inhibition of NF-κB and STAT6 activation using a chimeric decoy strategy. Mol Ther Nucleic Acids 2018; 10: 159-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Miyake T, Aoki M, Morishita R. Inhibition of anastomotic intimal hyperplasia using a chimeric decoy strategy against NFκB and E2F in a rabbit model. Cardiovasc Res 2008; 79: 706-14. [DOI] [PubMed] [Google Scholar]
- 79).Lee WR, Kim KH, An HJ, et al. Effects of chimeric decoy oligodeoxynucleotide in the regulation of transcription factors NF-κB and Sp1 in an animal model of atherosclerosis. Basic Clin Pharmacol Toxicol 2013; 112: 236-43. [DOI] [PubMed] [Google Scholar]
- 80).Kim KH, Park JH, Lee WR, et al. The inhibitory effect of chimeric decoy oligodeoxynucleotide against NF-κB and Sp1 in renal interstitial fibrosis. J Mol Med (Berl) 2013; 91: 573-86. [DOI] [PubMed] [Google Scholar]
- 81).Sung WJ, Kim KH, Kim YJ, et al. Antifibrotic effect of synthetic Smad/Sp1 chimeric decoy oligodeoxynucleotide through the regulation of epithelial mesenchymal transition in unilateral ureteral obstruction model of mice. Exp Mol Pathol 2013; 95: 136-43. [DOI] [PubMed] [Google Scholar]
- 82).Yuan HF, Huang H, Li XY, et al. A dual AP-1 and SMAD decoy ODN suppresses tissue fibrosis and scarring in mice. J Invest Dermatol 2013; 133: 1080-7. [DOI] [PubMed] [Google Scholar]
- 83).Miyake T, Aoki M, Nakashima H, et al. Prevention of abdominal aortic aneurysms by simultaneous inhibition of NFκB and ets using chimeric decoy oligonucleotides in a rabbit model. Gene Ther 2006; 13: 695-704. [DOI] [PubMed] [Google Scholar]
- 84).Hagedorn PH, Persson R, Funder ED, et al. Locked nucleic acid: modality, diversity, and drug discovery. Drug Discov Today 2018; 23: 101-14. [DOI] [PubMed] [Google Scholar]
- 85).Osako MK, Tomita N, Nakagami H, et al. Increase in nuclease resistance and incorporation of NF-κB decoy oligodeoxynucleotides by modification of the 3′-terminus. J Gene Med 2007; 9: 812-9. [DOI] [PubMed] [Google Scholar]
- 86).Sotobayashi D, Kawahata H, Anada N, et al. Therapeutic effect of intra-articular injection of ribbon-type decoy oligonucleotides for hypoxia inducible factor-1 on joint contracture in an immobilized knee animal model. J Gene Med 2016; 18: 180-92. [DOI] [PubMed] [Google Scholar]
- 87).Miyake T, Aoki M, Osako MK, et al. Systemic administration of ribbon-type decoy oligodeoxynucleotide against nuclear factor κB and ets prevents abdominal aortic aneurysm in rat model. Mol Ther 2011; 19: 181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).De Stefano D, De Rosa G, Maiuri MC, et al. Oligonucleotide decoy to NF-κB slowly released from PLGA microspheres reduces chronic inflammation in rat. Pharmacol Res 2009; 60: 33-40. [DOI] [PubMed] [Google Scholar]
- 89).Miyake T, Ihara S, Miyake T, et al. Prevention of neointimal formation after angioplasty using nuclear factor-κB decoy oligodeoxynucleotide-coated balloon catheter in rabbit model. Circ Cardiovasc Interv 2014; 7: 787-96. [DOI] [PubMed] [Google Scholar]
- 90).Panyam J, Zhou WZ, Prabha S, et al. Rapid endo-lysosomal escape of poly(dl-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J 2002; 16: 1217-26. [DOI] [PubMed] [Google Scholar]
- 91).Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 375: 998-1006. [DOI] [PubMed] [Google Scholar]
- 92).Stein EA, Dufour R, Gagne C, et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation 2012; 126: 2283-92. [DOI] [PubMed] [Google Scholar]
- 93).Gibson I. Antisense approaches to the gene therapy of cancer—‘Recnac’. Cancer Metastasis Rev 1996; 15: 287-99. [DOI] [PubMed] [Google Scholar]
- 94).Henry SP, Bolte H, Auletta C, et al. Evaluation of the toxicity of ISIS 2302, a phosphorothioate oligonucleotide, in a four-week study in cynomolgus monkeys. Toxicology 1997; 120: 145-55. [DOI] [PubMed] [Google Scholar]
- 95).Srinivasan SK, Iversen P. Review of in vivo pharmacokinetics and toxicology of phosphorothioate oligonucleotides. J Clin Lab Anal 1995; 9: 129-37. [DOI] [PubMed] [Google Scholar]
- 96).PREVENT IV Investigators; Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA 2005; 294: 2446-54. [DOI] [PubMed] [Google Scholar]
- 97).Suzuki J, Tezuka D, Morishita R, et al. An initial case of suppressed restenosis with nuclear factor-kappa B decoy transfection after percutaneous coronary intervention. J Gene Med 2009; 11: 89-91. [DOI] [PubMed] [Google Scholar]
- 98).Fukasawa M, Isobe M, Nanto S, et al. NF-κB decoy oligodeoxynucleotide-coated balloon catheter for arteriovenous fistula in hemodialysis. Kidney Int Rep 2019; 4: 126-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Zhao G, Fu Y, Cai Z, et al. Unspliced XBP1 confers VSMC homeostasis and prevents aortic aneurysm formation via FoxO4 interaction. Circ Res 2017; 121: 1331-45. [DOI] [PubMed] [Google Scholar]