Abstract
Objective: Open revascularization of the lower extremity in patients with chronic limb-threatening ischemia (CLTI) does not guarantee limb salvage. Due to the high prevalence of frailty among these patients, we hypothesized that sarcopenia negatively affects limb prognosis.
Methods: Seventy-five CLTI patients who underwent open revascularization between 2011 and 2015 were retrospectively reviewed. The lumbar psoas index, which is the ratio of the cross-sectional area of the psoas major muscles to the patients’ height squared, was used as a surrogate marker for sarcopenia. Male and female patients were stratified separately according to lumbar psoas index values. The lower two-thirds of the population for each sex were defined as the sarcopenia group, with the higher third defined as the non-sarcopenia group.
Results: Comorbidities and ambulatory status did not differ between the sarcopenia (n=50) and non-sarcopenia (n=25) groups. The sarcopenia group had significantly lower overall survival rates than the non-sarcopenia group (60% vs 87% at 3 years, P<0.05). Moreover, the limb salvage rates were significantly lower in the sarcopenia group than in the non-sarcopenia group (73% vs 100% at 2 years, P<0.05).
Conclusion: Sarcopenia, as measured by the lumbar psoas index, may predict poor limb prognosis in CLTI patients undergoing open revascularization.
Keywords: sarcopenia, frailty, chronic limb-threatening ischemia (CLTI), psoas major muscle, limb salvage
Introduction
Patients with chronic limb-threatening ischemia (CLTI) tend to have a frail clinical condition due to the presence of multiple comorbidities. Frailty is defined as “the biologic syndrome of decreased reserve and resistance to stressors, resulting from cumulative declines across multiple physiologic systems, and causing vulnerability to adverse outcomes.”1) Over the years, hematological, physiological, and radiographic tests have been performed to evaluate the patient’s “vulnerability” prior to surgical interventions. Since revascularization of the lower extremity does not guarantee limb salvage,2) patient selection for intervention is crucial, especially for open surgery candidates. However, as noted in the BASIL (Bypass versus Angioplasty in Severe Ischemia of the Leg) trial, no definite criteria for choosing either open surgery or less invasive endovascular therapy have been established.3)
The PREVENT III risk score for CLTI stratifies patients into three distinct categories of expected amputation-free survival.4) Considering the multiple comorbidities of CLTI patients, a substantial portion may fall into the high-risk group, especially dialysis dependent patients. Studies have shown that the prevalence of frailty in peripheral arterial disease (PAD) patients undergoing infrainguinal bypass is approximately 50%–60%.5,6) Therefore, a novel predictor reflecting the patients’ general status and frailty, not by assessing each organ, is needed to optimize the clinical benefits of revascularization.
One of the core factors of frailty is sarcopenia, defined as the depletion of skeletal muscle.1,7) The negative correlation between sarcopenia and overall survival has been discussed and shown in various diseases, including malignancies, post-liver transplantation, abdominal aortic aneurysms, and without exception, PAD.8–14) Nevertheless, the impact of sarcopenia on limb salvage in the CLTI cohort has not been satisfactorily discussed.
When diagnosing sarcopenia, muscle mass can be measured using body imaging techniques such as computed tomography (CT), magnetic resonance imaging, and dual energy X-ray absorptiometry (DXA). A recent study has shown that CT analysis of skeletal muscle at the level of the 3rd lumbar vertebra (L3) is strongly related to whole-body fat-free mass, and to appendicular skeletal muscle mass as measured by DXA.15) Therefore, this simplified method is used in many studies investigating the effect of sarcopenia.8–14)
In the present retrospective, cohort study, the aim was to elucidate the impact of sarcopenia on limb salvage in CLTI patients undergoing open revascularization. Other primary endpoints were overall survival and primary and secondary patency rates.
Methods
This study conformed to the guidelines established by the Declaration of Helsinki, and was approved by the Institutional Review Board at Saitama Medical Center, Saitama Medical University (No. 2008). Informed consent was obtained from all patients prior to revascularization.
Patient population
Patients with tissue loss due to atherosclerotic occlusive disease who underwent open revascularization at our tertiary institution between January 2011 and December 2015 were included in the study. Patients who did not have a diagnostic CT scan within the 6-month period before revascularization or the 1-month period after revascularization were excluded from the study. Revision cases were also excluded. Seventy-five patients who fulfilled the necessary requirements were analyzed retrospectively.
Clinical characteristics
Patients were assumed to have comorbidities including diabetes mellitus (DM), hypertension, or dyslipidemia based on their medications. Cerebrovascular disease (CVD) was diagnosed from a medical history of ischemic stroke or transient ischemic attack. Coronary artery disease was diagnosed from a medical history of percutaneous coronary intervention, coronary artery bypass graft surgery, or previous myocardial infarction. Foot infection was determined with the presence of more than two of the following items: local swelling or induration, erythema, local tenderness or pain, local warmth, and purulent discharge. Paramalleolar bypass was defined as any infrainguinal revascularization in which the distal anastomosis was 10 cm or less above the malleoli.16) Bypass to the tibial or peroneal artery within this criterion was classified as paramalleolar bypass instead of tibial/peroneal bypass.
L3 psoas index (LPI, mm2/m2)
The cross-sectional area of the bilateral psoas major muscles (mm2) at the caudal end of L3 was measured in a semi-automated fashion by manual outlining of CT scans using GE Centricity PACS software (GE Healthcare, Chicago, IL, USA). The LPI (mm2/m2) was calculated by dividing the muscle area by the square of the patients’ height, as per the convention for body composition measurements.17)
Definition of sarcopenia
Patients were stratified according to their LPI values. The sarcopenia group was defined as the population having the lower 2/3 values for each sex, with the population having the higher 1/3 values as the non-sarcopenia group. The clinical characteristics and outcomes after open revascularization of these two groups were compared.
Statistical analysis
All statistical evaluations were performed with standard software programs (JMP Pro 14.0.0, SAS Institute, Cary, NC, USA). The unpaired t-test was used to compare continuous variables, and the Chi-squared test was used for categorical variables. For the latter comparisons, the two-tailed Fisher’s exact probability test was used instead, if there was a variable with n≤5. Kaplan–Meier life-table analysis was performed for overall survival, limb salvage, and patency rates. The effects of clinicopathological factors on limb salvage were analyzed using the Cox proportional hazards model. Values are reported as means±standard deviation, unless otherwise specified. A P value of <0.05 was considered significant.
Results
Study population
The patients’ mean age was 72.9 years, and there was a male predominance (70.7%). Median follow-up was 13.7 months (interquartile range, 4.2–22.7 months). The baseline characteristics of the study population are presented in Table 1.
Table 1 Patients’ demographics.
| Variables | Total | Subgroups* | ||
|---|---|---|---|---|
| (n=75) | Non-sarcopenia (n=25) | Sarcopenia (n=50) | P value | |
| Age, y | 72.9±10.3 | 67.9±13 | 75.4±8 | <0.01 |
| Male sex, n (%) | 53 (70.7) | 18 (72.0) | 35 (70.0) | 0.86 |
| Body mass index, kg/m2 | 22.1±3.7 | 23.5±3.1 | 21.4±3.8 | <0.05 |
| Rutherford classification 5/6, n | 67/8 | 22/3 | 45/5 | 1.00 |
| Foot infection, n (%) | 42 (56.0) | 14 (56.0) | 28 (56.0) | 1.00 |
| Ejection fraction, % | 63.9±12.6 | 66.8±9.7 | 62.4±13.7 | 0.16 |
| Current or ex-smoking, n (%) | 60 (80.0) | 20 (80.0) | 40 (80.0) | 1.00 |
| Ambulatory, n (%) | 59 (78.7) | 21 (84.0) | 38 (76.0) | 0.56 |
| Comorbidity, n (%) | ||||
| Hypertension | 60 (80.0) | 17 (68.0) | 43 (86.0) | 0.07 |
| Dyslipidemia | 16 (21.3) | 6 (24.0) | 10 (20.0) | 0.69 |
| Diabetes mellitus | 54 (72.0) | 19 (76.0) | 35 (70.0) | 0.59 |
| Dialysis dependent | 24 (32.0) | 9 (36.0) | 15 (30.0) | 0.60 |
| Cerebrovascular disease | 22 (29.3) | 5 (20.0) | 17 (34.0) | 0.28 |
| Coronary artery disease | 23 (30.7) | 6 (24.0) | 17 (34.0) | 0.38 |
| Dementia | 8 (10.7) | 3 (12.0) | 5 (10.0) | 1.00 |
| Type of open revascularization, n (%) | ||||
| Bypass by outflow artery | ||||
| Supragenicular popliteal | 17 (22.7) | 5 (20.0) | 12 (24.0) | 0.78 |
| Infragenicular popliteal | 15 (20.0) | 5 (20.0) | 10 (20.0) | 1.00 |
| Tibial/peroneal | 10 (13.3) | 3 (12.0) | 7 (14.0) | 1.00 |
| Paramalleolar/pedal/plantar | 21 (28.0) | 9 (36.0) | 12 (24.0) | 0.28 |
| Others | 12 (16.0) | 3 (12.0) | 9 (18.0) | 0.74 |
*Percentage of each variable for the non-sarcopenia and the sarcopenia groups are those within each subgroup.
LPI differences by sex
LPI values were significantly higher for males than for females (866±272 vs 638±227 mm2/m2, P<0.01).
Sarcopenia and clinical characteristics
The sarcopenia group (n=50) and the non-sarcopenia group (n=25) had the same male to female sex ratios (72% and 70%, P=0.86). The sarcopenia group was significantly older and leaner than the non-sarcopenia group (P<0.01 and P<0.05, respectively). There were no significant differences between the groups for other variables, including Rutherford classification, foot infection, ambulatory status, comorbidities, and type of open surgery (Table 1).
Sarcopenia, overall survival, and limb salvage
The sarcopenia group had a significantly worse overall survival rate than the non-sarcopenia group (60% vs 87% at 3 years, P<0.05, Fig. 1A). Furthermore, the limb salvage rate of the sarcopenia group was significantly lower than that of the non-sarcopenia group (73% vs 100% at 2 years, P<0.05, Fig. 1B).
Fig. 1 (A) Kaplan–Meier curves of overall survival for the sarcopenia group and the non-sarcopenia group. P<0.05. (B) Kaplan–Meier curves of limb salvage for the sarcopenia group and the non-sarcopenia group. P<0.05. Lines are truncated when the standard error exceeds 10%.
Sarcopenia and patency rates
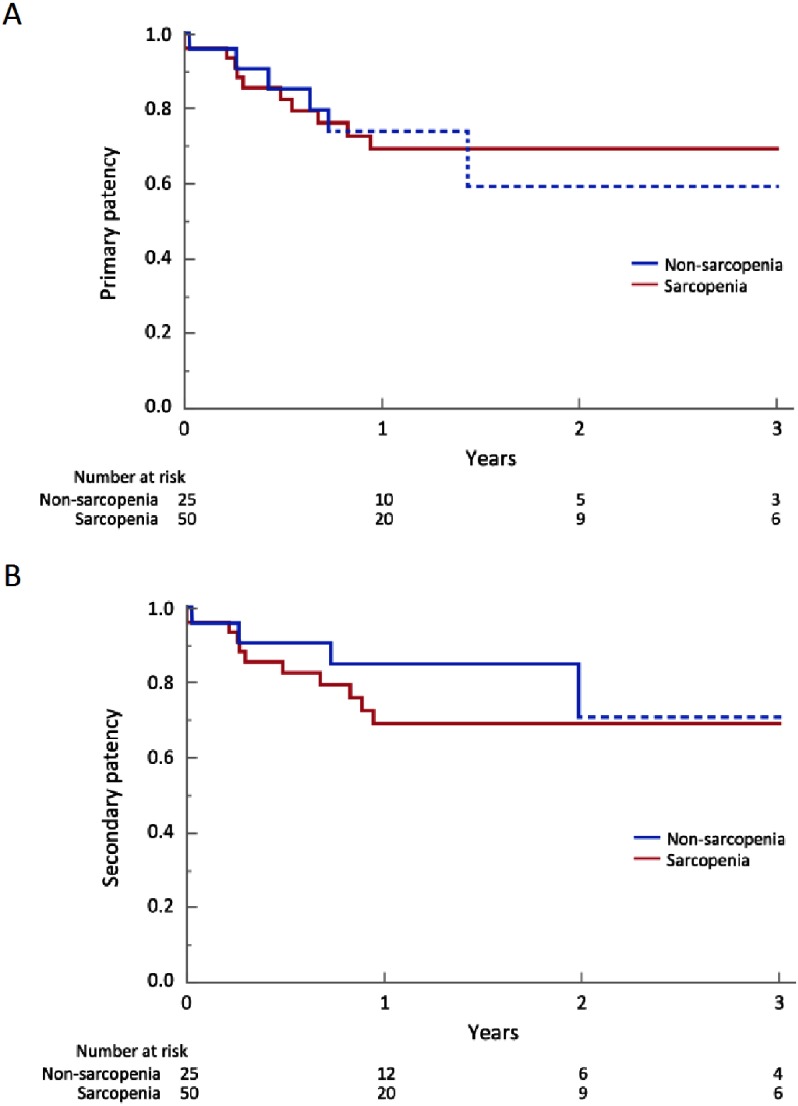
There was no significant difference between the sarcopenia and non-sarcopenia groups in the primary patency rate (69% vs 74% at 1 year, P=0.88, Fig. 2A). As for the secondary patency rate, the sarcopenia group showed inferior results, but the difference was not significant (69% vs 85% at 1 year, P=0.39, Fig. 2B).
Fig. 2 (A) Kaplan–Meier curves of primary patency for the sarcopenia group and the non-sarcopenia group. P=0.88. (B) Kaplan–Meier curves of secondary patency for the sarcopenia group and the non-sarcopenia group. P=0.39. Lines are truncated when the standard error exceeds 10%.
Clinicopathological factors and limb salvage
Univariate analysis showed that sarcopenia was the only significant factor related to worse limb salvage (Hazard ratio, 5.49; 95% confidence interval, 1.03–101; P<0.05, Table 2).
Table 2 Univariate analysis of clinicopathological factors and limb salvage.
| Variable | HR (95%CI) | P value |
|---|---|---|
| Age, y | 1.38 (0.07–43.5) | 0.84 |
| Male sex | 1.88 (0.47–12.4) | 0.40 |
| Sarcopenia, Y/N | 5.49 (1.03–101) | <0.05 |
| Rutherford, 6/5 | 2.57 (0.39–10.3) | 0.28 |
| Foot infection, Y/N | 2.38 (0.66–11.1) | 0.19 |
| Current or ex-smoking, Y/N | 1.13 (0.28–7.48) | 0.88 |
| Ambulatory, N/Y | 2.17 (0.47–7.90) | 0.29 |
| Comorbidity, Y/N | ||
| Hypertension | 3.10 (0.58–57.3) | 0.22 |
| Dyslipidemia | 1.38 (0.30–4.98) | 0.65 |
| Diabetes mellitus | 1.01 (0.28–4.71) | 0.99 |
| Dialysis dependent | 0.92 (0.29–3.32) | 0.90 |
| Cerebrovascular disease | 2.10 (0.53–7.38) | 0.27 |
| Coronary artery disease | 0.93 (0.20–3.36) | 0.92 |
| Dementia | 1.44 (0.08–7.76) | 0.74 |
HR: hazard ratio; CI: confidence interval; Y: yes; N: no
Discussion
This retrospective, observational study showed that sarcopenia might have a negative effect on limb salvage. Surprisingly, univariate analysis showed that only sarcopenia affected limb salvage versus other variables, including classical negative predictors such as extensive necrosis (Rutherford classification 6), foot infection, DM, and dialysis dependence. Moreover, there were no significant differences in the prevalence of these factors between the present sarcopenia and non-sarcopenia groups.
In terms of the patency rates, the gap between the sarcopenia group and the non-sarcopenia group became more conspicuous for secondary patency than for primary patency, although not significantly. Had there been more patients in this study, the difference may have become significant, thereby leading to a hypothesis that limb salvage rates might be affected simply by patency rates. Another interpretation of the result is that the patients’ frailty may have restricted additional interventions potentially contributing to secondary patency, thereby leading to poor limb salvage rates. Furthermore, the non-significant result for the primary patency rate itself suggests that classical factors, such as the quality and type of conduits or the distal anastomotic site, may have more impact.18,19)
In the present study, sarcopenia had a significant impact on the overall survival of CLTI patients, which is compatible with previous studies.8,10–12) However, the sarcopenia group was approximately 7 years older than the non-sarcopenia group. In the general Japanese population, the life expectancy for the mean ages of the two groups is approximately 13 and 18 years, respectively.20) This 5-year difference may need to be considered when observing the Kaplan–Meier curves of overall survival.
Interestingly, a recent report, with opposite results from the present study, concluded that the psoas index could not be used to predict amputation-free survival.14) However, there was a slight difference in the methodology for measuring the psoas index. In their study, the cross-sectional psoas area was divided by the cross-sectional area of the L4 vertebral body and not the square of the subject’s height, as in the present study. Another crucial factor for the opposite results may be that their Kaplan–Meier analysis of amputation-free survival was based on comparing the upper and lower halves of the psoas index. In the present study, the upper 1/3 and the lower 2/3 values of the psoas index were compared. The cut-off value of 2/3 was determined based on the prevalence of frailty among PAD patients in studies with large populations,5,6) and the relatively large proportion of the present patients with comorbid DM and end-stage renal disease.
There is no absolute standard for sarcopenia based on cross-sectional area measurements to date. Indeed, other studies have also done relative comparisons,10,11,14) or in studies with larger populations, cut-off values were established according to optimum stratification within their own population.8,9) Another aspect of LPI that needs to be taken into consideration is that males have greater skeletal muscle volume. An analysis based on the absolute value of LPI will need to be executed separately for each sex. In the present study, this was obviated by taking the same proportion of patients from each sex as the sarcopenia group.
Another interesting finding in the present study was that sarcopenia was not significantly correlated with the patient’s non-ambulatory status, although there was a trend toward it. The psoas major muscle contributes to flexion and outer rotation of the hip joint, and stabilizes the lumbar vertebrae. It is the primary contributor to positive fiber work during the swing phase in the gait cycle.21) During the stance phase, plantar flexors such as the soleus and gastrocnemius muscles are the primary contributors. Therefore, taking into account that there are muscles working as negative contributors during both phases, we must consider not just the psoas muscle when attempting to understand ambulation. However, the present results do not conflict with a previous report showing poor outcomes after below-knee bypass surgeries in non-ambulatory patients,22) or with reports showing poor overall survival in CLTI patients with decreased activities of daily living undergoing bypass surgery.23,24)
In the present study, the diagnosis of sarcopenia was made by measuring the cross-sectional area of the psoas major muscle in a single CT slice using analysis software that is standard in the medical imaging reference system. There are other studies measuring the area of the total skeletal muscle including the psoas, paraspinal muscles (erector spinae, quadratus lumborum), and the abdominal wall muscles (transversus abdominis, external and internal obliques, rectus abdominis).8,12,13) Furthermore, there were studies excluding adipose tissues within the muscles using Hounsfield units.8,9) The simple method used in the present study saves time compared to these methods requiring additional processes. Most importantly, the present method of using LPI as a surrogate for sarcopenia was feasible, considering the results that the LPI was greater in men and that the sarcopenia group was older, both of which are consistent with previous studies.6,8,25)
The limitations of the present study are its retrospective nature and the small population. With a larger population, other comorbidities that differed between the two groups may have become significant. Furthermore, other elements of sarcopenia, such as walking speed and grip strength, were not assessed in the present study.7) However, it may be difficult to evaluate gait speed, since the population of the present study composed of CLTI patients with toe or foot impairments. As for the methodology, adipose tissues within the psoas muscles were not excluded using Hounsfield units. This may have an effect when expanding the cohort outside of the Japanese population, a relatively lean population. Further studies are needed to construct a gold standard definition of sarcopenia using this modality. An upcoming absolute cut-off value for sarcopenia might be able to present a more accurate prognostic index and provide CTLI patients with (information about) the preferred choice of treatment.
Conclusion
Sarcopenia is an important factor in the multi-dimensional pathophysiology of CLTI. Measuring the LPI to evaluate comorbid sarcopenia is a feasible method, and it may predict poor limb prognosis of patients undergoing open revascularization.
Disclosure Statement
All authors have no conflict of interest.
Author Contributions
Study conception: RT, JD, OS
Data collection: RT, JD, TH
Analysis: RT
Investigation: RT, JD
Writing: RT, JD, TH
Funding acquisition: none
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146-56. [DOI] [PubMed] [Google Scholar]
- 2).Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45 Suppl S: S5-67. [DOI] [PubMed] [Google Scholar]
- 3).Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005; 366: 1925-34. [DOI] [PubMed] [Google Scholar]
- 4).Schanzer A, Mega J, Meadows J, et al. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg 2008; 48: 1464-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Arya S, Long CA, Brahmbhatt R, et al. Preoperative frailty increases risk of nonhome discharge after elective vascular surgery in home-dwelling patients. Ann Vasc Surg 2016; 35: 19-29. [DOI] [PubMed] [Google Scholar]
- 6).Brahmbhatt R, Brewster LP, Shafii S, et al. Gender and frailty predict poor outcomes in infrainguinal vascular surgery. J Surg Res 2016; 201: 156-65. [DOI] [PubMed] [Google Scholar]
- 7).Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008; 9: 629-35. [DOI] [PubMed] [Google Scholar]
- 9).Peng PD, van Vledder MG, Tsai S, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011; 13: 439-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010; 211: 271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Lee JS, He K, Harbaugh CM, et al. Frailty, core muscle size, and mortality in patients undergoing open abdominal aortic aneurysm repair. J Vasc Surg 2011; 53: 912-7. [DOI] [PubMed] [Google Scholar]
- 12).Matsubara Y, Matsumoto T, Aoyagi Y, et al. Sarcopenia is a prognostic factor for overall survival in patients with critical limb ischemia. J Vasc Surg 2015; 61: 945-50. [DOI] [PubMed] [Google Scholar]
- 13).Matsubara Y, Matsumoto T, Inoue K, et al. Sarcopenia is a risk factor for cardiovascular events experienced by patients with critical limb ischemia. J Vasc Surg 2017; 65: 1390-7. [DOI] [PubMed] [Google Scholar]
- 14).Nyers ES, Brothers TE. Perioperative psoas to lumbar vertebral index does not successfully predict amputation-free survival after lower extremity revascularization. J Vasc Surg 2017; 66: 1820-5. [DOI] [PubMed] [Google Scholar]
- 15).Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008; 33: 997-1006. [DOI] [PubMed] [Google Scholar]
- 16).Plecha EJ, Lee C, Hye RJ. Factors influencing the outcome of paramalleolar bypass grafts. Ann Vasc Surg 1996; 10: 356-60. [DOI] [PubMed] [Google Scholar]
- 17).Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004; 97: 2333-8. [DOI] [PubMed] [Google Scholar]
- 18).Wengerter KR, Veith FJ, Gupta SK, et al. Influence of vein size (diameter) on infrapopliteal reversed vein graft patency. J Vasc Surg 1990; 11: 525-31. [DOI] [PubMed] [Google Scholar]
- 19).Rutherford RB, Jones DN, Bergentz SE, et al. Factors affecting the patency of infrainguinal bypass. J Vasc Surg 1988; 8: 236-46. [PubMed] [Google Scholar]
- 20).Director-General for Statistics and Information Policy, Ministry of Health, Labour and Welfare, Government of Japan. Life expectancies at specified ages. In: Abridged Life Tables for Japan 2017. Tokyo: Ministry of Health, Labour and Welfare, 2018: 1. Retrieved from: https://www.mhlw.go.jp/english/database/db-hw/lifetb17/
- 21).Neptune RR, Sasaki K, Kautz SA. The effect of walking speed on muscle function and mechanical energetics. Gait Posture 2008; 28: 135-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Yamamoto K, Kitaoka T, Matsumoto H, et al. Preoperative non-ambulatory status predicts poor outcome after below knee bypass surgery. Ann Vasc Dis 2011; 4: 204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Kodama A, Koyama A, Sugimoto M, et al. Association between preoperative frailty and mortality in patients with critical limb ischemia following infrainguinal bypass surgery—usefulness of the Barthel index. Circ J 2018; 82: 267-74. [DOI] [PubMed] [Google Scholar]
- 24).Mii S, Guntani A, Kawakubo E, et al. Barthel index and outcome of open bypass for critical limb ischemia. Circ J 2018; 82: 251-7. [DOI] [PubMed] [Google Scholar]
- 25).Yoshizumi T, Shirabe K, Nakagawara H, et al. Skeletal muscle area correlates with body surface area in healthy adults. Hepatol Res 2014; 44: 313-8. [DOI] [PubMed] [Google Scholar]