Mucosal vaccination is proposed as a method of immunization able to induce protection against mucosal pathogens that is superior to protection provided by parenteral immunization. However, mucosal vaccination often induces serum antigen-specific immune responses of lower magnitude than those induced by parenteral immunization, making the comparison of mucosal and parenteral immunization difficult. We identified vaccine parameters that allowed an immunization regimen consisting of an i.n. prime followed by boosters administered by both i.n. and i.m. routes to induce serum antibody responses similar to those induced by i.m. prime/boost vaccination. Additional studies are needed to determine the potential benefit of mucosal immunization for HIV-1 and other mucosally transmitted pathogens.
KEYWORDS: HIV-1 vaccine, mucosal adjuvants, mucosal vaccines
ABSTRACT
The benefits of mucosal vaccines over injected vaccines are difficult to ascertain, since mucosally administered vaccines often induce serum antibody responses of lower magnitude than those induced by injected vaccines. This study aimed to determine if mucosal vaccination using a modified vaccinia virus Ankara expressing human immunodeficiency virus type 1 (HIV-1) gp120 (MVAgp120) prime and a HIV-1 gp120 protein boost could be optimized to induce serum antibody responses similar to those induced by an intramuscularly (i.m.) administered MVAgp120 prime/gp120 boost to allow comparison of an i.m. immunization regimen to a mucosal vaccination regimen for the ability to protect against a low-dose rectal simian-human immunodeficiency virus (SHIV) challenge. A 3-fold higher antigen dose was required for intranasal (i.n.) immunization with gp120 to induce serum anti-gp120 IgG responses not significantly different than those induced by i.m. immunization. gp120 fused to the adenovirus type 2 fiber binding domain (gp120-Ad2F), a mucosal targeting ligand, exhibited enhanced i.n. immunogenicity compared to gp120. MVAgp120 was more immunogenic after i.n. delivery than after gastric or rectal delivery. Using these optimized vaccines, an i.n. MVAgp120 prime/combined i.m. (gp120) and i.n. (gp120-Ad2F) boost regimen (i.n./i.m.-plus-i.n.) induced serum anti-gp120 antibody titers similar to those induced by the intramuscular prime/boost regimen (i.m./i.m.) in rabbits and nonhuman primates. Despite the induction of similar systemic anti-HIV-1 antibody responses, neither the i.m./i.m. nor the i.n./i.m.-plus-i.n. regimen protected against a repeated low-dose rectal SHIV challenge. These results demonstrate that immunization regimens utilizing the i.n. route are able to induce serum antigen-specific antibody responses similar to those induced by systemic immunization.
IMPORTANCE Mucosal vaccination is proposed as a method of immunization able to induce protection against mucosal pathogens that is superior to protection provided by parenteral immunization. However, mucosal vaccination often induces serum antigen-specific immune responses of lower magnitude than those induced by parenteral immunization, making the comparison of mucosal and parenteral immunization difficult. We identified vaccine parameters that allowed an immunization regimen consisting of an i.n. prime followed by boosters administered by both i.n. and i.m. routes to induce serum antibody responses similar to those induced by i.m. prime/boost vaccination. Additional studies are needed to determine the potential benefit of mucosal immunization for HIV-1 and other mucosally transmitted pathogens.
INTRODUCTION
A human immunodeficiency virus (HIV) vaccine would be of significant benefit for ending the HIV/AIDS epidemic (1). To date, the only HIV vaccine clinical trial to demonstrate protective efficacy was a poxvirus prime/recombinant gp120 boost RV144 trial (NCT00223080) (2). Since more than 90% of HIV infections are transmitted across mucosal tissues, with sexual transmission being the most common route (3), enhancing mucosal immunity with the use of mucosal immunization may enhance protection against sexual transmission of HIV (4). The benefit of mucosal immunity for protection against mucosal transmission of simian-human immunodeficiency virus (SHIV)/simian immunodeficiency virus (SIV) has been demonstrated using passive and active immunization. For example, passive transfer of the combination of systemically administered anti-HIV IgG1 and mucosally administered anti-HIV dimeric IgA2 provided complete protection against a high-dose rectal SHIV challenge, while transfer of anti-HIV IgG1 alone was not protective (5), suggesting that the combination of systemic and mucosal anti-HIV IgG and IgA, respectively, may be required for maximum protection. Another recent study demonstrated that systemic (intramuscular) and mucosal (aerosol) immunizations with the same vaccine induced equivalent levels of protection against SIV challenge (6). However, evaluation of vaccine-induced immune responses determined that protection induced by intramuscular immunization was associated with serum IgG-mediated immune responses while protection induced by aerosol immunization was associated with serum IgA-mediated immune responses (6). Therefore, optimizing both systemic and mucosal immunization regimens may be required to provide consistent protection against mucosal HIV transmission (7).
HIV-1 vaccine regimens utilizing a mucosal route of vaccination have been described in the literature, but evaluating the potency of a mucosally administered vaccine in comparison to a similar vaccine delivered parenterally is often not discussed. For example, mucosal immunization often fails to induce antigen-specific serum IgG responses comparable to those induced by parenteral immunization with the same antigen (8, 9). Our previous study that compared i.m. MVA priming with i.m. gp120 boosting (i.m./i.m.) to i.m. MVA priming with i.n. gp120 boosting (i.m./i.n.) demonstrated unique immune responses depending on the route of immunization used (8). The i.m. boosted group had a trend toward higher plasma HIV Env-specific IgG (P = 0.1), higher tier 1 neutralization in plasma (P = 0.2) and milk (P = 0.06), and higher antibody-dependent cell-mediated cytotoxicity (ADCC) activity in plasma (P = 0.06) than i.n. boosting, indicating that i.m./i.m. systemic immunization may be more effective than i.m./i.n. mucosal vaccination at eliciting functional systemic antibody responses (8). Others have reported that i.n. immunization of humans with 100 μg of HIV-1 gp140 was not immunogenic while i.m. immunization with 20 or 100 μg of the same antigen induced elevated serum anti-gp140 IgG responses (9). This observation highlights the difficulty in comparing the immunogenicities of the same HIV-1 antigen dose delivered parenterally versus mucosally. If the mucosal immunization strategy is not immunogenic, it is impossible to evaluate correlates of protection for the mucosal immunization strategy compared to the parenteral immunization strategy, as described above (8). We therefore propose that one criterion to identify an optimized mucosal immunization regimen is the expectation that the mucosal immunization regimen will induce systemic anti-HIV binding and functional antibody responses, such as virus neutralization or ADCC, comparable to those induced by systemic immunization while utilizing a mucosal route of immunization that may enhance the induction of protective serum (6) or mucosal (5) anti-HIV IgA responses.
A major impediment to developing any mucosal vaccine is the lack of an approved mucosal-vaccine adjuvant. Of all the mucosal-vaccine adjuvants tested to date, cholera toxin (CT) has been one of the most efficacious and has been used as a “gold standard” for mucosal adjuvant efficacy (10). However, safety concerns, including nerve toxicity, will likely prevent it from being used in humans as a nasal vaccine adjuvant (10). Monophosphoryl lipid A (MPL) is a Toll-like receptor 4 (TLR4) agonist that is approved for use in injected vaccines when it is combined with alum, and clinical trials indicate MPL is safe for use as a nasal vaccine adjuvant (11). Since mucosal vaccines need to be both safe and efficacious, newer compounds have been investigated as mucosal-vaccine adjuvants, including mast cell-activating compounds and cationic peptides, which can bridge innate and adaptive immune responses (12, 13). Compound 48/80 (C48/80) is a mast cell-activating compound with adjuvant activity capable of inducing antibody responses similarly to CT-adjuvanted vaccines when used as an adjuvant for mucosal or systemic immunization (14–17). However, C48/80 is a mixture of polymer species (18), and therefore, it may not be an ideal adjuvant, as regulatory agencies would most likely require a single active compound to provide the described adjuvant activity. To identify a potent adjuvant for nasally administered HIV-1 gp120, we compared the abilities of several adjuvants—CT, MPL, and C48/80, as well as mastoparan 7 (M7), a cationic antimicrobial mast cell-activating peptide (19, 20)—to enhance serum anti-gp120 antibody responses.
In addition to immunostimulatory adjuvants, adjuvants that enhance vaccine retention at mucosal sites may be beneficial. Several studies have demonstrated that increased nasal clearance correlates with diminished immunogenicity of nasal vaccines (21–25). To enhance nasal vaccine retention, we tested a fused protein consisting of gp120 and the adenovirus type 2 fiber protein (Ad2F). Ad2F binds the coxsackievirus and adenovirus receptor (CAR) expressed on epithelial and endothelial cells and thus can act as a mucosal targeting ligand to enhance antigen binding to the epithelial surfaces in the nasal cavity (26). In addition to enhancing vaccine retention, Ad2F binding can induce the release of proinflammatory cytokines and chemokines believed to exert a localized adjuvant effect (27, 28). The enhanced immunogenicity of fusion proteins consisting of botulinum neurotoxin vaccine antigens and Ad2F have been reported previously (17, 26, 29). However, the ability of Ad2F to enhance the nasal immunogenicity of HIV-1 gp120 has not been evaluated. Here, we test a fusion protein composed of HIV-1 gp120 and Ad2F to determine if the addition of Ad2F enhances the nasal immunogenicity of HIV-1 gp120.
Viral vectored vaccines are another vaccine/adjuvant system that may enhance the immunogenicity of mucosal-vaccine regimens. Viruses that can infect the host through mucosal tissues, such as modified vaccinia virus Ankara (MVA), have mechanisms to overcome the innate immune defenses at mucosal sites and may be more easily adapted to mucosal administration than subunit vaccines. Injected MVA vectored vaccines have been used in several HIV vaccine clinical trials, but they have shown various degrees of efficacy (30–36). Strategies that enhance insert-specific immune responses and/or minimize vector-specific responses may enhance the efficacy of MVA vaccine vectors. Thus, replication-defective MVA vectors may offer advantages over conventional MVA vectors. For example, MVA vectors lacking the ability to express late genes or immunomodulatory proteins or lacking the ability to replicate may induce elevated insert-specific immune responses compared to conventional MVA vectors (37–42). In this study, we evaluated the immunogenicity of traditional MVA and an MVA vector lacking a gene (udg) necessary for viral replication, both expressing HIV-1 gp120, when delivered intramuscularly or mucosally (intranasally, intragastrically, or rectally).
Here, we identify an HIV-1 vaccination regimen that utilizes a mucosal route of immunization and induces serum gp120-specific IgG titers similar to those induced by an injected vaccine regimen. Using the adjuvant systems discussed above, we optimized a vaccination regimen consisting of a prime with conventional MVA expressing gp120 (MVAgp120) followed by adjuvanted gp120 booster immunizations. Rabbits were used as a small-animal model for the optimization of mucosal vaccines. We demonstrate the translatability of our optimized mucosal vaccination regimen from rabbits to nonhuman primates (NHPs) by comparing the efficacy of the optimized mucosal vaccination regimen to that of the systemic regimen in rhesus macaques. Since the NHP model allows us to begin investigating the potential protective benefit of including mucosal vaccines in an HIV vaccination regimen, we rectally challenged both the mucosally and systemically vaccinated macaques with a heterologous tier 2 SHIV.
(This article was submitted to an online preprint archive [43].)
RESULTS
Evaluation of nontoxin adjuvants for use with nasally administered HIV-1 gp120.
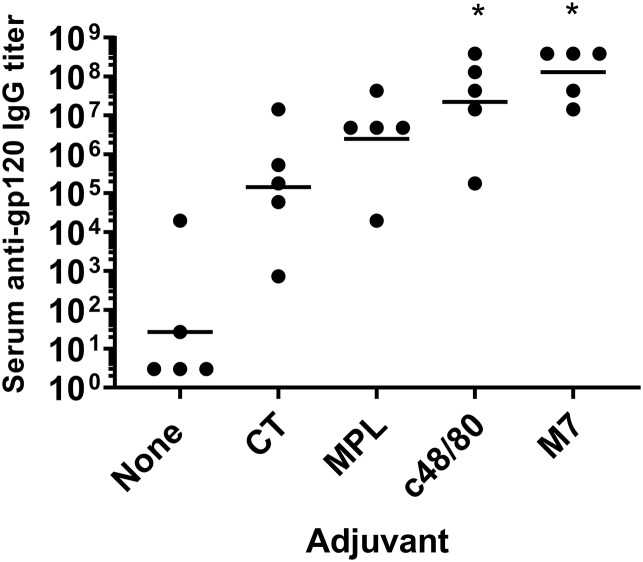
The lack of an approved mucosal-vaccine adjuvant is a major deterrent to mucosal-vaccine development. To determine if a nontoxin adjuvant could provide adjuvant activity similar or superior to that of CT, we compared the abilities of MPL, C48/80, and M7 to enhance serum antibody responses to HIV-1 gp120 when i.n. administered to C57BL/6 mice to that of the gold standard, CT. On day 14, all of the mice (5/5) that were vaccinated with gp120 plus C48/80 or M7 had detectable serum anti-gp120 IgG responses compared to 4/5 mice vaccinated with gp120 plus MPL, 3/5 mice vaccinated with gp120 plus CT, and 2/5 mice that received gp120 alone (Table 1). Only the mice that received gp120 plus M7 had serum anti-gp120 IgG titers significantly (P = 0.005) different than those of the mice that received gp120 alone (geometric mean titers [GMT], 1:4,782,969 versus 1:33.63, respectively).
TABLE 1.
Murine gp120-specific antibody responses after intranasal vaccination
| Day | Antibody | Unadjuvanted |
M7 |
C48/80 |
CT |
MPL |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. responders/total | GMTa | No. responders/total | GMT | No. responders/total | GMT | No. responders/total | GMT | No. responders/total | GMT | ||
| 14 | Serum IgG | 2/5 | 1:33 ± 72 | 5/5 | 1:4,782,969 ± 6b | 5/5 | 1:1,756 ± 5 | 3/5 | 1:729 ± 243 | 4/5 | 1:5,267 ± 187 |
| 35 | Serum IgG | 2/5 | 1:27 ± 45 | 5/5 | 1:129,140,163 ± 5b | 5/5 | 1:22,267,288 ± 19b | 5/5 | 1:142,203 ± 19 | 5/5 | 1:2,474,143 ± 5 |
| Serum IgA | 0/5 | 1:3 ± 1 | 4/5 | 1:2,187 ± 45b | 0/5 | 1:3 ± 1 | 2/5 | 1:22 ± 15 | 1/5 | 1:6 ± 4 | |
| Vaginal IgG | 1/5 | 1:12 ± 3 | 5/5 | 1:588 ± 3b | 5/5 | 1:1,176 ± 7b | 2/5 | 1:21 ± 5 | 4/5 | 1:97 ± 5 | |
| Vaginal IgA | 0/5 | 1:8 ± 1 | 4.5 | 1:74 ± 7b | 1/5 | 1:9 ± 1 | 1/5 | 1:18 ± 6 | 0/5 | 1:8 ± 1 | |
GMT, geometric mean titer ± geometric standard deviation factor.
Significantly different from mice receiving unadjuvanted gp120.
By day 35, all the mice (5/5) that were vaccinated with an adjuvanted vaccine developed detectable serum anti-gp120 IgG titers compared to only 2 of 5 mice vaccinated with gp120 alone. However, only gp120 adjuvanted with C48/80 or M7 induced serum anti-gp120 IgG titers significantly greater than the anti-gp120 IgG titers induced by the unadjuvanted vaccine (GMT, 1:22,267,288 and 1:129,140,163 versus 1:27, respectively) (Fig. 1). Mice vaccinated with gp120 plus M7 had significantly elevated serum anti-gp120 IgA titers (GMT, 1:2,187; 4 of 5 mice developed serum anti-gp120 IgA titers), while only 2 of 5 mice vaccinated with gp120 plus CT and 1 of 5 mice vaccinated with gp120 plus MPL developed serum anti-gp120 IgA titers (Table 1). Serum anti-gp120 IgA was not detected in mice that received gp120 alone or gp120 plus C48/80. Additionally, only mice that received vaccines adjuvanted with C48/80 or M7 developed significantly elevated vaginal gp120-specific IgG responses (1:1,176 and 1:588, respectively), and only mice vaccinated with gp120 plus M7 developed significantly elevated vaginal gp120-specific IgA titers (GMT, 1:74; 4 of 5 mice developed detectable vaginal anti-gp120 IgA) (Table 1). Based on these results, M7 was selected as the nasal vaccine adjuvant for subsequent experiments in rabbits and NHPs.
FIG 1.
Intranasal vaccines adjuvanted with the mast cell-activating adjuvant compound 48/80 or mastoparan 7 induce serum gp120-IgG titers stronger than those induced by cholera toxin or monophospholipid A. C57BL/6 mice (n = 5/group) were vaccinated on days 0, 7, and 21 with 10 μg gp120 and either no adjuvant, 7 μg M7, 15 μg C48/80, 1 μg CT, or 10 μg MPL. Serum was collected on day 35, and gp120-specific IgG titers were determined by ELISA. *, mice receiving vaccines adjuvanted with C48/80 or M7 had significantly higher serum anti-gp120 IgG titers than mice vaccinated with gp120 alone (P = 0.01 and 0.001, respectively). The horizontal lines indicate the geometric mean titers.
Intranasal immunogenicity of HIV-1 gp120 and gp120-Ad2F.
One goal of this study was to evaluate mucosal immunization for its ability to induce serum anti-HIV-1 antibody titers similar to those induced by i.m. immunization. For mucosal immunization with HIV-1 gp120, i.n. immunization was utilized, due to our previous success using this route in nonhuman primates (8, 44). Since rabbit nasopharyngeal lymphoid tissues are similar to those in primates and humans (65), results from the rabbit model may be more likely to translate to NHPs and humans than results obtained from mice. Thus, rabbits were used as the animal model to evaluate intranasal immunization with HIV-1 gp120 combined with the cationic antimicrobial mast cell-activating peptide M7.
Higher antigen doses are likely required for intranasal immunization to induce circulating antibody responses similar to those induced by injected vaccines (9). Therefore, we tested i.n. vaccination with 100, 200, and 300 μg of gp120 adjuvanted with the M7 peptide to determine what dose of gp120 administered i.n. was required to achieve serum anti-gp120 IgG titers similar to those induced by i.m. vaccination with 100 μg of HIV-1 gp120 adjuvanted with AddaVax, a squalene-based oil-in-water emulsion (experiments are shown in Table 2, i.n. 1). Squalene oil-in-water-adjuvanted vaccines induce titers similar to or higher than those induced by alum-adjuvanted vaccines (45, 46), and our previous success using MF59 (a squalene-based adjuvant) in a prime/boost vaccine regimen led to our use of AddaVax in the current study (8).
TABLE 2.
Animal characteristics and vaccine specifications for optimization experiments in mice and rabbits
| Expt | Species | Supplier | Sex | Bleed days | Vaccination days | Vaccination route | Antigen | Adjuvant | Total vaccine vol (μl) | μl/nostril if IN; μl/leg if IM |
|---|---|---|---|---|---|---|---|---|---|---|
| Adjuvants | C57BL/6 mice stock no. 000664 |
Jackson Labs |
Female | −4, 14, 35 | 0, 7, 21 | i.n. | 10 μg gp120 | None; 7 μg M7-NH2; 15 μg C48/80; 1 μg CT | 15 | 7.5 |
| 10 μg MPL | 21 | 10.5 | ||||||||
| IN 1 | NZW rabbitsa | Robinson | Male | 0, 21, 42 | 0, 28 | i.m. | 100 μg gp120 | Addavax | 400 | 200 |
| i.n. | 100 μg gp120, 200 μg gp120, 300 μg gp120 | 64 μg M7-NH2 | 340 | 85; 20-s restb | ||||||
| IN 2 | NZW rabbits | Robinson | Male | −6, 21, 48 | 0, 34 | i.m. | 100 μg gp120 | Addavax | 400 | 200 |
| i.n. | 300 μg gp120; 145 μg gp120-Ad2F; 290 μg gp120-Ad2F; 435 μg gp120-Ad2F | 64 μg M7-NH2 | 440 | 73.33; 15-s rest | ||||||
| IN 3 | NZW rabbits | Covance | Male | −15, 21, 42 | 0, 28 | i.m. | 100 μg gp120 | Addavax | 400 | 200 |
| i.n. | 300 μg gp120; 145 μg gp120-Ad2F; 435 μg gp120-Ad2F | 64 μg M7-NH2 | 440 | 73.33; 15-s rest | ||||||
| MVA 1 | NZW rabbits | Robinson | Female | 0, 21, 35 | 0, 21 | i.m. | 1 × 108 PFU MVAgp120, 5 × 106 PFU MVAgp120; 1 × 108 PFU MVAdelta5gp120, 5 × 106 PFU MVAdelta5gp120 | 200 | 200 | |
| MVA 2 | NZW rabbits | Covance | Female | 0, 21, 35 | 0, 21 | i.m. | 1 × 108 PFU MVAgp120, 1 × 108 PFU MVAdelta5gp120, 1 × 109 PFU MVAdelta5gp120, 1 × 1010 PFU MVAdelta5gp120 | 230 | 230 | |
| i.n. | 1 × 109 PFU MVAdelta5gp120 | 200 | 50; 30-s rest | |||||||
| MVA 3 | NZW rabbits | Robinson | Female | 0, 21, 35 | 0, 28 | i.m. | 1 × 108 PFU MVAgp120 | 200 | ||
| i.n. | 3 × 108 PFU MVAgp120 | 200 | 50; 30-s rest | |||||||
| Rectal | 3 × 108 PFU MVAgp120 | 200 | ||||||||
| Gastric | 3 × 108 PFU MVAgp120 | 1,000 |
NZW rabbits, New Zealand White rabbits.
rest, number of seconds the animal is rested after an aliquot of the vaccine was delivered to both the left and right nostril prior to receiving another aliquot of the vaccine. The animal was maintained in dorsal recumbency with isoflurane administered as needed to maintain surgical anesthesia for the indicated number of seconds prior to receiving another aliquot of vaccine to each nostril or being placed in sternal recumbency for recovery.
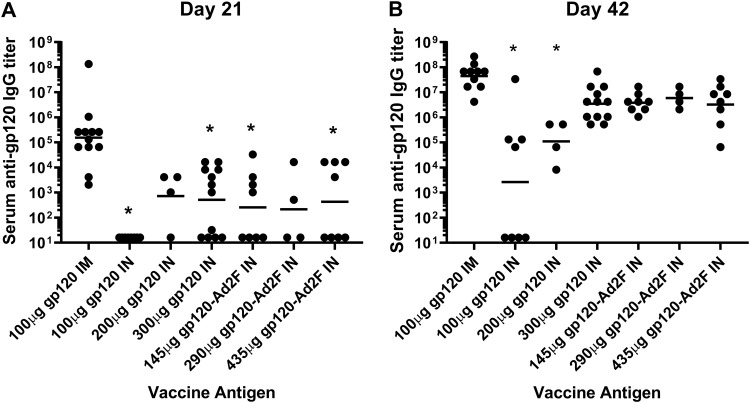
We also tested gp120-Ad2F at doses (145, 290, and 435 μg) that were equimolar to the i.n. doses of gp120 tested to determine if addition of the mucosal targeting ligand Ad2F to gp120 enhanced the immunogenicity of gp120 when i.n. delivered (Table 2, experiments i.n. 2 and 3). Regardless of the dose of gp120 or gp120-Ad2F used for i.n. immunization, all i.n. immunization groups had some rabbits that did not develop detectable anti-gp120 IgG responses, while all the rabbits immunized by the i.m. route with 100 μg of gp120 developed detectable serum anti-gp120 IgG responses (Fig. 2A).
FIG 2.
Three hundred micrograms of gp120 and gp120-Ad2F (doses equimolar to 100 to 300 μg gp120) administered i.n. induce serum anti-gp120 IgG titers similar to those induced by intramuscular administration of 100 μg of gp120. Male New Zealand White rabbits were vaccinated intramuscularly with 100 μg gp120 adjuvanted with AddaVax or intranasally with gp120 or gp120-Ad2F adjuvanted with 64 μg M7. The intranasal vaccines contained either 100, 200, or 300 μg gp120 or 145, 290, or 435 μg of gp120-Ad2F (doses equimolar to gp120). Serum was collected 2 weeks after the first vaccination (day 21) (A) and the second vaccination (day 42) (B) and assessed by ELISA for gp120-specific IgG responses. Experiments for each group (n = 4) were repeated 0 to 2 times (n = 4 to 12). (A) After a single vaccination, serum anti-gp120 IgG responses induced by 100 and 300 μg of gp120 or equimolar doses of Ad2F were significantly lower than anti-gp120 IgG responses induced by 100 μg gp120 i.m. (*, P < 0.05). (B) After administration of two vaccines, only 100 μg and 200 μg gp120 administered intranasally induced serum anti-gp120 IgG responses significantly lower than serum anti-gp120 IgG responses induced in the 100 μg gp120 i.m. group (*, P < 0.0001 and 0.001, respectively). The horizontal lines indicate the geometric mean titers.
As shown in Fig. 2B, while only 4 of 8 animals that received 100 μg of gp120 i.n. developed detectable serum anti-gp120 IgG titers on day 42 after two vaccines, all the rabbits in the remaining groups at that time point exhibited detectable serum gp120-specific IgG. Rabbits that received 100 μg or 200 μg gp120 i.n. developed serum anti-gp120 IgG titers that were significantly different from those receiving 100 μg i.m. (P < 0.0001 and P = 0.0015, respectively). Thus, we considered 300 μg gp120 administered i.n. to induce serum gp120-specific IgG titers similar to those induced by 100 μg gp120 administered i.m. In contrast to the results obtained with gp120, none of the gp120-Ad2F vaccine groups induced serum anti-gp120 IgG titers that were significantly different from the anti-gp120 IgG titers induced by the gp120 i.m. vaccine group after two immunizations (Fig. 2B). However, the geometric mean gp120-specific IgG titer in all of the gp120-Ad2F vaccine groups was 1 log unit lower than that in the i.m. group. Since gp120-Ad2F was more immunogenic than gp120 by more than 2 orders of magnitude at the 100-μg dose, gp120-Ad2F was used as the antigen for the remaining intranasal immunization studies.
Evaluation of the mucosal immunogenicity of MVA expressing gp120.
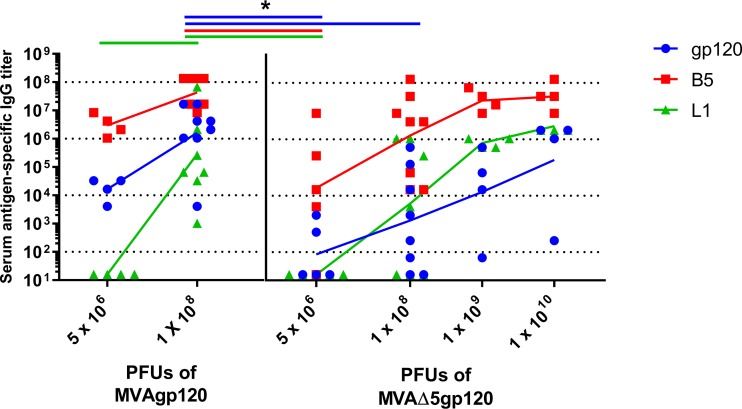
We tested various forms of MVA vectors expressing gp120 to determine if vector modifications enhanced their mucosal immunogenicity as determined by the serum anti-gp120 IgG responses induced. The immunogenicity of the replication-defective MVAdelta5 vector expressing gp120 (MVAdelta5gp120), derived from that developed by Garber et al. (42), was first compared to the immunogenicity of conventional MVA expressing gp120 (MVAgp120) when delivered by the i.m. route (Table 2, MVA 1 and MVA 2). Dose responses for both anti-gp120 IgG and vector-specific IgG (anti-B5 and anti-L1) were observed with escalating doses of MVAdelta5gp120 (Fig. 3). MVAdelta5gp120 (1 × 1010 PFU) induced the anti-gp120 IgG titers most similar to the anti-gp120 IgG titers induced by immunization with 1 × 108 PFU of MVAgp120. However, MVAgp120 induced a more consistent gp120-specific IgG response than MVAdelta5gp120, potentially indicating an even higher dose of MVAdelta5gp120 might be needed to induce consistently high anti-gp120-specific titers in all individuals.
FIG 3.
Nonreplicating MVAdelta5gp120 is not superior to conventional MVAgp120 for induction of serum gp120-specific IgG. Female rabbits were intramuscularly vaccinated with MVAgp120 or MVAdelta5gp120 at the doses indicated on days 0 and 21. The rabbits were bled on day 35, and gp120-specific and vector-specific (B5 and L1) IgG titers were determined by ELISA. *, significantly different from 1 × 108 PFU of conventional MVAgp120 (P < 0.05). The connecting lines represent the geometric mean titers.
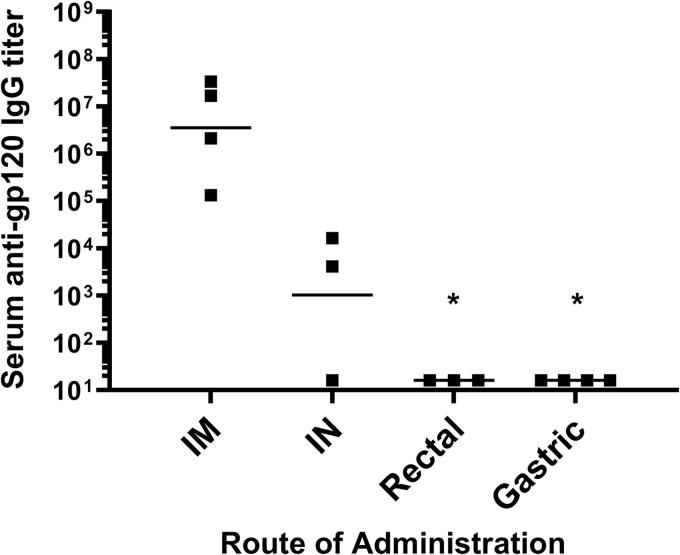
After determining that MVAgp120 was the most immunogenic form of MVA to use as a priming vaccine, different mucosal routes of immunization with MVAgp120 were evaluated for the ability to induce mucosal and systemic anti-HIV-1 gp120 antibody responses. Based on the requirement for three times the protein dose for gp120 i.n. vaccines to induce serum antibody titers similar to those induced by i.m. vaccination, a dose of 3 × 108 PFU of MVAgp120 administered by various mucosal routes (nasal, rectal, and gastric) was used for comparison to 1 × 108 PFU i.m. (Table 2, MVA 3). After the second vaccination, all i.m. vaccinated rabbits had detectable gp120-specific IgG titers while only 2/3 of the i.n. vaccinated rabbits had detectable gp120-specific IgG titers (Fig. 4). No rabbit that was rectally or gastrically immunized developed detectable serum gp120-specific IgG titers (<1:32 titer). Based on these data, an i.n. prime with MVAgp120 was selected for use in the prime/boost experiments.
FIG 4.
Intranasal, but not rectal or gastric, administration of MVAgp120 induced serum gp120-specific IgG titers. Female New Zealand White rabbits were vaccinated with MVAgp120 by the intramuscular, intranasal, intragastric, or intrarectal route (n = 3 or 4/group) on days 0 and 28. Serum was collected on day 42, and gp120-specific IgG titers were determined by ELISA. Rectal and gastric administration of MVAgp120 resulted in serum gp120-specific titers that were significantly lower than those from i.m. administration (P = 0.01 and 0.008, respectively). The horizontal lines indicate the geometric mean titers.
Evaluation of the immunogenicity of MVAgp120 prime/gp120 boost immunization regimens in rabbits.
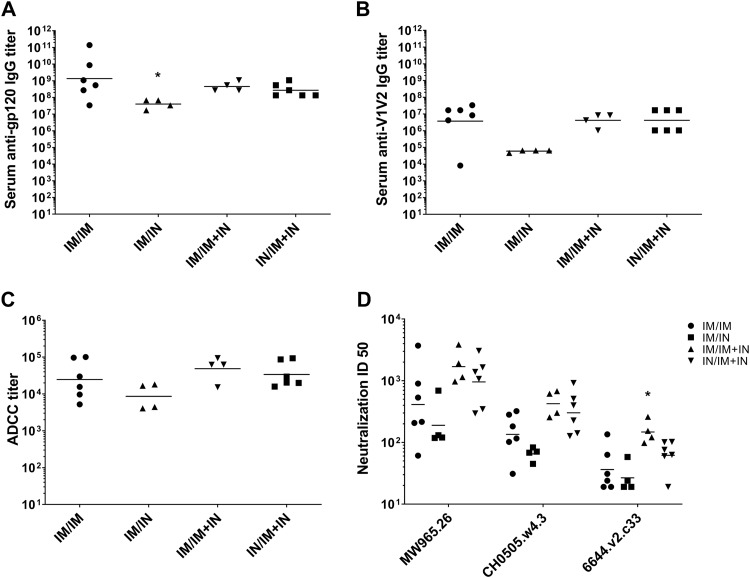
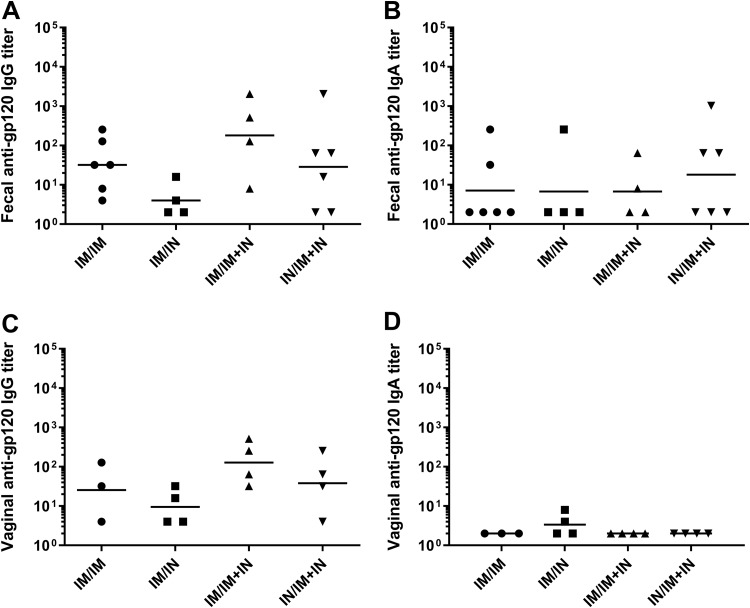
Three different prime/boost regimens that included nasal vaccination were compared to an intramuscular prime/boost regimen to determine if a prime/boost regimen utilizing i.n. immunization could be developed that induced serum anti-gp120 IgG responses similar to those induced by the i.m. prime/boost regimen. We also evaluated the use of a boosting regimen that utilized a combination of both the i.m. and i.n. routes, since combining the two routes of administration enhanced mucosal antibodies and enhanced serum viral neutralization activity in previously vaccinated NHPs (47). The prime/boost regimens evaluated that included nasal immunization were i.m. MVAgp120 prime and i.n. gp120-Ad2F boost (i.m./i.n.), i.m. MVAgp120 prime and i.n. gp120-Ad2F plus i.m. gp120 boost (i.m./i.m.-plus-i.n.), and i.n. MVAgp120 prime with i.n. gp120-Ad2F plus i.m. gp120 boost (i.n./i.m.-plus-i.n.) (Table 3). At the end of the prime/boost regimen (week 19), only the i.m./i.n. group had significantly lower (P = 0.01) serum gp120-specific IgG titers than the control i.m./i.m. group (Fig. 5A). We also monitored vaccine-induced serum IgG antibodies to variable regions 1 and 2 (V1V2) of HIV-1 gp120, since titers of IgG specific for V1V2 correlated inversely with the rate of HIV-1 infection in the RV144 efficacy trial (48). There were no significant differences in serum V1V2-specific IgG titers (Fig. 5B) between groups. Additionally, there were no significant differences in the gp120-specific ADCC titers between groups (P = 0.19). However, the geometric mean ADCC titers of the i.m./i.m. (1:24,705) and the i.n./i.m.-plus-i.n. (1:33,921) groups were more similar than those of the i.m./i.n. (1:8,591) and i.m./i.m.-plus-i.n. (1:48,626) groups (Fig. 5C). However, the i.m./i.m.-plus-i.n.-vaccinated rabbits developed significantly higher neutralization of 6644.v2.c33 (P = 0.04), a tier 1b pseudovirus, than the i.m./i.m. group (Fig. 5D). There were no significant differences in gp120-specific fecal (Fig. 6A and B) or vaginal (Fig. 6C and D) IgG or IgA titers between the different groups. The i.n./i.m.-plus-i.n. vaccination regimen was employed for further comparison to the i.m./i.m. regimen in NHPs, as these groups had the most similar functional serum antibody responses in rabbits and allowed a comparison of i.m. prime/boost to a vaccine regimen that utilized i.n. priming and boosting to maximize the use of mucosal immunization.
TABLE 3.
Animal characteristics and vaccine specifications for prime/boost experiments
| Expt | Species | Sex | Supplier | Bleed wks | Prime |
Boost |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk | Route | MVAgp120 amt (PFU) | Wks | Route | Antigen | Adjuvant | |||||
| Prime/boost 1 | NZW rabbits | Both | Robinson | 0, 6, 15, 19 | 0 | i.m. | 1 × 108 | 12, 16 | i.m. | 100 μg gp120 | Addavax |
| NZW rabbits | Female | Robinson | 0, 6, 15, 19 | 0 | i.m. | 1 × 108 | 12, 16 | i.n. | 435 μg gp120-Ad2F | 64 μg M7-NH2 | |
| NZW rabbits | Female | Robinson | 0, 6, 15, 19 | 0 | i.m. | 1 × 108 | 12, 16 | i.m. + i.n. | 100 μg gp120 (i.m.); 435 μg gp120-Ad2F (i.n.) | Addavax (i.m.); 64 μg M7-NH2 (i.n.) | |
| NZW rabbits | Both | Robinson | 0, 6, 15, 19 | 0 | i.n. | 3 × 108 | 12, 16 | i.m. + i.n. | 100 μg gp120 (i.m.); 435 μg gp120-Ad2F (i.n.) | Addavax (i.m.); 64 μg M7-NH2 (i.n.) | |
| Prime/boost 2 | Rhesus macaques | Both | New England Primate Research Center | −2, 8, 15, 19, 23, 27 | 0 | i.m. | 1 × 108 | 12, 16, 24 | i.m. | 100 μg gp120 | Addavax |
| Rhesus macaques | Both | New England Primate Research Center | −2, 8, 15, 19, 23, 27 | 0 | i.n. | 3 × 108 | 12, 16, 24 | i.m. + i.n. | 100 μg gp120 (i.m.); 435 μg gp120-Ad2F (i.n.) | Addavax (i.m.); 64 μg M7-NH2 (i.n.) | |
FIG 5.
Combined intramuscular and intranasal boosting, regardless of intramuscular or intranasal prime, induces serum anti-HIV antibodies similar to those induced by an intramuscular prime/boost regimen in rabbits. New Zealand White rabbits (n = 4 to 6/group) were primed i.m. or i.n. with MVAgp120 in week 0 and boosted with 100 μg gp120 adjuvanted with AddaVax i.m. and/or with 435 μg gp120-Ad2F adjuvanted with 64 μg M7 i.n. in weeks 12 and 16. Serum antibody titers and viral neutralization from blood drawn in week 19 are shown. (A) Serum gp120-specific IgG titers. Serum anti-gp120 IgG titers induced i.m./i.n. were significantly lower than serum anti-gp120 IgG titers induced i.m./i.m (P = 0.01). (B) Serum V1V2-specific IgG titers were not significantly different between groups. A Kruskal-Wallis test result was not significant (P = 0.057), so multiple comparisons were not made. (C) ADCC activity using human effector cells against HIV gp120-coated target cells. There were no significant differences between groups (P = 0.19). (D) Neutralization of tier 1a (MW965.26 and CH0505.w4.3) and tier 1b (6644.v2.c33) pseudoviruses. I.m./i.m.-plus-i.n. immunization induced significantly greater neutralization of 6644.v2.c33 (P = 0.04) than immunization by the i.m./i.m. route. The horizontal lines indicate the geometric mean titers.
FIG 6.
Rabbits developed undetectable or low mucosal antibody responses to all of the prime/boost regimens. New Zealand White rabbits received a priming immunization of MVAgp120 either i.m. or i.n. in week 0 and gp120 boosts i.m. and/or i.n. in weeks 12 and 16. Feces were collected from all individuals (n = 4 to 6/group), and vaginal lavage was performed on the females (n = 3 or 4/group). The results shown are from week 19, 3 weeks after administration of the second booster vaccine. (A) Fifty to 100% of the rabbits in each group developed detectable fecal gp120-specific IgG titers. (B) Twenty-five to 50% of the rabbits in each group developed fecal gp120-specific IgA titers. (C) Vaginal gp120-specific IgG was detectable in 50 to 100% of the female rabbits in each group but tended to be lower than the fecal gp120-specific IgG titers observed. (D) Vaginal gp120-specific IgA was detectable in only 2 of the 4 i.m./i.n.-vaccinated females. The horizontal lines indicate the geometric mean titers.
Evaluation of the immunogenicity of the i.n./i.m.-plus-i.n. prime/boost vaccine regimen in NHPs.
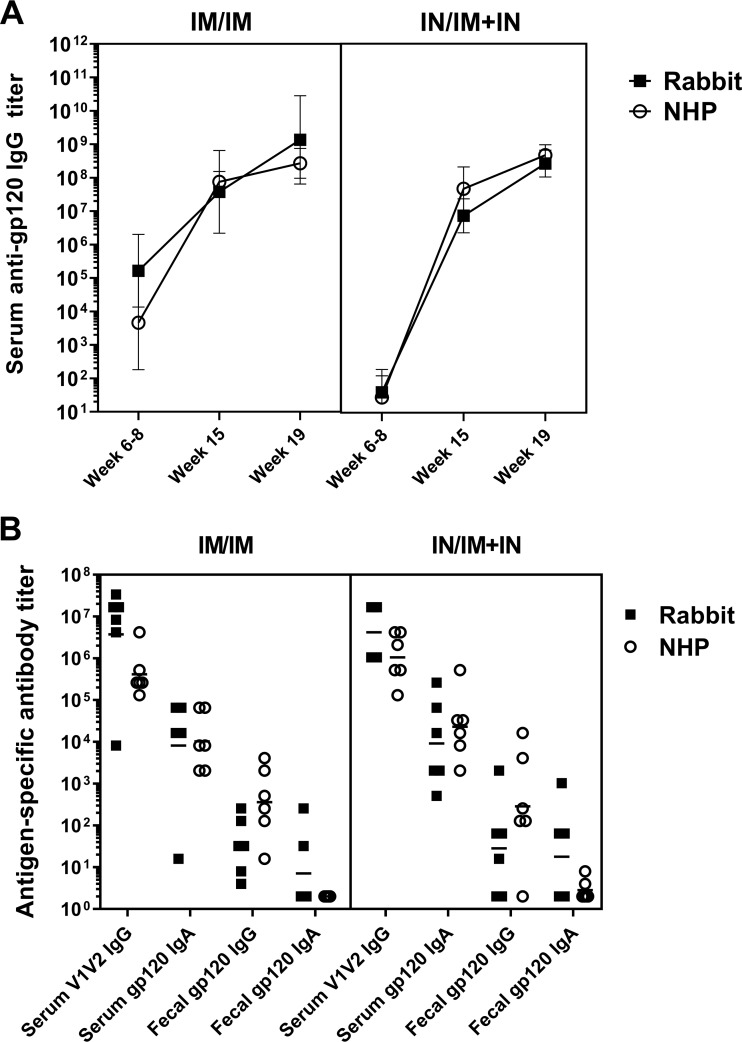
To determine if a combined mucosal-systemic vaccine regimen optimized in rabbits is translatable, NHPs were vaccinated with the control i.m./i.m. regimen (n = 6) or with the i.n./i.m.-plus-i.n. regimen (n = 6) developed in rabbits to determine if the i.n./i.m.-plus-i.n. regimen was able to induce serum anti-gp120 IgG responses similar to those induced by the i.m./i.m. regimen (Table 3). The vaccination regimens induced antibody responses in the NHPs that were similar to the antibody responses induced by the same vaccine regimens in rabbits (Fig. 7). There was no significant difference in week 19 serum gp120-specific IgG or IgA, serum V1V2-specific IgG, or fecal gp120-specific IgG or IgA titers or in virus neutralization between the i.m./i.m. and i.n./i.m.-plus-i.n. regimens in the NHPs (Fig. 7 and 8). As reported by Pollara et al. (49), there were no significant differences in ADCC activity against gp120-coated target cells or antibody-dependent cellular phagocytosis (ADCP) of HIV-1 virions between the two vaccination regimens.
FIG 7.
Rabbit and NHP antibody responses to both prime/boost regimens were similar. New Zealand White rabbits and rhesus macaques received a priming immunization of MVAgp120 in week 0 and gp120 boosts in weeks 12 and 16. All the vaccines were either administered intramuscularly (i.m. prime/i.m. boost) or the MVAgp120 prime was administered intranasally and boosting consisted of both intramuscular and intranasal components (i.n./i.m.-plus-i.n.) (n = 6/group). The animals were bled at the time points indicated, and antibody responses were determined by ELISA. (A) Serum gp120-specific IgG responses after administration of the MVAgp120 prime (weeks 6 to 8), the first gp120 boost (week 15), and the second gp120 boost (week 19). The connecting lines and error bars represent the geometric mean titers and the 95% confidence intervals. (B) After administration of the second booster vaccine (week 19), rabbits and NHPs had similar serum V1V2-specific IgG and serum gp120-specific IgA responses. Fecal gp120-specific responses were also similar between the species. The horizontal lines represent the geometric mean titers.
FIG 8.
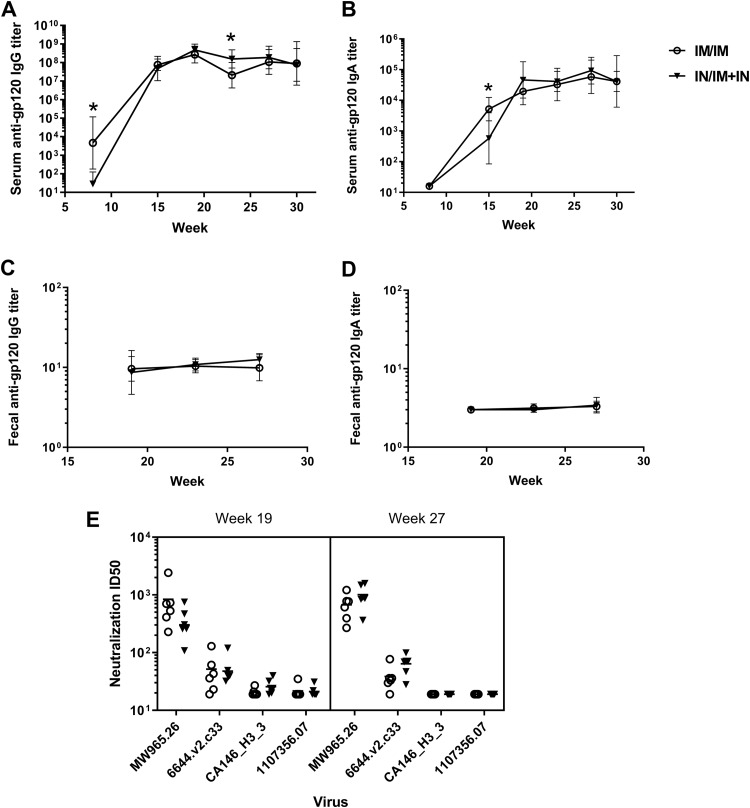
Intranasal prime with combined intranasal and intramuscular boosting induces serum antibodies and viral neutralization similar to those induced by the parenteral i.m. prime/i.m. boost regimen. Rhesus macaques (n = 6/group) were vaccinated with the same i.n./i.m.-plus-i.n. or i.m./i.m. regimen described for rabbits. An additional booster vaccine was given in week 24. Serum was collected at the indicated time points, and antigen-specific antibody titers were determined by ELISA. Antibody responses were compared between the two vaccination regimens at each time point using a Mann-Whitney test. (A) Time course of serum gp120-specific IgG titers during the vaccination regimen. The i.m./i.m. group had significantly higher serum gp120-specific IgG titers after administration of the priming immunization (week 8), while the i.n./i.m.-plus-i.n. group had significantly higher titers 7 weeks after administration of the second booster vaccination (week 27) (P = 0.01 and 0.03, respectively). The connecting lines and error bars represent the geometric mean titers and the 95% confidence intervals. (B) Time course of serum gp120-specific IgA titers. The i.m./i.m. regimen resulted in significantly higher anti-gp120 IgA titers than the i.n./i.m.-plus-i.n. regimen at week 15 (P = 0.04). The connecting lines and error bars represent the geometric mean titers and the 95% confidence intervals. (C) Fecal gp120-specific IgG titers were not significantly different between vaccination regimens at any time point tested. The connecting lines and error bars represent the geometric mean titers and the 95% confidence intervals. (D) While all NHPs had undetectable fecal gp120-specific IgA at a 1:16 dilution at week 19, low levels of fecal gp120-specific IgA were detected in a few NHPs in each vaccination regimen on week 27. The difference between time points was not significant (P = 0.6 for i.m./i.m.; P = 0.3 for i.n./i.m.-plus-i.n.). The connecting lines and error bars represent the geometric mean titers and the 95% confidence intervals. (E) Neutralization of tier 1a (MW965.26) and tier 1b (all others) pseudoviruses was not significantly different between the vaccination regimens after both the 2nd and 3rd boosts. The horizontal lines represent the geometric mean titers.
>NHPs received a third booster vaccine corresponding to their assigned vaccination regimens (thus, i.m. or i.m. plus i.n.) in week 24. Prior to administration of the 3rd booster, serum gp120-specific IgG titers were significantly greater (P = 0.03) in the i.n./i.m.-plus-i.n. group than in the i.m./i.m. group, potentially indicating that i.n./i.m.-plus-i.n.-induced serum anti-gp120 IgG responses are more durable than i.m./i.m.-induced responses. However, there was no significant difference in serum gp120-specific IgG or IgA titers after the 3rd boost (weeks 27 and 30) (Fig. 8A and B). There was also no significant change in fecal anti-gp120 IgG titers after the third boost (Fig. 8C). Fecal gp120-specific IgA was undetectable in any week 19 sample at a 1:16 dilution, but it was detected in week 27 samples at dilutions of ≤1:32 for 2/6 primates in each vaccine group (Fig. 8D). Our results indicate that a prime/boost vaccine regimen utilizing i.n. immunization that induces anti-HIV-1 serum IgG and IgA and fecal IgG responses similar to those induced by i.m./i.m. immunization can be developed.
Evaluation of protective immunity using a repeated low-dose rectal SHIV challenge.
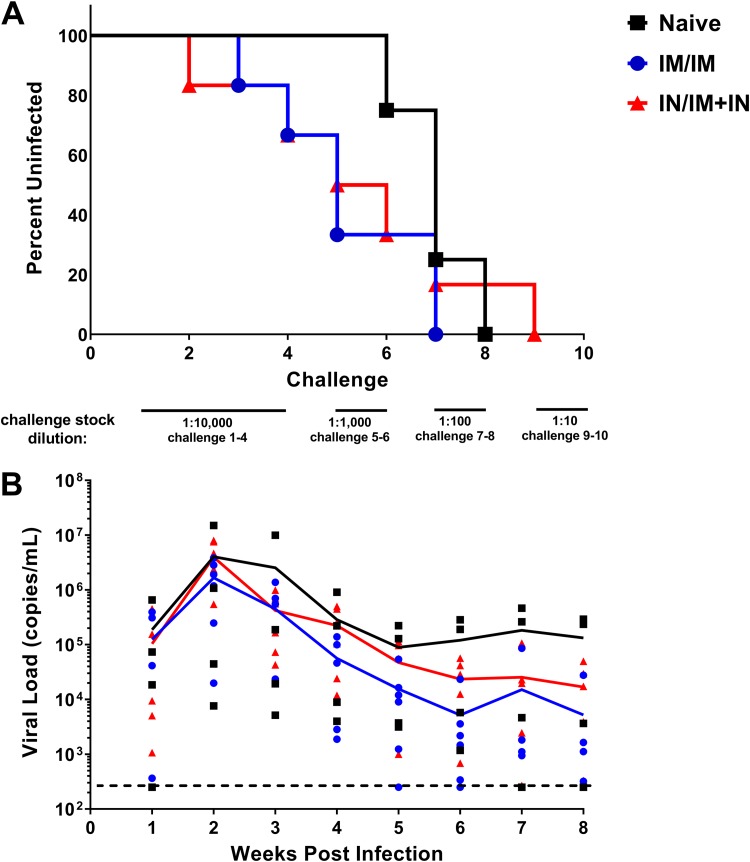
The NHPs vaccinated using the i.m./i.m. and i.n./i.m.-plus-i.n. regimens received a repeated escalating-dose rectal challenge with a heterologous tier 2 SHIV in week 30 to determine if the use of mucosal immunization routes provided any protective benefit versus the i.m./i.m. vaccine regimen. Four unvaccinated NHPs were challenged as negative controls. Following the four low-dose challenges, the challenge doses were increased by an order of magnitude every other week to determine if the vaccination regimens resulted in protection. All the unvaccinated animals became infected by the 7th ± 1 challenge (Fig. 9A). Two of the six NHPs in both the i.m./i.m. and i.n./i.m.-plus-i.n. groups became infected during the initial low-dose challenges. All of the i.m./i.m. group and 5/6 individuals in the i.n./i.m.-plus-i.n. group became infected at or by the 7th challenge. Thus, there was no significant difference in the numbers of challenges until infection between vaccine groups. Additionally, similar viral loads were present in the immunized and unimmunized animals, with the peak viral load 2 weeks postinfection (Fig. 9B). The lack of protection seen in both the i.m./i.m. and i.n./i.m.-plus-i.n. vaccines prevents us from determining if the use of an optimized mucosal-vaccine regimen provides superior protection against a mucosal SHIV challenge.
FIG 9.
Neither the i.m./i.m. vaccine regimen nor the optimized i.n./i.m.-plus-i.n. regimen protected NHPs from SHIV challenge. Rhesus macaques (n = 6/vaccine group; n = 4/naive group) were rectally challenged weekly with an escalating dose of heterologous tier 2 SHIV-1157QNE Y173H. All animals received two additional challenges after the infective challenge, or a maximum of 10 challenges. Blood was collected 1 week after challenge (the same day as administration of the next challenge) and assessed for viral RNA by PCR. (A) SHIV infection status after administration of each challenge. After five challenges, 4 of 6 individuals that received the i.m./i.m. regimen and 3 of 6 individuals that received the i.n./i.m.-plus-i.n. regimen were infected, while 0 of 4 unvaccinated individuals were infected. There was no statistical difference between vaccinated and naive animals (P = 0.08; Fisher’s exact test) even at that time point. (B) Viral loads after infection as determined by PCR. The lines represent the mean viral loads for the vaccine groups. The data points represent individuals’ viral loads for that time point. The limit of detection was 250 copies/ml.
DISCUSSION
The use of mucosal immunization may induce antigen-specific mucosal IgA responses that are significantly greater than the antigen-specific mucosal IgA responses induced by parenteral immunization (8), while the responses of serum antigen-specific IgG binding antibodies (9) and functional antibodies (8) induced by mucosal immunization are often lower than those induced by parenteral immunization. Since protection against HIV-1 mucosal transmission may require optimum systemic and mucosal anti-HIV-1 antibody responses (7), we propose that HIV-1 vaccine regimens utilizing a mucosal route of immunization should be optimized to ensure induction of elevated serum anti-HIV-1 binding and functional antibody responses. In this study we demonstrate that a prime/boost HIV-1 immunization regimen utilizing i.n. immunization may be optimized to induce serum anti-HIV-1 binding and functional antibodies in a quantity similar to that induced by a prime/boost immunization regimen delivered by the i.m. route.
Here, we report for the first time the use of M7, a cationic antimicrobial and mast cell-activating peptide, as an adjuvant in an HIV vaccine formulation. M7 provided more potent adjuvant activity than CT, the gold standard for nasal vaccines. The efficacy of M7 is not surprising, as vaccines adjuvanted with C48/80, another mast cell activator, have induced antibody titers in mice and rabbits similarly to CT-adjuvanted vaccines (14, 17, 50). Others have demonstrated that a vaccine formulation including an antimicrobial peptide adjuvant, IC31, was an effective adjuvant for the induction of potent anti-HIV-1 immune responses in mice after parenteral immunization (51). Our results suggest that M7 is an effective nasal vaccine adjuvant that could be evaluated in future human i.n. HIV-1 vaccine studies, although additional safety studies are needed with M7 prior to its use in clinical studies.
It has been documented in the mucosal-vaccine field that higher antigen doses are needed for mucosal vaccines to induce serum antibody responses similar to those induced by injected vaccines (52–54). Consistent with this, we demonstrated that i.n. administration of 300 μg of gp120 was required to induce serum gp120-specific IgG titers not significantly different from those induced by i.m. administration of 100 μg of gp120. Others have reported that the use of 3-fold higher antigen doses for i.n. vaccination did not induce serum antibody responses similar to those induced by parenteral immunization. For example, an NHP study that included five immunizations with either 300 μg of gp140 adjuvanted with LTK63 administered i.n. or 100 μg of gp140 adjuvanted with MF59 administered i.m. failed to achieve similar serum gp140-specific IgG titers in the two groups (55). Other studies have compared i.n. to systemic vaccination using the same (9, 56, 57), 2-fold higher (8), 3-fold higher (55), or 5-fold higher (9) antigen doses. Differences in the immunogenicity of different nasal vaccine regimens may be attributable to the antigen (specific antigen and dose), the adjuvant (specific adjuvant and dose), the method of nasal vaccine delivery (58), and the vaccine volume (59) administered to a nostril at a given time.
It is important to note that we did not determine that 100 μg gp120 i.m. and 300 μg gp120 i.n. are equivalent, but rather, we failed to detect a significant difference. Increasing statistical power by increasing the sample size and meeting the assumptions for parametric statistics would increase the likelihood of detecting a difference between the two vaccines. We consider the variation in individual responses observed with the use of an outbred-rabbit model beneficial for translation to real-world use, where vaccines are administered by a large number of individuals to people with different immune statuses, genetic backgrounds, and environmental exposures. For example, the extremely large variation in gp120-specific titers after vaccination with 100 μg of gp120 is consistent with a response along the linear portion of a dose-response curve, indicating that slight differences in administration or in immune responses between animals could determine if the vaccine induced an immune response or if the animal was a nonresponder. Notably, the 100-μg gp120 group experiment was repeated, with 4 rabbits per replicate and different personnel administering the i.n. vaccines in each replicate, and there were responders and nonresponders in each replicate. Since statistical power and sources of variance are important and often overlooked factors when evaluating comparisons of nasal and systemic vaccines, our results may more accurately be viewed as demonstrating a difference between 100 μg gp120 i.m. and 100 μg or 200 μg gp120 i.n.
The inclusion of the cell adhesin Ad2F greatly improved the immunogenicity of gp120. I.n. vaccination with 145 μg of gp120-Ad2F, which is equimolar to 100 μg gp120, induced serum antibody titers similar to those induced by the 100-μg gp120 administered i.m., while i.n. vaccination with 100 μg gp120 induced much lower and more variable anti-HIV-1 IgG responses. The approximately 100- to 1,000-fold increase in serum gp120-IgG titers induced by using gp120-Ad2F versus gp120 is similar to that observed with other Ad2F-coupled immunogens delivered by the nasal route in rabbits (17). While we did not see a dose-response effect when increasing to doses equimolar to 200 μg gp120 or 300 μg gp120, we hypothesize that this was due to reaching the upper plateau of the dose-response curve. If this is indeed the case, it is possible that even doses lower than 145 μg of gp120-Ad2F could be used and still achieve high serum antibody titers, allowing antigen sparing. Conversely, there may be a benefit to using the gp120-Ad2F dose equimolar to 300 μg gp120—for example, reduced variation in titers—that this study did not have sufficient power to detect. Recombinant adenovirus vectors have been safely delivered to humans by the nasal route (60, 61), demonstrating that exposure to Ad2F, at least in the context of adenovirus exposure, is safe for humans.
To optimize the MVA-vectored priming immunization, the effects of modifications to the MVA vectors through the use of a replication-defective MVA containing deletions of four genes encoding immune-modulatory proteins were evaluated. Although others have reported the induction of similar vector-specific and insert-specific antibody titers with replication-defective and conventional MVA vectors (39), in this study, 1 × 108 PFU of MVAdelta5gp120 induced significantly lower serum gp120-specific IgG responses than an equivalent dose of the conventional MVAgp120 vector. The lower response may be explained by differences between studies, including animal models (NHPs versus rabbits); route of administration (intradermal [i.d.] plus i.m. versus i.m.); HIV antigen (gag versus gp120); how enzyme-linked immunosorbent assay (ELISA) titers were determined (the optical density [OD] of a single serum dilution versus the endpoint titer determined from serial dilutions); and, most notably, differences in the promoters used to drive expression of HIV antigens. In the previous studies a modified H5 promoter was used to provide early transcription and reduced late transcription (42), whereas in this study, gp120 expression was controlled by a synthetic promoter designed to promote strong late gene expression, as well as early gene expression. Thus, in the present study, the replication-defective MVA vector would be expected to express less gp120 protein than the conventional MVA, because in the absence of the viral uracil-DNA-glycosylase and DNA replication, late expression would be abrogated.
The intranasal route was identified as the most effective mucosal route for priming with MVA, as determined by serum anti-gp120 IgG responses similar to those of the intramuscular regimen after administration of the first protein booster vaccine when combined i.n.-i.m. gp120 boosts were administered. This result is similar to what was observed with an HIV peptide vaccine, as in both studies, nasal vaccination was the most immunogenic mucosal route (62). Even though i.n. administration of MVA was effective for priming, similar immunogenicities between the i.m. and i.n. priming immunizations were not achieved, even when three times more MVA was i.n. administered. Regardless, there was no significant difference in serum antibody titers after administration of the first boost, indicating that i.n. administration of MVA was an effective priming immunization. Our results are consistent with a previous study in NHPs, where there was a significant difference in serum IgG titers when MVA was administered i.m. versus i.n.; however, nasal vaccination resulted in higher T cell responses and a longer interval until progression to AIDS than i.m. vaccination (63).
Using the MVA prime (i.m. or i.n.) and i.n. gp120-Ad2F and i.m. gp120 vaccine delivery, we tested various combinations of systemic and i.n. prime and boost vaccines, including i.m. MVA prime plus i.m. gp120 boost (i.m./i.m.), i.m. MVA prime plus i.n. gp120-Ad2F boost (i.m./i.n.), i.m. MVA prime plus i.m. gp120 and i.n. gp120-Ad2F combination boost (i.m./i.m.-plus-i.n.), and i.n. MVA prime plus i.m. gp120 and i.n. gp120-Ad2F combination boost (i.n./i.m.-plus-i.n.). In our rabbit model, only the i.m./i.n. regimen resulted in significantly lower serum gp120-specific IgG titers than the i.m./i.m. regimen. A previous NHP study did not observe a significant difference in plasma anti-gp120 IgG binding responses between a similar vaccination regimen consisting of an MVA prime and two gp120 boosts administered either i.m. or i.n. (8). However, differences in plasma virus neutralization between the i.m./i.m. and the i.m./i.n. vaccinees indicated that the i.m./i.n. regimen induced lower functional IgG responses than the i.m./i.m. regimen, congruent with our results. Additionally, NHPs from the i.m./i.n. regimen in the previous study went on to receive a combined i.m.-plus-i.n. boost almost a year after the MVA prime. The combined i.m.-plus-i.n. boost was able to enhance plasma anti-HIV-1 IgG, virus neutralization or ADCC titers, milk anti-HIV-1 IgA titers, and antibody epitope diversity (47). Likewise, we found that combined i.m.-plus-i.n. boosting increased serum IgG titers, viral neutralization, and ADCC activity in the rabbit model. In fact, combined i.m.-plus-i.n. boosting resulted in significantly higher virus-neutralizing responses than the i.m./i.m regimen. To prevent functional differences in serum IgG from impacting the challenge outcome, we performed NHP studies with the i.n./i.m.-plus-i.n. regimen, as it was the vaccination regimen that induced serum anti-HIV antibody responses most similar to the i.m./i.m. regimen while also utilizing the highest number of mucosal immunizations to determine if the use of optimized mucosal immunization would influence acquisition of SHIV after repeated low-dose rectal challenge.
In NHPs, both the i.m./i.m. and i.n./i.m.-plus-i.n. regimens induced high serum gp120-specific IgG titers, demonstrating that intranasal vaccines can be included in a vaccination regimen while inducing serum antibody titers similar to those achieved with systemic vaccination regimens. Furthermore, serum gp120-specific IgA titers were also similar between the vaccine groups after the second and third booster vaccines, with the i.n./i.m.-plus-i.n. regimen inducing lower IgA responses after administration of the first booster vaccine. Since serum gp120-specific IgA can interfere with protective gp120-specific IgG responses (48) and mucosal vaccination is associated with the production of IgA at mucosal surfaces, the lack of enhancement of serum gp120-specific IgA after the inclusion of intranasal vaccines in the i.n./i.m.-plus-i.n. regimen demonstrates that mucosal vaccines could potentially be incorporated into HIV vaccination regimens without detrimental IgA-mediated competition with protective IgG for binding to antigens.
While mucosal vaccination is thought to be a better activator of the mucosal immune system than systemic vaccination, the i.n./i.m.-plus-i.n. vaccine regimen did not induce anti-HIV fecal IgG or IgA responses greater than those induced by the i.m./i.m. regimen. Additionally, fecal gp120-specific IgG responses were more prevalent and higher titer than fecal gp120-specific IgA responses regardless of the vaccination regimen. There are other examples in the literature where HIV vaccination of NHPs induced antigen-specific mucosal IgG responses but not IgA responses in multiple mucosal samples (44). Our results are consistent with the conclusion that no HIV vaccination regimen developed thus far has induced sustained mucosal IgA responses in humans or NHPs (63).
Rabbits were used to optimize intranasal vaccines to induce serum antibody responses similar to those induced by i.m. vaccination. Rabbits have a nasal cavity anatomy similar to that of primates (64–66), making them ideal for the development of nasal vaccines. We demonstrated that i.m./i.m. and i.n./i.m.-plus-i.n. vaccination regimens induced similar serum and mucosal antibody responses in NHPs and rabbits (Fig. 7). Even though the vaccine regimens evaluated in this study elicited high-titer serum antibody responses and detectable fecal/rectal anti-gp120 IgG titers in both rabbits and NHPs, we did not detect mucosal anti-gp120 IgA in the majority of individuals, although anti-gp120 vaginal IgA responses were observed in 4 of 5 mice nasally immunized with gp120 plus M7. Others have also been unable to detect mucosal antigen-specific IgA responses in rabbits after vaccination regimens that resulted in mucosal IgA titers in mice (67). The relatively prolonged intervals between vaccine doses may also have limited our ability to induce mucosal IgA responses in rabbits and NHPs. For example, we have used nasal immunization schedules of days 0, 7, and 21 (16); days 0, 7, 14, and 28 (56); and days 0, 21, and 42 (21) in mice that induced vaginal antigen-specific IgA, while rabbits and NHPs were immunized on schedules of 0, 12, and 16 weeks (with a boost at week 24 for the NHPs).In addition to their use as an effective animal model for the development of nasally administered vaccines, rabbits are also a useful model for testing Fc-dependent functional antibody responses induced by HIV vaccines (49). Our work highlights the ability of rabbits to serve as a valuable small-animal model system for the development of mucosal HIV vaccination strategies, although more studies are needed to better define vaccination regimens that enhance the induction of antigen-specific mucosal IgA responses.
We chose to model our prime/boost regimen on the canarypox prime/gp120 boost used in the RV144 clinical trial, as that prime/boost regimen showed moderate protection from challenge (2). A vaccine regimen that is partially protective should be the most sensitive to any beneficial effects from mucosal vaccines. Unfortunately, no protection from challenge was observed in either vaccine group tested in the present study. Several factors likely played a role in the lack of protection observed. First, others have seen a lack of protection against the same SHIV strain utilized in our study even after several additional boosts of gp120 (68). However, protection was observed when a pentavalent gp120 vaccine was used, and the protective efficacy was correlated with nonneutralizing antibody responses (6). ADCC responses were induced by our prime/boost regimens in rabbits and NHPs, but both the lack of protective efficacy and the ADCC responses observed in our study are similar to what Bradley et al. (68) observed when using a bivalent gp120 vaccine regimen.
The decision to use AddaVax, an oil-in-water emulsion, as the adjuvant in our i.m.-administered vaccines instead of alum, which was used in the RV144 trials, may have resulted in lower vaccine efficacy. Vaccari et al., reported that a prime/boost vaccination regimen consisting of priming with the canarypox vector ALVAC and boosting with an adjuvanted gp120 vaccine was effective at preventing SIV acquisition only when the boosting vaccines contained alum (46). The lack of efficacy of booster vaccines adjuvanted with MF-59, another oil-in-water adjuvant, was correlated with mucosal innate lymphoid cells, mucosal V2 antibodies, and RAS activation. Additional studies are needed to determine if mucosal vaccination can overcome any skewing induced by the use of oil-in-water adjuvants for systemic vaccines.
In conclusion, optimized nasal HIV vaccination regimens are capable of inducing high-titer, functional serum antibody responses similar to those induced by i.m. vaccination regimens. More work is needed to optimize mucosal immunization for the induction of durable, elevated anti-HIV-1 IgA titers in mucosal secretions and to determine if mucosal anti-HIV IgA influences protection against mucosal SHIV challenge.
MATERIALS AND METHODS
MVA vaccines.
For experiment MVA 1 (Table 2), MVA expressing HIV-1 C.1086 gp140 was used. MVA for all other vaccinations expressed HIV-1 C.1086 gp120 (MVAgp120). gp120 or gp140 was placed under the control of a synthetic promoter capable of high levels of transcription in both early and late stages of viral replication and inserted between two essential genes, as previously described (8, 69).
The MVA used to construct conventional MVA vectors was kindly provided by B. Moss (NIH). This MVA (designated A660) was purified through three rounds of plaque purification in BHK21 cells maintained in minimal essential medium alpha (MEM-alpha) with 5% fetal bovine serum (Gibco), cultured, purified, and quantified in these cells as described previously (70). A derivative of the virus (designated A681) lacking the remnant of the K1L gene and the viral gene (MVA open reading frame [ORF] 184R) encoding the soluble secreted interleukin-1 receptor (71) was constructed as described previously (8). This virus was used as the base vector for the conventional MVA vectors used in this study. Replication-competent MVAs, including A703, expressing HIV 1086 C gp140 (8), and other MVAs derived from conventional MVA A681, were cultured in BHK-21 cells. MVAs rendered replication defective by deletion of the udg gene (39), a gene essential for virus replication, were cultured in duck fibroblasts modified to express the vaccinia virus udg gene (Can20 cells) as described previously (39). The Can20 cells were kindly provided by David Garber (Centers for Disease Control and Prevention, Atlanta, GA).
The replication-defective MVA Δ5-HIV (42) was kindly provided by David Garber (Centers for Disease Control and Prevention, Atlanta, GA). The virus contained HIV subtype C consensus gag and env genes inserted into the MVA deletion III locus. An insertion vector to enable the removal of these genes was constructed as follows. An 802-bp ApaI-BamHI DNA fragment was synthesized, containing a gene encoding a red fluorescent protein (RFP), the pDsRed-Express gene (Clontech Laboratories), under the control of an early/late promoter from vaccinia virus, flanked by lox66/lox71 sequences (72), and inserted within plasmid pUC57 to generate plasmid p2648. The ApaI-BamHI 802-bp fragment from p2648 was ligated to the 5,123-bp ApaI-BamHI fragment of the MVA deletion site III vector pG06dH5gz (42). The DNA of the plasmid was cleaved with MluI and SpeI, and the 2,298-bp fragment containing the Red Xpress gene was transfected into Can20 cells infected with MVA Δ5-HIV. RFP-expressing recombinants were isolated and plaque purified three times. Next, Can20 cells that were infected with the virus were transfected with plasmid DNA of pCAG-Cre:GFP, which was a gift from Connie Cepko (Addgene plasmid no. 13776), to drive the Cre-mediated deletion of the RFP marker gene (73). A recombinant virus (A721) that did not express RFP was isolated and plaque purified. The deletion of the marker gene was confirmed by PCR using primer pairs flanking the deletion III locus, as well as primer pairs within the RFP gene. The A721 virus was cultured in Can20 cells and quantified by plaque assay on Can20 cells employing polyclonal rabbit antisera against vaccinia virus (Meridian Life Sciences) to visualize foci of infected cells, as described previously (74).
A replication-defective MVA that expressed the HIV C.1086 gp120 protein, as described previously (75), was constructed as follows. DNA containing the pDs Red-Express RFP gene, as described above but synthesized to be flanked by wild-type lox sequences, was inserted into plasmid p2628, a derivative of plasmid p2614 (8) expressing gp120 in place of gp140, to replace the K1L-GUS marker genes. To generate recombinant virus expressing gp120, DNA from this plasmid (p2650) was transfected into Can20 cells infected with virus A721. RFP-expressing viral recombinants were isolated and plaque purified. Can20 cells that were infected with this RFP-expressing virus were transfected with plasmid DNA of pCAG-Cre:GFP to drive the Cre-mediated deletion of the RFP marker gene. A recombinant virus (A722) that did not express RFP was isolated and plaque purified. The deletion of the marker gene was confirmed by PCR using primer pairs within the RFP gene. Monoclonal antibodies (VRC C 16H3) were used in Western blot analyses of proteins extracted from A722-infected Can20 cells to confirm expression of the gp120 protein. The A722 virus was cultured in Can20 cells and then partially purified by ultracentrifugation through a 36% sucrose cushion. The virus was quantified by plaque assay on Can20 cells as described above.
Protein vaccines and adjuvants.
The antigen used for booster immunizations consisted of either C.1086 delta7gp120K160N (gp120) (47, 76) or a fusion protein between C.1086 delta7gp120K160N and the Ad2F adhesin (gp120-Ad2F) using a method described previously (26). Briefly, the C-terminal region of Ad2F, from G378 to E582, was used as the transporter/targeting domain. This region encompasses (i) a short stretch of amino acids rich in Gly (4 of 15); (ii) a trimerizing domain; and (iii) a globular domain, commonly referred to as the “knob,” that is important for interacting with the coxsackievirus/adenovirus receptor on the cell surface. The C.1086 delta7gp120K160N ATG codon was embedded in an ideal Kozak sequence, cloned upstream of the Ad2F transporter, and the carboxy terminus of the fusion contained a His tag to allow protein purification. At both ends of the synthetic gene, two XbaI sites allowed excision and cloning into the mammalian expression vector pcDNA3.1+ (Invitrogen). The orientation of the clones was determined by restriction analysis and confirmed by DNA sequencing. The resulting construct, named pGnMM9, was used for protein expression by Paragon Bioservices. Briefly, 293T cells were transfected with a synthetic gene encoding the fusion protein gp120-Ad2F. Secreted gp120-Ad2F was affinity purified by lectin affinity columns, and its purity was assessed by SDS-PAGE and Western blotting.
Intramuscular vaccines adjuvanted with AddaVax (Invivogen; catalog no. vac-adx-10) were mixed 1:1 with antigen for the total vaccine volume. Intranasal vaccines tested in rabbits and macaques were adjuvanted with 64 μg of the antimicrobial mast cell-activating peptide mastoparan 7 (INLKALAALAKALL-NH2; CPC Scientific, Sunnyvale, CA). In mice, intranasal vaccines were adjuvanted with 7 μg M7-NH2 (CPC), 15 μg compound 48/40 (Sigma), 1 μg cholera toxin (Enzo Life Sciences), or 10 μg MPL (Enzo Life Sciences). USP grade 0.9% saline (APP Pharmaceuticals) was added as needed to create the total vaccine volume (listed in Table 2).
Animal husbandry and procedures.
All experimental procedures were performed in accordance with IACUC policies. Summaries describing the numbers of experiments conducted, animal characteristics, and vaccine formulations are provided in Tables 2 and 3.
Mouse experiments.
Female C57BL/6J mice (Jackson Laboratory; 8 weeks old) were acclimated upon arrival at the facility. The mice were housed in a barrier facility free from Helicobacter, Pasteurella, and norovirus. They were fed PMI 5053 Picolab rodent diet 20 (LabDiet). Housing was in individually ventilated microisolator caging with up to 5 mice per cage, and the corn cob bedding was changed biweekly. A 12-h/12-h light cycle was used, with the light coming on at 6 a.m.
Mice were vaccinated by the i.n. route on days 0, 7, and 21. Serum was collected on days −4, 14, and 35. Vaginal lavage fluid was collected as previously described (62, 77) on days −4 and 35. All procedures were performed under isoflurane anesthesia. The mice were anesthetized in a group using an induction chamber. The mice were removed from the induction chamber and placed in dorsal recumbency. The vaccine was applied to the external nares with a micropipette. All procedures occurred in the room where the mice were housed.
Rabbit experiments.
New Zealand White rabbits weighing more than 2 kg were obtained from Robinson Services (Mocksville, NC) or Covance Research Products (Denver, PA). The rabbits were allowed to acclimate for at least 1 week upon arrival at the facility. The rabbits used in the experiments MVA 2 and i.n. 3 and the male rabbits in the prime/boost experiment were previously used in other vaccine experiments with a different antigen or were used as breeders.
The rabbits were fed LabDiet laboratory rabbit diet HF and timothy hay. The amount of feed was constrained on an individual basis to maintain a healthy body condition. Rabbit cage waste pans were changed twice a week. They received weekly enrichment consisting of both exercise in a playpen and food treats. The rabbits were housed singly during all the studies; however, an attempt was made during some studies to socialize the rabbits during playtime. All the abbits were maintained in the same room in the animal facility as the other treatment groups. All procedures occurred in the room where they were housed.
For vaccine optimization experiments, two vaccines were administered 3 to 5 weeks apart. The rabbits were bled prior to the first vaccination, 3 weeks after the first vaccination, and 1 to 2 weeks after the second vaccination. For prime/boost experiments, the rabbits received the MVAgp120 prime in week 0 and gp120 protein boosts in weeks 12 and 16. They were bled in weeks 0, 6, 15, and 19. Intramuscular immunizations were injected bilaterally or unilaterally into the thigh using a 25-gauge needle.
Intranasally vaccinated rabbits were sedated with acepromazine as described for blood collection and then anesthetized with isoflurane (up to 4% at 4 liters of O2/min) administered via a nose cone. Once a rabbit was unresponsive to handling, it was placed in dorsal recumbency with its nose at about a 90° angle to the table, and the vaccine was administered via a laboratory pipette. The intranasal vaccine was administered in aliquots (the volumes are listed in Table 2) to each nostril with a rest period as noted before an additional aliquot was applied to the same nostril (e.g., left nostril, right nostril, rest; left nostril, right nostril, rest). Isoflurane was administered as needed during the rest periods. After the total volume was administered, the rabbits were maintained in dorsal recumbency for the same amount of time as a rest period before being placed in sternal recumbency. If blood was collected that day, it was obtained while the rabbit was recovering from anesthesia. If simultaneous i.m. vaccination occurred, the i.m. vaccines were administered while the rabbit was under anesthesia or while the rabbit was recovering from anesthesia.
Gastrically immunized rabbits were sedated with ketamine-xylazine (35 and 5 mg/kg of body weight) administered subcutaneously. The rabbits were placed in sternal recumbency, and a no. 12 French catheter was passed into the stomach. Five milliliters of a 12% (wt/vol) sodium bicarbonate solution and 10 ml of room air were administered, and the catheter was removed. Twenty to 30 min afterward, the catheter was replaced and the vaccine was administered, followed by 10 ml of room air. Anesthesia was not reversed, and the rabbits were maintained in a pediatric incubator until they recovered from the anesthesia.
Rectally immunized rabbits were sedated with ketamine-xylazine at half the dose used for gastrically immunized rabbits. The rabbits were placed in dorsal recumbency, and the vaccine was administered via a round-tipped oral-gavage needle (Cadence; catalog no. 9915; 15 gauge by 3 in. with a 2.9-mm tip) that was inserted 1.5 in. into the rectum; 500 μl of room air was administered prior to removal of the gavage needle from the rectum. The rabbits were maintained in a pediatric incubator until they recovered from anesthesia.
For blood collection, rabbits were sedated with 1 mg/kg acepromazine administered subcutaneously between the shoulder blades. Lidocaine was applied topically to the ear, and a 21- to 25-gauge butterfly catheter was placed in the central auricular artery to collect 5 to 10 ml of blood. Feces were collected after spontaneous voiding during procedures or by expression from the rectum. If only cecotropes could be obtained and not true feces, the cecotropes were collected and this change was noted. Vaginal lavage was performed under isoflurane anesthesia. A pediatric nasal speculum or manual manipulation was used to open the vaginal vault, and 0.5 to 1 ml of sterile phosphate-buffered saline (PBS) was used to perform lavage.
NHP experiments.
Sixteen 1- to 3-year-old rhesus macaques were obtained from the New England Primate Research Center. The rhesus macaques had more than 6 months to acclimate upon arrival. They were socially housed when possible until 11 weeks prior to administration of the SHIV challenge, at which point they were individually housed. Access to a “play cage” was rotated between individuals and groups until SHIV challenge began, at which point access was rotated at each cage change to prevent exposure to bodily fluids that might contain SHIV. The NHPs were maintained in a single animal room until the unvaccinated individuals were to begin SHIV challenges. At that time, the unvaccinated NHPs were moved into an adjoining room. Semiannual veterinary examinations were performed. Brief physical examinations were performed prior to every procedure. A complete blood count was performed weekly once SHIV challenge began.
The NHPs received the MVAgp120 prime in week 0 and gp120 protein boosts in weeks 12, 16, and 24. They were bled in weeks −2, 8, 15, 19, 23, and 27. SHIV challenges began in week 30 (Table 3). The animals were anesthetized for all procedures, and food was withheld for 12 h prior to anesthesia. Ondansetron was administered to individuals with a history of vomiting. The animals were anesthetized with 0.075 to 0.15 mg/kg dexmedetomidine and 3 mg/kg ketamine administered intramuscularly. A physical examination was performed while under anesthesia. Once SHIV rectal challenges began, temperatures were taken orally, and nothing was placed in the rectum other than the challenge. Blood was collected via the femoral vein. Intramuscular and intranasal vaccines were administered as described for rabbits. The NHPs were reversed with atipamezole upon being returned to their home cages.
Mucosal secretions (saliva and rectal with or without vaginal) were collected atraumatically (78) with premoistened ophthalmic spears (BVI Weck-Cel, reference no. 0008680; BVI Merocel, reference no. 400101) in weeks −2, 8, 12, 15, 16, 19, and 27. The Weck-Cels were premoistened the day before with 100 μl of PBS for saliva and vaginal secretion collection and with 300 μl PBS for rectal secretion collection. The Weck-Cels were inserted 1 to 1.5 in. into the respective mucosal compartment and left for 5 min. Rectal secretions were collected as described previously (78), but with a 5-ml laboratory pipette inserted 1 inch into the rectum. Rectal and vaginal samples were processed as described previously (44) with the following modifications. A total of 250 μl elution buffer (1% protease inhibitor cocktail set V [Sigma; catalog no. P8340] and 0.5% IGEPAL [Sigma; catalog no. I8896] in 0.25% bovine serum albumin [BSA]-PBS) was added to samples collected <6 h previously and stored on ice, and the samples were centrifuged through a 30-μm filter (Thermo; catalog no. 69725) first due to the presence of mucus, which would impede filtration through smaller filters. The eluted secretions were then centrifuged through a 0.22-μm filter. As saliva samples did not contain the thick mucus present in other samples, the 30-μm filtration step was not required, and the samples were centrifuged through only a 0.22-μm filter.
SHIV challenge.
Rectal challenges with a heterologous tier 2 SHIV-1157QNE Y173H (68, 79) (2014 stock, provided by Sampa Santra) were administered weekly for up to 10 weeks or until infection was detected. For the first four challenges, virus stock was diluted 1:10,000. After the fourth challenge, the virus stock dilution decreased 10-fold every other challenge (challenges 5 and 6, 1:1,000; challenges 7 and 8, 1:100; challenges 9 and 10, 1:10). All the challenge virus was diluted with 0.5% human serum albumin (30% solution; Calbiochem) in PBS and administered in a 1-ml volume. Four unvaccinated macaques were used as naive controls. The naïve NHPs were challenged with SHIV in the months immediately preceding challenge of the vaccinated NHPs.
For challenge administration, anesthetized animals were positioned in ventral recumbency with their hindquarters elevated by a 6-in. PVC tube in their home cages. A 1-ml tuberculin syringe was inserted up to the plunger. The plunger was slowly depressed over 20 to 30 s and remained in the rectal vault for 15 min before the syringe was withdrawn slowly, followed by reversal of anesthesia. All the challenged animals received a weekly complete blood count and had viral loads determined weekly for 8 weeks after infection prior to being humanely euthanized. Since the animals were bled and challenged on the same day, and due to processing time for viral loads, all the animals received an additional 1 or 2 challenges after the infective challenge.
Animal health events.
In our rabbit studies, local inflammation and irritation did occasionally occur associated with acepromazine injection. Rabbits were removed from the study if there was an obvious break in skin integrity as evidenced by weeping/oozing. Additionally, following the MVA prime in the prime/boost study, several rabbits developed inappetence, which progressed to gastrointestinal (GI) stasis. Animals that were symptomatic for GI stasis for more than 2 days were removed from the study. Clostridium spp. were isolated from some of the affected rabbits. The experiments with the i.m./i.m. and i.n./i.m.-plus-i.n. groups in the rabbit prime/boost study were repeated to ensure that the results were similar in unexposed individuals. In the NHP study, a few individuals had trauma to digits that required veterinary medical attention.
Study design.
Intramuscular vaccines were used as the positive control. Unvaccinated NHPs were used as a control for SHIV infection. Animals were not assigned to groups randomly, but rather systematically, either by animal identifier (ID) (rabbits) or by cage (mice) to distribute experimental groups from a previous experiment across vaccine groups in this experiment (rabbits) or using a combination of age, sex, and weight (NHPs). Female rabbits in the prime/boost regimen that received an i.m. prime with MVAgp120 were redistributed among the i.m. prime groups after the Clostridium outbreak.
Animal caretakers had access to information indicating which animals were in the same treatment group but did not know what vaccine each group was receiving. Veterinary personnel were blinded to the treatment group unless there was a need to know. Laboratory personnel performing ELISAs were blinded to the treatment group until after the titer for each sample was determined. Personnel performing neutralization and ADCC assays had access to treatment group designations prior to performing the assays. Bleeding and vaccinations were performed in room order if all the animals were receiving the same sedation/anesthesia protocol. If there was a subset of animals that needed sedation/anesthesia, typically they were premedicated, the procedures were performed on unsedated/unanesthetized individuals, and then the procedures were performed on the premedicated individuals. During SHIV challenges, the individuals known to be infected were bled after individuals who had not yet tested positive. This minimized the risk of transmission via fomites, as well as minimizing the anesthesia duration for the animals that needed to be tested and challenged. Procedures typically began at 10 a.m.
Animals were removed from a study if they did not receive all the vaccines. This occurred only in the rabbit experiments, where rabbits were euthanized due to either GI stasis or Clostridium infection prior to completion of the vaccine regimen or when an acepromazine reaction was moderate or severe enough at the time of the next vaccination that sedation with acepromazine was contraindicated. Animals remained in the study even if there was evidence that they did not receive the full vaccine dose (for example, fluid was evident externally after i.m. vaccination or sneezing after i.n. vaccination). If there was evidence that the entire amount to be administered to the animal was unsuccessfully administered, a second attempt was made to vaccinate the animal with the desired vaccine amount allocated to that animal. All sample collections, anesthesia, and vaccinations occurred in the same room the animal was housed in. Procedures typically began between 9 and 10 a.m.
ELISA.
ELISAs were performed as described previously (80) with the following exceptions. The coating antigen was C.1086 K160N gp120 or V1V2 tags (NIH AIDS Reagent Program; catalog no. 12568) at 2 μg/ml. To detect antibody responses to the viral vector in animals immunized with MVA, the vaccinia virus proteins L1 and B5 (BEI; catalog no. NR-2625 and NR-546) were used as coating antigens at 2 μg/ml. Serum samples were tested starting at a 1:32 dilution, while mucosal samples were tested starting at a 1:16 dilution. Serial 2-fold dilutions were performed. If any individual animal had titers greater than 227 at a given time point, samples from all the animals in the experiment were retested using serial 3-fold dilutions. Murine antibodies were detected using alkaline phosphatase-conjugated goat anti-mouse IgG or IgA (Southern Biotech; catalog no. 1030-04 and 1040-04). Rabbit antibodies were detected using alkaline phosphatase-conjugated goat anti-rabbit IgG (Southern Biotech; catalog no. 4030-04) or IgA (Novus; catalog no. NB7167). For NHP studies, the secondary antibodies used were anti-NHP IgG and anti-NHP IgA (Rockland; catalog no. 617-105-012 and 617-105-006). The fluorescent substrate used, Attophos (Promega), increases the sensitivity of the assay compared to colorimetric substrates (81). The endpoint titers were defined as the last immune sample dilution with a relative light unit (RLU) value 3-fold higher than the RLU value of the animal’s preimmune sample at the same dilution. Since the mice were inbred, the average of two preimmune samples was used instead of a preimmune sample from each individual. Immune samples that were not 3-fold higher than the naive sample in the tested dilution series were assigned a value of one dilution lower than the starting dilution for statistical analysis.
Antibody-dependent cellular cytotoxicity.
ADCC activity was measured using the GranToxiLux (GTL) assay as previously described (82). A clonal isolate of the CEM.NKRCCR5 CD4+ T cell line (from Alexandra Trkola [83], NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH) was used as the source of target cells after coating with the recombinant HIV-1 gp120 protein used as the boost component of the vaccine regimen (HIV-1 isolate C.1086). Effector cells were human peripheral blood mononuclear cells (PBMC) obtained from an HIV-seronegative donor heterozygous for Fcγ receptor IIIa at position 158 (158F/V) (84, 85). PBMC and target cells were combined at an effector cell-to-target cell ratio of 30:1. Samples were tested after 4-fold serial dilutions starting at 1:100. ADCC activity was measured as the proportion of cells positive for proteolytically active granzyme B (GzB) out of the total viable target cell population (percent GzB activity) after subtracting the background activity observed in wells containing effector and target cells in the absence of plasma. ADCC endpoint titers were determined by interpolating the dilutions of plasma that intercepted the previously established positive cutoff for the assay (8% GzB activity) using GraphPad Prism software version 7.0b (GraphPad Software, Inc.) and are reported as reciprocal dilutions.
Neutralization.
Neutralizing antibody activity was measured in 96-well culture plates by using Tat-regulated luciferase (Luc) reporter gene expression to quantify reductions in virus infection in TZM-bl cells. TZM-bl cells were obtained from the NIH AIDS Research and Reference Reagent Program and were contributed by John Kappes and Xiaoyun Wu. Assays were performed with HIV-1 Env-pseudotyped viruses as described previously (86, 87). Test samples were diluted over a range of 1:20 to 1:43,740 in cell culture medium and preincubated with virus (∼150,000 RLU equivalents) for 1 h at 37°C before addition of cells. Following a 48-h incubation, the cells were lysed, and Luc activity was determined using a microtiter plate luminometer and BriteLite Plus reagent (Perkin Elmer). Neutralization titers are the sample dilution at which RLU were reduced by 50% compared to the RLU in virus control wells after subtraction of background RLU in cell control wells. Serum samples were heat inactivated at 56°C for 45 min (rabbits) or 30 min (NHP) prior to the assay.
Viral load quantification.
Quantitative PCR (qPCR) was used to quantify the SHIV RNA present in serum collected 1 week after each challenge. Viral RNA measurements were performed by the Immunology Virology Quality Assessment Center Laboratory Shared Resource, Duke Human Vaccine Institute, Durham, NC, as previously described (88). The lower limit of detection was 250 copies/ml.
Statistical methods.
Mouse and rabbit data were analyzed by Kruskal-Wallis test with Dunn’s multiple-comparison test. NHP ELISA data were analyzed by Mann-Whitney test. Graphs were prepared and all nonparametric comparisons were performed in GraphPad Prism (GraphPad Software, San Diego, CA). Values from individual animals, as well as the geometric mean titer, are shown with the geometric mean of each group depicted on the graph as a horizontal line unless otherwise indicated.
ACKNOWLEDGMENTS
We thank the animal husbandry staff, veterinary technicians, veterinarians, and diagnostic laboratory personnel at Duke’s Division of Laboratory Animal Resources for the care they provided our animals and for assistance with animal procedures. We also recognize Moses Wanyonyi and Michael Peace for assistance with rabbit procedures and Carolyn Weinbaum for help obtaining the primates. For assay and reagent support, we thank Gray Monroe for help performing ELISAs, Raul Louzao and the Immunology and Virology Quality Assessment Center and the Duke Human Vaccine Institute for performing SIV loads, Sampa Santra for providing SHIV 1157QNE Y173H, Nicole Rodgers for construction and preparation of MVAs, Kevin Saunders and Barton Haynes for providing protein antigens for BAMA, Jamie Peacock and the Duke Human Vaccine Institute Research Protein Production Facility for C.1086 gp120 protein, and the David Montefiori laboratory for neutralizing antibody assays. The following reagents were obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: vaccinia virus (WR) B5R protein with N-terminal histidine tag, recombinant from baculovirus, NR-546 and vaccinia virus, Western Reserve, L1R protein with C-terminal histidine tag, recombinant from baculovirus, NR-2625.
Herman F. Staats and Soman N. Abraham are coinventors on an issued patent that describes mast cell-activating peptides that are useful as vaccine adjuvants. However, none of the patented peptides were utilized in the studies described in this paper.
This work was funded by the National Institute of Allergy and Infectious Diseases (1R01AI102747 and 1P01AI117915). Publication was made possible with help from the Duke University Center for AIDS Research (CFAR), an NIH-funded program (5P30 AI064518). the Duke Human Vaccine Institute Research Protein Production Facility received funding support from the Collaboration for AIDS Vaccine Research Bill and Melinda Gates Foundation (OPP1066832). The David Montefiori laboratory received funding support through NIH/NIAID contract no. HHSN27201100016C. The funders had no role in study design, data collection and interpretation, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Fauci AS, Folkers GK, Marston HD. 2014. Ending the global HIV/AIDS pandemic: the critical role of an HIV vaccine. Clin Infect Dis 59 (Suppl 2):S80–4. doi: 10.1093/cid/ciu420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.Demberg T, Robert-Guroff M. 2009. Mucosal immunity and protection against HIV/SIV infection: strategies and challenges for vaccine design. Int Rev Immunol 28:20–48. doi: 10.1080/08830180802684331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni V, Ruprecht RM. 2017. Mucosal IgA responses: damaged in established HIV infection-yet, effective weapon against HIV transmission. Front Immunol 8:1581. doi: 10.3389/fimmu.2017.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sholukh AM, Watkins JD, Vyas HK, Gupta S, Lakhashe SK, Thorat S, Zhou M, Hemashettar G, Bachler BC, Forthal DN, Villinger F, Sattentau QJ, Weiss RA, Agatic G, Corti D, Lanzavecchia A, Heeney JL, Ruprecht RM. 2015. Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: complete protection of rhesus monkeys from mucosal SHIV challenge. Vaccine 33:2086–2095. doi: 10.1016/j.vaccine.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackerman ME, Das J, Pittala S, Broge T, Linde C, Suscovich TJ, Brown EP, Bradley T, Natarajan H, Lin S, Sassic JK, O’Keefe S, Mehta N, Goodman D, Sips M, Weiner JA, Tomaras GD, Haynes BF, Lauffenburger DA, Bailey-Kellogg C, Roederer M, Alter G. 2018. Route of immunization defines multiple mechanisms of vaccine-mediated protection against SIV. Nat Med 24:1590–1598. doi: 10.1038/s41591-018-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belyakov IM, Ahlers JD. 2009. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol 183:6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 8.Fouda GGA, Amos JD, Wilks AB, Pollara J, Ray CA, Chand A, Kunz EL, Liebl BE, Whitaker K, Carville A, Smith S, Colvin L, Pickup DJ, Staats HF, Overman G, Eutsey-Lloyd K, Parks R, Chen H, LaBranche C, Barnett S, Tomaras GD, Ferrari G, Montefiori DC, Liao H-X, Letvin NL, Haynes BF, Permar SR. 2013. Mucosal immunization of lactating female rhesus monkeys with a transmitted/founder HIV-1 envelope induces strong Env-specific IgA antibody responses in breast milk. J Virol 87:6986–6999. doi: 10.1128/JVI.00528-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosgrove CA, Lacey CJ, Cope AV, Bartolf A, Morris G, Yan C, Baden S, Cole T, Carter D, Brodnicki E, Shen X, Joseph S, DeRosa SC, Peng L, Yu X, Ferrari G, Seaman M, Montefiori DC, Frahm N, Tomaras GD, Stohr W, McCormack S, Shattock RJ. 2016. Comparative immunogenicity of HIV-1 gp140 vaccine delivered by parenteral, and mucosal routes in female volunteers; MUCOVAC2, a randomized two centre study. PLoS One 11:e0152038. doi: 10.1371/journal.pone.0152038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lycke N, Lebrero-Fernández C. 2018. ADP-ribosylating enterotoxins as vaccine adjuvants. Curr Opin Pharmacol 41:42–51. doi: 10.1016/j.coph.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 11.El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, Ferreira J, Chen WH, Sublett R, Richardson C, Bargatze RF, Sztein MB, Tacket CO. 2010. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis 202:1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, Staats HF, Abraham SN. 2008. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med 14:536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 13.Kurashima Y, Kiyono H. 2014. New era for mucosal mast cells: their roles in inflammation, allergic immune responses and adjuvant development. Exp Mol Med 46:e83. doi: 10.1038/emm.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGowen AL, Hale LP, Shelburne CP, Abraham SN, Staats HF. 2009. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine 27:3544–3552. doi: 10.1016/j.vaccine.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bento D, Staats HF, Goncalves T, Borges O. 2015. Development of a novel adjuvanted nasal vaccine: C48/80 associated with chitosan nanoparticles as a path to enhance mucosal immunity. Eur J Pharm Biopharm 93:149–164. doi: 10.1016/j.ejpb.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Gwinn WM, Johnson BT, Kirwan SM, Sobel AE, Abraham SN, Gunn MD, Staats HF. 2013. A comparison of non-toxin vaccine adjuvants for their ability to enhance the immunogenicity of nasally-administered anthrax recombinant protective antigen. Vaccine 31:1480–1489. doi: 10.1016/j.vaccine.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staats HF, Fielhauer JR, Thompson AL, Tripp AA, Sobel AE, Maddaloni M, Abraham SN, Pascual DW. 2011. Mucosal targeting of a BoNT/A subunit vaccine adjuvanted with a mast cell activator enhances induction of BoNT/A neutralizing antibodies in rabbits. PLoS One 6:e16532. doi: 10.1371/journal.pone.0016532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koibuchi Y, Ichikawa A, Nakagawa M, Tomita K. 1985. Binding of active components of compound 48/80 to rat peritoneal mast cells. Eur J Pharmacol 115:171–177. doi: 10.1016/0014-2999(85)90688-0. [DOI] [PubMed] [Google Scholar]
- 19.Nakao S, Komagoe K, Inoue T, Katsu T. 2011. Comparative study of the membrane-permeabilizing activities of mastoparans and related histamine-releasing agents in bacteria, erythrocytes, and mast cells. Biochim Biophys Acta 1808:490–497. doi: 10.1016/j.bbamem.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Sample CJ, Hudak KE, Barefoot BE, Koci MD, Wanyonyi MS, Abraham S, Staats HF, Ramsburg EA. 2013. A mastoparan-derived peptide has broad-spectrum antiviral activity against enveloped viruses. Peptides 48:96–105. doi: 10.1016/j.peptides.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwinn WM, Kirwan SM, Wang SH, Ashcraft KA, Sparks NL, Doil CR, Tlusty TG, Casey LS, Hollingshead SK, Briles DE, Dondero RS, Hickey AJ, Foster WM, Staats HF. 2010. Effective induction of protective systemic immunity with nasally administered vaccines adjuvanted with IL-1. Vaccine 28:6901–6914. doi: 10.1016/j.vaccine.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito S, Ainai A, Suzuki T, Harada N, Ami Y, Yuki Y, Takeyama H, Kiyono H, Tsukada H, Hasegawa H. 2016. The effect of mucoadhesive excipient on the nasal retention time of and the antibody responses induced by an intranasal influenza vaccine. Vaccine 34:1201–1207. doi: 10.1016/j.vaccine.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Fukuyama Y, Yuki Y, Katakai Y, Harada N, Takahashi H, Takeda S, Mejima M, Joo S, Kurokawa S, Sawada S, Shibata H, Park EJ, Fujihashi K, Briles DE, Yasutomi Y, Tsukada H, Akiyoshi K, Kiyono H. 2015. Nanogel-based pneumococcal surface protein A nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques. Mucosal Immunol 8:1144–1153. doi: 10.1038/mi.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabbal-Gill I, Watts P, Smith A. 2012. Chitosan-based delivery systems for mucosal vaccines. Expert Opin Drug Deliv 9:1051–1067. doi: 10.1517/17425247.2012.697455. [DOI] [PubMed] [Google Scholar]
- 25.Wang SH, Thompson AL, Hickey AJ, Staats HF. 2012. Dry powder vaccines for mucosal administration: critical factors in manufacture and delivery. Curr Top Microbiol Immunol 354:121–156. doi: 10.1007/82_2011_167. [DOI] [PubMed] [Google Scholar]
- 26.Maddaloni M, Staats HF, Mierzejewska D, Hoyt T, Robinson A, Callis G, Kozaki S, Kiyono H, McGhee JR, Fujihashi K, Pascual DW. 2006. Mucosal vaccine targeting improves onset of mucosal and systemic immunity to botulinum neurotoxin A. J Immunol 177:5524–5532. doi: 10.4049/jimmunol.177.8.5524. [DOI] [PubMed] [Google Scholar]
- 27.Gibson M, Tiensiwakul P, Khoobyarian N. 1982. Adenovirus fiber protein (FP) functions as a mitogen and an adjuvant. Cell Immunol 73:397–403. doi: 10.1016/0008-8749(82)90466-X. [DOI] [PubMed] [Google Scholar]
- 28.Tamanini A, Nicolis E, Bonizzato A, Bezzerri V, Melotti P, Assael BM, Cabrini G. 2006. Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J Virol 80:11241–11254. doi: 10.1128/JVI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clapp B, Golden S, Maddaloni M, Staats HF, Pascual DW. 2010. Adenovirus F protein as a delivery vehicle for botulinum B. BMC Immunol 11:36. doi: 10.1186/1471-2172-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Currier JR, Ngauy V, de Souza MS, Ratto-Kim S, Cox JH, Polonis VR, Earl P, Moss B, Peel S, Slike B, Sriplienchan S, Thongcharoen P, Paris RM, Robb ML, Kim J, Michael NL, Marovich MA. 2010. Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS One 5:e13983. doi: 10.1371/journal.pone.0013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goepfert PA, Elizaga ML, Sato A, Qin L, Cardinali M, Hay CM, Hural J, DeRosa SC, DeFawe OD, Tomaras GD, Montefiori DC, Xu Y, Lai L, Kalams SA, Baden LR, Frey SE, Blattner WA, Wyatt LS, Moss B, Robinson HL. 2011. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 203:610–619. doi: 10.1093/infdis/jiq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guimaraes-Walker A, Mackie N, McCormack S, Hanke T, Schmidt C, Gilmour J, Barin B, McMichael A, Weber J, Legg K, Babiker A, Hayes P, Gotch F, Smith C, Dally L, Dorrell L, Cebere I, Kay R, Winstone N, Moore S, Goonetilleke N, Fast P. 2008. Lessons from IAVI-006, a phase I clinical trial to evaluate the safety and immunogenicity of the pTHr.HIVA DNA and MVA.HIVA vaccines in a prime-boost strategy to induce HIV-1 specific T-cell responses in healthy volunteers. Vaccine 26:6671–6677. doi: 10.1016/j.vaccine.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Jaoko W, Nakwagala FN, Anzala O, Manyonyi GO, Birungi J, Nanvubya A, Bashir F, Bhatt K, Ogutu H, Wakasiaka S, Matu L, Waruingi W, Odada J, Oyaro M, Indangasi J, Ndinya-Achola J, Konde C, Mugisha E, Fast P, Schmidt C, Gilmour J, Tarragona T, Smith C, Barin B, Dally L, Johnson B, Muluubya A, Nielsen L, Hayes P, Boaz M, Hughes P, Hanke T, McMichael A, Bwayo J, Kaleebu P. 2008. Safety and immunogenicity of recombinant low-dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Vaccine 26:2788–2795. doi: 10.1016/j.vaccine.2008.02.071. [DOI] [PubMed] [Google Scholar]
- 34.Keefer MC, Frey SE, Elizaga M, Metch B, De Rosa SC, Barroso PF, Tomaras G, Cardinali M, Goepfert P, Kalichman A, Philippon V, McElrath MJ, Jin X, Ferrari G, Defawe OD, Mazzara GP, Montefiori D, Pensiero M, Panicali DL, Corey L. 2011. A phase I trial of preventive HIV vaccination with heterologous poxviral-vectors containing matching HIV-1 inserts in healthy HIV-uninfected subjects. Vaccine 29:1948–1958. doi: 10.1016/j.vaccine.2010.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mwau M, Cebere I, Sutton J, Chikoti P, Winstone N, Wee EG, Beattie T, Chen YH, Dorrell L, McShane H, Schmidt C, Brooks M, Patel S, Roberts J, Conlon C, Rowland-Jones SL, Bwayo JJ, McMichael AJ, Hanke T. 2004. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J Gen Virol 85:911–919. doi: 10.1099/vir.0.19701-0. [DOI] [PubMed] [Google Scholar]
- 36.Vasan S, Schlesinger SJ, Chen Z, Hurley A, Lombardo A, Than S, Adesanya P, Bunce C, Boaz M, Boyle R, Sayeed E, Clark L, Dugin D, Boente-Carrera M, Schmidt C, Fang Q, Lei B, Huang Y, Zaharatos GJ, Gardiner DF, Caskey M, Seamons L, Ho M, Dally L, Smith C, Cox J, Gill D, Gilmour J, Keefer MC, Fast P, Ho DD. 2010. Phase 1 safety and immunogenicity evaluation of ADMVA, a multigenic, modified vaccinia Ankara-HIV-1 B'/C candidate vaccine. PLoS One 5:e8816. doi: 10.1371/journal.pone.0008816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyatt LS, Xiao W, Americo JL, Earl PL, Moss B. 2017. Novel nonreplicating vaccinia virus vector enhances expression of heterologous genes and suppresses synthesis of endogenous viral proteins. mBio 8:e00790-17. doi: 10.1128/mBio.00790-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sánchez-Sampedro L, Perdiguero B, Mejías-Pérez E, García-Arriaza J, Di Pilato M, Esteban M. 2015. The evolution of poxvirus vaccines. Viruses 7:1726–1803. doi: 10.3390/v7041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garber DA, O'Mara LA, Zhao J, Gangadhara S, An I, Feinberg MB. 2009. Expanding the repertoire of modified vaccinia Ankara-based vaccine vectors via genetic complementation strategies. PLoS One 4:e5445. doi: 10.1371/journal.pone.0005445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Arriaza J, Arnaez P, Gomez CE, Sorzano CO, Esteban M. 2013. Improving adaptive and memory immune responses of an HIV/AIDS vaccine candidate MVA-B by deletion of vaccinia virus genes (C6L and K7R) blocking interferon signaling pathways. PLoS One 8:e66894. doi: 10.1371/journal.pone.0066894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Arriaza J, Gomez CE, Sorzano CO, Esteban M. 2014. Deletion of the vaccinia virus N2L gene encoding an inhibitor of IRF3 improves the immunogenicity of modified vaccinia virus Ankara expressing HIV-1 antigens. J Virol 88:3392–3410. doi: 10.1128/JVI.02723-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garber DA, O'Mara LA, Gangadhara S, McQuoid M, Zhang X, Zheng R, Gill K, Verma M, Yu T, Johnson B, Li B, Derdeyn CA, Ibegbu C, Altman JD, Hunter E, Feinberg MB. 2012. Deletion of specific immune-modulatory genes from modified vaccinia virus Ankara-based HIV vaccines engenders improved immunogenicity in rhesus macaques. J Virol 86:12605–12615. doi: 10.1128/JVI.00246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones DI, Pollara JJ, Johnson-Weaver BT, LaBranche CC, Montefiori DC, Pickup DJ, Permar SR, Abraham SN, Maddaloni M, Pascual DW, Staats HF. 2019. Optimized mucosal modified vaccinia virus Ankara prime/soluble gp120 boost HIV vaccination regimen induces antibody responses similar to those of an intramuscular regimen. bioRxiv 10.1101/573394. [DOI] [PMC free article] [PubMed]
- 44.Egan MA, Chong SY, Hagen M, Megati S, Schadeck EB, Piacente P, Ma BJ, Montefiori DC, Haynes BF, Israel ZR, Eldridge JH, Staats HF. 2004. A comparative evaluation of nasal and parenteral vaccine adjuvants to elicit systemic and mucosal HIV-1 peptide-specific humoral immune responses in cynomolgus macaques. Vaccine 22:3774–3788. doi: 10.1016/j.vaccine.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Alving CR. 2002. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine 20:S56–S64. doi: 10.1016/S0264-410X(02)00174-3. [DOI] [PubMed] [Google Scholar]
- 46.Vaccari M, Gordon SN, Fourati S, Schifanella L, Liyanage NPM, Cameron M, Keele BF, Shen X, Tomaras GD, Billings E, Rao M, Chung AW, Dowell KG, Bailey-Kellogg C, Brown EP, Ackerman ME, Vargas-Inchaustegui DA, Whitney S, Doster MN, Binello N, Pegu P, Montefiori DC, Foulds K, Quinn DS, Donaldson M, Liang F, Loré K, Roederer M, Koup RA, McDermott A, Ma Z-M, Miller CJ, Phan TB, Forthal DN, Blackburn M, Caccuri F, Bissa M, Ferrari G, Kalyanaraman V, Ferrari MG, Thompson D, Robert-Guroff M, Ratto-Kim S, Kim JH, Michael NL, Phogat S, Barnett SW, Tartaglia J, Venzon D, Stablein DM, Alter G, Sekaly R-P, Franchini G. 2016. Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat Med 22:762–770. doi: 10.1038/nm.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson CS, Pollara J, Kunz EL, Jeffries TL Jr, Duffy R, Beck C, Stamper L, Wang M, Shen X, Pickup DJ, Staats HF, Hudgens MG, Kepler TB, Montefiori DC, Moody MA, Tomaras GD, Liao HX, Haynes BF, Ferrari G, Fouda GG, Permar SR. 2016. Combined HIV-1 envelope systemic and mucosal immunization of lactating rhesus monkeys induces robust IgA-isotype B cell response in breast milk. J Virol doi: 10.1128/jvi.00335-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollara J, Jones DI, Huffman T, Edwards RW, Dennis M, Li SH, Jha S, Goodman D, Kumar A, LaBranche CC, Montefiori DC, Fouda GG, Hope TJ, Tomaras GD, Staats HF, Ferrari G, Permar SR. 2019. Bridging vaccine-induced HIV-1 neutralizing and effector antibody responses in rabbit and rhesus macaque animal models. J Virol 93:e02119-18. doi: 10.1128/JVI.02119-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng L, Liu Y, Wang H, Liao P, Song Z, Gao S, Wu Y, Zhang X, Yin Y, Xu W. 2015. Compound 48/80 acts as a potent mucosal adjuvant for vaccination against Streptococcus pneumoniae infection in young mice. Vaccine 33:1008–1016. doi: 10.1016/j.vaccine.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Pattacini L, Mize GJ, Graham JB, Fluharty TR, Graham TM, Lingnau K, Wizel B, Perdiguero B, Esteban M, Pantaleo G, Shen M, Spies GA, McElrath MJ, Lund JM. 2012. A novel HIV vaccine adjuvanted by IC31 induces robust and persistent humoral and cellular immunity. PLoS One 7:e42163. doi: 10.1371/journal.pone.0042163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Czerkinsky C, Anjuere F, McGhee JR, George-Chandy A, Holmgren J, Kieny MP, Fujiyashi K, Mestecky JF, Pierrefite-Carle V, Rask C, Sun JB. 1999. Mucosal immunity and tolerance: relevance to vaccine development. Immunol Rev 170:197–222. doi: 10.1111/j.1600-065X.1999.tb01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vajdy M, Srivastava I, Polo J, Donnelly J, O'Hagan D, Singh M. 2004. Mucosal adjuvants and delivery systems for protein-, DNA- and RNA-based vaccines. Immunol Cell Biol 82:617–627. doi: 10.1111/j.1440-1711.2004.01288.x. [DOI] [PubMed] [Google Scholar]
- 54.Atmar RL, Keitel WA, Cate TR, Munoz FM, Ruben F, Couch RB. 2007. A dose-response evaluation of inactivated influenza vaccine given intranasally and intramuscularly to healthy young adults. Vaccine 25:5367–5373. doi: 10.1016/j.vaccine.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barnett SW, Srivastava IK, Kan E, Zhou F, Goodsell A, Cristillo AD, Ferrai MG, Weiss DE, Letvin NL, Montefiori D, Pal R, Vajdy M. 2008. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS 22:339–348. doi: 10.1097/QAD.0b013e3282f3ca57. [DOI] [PubMed] [Google Scholar]
- 56.Bradney CP, Sempowski GD, Liao HX, Haynes BF, Staats HF. 2002. Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization. J Virol 76:517–524. doi: 10.1128/JVI.76.2.517-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolton DL, Song K, Tomaras GD, Rao S, Roederer M. 2017. Unique cellular and humoral immunogenicity profiles generated by aerosol, intranasal, or parenteral vaccination in rhesus macaques. Vaccine 35:639–646. doi: 10.1016/j.vaccine.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bryant ML, Brown P, Gurevich N, McDougall IR. 1999. Comparison of the clearance of radiolabelled nose drops and nasal spray as mucosally delivered vaccine. Nucl Med Commun 20:171–174. doi: 10.1097/00006231-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Romeo VD, deMeireles J, Sileno AP, Pimplaskar HK, Behl CR. 1998. Effects of physicochemical properties and other factors on systemic nasal drug delivery. Adv Drug Deliv Rev 29:89–116. [DOI] [PubMed] [Google Scholar]
- 60.Hay JG, McElvaney NG, Herena J, Crystal RG. 1995. Modification of nasal epithelial potential differences of individuals with cystic fibrosis consequent to local administration of a normal CFTR cDNA adenovirus gene transfer vector. Hum Gene Ther 6:1487–1496. doi: 10.1089/hum.1995.6.11-1487. [DOI] [PubMed] [Google Scholar]
- 61.Van Kampen KR, Shi Z, Gao P, Zhang J, Foster KW, Chen DT, Marks D, Elmets CA, Tang DC. 2005. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine 23:1029–1036. doi: 10.1016/j.vaccine.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 62.Staats HF, Montgomery SP, Palker TJ. 1997. Intranasal immunization is superior to vaginal, gastric, or rectal immunization for the induction of systemic and mucosal anti-HIV antibody responses. AIDS Res Hum Retroviruses 13:945–952. doi: 10.1089/aid.1997.13.945. [DOI] [PubMed] [Google Scholar]
- 63.Manrique M, Kozlowski PA, Wang SW, Wilson RL, Micewicz E, Montefiori DC, Mansfield KG, Carville A, Aldovini A. 2009. Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal Immunol 2:536–550. doi: 10.1038/mi.2009.103. [DOI] [PubMed] [Google Scholar]
- 64.Harkema JR, Carey SA, Wagner JG. 2006. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol 34:252–269. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- 65.Casteleyn C, Broos AM, Simoens P, Van den Broeck W. 2010. NALT (nasal cavity-associated lymphoid tissue) in the rabbit. Vet Immunol Immunopathol 133:212–218. doi: 10.1016/j.vetimm.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Debertin AS, Tschernig T, Tonjes H, Kleemann WJ, Troger HD, Pabst R. 2003. Nasal-associated lymphoid tissue (NALT): frequency and localization in young children. Clin Exp Immunol 134:503–507. doi: 10.1111/j.1365-2249.2003.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cranage MP, Fraser CA, Stevens Z, Huting J, Chang M, Jeffs SA, Seaman MS, Cope A, Cole T, Shattock RJ. 2010. Repeated vaginal administration of trimeric HIV-1 clade C gp140 induces serum and mucosal antibody responses. Mucosal Immunol 3:57–68. doi: 10.1038/mi.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bradley T, Pollara J, Santra S, Vandergrift N, Pittala S, Bailey-Kellogg C, Shen X, Parks R, Goodman D, Eaton A, Balachandran H, Mach LV, Saunders KO, Weiner JA, Scearce R, Sutherland LL, Phogat S, Tartaglia J, Reed SG, Hu SL, Theis JF, Pinter A, Montefiori DC, Kepler TB, Peachman KK, Rao M, Michael NL, Suscovich TJ, Alter G, Ackerman ME, Moody MA, Liao HX, Tomaras G, Ferrari G, Korber BT, Haynes BF. 2017. Pentavalent HIV-1 vaccine protects against simian-human immunodeficiency virus challenge. Nat Commun 8:15711. doi: 10.1038/ncomms15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wyatt LS, Earl PL, Xiao W, Americo JL, Cotter CA, Vogt J, Moss B. 2009. Elucidating and minimizing the loss by recombinant vaccinia virus of human immunodeficiency virus gene expression resulting from spontaneous mutations and positive selection. J Virol 83:7176–7184. doi: 10.1128/JVI.00687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Staib C, Drexler I, Sutter G. 2004. Construction and isolation of recombinant MVA. Methods Mol Biol 269:77–100. doi: 10.1385/1-59259-789-0:077. [DOI] [PubMed] [Google Scholar]
- 71.Staib C, Kisling S, Erfle V, Sutter G. 2005. Inactivation of the viral interleukin 1beta receptor improves CD8+ T-cell memory responses elicited upon immunization with modified vaccinia virus Ankara. J Gen Virol 86:1997–2006. doi: 10.1099/vir.0.80646-0. [DOI] [PubMed] [Google Scholar]
- 72.Lambert JM, Bongers RS, Kleerebezem M. 2007. Cre-lox-based system for multiple gene deletions and selectable-marker removal in Lactobacillus plantarum. Appl Environ Microbiol 73:1126–1135. doi: 10.1128/AEM.01473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsuda T, Cepko CL. 2007. Controlled expression of transgenes introduced by in vivo electroporation. Proc Natl Acad Sci U S A 104:1027–1032. doi: 10.1073/pnas.0610155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carroll MW, Moss B. 1997. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology 238:198–211. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 75.Phillips B, Fouda GG, Eudailey J, Pollara J, Curtis AD II, Kunz E, Dennis M, Shen X, Bay C, Hudgens M, Pickup D, Alam SM, Ardeshir A, Kozlowski PA, Van Rompay KKA, Ferrari G, Moody MA, Permar S, De Paris K. 2017. Impact of poxvirus vector priming, protein coadministration, and vaccine intervals on HIV gp120 vaccine-elicited antibody magnitude and function in infant macaques. Clin Vaccine Immunol 24:e00231-17. doi: 10.1128/CVI.00231-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mutucumarana CP, Eudailey J, McGuire EP, Vandergrift N, Tegha G, Chasela C, Ellington S, van der Horst C, Kourtis AP, Permar SR, Fouda GG. 2017. Maternal humoral immune correlates of peripartum transmission of clade c HIV-1 in the setting of peripartum antiretrovirals. Clin Vaccine Immunol 24:e00062-17. doi: 10.1128/CVI.00062-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Staats HF, Nichols WG, Palker TJ. 1996. Mucosal immunity to HIV-1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A). J Immunol 157:462–472. [PubMed] [Google Scholar]
- 78.Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR. 2000. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr 24:297–309. doi: 10.1097/00126334-200008010-00001. [DOI] [PubMed] [Google Scholar]
- 79.Asmal M, Luedemann C, Lavine CL, Mach LV, Balachandran H, Brinkley C, Denny TN, Lewis MG, Anderson H, Pal R, Sok D, Le K, Pauthner M, Hahn BH, Shaw GM, Seaman MS, Letvin NL, Burton DR, Sodroski JG, Haynes BF, Santra S. 2015. Infection of monkeys by simian-human immunodeficiency viruses with transmitted/founder clade C HIV-1 envelopes. Virology 475:37–45. doi: 10.1016/j.virol.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang SH, Kirwan SM, Abraham SN, Staats HF, Hickey AJ. 2012. Stable dry powder formulation for nasal delivery of anthrax vaccine. J Pharm Sci 101:31–47. doi: 10.1002/jps.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blais BW, Leggate J, Bosley J, Martinez-Perez A. 2004. Comparison of fluorogenic and chromogenic assay systems in the detection of Escherichia coli O157 by a novel polymyxin-based ELISA. Lett Appl Microbiol 39:516–522. doi: 10.1111/j.1472-765X.2004.01616.x. [DOI] [PubMed] [Google Scholar]
- 82.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. 2011. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 79:603–612. doi: 10.1002/cyto.a.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trkola A, Matthews J, Gordon C, Ketas T, Moore JP. 1999. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J Virol 73:8966–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koene HR, Kleijer M, Algra J, Roos D, von Dem Borne AE, de Haas M. 1997. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 90:1109–1114. [PubMed] [Google Scholar]
- 85.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. 2009. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 86.Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin CL, Bilska M, Greene KM, Gao H, Todd CA, Ozaki DA, Seaman MS, Mascola JR, Montefiori DC. 2014. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods 409:131–146. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Montefiori DC. 2009. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 88.Santra S, Tomaras GD, Warrier R, Nicely NI, Liao H-X, Pollara J, Liu P, Alam SM, Zhang R, Cocklin SL, Shen X, Duffy R, Xia S-M, Schutte RJ, Pemble CW IV, Dennison SM, Li H, Chao A, Vidnovic K, Evans A, Klein K, Kumar A, Robinson J, Landucci G, Forthal DN, Montefiori DC, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, Michael NL, Kim JH, Soderberg KA, Giorgi EE, Blair L, Korber BT, Moog C, Shattock RJ, Letvin NL, Schmitz JE, Moody MA, Gao F, Ferrari G, Shaw GM, Haynes BF. 2015. Human NOn-neutralizing HIV-1 envelope monoclonal antibodies limit the number of founder viruses during SHIV mucosal infection in rhesus macaques. PLoS Pathog 11:e1005042. doi: 10.1371/journal.ppat.1005042. [DOI] [PMC free article] [PubMed] [Google Scholar]