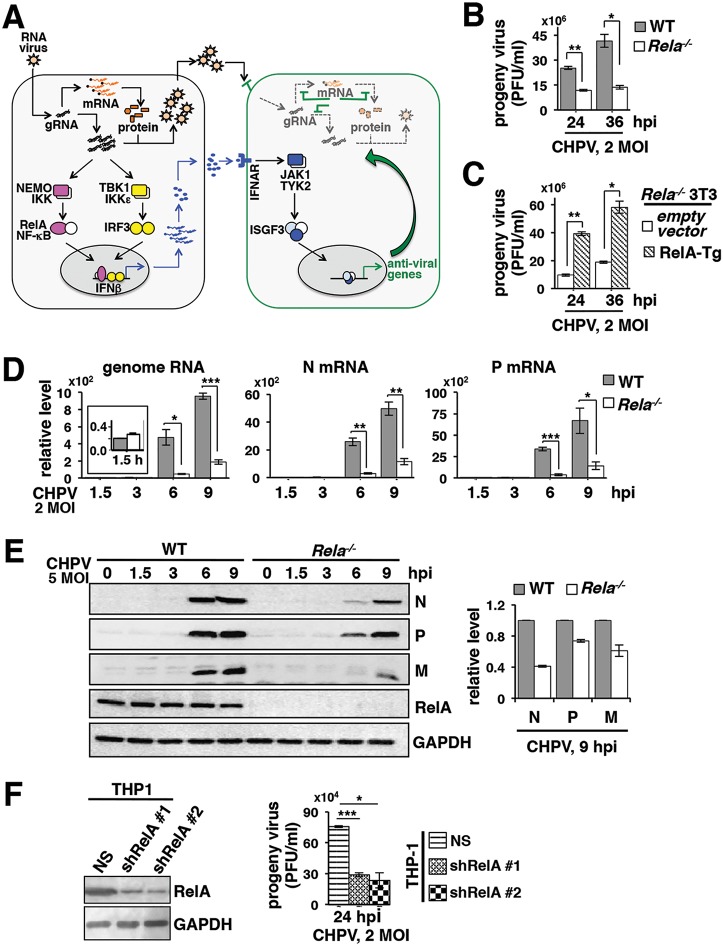
RelA/NF-κB participates in a wide spectrum of physiological processes, including shaping immune responses against invading pathogens. In virus-infected cells, RelA typically induces the expression of IFN-β, which restrains viral propagation in neighboring cells involving paracrine mechanisms. Our study suggested that RelA might also play a proviral role. A cell-autonomous RelA activity amplified the yield of Chandipura virus, a cytopathic RNA virus associated with human epidemics, by extending the life span of infected cells. Our finding necessitates a substantial revision of our understanding of host-virus interactions and indicates a dual role of NF-κB signaling during the course of RNA virus infections. Our study also bears significance for therapeutic regimes which alter NF-κB activities while alleviating the viral load.
KEYWORDS: cytopathic, interferon, NF-κB, RNA virus, RelA, burst size, cell death
ABSTRACT
Chandipura virus (CHPV), a cytoplasmic RNA virus, has been implicated in several outbreaks of acute encephalitis in India. Despite the relevance of CHPV to human health, how the virus interacts with the host signaling machinery remains obscure. In response to viral infections, mammalian cells activate RelA/NF-κB heterodimers, which induce genes encoding interferon beta (IFN-β) and other immune mediators. Therefore, RelA is generally considered to be an antiviral transcription factor. However, RelA activates a wide spectrum of genes in physiological settings, and there is a paucity of direct genetic evidence substantiating antiviral RelA functions. Using mouse embryonic fibroblasts, we genetically dissected the role of RelA in CHPV pathogenesis. We found that CHPV indeed activated RelA and that RelA deficiency abrogated the expression of IFN-β in response to virus infections. Unexpectedly, infection of Rela−/− fibroblasts led to a decreased CHPV yield. Our investigation clarified that RelA-dependent synthesis of prosurvival factors restrained infection-inflicted cell death and that exacerbated cell death processes prevented multiplication of CHPV in RelA-deficient cells. Chikungunya virus, a cytopathic RNA virus associated also with epidemics, required RelA, and Japanese encephalitis virus, which produced relatively minor cytopathic effects in fibroblasts, circumvented the need of RelA for their propagation. In sum, we documented a proviral function of the pleiotropic factor RelA linked to its prosurvival properties. RelA promoted the growth of cytopathic RNA viruses by extending the life span of infected cells, which serve as the replicative niche of intracellular pathogens. We argue that our finding bears significance for understanding host-virus interactions and may have implications for antiviral therapeutic regimes.
IMPORTANCE RelA/NF-κB participates in a wide spectrum of physiological processes, including shaping immune responses against invading pathogens. In virus-infected cells, RelA typically induces the expression of IFN-β, which restrains viral propagation in neighboring cells involving paracrine mechanisms. Our study suggested that RelA might also play a proviral role. A cell-autonomous RelA activity amplified the yield of Chandipura virus, a cytopathic RNA virus associated with human epidemics, by extending the life span of infected cells. Our finding necessitates a substantial revision of our understanding of host-virus interactions and indicates a dual role of NF-κB signaling during the course of RNA virus infections. Our study also bears significance for therapeutic regimes which alter NF-κB activities while alleviating the viral load.
INTRODUCTION
Cytoplasmic RNA viruses continue to pose a threat to public health (1, 2). In particular, Chandipura virus (CHPV) has been implicated in several recent outbreaks of acute encephalitis in India (3). CHPV, which belongs to the Rhabdoviridae family and the genus Vesiculovirus, possesses a single-stranded negative-sense RNA genome (4). The viral life cycle involves entry into host cells, the release of genetic material, synthesis of viral proteins as well as progeny genome RNA, assembly of mature virus particles, and their exit from infected cells. CHPV also produces cytopathic effects and causes cell death. It was, in fact, suggested that neuronal death triggered by CHPV leads to neuropathogenesis in human patients (5).
Mammalian cells engage innate immune pathways for limiting cytoplasmic RNA virus infections (Fig. 1A). Previous biochemical studies involving vesicular stomatitis virus (VSV) and other prototype RNA viruses defined broadly the biochemical mechanism underlying antiviral host responses (6, 7). In brief, viral nucleic acids stimulate the activity of TANK-binding kinase 1 (TBK1) and IκB kinase ε (IKKε), which phosphorylate interferon regulatory factor 3 (IRF3), leading to its nuclear translocation. Moreover, viral infections activate the canonical nuclear factor-κB (NF-κB) pathway. In uninfected cells, RelA:p50 NF-κB heterodimers are sequestered in the cytoplasm by NF-κB inhibitor proteins (IκBs), IκBα, IκBβ, and IκBε. Canonical signaling recruits a kinase complex consisting of NF-κB essential modulator (NEMO) and IKK2 (also known as IKKβ), which phosphorylates IκBs. Consequent proteasomal degradation of IκBs liberates RelA:p50 into the nucleus. RelA:p50 and IRF3 cooperatively induce the expression of type I interferon (IFN) genes, particularly the one encoding IFN-β. IFN-β provides autocrine and paracrine signals through IFN-alpha/beta receptor (IFNAR) that upregulates hundreds of IFN-stimulated genes (ISGs), producing a robust antiviral cell state.
FIG 1.
Genetically dissecting the role of RelA in regulating CHPV propagation. (A) A current model for the cellular defense to RNA virus infections. In general, viral sensing activates the RelA NF-κB heterodimer, which in collaboration with IRF3 induces the expression of type I interferons, including IFN-β. Autocrine and paracrine signals of IFN-β through the cognate IFNAR restrict viral multiplication and produce an antiviral state in neighboring cells. Despite the relevance of CHPV to human health, immune signaling pathways activated by CHPV per se have not been characterized. (B) Primary MEFs derived from WT and Rela−/− mice were infected with CHPV at an MOI of 2; culture supernatants were collected at the indicated time points, and progeny virus yield was measured using a plaque assay. Data represent means of three biological replicates ± SEM. (C) Rela−/− MEFs immortalized using the NIH 3T3 protocol were transduced with retrovirus expressing RelA from a transgene (RelA-Tg). Subsequently, cells were infected with CHPV, and the progeny virus yield was measured. Data represent means of three biological replicates ± SEM. (D) RT-qPCR revealing the relative abundance of viral genomic RNA as well as N and P mRNAs in WT and Rela−/− primary MEFs infected with CHPV an MOI of 2 for the indicated times. The abundance of viral RNAs was normalized to that of beta-actin mRNA. Data represent means of four biological replicates ± SEM. The inset shows the relative level of genome RNA subsequent to viral absorption in WT and RelA-deficient cells. (E) WT and Rela−/− MEFs were infected with CHPV at an MOI of 5 and harvested at the indicated times postinfection, and whole-cell extracts were subjected to immunoblot analyses using antibodies against the indicated viral proteins. GAPDH served as a loading control. The panel on the right shows a densitometric analysis of the relative abundances of N, P, and M proteins quantified from three independent experiments. (F) Effect of shRNA-mediated knockdown of RelA in THP-1 cells on the progeny CHPV titer. Data represent means of three experimental repeats ± SEM. The immunoblot on the left reveals the effect of shRNA-mediated knockdown of RelA expressions. NS, nonsilencing shRNA. Statistical significance was determined using two-tailed Student's t test. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Genetic analyses causally linked the IRF pathway to cellular defense in relation to a number of cytoplasmic RNA viruses. For example, deficiency of Irf3 diminished the production of IFN-β by cells infected with VSV, Newcastle disease virus (NDV), or dengue virus (DENV) (8, 9). Similarly, knockout studies established a role of TBK1 and IKKε in phosphorylating IRF3 and activating the expression of IFN-β in response to VSV, Sendai virus (SeV), and West Nile virus (WNV) (10–13). Consistently, a lack of IKKε or IRF3 potentiated the propagation of VSV, WNV, or DENV ex vivo (9, 10, 13).
Canonical NF-κB signaling regulates a diverse range of cellular functions and mediates also the expression of prosurvival factors. Targeted deletion of the gene encoding RelA in mice resulted in cellular apoptosis as well as necroptosis and caused embryonic lethality (14, 15). Unavailability of adult mice lacking components of the canonical pathway largely impeded genetic dissection of NF-κB signaling in the context of virus infections. In a solitary study, Wang et al. infected mouse embryonic fibroblasts (MEFs) devoid of RelA with VSV or NDV at low multiplicities of infection (MOIs) (16). Their study indicated that the canonical NF-κB pathway is important for early, but not late, expression of IFN-β in infected cells and for suppressing the growth of these viruses. Of note, mainstay immune pathways are often modulated by additional virus-specific interventions (17). Despite the relevance of CHPV to human health, how CHPV per se interacts with the cellular signaling machinery has not been investigated.
Utilizing genetically tractable MEFs, here we examined the role of RelA in antiviral host responses to CHPV. CHPV induced nuclear translocation of RelA:p50 via the canonical NF-κB pathway. Indeed, RelA deficiency abrogated the expression of IFN-β in response to CHPV infection. Unexpectedly, infection of Rela−/− MEFs led to a decreased yield of progeny CHPV particles. Our combined experimental and mathematical analyses suggested that this proviral NF-κB function was linked to the ability of RelA to suppress CHPV-induced cell death. Furthermore, exacerbated cell death processes in Rela−/− MEFs adversely affected the multiplication of cytopathic Chikungunya virus (CHIKV) but did not impact the growth of Japanese encephalitis virus (JEV), which produced relatively minor cytopathic effects in MEFs. In sum, we provide evidence that the pleiotropic transcription factor RelA may have proviral functions.
(This article was submitted to an online preprint archive [18].)
RESULTS AND DISCUSSION
Diminished multiplication of CHPV in RelA-deficient cells.
In general, mammalian cells engage NF-κB and IRF factors for inducing the expression of type I interferons, which mediate antiviral functions (outlined in the introduction and Fig. 1A). Given that RelA participates in diverse cellular processes, we asked if genetic deficiency of the pleiotropic factor RelA indeed led to an overall increase in the production of CHPV. To this end, we infected primary MEFs derived from wild-type (WT) and Rela−/− mice with CHPV and then measured the titer of progeny virus particles in the culture supernatant using a plaque assay (see Materials and Methods). Surprisingly, Rela−/− MEFs infected with CHPV at an MOI of 2 produced 2.2-fold fewer progeny virus particles at 24 h postinfection (hpi) than corresponding CHPV-infected WT cells; there was a further 3.1-fold reduction in the virus titer at 36 hpi (Fig. 1B). Progeny virus particles were not discernible before the 24-hpi time point. We also utilized Rela−/− MEFs immortalized using an NIH 3T3 protocol in our experiments. We transduced Rela−/− 3T3 cells with retrovirus expressing RelA and subsequently infected these cells with CHPV. Our results confirmed that reconstitution of Rela−/− cells with RelA was sufficient for enhancing CHPV yield (Fig. 1C). Next, we measured the abundance of viral genome RNA and mRNAs in infected cells using reverse transcription-quantitative PCR (RT-qPCR). We found that CHPV genome RNAs were abundant equivalently in WT and Rela−/− primary MEFs immediately after viral adsorption (Fig. 1D, inset in the left panel). Ongoing viral replication in WT cells led to a robust increase in the abundance of CHPV genome RNA at 9 hpi, whereas the increase was less obvious in Rela−/− MEFs (Fig. 1D). Moreover, the abundance of mRNAs encoding viral nucleocapsid (N) protein as well as phosphoprotein (P) was markedly low in Rela−/− cells in relation to that in WT MEFs at both 6 hpi and 9 hpi. Our quantitative immunoblot analyses consistently showed a substantially diminished abundance of viral N, P, and matrix (M) protein in Rela−/− MEFs at these time points (Fig. 1E).
Cells of the myeloid lineage, particularly macrophages, not only account for effective antiviral responses but also represent an important niche for RNA virus replication in vivo (19). We asked if RelA was similarly important for the propagation of CHPV, a human pathogen, in human-derived myeloid cells. To address this question, we first subjected THP-1 monocytic cells to short hairpin RNA (shRNA)-mediated depletion of RelA (Fig. 1F, top blot). As a control, we used a nonsilencing shRNA. We then infected these cells with CHPV at an MOI of 2 and measured the progeny virus yield. Our results substantiated that depletion of RelA led to a 3-fold reduction in the progeny virus titer in human-derived myeloid cells (Fig. 1F, bar plot). Taking these results together, our study suggested that RelA promoted the production of progeny CHPV particles and positively impacted viral RNA synthesis ex vivo.
Activation of canonical RelA NF-κB signaling in CHPV-infected cells.
The NF-κB family consists of more than a dozen dimeric factors, with RelA:p50 and RelB:p52 heterodimers being the most prevalent in the majority of cell types (20). RelA:p50 and RelB:p52 are activated by the canonical and noncanonical NF-κB pathways, respectively. As such, RNA viruses trigger the canonical RelA:p50 activity, which along with IRF3 contributes to antiviral IFN-β expression. However, VSV also stimulates noncanonical RelB:p52 activity, which actually inhibits the expression of IFN-β (21). Of note, RelB is encoded by an RelA target gene (22). Indeed, RelA deficiency diminishes basal expressions of RelB and impairs noncanonical RelB:p52 activation downstream of lymphotoxin-β receptor (22). We asked if CHPV engaged primarily the interferon-inhibitory noncanonical NF-κB pathway, whose weakening led to reduced CHPV yield in RelA-deficient cells.
We infected MEFs with CHPV at an MOI of 2 and then measured nuclear NF-κB (NF-κBn) DNA binding activity using an electrophoretic mobility shift assay (EMSA). WT MEFs elicited the NF-κBn activity within 3 h of infection, which was further augmented in a time course until 9 h (Fig. 2A). The onset of cytopathic effects dissuaded us from biochemically analyzing infected cells beyond the 9-h time point. Our supershift analyses confirmed that this NF-κBn activity induced in WT MEFs consisted of RelA:p50 (Fig. 2B). Accordingly, Rela−/− cells as well as Rela−/− Nfkb1−/− 3T3 cells, which lacked expression of both RelA and p50, were unable to produce the NF-κBn activity upon CHPV infection (Fig. 2C). Moreover, this CHPV-induced NF-κB activity temporally coincided with the NEMO-IKK activity and IκBα degradation in WT MEFs (Fig. 2D and E). Consistently, genetic deficiency of the canonical signal transducer NEMO or IKK2 abrogated completely NF-κBn induction upon CHPV infection in immortalized 3T3 cells (Fig. 2F). Our immunoblot analyses further revealed that CHPV infection stimulated IRF3 phosphorylation in WT MEFs and that RelA deficiency did not discernibly impact the virus-induced IRF3 activity (Fig. 2G). Our analyses formally established that CHPV, akin to most other prototypical RNA viruses, engaged the canonical NF-κB pathway and triggered RelA:p50 activity.
FIG 2.
CHPV infection triggers the canonical RelA NF-κB signaling pathway. (A) EMSA revealing the nuclear NF-κB activity (top panel, indicated by an arrow) induced in a time course in WT MEFs infected with CHPV at an MOI of 2. Briefly, cells were harvested at the indicated times postinfection, and nuclear extracts were prepared and examined for the presence of NF-κB DNA binding activity using a DNA probe containing a κB site. Oct1 DNA binding served as a loading control (bottom panel). The nuclear extract from tumor necrosis factor (TNF)-treated cells was used as a positive control. The NF-κB activity from four independent experiments was quantified by densitometric analyses and is presented in the bar plot as means ± SEM. (B) Supershift analysis revealing the composition of the NF-κB dimers activated at 9 h post-CHPV infection in WT MEFs. #, supershifted bands. Anti-p50 antibody actually ablated the NF-κB DNA binding activity in an EMSA. (C and F) NF-κB activities induced upon CHPV infection in WT 3T3 cells and in various knockout 3T3 cells devoid of one or more constituents of the canonical NF-κB pathway. The NF-κB activity from three independent experiments was quantified by densitometric analyses and is presented in the accompanying bar plots as means ± SEM. (D) Time course analyses revealing the NEMO-IKK2 activity induced upon CHPV infection at an MOI of 5 in primary WT MEFs. Extracts from tumor necrosis factor (TNF)-treated cells were used as positive controls. Briefly, NEMO coimmunoprecipitates derived from CHPV-infected MEFs were incubated with recombinant glutathione S-transferase-IκBα and [γ-32P]ATP; the resultant mixture was resolved by SDS-PAGE. Coimmunoprecipitated IKK1 served as a loading control. (E) WT and Rela−/− MEFs were infected with CHPV at an MOI of 5 and harvested at the indicated times, and whole-cell extracts were subjected to immunoblot analyses. GAPDH served as a loading control. The bottom panel shows densitometric analysis of the relative abundance of IκB proteins quantified from three independent experiments. (G) Whole-cell extracts derived from CHPV-infected (MOI of 5) WT and Rela−/− MEFs were analyzed for the abundance of phospho-IRF3 (p-IRF3) and IRF3 by immunoblotting. The bottom panels show the abundances of p-IRF3 and IRF3 proteins quantified from three independent experiments; data are presented as means ± SEM. IP, immunoprecipitation; IB, immunoblotting; U, untreated; ss-Ab, supershift antibody; KA, kinase assay.
Lack of RelA prevents antiviral gene expression in response to CHPV infection.
RelA is capable of activating genes with opposing biological functions. For example, RelA generally induces the expression of proinflammatory cytokines. However, RelA is also known to activate the expression of anti-inflammatory cytokines, such as interleukin-10, in specific cell types (23). Similarly, RelA stimulates the transcription of genes encoding important prosurvival factors, including Bcl2, cellular FLIP (cFLIP), and cellular inhibitor of apoptosis proteins (cIAPs), in a wide variety of cells, including cancerous cells. Curiously, RelA also upregulates the expression of FAS receptor and sensitizes spontaneous sarcoma and colon carcinoma cells to FasL-mediated apoptosis (24). It is thought that the physiological and cellular context tunes NF-κB-driven gene expression. We asked if CHPV altered RelA-mediated controls of immune response genes, including those encoding interferons.
Our RT-qPCR analyses revealed a robust, 3-log increase in the abundance of IFN-β mRNA in WT MEFs at 9 h post-CHPV infection (Fig. 3A), whereas RelA deficiency severely diminished IFN-β expression. Autocrine and paracrine signals by type I interferons activate the expression of ISGs in virus-infected cells. Accordingly, CHPV infection led to gradual accumulation of mRNAs encoding ISG15 and OAS-1 in WT MEFs (Fig. 3B). On the other hand, Rela−/− MEFs were defective for the expression of these genes. We also measured the abundance of mRNA encoding the antiviral protein ISG56, whose expression is induced by IRF3 but not by RelA (25). Type I interferons also act as potent inducers of ISG56 expression. We indeed noticed an almost equivalent abundance of ISG56 mRNA in WT and Rela−/− MEFs at 6 h post-CHPV infection (Fig. 3B). However, the ISG56 mRNA level was significantly reduced in RelA-deficient cells at 9 h postinfection, presumably owing to weakened autocrine type I IFN signaling. Virus-infected cells also produce proinflammatory chemokines and cytokines, which direct effector immune cells, including CD8+ T cells and natural killer T cells, at the site of infection. Corroborating earlier studies showing a role of RelA in proinflammatory gene expression, genes encoding CXCL10 and CXCL16 were induced in WT but not in RelA-deficient MEFs (Fig. 3C). Furthermore, WT MEFs infected with CHPV upregulated mRNAs encoding various prosurvival factors, such as cIAP2, MnSOD, and TRAF1; these genes were not activated in Rela−/− cells (Fig. 3D). Of note, the abundances of cFOS mRNA, whose expression involves NF-κB-independent mechanisms, were not discernibly different between CHPV-infected WT and RelA-deficient MEFs (Fig. 3E). Taking these results together, well-articulated transcriptional properties of RelA linked to its immune-activating and prosurvival functions were preserved in CHPV-infected cells.
FIG 3.

Investigating RelA NF-κB driven gene expression in CHPV-infected cells. RT-qPCR analyses revealed CHPV-induced expression levels of mRNAs encoding IFN-β (A) or various ISGs (B), immune-activating chemokines (C), and prosurvival factors (D) in a time course in WT and Rela−/− MEFs infected at an MOI of 2. (E) The expression of cFOS mRNA, which is encoded by an NF-κB-insensitive gene, was also scored. The abundance of mRNAs was normalized to that of beta-actin mRNA. Data represent means of four biological replicates ± SEM. The data corresponding to c-FOS, ISG15, and OAS-1 mRNAs represent three replicates. *, P ≤ 0.05; **, P ≤ 0.01; ns, not significant (paired two-tailed Student's t test).
RelA deficiency exacerbates cell death processes in CHPV-infected MEFs.
Cell death abolishes the replicative niche of viruses and thereby serves as a host defense mechanism (26). Cells infected with VSV and SeV activate the intrinsic apoptotic pathway where mitochondrial translocation of an IRF3-Bax complex causes cytochrome c release, caspase 9 activation, and subsequent caspase 3-mediated cell death (27). This cell death mechanism does not require transcriptionally active IRF3 and yet restrains effectively viral multiplication in vivo. RNA viruses also engage the extrinsic cell death pathway, which involves FAS-associated death domain (FADD)-mediated caspase 8 activation (5, 28). In addition, recognition of influenza A virus (IAV) by cytosolic DNA-dependent activator of IRFs (DAI) stimulates receptor-interacting serine/threonine-protein kinase 3 (RIPK3) (29–31). RIPK3 engages parallel cell death pathways: a kinase activity-independent RIPK3 function reinforces extrinsic cell death signaling, while RIPK3 also phosphorylates mixed-lineage kinase domain-like pseudokinase (MLKL), which promotes necroptosis. DAI-deficient mice fail to confine IAVs and succumb to infections (30). VSV infections also trigger MLKL-dependent necroptosis (32). Viruses tend to counteract infection-induced cell death for promoting their growth. In our experiments, RelA upregulated prosurvival factors in CHPV-infected cells. We asked if RelA indeed suppressed cell death processes in CHPV-infected cells.
Our immunoblot analyses demonstrated that CHPV induced processing of procaspase 3 to caspase 3 in WT MEFs at 9 hpi (Fig. 4A). CHPV also promoted phosphorylation of MLKL at this time point. RelA deficiency not only accelerated the accumulation of caspase 3 and phosphorylation of MLKL in infected cells but also augmented their abundance. CHPV did not alter the abundance of total MLKL, RIPK1, and RIPK3 in either WT or Rela−/− MEFs. Our fluorescence-activated cell sorting (FACS) analyses revealed that CHPV infection of WT MEFs for 12 h only subtly increased the frequency of annexin V-positive (annexin V+) cells, which bear compromised membrane structures (Fig. 4B). RelA deficiency produced ∼12.0% annexin V+ cells within 9 h of infection and more than 20% annexin V+ cells at 12 hpi. At these early time points, however, we were unable to detect either propidium iodide-positive (PI+) or annexin V+ PI+ cells that completely lack membrane integrity. Nonetheless, microscopic examination demonstrated discernible cytopathic effects at 12 hpi in Rela−/−, but not WT, MEFs (Fig. 4C). Crystal violet staining confirmed that CHPV triggered relatively rapid death of Rela−/− MEFs with less than 25% viable cells at 24 hpi and ∼15% live cells at 36 hpi (Fig. 4D). WT MEFs showed substantial delay in infection-induced death with close to 70% viable cells at 24 hpi; however, prolonged infection reduced the cell viability to ∼20% at 36 hpi. Collectively, CHPV infection activated both apoptotic and necroptotic pathways that culminated in cell death, and RelA suppressed these infection-induced cell death processes.
FIG 4.
Charting cell death processes in CHPV-infected WT and Rela−/− MEFs. (A) Immunoblot analysis of whole-cell extracts derived from WT and Rela−/− MEFs infected with CHPV at an MOI of 5 for the indicated times using antibodies against caspase 3, p-MLKL, MLKL, RIPK1, RIPK3, and GAPDH. (B) CHPV-infected (MOI of 2) WT and Rela−/− MEFs were stained with FITC/annexin V and PI before being subjected to FACS analysis. Uninfected cells were used as controls. In the top panel, representative dot plots show the prevalence of annexin V+, PI+, and annexin V+ PI+ cells in the uninfected and infected populations. In the bottom panel, the bar diagram reveals early cell death quantified from six biological replicates, corrected for corresponding basal cell death and presented as means ± SEM. The data corresponding to the 3-h and 6-h time points represent three replicates. (C) Representative light microscope images capturing CHPV-mediated cell-rounding effects in WT and Rela−/− MEFs. (D) Infection-induced cell death at late time points was determined using crystal violet staining, and results are presented relative to basal cell death measured using uninfected MEFs. The plot represents the average of three biological replicates ± SEM. *, P ≤ 0.05; **, P ≤ 0.01 (paired two-tailed Student's t test).
Suppressing cell death processes rescue CHPV multiplication in RelA-deficient MEFs.
Next, we examined if suppressing apoptotic and necroptotic cell death processes could restore CHPV propagation in Rela−/− MEFs. To this end, we treated RelA-deficient cells for 1 h with the pan-caspase inhibitor zVAD (benzyloxycarbonyl-Val-Ala-Asp- fluoromethylketone) either alone or in combination with inhibitors of the RIPK3 kinase activity GSK843 or GSK872. Then these cells were infected with CHPV in the continuing presence of these inhibitors. Our immunoblot analyses revealed that zVAD treatment prevented the accumulation of mature caspase 3 in Rela−/− MEFs (Fig. 5A). As described previously, inhibition of caspases enhanced the phosphorylation of MLKL in these cells (33). Use of RIPK3 kinase inhibitors along with zVAD abrogated both caspase activation and MLKL phosphorylation. Of note, the RIPK3 kinase pathway was shown to produce both annexin V+ and annexin V+ PI+ cells during the course of Staphylococcus aureus infection (34). We found that zVAD alone was insufficient and that a combinatorial treatment with zVAD and GSK843 was necessary for preventing the accumulation of annexin V+ cells undergoing death at 12 h post-CHPV infection of RelA-deficient MEFs (Fig. 5B and C). Notably, this combinatorial regime enhanced viral gene expression, augmenting the abundance of genome RNA as well as N and P mRNAs at 9 hpi (Fig. 5D), and CHPV propagation in Rela−/− MEFs (Fig. 5E). Our analyses suggested broadly that caspases and MLKL cooperatively induce death of CHPV-infected cells and that RelA promoted viral RNA syntheses and multiplication by restraining infection-inflicted cell death processes.
FIG 5.
Investigating the effect of cell death inhibitors on CHPV growth in Rela−/− MEFs. (A) WT and Rela−/− MEFs were infected in the presence of zVAD(OH)-FMK or in the concomitant presence of zVAD(OH)-FMK and GSK843 or zVAD(OH)-FMK and GSK872. Cells were harvested at 12 hpi and subjected to immunoblot analyses using indicated antibodies. (B and C) Primary MEFs were infected as described for panel A and then were examined for the presence of annexin V+ cells or PI+ cells or both using FACS. A representative dot plot of FACS analysis is shown in panel B. The bar diagram represents means of quantified data from four independent biological replicates ± SEM. (D and E) MEFs of indicated genotypes were infected with CHPV at an MOI of 2 in the absence or presence of cell death inhibitors. The relative abundance of viral genomic RNA as well as N and P mRNAs was determined using RT-qPCR (D). Progeny virus yield in the culture supernatant at 24 hpi was measured by the plaque assay (E). The data represent the average of four (D) or three (E) biological replicates ± SEM. **, P ≤ 0.01; ***, P ≤ 0.001 (paired two-tailed Student's t test). DMSO, dimethyl sulfoxide.
Although CHPV infection activated caspase 3 and MLKL in Rela−/− MEFs within 6 h (Fig. 4A), it did not produce detectable PI+ dead cells at early time points (Fig. 4B). However, transcription and replication of the CHPV genome were substantially attenuated in RelA-null cells even at 6 hpi (Fig. 1C). We propose that RelA potentiated CHPV growth involving two related mechanisms. For one, RelA safeguarded the replicative niche of this intracellular pathogen by inhibiting cell death per se. RelA also modulated viral RNA syntheses presumably by protecting viral gene expression machinery from the detrimental effects of caspases and MLKL. Indeed, cellular caspases were shown to cleave Crimean-Congo hemorrhagic fever virus nucleoproteins, which play an essential role in viral replication (35). Despite the reduced abundance of CHPV N and P proteins in RelA-deficient cells, we were unable to detect fragmented viral proteins in our experiments. Future studies should further characterize how cell death mediators regulate CHPV gene expression at early time points in an infection time course.
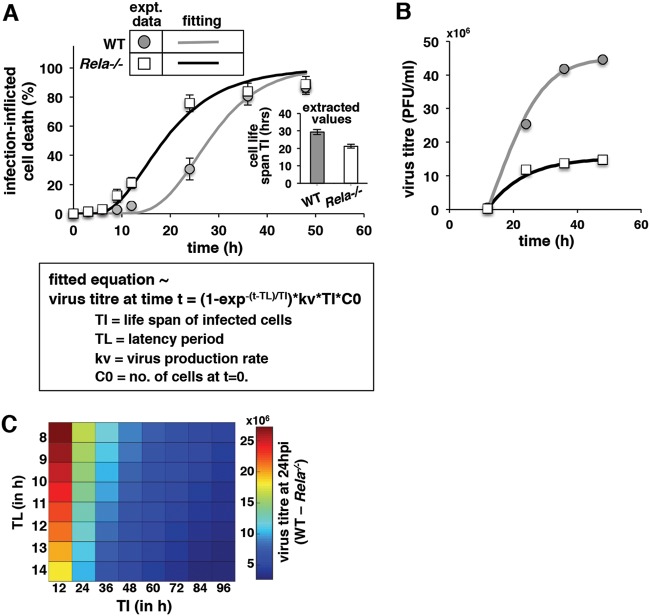
Mathematical studies revealing a quantitative constraint underlying RelA-dependent viral propagation.
Mathematical analyses of viral growth kinetics often offer valuable mechanistic insight into RNA virus pathogenesis (36, 37). Using mathematical formalism, we set out to probe quantitatively the dependence of CHPV growth on RelA-sensitive cell death events. Given that our study involved high MOIs, we assumed that all cells were infected by viruses at the start of the experiment. We also assumed that the time to virus-induced cell death from the infection (TI) followed a lognormal distribution, with mean TI and standard deviation σI. Fitting the cumulative density function of the lognormal distribution to the experimental data related to the fraction of cell death measured over time, we estimated a TI of 29 ± 0.93 h for WT cells and a TI of 21 ± 0.75 h for RelA-deficient cells (Fig. 6A). To compute virus production, we considered a latent period, TL, from the start of the infection when there was no virus production and assumed that virus production subsequently occurred with a constant rate, kv, until the time of cell death, TI. Using experimental data related to virus titer measured over time in our fitting exercise, we estimated a TL of 10.5 h for WT as well as RelA-deficient cells, and kv values of 2.6 ± 0.17 PFU/cell/h and of 1.6 ± 0.21 PFU/cell/h for WT and RelA-deficient cells, respectively (Fig. 6B). Extracted values of kv and TI reinforced the notion that both reduced CHPV production and exacerbated cell death diminished viral yield in RelA-null cells. Finally, we computed virus titer at 24 hpi for a range of values corresponding to the mean time to virus-induced cell death, TI. and the latency period, TL. For that we assumed that the TI values of WT and RelA-null cells varied proportionately. Given that accelerated virus production is often associated with reduced life span of infected cells, the product of TI and kv was also assumed to be constant for individual WT and RelA-null systems. Intriguingly, our analyses indicated that increased values of TI, and less prominently of TL, diminished the impact of RelA deficiency on viral yield (Fig. 6C). Because moderately cytopathic viruses typically cause delayed cell death, we reasoned that RelA would be largely superfluous for the growth of moderately cytopathic viruses but would be required for the growth of highly cytopathic viruses.
FIG 6.
Mathematically probing the kinetics of CHPV propagation. (A and B) Fitting (solid line) mathematical formulations with experimental time course data (discrete points) related to infection-inflicted cell death and virus titer. The best data fit has been presented in both cases. The corresponding equation is also depicted. As discussed, cell death up to 12 hpi was estimated by FACS, and the data point beyond 12 h was acquired using a crystal violet assay. (C) Computing the difference in the virus titers at 24 hpi between WT and RelA-deficient cells as a function of the time to infection-induced cell death (TI) and the latency period (TL). We assumed that the TI values of WT and RelA-null cells varied proportionately; the TI values of the WT have been plotted in the x axis. In addition, the product of TI and kv was kept constant for the individual WT and RelA-null cells.
RelA selectively promotes the growth of highly cytopathic RNA viruses.
We then investigated if RelA indeed augmented the yield of other cytopathic RNA viruses. To this end, we first compared two important human pathogens, CHIKV and JEV (38–40), for their ability to induce cell death. Our analyses revealed that CHIKV infection at an MOI of 2 caused ∼25% death of WT MEFs and a robust 70% death of Rela−/− cells within 24 hpi (Fig. 7A). However, JEV infections at an MOI of 2 did not trigger discernible cell death at 24 hpi in either WT or RelA-deficient MEFs. Therefore, CHIKV, but not JEV, showed substantial cytopathic effects in our MEF-based analyses. Corroborating our studies involving CHPV, we noticed a significant reduction in the titer of progeny CHIKV particles in Rela−/− MEFs compared to that in WT cells (Fig. 7B). RelA deficiency had no impact on the progeny JEV yield in MEFs. Low-MOI regimes, such as those used previously in VSV infection studies (16), entail the transmission of viruses from the infected cells to neighboring cells that is inhibited by IFN-β. Not surprisingly, those studies identified interferon-inducing RelA as an antiviral molecule. Indeed, when we infected MEFs with CHPV at the lower MOI of 0.01, the progeny virus titers were not significantly different between WT and Rela−/− MEFs at 24 hpi, and, if anything, RelA-deficient cells produced close to 10-fold more virus at 36 hpi (Fig. 7C). When used at a high MOI, however, RNA viruses infected almost all the cells in a culture shortly after inoculation. We argue that high-MOI regimes rendered type I interferons less relevant and interferon-regulatory antiviral properties of RelA unimportant. Instead, prosurvival RelA functions played a dominant role and supported the growth of RNA viruses by delaying infection-inflicted cell death. However, RelA was selectively required for the propagation of cytopathic RNA viruses, and moderately cytopathic RNA viruses did not require prosurvival RelA functions for their growth. In other words, cell death, and consequently RelA, played only a minor role in the propagation of moderately cytopathic viruses. Future studies ought to compare side-by-side cytopathicity levels of different viruses in physiologically relevant cell types, including macrophages and neurons, and determine critically the role of RelA in viral pathogenicity.
FIG 7.
Studying CHIKV and JEV infections in RelA-deficient cells. (A and B) WT and Rela−/− MEFs were infected with CHIKV or JEV at an MOI of 2. At 24 hpi, infection-induced cell death was determined using crystal violet staining, and progeny virus yield was measured by plaque assay. The data represent the average of three biological replicates ± SEM. (C) WT and Rela−/− MEFs were infected with CHPV at an MOI of 0.01, and progeny virus yield was measured. Data represent means of three biological replicates ± SEM. (D) The proposed model explaining how RelA NF-κB promotes the multiplication of cytopathic RNA viruses, particularly CHPV, by restraining infection-induced cell death processes. *, P ≤ 0.05; **, P ≤ 0.01; ns, not significant.
RNA viruses and pleiotropic transcription factors.
Viruses have evolved mechanisms for preventing the death of infected cells, which serve as their replicative niche. DNA viruses typically encode prosurvival factors in their genome. For example, poxviruses express serpins, which inhibit the activity of cellular caspases (41). Cytomegaloviruses utilize virally encoded inhibitors of Bax and Bak, which trigger the intrinsic apoptotic pathway, for preventing cell death (42). Additionally, murine cytomegalovirus (MCMV)-encoded M45 protein diminishes RIPK3-dependent necroptosis of infected cells (43). Oncogenic human herpesvirus 8, which is associated with Kaposi's sarcoma, recruits a viral analogue of prosurvival factor cFLIP (44). RNA viruses normally do not encode specialized prosurvival factors, presumably owing to relatively shorter genome lengths. Cells infected with viruses, including RNA viruses, elicit RelA NF-κB activity, which upregulates the expression of antiviral type I interferons. Our analyses revealed a rather counterintuitive proviral function of RelA, particularly in the context of high-MOI infection with cytopathic RNA viruses, such as CHPV and CHIKV. As such, RelA represents a pleiotropic transcription factor. In addition to activating the expression of IFN-β, RelA also mediates the expression of genes encoding prosurvival factors. Indeed, RelA was shown to be capable of suppressing apoptotic as well as necroptotic cell death in response to a variety of death-inducing agents (14, 20). Our investigation revealed that CHPV engaged both apoptotic and necroptotic pathways for inducing cell death and that RelA restrained death of CHPV-infected cells. We further identified that RelA-mediated suppression of infection-inflicted cell death augmented the yield of CHPV and CHIKV (Fig. 7D). Therefore, RNA viruses, which do not encode specialized prosurvival factors, appear to exploit pleiotropic transcription factors for extending the life span of infected cells that promote the growth of these viruses as well. Our study also suggested a contrast between two seemingly related transcription factors, RelA and IRF3, in the context of RNA virus pathogenesis. IRF3 employs two distinct mechanisms for preventing viral propagation. It not only induces the expression of type I interferons in collaboration with RelA but also triggers Bax-dependent cell death. RelA seems to play a dual role in the context of RNA virus infections. In a cell-extrinsic mechanism, RelA prevents infection of neighboring cells by activating the expression of IFN-β. On the other hand, cell-autonomous RelA activity amplifies the viral yield by extending the life span of infected cells. In conclusion, our finding not only necessitates a revision of our understanding of NF-κB functions during the course of RNA virus infections but also bears significance for therapeutic regimes which alter NF-κB activities while alleviating the viral load.
MATERIALS AND METHODS
Cells and viruses.
WT and gene-deficient C57BL/6 mice were used in accordance with the guidelines of the Institutional Animal Ethics Committee of NII (approval no. 380/15). As described earlier (45), MEFs generated from embryonic day 13.5 (E13.5) embryos were used either as primary cells or subsequent to their immortalization by the NIH 3T3 protocol. Unless otherwise mentioned, experiments were performed using primary MEFs. The following RNA virus strains were used, adhering to the guidelines of the institutional biosafety committees: CHPV strain 653514 (obtained from the National Institute of Virology, Pune), JEV strain Vellore-P20778, and a clinical isolate of CHIKV strain 119067 (40). CHPV and JEV were propagated in Vero cells, and BHK21 cells were used for growing CHIKV. The RelA transgene was expressed from pBabe.puro upon retroviral transduction of Rela−/− 3T3 cells. Lentivirus particles were produced in 293T cells using shRNA constructs from GE Dharmacon, USA. We used V3LHS_633767 (shRNA 1) and V3LHS_300422 (shRNA 2) lentiviral short hairpin microRNA-adapted RNA (shRNAmir) constructs for the knockdown of RelA expression. We used the RHS4346 GIPZ lentiviral shRNAmir construct as a nonsilencing control. THP-1 cells were transduced with lentiviral particles and selected with puromycin, as described earlier (46).
Virus infection studies.
Semiconfluent cultures of MEFs were incubated with CHPV, JEV, or CHIKV for 1.5 h in serum-free medium. Subsequent to viral adsorption, cells were washed and replenished with medium containing 10% bovine calf serum (BCS). At various time points postinfection, either the culture supernatants were collected, or the cells were harvested for further analyses. THP-1 cells were infected similarly with CHPV, albeit 2 weeks after lentiviral transduction.
Viral plaque assay.
Culture supernatants were collected from infected cells at various times postinfection and analyzed for the presence of progeny virus particles using the plaque assay. Briefly, monolayers of Vero E6 (for CHPV and CHIKV) or PS (for JEV) cells were infected with serial dilutions of the culture supernatants. After 60 min of incubation, inocula were removed, and cells were washed with phosphate-buffered saline (PBS) and overlaid with Dulbecco’s modified Eagle’s medium (DMEM) containing 1% low-melting-point agarose and 5% fetal calf serum (FCS). After another 24 to 72 h of incubation, cells were fixed with formaldehyde and stained with crystal violet for visualizing viral plaques.
Biochemical analyses.
MEFs were harvested at different times postinfection; whole-cell extracts and nuclear extracts were analyzed by immunoblotting and EMSA, respectively (45). NF-κB-related antibodies have been described earlier (45). CHPV-related anti-N, anti-M, and anti-P antibodies were obtained as a gift from National Institute of Virology (NIV), Pune, India. Antibodies against p-IRF3 (catalog no. 4947), IRF3 (4302), caspase 3 (9665), p-MLKL (62233), MLKL (37705), RIPK3 (95702), RIPK1 (3493), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (2118) were from Cell Signaling Technologies (Beverly, MA, USA). The gel images were acquired using PhosphorImager (GE Amersham, UK) and quantified using ImageQuant, version 5.2. The IKK2 kinase assay was performed as described previously (47).
RNA analyses.
Total RNA was isolated from infected MEFs, and RT-qPCR analyses were conducted, as described previously (45). The following primers were used in this study: for the CHPV genome, CGAGTGAACTCAGTTGCAGAG (forward) and GAATCGAGAGTGTCCTGAAGC (reverse); CHPV-N, GATTTGTTGCGGATGATGAC (forward) and CCAGAAATGGAAACTGGGAT (reverse); CHPV-P, CTCTCCGTCTGATCCACCTT (forward) and TCAATCCAGCAATGACCAGT (reverse); mIFNβ, CCGGACTTCAAGATCCCTATGGA (forward) and TGGCAAAGGCAGTGTAACTCTTC (reverse); ISG-15, AGCTCCATGTCGGTGTCAG (forward) and GAAGGTCAGCCAGAACAGGT (reverse); OAS-1, AGGGGCATTTGCTGCTCTGC (forward) and GGGCACCTGCTGTGGTTTATTG (reverse); CXCL10, AGACATCCCGAGCCAACCTT (forward) and GTTAAGGAGCCCTT TTAGAC (reverse); CXCL16, TGGAACTGGTCATGGGAAGAG (forward) and GGGTACTGGCTTGAGGCAAA (reverse); cIAP2, GAAGTGGGCTGCGGTATCA (forward) and GCGCTGTCTTGAACCATGTTC (reverse); MnSOD, TCAATGGTGGGGGACATATT (forward) and GCTTGATAGCCTCCAGCAAC (reverse); TRAF1, GGAGGCATCCTTTGATGGTA (forward) and AGGGACAGGTGGGTCTTCTT (reverse); cFOS, CCTTCGGATTCTCCGTTTCTCT (forward) and TGGTGAAGACCGTGTCAGGA (reverse); ISG56, GTCCGGTTAAATCCAGAAGATCC (forward) and TAGCTTTGGCAAGATGTGCTG (reverse); actin, CCAACCGTGAAAAGATGAC (forward) and GTACGACCAGAGGCATACAG (reverse).
Cell death studies.
Infection-inflicted early cell death was quantified by FACS analyses subsequent to staining of MEFs with fluorescein isothiocyanate (FITC)/annexin V and PI using an apoptosis detection kit from BD Biosciences (catalog no. 556547). Data were obtained using VERSE (BD Biosciences, NJ, USA) and analyzed in FlowJo, version 9.8.3. Infection-induced cell death at late time points was determined by crystal violet staining, as described earlier (48). In certain experiments, cells were incubated with various cell death inhibitors, including zVAD(OH)-FMK [Z-Val-Ala-Asp-(OH)-fluoromethyl ketone, catalog no. 14467; Cayman Chemicals, MI, USA], GSK843 (4898; Aobious, MA, USA), and GSK872 (2673; Biovision, CA, USA) for 1 h before virus infections.
Statistical analysis.
Error bars are shown as standard errors of the means (SEM) of three or more experimental replicates. Quantified data are means ± SEM, and two-tailed a Student's t test was used for verifying statistical significance.
ACKNOWLEDGMENTS
We thank D. Chattopadhyay, Amity University, Kolkata, for insightful discussions and help with reagents. We thank V. Kumar, Systems Immunology Laboratory (SIL), National Institute of Immunology (NII), for technical help, and P. Nagarajan from SAF, NII, for help with animal husbandry.
Virus research in the principal investigator’s laboratory is funded by a research grant from the Department of Biotechnology (DBT) and support from NII-core. S.B. is a recipient of the S. N. Ramachandran National Bioscience Award for Career Development award. S.S.B. and Y.R. thank the Council of Scientific and Industrial Research (CSIR) and DBT, respectively, for research fellowships.
S.S.B. carried out the wet laboratory experiments with the help from Y.R., N.A.K., and P.K.K. and supervision from S.T., G.M., and S.B. A.S. conducted the in silico analyses with the help from R.P. S.B. conceived and supervised the overall research. S.S.B. wrote the manuscript with S.B.
We have no conflicts of interest to declare.
REFERENCES
- 1.Dye C. 2014. After 2015: infectious diseases in a new era of health and development. Philos Trans R Soc Lond B Biol Sci 369:20130426. doi: 10.1098/rstb.2013.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marston HD, Folkers GK, Morens DM, Fauci AS. 2014. Emerging viral diseases: confronting threats with new technologies. Sci Transl Med 6:253ps10. doi: 10.1126/scitranslmed.3009872. [DOI] [PubMed] [Google Scholar]
- 3.Menghani S, Chikhale R, Raval A, Wadibhasme P, Khedekar P. 2012. Chandipura virus: an emerging tropical pathogen. Acta Trop 124:1–14. doi: 10.1016/j.actatropica.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Basak S, Mondal A, Polley S, Mukhopadhyay S, Chattopadhyay D. 2007. Reviewing Chandipura: a vesiculovirus in human epidemics. Biosci Rep 27:275–298. doi: 10.1007/s10540-007-9054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh S, Dutta K, Basu A. 2013. Chandipura virus induces neuronal death through Fas-mediated extrinsic apoptotic pathway. J Virol 87:12398–12406. doi: 10.1128/JVI.01864-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlier D, Lyles DS. 2011. Interplay between innate immunity and negative-strand RNA viruses: towards a rational model. Microbiol Mol Biol Rev 75:468–490. doi: 10.1128/MMBR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scutigliani EM, Kikkert M. 2017. Interaction of the innate immune system with positive-strand RNA virus replication organelles. Cytokine Growth Factor Rev 37:17–27. doi: 10.1016/j.cytogfr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 13:539–548. doi: 10.1016/S1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen H-W, King K, Tu J, Sanchez M, Luster AD, Shresta S. 2013. The roles of IRF-3 and IRF-7 in innate antiviral immunity against dengue virus. J Immunol 191:4194–4201. doi: 10.4049/jimmunol.1300799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, TenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. 2003. IKKepsilon and TBKI are essential components of the IRF3 signalling pathway. Nat Immunol 4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin R, Sharma S, Zou W, Sun Q, Grandvaux N, Julkunen I, Hemmi H, Akira S, Yeh W, Lin R, Hiscott J, Yamamoto M. 2004. Activation of TBK1 and IKK ε kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J Virol 78:10636–10649. doi: 10.1128/JVI.78.19.10636-10649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredericksen BL, Smith M, Katze MG, Shi P-Y, Gale MJ. 2004. The host response to West Nile virus infection limits viral spread through the activation of the interferon regulatory factor 3 pathway. J Virol 78:7737–7747. doi: 10.1128/JVI.78.14.7737-7747.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu C, Wu X, Zhang X, Xie Q, Fan C, Zhang H. 2018. Embryonic lethality and host immunity of RelA-deficient mice are mediated by both apoptosis and necroptosis. J Immunol 200:271–285. doi: 10.4049/jimmunol.1700859. [DOI] [PubMed] [Google Scholar]
- 15.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Basagoudanavar SH, Wang X, Hopewell E, Albrecht R, Garcia-Sastre A, Balachandran S, Beg AA. 2010. NF- κB RelA subunit is crucial for early IFN-β expression and resistance to RNA virus replication. J Immunol 185:1720–1729. doi: 10.4049/jimmunol.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor KE, Mossman KL. 2013. Recent advances in understanding viral evasion of type I interferon. Immunology 138:190–197. doi: 10.1111/imm.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bais SS, Ratra Y, Kushawaha PK, Basak S. 2019. Chandipura virus requires pro-survival RelA NF-κB function for its propagation. bioRxiv 10.1101/509893. [DOI] [PMC free article] [PubMed]
- 19.Nikitina E, Larionova I, Choinzonov E, Kzhyshkowska J. 2018. Monocytes and macrophages as viral targets and reservoirs. Int j Mol Sci 19:E2821. doi: 10.3390/ijms19092821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell S, Vargas J, Hoffmann A. 2016. Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med 8:227–241. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin J, Hu H, Li HS, Yu J, Xiao Y, Brittain GC, Zou Q, Cheng X, Mallette FA, Watowich SS, Sun SC. 2014. Noncanonical NF-κB pathway controls the production of type I interferons in antiviral innate immunity. Immunity 40:342–354. doi: 10.1016/j.immuni.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basak S, Shih V-S, Hoffmann A. 2008. Generation and activation of multiple dimeric transcription factors within the NF-κB signaling system. Mol Cell Biol 28:3139–3150. doi: 10.1128/MCB.01469-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosser DM, Zhang X. 2008. Interleukin-10: new perspectives on an old cytokine. Immunol Rev 226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, Bardhan K, Yang D, Thangaraju M, Ganapathy V, Waller JL, Liles GB, Lee JR, Liu K. 2012. NF-κB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression. J Biol Chem 287:25530–25540. doi: 10.1074/jbc.M112.356279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond MS, Farzan M. 2013. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol 13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orzalli MH, Kagan JC. 2017. Apoptosis and necroptosis as host defense strategies to prevent viral infection. Trends Cell Biol 27:800–809. doi: 10.1016/j.tcb.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chattopadhyay S, Kuzmanovic T, Zhang Y, Wetzel JL, Sen GC. 2016. Ubiquitination of the transcription factor IRF-3 activates RIPA, the apoptotic pathway that protects mice from viral pathogenesis. Immunity 44:1151–1161. doi: 10.1016/j.immuni.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearce AF, Lyles DS. 2009. Vesicular stomatitis virus induces apoptosis primarily through Bak rather than Bax by inactivating Mcl-1 and Bcl-XL. J Virol 83:9102–9112. doi: 10.1128/JVI.00436-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesavardhana S, Kuriakose T, Guy CS, Samir P, Malireddi RKS, Mishra A, Kanneganti T-D. 2017. ZBP1/DAI ubiquitination and sensing of influenza vRNPs activate programmed cell death. J Exp Med 214:2217–2229. doi: 10.1084/jem.20170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thapa RJ, Ingram JP, Ragan KB, Nogusa S, Boyd DF, Benitez AA, Sridharan H, Kosoff R, Shubina M, Landsteiner VJ, Andrake M, Vogel P, Sigal LJ, TenOever BR, Thomas PG, Upton JW, Balachandran S. 2016. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe 20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Kumari S, Kim C, Van TM, Wachsmuth L, Polykratis A, Pasparakis M. 2016. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 540:124–128. doi: 10.1038/nature20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Jiang W, Yan Y, Gong T, Han J, Tian Z, Zhou R. 2014. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol 15:1126–1133. doi: 10.1038/ni.3015. [DOI] [PubMed] [Google Scholar]
- 33.Nogusa S, Thapa RJ, Dillon CP, Liedmann S, Oguin TH, Ingram JP, Rodriguez DA, Kosoff R, Sharma S, Sturm O, Verbist K, Gough PJ, Bertin J, Hartmann BM, Sealfon SC, Kaiser WJ, Mocarski ES, López CB, Thomas PG, Oberst A, Green DR, Balachandran S. 2016. RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against influenza A virus. Cell Host Microbe 20:13–24. doi: 10.1016/j.chom.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenlee-Wacker MC, Kremserová S, Nauseef WM. 2017. Lysis of human neutrophils by community-associated methicillin-resistant Staphylococcus aureus. Blood 129:3237–3244. doi: 10.1182/blood-2017-02-766253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlberg H, Tan YJ, Mirazimi A. 2011. Induction of caspase activation and cleavage of the viral nucleocapsid protein in different cell types during Crimean-Congo hemorrhagic fever virus infection. J Biol Chem 286:3227–3234. doi: 10.1074/jbc.M110.149369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahasa KJ, Eladdadi A, De Pillis L, Ouifki R. 2017. Oncolytic potency and reduced virus tumor-specificity in oncolytic virotherapy. A mathematical modelling approach. PLoS One 12:e0184347. doi: 10.1371/journal.pone.0184347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boianelli A, Nguyen VK, Ebensen T, Schulze K, Wilk E, Sharma N, Stegemann-Koniszewski S, Bruder D, Toapanta FR, Guzmán CA, Meyer-Hermann M, Hernandez-Vargas EA. 2015. Modeling influenza virus infection: a roadmap for influenza research. Viruses 7:5274–5304. doi: 10.3390/v7102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turtle L, Solomon T. 2018. Japanese encephalitis—the prospects for new treatments. Nat Rev Neurol 14:298–313. doi: 10.1038/nrneurol.2018.30. [DOI] [PubMed] [Google Scholar]
- 39.Silva LA, Dermody TS. 2017. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest 127:737–749. doi: 10.1172/JCI84417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh H, Mudgal R, Narwal M, Kaur R, Singh VA, Malik A, Chaudhary M, Tomar S. 2018. Chikungunya virus inhibition by peptidomimetic inhibitors targeting virus-specific cysteine protease. Biochimie 149:51–61. doi: 10.1016/j.biochi.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Nichols DB, De Martini W, Cottrell J. 2017. Poxviruses utilize multiple strategies to inhibit apoptosis. Viruses 9:E215. doi: 10.3390/v9080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brune W, Andoniou CE. 2017. Die another day: inhibition of cell death pathways by cytomegalovirus. Viruses 9:249. doi: 10.3390/v9090249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Upton JW, Kaiser WJ, Mocarski ES. 2010. Virus Inhibition of RIP3-dependent necrosis. Cell Host Microbe 7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thurau M, Marquardt G, Gonin-Laurent N, Weinländer K, Naschberger E, Jochmann R, Alkharsah KR, Schulz TF, Thome M, Neipel F, Stürzl M. 2009. Viral inhibitor of apoptosis vFLIP/K13 protects endothelial cells against superoxide-induced cell death. J Virol 83:598–611. doi: 10.1128/JVI.00629-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roy P, Mukherjee T, Chatterjee B, Vijayaragavan B, Banoth B, Basak S. 2017. Non-canonical NFκB mutations reinforce pro-survival TNF response in multiple myeloma through an autoregulatory RelB:p50 NF κB pathway. Oncogene 36:1417–1429. doi: 10.1038/onc.2016.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Migliavacca J, Percio S, Valsecchi R, Ferrero E, Spinelli A, Ponzoni M, Tresoldi C, Pattini L, Bernardi R, Coltella N. 2016. Hypoxia inducible factor-1a regulates a pro-invasive phenotype in acute monocytic leukemia. Oncotarget 7:53540–53557. doi: 10.18632/oncotarget.10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banoth B, Chatterjee B, Vijayaragavan B, Prasad M, Roy P, Basak S. 2015. Stimulus-selective crosstalk via the NF-κB signaling system reinforces innate immune response to alleviate gut infection. Elife 4:e05648. doi: 10.7554/eLife.05648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zong W-x, Bash J, Gélinas C. 1998. Rel blocks both anti-Fas- and TNFα-induced apoptosis and an intact Rel transactivation domain is essential for this effect. Cell Death Differ 5:963–972. doi: 10.1038/sj.cdd.4400441. [DOI] [PubMed] [Google Scholar]