HIV-1 broadly neutralizing antibodies (bNAbs) and engineered antibody-like inhibitors have been compared for their breadths, potencies, and in vivo half-lives. However, a key limitation in the use of antibodies to treat an established HIV-1 infection is the rapid emergence of fully resistant viruses. Entry inhibitors of similar breadths and potencies can differ in the ease with which viral escape variants arise. Here we show that HIV-1 escape from the potent and exceptionally broad entry inhibitor eCD4-Ig is more difficult than that from CD4-Ig or the bNAb NIH45-46. Indeed, full escape was not observed under conditions under which escape from CD4-Ig or NIH45-46 was readily detected. Moreover, viruses that were partially resistant to eCD4-Ig were markedly less infective and more sensitive to antibodies in the serum of an infected person. These data suggest that eCD4-Ig will be more difficult to escape and that even partial escape will likely extract a high fitness cost.
KEYWORDS: CCR5, CD4, HIV-1, eCD4-Ig, viral entry
ABSTRACT
The engineered antibody-like entry inhibitor eCD4-Ig neutralizes every human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus isolate it has been tested against. The exceptional breadth of eCD4-Ig derives from its ability to closely and simultaneously emulate the HIV-1 receptor CD4 and coreceptors, either CCR5 or CXCR4. Here we investigated whether viral escape from eCD4-Ig is more difficult than that from CD4-Ig or the CD4-binding site antibody NIH45-46. We observed that a viral swarm selected with high concentrations of eCD4-Ig was increasingly resistant to but did not fully escape from eCD4-Ig. In contrast, viruses selected under the same conditions with CD4-Ig or NIH45-46 fully escaped from those inhibitors. eCD4-Ig-resistant viruses acquired unique changes in the V2 apex, V3, V4, and CD4-binding regions of the HIV-1 envelope glycoprotein (Env). Most of the alterations did not directly affect neutralization by eCD4-Ig or neutralizing antibodies. However, alteration of Q428 to an arginine or lysine resulted in markedly greater resistance to eCD4-Ig and CD4-Ig, with correspondingly dramatic losses in infectivity and greater sensitivity to a V3 antibody and to serum from an infected individual. Compensatory mutations in the V3 loop (N301D) and in the V2 apex (K171E) partially restored viral fitness without affecting serum or eCD4-Ig sensitivity. Collectively, these data suggest that multiple mutations will be necessary to fully escape eCD4-Ig without loss of viral fitness.
IMPORTANCE HIV-1 broadly neutralizing antibodies (bNAbs) and engineered antibody-like inhibitors have been compared for their breadths, potencies, and in vivo half-lives. However, a key limitation in the use of antibodies to treat an established HIV-1 infection is the rapid emergence of fully resistant viruses. Entry inhibitors of similar breadths and potencies can differ in the ease with which viral escape variants arise. Here we show that HIV-1 escape from the potent and exceptionally broad entry inhibitor eCD4-Ig is more difficult than that from CD4-Ig or the bNAb NIH45-46. Indeed, full escape was not observed under conditions under which escape from CD4-Ig or NIH45-46 was readily detected. Moreover, viruses that were partially resistant to eCD4-Ig were markedly less infective and more sensitive to antibodies in the serum of an infected person. These data suggest that eCD4-Ig will be more difficult to escape and that even partial escape will likely extract a high fitness cost.
INTRODUCTION
In the absence of an effective conventional human immunodeficiency virus type 1 (HIV-1) vaccine, passive immunization strategies have become an increasingly attractive approach for limiting HIV-1 transmission (1–5). Broadly neutralizing antibodies (bNAbs) and engineered antibody-like inhibitors can effectively prevent new HIV-1 or simian-human immunodeficiency virus (SHIV) infections (6–10). They may also be useful for controlling an established infection or removing transiently activated reservoir cells (11–15). However, the diversity of HIV-1 quasispecies in vivo and the ease with which HIV-1 typically escapes single bNAbs or even some bNAb combinations present major hurdles to the use of bNAbs in therapeutic or eradication strategies (6, 14–18). As a result, initial escape from antibody neutralization regularly emerges rapidly, if occasionally linked to fitness costs (14, 19) that could be overcome through additional compensator mutations (17, 20). This rapid escape is a consequence of the high rate of mutation of HIV-1, the plasticity of the heavily glycosylated envelope glycoprotein (Env) surface, and the size of antibody epitopes, which are typically larger than functionally important receptor-binding regions of Env (21, 22).
Multivalent antibody-like inhibitors, particularly those that bind functionally critical regions of Env, can neutralize a wider range of viruses. Increased breadth may also associated with greater difficulty of escape, although these properties are not necessarily linked (16, 20, 23). For example, an antibody may be broad because it is rare in the human population and does not exert selective pressure on its Env epitope. Several multivalent entry inhibitors have been developed (24–29), the broadest of which is the antibody-like molecule eCD4-Ig, a fusion of the well-characterized inhibitor CD4-Ig and a short tyrosine-sulfated coreceptor-mimetic peptide (30). eCD4-Ig neutralized all 270 HIV-1, HIV-2, and simian immunodeficiency virus (SIV) isolates it has been tested against, each with 80% inhibitory concentration (IC80) values of less than 10 μg/ml. This breadth has also been confirmed in vivo; vectored expression of eCD4-Ig fully protected rhesus macaques from SHIV-AD8 and SIVmac239 for more than 1 year, with challenges that infected all control animals (30, 31). The carboxy-terminal coreceptor-mimetic peptide is critical for the breadth and potency of eCD4-Ig. This peptide increases the avidity of eCD4-Ig for HIV-1 Env, and it directly blocks Env association with the coreceptor, impeding the dominant pathway of escape from CD4-Ig.
Therefore, we hypothesized that it would be more difficult to escape eCD4-Ig than CD4-Ig or a CD4-binding-site bNAb. We observed that a SHIV-SF162P3 swarm could not fully escape eCD4-Ig under conditions in which escape variants fully resistant to CD4-Ig and the bNAb NIH45-46 could be readily selected. Moreover, Env mutations that conferred partial resistance to eCD4-Ig were associated with markedly decreased infectivity and greater susceptibility to patient serum. These data highlight an apparently unique property of eCD4-Ig among HIV-1 entry inhibitors that may be critical to its therapeutic applications.
RESULTS
SHIV-SF162P3 escapes NIH45-46 and CD4-Ig more easily than it does eCD4-Ig.
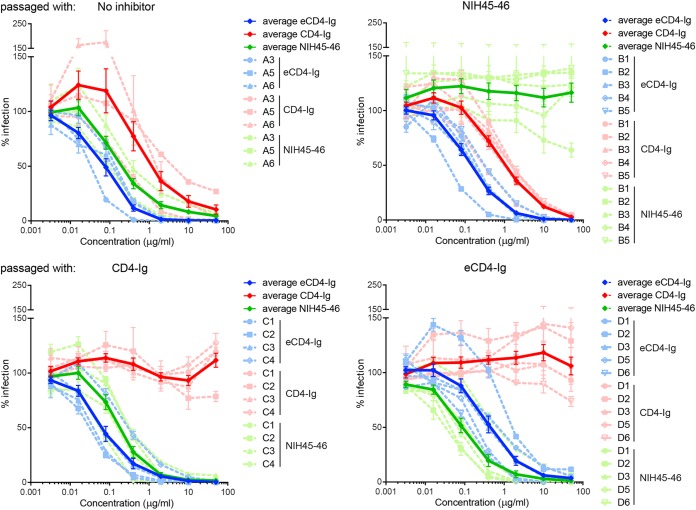
To investigate how HIV-1 escapes from eCD4-Ig, a swarm of SHIV-SF162P3 was passaged on GHOST CCR5+ cells three times over a 9-day period, in the absence of any inhibitor. The resulting diversified viral swarm was subsequently divided into multiple wells and passaged on the same cell line in the presence of eCD4-Ig, CD4-Ig, the bNAb NIH45-46, or no inhibitor. Selection was initiated in the presence of the IC90 of the respective inhibitor, and concentrations were gradually increased to a maximum of 100 μg/ml. After each passage, virus was briefly expanded in the absence of inhibitor to generate sufficient viral titers for each subsequent selection step. SHIV-SF162P3 infection was monitored in all cases by green fluorescent protein (GFP) expression of infected GHOST CCR5+ cells. After 60 such rounds of selection, samples from each well were characterized for their resistance to each entry inhibitor using TZM-bl neutralization assays (Fig. 1). Viral swarms from all three wells passaged in the absence of inhibitor retained the properties of the original swarm. Specifically, eCD4-Ig neutralized this swarm more efficiently than NIH45-46, which in turn neutralized more efficiently than CD4-Ig. As expected, all five wells of SHIV-SF162P3 passaged in the presence of NIH45-46 were largely or completely resistant to this bNAb, but this resistance did not substantially alter swarm sensitivity to CD4-Ig or eCD4-Ig. Similarly, swarms in all four wells passaged in the presence of CD4-Ig became substantially resistant to this inhibitor, but these swarms remained sensitive to NIH45-46 and, surprisingly, to eCD4-Ig. However, all five swarms passaged in the presence of eCD4-Ig became completely resistant to CD4-Ig. The IC50s for eCD4-Ig in these swarms were 5-fold higher than those in the absence of a selecting inhibitor, but complete resistance was not observed. These swarms also became modestly more sensitive to NIH45-46. These data show that SHIV-SF162P3 could not escape eCD4-Ig under selection conditions in which complete escape from NIH45-46 and CD4-Ig was consistently observed. They also show that full escape from CD4-Ig does not result in escape from eCD4-Ig but one pathway for eCD4-Ig resistance includes full CD4-Ig escape.
FIG 1.
Neutralization of SHIV-SF162P3 swarms after extensive passage in the presence of NIH45-46, CD4-Ig, or eCD4-Ig. SHIV-SF162P3 was amplified for three passages on GHOST CCR5+ cells in the absence of inhibitor and then was passaged for 60 rounds of selection in the presence of NIH45-46, CD4-Ig, or eCD4-Ig or in the absence of any inhibitor, as indicated. Inhibitor concentrations were determined from the estimated IC90 values for the swarm at a given passage, up to 100 μg/ml. Passages were conducted in 3 (no inhibitor control), 4 (CD4-Ig), or 5 (NIH45-46 and eCD4-Ig) independent wells. Neutralization of each selected swarm was measured for each inhibitor, as indicated at the top of each panel. Dashed lines indicate neutralization curves for individual wells, and solid lines indicate averages among wells selected with the same inhibitor. Note that swarms selected with NIH45-46 (top right, green) and CD4-Ig (bottom left, red) were fully resistant to the respective inhibitors, whereas virus selected with eCD4-Ig remained partially sensitive to this inhibitor (bottom right, blue) but was fully resistant to CD4-Ig (bottom right, red). Error bars represent standard errors of the means (SEM).
eCD4-Ig-resistant SHIV-SF162P3 Envs accumulated mutations in the V2 apex region, V3 loop, V4 region, and CD4-binding site.
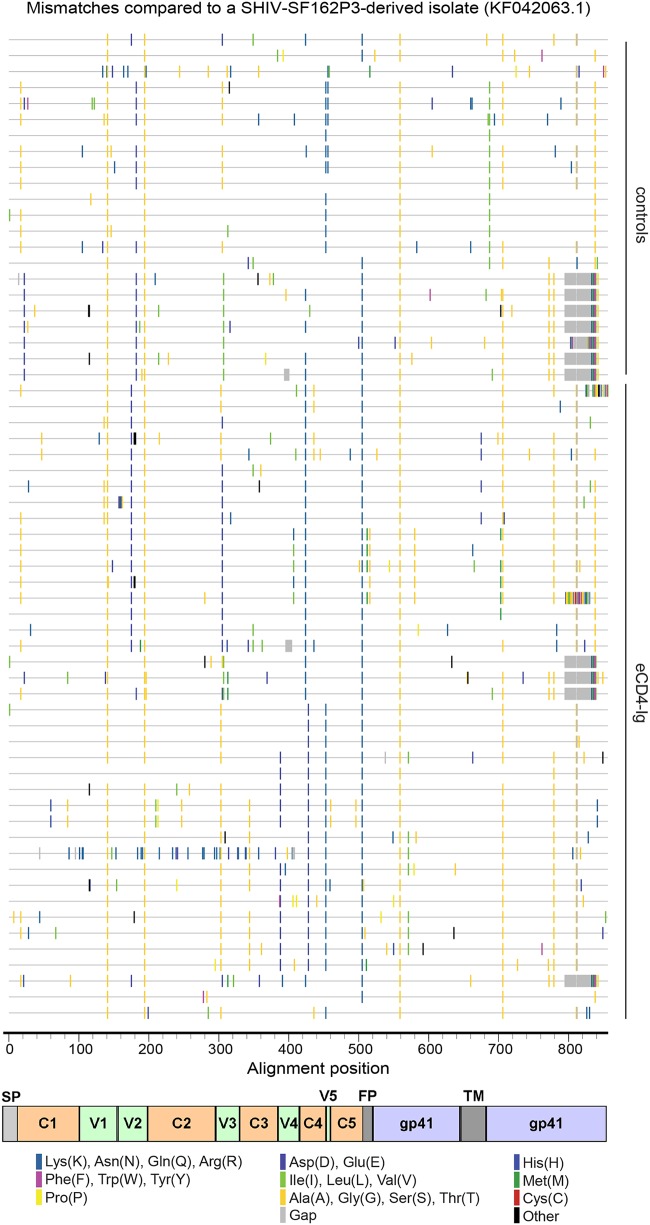
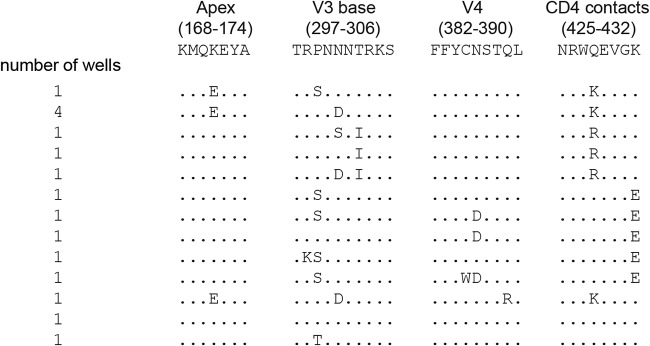
To identify eCD4-Ig-resistance-conferring mutations, we cloned and sequenced gp160 Env from virus passaged in the presence of eCD4-Ig. Comparison of these Env sequences to the sequences of a previously isolated SHIV-SF162P3 Env (GenBank accession no. KF042063.1) and those from viruses passaged in the absence of any inhibitor identified two sets of mutations (Fig. 2). First, many mutations emerged independently of inhibitor or were present in the original diversified SHIV-SF162P3 swarm. Second, another class of mutations were present only in eCD4-Ig-selected swarms. This second class of mutations included multiple instances of changes to the V2 apex region (K171E), at the base of the V3 loop (P299S, N301D, and T303I), in the V4 region (N386D), and at the CD4-binding site (residues Q428K, Q428R, and K432E), each in various combinations (Fig. 3). N301D, T303I, and N386D each eliminate an Env glycosylation site. Note that changes of Q428 were associated in multiple independent instances with K171E and/or N301D mutations. Similarly, K432E was invariably associated with P299S and/or N386D mutations.
FIG 2.
Env substitutions found in SHIV-162P3 swarms selected by eCD4-Ig. A plot of Env amino acid sequences from viruses passaged in the absence of inhibitor or in the presence of eCD4-Ig was generated with the Highlighter utility. A single SHIV-SF162P3 isolate (GenBank accession no. KF042063.1) was used as a reference sequence. Bars indicate changes from this reference sequence to the amino acids indicated. The corresponding regions of Env are indicated at the bottom of the figure. C1 to C5, gp120 constant regions 1 to 5; V1 to V5, gp120 variable regions 1 to 5; SP, signal peptide; FP, fusion peptide; TM, transmembrane region.
FIG 3.
Repeated substitutions in key regions of Env unique to eCD4-Ig-selected swarms. Env mutations present in several individual isolates, not found in control swarms, are indicated. Sequences from the SHIV-SF162P3 reference sequence found in the apex region (residues 168 to 171), the base of V3 (residues 296 to 306), V4 (residues 382 to 390), and the CD4-binding site (residues 425 to 432) are shown. Changes from this reference sequence are shown, along with the number of independent wells in which a given pattern was observed. Note that K171E and Q428K emerged independently in several wells in combination with a mutation in the V3 base.
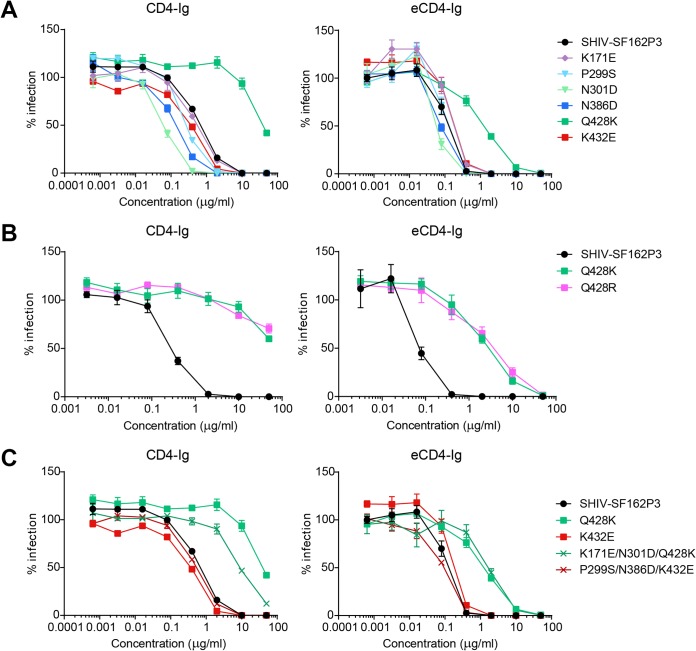
Resistance to eCD4-Ig is associated with changes at Env residue 428.
To determine which of these mutations facilitated escape from eCD4-Ig, we introduced the six most frequent mutations (K171E, P299S, N301D, N386D, Q428K, and K432E) into the Env of a specific parental SHIV-SF162P3 isolate (clone 11) (32), denoted hereafter as SHIV-SF162P3. Individual point mutations were introduced into this Env, the resulting Env variants were used to pseudotype an Env-negative HIV-1, and these pseudoviruses were characterized for their resistance to CD4-Ig or eCD4-Ig in neutralization assays (33). Most individual mutations did not significantly alter sensitivity to CD4-Ig or eCD4-Ig, but a Q428K variant was significantly more resistant to eCD4-Ig and, even more so, to CD4-Ig (Fig. 4A). Variants carrying the less frequently selected Q428R substitution exhibited a nearly identical phenotype (Fig. 4B). This CD4-Ig-resistant phenotype remained consistent despite the introduction of V2 apex and V3 mutations (K171E and N301D) that were associated with residue 428 mutations in multiple independent wells (Fig. 4C). We conclude that mutations of residue 428 of the CD4-binding site conferred at least some of the partial resistance to eCD4-Ig observed in eCD4-Ig-selected swarms.
FIG 4.
Neutralization of Env variants bearing eCD4-Ig-selected substitutions. HIV-1 pseudotyped with Env of the SHIV-SF162P3 sequence or the same Env modified to include the indicated eCD4-Ig-selected substitutions was used to infect TZM-bl cells in the presence of the indicated concentrations of CD4-Ig or eCD4-Ig. Luciferase activity, normalized to that for each variant in the absence of inhibitor, is shown. Error bars represent SEM. The experiment is representative of two or three with similar results. Note that only variants bearing a substitution at Q428 were more resistant to these inhibitors.
Changes in the CD4-binding site decrease the ability of SHIV-SF162P3 Env to infect cells.
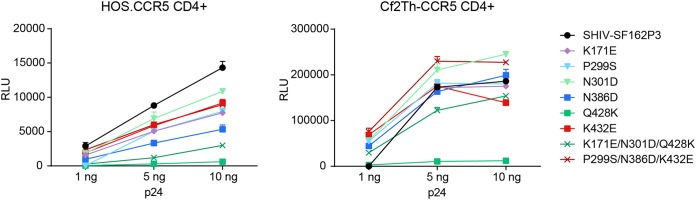
We hypothesized that changes that interfered with CD4 binding would also impair Env infectivity. To test this possibility, equivalent titers of luciferase-expressing viruses pseudotyped with Env variants, normalized by p24 enzyme-linked immunosorbent assay (ELISA), were assayed for their ability to infect HOS or Cf2Th cells expressing CD4 and CCR5 (Fig. 5). In the case of CD4+ HOS.CCR5 cells, all variant Envs infected less efficiently than virus pseudotyped with SHIV-SF162P3 Env. Among Env variants, those with the Q428K substitution exhibited the least efficient infectivity, although inclusion of K171E and N301D improved infectivity, relative to an Env with the Q428K substitution alone. In the case of CD4+ Cf2Th.CCR5 cells, several Env variants, notably those with a P299S substitution, infected more efficiently than unmodified SHIV-SF162P3. Again, variants with the Q428K substitution exhibited the lowest infectivity, but the K171E and N301D mutations more dramatically complemented the Q428K change. We conclude that the Q428K change, which is responsible for partial eCD4-Ig escape, results in a substantial drop in infectivity that can be partially rescued by changes in the V3 base region and/or the V2 apex region. Modest differences between HOS and Cf2Th cells may reflect the relatively higher levels of CD4 on the latter cells (34) or differences in CCR5 expression between these cell lines.
FIG 5.
Lower infectivity of partially eCD4-Ig-resistant Env variants. A luciferase-encoding HIV-1 pseudovirus pseudotyped with Env of the SHIV-SF162P3 reference sequence or the same Env modified to include the indicated eCD4-Ig-selected substitutions was incubated at the indicated p24 amounts with either HOS.CCR5 or Cf2Th-CCR5 cells stably expressing human CD4. Luciferase activity was measured 36 to 48 h postinfection. Error bars represent SEM, and the results are representative of two independent experiments. Note that variants bearing the Q428K substitution were markedly less infective than the reference SHIV-SF162p3 Env sequence or other Env variants.
eCD4-Ig-selected substitutions alter sensitivity to nonneutralizing antibodies and patient serum.
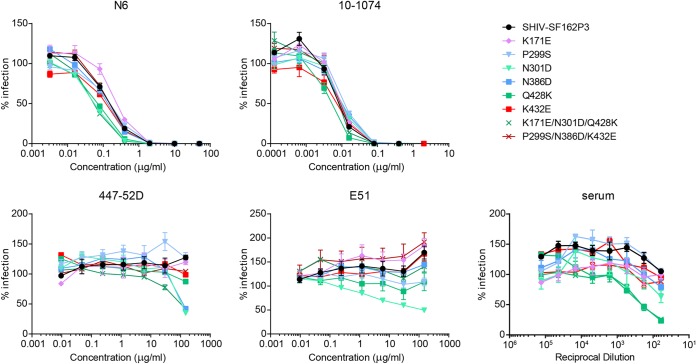
To determine whether eCD4-Ig-selected substitutions could impair viral fitness through other means in vivo, we assessed the impact of these Env substitutions on neutralization by various antibodies and by serum from an infected individual (Fig. 6). Three variants, namely, N301D, Q428K, and K171E/N301D/Q428K, were modestly but reproducibly more susceptible to the CD4-binding site bNAb N6, but these substitutions did not affect sensitivity to the V3 glycan antibody 10-1074. Variants including the N301D substitution were rendered more susceptible to the V3 loop antibody 447-52D and to the CD4-induced antibody E51. The epitopes for these antibodies are usually occluded in primary isolates, and their exposure suggests that N301D-containing Envs have a more open conformation or these epitopes are more accessible due to the loss of the glycosylation site at position 301. Finally, variants containing Q428K were more susceptible to neutralization by serum from an HIV-1-positive individual. N301D-containing variants also displayed increased sensitivity to serum neutralization, again consistent with greater exposure of V3 epitopes. Thus, some Envs selected in vitro in the presence of eCD4-Ig are modestly more sensitive to neutralizing and nonneutralizing antibodies.
FIG 6.
Characterization of eCD4-Ig-induced residue changes with monoclonal and polyclonal antibodies. TZM-bl cells were incubated with HIV-1 pseudoviruses pseudotyped with Env of the SHIV-SF162P3 sequence or variants including eCD4-Ig-selected substitutions, in the presence of the indicated concentrations of the neutralizing antibodies N6 (CD4-binding site), 10-1074 (V3 glycan), 447-52D (V3 loop), or E51 (CD4-induced) or with serum from an HIV-1-positive person. Results are representative of two independent experiments. Error bars represent SEM. Note that V3 loop substitutions increased sensitivity to 447-52D and E51 and variants with the Q428K substitution were more susceptible to serum neutralization.
DISCUSSION
HIV-1 may have greater difficulty escaping from eCD4-Ig than from NIH45-46 for two reasons. First, eCD4-Ig differs from antibodies because its two binding sites on Env coincide with the CD4- and coreceptor-binding sites. In contrast, the footprints of every bNAb include residues outside these functionally necessary sites (35, 36). This unique property of eCD4-Ig suggests that virus selected for resistance to eCD4-Ig would less efficiently associate with its native receptors and thus escape from eCD4-Ig would be slower than with antibodies, because the resulting virus would be less fit. A second reason why escape from eCD4-Ig may be more difficult than escape from bNAbs is that HIV-1 has been exposed in its recent past to antibodies recognizing every key epitope, and thus there are readily accessible pathways of escape (often simple shifting of a glycosylation site) from every class of antibodies (37–39). Of course, HIV-1 has not been previously exposed to eCD4-Ig. Thus, even if full escape were possible, it might be harder for the virus to access the changes. Here we demonstrate that escape from eCD4-Ig is indeed more difficult than escape from a CD4-binding site antibody or from CD4-Ig in vitro. Specifically, after extensive passaging of SHIV-SF162P3 in the presence of eCD4-Ig, CD4-Ig, or the CD4-binding site antibody NIH45-46, we observed rapid escape from the latter two inhibitors but only partial resistance to eCD4-Ig. Thus, at least in this context, escape from eCD4-Ig is clearly more difficult than escape from CD4-Ig or NIH45-46.
To better characterize partial escape from eCD4-Ig, we sequenced virus from six independent passages of SHIV-SF162P3 selected in the presence of up to 100 μg/ml eCD4-Ig. We observed several mutations that emerged independently in more than one passage, possibly because progenitor virus bearing these mutations was present in the original viral swarm. Specifically, changes in the apex (K171E) (Fig. 7A), V3 (P299S and N301D) (Fig. 7B), V4 (N386D), and CD4-binding site (Q428K, Q428R, and K432K) (Fig. 7C) regions of Env were common in several passages. Among these mutations, only Q428K and Q428R in the CD4-binding site could confer partial eCD4-Ig resistance, suggesting that the resistance to eCD4-Ig observed in Fig. 1 was derived from mutations at this position. Notably, the Q428K mutation also resulted in a dramatic loss of viral infectivity (Fig. 5). Not unexpectedly, these changes also increased resistance to CD4-Ig. Q428 mutations were observed in 4 of 5 independent eCD4-Ig-selected wells and likely accounted for most of the resistance observed in those wells. Indeed, none of the remaining mutations characterized increased resistance to eCD4-Ig or CD4-Ig. However, the combination of K171E and N301D partially rescued the loss in infectivity associated with the Q428K mutation, perhaps because they increased exposure of the V3 loop and thus promoted more direct coreceptor association. Consistent with this possibility, Env variants bearing the N301D mutation were better neutralized by the anti-V3-loop antibody 447-52D, and the N301D mutation by itself increased neutralization by the CD4-inducible antibody E51, indicating that the coreceptor-binding site is more exposed in the N301D variant (Fig. 6).
FIG 7.
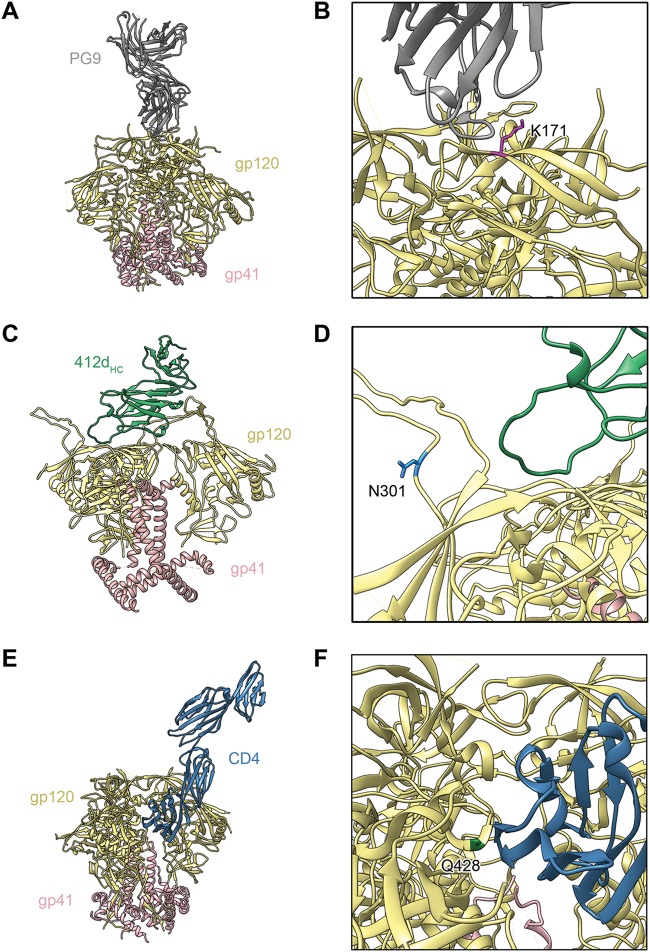
Modeling of Env trimers, highlighting some of the eCD4-Ig selected mutated residues. (A) Model of apex V2 glycan bNAb PG9 in complex with the BG505 SOSIP.664 trimer (PDB accession no. 5VJ6) (67). gp120 is depicted in tan, gp41 in pink, and PG9 in gray. (B) Detailed view of the structure in panel A. Apex residue K171, which is altered to glutamic acid in some eCD4-Ig-selected Env variants, is highlighted in magenta. (C) Model of CD4-induced bNAb 412d heavy chain (412dHC) in complex with an Env trimer in a partially open conformation, created by combining the crystal structure of BG505 SOSIP gp140 with that of 412d complexed with HIV-1 YU2 gp120 (PDB accession no. 4NCO and accession no. 2QAD) (68, 69). 412dHC is depicted in green. (D) Detailed view of the structure in panel C. Residue N301, which is altered to aspartic acid in some eCD4-Ig-selected Env variants, is shown in light blue. (E) Model of the BG505 DS-SOSIP trimer in complex with CD4 (PDB accession no. 5U1F) (70). CD4 is depicted in dark blue. (F) Detailed view of the structure in panel E. CD4-binding site residue Q428, which is altered to lysine or arginine in some eCD4-Ig-selected Env variants, is highlighted in green.
The unexpected and frequent emergence of a K171E mutation in the Env V2 apex region remains an unexplained puzzle, even more so because similar changes were observed in the Env genes isolated from SIVmac293-infected macaques expressing a rhesus form of eCD4-Ig (31). This observation raises the possibility that there is a conserved tyrosine sulfate-binding pocket at the apex that is bound by eCD4-Ig, perhaps only before induction of the CD4-bound conformation of Env. Consistent with this speculation, nearly every V2 apex bNAb, including PG9, PG16, PGT145, PGDM1400, and Cap256-VRC26.25, incorporates rare sulfotyrosines in their heavy-chain CDR3 that directly contact the V2 apex, including residue 171 (40–45). Nonetheless, K171E did not detectably alter the neutralization sensitivity of SHIV-SF162P3, alone or in combination with other gp120 changes, and it did not affect the fitness of Envs so modified. Despite the absence of an obvious phenotype, Envs bearing this mutation emerged only in eCD4-Ig-selected swarms. Therefore, it is possible that our selection regimen and standard neutralization assays are subtly different, and early association of the sulfopeptide with Env before CD4 binding may be more useful in the former system. For example, standard neutralization assays do not model cell-to-cell transfer of virus or indeed any condition in which the inhibitor directly competes in a short time window with cellular CD4 and CCR5. Further work will be important to determine whether modification of the apex can promote resistance to eCD4-Ig in vivo.
Another question that these data raise is how a virus can be fully resistant to CD4-Ig but still retain full sensitivity to eCD4-Ig. This question is more puzzling since the coreceptor-binding site is not typically exposed until after CD4 engagement. One explanation is based on the observation that CD4-Ig actually promotes infection at low concentrations, a phenomenon that is masked by its neutralization activity at higher concentrations (46); it does so presumably due to its well-established ability to promote direct association with the coreceptor CCR5 in particular. In this model, the sulfopeptide of eCD4-Ig competes with the coreceptor amino terminus and blocks the major pathway for escape from CD4-Ig.
Regardless, the data presented here make clear that HIV-1 Env escapes more slowly from eCD4-Ig than from CD4-Ig or NIH45-46, perhaps in part because the virus does not have an accessible pathway for escape. Moreover, Env mutations that conferred partial resistance to eCD4-Ig, notably those at residue 428, were associated with a high fitness cost. Mutations that partially compensated for this fitness loss in vitro, especially N301D, may be associated with an in vivo fitness cost because they help expose the V3 and CD4i epitopes on Env, making them more susceptible to common but usually ineffective serum antibodies. Consistent with this speculation, we have not observed mutations in the V3 loop of Envs isolated from macaques expressing eCD4-Ig (31). Even without the emergence of eCD4-Ig resistance, eCD4-Ig uniquely collaborates with such serum antibodies. Specifically, its CD4 domain induces exposure of the same gp120 epitopes on Env. While this property may not increase its already potent neutralization, we showed previously that eCD4-Ig markedly enhances serum antibody-mediated killing of infected cells (47). In short, eCD4-Ig is qualitatively different from bNAbs in several useful ways; it is exceptionally broad, it is more difficult to escape, even partial resistance is associated with loss of fitness, and it collaborates with common antibodies in the serum to eliminate infected cells. Further studies will help establish whether these properties make eCD4-Ig more effective as a monotherapy than any bNAb.
MATERIALS AND METHODS
Cells, plasmids, and viruses.
HEK293T (human embryonic kidney; ATCC CRL-3216), TZM-bl, Cf2Th-CCR5, HOS.CCR5, and GHOST CCR5+ cells (NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH) were grown at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS). Cf2Th-CCR5 cells were supplemented with 500 μg/ml G418, 500 μg/ml zeocin, and 3 μg/ml puromycin. HOS.CCR5 cells were further supplemented with 1 μg/ml puromycin. GHOST CCR5+ cells were supplemented with 500 μg/ml G418 (VWR), 100 μg/ml hygromycin B (Fisher Scientific), and 1 μg/ml puromycin (Gibco). TZM-bl cells originated from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc. (48–52), Cf2Th-CCR5 cells from Joseph Sodroski (53), and GHOST CCR5+ cells from Vineet N. KewalRamani and Dan R. Littman (54). HOS.CCR5 and pNL4-3.Luc.R−E− were supplied by Nathaniel Landau (55–58). Expi293 cells were grown in Expi293 expression medium (Thermo Fisher Scientific). Plasmids encoding CD4-Ig, eCD4-Ig, and tyrosylprotein sulfotransferase 2 (TPST2) have been described previously (30, 34). Vectors expressing NIH45-46 and 10-1074 were provided by Michel Nussenzweig. Vectors expressing N6 and 35O22 were obtained through the NIH AIDS Reagent Program (Division of AIDS, NIAID, NIH) (N6 monoclonal antibody [MAb] heavy chain expression vector, catalogue no. 12967; N6 MAb light chain expression vector, catalogue no. 12966; MAb 35O22 heavy chain expression vector [CMVR], catalogue no. 12584; MAb 35O22 light chain, from Jinghe Huang and Mark Connors, catalogue no. 12585) (59, 60). Vectors expressing 447-52D were provided by Meredith Davis-Gardner. Vectors expressing E51 were described previously (61). SHIV-SF162P3 was acquired from Janet Harouse, Cecilia Cheng-Mayer, Ranajit Pal, and the Division of AIDS, NIAID, through the NIH AIDS Reagent Program (62, 63). The SF162P3N11 Env expression vector was provided by Cecilia Cheng-Mayer. The SF162P3N11 env gene was cloned from a full-length vector into pcDNA3.1(+) (Thermo Fisher Scientific), using Gibson assembly (New England Biolabs). Nucleotide changes encoding amino acid changes were introduced into the pcDNA3.1(+) Env expression plasmid using PCR site-directed mutagenesis.
HIV-1-positive serum.
Deidentified HIV-1-positive serum was obtained from Boston Biomedical Inc. (Boston, MA) and has been described previously. Material was handled in accordance with the regulations established by the Scripps Office for the Protection of Research Subjects.
eCD4-Ig, CD4-Ig, and antibody production.
To produce eCD4-Ig and CD4-Ig, Expi293 cells (Thermo Fisher Scientific) were grown to a density of 3 × 106 cells/ml in 250 ml Expi293 expression medium (Thermo Fisher Scientific), and 140 μg of vector expressing CD4-Ig was transfected with ExpiFectamine (Thermo Fisher Scientific), according to the manufacturer’s instructions. For eCD4-Ig production, 112 μg of plasmid encoding eCD4-Ig was cotransfected with 28 μg of plasmid encoding TPST2; eCD4-Ig and CD4-Ig were harvested after 5 days. To produce the antibodies NIH45-46, N6, 35O22, 10-1074, and 447-52D, Expi293 cells were transfected with a total of 80 μg of two plasmids encoding heavy and light chains, at a 1:1 ratio. E51 was produced by transfection of 32 μg of plasmid encoding the heavy chain, 32 μg of plasmid encoding the light chain, and 16 μg of plasmid encoding TPST2. Antibodies were also harvested after 5 days. To harvest protein, medium was collected, centrifuged at 4,000 × g for 10 min, and filtered with a 0.45-μm filter flask (Millipore). Protein was isolated with HiTrap MabSelect SuRe columns (GE Healthcare) and eluted with IgG elution buffer (Thermo Fisher Scientific) into 1 M Tris-HCl buffer (pH 9) (G-Biosciences). Buffer exchange was performed with Amicon Ultra-15 centrifugal filter units (Millipore) and phosphate-buffered saline.
Viral passage in vitro.
First, SHIV-SF162P3 was passaged three times on GHOST CCR5+ cells in the absence of inhibitor. Then, we established the IC90 of eCD4-Ig, CD4-Ig, and NIH45-46 against the diversified viral swam in TZM-bl neutralization assays. The virus was divided into multiple wells and passaged on GHOST CCR5+ cells in the presence of eCD4-Ig, CD4-Ig, NIH45-46, or no inhibitor. Initial inhibitor concentrations were the previously determined IC90 values, and concentrations were increased over time to a maximum of 100 μg/ml. Passaging was performed in the following manner. The day before each supernatant transfer, GHOST CCR5+ cells were plated into a 24-well plate at a concentration of 30,000 cells/well. The following day, virus-containing supernatant was transferred onto the new cells. Approximately 8 h after transfer, supernatant was aspirated and replaced with fresh medium containing inhibitor. Sixty-four hours later, supernatant was transferred to a new plate with GHOST CCR5+ cells. Eight hours after transfer, supernatant was again replaced with fresh medium without inhibitor. Sixty-four hours later, supernatant was transferred to a new plate and the cycle was repeated. Thus, after each selective passage, virus was expanded in the absence of any inhibitor to generate sufficient viral titers for the following selection step. Viral infection was monitored by GFP expression of the infected GHOST CCR5+ cells. After 60 passages, an inhibitor concentration of 100 μg/ml was reached and viral samples from each well were characterized for their resistance to entry inhibitors.
Neutralization assays.
TZM-bl neutralizations assays were performed as described previously (33, 61). Briefly, pseudotyped HIV-1 was produced in 175-cm2 flasks by transfecting 293T cells with a mixture of an HIV-1 expression vector lacking a functional env gene (45 μg DNA), a plasmid encoding the desired Env (25 μg), a plasmid expressing the tat gene (5 μg), and a plasmid expressing the rev gene (5 μg). Viral supernatants were passed through a 0.45-μm syringe filter, stored at −80°C, and normalized for infectivity. In most cases, 150,000 relative light units (RLU) of luciferase activity was used to perform neutralization studies; in the case of low-infectivity viruses, however (specifically, viruses bearing mutations at residue 428), levels as low as 40,000 RLU were used. For the assay, pseudoviruses were preincubated for 1 h at 37°C with titrated amounts of inhibitor or serum in DMEM with 10% FBS. TZM-bl cells were detached by trypsin and diluted to 100,000 cells/ml in DMEM with 10% FBS. Cells were then added to the pseudovirus-inhibitor mixture and incubated for 36 to 48 h at 37°C. Viral entry was determined using Britelite Plus (PerkinElmer), and luciferase expression was measured using a Victor X3 plate reader (PerkinElmer). Data were analyzed with Prism software (GraphPad).
Infection assays.
HIV-1 pseudoviruses were generated as described above except that pNL4-3.Luc.R−E− was used as the HIV-1 expression vector (64). The generation of Cf2Th-CCR5 cells stably expressing rhesus CD4 has been described before (34). HOS CD4+ CCR5+ cells and Cf2Th-CCR5 cells stably expressing rhesus CD4 were harvested, diluted to 100,000 cells/ml in DMEM with 10% FBS, and incubated with virus dilutions at 37°C. Viral entry was analyzed after 48 to 72 h using Britelite Plus (PerkinElmer), and luciferase expression was measured using a Victor X3 plate reader (PerkinElmer). Data were analyzed with Prism software (GraphPad).
Env sequencing.
Sequencing of the env gene of SHIV-SF162P3 was performed as follows. Viral RNA was isolated from 200 μl of supernatant using the QIAamp MinElute virus spin kit (Qiagen) or the PureLink viral RNA/DNA minikit (Thermo Fisher Scientific). Virus-specific primers (5′-TGTAATAAATCCCTTCCAGTCCCCCC-3′ or 5′-AGTCTACTCGAGAGAAAGTGGGCGTTCCCGACC-3′) were used for cDNA synthesis with SuperScript III or IV reverse transcriptase (Thermo Fisher Scientific). The env gene sequences were amplified by nested PCRs using Platinum Taq DNA polymerase (Thermo Fisher Scientific) or iProof polymerase (Bio-Rad). First-round primers were 5′-TACCGAGCTCGGATCCCAGAAAGAGCAGAAGACAGTGG-3′ and 5′-GATATCTGCAGAATTCGCTCCACCCATATTGTAGGTAGG-3′; second-round primers were 5′-AAAGAGCAGAAGACAGTGGCAATGAGAGTGAAGGGGATCAGGAAG-3′ and 5′-ACCACTTGCCCCCCATGTTATAGCAAAGTCCTTTCAAGGCCC-3′. Amplified DNA was extracted from a gel (QIAquick gel extraction kit; Qiagen) and cloned into the pcDNA3.1/V5-His TOPO or pCR4-TOPO vector (Thermo Fisher Scientific). One Shot Mach1-T1 or One Shot Stbl3 cells (Thermo Fisher Scientific) were transformed and grown at 30°C. Single colonies were sequenced using primers annealing to the env sequence and were compared to the sequences of GenBank accession no. KF042063.1. Sequence analysis was performed using Lasergene SeqMan Pro (DNASTAR) and the online tools Clustal Omega (EMBL-EBI) and Highlighter (Los Alamos National Laboratory) (65, 66). Sequence positions were annotated based on the HXB2 numbering system. A total of 40 full and distinct Env sequences were obtained from the 5 wells incubated with eCD4-Ig; the values ranged from 1 to 13 distinct sequences, depending on the diversity and the number of partial sequences found in each well.
ACKNOWLEDGMENTS
This project was supported by National Institutes of Health grants R37-AI091476, R01-AI129868, and UM1-AI126623.
We thank Meredith Davis-Gardner for her careful readings of the manuscript and thoughtful comments.
M.F. and M.R.G. are cofounders of Emmune, Inc., a company that has licensed eCD4-Ig from the Scripps Research Institute.
C.H.F., M.R.G., and M.F. designed the experiments, C.H.F., J.A.W., B.A., and A.S.Z. performed the experiments, and C.H.F. and M.F. analyzed the data and wrote the manuscript, with input and approval from the other authors.
REFERENCES
- 1.Liao H-X, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, NISC Comparative Sequencing Program, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia S-M, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BTM, Kwong PD, Mascola JR, Haynes BF. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonsignori M, Liao HX, Gao F, Williams WB, Alam SM, Montefiori DC, Haynes BF. 2017. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol Rev 275:145–160. doi: 10.1111/imr.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonsignori M, Zhou T, Sheng Z, Chen L, Gao F, Joyce MG, Ozorowski G, Chuang G-Y, Schramm CA, Wiehe K, Alam SM, Bradley T, Gladden MA, Hwang K-K, Iyengar S, Kumar A, Lu X, Luo K, Mangiapani MC, Parks RJ, Song H, Acharya P, Bailer RT, Cao A, Druz A, Georgiev IS, Kwon YD, Louder MK, Zhang B, Zheng A, Hill BJ, Kong R, Soto C, Mullikin JC, Douek DC, Montefiori DC, Moody MA, Shaw GM, Hahn BH, Kelsoe G, Hraber PT, Korber BT, Boyd SD, Fire AZ, Kepler TB, Shapiro L, Ward AB, Mascola JR, Liao H-X, Kwong PD, Haynes BF. 2016. Maturation pathway from germline to broad HIV-1 neutralizer of a CD4-mimic antibody. Cell 165:449–463. doi: 10.1016/j.cell.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton DR, Hangartner L. 2016. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu Rev Immunol 34:635–659. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwong PD, Mascola JR. 2018. HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity 48:855–871. doi: 10.1016/j.immuni.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio PM, Incesu R-B, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. 2012. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs SP, Martinez-Navio JM, Piatak M Jr, Lifson JD, Gao G, Desrosiers RC. 2015. AAV-delivered antibody mediates significant protective effects against SIVmac239 challenge in the absence of neutralizing activity. PLoS Pathog 11:e1005090. doi: 10.1371/journal.ppat.1005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M Jr, Lifson JD, Dimitrov DS, Nussenzweig MC, Martin MA. 2013. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura Y, Gautam R, Chun TW, Sadjadpour R, Foulds KE, Shingai M, Klein F, Gazumyan A, Golijanin J, Donaldson M, Donau OK, Plishka RJ, Buckler-White A, Seaman MS, Lifson JD, Koup RA, Fauci AS, Nussenzweig MC, Martin MA. 2017. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543:559–563. doi: 10.1038/nature21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun T-W, Murray D, Justement JS, Blazkova J, Hallahan CW, Fankuchen O, Gittens K, Benko E, Kovacs C, Moir S, Fauci AS. 2014. Broadly neutralizing antibodies suppress HIV in the persistent viral reservoir. Proc Natl Acad Sci U S A 111:13151–13156. doi: 10.1073/pnas.1414148111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halper-Stromberg A, Lu C-L, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich TR, Liu C, Gazumyan A, Schaefer U, Furze RC, Seaman MS, Prinjha R, Tarakhovsky A, Ravetch JV, Nussenzweig MC. 2014. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu C-L, Lorenzi JCC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, Shimeliovich I, Patel R, Burke L, Cohen YZ, Hadrigan S, Settler A, Witmer-Pack M, West AP, Juelg B, Keler T, Hawthorne T, Zingman B, Gulick RM, Pfeifer N, Learn GH, Seaman MS, Bjorkman PJ, Klein F, Schlesinger SJ, Walker BD, Hahn BH, Nussenzweig MC, Caskey M. 2016. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535:556–560. doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Casazza J, Conan-Cibotti M, Migueles SA, Tressler R, Bailer RT, McDermott A, Narpala S, O'Dell S, Wolf G, Lifson JD, Freemire BA, Gorelick RJ, Pandey JP, Mohan S, Chomont N, Fromentin R, Chun TW, Fauci AS, Schwartz RM, Koup RA, Douek DC, Hu Z, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE. 2015. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PubMed] [Google Scholar]
- 15.Caskey M, Klein F, Lorenzi JCC, Seaman MS, West AP, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fätkenheuer G, Schlesinger SJ, Nussenzweig MC. 2015. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diskin R, Klein F, Horwitz JA, Halper-Stromberg A, Sather DN, Marcovecchio PM, Lee T, West AP Jr, Gao H, Seaman MS, Stamatatos L, Nussenzweig MC, Bjorkman PJ. 2013. Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies. J Exp Med 210:1235–1249. doi: 10.1084/jem.20130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch RM, Wong P, Tran L, O'Dell S, Nason MC, Li Y, Wu X, Mascola JR. 2015. HIV-1 fitness cost associated with escape from the VRC01 class of CD4 binding site neutralizing antibodies. J Virol 89:4201–4213. doi: 10.1128/JVI.03608-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, Manrique A, Huber M, Rehr M, Oxenius A, Weber R, Stiegler G, Vcelar B, Katinger H, Aceto L, Gunthard HF. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med 11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 19.Klein F, Nogueira L, Nishimura Y, Phad G, West AP Jr, Halper-Stromberg A, Horwitz JA, Gazumyan A, Liu C, Eisenreich TR, Lehmann C, Fatkenheuer G, Williams C, Shingai M, Martin MA, Bjorkman PJ, Seaman MS, Zolla-Pazner S, Karlsson Hedestam GB, Nussenzweig MC. 2014. Enhanced HIV-1 immunotherapy by commonly arising antibodies that target virus escape variants. J Exp Med 211:2361–2372. doi: 10.1084/jem.20141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otsuka Y, Schmitt K, Quinlan BD, Gardner MR, Alfant B, Reich A, Farzan M, Choe H. 2018. Diverse pathways of escape from all well-characterized VRC01-class broadly neutralizing HIV-1 antibodies. PLoS Pathog 14:e1007238. doi: 10.1371/journal.ppat.1007238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward AB, Wilson IA. 2017. The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunol Rev 275:21–32. doi: 10.1111/imr.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell RLR, Totrov M, Itri V, Liu X, Fox A, Zolla-Pazner S. 2017. Plasticity and epitope exposure of the HIV-1 envelope trimer. J Virol 91:e00410-17. doi: 10.1128/JVI.00410-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnus C, Reh L, Trkola A. 2016. HIV-1 resistance to neutralizing antibodies: determination of antibody concentrations leading to escape mutant evolution. Virus Res 218:57–70. doi: 10.1016/j.virusres.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, McKee K, Lord DM, Wei RR, Deng G, Louder M, Schmidt SD, Mankoff Z, Wu L, Asokan M, Beil C, Lange C, Leuschner WD, Kruip J, Sendak R, Do Kwon Y, Zhou T, Chen X, Bailer RT, Wang K, Choe M, Tartaglia LJ, Barouch DH, O’Dell S, Todd J-P, Burton DR, Roederer M, Connors M, Koup RA, Kwong PD, Yang Z-y, Mascola JR, Nabel GJ. 2017. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 358:85–90. doi: 10.1126/science.aan8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan SN, Sok D, Tran K, Movsesyan A, Dubrovskaya V, Burton DR, Wyatt RT. 2018. Targeting the HIV-1 spike and coreceptor with bi- and trispecific antibodies for single-component broad inhibition of entry. J Virol 92:e00384-18. doi: 10.1128/JVI.00384-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinhardt JJ, Guenaga J, Turner HL, McKee K, Louder MK, O'Dell S, Chiang CI, Lei L, Galkin A, Andrianov AK, Doria-Rose NA, Bailer RT, Ward AB, Mascola JR, Li Y. 2018. Rational design of a trispecific antibody targeting the HIV-1 Env with elevated anti-viral activity. Nat Commun 9:877. doi: 10.1038/s41467-018-03335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun M, Pace CS, Yao X, Yu F, Padte NN, Huang Y, Seaman MS, Li Q, Ho DD. 2014. Rational design and characterization of the novel, broad and potent bispecific HIV-1 neutralizing antibody iMabm36. J Acquir Immune Defic Syndr 66:473–483. doi: 10.1097/QAI.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bournazos S, Gazumyan A, Seaman MS, Nussenzweig MC, Ravetch JV. 2016. Bispecific anti-HIV-1 antibodies with enhanced breadth and potency. Cell 165:1609–1620. doi: 10.1016/j.cell.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner MR, Farzan M. 2017. Engineering antibody-like inhibitors to prevent and treat HIV-1 infection. Curr Opin HIV AIDS 12:294–301. doi: 10.1097/COH.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, Chiang JJ, Haworth KG, Decker JM, Alpert MD, Bailey CC, Neale ES, Fellinger CH, Joshi VR, Fuchs SP, Martinez-Navio JM, Quinlan BD, Yao AY, Mouquet H, Gorman J, Zhang B, Poignard P, Nussenzweig MC, Burton DR, Kwong PD, Piatak M, Lifson JD, Gao G, Desrosiers RC, Evans DT, Hahn BH, Ploss A, Cannon PM, Seaman MS, Farzan M. 2015. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardner MR, Fellinger CH, Kattenhorn LM, Davis-Gardner ME, Weber JA, Alfant B, Zhou AS, Prasad NR, Kondur HR, Newton WA, Weisgrau KL, Rakasz EG, Lifson JD, Gao G, Schultz-Darken N, Farzan M. AAV-delivered eCD4-Ig protects rhesus macaques from high-dose SIVmac239 challenges. Sci Transl Med, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren W, Mumbauer A, Zhuang K, Harbison C, Knight H, Westmoreland S, Gettie A, Blanchard J, Cheng-Mayer C. 2013. Mucosal transmissibility, disease induction and coreceptor switching of R5 SHIVSF162P3N molecular clones in rhesus macaques. Retrovirology 10:9. doi: 10.1186/1742-4690-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fellinger CH, Gardner MR, Bailey CC, Farzan M. 2017. Simian immunodeficiency virus SIVmac239, but not SIVmac316, binds and utilizes human CD4 more efficiently than rhesus CD4. J Virol 91:e00847-17. doi: 10.1128/JVI.00847-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Kwon YD, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou T, Lynch RM, Chen L, Acharya P, Wu X, Doria-Rose NA, Joyce MG, Lingwood D, Soto C, Bailer RT, Ernandes MJ, Kong R, Longo NS, Louder MK, McKee K, O’Dell S, Schmidt SD, Tran L, Yang Z, Druz A, Luongo TS, Moquin S, Srivatsan S, Yang Y, Zhang B, Zheng A, Pancera M, Kirys T, Georgiev IS, Gindin T, Peng H-P, Yang A-S, Mullikin JC, Gray MD, Stamatatos L, Burton DR, Koff WC, Cohen MS, Haynes BF, Casazza JP, Connors M, Corti D, Lanzavecchia A, Sattentau QJ, Weiss RA, West AP, Bjorkman PJ, Scheid JF, Nussenzweig MC, Shapiro L, Mascola JR, Kwong PD. 2015. Structural repertoire of HIV-1-neutralizing antibodies targeting the CD4 supersite in 14 donors. Cell 161:1280–1292. doi: 10.1016/j.cell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong C-H, Phogat S, Wrin T, Simek MD, Protocol G. Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 39.Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB. 2013. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLellan JS, Pancera M, Carrico C, Gorman J, Julien J-P, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O’Dell S, Patel N, Shahzad-Ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang G-Y, Diwanji D, Georgiev I, Do Kwon Y, Lee D, Louder MK, Moquin S, Schmidt SD, Yang Z-Y, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang L-X, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Julien J-P, Lee JH, Cupo A, Murin CD, Derking R, Hoffenberg S, Caulfield MJ, King CR, Marozsan AJ, Klasse PJ, Sanders RW, Moore JP, Wilson IA, Ward AB. 2013. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proc Natl Acad Sci U S A 110:4351–4356. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pejchal R, Walker LM, Stanfield RL, Phogat SK, Koff WC, Poignard P, Burton DR, Wilson IA. 2010. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc Natl Acad Sci U S A 107:11483–11488. doi: 10.1073/pnas.1004600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sok D, van Gils MJ, Pauthner M, Julien J-P, Saye-Francisco KL, Hsueh J, Briney B, Lee JH, Le KM, Lee PS, Hua Y, Seaman MS, Moore JP, Ward AB, Wilson IA, Sanders RW, Burton DR. 2014. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Natl Acad Sci U S A 111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JH, Andrabi R, Su C-Y, Yasmeen A, Julien J-P, Kong L, Wu NC, McBride R, Sok D, Pauthner M, Cottrell CA, Nieusma T, Blattner C, Paulson JC, Klasse PJ, Wilson IA, Burton DR, Ward AB. 2017. A broadly neutralizing antibody targets the dynamic HIV envelope trimer apex via a long, rigidified, and anionic β-hairpin structure. Immunity 46:690–702. doi: 10.1016/j.immuni.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doria-Rose NA, Bhiman JN, Roark RS, Schramm CA, Gorman J, Chuang G-Y, Pancera M, Cale EM, Ernandes MJ, Louder MK, Asokan M, Bailer RT, Druz A, Fraschilla IR, Garrett NJ, Jarosinski M, Lynch RM, McKee K, O'Dell S, Pegu A, Schmidt SD, Staupe RP, Sutton MS, Wang K, Wibmer CK, Haynes BF, Abdool-Karim S, Shapiro L, Kwong PD, Moore PL, Morris L, Mascola JR. 2016. New member of the V1V2-directed CAP256-VRC26 lineage that shows increased breadth and exceptional potency. J Virol 90:76–91. doi: 10.1128/JVI.01791-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenten D, Marcon L, Karlsson GB, Parolin C, Kodama T, Gerard N, Sodroski J. 1999. Effects of soluble CD4 on simian immunodeficiency virus infection of CD4-positive and CD4-negative cells. J Virol 73:5373–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis-Gardner ME, Gardner MR, Alfant B, Farzan M. 2017. eCD4-Ig promotes ADCC activity of sera from HIV-1-infected patients. PLoS Pathog 13:e1006786. doi: 10.1371/journal.ppat.1006786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Platt EJ, Bilska M, Kozak SL, Kabat D, Montefiori DC. 2009. Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J Virol 83:8289–8292. doi: 10.1128/JVI.00709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 72:2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeuchi Y, McClure MO, Pizzato M. 2008. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J Virol 82:12585–12588. doi: 10.1128/JVI.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol 74:8358–8367. doi: 10.1128/JVI.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirzabekov T, Bannert N, Farzan M, Hofmann W, Kolchinsky P, Wu L, Wyatt R, Sodroski J. 1999. Enhanced expression, native purification, and characterization of CCR5, a principal HIV-1 coreceptor. J Biol Chem 274:28745–28750. doi: 10.1074/jbc.274.40.28745. [DOI] [PubMed] [Google Scholar]
- 54.Morner A, Bjorndal A, Albert J, Kewalramani VN, Littman DR, Inoue R, Thorstensson R, Fenyo EM, Bjorling E. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J Virol 73:2343–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 56.Landau NR, Littman DR. 1992. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J Virol 66:5110–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol 69:6705–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Connor RI, Chen BK, Choe S, Landau NR. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 59.Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Imamichi H, Georgiev IS, Chuang GY, Druz A, Doria-Rose NA, Laub L, Sliepen K, van Gils MJ, de la Pena AT, Derking R, Klasse PJ, Migueles SA, Bailer RT, Alam M, Pugach P, Haynes BF, Wyatt RT, Sanders RW, Binley JM, Ward AB, Mascola JR, Kwong PD, Connors M. 2014. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature 515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J, Kang BH, Ishida E, Zhou T, Griesman T, Sheng Z, Wu F, Doria-Rose NA, Zhang B, McKee K, O’Dell S, Chuang G-Y, Druz A, Georgiev IS, Schramm CA, Zheng A, Joyce MG, Asokan M, Ransier A, Darko S, Migueles SA, Bailer RT, Louder MK, Alam SM, Parks R, Kelsoe G, Von Holle T, Haynes BF, Douek DC, Hirsch V, Seaman MS, Shapiro L, Mascola JR, Kwong PD, Connors M. 2016. Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth. Immunity 45:1108–1121. doi: 10.1016/j.immuni.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gardner MR, Fellinger CH, Prasad NR, Zhou AS, Kondur HR, Joshi VR, Quinlan BD, Farzan M. 2016. CD4-induced antibodies promote association of the HIV-1 envelope glycoprotein with CD4-binding site antibodies. J Virol 90:7822–7832. doi: 10.1128/JVI.00803-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 63.Harouse JM, Gettie A, Eshetu T, Tan RC, Bohm R, Blanchard J, Baskin G, Cheng-Mayer C. 2001. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIVSF162P3. J Virol 75:1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choe H, Li W, Wright PL, Vasilieva N, Venturi M, Huang CC, Grundner C, Dorfman T, Zwick MB, Wang L, Rosenberg ES, Kwong PD, Burton DR, Robinson JE, Sodroski JG, Farzan M. 2003. Tyrosine sulfation of human antibodies contributes to recognition of the CCR5 binding region of HIV-1 gp120. Cell 114:161–170. doi: 10.1016/S0092-8674(03)00508-7. [DOI] [PubMed] [Google Scholar]
- 65.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H, Gristick HB, Scharf L, West AP, Galimidi RP, Seaman MS, Freund NT, Nussenzweig MC, Bjorkman PJ. 2017. Asymmetric recognition of HIV-1 envelope trimer by V1V2 loop-targeting antibodies. Elife 6:e27389. doi: 10.7554/eLife.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Julien J-P, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse P-J, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. 2013. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang C-C, Lam SN, Acharya P, Tang M, Xiang S-H, Hussan SS-U, Stanfield RL, Robinson J, Sodroski J, Wilson IA, Wyatt R, Bewley CA, Kwong PD. 2007. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science 317:1930–1934. doi: 10.1126/science.1145373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Q, Acharya P, Dolan MA, Zhang P, Guzzo C, Lu J, Kwon A, Gururani D, Miao H, Bylund T, Chuang GY, Druz A, Zhou T, Rice WJ, Wigge C, Carragher B, Potter CS, Kwong PD, Lusso P. 2017. Quaternary contact in the initial interaction of CD4 with the HIV-1 envelope trimer. Nat Struct Mol Biol 24:370–378. doi: 10.1038/nsmb.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]