Abstract
Microbial resistance to drugs is an unresolved global concern, which is present in every country. Developing new antibiotics is one of the guidelines of the Centers for Disease Control and Preventions (CDC) to combat bacterial resistance to drugs. Based on our lead molecules, we report the synthesis and antimicrobial studies of 27 new pyrazole derivatives. These new coumarin-pyrazole-hydrazone hybrids are readily synthesized from commercially available starting materials and reagents using benign reaction conditions. All the synthesized molecules were tested against 14 Gram-positive and Gram-negative bacterial strains. Several of these molecules have been found to be potent growth inhibitors of several strains of these tested bacteria with minimum inhibitory concentrations as low as 1.56 μg/mL. Furthermore, active molecules are non-toxic in in vitro and in vivo toxicity studies.
Keywords: Coumarin, pyrazole, hydrazone, MRSA, drug-resistant, Acinetobacter baumannii, antimicrobials, ESKAPE pathogen, Staphylococcus aureus
1. Introduction
Microbial resistance to antibiotics is a growing public health concern worldwide [1]. Existing antibiotics are losing their effect. There is insufficient investment and research to develop new antibiotics. If this trend continues, the tools to combat antimicrobial resistance will be depleted and many modern medical breakthroughs will be ineffective [2]. Two-thirds of nosocomial drug-resistant infections in the United States are caused by six bacteria informally called the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogens. A. baumannii, a member of the ESKAPE pathogens, can live in tracheostomy sites or open wounds for several days without causing infection, and this bacterium is life threatening for people with weakened immune systems [3]. Recently, the World Health Organization (WHO) has listed 12 drug resistant bacteria for which new antibiotics are urgently needed. Carbapenem-resistant A. baumannii is at the top of the list with critical priority and drug resistant S. aureus is in the high priority category [4,5]. S. aureus, a Gram-positive bacterium, is found in the nares (nostrils) of about 30% of the population. This bacterium may cause sepsis, pneumonia, endocarditis, and osteomyelitis. S. aureus resistance to different antibiotics has evolved into different strains such as methicillin-resistant S. aureus (MRSA), vancomycin-intermediate S. aureus (VISA), and vancomycin-resistant S. aureus (VRSA) [6]. Two percent of the world population are nasal carriers of MRSA. Invasive infection caused by S. aureus and its drug resistant strains has decreased over the years but MRSA and other drug resistant strains are still a major threat in healthcare settings [7].
Coumarin derivatives are well-known oxygenated fused bicyclic molecules. These molecules are known for their wide range of biological activities [8,9,10] including activity against Gram-positive [11] bacteria (S. aureus, [12] Bacillus subtilis [13,14]) and Gram-negative [11] (E. coli, [12,14] P. aeruginosa [15] and other bacterial species [16]). Coumarin derivatives as anti-A. baumannii agents are rare [17]. Pyrazole derivatives are another class of pharmacologically important molecules known for their wide range of therapeutic properties including antimicrobial activities [18,19,20]. Likewise, hydrazone derivatives are known for their wide range of biological activities including antibacterial properties; e.g., rifampicin, an approved drug to treat tuberculosis [21,22].
2. Results and Discussion
2.1. Chemistry
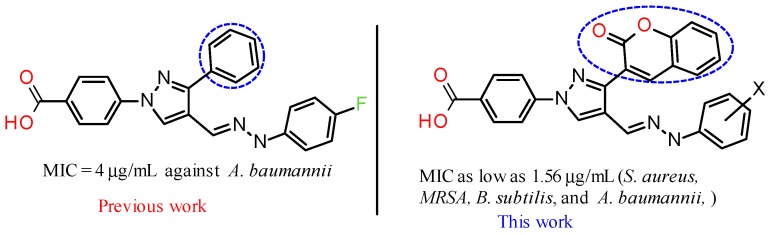
In our quest to develop novel methodologies to synthesize bioactive molecules [23,24,25,26], we have reported the synthesis of novel pyrazole derivatives as potent antimicrobial agents [27,28,29]. Based on our lead molecules and known pharmacological properties of coumarin derivatives, we designed the novel molecules hoping to produce potent antimicrobial agents (Figure 1).
Figure 1.
Pyrazole-derived hydrazones as potent antimicrobial agents.
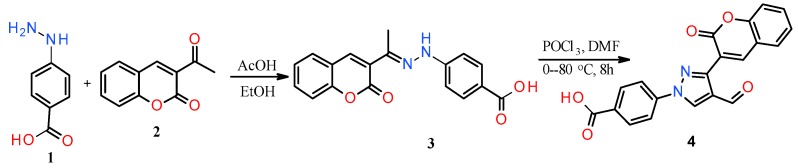
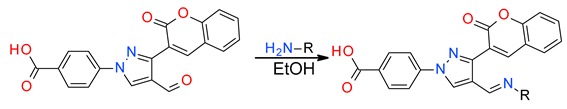
The ease of preparation of the aldehyde derivative (4) in multi-gram scale without work-up and column purification (Scheme 1) is the key to synthesizing a number of hydrazone derivatives (5–31) for the study of structure activity relationship (SAR). The reaction of hydrazinobenzoic acid (1) with 3-acetylcoumarin (2) formed the hydrazone derivative (3), which on reaction with in situ generated Vilsmeier reagent afforded the starting material (4).
Scheme 1.
Synthesis of coumarin-substituted pyrazole aldehyde.
Designed molecules were synthesized by the reaction of the aldehyde derivative (4) with commercially available substituted hydrazines in ethanol to afford products in very good average yield. All the molecules are characterized by 1H- and 13C-NMR spectroscopy and their structures are confirmed by high resolution mass spectrometry (see Supplementary Materials). Due to the labile nature of the carboxylic acid proton, it does not appear in most of the 1H-NMR spectra. All other hydrogens and carbons are accounted for in the spectra. These novel molecules were tested against 14 bacterial strains including ESKAPE pathogens.
2.2. Biology
2.2.1. Antimicrobial Studies
The phenyl substituted derivative (5) did not show any significant activity against the tested bacteria. N-Methyl-N-phenyl derivative (6) also failed to show any significant activity. N-Benzyl-N-phenyl derivative (7) inhibited the growth of tested Gram-positive strains: S. aureus and B. subtilis with an MIC value of 6.25 μg/mL. N,N-Diphenyl substitution (8) showed improved activity against B. subtilis with an MIC value as low as 3.125 μg/mL. Electron donating substituents such as ethyl (9) did not improve the growth inhibition ability of the molecules. Methoxy substitution completely ceased the potency of the resultant compound (10). Fluoro substitution showed moderate activity of the products (11, 12, and 13). Chloro substitution showed mixed results; the meta-chloro derivative (14) did not inhibit the bacterial growth significantly but the para-substitution (15) showed very good potency of the resultant molecule with MIC values as low as 3.125 μg/mL concentration. A similar pattern was seen for the bromo-substituted compounds (16 and 17). Bisfluoro and polyfluorinated compounds (18, 19, and 20) failed to show any noteworthy activity against the tested bacterial strains. The bischloro substituted derivative (21) inhibited the growth of Gram-positive strains with an MIC value of 3.125 μg/mL. The 2-fluoro-3-chloro derivative (22) failed to show any remarkable activity while the 3-chloro-4-fluoro substituted compound (23) showed moderated activity against S. aureus, B. subtilis, and A. baumannii. The 4-trifluormethyl phenyl hydrazone derivative (24) showed selective and potent activity against Gram-positive strains with MIC values as low as 3.125 μg/mL against B. subtilis. Other, strong electron withdrawing groups such as nitro (25), carboxylic acid (26 and 27), and cyano (28) terminated the activity of the resultant hydrazones. Alkyl substituted compounds (29, 30, and 31) also failed to show any activity against the tested strains of bacteria (Table 1).
Table 1.
Synthesis and antimicrobial activity of coumarin-substituted hydrazone derivatives, Gram-positive bacteria: S. aureus ATCC 25923 (Sa) and B. subtilis ATCC 6623 (Bs), Gram-negative bacteria: Escherichia coli ATCC 25922 (Ec), E. aerogenes ATCC 13048 (Ea), A. baumannii ATCC 19606 (type strain), Pseudomonas aeruginosa 27833 (Pa), Klebsiella pneumoniae ATCC 700603 (Kp), and NA = no activity (compounds did not show any noticeable activity up to 50 μg/mL concentration). Values are the average of two closely related experimental values.
| SN | Structure | Sa | Bs | Ec | Ea | Ab | Pa | Kp |
|---|---|---|---|---|---|---|---|---|
| 5 |
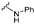
|
NA | NA | NA | NA | 25 | NA | NA |
| 6 |
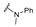
|
NA | >25 | NA | NA | NA | NA | NA |
| 7 |
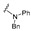
|
6.25 | 6.25 | NA | NA | NA | NA | NA |
| 8 |
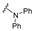
|
6.25 | 3.125 | NA | NA | NA | NA | NA |
| 9 |
|
>25 | 12.5 | NA | NA | NA | NA | NA |
| 10 |
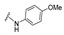
|
NA | NA | NA | NA | NA | NA | NA |
| 11 |
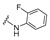
|
>25 | >25 | NA | NA | 25 | NA | NA |
| 12 |
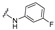
|
25 | 25 | NA | NA | 6.25 | NA | NA |
| 13 |
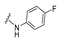
|
>25 | 25 | NA | NA | 25 | NA | NA |
| 14 |
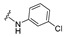
|
>25 | >25 | NA | NA | 25 | NA | NA |
| 15 |
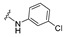
|
25 | 12.5 | NA | NA | 3.125 | NA | NA |
| 16 |
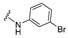
|
NA | NA | NA | NA | 12.5 | NA | NA |
| 17 |
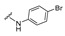
|
25 | 12.5 | NA | NA | 6.25 | NA | NA |
| 18 |
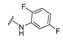
|
12.5 | NA | NA | NA | 12.5 | NA | NA |
| 19 |
|
NA | NA | NA | NA | NA | NA | NA |
| 20 |
|
NA | NA | NA | NA | NA | NA | NA |
| 21 |
|
3.125 | 3.125 | NA | NA | NA | NA | NA |
| 22 |
|
NA | NA | NA | NA | >25 | NA | NA |
| 23 |
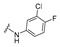
|
25 | 25 | NA | NA | 25 | NA | NA |
| 24 |
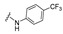
|
6.25 | 3.125 | NA | NA | NA | NA | NA |
| 25 |
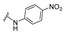
|
NA | NA | NA | NA | 25 | NA | NA |
| 26 |
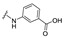
|
NA | NA | NA | NA | NA | NA | NA |
| 27 |
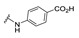
|
NA | NA | NA | NA | NA | NA | NA |
| 28 |
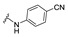
|
NA | NA | NA | NA | NA | NA | NA |
| 29 |
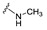
|
NA | NA | NA | NA | >25 | NA | NA |
| 30 |
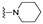
|
NA | NA | NA | NA | NA | NA | NA |
| 31 |
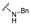
|
NA | NA | NA | NA | NA | NA | NA |
| Vancomycin | 0.78 | |||||||
| Colistin | 0.78 | |||||||
Bacillus species are spore-forming bacteria [30]. Compounds showing activity against B. subtilis could have the potential to treat anthrax. Potent compounds such as 8, 21, and 24 will be tested against B. anthracis Sterne strain in due course.
Molecules showing activity with MIC values as low as 25 μg/mL against S. aureus and A. baumannii type strain were tested against four other strains of Staphylococcus, three MRSA strains: S. aureus ATCC 33591 (MRSA), S. aureus ATCC 33592, and S. aureus ATCC 43300 (MRSA), plus S. epidermidis 700296, and two strains of A. baumannii: A. baumannii ATCC 747 (human clinical specimen) and A. baumannii ATCC BAA-1605 (isolated from a military personnel). It is worth noting that the three MRSA strains possess the SCC mec II or III genomic islands conferring multidrug resistance [31]. The N-benzyl-N-phenyl derivative (7) showed potent activity against two methicillin resistant strains of S. aureus with MIC values as low as 3.125 μg/mL. The bisphenyl substituted compound (8) inhibited the growth of all the tested strains of Staphylococcus with MIC values of 3.125 μg/mL against S. aureus ATCC 33592. Potent growth inhibition of this strain is very significant as it is not only methicillin resistant but also gentamicin resistant. Fluoro substituted compounds (11, 12, and 13) inhibited the tested strains with MIC values as low as 6.25 μg/mL. These molecules showed better growth inhibition activity against Gram-negative bacteria than the Gram-positive strains. Chloro and bromo substitution (14, 15, 16, and 17) produced potent activity; para-substituted products showed several times more activity than the meta products with MIC values as low as 1.56 μg/mL. Bis-halo products showed expected activity against the tested strains. Para-chloro and bromo substituted compounds (15 and 17) were the best in the series. The bisfluoro derivative (18) failed to show any significant activity against these additional strains. Bischloro derivatives (21) were found to be potent growth inhibitors of drug resistant S. aureus strains with MIC values as low as 3.125 μg/mL. The 3-chloro-2-fluoro derivative (22) was active against two strains of S. aureus with an MIC value of 3.125 µg/mL. 3-Chloro-4-fluoro substituted compound (23) showed broad-spectrum antibiotic activity but with less potency. Last but not least, we found a potent and selective inhibitor of drug resistant Gram-positive strains of S. aureus, the 4-trifluoromethyl phenyl derivative (24), with an MIC value of 6.25 µg/mL (Table 2).
Table 2.
Antimicrobial properties of the lead molecules against S. aureus ATCC 33591 (Sa91), S. aureus ATCC 33592 (Sa92), S. aureus ATCC 43300 (Sa00), S. epidermidis 700296 (Se), A. baumannii ATCC BAA-1605 (Ab05) and A. baumannii ATCC 747 (Ab47). NA = no activity (compounds did not show any noticeable activity up to 50 μg/mL concentration).
| SN | Sa91 | Sa92 | Sa12 | Sa00 | Se | Ab05 | Ab47 |
|---|---|---|---|---|---|---|---|
| 7 | NA | 3.125 | NA | 3.125 | NA | NA | >25 |
| 8 | 6.25 | 3.125 | 6.25 | 6.25 | 6.25 | NA | NA |
| 11 | NA | >25 | NA | >25 | NA | >25 | 12.5 |
| 12 | 25 | 25 | >25 | 25 | 25 | 25 | 6.25 |
| 13 | >25 | 25 | >25 | >25 | NA | 12.5 | 12.5 |
| 14 | NA | 25 | NA | >25 | >25 | >25 | >25 |
| 15 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 3.125 | 3.125 |
| 16 | NA | 25 | >25 | 25 | 25 | 12.5 | 12.5 |
| 17 | 12.5 | 12.5 | NA | 25 | 25 | 6.25 | 1.56 |
| 18 | NA | >25 | NA | NA | NA | NA | >25 |
| 21 | NA | 3.125 | NA | 3.125 | NA | NA | NA |
| 22 | NA | 6.25 | NA | 3.125 | NA | NA | NA |
| 23 | 25 | 25 | 12.5 | 12.5 | 25 | 25 | >25 |
| 24 | NA | 6.25 | 6.25 | 6.25 | 6.25 | NA | NA |
| Vancomycin | 0.78 | ||||||
| Colistin | 0.78 | ||||||
2.2.2. In vitro Toxicity Studies
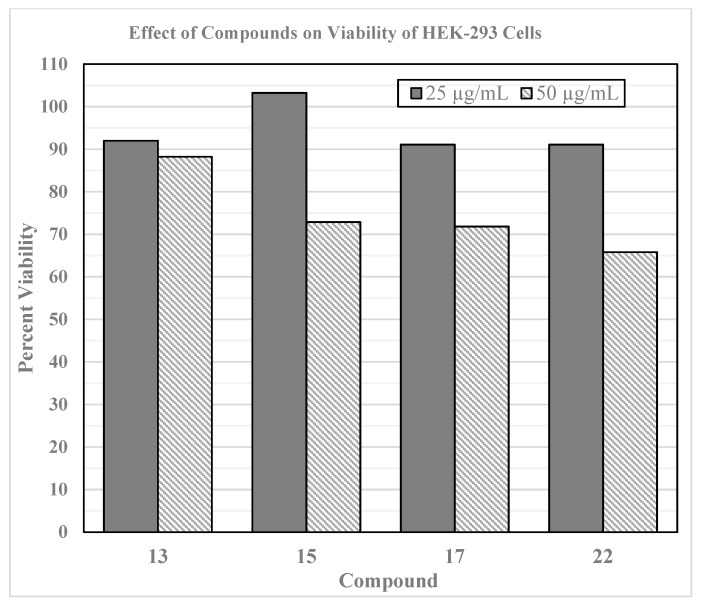
Active molecules were tested against a human embryonic kidney (HEK293) cell line at 25 µg/mL and 50 µg/mL concentrations for in vitro toxicity studies (Figure 2). Potent compounds (15 and 17) did not show any significant cytotoxicity at 25 µg/mL concentration. All of the synthesized compounds were submitted to the National Cancer Institute (NCI) for cytotoxicity studies. None of the compounds showed any significant cytotoxicity against NCI-60 cancer cell lines. Thus, these compounds are non-toxic to mammalian cell lines.
Figure 2.
Cytotoxic assay of active compounds on HEK293 cell line by using resazurin assay.
2.2.3. Calculated Physicochemical Properties
We calculated the physicochemical properties of the two most potent antimicrobial compounds (15 and 17) which are non-toxic to mammalian cell lines (Table 3). The n-octanol/water partition coefficient (ilogP) is a key parameter for drug design and development. The ilogP values are 3.07 and 3.26 for compounds 15 and 17 respectively [32]. Topological total surface area of these molecules is less than 140 Å, which indicates the potential for significant passive transport through the cell membrane [33].
Table 3.
Calculated physicochemical properties.
| Property | 15 | 17 |
|---|---|---|
| ilogP | 3.07 | 3.26 |
| TPSA (A2) | 109.72 | 103.59 |
2.2.4. In vivo Toxicity Studies
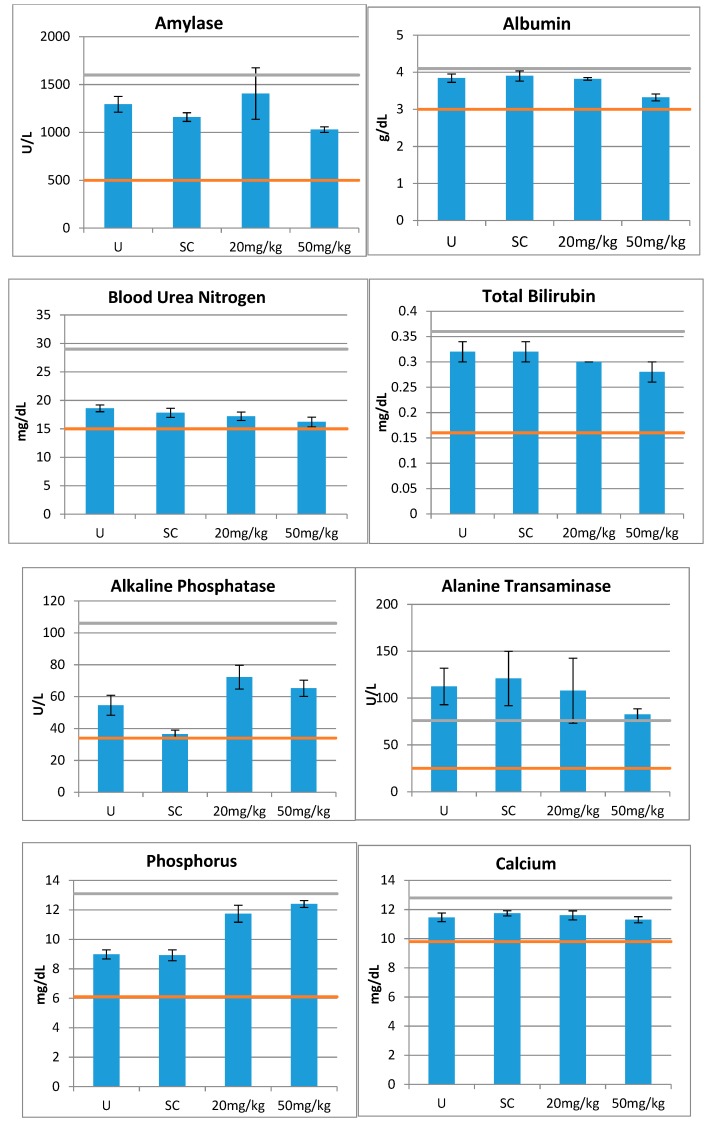
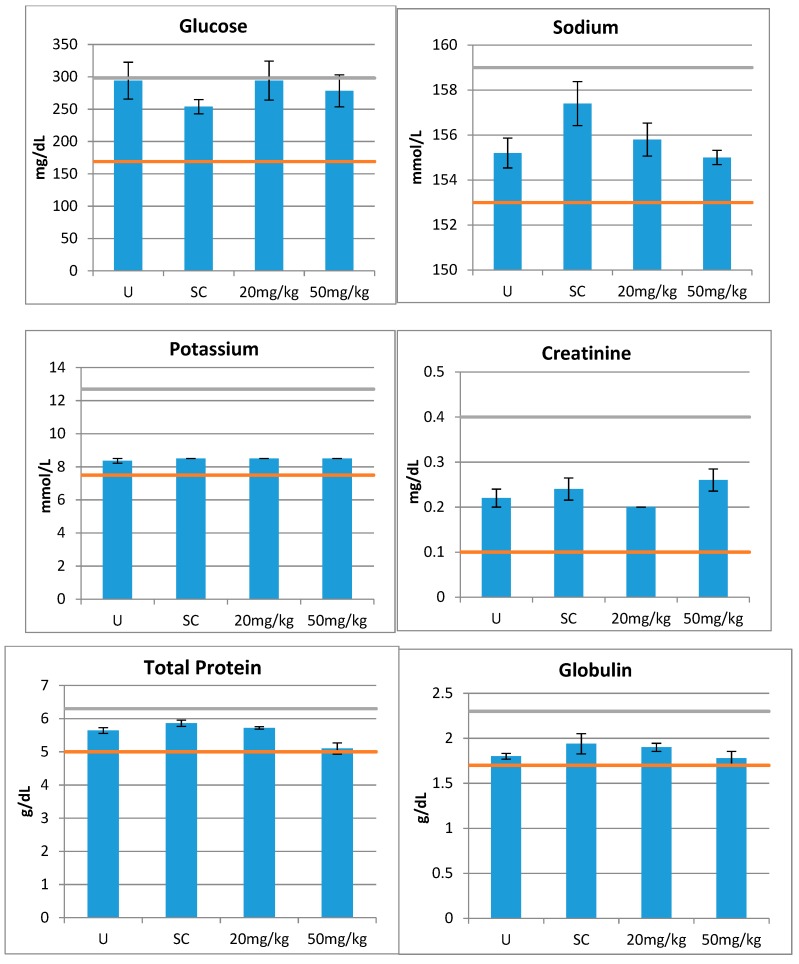
An important condition for eventual use of this compound in humans is the absence of toxicity in vivo animal model studies. Therefore, after demonstrating the absence of toxicity in vitro, we next examined whether compound 15 is toxic to mice. This compound was selected for its potent anti-A. baumannii activity, low ilogP, and non-toxicity in in vitro studies. In vivo effects of a single IP injection of the compound were assessed by 14 different parameters for organs’ functions as shown in Figure 3. This measurement clearly showed that none of the organ function markers indicated a toxicity by the used criteria. The majority of tests after the administration showed no significant difference from control samples, and none of them were beyond the normal ranges.
Figure 3.
In vivo toxicity of compound 15. Two doses (20 and 50 mg/kg) were administered IP in CD-1 mice (n = 5 for each dose). Blood samples were collected 24 hours later and tested by 14 parameters for organ functions. The orange and green lines indicate the normal ranges for the assays. U = Untreated and SC = Saline Control.
We tested 14 blood markers of organ functions that gave us a toxicity profile of 15 indicative of the complete absence of toxicity at the tested doses. Therefore, we can conclude that this compound is likely to be non-toxic in therapeutic doses. Colistin, the drug used to treat drug-resistant A. baumannii infection, will be extremely toxic at these doses [34]. This is a very important discovery, having in mind further potential application of this agent in humans. Certainly, for the development of a final pharmaceutical product, additional tests would be needed to detect pharmacological responses such as absorption, distribution, metabolism, and excretion studies.
3. Experimental Section
3.1. General Procedures
All the chemicals were purchased from Fisher Chemical (Hanover Park, IL, USA.) and Oakwoochemical (Estill, SC, USA). Commercially available solvents were used without drying. 1H- and 13C-NMR spectra were obtained with a Varian Mercury-300 MHz in DMSO-d6 with TMS as internal standard. Purity of the compounds were determined by the 1H- and 13C-NMR spectroscopy. The ESI-FTMS Mass spectra were recorded Bruker ApexII-FTMS system (Bremen, Germany).
3.1.1. Synthesis of Hydrazone Derivatives
A mixture of 4-hydrazinobenzoic acid hydrochloride (1, 1.980 g, 10.5 mmol), 3-acetylcoumarin (1.881 g, 10 mmol), and sodium acetate (0.82 g, 10 mmol) in ethanol. Ethanol was evaporated under reduced pressure and the hydrazone derivative was dried in vacuum. The dried material was subjected to further reaction without isolation or purification. The coumarin-derived hydrazone was dissolved in anhydrous N,N-dimethyl formamide (30 mL) and sealed by a rubber septum. The solution was cooled under ice for ~10 min, followed by the dropwise addition of phosphorous oxychloride (POCl3, 4.67 mL, 50 mmol). The reaction mixture was brought to room temperature and heated 80 °C for eight hours. After completion of the reaction, the reaction mixture was poured onto ice and was stirred for 12 h. The solid product was filtered and washed with water repeatedly followed by drying the product under vacuum to get the pure product in a very good overall yield [27,28,29].
3.1.2. Synthesis of Hydrazones
A mixture of the coumarin-derived aldehyde (360 mg, 1 mmol), hydrazine derivative (1.05 mmol) and sodium acetate (86 mg, 1.05 mmol), in case of the hydrochloride salt of the hydrazine derivatives, in anhydrous ethanol was refluxed for 8 h. The solid product was filtered and washed with water (~20 mL), followed by washing with ethanol (~15 mL) to get the pure product, which was dried under vacuum for biological studies.
3.2. MIC Studies
The Minimum Inhibitory Concentration (MIC) of the synthesized compounds was determined by a broth microdilution plate-based technique as per Clinical and Laboratory Standards Institute (CLSI) procedures for antimicrobial susceptibility testing of aerobic bacteria. In brief, bacteria were streaked onto Tryptic Soy Agar (TSA) or TSA with 5% sheep blood and incubated at 35 °C overnight. Colonies were suspended in sterile saline (0.9% NaCl) to match a 0.5 MacFarland standard then diluted 1:100 with Mueller Hinton broth to an estimated 1 × 106 CFU/mL concentration. Compounds to be tested were dissolved in DMSO to a 2 mg/mL concentration and diluted serially 1:2 in microplates such that after the addition of bacteria in broth the final concentrations began at 50 µg/mL. Inhibition of bacterial growth was determined using resazurin as a marker for cell viability [35]. Resazurin was added with bacteria to 8 µg/mL final concentration, and plates were incubated at 35 °C for 20–24 h and read visually. Negative controls (bacteria without inhibitors) and positive controls (bacteria plus a serially diluted antibiotic) were included on every plate. The MIC was determined as the lowest concentration where there was no color change of resazurin from purple to red (evidence of bacterial growth) [28,29].
3.3. Cytotoxicity Studies
In vitro toxicity of the active compounds were carried out according to our recently reported modified procedure [28,29]. HEK293 cells were counted using a Countess automated cell counter and 5000 cells/well were plated in black 96 well plates. Compounds were added in triplicate at 50 µg/mL and 25 µg/mL concentrations and incubated for 24 h followed by adding the 5% resazurin solution for a final volume of 200 µL in each well. Resazurin containing plates were incubated for four and six hours before the taking the readings for cell viability. Excitation and emission for fluorescence were measured at 544 nm and 590 nm respectively using a BMG Labtech Fluostar Optima plate reader.
3.4. In Vivo Toxicity Assessment
All animal experiments were performed at the Central Arkansas Veterans Healthcare System (John L. McClellan Memorial Veterans Hospital in Little Rock, AR, USA) and have been approved by the Institutional Animal Care and Use Committee. CD1 male mice (8 weeks old, 33–37 g) were purchased from Charles River Laboratories (Wilmington, MA, USA). Compound (15) was freshly dissolved in 0.9% saline, sterilized by ultrafiltration, and injected intraperitoneally (IP) in mice at two doses of 20 or 50 mg/kg (n = 5 per dose). The two control groups (n = 5/group) were untreated of administered with the vehicle (saline). The mice were euthanized 24 h after the injection, and blood was collected by cardiac puncture. Toxicity was assessed by measuring 14 blood markers of various organ function available in the Comprehensive Diagnosis Kit (Abaxis, Union City, CA, USA) and using VetScan VS2 instrument (Abaxis). The markers included: alanine aminotransferase (ALT), albumin (ALB), alkaline phosphatase (ALP), amylase (AMY), calcium (CA), creatinine (CRE), globulin (GLOB), glucose (GLU), phosphorus (PHOS), potassium (K+), sodium (Na+), total bilirubin (TBIL), total protein (TP), and blood urea nitrogen (BUN) measured by VetScan VS2 (Abaxis). Our criteria for toxicity included measurements being beyond the normal ranges, plus statistically significantly different from the untreated and vehicle (saline) controls.
3.5. Experimental Data
4-[4-Formyl-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (4). Whitish solid, yield: 92% (3.31 g) 1H-NMR (300 MHz, DMSO-d6): δ 9.93 (s, 1H), 9.35 (s, 1H), 8.47 (s, 1H), 8.12–8.05 (m, 4H), 7.87 (d, J = 6.7 Hz, 1H), 7.72–7.68 (m, 1H), 7.51 (d, J = 8.2 Hz, 1H), 7.45–7.40 (m, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 186.0, 167.3, 159.8, 154.0, 148.2, 143.7, 141.4, 133.5, 133.2, 131.3, 129.6, 125.4, 124.2, 120.1, 119.3, 119.2, 116.7, 115.2. HRMS (ESI-FTMS Mass (m/z): calcd for C20H12N2O5 [M + H]+ = 361.0819, found 361.0816.
4-[3-(2-Oxochromen-3-yl)-4-[(E)-(phenylhydrazono)methyl]pyrazol-1-yl]benzoic acid (5). Yellowish; yield: 82% (369 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.20 (s, 1H), 8.97 (s, 1H), 8.35 (s, 1H), 8.08 (s, 5H), 7.87–7.83 (m, 2H), 7.71–7.68 (m, 1H), 7.54–7.51 (m, 1H), 7.43 (t, J = 6.8 Hz, 1H), 7.00–6.95 (m, 2H), 6.75 (d, J = 7.4 Hz, 2H), 6.62 (t, J = 6.3 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.4, 153.9, 146.3, 145.6, 143.0, 142.4, 132.8, 131.4, 129.3, 129.2, 129.0, 127.2, 125.3, 122.1, 121.3, 119.3, 118.7, 118.3, 116.5, 112.0. HRMS (ESI-FTMS Mass (m/z): calcd for C26H19N4O4 [M + H]+ = 451.1401, found 451.1395.
4-[4-[(E)-[Methyl(phenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (6). Shiny yellow; yield: 90% (417 mg). 1H-NMR (300 MHz, DMSO-d6): δ 8.93 (s, 1H), 8.35 (s, 1H), 8.09 (s, 4H), 7.84–7.73 (m, 1H), 7.71–7.68 (m, 1H), 7.62 (s, 1H), 7.51–7.41 (m, 2H), 7.08–7.05 (m, 2H), 6.96–6.72 (m, 2H), 6.72 (t, J = 7.1 Hz, 1H), 3.30 (s, 3H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.5, 153.9, 147.5, 146.3, 142.9, 142.5, 132.6, 131.4, 129.2, 129.0, 128.8, 127.2, 125.4, 125.2, 122.6, 122.1, 120.0, 119.4, 118.3, 116.6, 114.5, 32.7. HRMS (ESI-FTMS Mass (m/z): calcd for C27H20N4O4 [M + H]+ = 465.1557, found 465.1545.
4-[4-[(E)-[Benzyl(phenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid(7). Yellow solid; yield: 92% (496 mg). 1H-NMR (300 MHz, DMSO-d6): δ 8.87 (s, 1H), 8.31 (s, 1H), 8.09-8.04 (m, 4H), 7.74 (d, J = 7.3 Hz, 1H), 7.74–7.69 (m, 1H), 7.58 (s, 1H), 7.51–7.42 (m, 2H), 7.30–7.21 (m, 3H), 7.13 (d, J = 7.0 Hz, 2H), 6.98–6.96 (m, 2H), 6.87–6.83 (m, 2H), 6.71 (t, J = 6.4 Hz, 1H), 5.19 (s, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.3, 153.8, 147.1, 146.2, 142.7, 142.4, 139.8, 136.2, 132.7, 131.4, 129.2, 129.1, 128.8, 127.8, 127.4, 126.6, 125.2, 122.8, 121.7, 120.1, 119.3, 118.3, 116.6, 113.9, 48.2. HRMS (ESI-FTMS Mass (m/z): calcd for C20H12N2O5 [M + H]+ = 541.1870, found 541.1860.
4-[4-[(E)-(Diphenylhydrazono)methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (8). Orange; yield: 91% (478 mg). 1H-NMR (300 MHz, DMSO-d6): δ 8.92 (s, 1H), 8.35 (s, 1H), 8.09–7.98 (m, 4H), 7.87 (d, J = 7.4 Hz, 1H), 7.75–7.70 (m, 1H), 7.52–7.43 (m, 2H), 7.27–7.22 (m, 5H), 7.16–7.09 (m, 2H), 6.93 (d, J = 7.7 Hz, 4H); 13C-NMR (75 MHz, DMSO-d6) δ 167.0, 159.3, 153.8, 146.1, 143.1, 142.9, 142.4, 132.8, 131.4, 130.2, 129.3, 128.9, 128.5, 128.0, 125.3, 124.8, 122.6, 122.2, 121.0, 119.3, 118.2, 116.6. HRMS (ESI-FTMS Mass (m/z): calcd for C32H22N4O4 [M + H]+ = 527.1714, found 527.1699.
4-[4-[(E)-[(2-Ethylphenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (9). Yellow solid; yield: 80% (382 mg). 1H-NMR (300 MHz, DMSO-d6): δ 9.48 (s, 1H), 8.98 (s, 1H), 8.53 (s, 1H), 8.12–8.09 (m, 6H), 7.86 (d, J = 7.6 Hz, 1H), 7.71 (t, J = 8.1 Hz, 1H), 7.52 (d, J = 8,2 Hz,1H), 7.44 (t, J = 7.4 Hz,1H), 6.96 (t, J = 7.2 Hz, 2H), 6.69–6.59 (m, 2H), 1.11 (t, J = 7.3 Hz, 3H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.4, 153.9, 146.4, 143.1, 142.8, 142.4, 132.8, 131.4, 130.3, 129.3, 128.6, 127.1, 126.5, 125.3, 122.2, 121.5, 119.3, 119.0, 118.3, 116.6, 112.3. HRMS (ESI-FTMS Mass (m/z): calcd for C28H22N4O4 [M + H]+ = 479.1714, found 479.1700.
4-[4-[(E)-[(4-Methoxyphenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (10). Orange solid; yield: 83% (398 mg). 1H-NMR (300 MHz, DMSO-d6): δ 9.96 (s, 1H), 8.93 (s,1H), 8.34 (s, 1H), 8.08 (s, 4H), 7.85 (d, J = 7.5 Hz, 1H), 7.77–7.69 (m, 2H), 7.53 (d, J = 8.2 Hz, 1H), 7.44 (t, J = 7.4 Hz,1H), 6.72–6.69 (m, 2H), 6.61–6.58 (m,1H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.4, 153.9, 152.7, 146.2, 143.0, 142.5, 139.7, 132.7, 131.4, 129.3, 128.9, 127.9, 126.8, 125.3, 122.2, 121.5, 119.4, 118.3, 116.6, 114.7, 113.0. HRMS (ESI-FTMS Mass (m/z): calcd for C27H20N4O5 [M + H]+ = 481.1506, found 481.1495.
4-[4-[(E)-[(2-Fluorophenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (11). Orange solid; yield: 80% (374 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.12 (s, 1H), 9.01 (s, 1H), 8.35 (s, 1H), 8.11–8.09 (m, 5H), 7.86 (d, J = 7.6 Hz, 1H), 7.71 (t, J = 7.7 Hz, 1H), 7.53 (d, J = 8.3 Hz, 1H), 7.44 (t, J = 7.3 Hz, 1H) 7.09–6.96 (m, 2H), 6.71–6.66 (m, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.4, 153.9, 150.9, 147.7, 146.4, 143.1, 142.3, 133.7 (d, J = 9.8 Hz), 132.4 (d, J = 64.2 Hz), 131.4, 129.3, 127.6, 125.3, 124.8, 122.1, 121.0, 119.3, 118.4, 116.5, 115.3 (d, J = 16.9 Hz), 113.8. HRMS (ESI-FTMS Mass (m/z): calcd for C26H17N4O4F [M + H]+ = 469.1307, found 469.1292.
4-[4-[(E)-[(3-Fluorophenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (12). Off orange; yield: 78% (365 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.42 (s, 1H), 9.02 (s, 1H), 8.36 (s, 1H), 8.09 (s, 4H), 7.89–7.84 (m, 2H), 7.69 (t, J = 7.8 Hz, 1H), 7.51–7.39 (m, 2H), 7.07–6.99 (m, 1H), 6.59–6.54 (m, 2H), 6.40 (t, J = 7.9 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 165.3, 162.1, 159.4, 153.8, 147.6 (d, J = 11.0 Hz), 146.4, 142.8 (d, J = 54.5 Hz), 132.8, 131.4, 130.8 (d, J = 9.9 Hz), 130.4, 129.3, 129.0, 127.6, 125.3, 121.9, 120.9, 119.3, 118.4, 116.6, 108.2, 104.8 (d, J = 21.3 Hz), 98.5 (d, J = 26.3 Hz). HRMS (ESI-FTMS Mass (m/z): calcd for C26H17N4O4F [M + H]+ = 469.1307, found 469.1294.
4-[4-[(E)-[(4-Fluorophenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (13). Yellow solid; yield: 75% (355 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.18 (s, 1H), 8.97 (s, 1H), 8.35 (s, 1H), 8.08 (s, 4H), 7.87–7.82 (m, 2H), 7.71 (t, J = 7.6 Hz, 1H), 7.54 (d, J = 8.3 Hz, 1H), 7.44 (t, J = 7.4 Hz, 1H), 6.84–6.78 (m, 4H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.4, 153.8, 146.3, 143.1, 142.4, 142.3, 132.8, 131.4, 129.3, 129.0, 127.1, 125.3, 122.0, 121.2, 119.3, 118.4, 116.6, 115.7 (d, J = 22.2 Hz), 112.9 (d, J = 7.2 Hz). HRMS (ESI-FTMS Mass (m/z): calcd for C26H17N4O4F [M + H]+ = 469.1307, found 469.1294.
4-[4-[(E)-[(3-Chlorophenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (14). Brown solid; yield: 81% (382 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.57 (s, 1H), 9.02 (s, 1H), 8.36 (s, 1H), 8.08 (s, 4H), 7.90 (s, 1H), 7.86 (d, J = 7.5 Hz, 1H), 7.71–7.66 (m, 1H), 7.50 (d, J = 8.2 Hz, 1H), 7.42 (t, J = 7.4 Hz, 1H), 7.02 (t, J = 7.9 Hz, 1H), 6.79 (s, 1H), 6.67 (d, J = 8.2 Hz, 1H), 6.63 (d, J = 7.7 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.4, 153.9, 147.0, 146.3, 143.1, 142.3, 134.2, 132.7, 131.4, 130.8, 130.6, 129.3, 129.2, 127.8, 125.3, 122.0, 120.9, 119.3, 118.4, 118.1, 116.8, 111.1, 110.8. HRMS (ESI-FTMS Mass (m/z): calcd for C26H17N4O4Cl [M + H]+ = 469.1307, found 469.1296.
4-[4-[(E)-[(4-Chlorophenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (15). Orange solid; yield: 77% (372 mg). 1H-NMR (300 MHz, DMSO-d6): δ 13.10 (br s, 1H), 10.35 (s, 1H), 9.00 (s, 1H), 8.37 (s,1H), 8.09 (s, 4H), 7.87–7.84 (m, 2H), 7.72 (t, J = 7.5 Hz, 1H), 7.54 (d, J = 8.2 Hz, 1H), 7.44 (t, J = 7.4 Hz, 1H), 7.02 (d, J = 8.7 Hz, 2H), 6.78 (d, J = 8.7 Hz, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 167.0, 159.4, 153.9, 146.4, 144.5, 143.2, 142.4, 132.9, 131.4, 130.1, 129.3, 129.05, 129.01, 127.4, 125.3, 121.94, 121.90, 121.0, 119.3,118.4, 116.6, 113.4. HRMS (ESI-FTMS Mass (m/z): calcd for C26H17N4O4Cl [M + H]+ = 469.1307, found 469.1292, Yield = 95%.
4-[4-[(E)-[(3-Bromo)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (16). Brown solid; yield: 83% (438 mg). 1H-NMR (300 MHz, DMSO-d6): δ 13.12 (br s, 1H), 10.40 (s, 1H), 9.03 (s, 1H), 8.37 (s, 1H), 8.09 (s, 4H), 7.87–7.84 (m, 2H), 7.68 (t, J = 7.7 Hz, 1H), 7.52 (d, J = 8.2 Hz, 1H), 7.42 (t, J = 7.4 Hz, 1H), 7.00–6.95 (m, 2H), 6.78 (d, J = 7.8 Hz, 1H), 6.72 (d, J = 8.1 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 167.0, 159.4, 153.9, 147.1, 146.4, 143.2, 142.4, 132.7, 131.4, 131.1, 130.8, 129.3, 129.0, 127.8, 125.3, 122.9, 121.9, 121.0, 120.8, 119.3, 118.4, 116.8, 114.0, 111.2. HRMS (ESI-FTMS Mass (m/z): calcd for C26H17N4O4Br [M + H]+ = 531.0487, found 531.0473.
4-[4-[(E)-[(4-Bromophenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (17). Yellow solid; yield: 81% (427 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.35 (s, 1H), 9.00 (s, 1H), 8.37 (s, 1H), 8.09 (s, 4H), 7.87–7.85 (m, 2H), 7.72 (t, J = 7.1 Hz, 1H), 7.55 (d, J = 8.2 Hz, 1H), 7.44 (t, J = 7.4 Hz, 1H), 7.14 (d, J = 8.7 Hz, 2H), 6.73 (d, J = 8.8 Hz, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 167.0, 159.4, 153.9, 146.4, 144.9, 143.2, 142.4, 132.9, 131.8, 131.4, 130.2, 129.3, 129.0, 127.4, 125.3, 121.8, 121.0, 119.3, 118.4, 116.6, 113.9, 109.5. HRMS (ESI-FTMS Mass (m/z): calcd for C26H17N4O4Br [M + H]+ = 531.0487, found 531.0471
4-[4-[(E)-[(2,5-Difluorophenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (18). Reddish solid; yield: 78% (377 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.36 (s,1H), 9.09 (s, 1H), 8.37 (s, 1H), 8.13–8.09 (m, 5H), 7.85 (d, J = 7.2 Hz, 1H), 7.68 (t, J = 7.2 Hz, 1H), 7.49–7.41 (m, 2H), 7.10 (br s, 1H), 6.77 (br s, 1H), 6.40 (br s, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 167.0, 159.5 (d, J = 235.7 Hz), 159.4, 153.8, 147.0, 146.5, 143.9, 143.3, 142.4, 135.1 (t, J = 11.8 Hz), 133.3, 132.9, 131.4, 129.3, 129.0, 128.0, 125.3, 121.7, 120.7, 119.2, 118.4, 116.6-116.0 (m), 103.8 (d, J = 24.0 Hz), 100.2 (d, J = 26.0 Hz). HRMS (ESI-FTMS Mass (m/z): calcd for C26H17N4O4F2 [M + H]+ = 487.1212, found 487.1199.
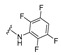
4-[3-(2-Oxochromen-3-yl)-4-[(E)-[(2,3,5,6-tetrafluorophenyl)hydrazono]methyl]pyrazol-1-yl]benzoic acid (19). Yellow solid; yield: 89% (464 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.28 (s, 1H), 8.94 (s, 1H), 8.31 (s, 1H), 8.17 (s, 1H), 8.05 (s, 3H), 7.82 (d, J = 7.5 Hz, 1H), 7.67 (t, J = 7.4 Hz, 1H), 7.46–7.37 (m, 2H), 7.14–7.02 (m, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 167.0, 159.4, 154.0, 148.1, 142.1, 142.3, 135.1, 132.6, 131.4, 129.3-129.2 (m), 128.1, 125.1, 121.7, 120.2, 119.3, 118.5, 116.4, 95.2 (t, J = 23.5 Hz). HRMS (ESI-FTMS Mass (m/z): calcd for C26H15N4O4F4 [M + H]+ = 523.1024, found 523.1008.
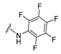
4-[3-(2-Oxochromen-3-yl)-4-[(E)-[(2,3,4,5,6-pentafluorophenyl)hydrazono]methyl]pyrazol-1-yl]benzoic acid (20). Yellow solid; yield: 90% (486 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.12 (s, 1H), 8.92 (s, 1H), 8.30 (s, 1H), 8.13–8.07 (m, 5H), 7.82 (d, J = 7.6 Hz, 1H), 7.67 (t, J = 7.6 Hz, 1H), 7.47–7.37 (m, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.4, 154.0, 146.6, 143.1, 142.3, 135.1, 132.6, 131.3, 129.5, 129.2, 128.0, 125.1, 121.6, 119.6, 119.3, 118.5, 116. HRMS (ESI-FTMS Mass (m/z): calcd for C26H14N4O4F5 [M + H]+ = 541.0930, found 541.0919.
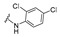
4-[4-[(E)-[(2,4-Dichlorophenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (21). Brownish solid; yield: 85% (440 mg). 1H-NMR (300 MHz, DMSO-d6): δ 9.94 (s, 1H), 9.05 (s, 1H), 8.37 (s, 1H), 8.27 (s, 1H), 8.09 (s, 4H), 7.86 (d, J = 7.7 Hz, 1H), 7.72 (t, J = 7.4 Hz, 1H), 7.54 (d, J = 8.1 Hz, 1H), 7.47–7.39 (m, 2H), 7.11 (d, J = 8.8 Hz, 1H), 6.89 (d, J = 8.8 Hz, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.4, 153.9, 146.6, 143.4, 142.3, 140.9 133.9, 132.9, 131.4, 129.4, 129.0, 127.8, 125.4, 122.2, 121.7, 120.7, 119.3, 118.5, 116.8, 116.6, 114.9. HRMS (ESI-FTMS Mass (m/z): calcd for C26H17N4O4Cl2 [M + H]+ = 519.0621, found 519.0615.
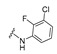
4-[4-[(E)-[(3-Chloro-2-fluoro-phenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (22). Orange solid; yield: 72% (361 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.37 (s, 1H), 9.04 (s, 1H), 8.35 (s, 1H), 8.14 (s, 1H), 8.09 (s, 4H), 7.86 (d, J = 7.3 Hz, 1H), 7.73–7.68 (m, 1H), 7.53 (d, J = 8.1 Hz, 1H), 7.45–7.41 (m, 1H), 6.98 (m, 1H), 6.78–6.67 (m, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.4, 153.8, 146.5, 144.68 (1JC-F = 239.8 Hz), 143.2, 142.4, 135.3, 135.1, 133.1 (2JC-F = 22.3 Hz), 131.4, 129.3 (2JC-F = 22.7 Hz), 127.9, 125.3, 121.8, 120.7, 120.0, 119.8, 119.3, 118.7, 118.4, 116.5, 112.5. HRMS (ESI-FTMS Mass (m/z): calcd for C26H17N4O4ClF [M + H]+ = 503.0917, found 503.0903.
4-[4-[(E)-[(3-chloro-4-fluoro-phenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (23). Yellow solid; yield: 76% (381 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.35 (s, 1H), 9.01 (s, 1H), 8.36 (s, 1H), 8.08–8.07 (m, 4H), 7.86–7.84 (m, 2H), 7.69 (t, J = 7.5 Hz, 1H), 7.51 (d, J = 8.2 Hz, 1H), 7.42 (t, J = 7.4 Hz, 1H), 7.09 (t, J = 8.9 Hz, 1H), 6.88–6.87 (m, 1H), 6.71–6.68 (m, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.4, 153.9, 152.4, 149.2, 146.3, 143.3, 142.3, 132.8, 131.4, 130.7, 129.4, 129.3, 127.7, 125.3, 121.9, 120.8, 120.2 (d, J = 18.3 Hz), 119.3, 118.4, 117.4 (d, J = 21.6 Hz), 116.8, 112.4, 111.8 (d, J = 6.4 Hz). HRMS (ESI-FTMS Mass (m/z): calcd for C26H17N4O4ClF [M + H]+ = 503.0917, found 503.0910.
4-[3-(2-oxochromen-3-yl)-4-[(E)-[[4-(trifluoromethyl)phenyl]hydrazono]methyl]pyrazol-1-yl]benzoic acid (24). Yellow solid; yield: 81% (419 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.68 (s, 1H), 9.03 (s, 1H), 8.37 (s, 1H), 8.09 (s, 4H), 7.92 (s, 1H), 7.87 (d, J = 7.5 Hz, 1H), 7.74–7.69 (m, 1H), 7.54 (d, J = 8.1 Hz, 1H), 7.46–7.41 (m, 1H), 7.31 (d, J = 8.3 Hz, 2H), 6.91 (d, J = 8.4 Hz, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.5, 153.9, 148.5, 146.5, 143.3, 142.4, 132.9, 131.9, 131.4, 129.4, 129.1, 127.7, 127.1, 126.6, 125.3, 121.7, 120.7, 119.3, 118.2, 116.6, 111.6. HRMS (ESI-FTMS Mass (m/z): calcd for C27H18N4O4F3 [M + H]+ = 519.1275, found 519.1262.
4-[4-[(E)-[(4-nitrophenyl)hydrazono]methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (25). Reddish; yield: 89% (440 mg). 1H-NMR (300 MHz, DMSO-d6): δ 11.23 (s, 1H), 9.13 (s, 1H), 8.42 (s, 1H), 8.11 (s, 4H), 8.04 (s, 1H), 7.96–7.87 (m, 3H), 7.74 (t, J = 7.3 Hz, 1H), 7.56 (d, J = 8.3 Hz, 1H), 7.46 (t, J = 7.4 Hz, 1H), 6.91 (d, J = 9.2, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 167.0, 159.5, 153.9, 150.8, 146.7, 143.4, 142.3, 138.4, 134.9, 133.0, 131.4, 129.4, 129.2, 128.3, 126.3, 125.4, 121.4, 120.2, 119.3, 118.5, 116.6, 111.2. HRMS (ESI-FTMS Mass (m/z): calcd for C26H18N5O6 [M + H]+ = 496.1252, found 496.1240.
4-[(2E)-2-[[1-(4-carboxyphenyl)-3-(2-oxochromen-3-yl)pyrazol-4-yl]methylene]hydrazino]benzoic acid (26). Brownish solid; yield: 83% (410 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.39 (s, 1H) 8.99 (s, 1H), 8.38 (s, 1H), 8.09 (s, 4H), 7.86–7.85 (m, 2H), 7.72–7.68 (m, 1H), 7.52 (d, J = 8.2 Hz, 1H), 7.45–7.42 (m, 2H), 7.24 (d, J = 7.3 Hz, 1H), 7.10–6.99 (m, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 167.9, 167.1, 159.5, 153.9, 146.4, 145.8, 143.3, 142.4, 132.9, 132.0, 131.4, 130.3, 129.4, 129.3, 129.2, 127.3, 125.3, 121.7, 120.9, 119.7, 119.3, 118.4, 116.6, 115.9, 112.9. HRMS (ESI-FTMS Mass (m/z): calcd for C27H19N4O6 [M + H]+ = 495.1299, found 495.1284.
4-[(2E)-2-[[1-(4-carboxyphenyl)-3-(2-oxochromen-3-yl)pyrazol-4-yl]methylene]hydrazino]benzoic acid (27). Orange solid; yield: 82% (405 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.73 (s, 1H), 8.97 (s, 1H), 8.37 (s, 1H), 8.08 (d, J = 8.5 Hz, 2H), 8.01 (d, J = 9.0 Hz, 3H), 7.87 (d, J = 7.1 Hz, 1H), 7.72 (t, J = 8.1 Hz, 1H), 7.62–7.52 (m, 3H), 7.44 (t, J = 7.5 Hz, 1H), 6.81 (d, J = 8.6 Hz, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 168.3, 167.9, 159.5, 153.9, 149.0, 146.0, 143.1, 141.0, 134.3, 132.8, 131.3, 131.1, 129.3, 127.6, 125.3, 122.1, 121.0, 120.5, 119.4, 118.0, 116.5, 111.1. HRMS (ESI-FTMS Mass (m/z): calcd for C27H19N4O6 [M + H]+ = 495.1299, found 495.1290.
4-[(2E)-2-[[1-(4-cyanophenyl)-3-(2-oxochromen-3-yl)pyrazol-4-yl]methylene]hydrazino]benzoic acid (28). Off yellow solid; yield: 78% (370 mg). 1H-NMR (300 MHz, DMSO-d6): δ 10.87 (s, 1H), 9.07 (s, 1H), 8.39 (s, 1H), 8.12-8.09 (m, 4H), 7.95 (s, 1H), 7.87 (d, J = 7.5 Hz, 1H), 7.75–7.70 (m, 1H), 7.56 (d, J = 8.2 Hz, 1H), 7.47–7.41 (m, 3H), 6.89 (d, J = 8.7 Hz, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.5, 153.9, 148.9, 146.6, 143.3, 142.3, 133.8, 133.0, 133.0, 131.4, 129.4, 129.2, 128.0, 125.4, 121.5, 120.5, 119.3, 118.5, 116.6, 112.1, 99.3. HRMS (ESI-FTMS Mass (m/z): calcd for C27H18N5O4 [M + H]+ = 476.1353, found 476.1349.
4-[4-[(E)-(methylhydrazono)methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (29). Brownish solid; yield: 69% (267 mg). 1H-NMR (300 MHz, DMSO-d6): δ 8.63 (s, 1H), 8.30 (s, 1H), 7.99 (d, J = 8.3 Hz, 2H), 7.84-7.81 (m, 3H), 7.66 (t, J = 7.5 Hz, 1H), 7.48–7.36 (m, 3H), 2.69 (s, 3H), 2.50 (s, 4H), 1.86 (s, 1H); 13C-NMR (75 MHz, DMSO-d6) δ 169.0, 159.4, 153.8, 154.3 142.9, 139.9, 138.3, 132.5, 130.7, 129.2, 125.4, 125.3, 125.1, 121.9, 121.8, 119.5, 117.5, 116.5. HRMS (ESI-FTMS Mass (m/z): calcd for C21H17N4O4 [M + H]+ = 389.1244, found 389.1239.
4-[3-(2-Oxochromen-3-yl)-4-[(E)-1-piperidyliminomethyl]pyrazol-1-yl]benzoic acid (30). Yellowish solid; yield: 86% (380 mg). 1H-NMR (300 MHz, DMSO-d6): δ 8.75 (s, 1H), 8.29 (s, 1H), 8.05 (s, 4H), 7.83 (d, J = 7.6 Hz, 1H), 7.68–7.63 (m, 1H), 7.50–7.46 (m, 2H), 7.42–7.37 (m, 1H), 2.94 (br s, 4H), 1.58 (br s, 4H), 1.43 (br s, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 13C-NMR (75 MHz, DMSO-d6) δ 167.1, 159.5, 153.9, 146.6, 142.8, 142.4, 132.6, 131.3, 129.2, 129.0, 127.4, 126.0, 125.1, 122.2, 121.8, 119.4, 118.4, 116.5, 51.9, 24.9, 24.0. HRMS (ESI-FTMS Mass (m/z): calcd for C25H23N4O4 [M + H]+ = 443.1714, found 443.1701.
4-[4-[(E)-(Benzylhydrazono)methyl]-3-(2-oxochromen-3-yl)pyrazol-1-yl]benzoic acid (31). Yellowish solid; yield: 84% (389 mg). 1H-NMR (300 MHz, DMSO-d6): δ 8.75 (s, 1H), 8.25 (s, 1H), 8.04 (s, 4H), 7.81 (s, 1H), 7.66 (s, 1H), 7.56 (s, 1H), 7.44 (d, J = 25.6 Hz, 2H), 7.22 (s, 5H), 4.18 (s, 2H); 13C-NMR (75 MHz, DMSO-d6) δ 167.0, 159.3, 153.9, 146.3, 143.2, 142.5, 139.6, 132.7, 131.3, 129.3, 128.8, 128.5, 128.3, 127.1, 125.9, 125.1, 121.9, 121.6, 119.3, 118.3, 116.5. HRMS (ESI-FTMS Mass (m/z): calcd for C27H21N4O4 [M + H]+ = 465.1557, found 465.1542.
4. Conclusions
We have reported the synthesis of new hydrazone derivatives of coumarin-derived pyrazoles. These new molecules are the potent growth inhibitors of Gram-positive and Gram-negative bacterial strains. We found several molecules that inhibited A. baumannii growth with an MIC value as low as 1.56 μg/mL. Some of them are also good inhibitors of MRSA and B. subtilis. Absence of toxicity in both in vitro and In vivo models are making them good candidates for further therapeutic development.
Supplementary Materials
Supplementary Materials are available online.
Author Contributions
Conceptualization, M.A.A.; methodology, J.W., C.D., A.S., S.A.C., R.A., and T.F,; Formal analysis, M.A.A., R.A., D.G., T.F., A.G.B.; writing-review and editing, M.A.A, D.G, and A.G.B; Funding acquisition, M.A.A, and D.G.
Funding
This publication was made possible by the Research Technology Core of the Arkansas INBRE program, supported by a grant from the National Institute of General Medical Sciences, (NIGMS), P20 GM103429 from the National Institutes of Health to record the Mass Spectrometry data. This publication was made possible by the Arkansas INBRE program, supported by a grant from the National Institute of General Medical Sciences, (NIGMS), P20 GM103429 from the National Institutes of Health, grant number P20 GM109005 (AGB); and VA Merit Review grant 2IO1BX002425 (AGB). ABI mini-grant 200136 and grant number P20 GM109005 (AGB); and VA Merit Review grant 2IO1BX002425 (AGB) helped to complete this project
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of all the compounds are available from the authors.
References
- 1.FDA Combating Antibiotic Resistance. [(accessed on 19 December 2018)]; Available online: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm092810.htm.
- 2.WHO Antimicrobial resistance. [(accessed on 6 February 2019)]; Available online: https://www.who.int/antimicrobial-resistance/en/
- 3.CDC Acinetobacter in Healthcare Settings Acinetobacter in Healthcare Settings. [(accessed on 22 September 2016)]; Available online: https://www.cdc.gov/hai/organisms/acinetobacter.html.
- 4.WHO Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. [(accessed on 28 February 2017)]; Available online: http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/
- 5.Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nature. 2017;543:15. doi: 10.1038/nature.2017.21550. [DOI] [PubMed] [Google Scholar]
- 6.CDC Staphylococcus aureus in Healthcare Settings. [(accessed on 28 October 2017)]; Available online: https://www.cdc.gov/hai/organisms/staph.html.
- 7.CDC Methicillin-resistant Staphylococcus aureus (MRSA) [(accessed on 28 October 2017)]; Available online: https://www.cdc.gov/mrsa/tracking/index.html.
- 8.Alam M.A., Reddy Y.S., Ali M.A. New and under explored epigenetic modulators in search of new paradigms. Med. Chem. 2015;11:271–285. doi: 10.2174/1573406410666140925150142. [DOI] [PubMed] [Google Scholar]
- 9.Stefanachi A., Leonetti F., Pisani L., Catto M., Carotti A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules. 2018;23:250. doi: 10.3390/molecules23020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurrapu S., Jonnalagadda S.K., Alam M.A., Ronayne C.T., Nelson G.L., Solano L.N., Lueth E.A., Drewes L.R., Mereddy V.R. Coumarin carboxylic acids as monocarboxylate transporter 1 inhibitors: In vitro and In vivo studies as potential anticancer agents. Bioorg. Med. Chem. Lett. 2016;26:3282–3286. doi: 10.1016/j.bmcl.2016.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Widelski J., Luca S.V., Skiba A., Chinou I., Marcourt L., Wolfender J.L., Skalicka-Wozniak K. Isolation and Antimicrobial Activity of Coumarin Derivatives from Fruits of Peucedanum luxurians Tamamsch. Molecules. 2018;23:1222. doi: 10.3390/molecules23051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangasuli S.N., Hosamani K.M., Devarajegowda H.C., Kurjogi M.M., Joshi S.D. Synthesis of coumarin-theophylline hybrids as a new class of anti-tubercular and anti-microbial agents. Eur. J. Med. Chem. 2018;146:747–756. doi: 10.1016/j.ejmech.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Walasek M., Grzegorczyk A., Malm A., Skalicka-Wozniak K. Bioactivity-guided isolation of antimicrobial coumarins from Heracleum mantegazzianum Sommier & Levier (Apiaceae) fruits by high-performance counter-current chromatography. Food Chem. 2015;186:133–138. doi: 10.1016/j.foodchem.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Valadbeigi E., Ghodsi S. Synthesis and Characterization of Some New Thiazolidinedione Derivatives Containing a Coumarin Moiety for their Antibacterial and Antifungal Activities. Med. Chem. 2017;7:178–185. doi: 10.4172/2161-0444.1000453. [DOI] [Google Scholar]
- 15.Rashdan H.R.M., Nasr S.M., El-Refai H.A., Abdel-Aziz M.S. A novel approach of potent antioxidant and antimicrobial agents containing coumarin moiety accompanied with cytotoxicity studies on the newly synthesized derivatives. J. Appl. Pharm. Sci. 2017;7:186–196. [Google Scholar]
- 16.Holiyachi M., Samundeeswari S., Chougala B.M., Naik N.S., Madar J., Shastri L.A., Joshi S.D., Dixit S.R., Dodamani S., Jalalpure S., et al. Design and synthesis of coumarin–imidazole hybrid and phenyl-imidazoloacrylates as potent antimicrobial and antiinflammatory agents. Monatsh Chem. 2018;149:595–609. doi: 10.1007/s00706-017-2079-5. [DOI] [Google Scholar]
- 17.Chusri S., Villanueva I., Voravuthikunchai S.P., Davies J. Enhancing antibiotic activity: A strategy to control Acinetobacter infections. J. Antimicrob. Chemother. 2009;64:1203–1211. doi: 10.1093/jac/dkp381. [DOI] [PubMed] [Google Scholar]
- 18.Faria J.V., Vegi P.F., Miguita A.G.C., dos Santos M.S., Boechat N., Bernardino A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017;25:5891–5903. doi: 10.1016/j.bmc.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Khan K.A., Faidallah H.M. 1-Substituted carbamoyl and thiocarbamoyl-4,5-dihydro-1H-pyrazoles as possible cytotoxic and antimicrobial agents. J. Enzym. Inhib. Med. Chem. 2016;31:619–627. doi: 10.3109/14756366.2015.1057717. [DOI] [PubMed] [Google Scholar]
- 20.Alam R., Wahi D., Singh R., Sinha D., Tandon V., Grover A., Rahisuddin Design, synthesis, cytotoxicity, HuTopoIIα inhibitory activity and molecular docking studies of pyrazole derivatives as potential anticancer agents. Bioorg. Chem. 2016;69:77–90. doi: 10.1016/j.bioorg.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Pyta K., Janas A., Szukowska M., Pecyna P., Jaworska M., Gajecka M., Bartl F., Przybylski P. Synthesis, docking and antibacterial studies of more potent amine and hydrazone rifamycin congeners than rifampicin. Eur. J. Med. Chem. 2019;167:96–104. doi: 10.1016/j.ejmech.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Lee J.Y., Jeong M.C., Jeon D., Lee Y., Lee W.C., Kim Y. Structure-activity relationship-based screening of antibiotics against Gram-negative Acinetobacter baumannii. Bioorg. Med. Chem. 2017;25:372–380. doi: 10.1016/j.bmc.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Okolo C., Ali M.A., Newman M., Chambers S.A., Whitt J., Alsharif Z.A., Day V.W., Alam M.A. Hexafluoroisopropanol-Mediated Domino Reaction for the Synthesis of Thiazolo-androstenones: Potent Anticancer Agents. ACS Omega. 2018;3:17991–18001. doi: 10.1021/acsomega.8b02840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali M.A., Okolo C., Alsharif Z.A., Whitt J., Chambers S.A., Varma R.S., Alam M.A. Benign Synthesis of Thiazolo-androstenone Derivatives as Potent Anticancer Agents. Org. Lett. 2018;20:5927–5932. doi: 10.1021/acs.orglett.8b02587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alam M.A., Alsharif Z., Alkhattabi H., Jones D., Delancey E., Gottsponer A., Yang T. Hexafluoroisopropyl alcohol mediated synthesis of 2,3-dihydro-4H-pyrido[1,2-a]pyrimidin-4-ones. Sci. Rep. 2016;6:36316. doi: 10.1038/srep36316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsharif Z., Ali M.A., Alkhattabi H., Jones D., Delancey E., Ravikumar P.C., Alam M.A. Hexafluoroisopropanol mediated benign synthesis of 2H-pyrido[1,2-a]pyrimidin-2-ones by using a domino protocol. New J. Chem. 2017;41:14862–14870. doi: 10.1039/C7NJ03376A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brider J., Rowe T., Gibler D.J., Gottsponer A., Delancey E., Branscum M.D., Ontko A., Gilmore D., Alam M.A. Synthesis and antimicrobial studies of azomethine and N-arylamine derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-methicillin-resistant Staphylococcus aureus agents. Med. Chem. Res. 2016;25:2691–2697. doi: 10.1007/s00044-016-1678-8. [DOI] [Google Scholar]
- 28.Allison D., Delancey E., Ramey H., Williams C., Alsharif Z.A., Al-khattabi H., Ontko A., Gilmore D., Alam M.A. Synthesis and antimicrobial studies of novel derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-Acinetobacter baumannii agents. Bioorg. Med. Chem. Lett. 2017;27:387–392. doi: 10.1016/j.bmcl.2016.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zakeyah A.A., Whitt J., Duke C., Gilmore D.F., Meeker D.G., Smeltzer M.S., Alam M.A. Synthesis and antimicrobial studies of hydrazone derivatives of 4-[3-(2,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid and 4-[3-(3,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid. Bioorg. Med. Chem. Lett. 2018;28:2914–2919. doi: 10.1016/j.bmcl.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aizawa S.-I. Bacillus subtilis—The Representative of Gram-Positive Bacteria. In: Aizawa S.-I., editor. The Flagellar World. Academic Press; Cambridge, MA, USA: 2014. pp. 22–23. [Google Scholar]
- 31.Saber H., Jasni A.S., Jamaluddin T.Z.M.T., Ibrahim R. A Review of Staphylococcal Cassette Chromosome mec (SCCmec) Types in Coagulase-Negative Staphylococci (CoNS) Species. Malays. J. Med Sci. MJMS. 2017;24:7–18. doi: 10.21315/mjms2017.24.5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daina A., Michielin O., Zoete V. iLOGP: A Simple, Robust, and Efficient Description of n-Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Modeling. 2014;54:3284–3301. doi: 10.1021/ci500467k. [DOI] [PubMed] [Google Scholar]
- 33.Palm K., Stenberg P., Luthman K., Artursson P. Polar molecular surface properties predict the intestinal absorption of drugs in humans. Pharm. Res. 1997;14:568–571. doi: 10.1023/A:1012188625088. [DOI] [PubMed] [Google Scholar]
- 34.Falagas M.E., Kasiakou S.K. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care (Lond. Engl.) 2006;10:R27. doi: 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarker S.D., Nahar L., Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods (San DiegoCalif.) 2007;42:321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.