SUMMARY
Optimization of fermentation processes requires monitoring the species composition of starter cultures and their growth during fermentation. Most starter cultures contain closely related species. Nowadays, high-resolution melting (HRM) analysis is extensively used for multiplex identification of closely related species. In the present paper, we applied real-time polymerase chain reaction (PCR) with HRM analysis for the detection and differentiation of Lactobacillus sakei and L. curvatus. A primer pair was selected for the site of the rpoA gene of Lactobacillus spp. Eleven starter cultures and fifteen fermented sausages with a known bacterial composition were successfully tested using real-time PCR with HRM analysis with the developed primer pair.
Key words: real-time PCR, HRM, starter cultures, fermented sausages, Lactobacillus sakei, Lactobacillus curvatus
INTRODUCTION
Technology used to produce raw smoked sausages is based on the fermentation of raw meat by indigenous microflora or introduced starter cultures. Lactic acid bacteria (LAB) play a significant role in the technological process, decreasing the pH value and causing the formation of a number of aromatic compounds. Starter cultures are one of the key factors in the microbiological safety of fermented meat products due to competitive growth with pathogenic microorganisms and causative agents of spoilage. Thus, optimization of fermentation processes requires monitoring the species composition of starter cultures and their growth rate during fermentation.
Analysis of the microbiota of raw smoked sausages (1-4), as well as of starter cultures in the Russian market, showed that several LAB species are important for control of the technological process: Lactobacillus sakei, L. curvatus, L. pentosus, L. plantarum, L. paraplantarum and Pediococcus pentosaceus.
Fast, reliable identification methods that do not necessitate obtaining isolates of microorganisms are of great importance for the control of endogenous or introduced starter cultures. One such method is polymerase chain reaction (PCR). The design of species-specific primers is a trivial task. However, determining an unknown microbiological composition is necessary, but conducting separate reactions with all primer pairs is costly. To identify Lactobacillus spp., multiplex PCR based on the band size differentiation in agarose gel after electrophoresis can be used (5, 6). However, the design of several primer pairs presents a number of difficulties, in particular, preventing their mutual complementarity. Restriction fragment length polymorphism is another method for species differentiation (7). In this approach, one universal primer pair is used; however, when performing an additional restriction step, manipulations with amplicons are necessary, which increases the risk of cross-contamination. The electrophoresis stage of both methods extends the process. The development of real-time PCR, including the use of TaqMan probes, allowed the quantitative counting of microorganisms in the sample without the isolation of isolates in both single (8, 9) and multiplex reactions (10, 11). The use of primer probe set accelerates the study and increases the specificity of the reaction, but the use of probes raises the cost of analysis due to the complexity in the design of multiplex reactions among other factors.
A number of fermentation-relevant organisms belong to two clades: L. sakei and L. plantarum (12). Thus, to differentiate among the species within these clades, it is possible to use homologous genome regions with single nucleotide polymorphisms (SNPs). High-resolution melting (HRM) analysis is suitable for this purpose. A thermocycler with an intercalating saturating dye is used to perform HRM after real-time PCR and does not require additional manipulation. Wittwer et al. (13) first proposed HRM analysis in 1997, and it is currently used primarily for detecting SNPs, genetic mosaicism, copy number variant confirmation and as an alternative to gel electrophoresis (14). Also, HRM is now widely used to identify subspecies and related species of plant (15), fungi (16), Phytophthora spp. (17), nematodes (18, 19), mosquitoes (20), Plasmodium spp. (21), cyanobacteria (22) and bacteria (23-31).
Various strategies have been used for species-level identification of LAB using the HRM method. Porcellato et al. (29) used universal primers targeting the V1 and V3 regions of a 16S rRNA gene for strain-level characterization of isolates of various LAB species. Iacumin et al. (25) used a system of three primer pairs aimed at the genes dnaJ and dnaK to differentiate among species of the phylogenetic group L. casei. However, PCR was successfully used for the same purpose with HRM analysis with one primer pair (29, 30). The use of one primer pair has also been successful in differentiating among species and subspecies within other genera of microorganisms (15, 17, 18, 25, 26).
Isolated cultures were used in most of the aforementioned studies. However, HRM provided reliable results in the study of complex samples, such as artificially contaminated food samples (26, 27), cheese during fermentation (30), and clinical samples (23). Thus, HRM analysis can be used when DNA is isolated from various objects with different contents of the target microorganism.
In the present study, we designed a primer pair for the multiplex detection of the closely related species L. sakei and L. curvatus in samples of starter cultures and fermented sausages by real-time PCR with HRM analysis.
MATERIALS AND METHODS
Control strains and growth conditions
The positive controls were strains of Lactobacillus sakei and L. curvatus from the laboratory collection. Strains of L. plantarum, Listeria monocytogenes and Staphylococcus curvatus from the laboratory collection were used as negative controls. LAB were grown on MRS agar (Liofilchem, Roseto degli Abruzzi, Italy) at 30 °C for 48 h. L. monocytogenes and S. curvatus were grown on plate count agar (PCA; Liofilchem) at 37 °C for 48 h.
Samples of products and microbiological identification
The subjects of this work were commercial starter cultures (code symbols A-1 to A-11) and semi-dry fermented sausages (code symbols B-1 to B-15). Samples A-1 to A-8 and B-1 to B-9 had L. sakei in their compositions, samples A-9, A-10 and B-10–B-12 had L. curvatus, while samples A-11 and B-13 to B-15 did not contain either of these species.
The presence or absence of L. sakei or L. curvatus in the samples was confirmed by microbiological methods. First, 10 g of each starter culture were added to 90 mL of buffered peptone water (Liofilchem), while fermented sausages were sampled according to GOST R ISO 6887-2:2013 (32) by preparing 10-fold dilutions of samples. Then, 1 mL of each 10-fold dilution was inoculated into two Petri dishes containing MRS agar. Cultivation conditions were 30 °C for 48 h. We took three isolated colonies for biochemical identification from each of the Petri dishes of the last dilutions in which growth was observed. We took only Gram-positive bacilli from starter culture samples. Identification was performed using the API 50 CHL test system (Merck, Darmstadt, Germany).
Isolation of DNA
The control strains were suspended in sterile 0.9% saline solution until an opacity rate of 5 was achieved according to the McFarland standard. Starter cultures were dissolved in saline solution in a ratio of 1:10. Next, 1 mL of each sample suspension was centrifuged at 3286×g for 5 min in a microcentrifuge MiniSpin (Eppendorf, Hamburg, Germany), followed by the removal of 800 μL of the supernatant. The remaining 200 μL were resuspended for further DNA isolation on the MagNA Pure LC 2.0 isolation station (Roche, Mannheim, Germany) using the MagNA Pure LC DNA Isolation Kit III (bacteria, fungi) (Roche).
To isolate DNA from fermented sausages, 50 mg of samples were taken. Then, DNA isolation was performed on the MagNA Pure LC 2.0 isolation station (Roche) using the MagNA Pure LC DNA Isolation Kit II (tissue) (Roche).
Primer design
Primer pairs for sequencing and specific identification were designed using the rpoA gene sequences of L. sakei and L. curvatus (Table 1) taken from the GenBank database (33). Primer-BLAST (34) and OligoAnalyzer v. 3.1 (35) were used for primer design. The melting points (Tm) of the final amplicons were evaluated using uMeltSM software according to the Blake and Delcourt thermodynamic model (36).
Table 1. Sequences and positions of the primers used in the study.
| Primer | Method | Primer sequence (5’–3’) | Amplification region | Amplification size/bp | Tm/°C* |
|---|---|---|---|---|---|
| LscHRM-F | PCR with HRM | CCGTGGTTATGTTGCTGCTG | rpoA | 97 | 78.7 for L. sakei 79.1 for L. curvatus |
| LscHRM-R | GTTGACACGACTGATTGGGGTA | ||||
| LscSeq-F | Sequencing | CAAAGATTGCCAAATGCTCTGTC | rpoA | 291 | |
| LscSeq-R | GTACAGTAGCTGAAGGCGGC |
bp=base pairs, *calculated by uMelt software (36), PCR=polymerase chain reaction, HRM=high-resolution melting analysis
Sequencing of the rpoA gene site of positive controls
The amplicon of the target site of the rpoA gene was obtained using primers for sequencing. Each 50 μL of the reaction mixture contained primers at a concentration of 300 nM, deoxynucleotide triphosphates at a concentration of 0.2 mM each, MgCl2 at a concentration of 2.5 mM, HF-Fuzz DNA polymerase at a concentration of 1 unit of activity, 20 μL of 2.5× amplification buffer (Dialat, Moscow, Russian Federation), and 5 μL of DNA. Conditions for PCR were preliminary denaturation at 98 °C for 120 s and 35 amplification cycles (60 °C for 30 s, 72 °C for 15 s, and 98 °C for 10 s).
The amplicons were sequenced using Sanger’s method (37) in Evrogen, JSC (Moscow, Russian Federation). The obtained sequences were aligned using Clustal Omega (38) with the corresponding sites of the genes of the L. sakei FAM18311 strain (GenBank: NZ_CP020459.1) and L. curvatus MRS6 strain (GenBank: NZ_CP022474.1).
Conditions for real-time PCR with HRM analysis
Real-time PCR with HRM analysis was performed on the LightCycler® 96 (Roche) and CFX96™ (Bio-Rad Laboratories Inc., Hercules, CA, USA) amplifiers. Efficacy and specificity were evaluated and the cycle cut-off was determined with the use of real-time PCR reagent (Syntol, Moscow, Russian Federation). We conducted the study of positive controls, their dilutions, and mixtures of positive and negative controls using the LightCycler 480 HRM Master kit (Roche) and real-time PCR reagent (Syntol). Study of the samples of starter cultures was performed using the LightCycler 480 HRM Master kit, while fermented sausages were studied using real-time PCR reagent.
A volume of 20 μL of the reaction mixture analysed using the LightCycler 480 HRM Master kit contained primers at a concentration of 300 nM, 10 μL Master Mix 2× with ResoLight dye (Roche), 2.5 mM MgCl2 and 2 μL DNA. The PCR conditions were preliminary denaturation at 95 °C for 10 min and 45 amplification cycles (57 °C for 15 s, 72 °C for 10 s, and 95 °C for 10 s).
A volume of 25 μL of the reaction mixture analysed using the real-time PCR reagent (Syntol) comprised primers at a concentration of 300 nM, 10 μL of EVA Green mixture (final concentration 2.5 mM MgCl2, dNTP at a concentration of 0.25 mM each (2.5 mM each nucleotide, SynTaq polymerase at a concentration of 2.5 unit of activity, 1× EVA Green)) and 2 μL of isolated DNA. PCR conditions were preliminary denaturation at 95 °C for 7 min and 45 amplification cycles (60 °C for 40 s and 95 °C for 15 s).
The HRM analysis on LightCycler 96 was performed at 0.05 °C, whereas on CFX96, it was at 0.2 °C. Melting curves were analysed using LightCycler 96 software v. 1.1.1 (39) and Precision Melt Analysis software (40). The values of Tm were taken from the LightCycler 96 software (39) and CFX Maestro™ data (41). All samples were studied in triplicate.
HRM of mixed samples
To test the possibility of detection in mixed samples, mixtures of DNA from standard L. sakei and L. curvatus samples were prepared in the ratios 1:1, 1:2, 2:1, 1:10 and 10:1. DNA concentrations of the positive controls were aligned earlier according to the real-time PCR data. The conditions for conducting real-time PCR with HRM analysis were the same as those for other experiments, and HRM of the mixed samples was conducted in parallel with HRM of the positive controls.
Statistical analysis
All data were statistically analyzed using Microsoft Excel 2010 (42) and R statistical software (43). A comparison of the threshold value and Tm values was performed, and a multiple comparison was done using Dunn’s test (44) to determine which values were significantly different (p<0.05 with Bonferroni adjustment).
RESULTS AND DISCUSSION
Selecting a gene site
For the design of primers specific to Lactobacillus sp. clades, various target genes were used: groEL (28), spxB (30) and rpoA (7, 45). We chose rpoA gene based on literature data. Two SNPs were identified on this site: T and A in L. sakei and C and T in L. curvatus at positions 1779480 and 1779517, respectively, according to the sequence of L. sakei FAM18311 strain (GenBank: NZ_CP020459.1).
We conducted PCR for positive controls and sequencing to confirm the practicability of the use of this site. The obtained amplification products were sequenced. Using Clustal Omega software (38), we aligned the sequences to each other and to the corresponding sections of the genomes of L. sakei FAM18311 (GenBank: NZ_CP020459.1) and L. curvatus MRS6 (GenBank: NZ_CP022474.1). The result of the alignment is shown in Fig. S1 (33).
The gene sites of the positive controls coincided with the corresponding sites of the L. sakei FAM18311 and L. curvatus MRS6, which confirms the practicability of using this site for the differentiation between these two species.
Evaluation of efficiency and specificity, and definition of the cycle cut-off
We used the primer pair LscHRM (Table 1) to perform PCR followed by HRM analysis. To assess the efficiency, specificity, and cycle cut-off of the reaction, the DNA of a positive control of L. curvatus, its 10-fold dilution, and the DNA of negative controls were used (results are not shown). The DNA of L. sakei was also investigated. The ratio of positive control quantities was determined by the threshold values. Their concentrations were aligned for further studies.
The calculated correlation coefficient of real-time PCR on LightCycler 96 was R2=1.0 and the efficiency was E=96.5%. The equation of its linear regression line was:
| y=−3.5095x+47.42 /1/ |
The calculated correlation coefficient of real-time PCR on CFX96 was R2=0.999 and the efficiency was E=95.5%. The equation of its linear regression line was:
| y=−3.435x+50.588 /2/ |
The penultimate dilution was 10-4, which gave a positive result. The threshold value for 10-4 dilution using LightCycler 96 was 32.9 cycles, whereas using CFX96, it was 35.89 cycles. These values were taken as cycle cut-offs. The threshold values for 10-5 dilution were 36.26 and 37.5 cycles respectively. Thus, the dynamic range of LscHRM primers was five orders of magnitude. Negative controls came out after 35 cycles on LightCycler 96 and after 37 cycles on CFX96. According to real-time PCR results, the concentration of DNA of positive controls was aligned for further studies.
Analysis of the effect of the concentration of target DNA on the HRM result
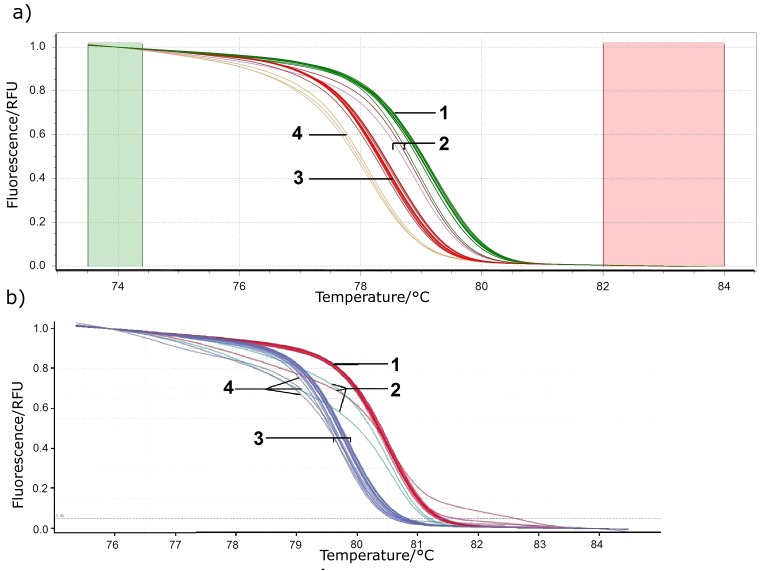
To analyse the effect of the initial DNA concentration on the HRM analysis, the DNA of positive controls and their four 10- -fold dilutions were studied. The melting curves of positive controls and their dilutions were divided into two clusters belonging to two species (L. sakei and L. curvatus). However, the melting curves of the fourth dilutions when using LightCycler 480 HRM Master Mix were isolated into their own clusters when analyzed on Precision Melt Analysis software (Fig. S2a). The result of analysis with LightCycler 96 software did not reveal a significant change in the shape of the curves (Fig. S2b). This is consistent with the literature data on the stability of the HRM results within four logarithms of the initial DNA concentration (46).
In this case, the Tm values of positive controls were close to those of all dilutions (Table 2 and Table S1), and varied depending on the amplifier and reagents. It should be noted that Tm values of one sample type may vary using separately (even if identically) prepared reagent master mixes within a single run (47), but the average melting points (ΔTm) of positive controls under all PCR conditions were similar and the overall average was 0.65 °C (σΔTm=0.034). Values Tm of dilutions could differ by 0.2 °C when working with data obtained with CFX96. However, the difference between the two groups remained reliable.
Table 2. Melting temperature (Tm) and threshold cycle values of positive controls and their dilutions obtained by LightCycler 96 (39).
| Lactobacillus sakei | Lactobacillus curvatus | |||||
|---|---|---|---|---|---|---|
| Dilution | Tm/°C | Threshold cycle | Dilution | Tm/°C | Threshold cycle | |
| LightCycler 480 HRM master kit | ||||||
| 1 | (79.70±0.08)a | (18.8±0.1)a | 1 | (80.46±0.03)a | (19.2±0.1)a | |
| 10-1 | (79.71±0.04)a | (22.50±0.04)a | 10-1 | (80.46±0.01)a | (22.6±0.1)a | |
| 10-2 | (79.8±0.1)a | (26.1±0.1)a | 10-2 | (80.43±0.04)a | (26.15±0.08)a | |
| 10-3 | (79.69±0.05)a | (29.7±0.1)a | 10-3 | (80.45±0.05)a | (29.67±0.09)a | |
| 10-4 | (79.69±0.05)a | (32.8±0.5)a | 10-4 | (80.44±0.05)a | (32.7±0.5)a | |
| Total | (79.73±0.07)b | Total | (80.45±0.03)b | |||
| Real-time PCR reagent | ||||||
| 1 | (80.63±0.03)a | (19.60±0.06)a | 1 | (81.29±0.03)a | (19.18±0.05)a | |
| 10-1 | (80.50±0.04)a | (22.89±0.05)a | 10-1 | (81.30±0.02)a | (22.8±0.1)a | |
| 10-2 | (80.56±0.08)a | (26.7±0.2)a | 10-2 | (81.28±0.03)a | (26.1±0.1)a | |
| 10-3 | (80.59±0.03)a | (30.0±0.2)a | 10-3 | (81.27±0.03)a | (29.5±0.1)a | |
| 10-4 | (80.55±0.03)a | (33.8±0.2)a | 10-4 | (81.25±0.03)a | (33.5±0.4)a | |
| Total | (80.58±0.05)b | Total | (81.28±0.03)b | |||
Data represent the mean value±standard deviation (aN=3, bN=15), PCR=polymerase chain reaction
Various data on the concentration of DNA and the threshold value at which the HRM result for a number of dilutions coincided are found in the literature. In a study by Winchell (31), the range of optimal threshold values was 15–35 cycles, whereas in the study by Koirala et al. (28), it was 21–28. The ranges of threshold values at which differentiation was possible are shown in Table 2.
In our study, we used a site with SNPs stable for each species and specific primers. Therefore, a discrepancy of 0.2 °C is permissible, and a change in the shape of the melting curve does not affect the result. Thus, the Tm value is sufficient for the differentiation of the two species of microorganisms at a wide range of microbial counts in a sample. It is necessary to compare Tm value of samples with the Tm value of positive controls of both species in one run for species differentiation by HRM. HRM result (ΔTm between L. sakei and L. curvatus groups) was the same using specialized reagent for HRM (LightCycler 480 HRM master kit) and common reagent for real-time PCR.
HRM of the studied samples
Real-time PCR was performed with HRM analysis of DNA samples of starter cultures and fermented sausages. Samples A-11, B-13, B-14 and B-15 gave negative results. Table 3 gives the Tm values of the samples.
Table 3. Melting temperature (Tm) of samples and positive controls.
| Lactobacillus sakei | Lactobacillus curvatus | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Tm/°C | Sample | Tm/°C | ||||||
| LightCycler 96 | CFX96 | LightCycler 96 | CFX96 | ||||||
| Starter culture | |||||||||
| Control strain | (79.79±0.08)a | (78.6±0.0)a | Control strain | (80.46±0.03)a | (79.2±0.0)a | ||||
| A-1 | (79.79±0.02)a | (78.6±0.0)a | A-9 | (80.49±0.01)a | (79.2±0.0)a | ||||
| A-2 | (79.66±0.09)a | (78.6±0.0)a | A-10 | (80.51±0.07)a | (79.0±0.0)a | ||||
| A-3 | (79.68±0.05)a | (78.6±0.0)a | |||||||
| A-4 | (79.70±0.08)a | (78.6±0.0)a | |||||||
| A-5 | (79.68±0.02)a | (78. 7±0.1)a | |||||||
| A-6 | (79.65±0.03)a | (78.5±0.1)a | |||||||
| A-7 | (79.68±0.04)a | (78.6±0.0)a | |||||||
| A-8 | (79.68±0.05)a | (78.7±0.1)a | |||||||
| Total | (79.70±0.07)b | (78.61±0.07)b | Total | (80.48±0.04)c | (79.1±0.1)c | ||||
| Fermented sausage | |||||||||
| Control strain | (80.8±0.1)a | (79.8±0.0)a | Control strain | (81.3±0.1)a | (80.4±0.0)a | ||||
| B-1 | (80.69±0.04)a | (79.9±0.1)a | B-10 | (81.20±0.08)a | (80.5±0.1)a | ||||
| B-2 | (80.66±0.03)a | (79.8±0.0)a | B-11 | (81.30±0.04)a | (80.6±0.0)a | ||||
| B-3 | (80.72±0.07)a | (79.8±0.0)a | B-12 | (81.30±0.04)a | (80.6±0.0)a | ||||
| B-4 | (80.7±0.1)a | (79.9±0.1)a | |||||||
| B-5 | (80.75±0.05)a | (79.7±0.1)a | |||||||
| B-6 | (80.59±0.08)a | (79.9±0.1)a | |||||||
| B-7 | (80.61±0.05)a | (79.8±0.0)a | |||||||
| B-8 | (80.55±0.09)a | (80.0±0.0)a | |||||||
| B-9 | (80.7±0.2)a | (79.9±0.1)a | |||||||
| Total | (80.7±0.1)d | (79.8±0.1)d | Total | (81.3±0.1)e | (80.5±0.1)e | ||||
Data represent the mean value±standard de nviation (aN=3, bN=27, cN=9, dN=30 and eN=12)
The Tm of the samples in each group (L. sakei or L. curvatus) was close to the Tm of the control samples. Moreover, the Tm of the groups significantly differed from each other.
In our study, the source of DNA (isolated cultures, starter cultures or fermented sausages) did not have a significant effect on Tm. However, Słomka et al. (47) observed a discrepancy in the data upon comparative HRM analysis of DNA of formalin-fixed and paraffin-embedded tissue samples.
The result of HRM was consistent with the data obtained by microbiological methods (Table S2). L. plantarum and L. rhamnosus were identified as dominant LAB species in sample A-11, L. plantarum was also identified in samples B-13 and B-14. P. pentosaceus was identified in sample B-15, L. sakei in samples A-1 to A-8 and B-1 to B-9, and L. curvatus in samples A-9, A-10 and B-10 to B-12.
Analysis of the dynamic range of HRM detection of both species in the sample
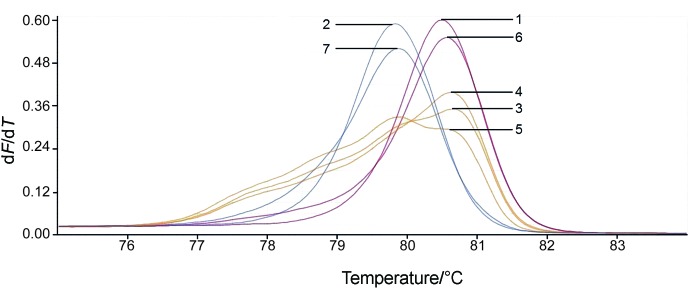
The normalized melting peaks of standard and mixed samples are shown in Fig. 1 (shown for one replication). HRM analysis with the primer pair LscHRM was only able to identify both species in a sample when their ratio was almost equal. Thus, faint peak for each species is seen in the DNA samples from mixtures of L. sakei and L. curvatus in ratios 2:1, 1:1 and 1:2. When the quantity of one species was ten times higher than that of the other, the HRM result was the same as for positive controls, which is in line with the literature (21). Detection of two species in one sample by the HRM method is not always possible even with the use of two primer pairs (21).
Fig. 1.
High-resolution melting analysis (HRM) of mixed samples. Normalized melt peaks analysed by LightCycler® 96 software (39). Samples: 1=Lactobacillus curvatus, 2=L. sakei, 3=L. curvatus and L. sakei mix in ratio 1:1, 4=L. curvatus and L. sakei mix in ratio 2:1, 5=L. curvatus and L. sakei mix in ratio 1:2, 6=L. curvatus and L. sakei mix in ratio 10:1, and 7=L. curvatus and L. sakei mix in ratio 1:10
CONCLUSIONS
In this study, real-time polymerase chain reaction (PCR) with high-resolution melting (HRM) analysis was successfully applied to quickly and accurately detect Lactobacillus sakei and L. curvatus in food samples. Real-time PCR with HRM is able to identify both species in the sample only when their ratio is equal. However, this limitation is not critical, as the dominant microflora is of technological importance. HRM differentiation is based on comparing Tm values of samples with Tm values of positive controls, which makes it possible to use different reagent kits for real-time PCR with saturating dye. In summary, HRM analysis is a simple, quick and cheap method. Real-time PCR with HRM analysis is a good alternative to multiplex PCR with fluorescent probes. It is relevant to the food industry, where closely related microbial species are widely used.
SUPPLEMENTARY MATERIAL
All supplementary material is available at www.ftb.com.hr.
Table S1. Melting temperature (Tm) and threshold cycle values of positive controls and their dilutions obtained by CFX96 (40).
| Lactobacillus sakei | Lactobacillus curvatus | |||||
|---|---|---|---|---|---|---|
| Dilution | Tm/°C | Threshold cycle | Dilution | Tm/°C | Threshold cycle | |
| LightCycler 480 HRM master kit | ||||||
| 1 | (78.5±0.1)a | (21.7±0.2)a | 1 | (79.1±0.1)a | (21.55±0.07)a | |
| 10-1 | (78.4±0.0)a | (25.09±0.03)a | 10-1 | (79.0±0.0)a | (25.23±0.08)a | |
| 10-2 | (78.4±0.0)a | (28.47±0.09)a | 10-2 | (79.1±0.1)a | (28.52±0.08)a | |
| 10-3 | (78.4±0.0)a | (32.2±0.2)a | 10-3 | (79.0±0.0)a | (32.32±0.03)a | |
| 10-4 | (78.2±0.0)a | (35.9±0.6)a | 10-4 | (79.0±0.0)a | (35.8±0.6)a | |
| Total | (78.4±0.1)b | Total | (80.45±0.03)b | |||
| Real-time PCR reagent | ||||||
| 1 | (79.4±0.0)a | (20.3±0.1)a | 1 | (80.0±0.0)a | (20.21±0.04)a | |
| 10-1 | (79.3±0.1)a | (24.07±0.08)a | 10-1 | (79.8±0.0)a | (24.15±0.05)a | |
| 10-2 | (79.2±0.0)a | (28.5±0.1)a | 10-2 | (79.8±0.0)a | (27.8±0.1)a | |
| 10-3 | (79.2±0.0)a | (31.6±0.3)a | 10-3 | (79.8±0.0)a | (31.15±0.04)a | |
| 10-4 | (79.2±0.0)a | (35.3±0.2)a | 10-4 | (79.8±0.0)a | (34.5±0.4)a | |
| Total | (79.25±0.09)b | Total | (79.84±0.08)b | |||
Data represent the mean value±standard deviation (aN=3, bN=15). PCR=polymerase chain reaction
Table S2. The comparison of high-resolution melting analysis (HRM) and biochemical identification results of samples.
| Sample code | Species included in the composition of the starter culture |
Microbiogical identification* | PCR with LscHRM primers |
HRM differentation |
|---|---|---|---|---|
| Starter culture | ||||
| A-1 | Lactobacillus sakei, Debaromyces hansenii, Pediococcus pentosaceus, Staphylococcus carnosus, S. xylosus | L. sakei | + | L. sakei |
| A-2 | L. sakei, S. carnosus | L. sakei | + | L. sakei |
| A-3 | L. sakei, S. carnosus | L. sakei | + | L. sakei |
| A-4 | L. sakei, S. carnosus | L. sakei | + | L. sakei |
| A-5 | L. sakei, S. carnosus | L. sakei | + | L. sakei |
| A-6 | L. sakei, S. carnosus | L. sakei | + | L. sakei |
| A-7 |
L. sakei, S. carnosus, S. xylosus, P. pentosaceus, Candida famata |
L. sakei | + | L. sakei |
| A-8 | L. sakei, S. carnosus | L. sakei | + | L. sakei |
| A-9 | L. curvatus, S. carnosus, S. xylosus, Lactococcus lactis, D. hansenii | L. curvatus | + | L. curvatus |
| A-10 | L. curvatus | L. curvatus | + | L. curvatus |
| A-11 |
L. plantarum, L. rhamnosus, Kocuria varians |
L. plantarum (5 colonies) L. rhamnosus (1 colony) |
- | |
| Fermented sausage | ||||
| B-1 | L. sakei | + | L. sakei | |
| B-2 | L. sakei | + | L. sakei | |
| B-3 | L. sakei | + | L. sakei | |
| B-4 | L. sakei | + | L. sakei | |
| B-5 | L. sakei | + | L. sakei | |
| B-6 | L. sakei | + | L. sakei | |
| B-7 | L. sakei | + | L. sakei | |
| B-8 | L. sakei | + | L. sakei | |
| B-9 | L. sakei | + | L. sakei | |
| B-10 | L. curvatus | + | L. curvatus | |
| B-11 | L. curvatus | + | L. curvatus | |
| B-12 | L. curvatus | + | L. curvatus | |
| B-13 | L. plantarum | - | ||
| B-14 | L. plantarum | - | ||
| B-15 | P. pentosaceus | - | ||
*According to API 50 CHL test system result of 6 isolated colonies from the Petri dishes of the last dilutions in which growth was observed; +=positive result, -=negative result, PCR=polymerase chain reaction
Fig. S1.
Alignment of the sequences of Lactobacillus sakei and L. curvatus laboratory strains with the respective sections of the rpoA gene from the strains in the NCBI database (L. sakei FAM18311 strain and L. curvatus MR56 strain) (33). SNPs are highlighted in red, primer (LscHRM-F and LscHRM-R) positions are marked
Fig. S2.
Normalized melting curves analysed by: a) Precision Melt Analysis software (40), and b) LightCycler® 96 software (39). PCR was performed using LightCycler 480 HRM MasterMix. Samples: 1=1-103 dilutions of Lactobacillus curvatus, 2=104 dilution of L. curvatus, 3=1-103 dilutions of L. sakei, and 4=104 dilution of L. sakei. RFU=relative fluorescence unit
Footnotes
FUNDING: Work of EAK was supported by research program of Russian Academy of Science no. 0112-2015-0015-2017.
REFERENCES
- 1.Aymerich T, Martín B, Garriga M, Hugas M. Microbial quality and direct PCR identification of lactic acid bacteria and nonpathogenic staphylococci from artisanal low-acid sausages. Appl Environ Microbiol. 2003;69(8):4583–94. 10.1128/AEM.69.8.4583-4594.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cocolin L, Alessandria V, Dolci P, Gorra R, Rantsiou K. Culture independent methods to assess the diversity and dynamics of microbiota during food fermentation. Int J Food Microbiol. 2013;167(1):29–43. 10.1016/j.ijfoodmicro.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 3.Daga ES. Traditional home-made dry sausages produced in Sardinia: a study of the microflora [PhD Thesis]. Sassari, Italy: The University of Sassari; 2008. [Google Scholar]
- 4.Kozačinski L, Drosinos E, Čaklovica F, Cocolin L, Gasparik-Reichardt J, Vesković S. Investigation of microbial association of traditionally fermented sausages. Food Technol Biotechnol. 2008;46(1):93–106. [Google Scholar]
- 5.Kwon HS, Yang EH, Yeon SW, Kang BH, Kim TY. Rapid identification of probiotic Lactobacillus species by multiplex PCR using species-specific primers based on the region extending from 16S rRNA through 23S rRNA. FEMS Microbiol Lett. 2004;239(2):267–75. 10.1016/j.femsle.2004.08.049 [DOI] [PubMed] [Google Scholar]
- 6.Song Y, Kato N, Liu CX, Matsumiya Y, Kato H, Watanabe K. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S–23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol Lett. 2000;187(2):167–73. 10.1111/j.1574-6968.2000.tb09155.x [DOI] [PubMed] [Google Scholar]
- 7.Torriani S, Felis GE, Dellaglio F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl Environ Microbiol. 2001;67(8):3450–4. 10.1128/AEM.67.8.3450-3454.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbel SR, Von Nickisch-Rosenegk M, Kuhn M, Murugaiyan J, Wieler LH, Guenther S. Specific TaqMan® probes for the identification and quantification of lactobacilli in pharmaceuticals. J Probiotics Health. 2014;2(1):115 10.4172/2329-8901.1000115 [DOI] [Google Scholar]
- 9.Martín B, Jofré A, Garriga M, Pla M, Aymerich T. Rapid quantitative detection of Lactobacillus sakei in meat and fermented sausages by real-time PCR. Appl Environ Microbiol. 2006;72(9):6040–8. 10.1128/AEM.02852-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balashov SV, Mordechai E, Adelson ME, Sobel JD, Gygax SE. Multiplex quantitative polymerase chain reaction assay for the identification and quantitation of major vaginal lactobacilli. Diagn Microbiol Infect Dis. 2014;78(4):321–7. 10.1016/j.diagmicrobio.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 11.Klocke M, Mundt K, Idler C, McEniry J, O’Kiely P, Barth S. Monitoring Lactobacillus plantarum in grass silages with the aid of 16S rDNA-based quantitative real-time PCR assays. Syst Appl Microbiol. 2006;29(1):49–58. 10.1016/j.syapm.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 12.Cousin FJ, Lynch SM, Harris HMB, McCann A, Lynch DB, Neville BA, et al. Detection and genomic characterization of motility in Lactobacillus curvatus: confirmation of motility in a species outside the Lactobacillus salivarius Clade. Appl Environ Microbiol. 2015;81(4):1297–308. 10.1128/AEM.03594-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wittwer CT, Herrmann MG, Moss AA, Rasmussen RP. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques. 1997;22(1):130–1. 10.2144/97221bi01 [DOI] [PubMed] [Google Scholar]
- 14.Vossen RHAM, Aten E, Roos A, den Dunnen JT. High-resolution melting analysis (HRMA)—more than just sequence variant screening. Hum Mutat. 2009;30(6):860–6. 10.1002/humu.21019 [DOI] [PubMed] [Google Scholar]
- 15.Song M, Li J, Xiong Ch, Liu H, Liang J. Applying high-resolution melting (HRM) technology to identify five commonly used Artemisia species. Sci Rep. 2016;6:34133. 10.1038/srep34133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu S, Mirchevska G, Phatak SS, Li D, Luka J, Calderone RA, et al. Dynamic time warping assessment of high-resolution melt curves provides a robust metric for fungal identification. PLoS One. 2017;12(3):e0173320. 10.1371/journal.pone.0173320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zambounis A, Samaras A, Xanthopoulou A, Osathanunkul M, Schena L, Tsaftaris A, et al. Identification of Phytophthora species by a high resolution melting analysis: an innovative tool for rapid differentiation. Plant Prot Sci. 2016;52(3):176–81. 10.17221/179/2015-PPS [DOI] [Google Scholar]
- 18.Filipiak A, Hasiów-Jaroszewska B. The use of real-time polymerase chain reaction with high resolution melting (real-time PCR-HRM) analysis for the detection and discrimination of nematodes Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Mol Cell Probes. 2016;30(2):113–7. 10.1016/j.mcp.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 19.Doyle SR, Armoo S, Renz A, Taylor MJ, Osei-Atweneboana MY, Grant WN. Discrimination between Onchocerca volvulus and O. ochengi filarial larvae in Simulium damnosum (s.l.) and their distribution throughout central Ghana using a versatile high-resolution speciation assay. Parasit Vectors. 2016;9:536. 10.1186/s13071-016-1832-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ajamma YU, Mararo E, Omondi D, Onchuru T, Muigai AWT, Masiga D, et al. Rapid and high throughput molecular identification of diverse mosquito species by high resolution melting analysis. F1000Res. 2016;5:1949. 10.12688/f1000research.9224.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chua KH, Lim SC, Ng CC, Lee PC, Lim YAL, Lau TP, et al. Development of high resolution melting analysis for the diagnosis of human malaria. Sci Rep. 2015;5:15671. 10.1038/srep15671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manali KM, Arunraj R, Kumar T, Ramya M. Detection of microcystin producing cyanobacteria in Spirulina dietary supplements using multiplex HRM quantitative PCR. J Appl Phycol. 2016;29(3):1279–86. 10.1007/s10811-016-1011-4 [DOI] [Google Scholar]
- 23.Banowary B, Dang VT, Sarker S, Connolly JH, Chenu J, Groves P, et al. Differentiation of Campylobacter jejuni and Campylobacter coli using multiplex-PCR and high resolution melt curve analysis. PLoS One. 2015;10(9):e0138808. 10.1371/journal.pone.0138808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chernukha IM, Minaev MY, Kurbakov KA, Bataeva DS. Detection and identification of S. carnosus in starter cultures using real time PCR and subsequent HRM analysis of amplification products. Procedia Food Sci. 2015;5:38–41. 10.1016/j.profoo.2015.09.010 [DOI] [Google Scholar]
- 25.Iacumin L, Ginaldi F, Manzano M, Anastasi V, Reale A, Zotta T, et al. High resolution melting analysis (HRM) as a new tool for the identification of species belonging to the Lactobacillus casei group and comparison with species-specific PCRs and multiplex PCR. Food Microbiol. 2015;46:357–67. 10.1016/j.fm.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 26.Jin D, Luo Y, Zhang Z, Fang W, Ye J, Wu F, et al. Rapid molecular identification of Listeria species by use of real-time PCR and high-resolution melting analysis. FEMS Microbiol Lett. 2012;330(1):72–80. 10.1111/j.1574-6968.2012.02535.x [DOI] [PubMed] [Google Scholar]
- 27.Kagkli DM, Folloni S, Barbau-Piednoir E, Van den Eede G, Van den Bulcke M. Towards a pathogenic Escherichia coli Detection Platform Using Multiplex SYBR®Green real-time PCR methods and high resolution melting analysis. PLoS One. 2012;7(6):e39287. 10.1371/journal.pone.0039287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koirala R, Taverniti V, Balzaretti S, Ricci G, Fortina MG, Guglielmetti S. Melting curve analysis of a groEL PCR fragment for the rapid genotyping of strains belonging to the Lactobacillus casei group of species. Microbiol Res. 2015;173:50–8. 10.1016/j.micres.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 29.Porcellato D, Østlie HM, Liland KH, Rudi K, Isaksson T, Skeie SB. Strain-level characterization of nonstarter lactic acid bacteria in Norvegia cheese by high-resolution melt analysis. J Dairy Sci. 2012;95(9):4804–12. 10.3168/jds.2012-5386 [DOI] [PubMed] [Google Scholar]
- 30.Savo Sardaro ML, Levante A, Bernini V, Gatti M, Neviani E, Lazzi C. The spxB gene as a target to identify Lactobacillus casei group species in cheese. Food Microbiol. 2016;59:57–65. 10.1016/j.fm.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 31.Winchell JM, Wolff BJ, Tiller R, Bowen MD, Hoffmaster AR. Rapid identification and discrimination of Brucella isolates by use of real-time PCR and high-resolution melt analysis. J Clin Microbiol. 2010;48(3):697–702. 10.1128/JCM.02021-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.GOST R ISO 6887-2:2013. Microbiology of food and animal feeding stuffs. Preparation of test samples, initial suspension and decimal dilutions for microbiological examination. Part 2. Specific rules for the preparation of meat and meat products. Moscow, Russian Federation: Standartinform; 2014 (in Russian).
- 33.GenBank®. Bethesda, MD, USA: National Center for Biotechnology Information (NCBI), US National Library of Medicine; 2017. Available from: http://www.ncbi.nlm.nih.gov/.
- 34.Primer-BLAST. Bethesda, MD, USA: National Center for Biotechnology Information, U.S. National Library of Medicine; 2017. Available from: https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi.
- 35.OligoAnalyzer 3.1, Integrated DNA Technologies, Inc., Coralville, IA, USA; 2017. Available from: http://eu.idtdna.com/calc/analyzer.
- 36.uMeltSM, University of Utah, Wittwer Lab, Salt Lake City, UT, USA; 2017. Available from: https://www.dna.utah.edu/umelt/um.php.
- 37.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74(12):5463–7. 10.1073/pnas.74.12.5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clustal Omega EMBL-EBI. Wellcome Genome Campus, Hinxton, Cambridgeshire, CB10 1SD, UK; 2017. Available from: https://www.ebi.ac.uk/Tools/msa/clustalo/.
- 39.LightCycler® 96 SW 1.1, v. 1.1.0.1320, Roche Diagnostics, Mannheim, Germany; 2011.
- 40.Precision Melt Analysis TM. Software, v. 1.3, Bio-Rad Laboratories, Inc., Hercules, CA, USA; 2010. Available from: http://www.bio-rad.com/ru-ru/product/precision-melt-analysis-software?ID=df190aee-f184-497e-bfb6-b6dd632c99b5.
- 41.CFX Maestro™, v. 1.1, Bio-Rad Laboratories, Inc., Hercules, CA, USA; 2010. Available from: http://www.bio-rad.com/ru-ru/product/cfx-maestro-software-for-cfx-real-time-pcr-instruments?ID=OKZP7E15.
- 42.Microsoft Excel. 2010, Microsoft, Redmond, WA, USA; 2010. [Google Scholar]
- 43.RStudio Desktop v. 1.1.463, RStudio, Boston, MA, USA; 2018. Available from: https://www.rstudio.com/products/rstudio/download/.
- 44.Dunn’s test, v. 1.3.5, Comprehensive R Archive Network (CRAN), Vienna University of Economics and Business, Vienna, Austria; 2017. Available from: https://cran.r-project.org/web/packages/dunn.test/index.html.
- 45.Huang CH, Chang MT, Huang MC, Lee FL. Application of the SNaPshot minisequencing assay to species identification in the Lactobacillus casei group. Mol Cell Probes. 2011;25(4):153–7. 10.1016/j.mcp.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 46.Wittwer CT. High-resolution DNA melting analysis: advancements and limitations. Hum Mutat. 2009;30(6):857–9. 10.1002/humu.20951 [DOI] [PubMed] [Google Scholar]
- 47.Słomka M, Sobalska-Kwapis M, Wachulec M, Bartosz G, Strapagiel D. High resolution melting (HRM) for high-throughput genotyping—limitations and caveats in practical case studies. Int J Mol Sci. 2017;18(11):2316. 10.3390/ijms18112316 [DOI] [PMC free article] [PubMed] [Google Scholar]