Abstract
Background: CD123-targeted chimeric antigen receptor (CAR) T cell (CART123) for the treatment of acute myeloid leukemia (AML) and blastic plasmacytoid dendritic cell neoplasm has exhibited potential in clinical trials. However, capillary leakage syndrome, which is associated with endothelial cells damage, is under intensive focus in CART123 therapy.
Purpose: The present study aimed to explore the change in CD123 in endothelial cells and the injury to endothelial cells caused by CART123.
Methods: The expression of CD123 and cytotoxicity were assessed by flow cytometry. Cytokine release was assessed by ELISA. An in vitro co-culture model was designed to mimic the status, wherein CART123 was stimulated and cytokines were released.
Results: In the current study, CART123 exhibited cytotoxicity and the effects of cytokine production on endothelium, and the upregulation of CD123 enhanced the cytotoxicity. The addition of interferon (IFN)-γ and tumor necrosis factor (TNF)-α neutralizing antibodies can effectively reverse the upregulation of CD123 on the endothelial cells caused by CART123, while the cytotoxicity of CART123 in AML cell lines was not affected in vitro. Second, we proved that CD123 expresses in CART123 and would be upregulated after activation, putatively causing an overactivated and fratricide effect.
Conclusion: In summary, this study identified that the expression of CD123 on endothelial cells could be upregulated when co-cultured with CART123. Furthermore, IFN-γ and TNF-α could aggravate endothelial damage caused by CART123 in vitro.
Keywords: chimeric antigen receptor, CD123, capillary leak syndrome, cytokine release syndrome, acute myeloid leukemia, endothelium
Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia, accounting for about 80% of the adult cases.1,2 However, in children <10 years of age, AML in cases of acute leukemia is <10%.3 The outcome remains bleak, with a median survival of only 5–10 months, and the high rate of relapse is yet the primary challenge for AML therapy.4,5 Chimeric antigen receptor (CAR) T cell therapy has made breakthroughs showing a satisfactory efficacy, especially in B-cell malignancies. However, unlike CD19 which is restricted to B cells, it is difficult to find such a specific target in AML. Interestingly, several targets for AML have been studied, and among these, Lewis Y, CD33, CD123, and NKG2D-ligands have been applied to clinical trials.6 Currently, CD123 is under intensive research focus. Previous studies demonstrated CD123 is highly expressed in both AML blasts and leukemia stem cells,7–9 whereas it is also expressed in kinds of cells, such as normal hematopoietic stem and progenitor cells (nHSPCs), dendritic cells, monocytes, and endothelial cells.5,7,10,11 Promising anti-leukemic activity was exhibited in preliminary clinical trials in both AML and blastic plasmacytoid dendritic cell neoplasm (BPDCN) without treatment-related cytopenia and other unreversible toxicity.12 At the end of 2018, the US FDA granted the orphan drug designation for BPDCN to Mustang’s MB-102 (CD123 CAR T).
Figure 2.

CART123 exerts potent anti-leukemia and anti-endothelium activities in vitro. (A) Schematic of CAR constructs, showing that CARs consisted of anti-CD123 or anti-CD19, a CD8a hinge region, CD8 transmembrane and cytoplasmic regions, and a CD3ζ cytoplasmic region. The anti-CD123 CAR connected with EGFRt via the P2A peptide. (B) CAR expression on CART123 and CART19 was detected by biotin-conjugated goat anti-mouse IgG,F(ab’)2 fragment polyclonal antibody followed by PE-conjugated streptavidin. (C) The expression of CD123 in K562, KG-1a, MOLM-13, and NALM-6 cell lines and primary AML cells from two patients (AML-2 and AML-3) was analyzed by flow cytometry. (D) Cytotoxicity at 24 hrs of CART123, or NT, when co-cultured with MOLM-13, K562, and HUVECs was detected using 7-AAD (mean ±SEM, n=3). Data show one representative experiment. (E) Cytokine production by CART123, CART19, or NT co-cultured with primary AML cells (AML-2), myeloid leukemia cell lines K562, MOLM-13, B-ALL cell line NALM-6, and HUVECs for 24 hrs was analyzed. The culture supernatants were harvested and analyzed by ELISA (mean±SEM of triplicate). *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Abbreviations: CAR, chimeric antigen receptor; NT, non-transduced T; HUVECs, human umbilical vein endothelial cells; 7-AAD, 7-Aminoactinomycin D; AML, acute myeloid leukemia; B-ALL, B-cell acute lymphoblastic leukemia; MFI, mean fluorescence intensity; EGFRt, truncated form of the human epidermal growth factor receptor; E:T, the effector to target cell ratio.
However, as such a potential therapy, the safety of CD123-targeted CAR T cell (CART123) is crucial. Preclinical studies demonstrated that CART123 causes severe cytopenia, and it could be recovered by hematopoietic stem cell transplantation after CART123 elimination.13,14 Other studies have reported that the hematopoietic toxicity of CART123 is quite limited in both pre-clinical and clinical studies.12,15 Nevertheless, a few studies investigated the expression of CD123 on endothelial cells. The endothelial injury plays a vital role in the development of cytokine release syndrome (CRS) and capillary leakage syndrome (CLS).16–19 CART123 has a low level of specific lysis to the endothelial cells in vitro.5,20 However, the premise of these studies is that normal tissues, especially endothelial cells, express a low level of CD123. A previous study exhibited that CD123 is lowly expressed in endothelial cells but could be upregulated by interferon (IFN)-γ and tumor necrosis factor (TNF)-α,21 which in turn, might aggravate the endothelial cell damage by CART123. Severe CRS and CLS occurred in the two patients in Cellectis’ UCART123 clinical trials. Also, Linda et al22 developed non-human primates to address the potential effects of targeting CD123 on endothelial cells that significantly expressed CD123. Therefore, injury to endothelial cells could be a major cause of concern in CART123 therapy.
Furthermore, CRS is the most common adverse effect after CAR T cell treatment, with 18–100% incidence, whereas severe CRS had an incidence of 8–46% in the previous major CART19 clinical studies.23 The levels of both IFN-γ and TNF-α are elevated in the CRS; IFN-γ remains continuously elevated in CRS.24–26 Therefore, we hypothesized that in the state of CRS, the expression of CD123 on endothelial cells is upregulated due to the elevated levels of IFN-γ and TNF-α in vivo, thereby enhancing the endothelium injured by CART123, aggravating CRS, and leading to CLS. In order to preliminarily validate the endothelial cell injury, especially in the case of abundant cytokines release, we conducted in vitro experiments. Next, we studied the CD123 expression on other normal cells, such as T cells, myeloid leukemia cell lines, and CD34+ cells.
Methods and materials
Cell lines and primary cells
AML cell lines KG-1a and MOLM-13 (ATCC, USA), chronic myelogenous leukemia (CML) cell line K562 (ATCC, USA), B-cell acute lymphoblastic leukemia (B-ALL) cell line NALM-6 (ATCC, USA), human umbilical vein endothelial cells (HUVECs; Cat #8000, ScienCell, USA) before passage 7, human dermal microvascular endothelial cells (HDMECs; Cat #2000, ScienCell, USA) before passage 5, primary AML cells (AML-2 and AML-3), and healthy donor-derived blood samples were used in the current study. Blood samples from healthy volunteers or AML patients were obtained using an approved protocol by the Ethics Committee on the Fifth Medical Center of Chinese PLA General Hospital. These studies were conducted following the Declaration of Helsinki. All subjects have provided written informed consent before participating in this study. MOLM-13, K562, and primary AML cells were cultured with Roswell Park Memorial Institute (RPMI)-1640 supplemented with 10% heat-inactivated FBS, and KG-1a cells with 20% FBS. HUVECs and HDMECs were maintained in endothelial cell medium (ECM; Cat #1001, ScienCell).
T-cell transduction
The retroviral vectors encoding anti-CD123 and anti-CD19 CARs were constructed based on a modified Moloney murine leukemia virus (Mo-MLV) vector described previously.27 The CAR123 consisted of anti-CD123 single chain fragment variable(clone 32,716), CD8a hinge region, CD8 transmembrane domain, 41BB costimulatory domain, and CD3ζ cytoplasmic region. CAR19 with 41BB costimulatory domain was described previously.28 Normal donor T cells were positively selected from leukapheresis packs using Dynabeads human T-expander CD3/CD28 (Life technology) at a bead: T cells ratio of 1:1 and expanded in vitro with 100 U/mL interleukin (IL)-2 for up to 15 days. The T cells were transduced with retrovirus vector at 48 hrs after activation as reported previously.28
Flow cytometry
Anti-human antibodies were purchased from Becton Dickinson, BioLegend, and Miltenyi Biotec. The Accuri C6 (Becton Dickinson, USA) and FACS Calibur (Becton Dickinson, USA) were used for the analysis of various samples. For the detection of CAR expression, cells were stained with biotin-conjugated goat anti-mouse IgG,F(ab’)2 fragment polyclonal antibody (Jackson ImmunoResearch Laboratories Inc., USA), followed by APC or PE-conjugated streptavidin (Jackson ImmunoResearch Laboratories Inc. or Invitrogen, USA).
Colony-forming assays
Mobilized peripheral blood (MPB) cells were sorted by the CD34+ cell isolation kit (Miltenyi Biotec, Germany). MPB CD34+ cells were incubated with IFN-γ and TNF-α in StemSpan (StemCell Technologies, Inc.) for 24 hrs and then, for an additional 12 hrs with CART123 or NT at an E: T ratio of 2:1 or 10:1, plated in a methylcellulose-based medium (StemCell Technologies Inc.). A total of 1,000 CD34+ cells/mL of media were plated in 35-mm Petri dishes in triplicate and incubated at 37°C, 5% CO2 for 14–16 days. The method by Gill et al.13 was employed to quantify the myeloid colonies (total amount of colony forming unit (CFU)-granulocyte (G), macrophage (M), and granulocyte macrophage) and erythroid colonies (total amount of burst-forming unit-erythroid (BFU-E) and CFU-E) by optical microscopy. For normalization, the average colony number of one group was set at 100% and the values from the other groups were adjusted as described previously.29
Killing assay
Briefly, carboxyfluorescein succinimidyl ester (CFSE)-labeled effector cells were incubated with targets at the indicated ratios for 6 to 24 hrs. The cells were harvested, stained for 7-ADD (BioLegend, USA) and (or) Annexin V (BioLegend, USA), and subjected to flow cytometry analysis, using the established protocol.30 In all of the studies, the effector to target cell ratio (E:T) was calculated by the number of total T cells and targeted cells.
mRNA microarray analysis
Total RNA was extracted using TRIzol. Hybridization and scanning of the chips (PrimeView™ Human Gene Expression Array, Thermo Fisher Scientific Inc., USA) were performed as outlined in the Affymetrix technical manual by CapitalBio Corp. (Beijing, People’s Republic of China). Differentially regulated mRNA expression was defined as >1.5-fold changes and the P-value threshold (P<0.05) as compared to the saline.
Cytokine release
Effector cells (5×104) and target cells (5×104) were incubated in a total volume of 1 mL RPMI-1640 medium (Lonza, USA) with 10% FBS in 24-well plate for 24 hrs. The supernatant was harvested and analyzed by ELISA (MultiSciences Biotech Co., Ltd., People's Republic of China) according to the manufacturer’s protocol. All experiments were carried out in triplicate.
In vitro co-culture model
The in vitro co-culture model was designed to mimic the effects of abundant cytokines release on the endothelium and other cells when incubated with CART123 and AML cells indirectly. The 0.4-μm pore size Transwell in a 12-well plate (Corning, Cat #3460) was used in the current study, which can allow the passage of cytokines but not cells. A total of 1×105 effector cells (CART123 or NT) and 5×105 target cells (MOLM-13, K562, and primary AML cells) were seeded in the upper chamber and objective HUVECs, CD34+ cells, or PBMC were seeded in the lower chamber. The cells were maintained in culture with RPMI-1640 supplemented with 10% FBS for 24−36 hrs before analysis by flow cytometry. The supernatant was harvested and analyzed by ELISA according to the manufacturer’s protocol.
In vitro live cell imaging
HUVECs were labeled with Paul Karl Horan 26 (PKH26; Sigma-Aldrich, USA), and effector cells (CART123 and NT) were labeled with Carboxyfluorescein succinimidyl ester CFSE (BioLegend, USA) according to the manufacturers’ instructions. First, PKH-26-labeled HUVECs were seeded in 48-well plates and maintained in culture with ECM. Then, IFN-γ or PBS (control group) added to wells. After 24 hrs, the medium was removed, and the cells were washed two times with PBS, and the media was replaced with RPMI-1640 supplemented with 10% FBS. CFSE-labeled effector cells were seeded at E:T of 5:1. Subsequently, the cells were observed and recorded by Nikon Ti-E Inverted Live Cell Imaging System Manuals (Japan) at 5% CO2 and 37°C for 24 hrs. A red and green fluorescent image was captured, respectively, every 2.5 mins for each point selected.
Statistics analysis
Statistical analyses were performed using Prism version 7.0 (GraphPad). The difference between the two groups was assessed using Student’s t-test.
Results
TNF-α and IFN-γ upregulate the CD123 expression on endothelial cells in vitro
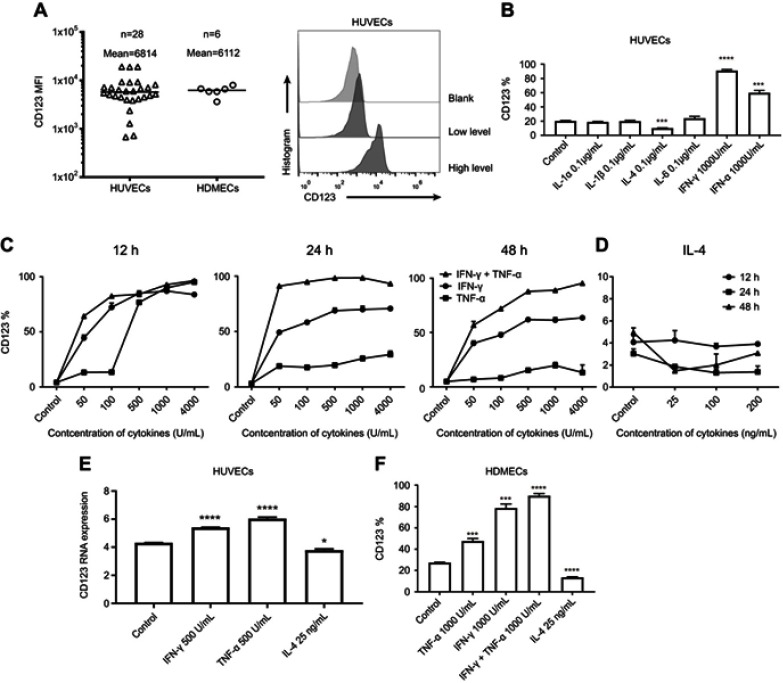
The expression of CD123 on HUVECs varied in vitro culture; we summarized the results of MFI expression with respect to CD123 on HUVECs and HDMECs (Figure 1A). As described previously, CD123 can be induced by IFN-γ and TNF-α on HUVECs.31 Firstly, the six cytokines (IL-1α, IL-1β, IL-4, IL-6, TNF-α, and IFN-γ) were incubated with HUVECs for 24 hrs, respectively. We confirmed that only TNF-α and IFN-γ upregulated CD123 and IL-4 downregulated CD123 (Figure 1B). At high levels of CD123 expression, the trend was consistent (data not shown). Furthermore, we studied the time- and dose-dependent changes of CD123 on HUVECs after incubation with different concentrations of IFN-γ, TNF-α (50–4000 U/mL), and IL-4 (25–200 ng/mL) for 12, 24, and 48 hrs (Figure 1C-D). 50 U/mL IFN-γ could sufficiently cause a significant change in CD123 expression within 12 hrs, whereas 500 U/mL was optimal for TNF-α. The upregulation of CD123 by IFN-γ was stronger than that by TNF-α, and the combination exerted a robust effect. When the concentration of cytokines >500 U/mL, the effect on the increased expression of CD123 was not apparent. The expression of CD123 was sufficiently downregulated by 25 ng/mL for 24 hrs. The mRNA level was confirmed by mRNA microarray analysis (Figure 1E). Also, CD123 expression on HDMECs shows similar results (Figure 1F). The results of mRNA microarray analysis showed that the inflammatory factors-related genes were significantly up-regulated after TNF-α treatment, including IL-6 (Figure S1).
Figure 1.
Expression of CD123 in HUVECs. (A) Surface expression of CD123 MFI on HUVECs and HDVECs was detected by staining with anti-CD123 APC and flow cytometry in Accuri C6. Representative plots are shown. (B) Expression of CD123 on HUVECs after treatment with cytokines (IL-1α, IL-1β, IL-4, IL-6, TNF-α, and IFN-γ) for 24 hrs was detected by flow cytometry (mean±SEM, n=3). Data show one representative experiment. (C-D) Expression of CD123 on HUVECs after treatment with IFN-γ, TNF-α (C), and IL-4 (D) at different concentrations for different time points (mean±SEM, n=3). Data show one representative experiment. (E) In HUVECs, the expression of CD123 after treated with IFN-γ, TNF-α, and IL-4 for 24 hrs was detected by mRNA Microarray Analysis (mean ± SEM of triplicate). (F) Expression of CD123 on HDMECs after treatment with IFN-γ, TNF-α, and IL-4 for 24h was detected by flow cytometry (mean±SEM, n=3). Data show one representative experiment. *P<0.05; ***P<0.001; ****P<0.0001.
Abbreviations: MFI, mean fluorescence intensity; IFN, interferon; TNF, tumor necrosis factor, HUVECs, human umbilical vein endothelial cells; HDMECs, human dermal microvascular endothelial cells.
CART123 damages the HUVECs and releases the cytokines after co-culturing
To evaluate the influence of HUVECs caused by CART123, we first constructed γ-retroviral vectors encoding the 41BB CD123-targeting CAR molecules with a truncated form of the human epidermal growth factor receptor (EGFRt) and the 41BB CD19-targeting CAR molecules (Figure 2A). Then, the expression of CAR was confirmed by flow cytometry (Figure 2B). Non-transducted T cells (NT) and CART19 were used as controls. The expression of CD123 and CD19 expression in these cell lines and primary AML blasts is presented in Figure 2C. These results showed that K562 and NALM-6 are CD123-negative; AML cell lines KG-1a and MOLM-13 are CD123-positive; only NALM-6 cells are CD19-positive. In primary AML cells from patients, AML-2 and AML-3 exhibited CD123 expression. Next, we assessed the cytotoxicity and cytokine production of CART123 in vitro. CART123 exhibited specific cytotoxic activities in MOLM-13 and HUVECs by flow cytometry; the cytotoxicity was not very strong in MOLM-13 than in HUVECs. Also, low cytotoxicity was exerted in K562 cells (Figure 2D). The difference between the adherent and suspension cells reduces the comparability of cytotoxicity. In addition, variable CD123 on HUVECs might be the reason for high cytotoxicity to HUVECs. Furthermore, when incubated with AML-2, K562, MOLM-13, and HUVECs in 24-well plate, CART123 produced more IFN-γ, TNF-α, and IL-3 compared to CART19, whereas CART19 produced more cytokines when cultured with NALM-6 as compared to CART123. Interestingly, the production of IL-2 and IL-4 was not obvious when CART123 was challenged with AML cells or HUVECs. Strikingly, abundant IL-6 was in both HUVEC+CART19 and HUVEC+CART123 cells, and cytokines production was high when challenged with K562. Also, the cytokine production by CART123 in MOLM-13 and AML-2 was specific but at a low level as compared to K562 (Figure 2E). The low cytokine release, when challenged with CD123hi AML cells, may be caused by the incubation system consisting of small cells (AML-2 and MOLM-13) and insufficient contact. This phenomenon also reduced the comparability between different target cells; however, this study also showed the specificity of CART123. Together, these results demonstrated that CART123 has specific killing and cytokine production effects on AML cells as well as HUVECs.
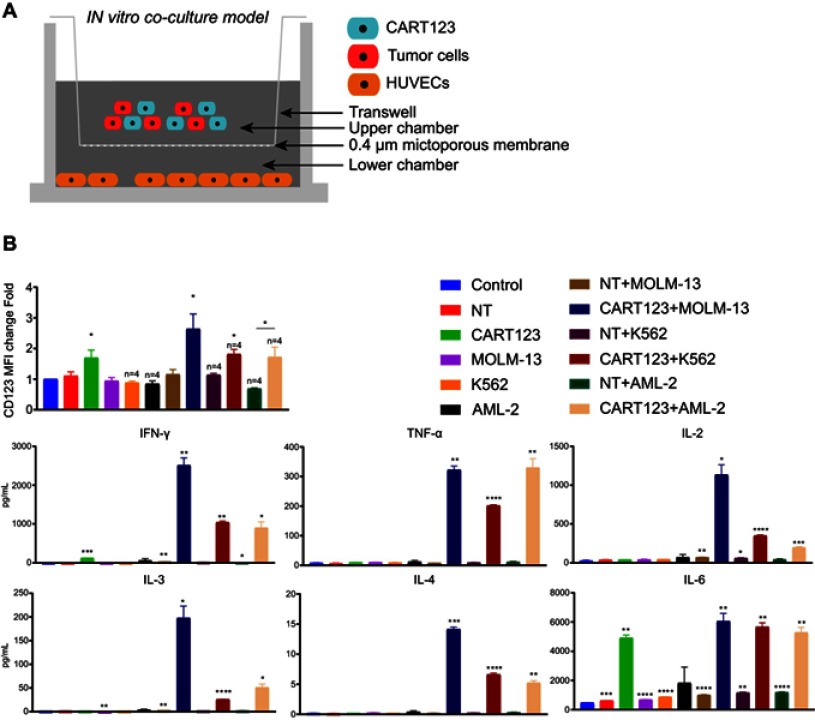
CART123 co-cultured with myeloid leukemia cells upregulated CD123 on HUVECs in the in vitro co-culture model
Although we confirmed the upregulation of IFN-γ- and TNF-α-mediated CD123 on HUVECs, the change when CART123 encounters AML cells and releases a large number of cytokines yet to be elucidated. To mimic the status that CART123 is stimulated by a large number of tumors and releases several cytokines in vitro, we designed the in vitro co-culture model for studying the altered expression of CD123 in cells (Figure 3A). Firstly, incubation of CART123 with MOLM-13, K562 cells resulted in a significant upregulation of CD123 MFI on HUVECs as compared to the control group as well as CART123 alone. Incubation of CART123 with AML-2 resulted in a significant upregulation as compared to NT+AML-2 group (Figure 3B). The evaluation of cytokine production revealed that IFN-γ, TNF-α, IL-2, IL-3, IL-4, and IL-6 were upregulated in the co-incubation group; among these, which IFN-γ, TNF-α, IL-2, and IL-6 were predominant. A moderate level of IFN-γ and significant production of IL-6 was observed in CART123 alone (Figure 3C). These findings indicated that CART123 co-cultured with myeloid leukemia cells and CART123 alone could release a large number of cytokines and induce the expression of CD123 on endothelium.
Figure 3.
CART123 co-cultured with myeloid leukemia cells upregulated CD123 on HUVECs in the in vitro co-culture model. (A) Schematic of the in vitro co-culture model. (B) Effector cells (CART123 or NT) and target cells (MOLM-13, K562, and AML-2) were seeded in the upper chamber, and HUVECs were seeded in the lower chamber of the in vitro co-culture model. After co-culturing for 36 hrs, HUVECs were digested with trypsin to obtain single cell suspension to assess the expression of CD123 by flow cytometry (mean±SEM). Data show the results of six independent experiments. (C) Also, the culture supernatants were harvested and used for analyzing cytokine production by ELISA. (mean±SEM of triplicate). *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Abbreviations: CAR, chimeric antigen receptor; NT, non-transduced T; HUVECs, human umbilical vein endothelial cells; AML, acute myeloid leukemia.
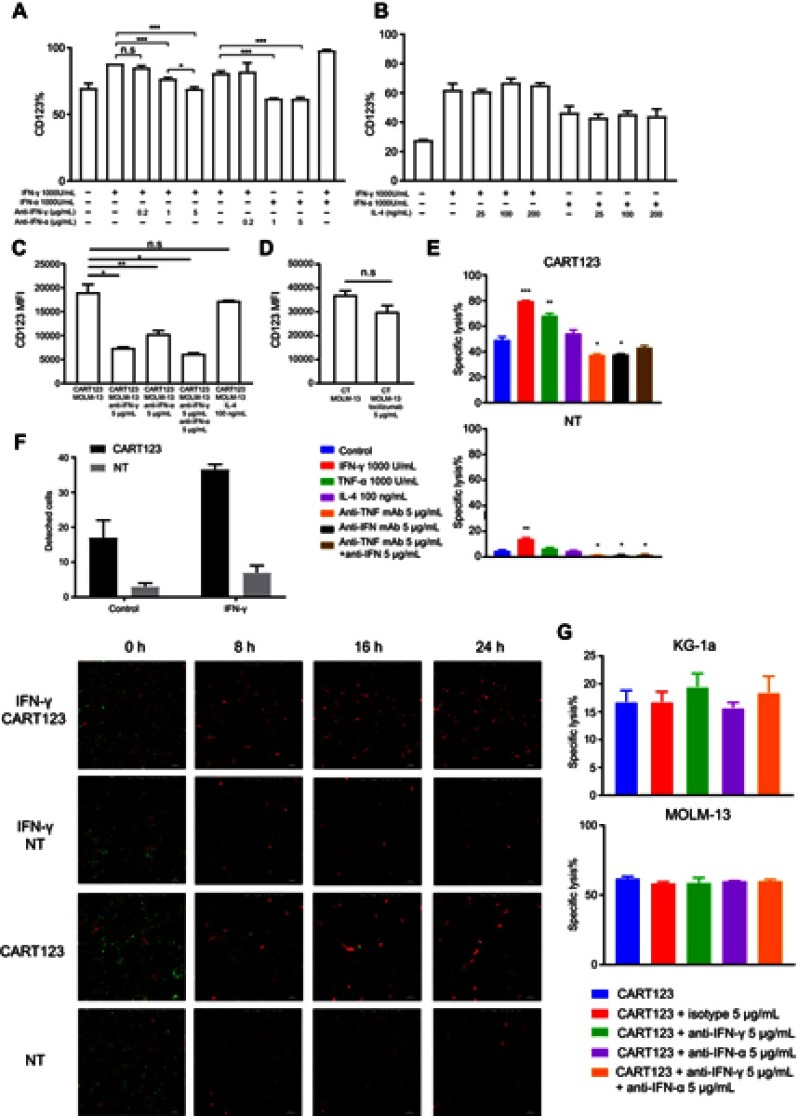
IFN-γ and TNF-α-pretreated HUVECs are vulnerable to CART123 in vitro
Next, we studied the impact of CD123 upregulation. The corresponding neutralizing antibody blocked the upregulation of CD123 of HUVECs caused by IFN-γ and TNF-α, respectively, but not by IL-4 (Figure 4A and B). To further verify the cause of CD123 upregulation and to block it in the co-culture model, we used IFN-γ and TNF-α neutralizing antibodies, IL-4, and tocilizumab, a humanized monoclonal antibody against the IL-6 receptor (IL-6R). Although activated T cells also release IL-4, the level is shallow. These results did not show any significant effect on the expression level of CD123 by the addition of IL-4 or tocilizumab. However, IFN-γ and TNF-α neutralizing antibodies could significantly downregulate the expression of CD123. Also, a combination of IFN-γ and TNF-α neutralizing antibodies reduced the level of CD123 expression to that of the baseline. (Figure 4C and D). These findings indicated that among the cytokines released in the co-culture model, IFN-γ and TNF-α specifically upregulate the endothelial CD123 level.
Figure 4.
IFN-γ- and TNF-α-pretreated HUVECs are vulnerable to CART123 in vitro. (A) Expression of CD123 on HUVECs after treatment with IFN-γ, TNF-α and neutralizing antibodies at different concentrations for 24 hrs (mean±SEM, n=3). Data show one representative experiment. (B) Expression of CD123 on HUVECs after treatment with IFN-γ, TNF-α, and IL-4 at different concentrations for 24 hrs (mean ±SEM, n=3). Data show one representative experiment. (C-D) In in vitro co-culture model, HUVECs were incubated with effector cells (CART123 or NT), target cells (MOLM-13), IFN-γ and TNF-α neutralizing antibodies, IL-4 (C) and tocilizumab (D) for 36 hrs and detected the CD123 expression by flow cytometry (mean±SEM, n=3). Data show one representative experiment. (E) Cytotoxicity of CART123 or NT at 6 hrs when co-cultured with HUVECs pretreated with cytokines (IFN-γ, TNF-α, and IL-4) for 24 hrs and treated with neutralizing antibodies at E:T of 5:1 was detected using 7-AAD and Annexin V (mean±SEM, n=3). Data show one representative experiment. (F) HUVECs (red) and CART123 or NT (green) were co-cultured for 24 hrs and recorded by Nikon Ti-E Inverted Live Cell Imaging System Manuals at 5% CO2 and 37°C. The number of detached cells after co-culture for 24 hrs is shown (mean±SEM, n=2). (G) Cytotoxicity at 14 hrs of CART123 or NT when co-cultured with AML cell lines KG-1a and MOLM-13 treated with neutralizing antibodies at 5:1 E:T was detected using 7-AAD and Annexin V (mean±SEM, n=3). Data show one representative experiment. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Abbreviations: IFN, interferon; TNF, tumor necrosis factor, HUVECs, human umbilical vein endothelial cells; CAR, chimeric antigen receptor; NT, non-transduced T; 7-AAD, 7-Aminoactinomycin D; AML, acute myeloid leukemia; E:T, the effector to target cell ratio.
Furthermore, to evaluate whether CD123 upregulation would enhance the specific cytotoxic activities in endothelial cells by CART123, we performed flow cytometry and live cell imaging system. After a 24-hr treatment with IFN-γ or TNF-α, the cytotoxicity of CART123 on HUVECs was significantly enhanced. However, the addition of IFN-γ and TNF-α neutralizing antibodies alone reduced the cytotoxicity, but combination during incubation did not exert a similar effect (Figure 4E). CD123 upregulation could embody a dual role due to anti-apoptosis effect of IL-3.32,33,34,35
We observed the dynamic changes of co-incubation of CART123 and HUVECs by in vitro live cell imaging. The endothelial cell lethality was assessed by the detachment of HUVECs. Notably, CART123 significantly enhanced the damage to HUVECs as compared with NT. Moreover, the killing was significantly enhanced after treatment with IFN-γ for 24 hrs, which was similar to the previous findings (Figure 4F and Videos S1; S2; S3; S4; S5; S6; S7; and S8). These findings suggested that upregulation of CD123 by IFN-γ or TNF-α leads to the enhanced killing of CART123 on HUVECs, and the addition of IFN-γ and TNF-α neutralizing antibodies decreased the cytotoxicity to HUVECs via blocking the upregulation of CD123.
Next, to verify whether neutralizing antibodies affect the efficacy of CART123 on AML cells, IFN-γ and TNF-α neutralizing antibodies were added when CART123 was incubated with MOLM-13 and KG-1a cells (Figure 4G). We found that the addition of IFN-γ and TNF-α neutralizing antibodies did not affect the cytotoxicity of CART123 on AML cell lines. In summary, these findings demonstrated that IFN-γ and TNF-α neutralizing antibodies could exert a protective effect on the endothelium in the treatment of CART123.
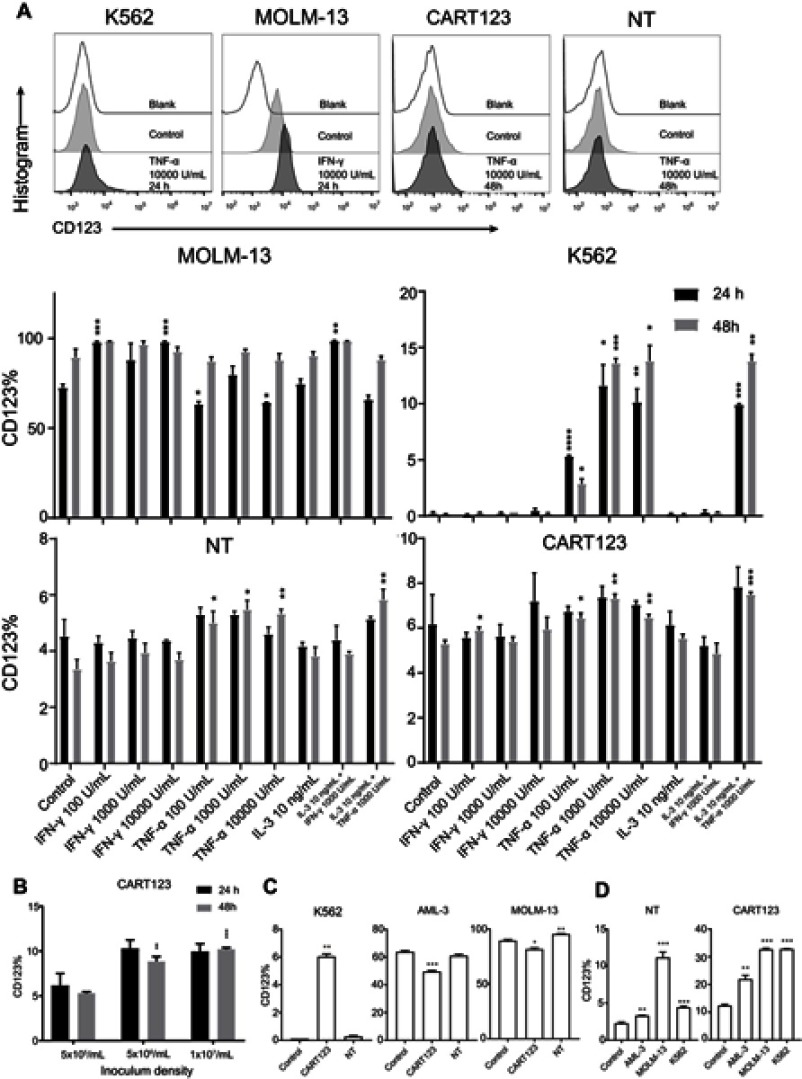
Induced expression of CD123 in T cells and myeloid leukemia cells in vitro
Based on the pattern of CD123 expression on endothelial cells, we explored whether CD123 expression on normal cells of the hematopoietic system and myeloid leukemia cells is also affected by cytokines. First, we incubated CART123, NT, K562, and MOLM-13 cells with different cytokines and found that CD123 was induced by TNF-α in K562 cells. Also, CD123 on MOLM-13 cells could be induced by IFN-γ, but CART123 and NT showed a slight upregulation after treatment with high concentrations of TNF-α (Figure 5A). Moreover, CART123 showed a high CD123 expression with increasing density of seeding cells (Figure 5B).
Figure 5.
Induced expression of CD123 in CAR T and myeloid leukemia cells in vitro. (A) Expression of CD123 on MOLM-13, K562, NT, and CART123 after treatment with cytokines (TNF-α, IFN-γ, and IL-3) for 24 and 48 hrs was detected by flow cytometry (mean±SEM, n=3). Data show one representative experiment. Representative plots are shown. (B) Expression of CD123 on CART123 in different density of seeding cells after in vitro culture for 24 and 48 hrs (mean±SEM, n=3). Data show one representative experiment. (C-D) CFSE-labeled effector cells (CART123 or NT) and target cells (MOLM-13, K562, and AML-3) were seeded in the upper chamber, and healthy donor-derived PBMC were seeded in the lower chamber of the in vitro co-culture model. After co-culturing for 24 hrs, target cells (C) and CFSE-labeled effector cells (D) and were analyzed for CD123 expression (mean±SEM, n=3). Data show one representative experiment. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Abbreviations: IFN, interferon; TNF, tumor necrosis factor, HUVECs, human umbilical vein endothelial cells; CAR, chimeric antigen receptor; NT, non-transduced T; 7-AAD, 7-Aminoactinomycin D; AML, acute myeloid leukemia; E:T, the effector to target cell ratio; PBMC, peripheral blood mononuclear cell; CFSE, carboxyfluorescein succinimidyl ester.
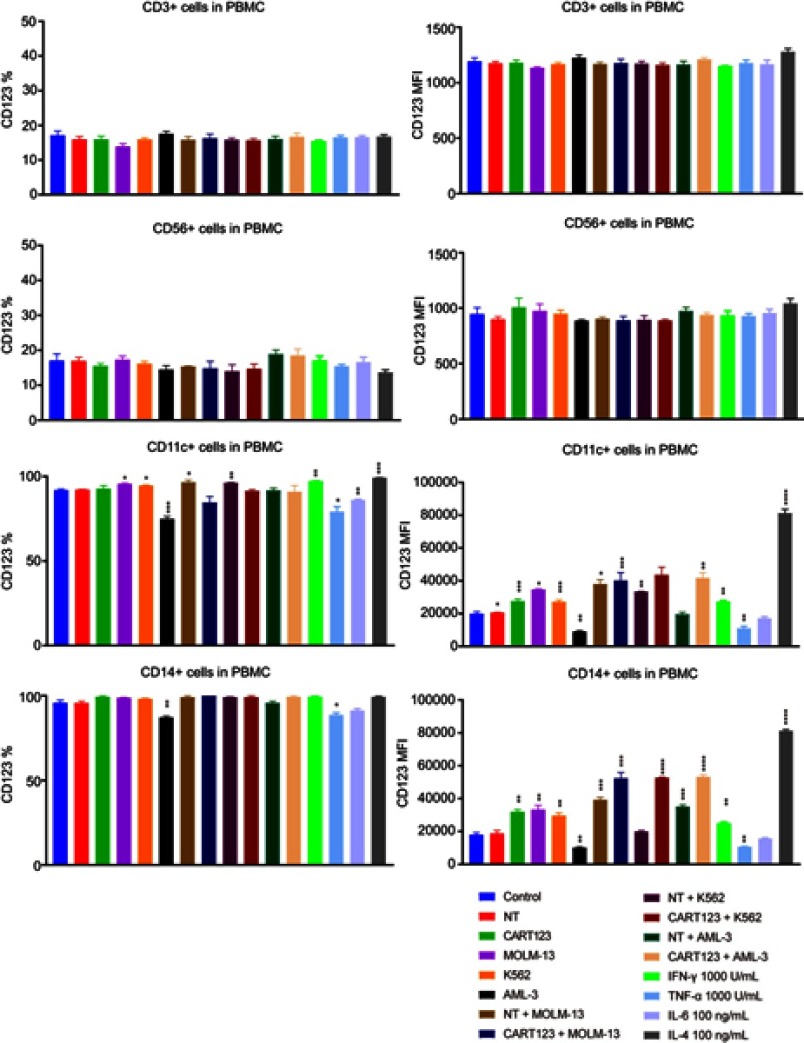
Furthermore, to investigate the changes in CD123 after different hematopoietic cells under the status that CART123 is stimulated, we used the in vitro co-culture model to study the PBMCs by staining the cells with CD56, CD3, CD11c, CD14, and CD123 fluorescent antibodies (Figure S2). In CD56+ and CD3+ cells, we did not find any significant differences in CD123 expression levels after incubation. Notably, in CD11c+ and CD14+ cells, the expression level of CD123 was significantly upregulated when NT was co-cultured with tumor cells. The maximal upregulation was detected in the IL-4-treated group, as IL-4 induces monocytic differentiation into DCs that highly express CD123.36 To further understand the changes in CD123 on T cells and AML cells, we examined the expression of CD123 on K562, MOLM-13, and AML-3 cells in the upper chamber of the co-culture model. Consequently, the expression of CD123 in K562 was upregulated after incubation with CART123. Conversely, AML-3 and MOLM-13 highly expressed CD123 that was downregulated after incubation with CART123 (Figure 5C). This phenomenon could be explained by the selective killing of CD123hi cells, excluding the CD123lo cells. The underlying mechanism needs further study; however, the induction of CD123 expression on K562 cells after incubation with CART123 could explain the low-level killing and cytokine production by CART123. Thus, K562 may be not an ideal negative control for CART123. In addition, expression of CD123 on CART123 and NT was assessed after incubation with myeloid leukemia cells. Consequently, CART123 showed a higher CD123 expression than NT. After incubation with leukemia cells, the expression level of CD123 was markedly upregulated on CART123 (Figure 5D).
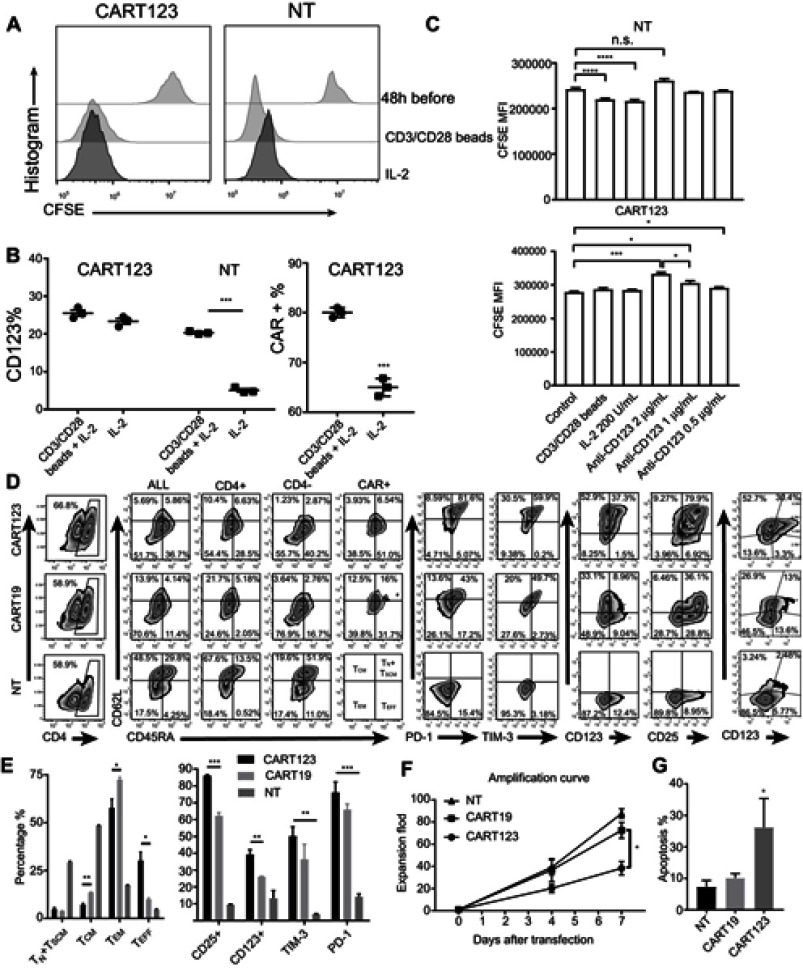
Upregulation of CD123 expression after activation of CAR T cell
To further verify the correlation between expression of CD123 on CART123 and T-cell activation, we performed a cell proliferation assay and found that after stimulation with CD3/28 Dynamic beads, the proliferation and expression of CD123 were increased. The proliferation and expression of CD123 remained unchanged after stimulation; however, the levels of CD123 expression were high (Figure 6A). Also, we observed a significant upregulation of CAR expression after stimulation with CD3/28 Dynamic beads, which could be attributed to the activation of CART123 under the stimulation of CD123 antigen (Figure 6B). When co-cultured with the anti-CD123 antibody, CFSE level of CART123 was significantly increased, coinciding with the decrease of cell proliferation rate. In contrast, no significant change was detected in the level of CFSE on NT (Figure 6C). These results indicated that CART123 expressed a higher level of CD123 than NT, putatively caused by CAR123 in cis or trans, leading to cell activation. In order to substantiate whether CART123 has to be susceptible to become activated, we detected the percentages of CD3, CD4, CD45RA, CD62L, CAR, CD123, CD25, PD-1, and TIM-3. The results exhibited that CART123 can be easily differentiated into the terminal state. Additionally, the expression of PD-1 and TIM-3 was upregulated and correlated with the expression of CAR compared to the NT group. Also, we found that the expression of CD123 and CD25 was distinct in CART123 as well as correlated with the expression of CAR (Figure 6D and E). These results demonstrated that CART123, especially CAR123hi T cells express abundant CD123 and an activated state, marked by a high level of PD-1, TIM-3, and CD25. In order to prove whether CART123 is vulnerable to apoptosis when cultured in vitro, we calculated the amplification fold and detected the level of apoptosis. The data showed that CART123 exhibited lower expansion folds compared to CART19 and a higher level of apoptosis compared to NT (Figure 6F and G).
Figure 6.
Upregulation of CD123 expression after activation of CAR T cell. (A) The proliferation of CART123 and NT after CFSE-stained CART123 and NT were stimulated with IL-2 100 U/mL and CD3/CD28 beads at 1:1+ IL-2 100 U/mL for 48 hrs; then, CFSE dilution was analyzed by flow cytometry. (B) Expression of CD123 and CAR after stimulation for 48 hrs was analyzed by flow cytometry (mean±SEM, n=3). Data show one representative experiment. (C) The proliferation of CART123 and NT were stimulated with IL-2 and CD3/CD28+ IL-2, or treated with different concentrations of anti-CD123 antibodies for 48 hrs (mean± SEM, n=3). Data show one representative experiment. (D-E) CART123, CART19 or NT cells from healthy donors were activated by CD3/CD28 beads at 1:1 and cultured in vitro with IL-2 100 U/mL. Six days after transduction, T lymphocytes were analyzed by flow cytometry. (D) Representative plots are shown. (E) (mean±SEM, n=3). Data show one representative experiment. (F) Proliferation curve of CART123, CART19, and NT within 7 days after transfection (mean±SEM, n=3). Data are depicted from three independent experiments; each point represented an average of triplicate. (G) Apoptosis detected by 7-AAD and Annexin V at day 7 after transfection (mean±SEM, n=3). Data are depicted from three independent experiments; each point represented an average of triplicate. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Abbreviations: CAR, chimeric antigen receptor; NT, non-transduced T; 7-AAD, 7-Aminoactinomycin D; CFSE, carboxyfluorescein succinimidyl ester.
In conclusion, the current results exhibited that CART123 has a higher level of CD123 and CD25, is more vulnerable to apoptosis as compared to CART19.
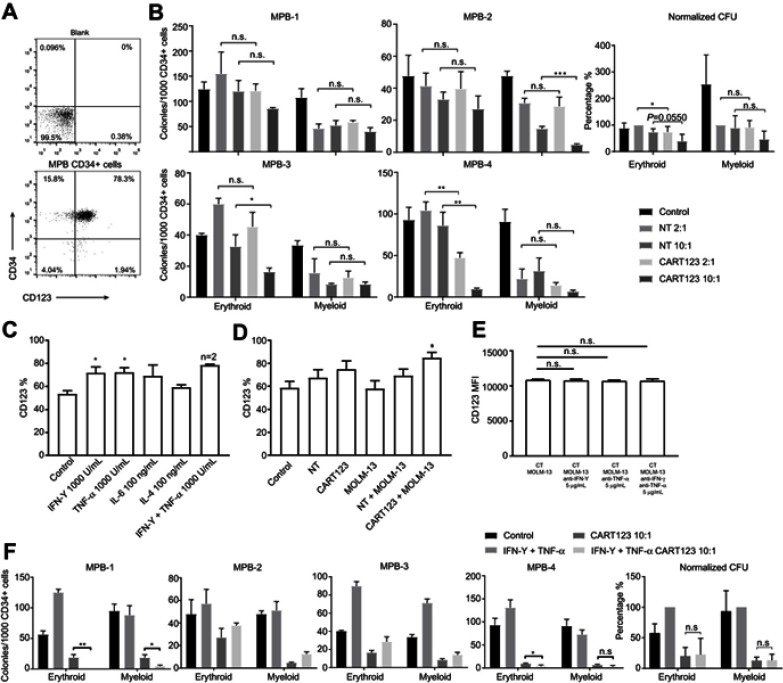
CART123 affects erythroid differentiation of CD34+ cells, and CD123 on CD34+ cells could be upregulated
Cytopenia is another possible side effect of CD123-targeted therapy. The MPB CD34+ cells highly expressed CD34 and CD123 (Figure 7A). Then, we assessed the effect of CART123 on the colony formation of MPB CD34+ samples. NT serves as the control group. Following a 12-hr co-culture with CART123 at an E:T of 2:1, 1/4 samples exhibit damaged erythroid colony formation. When the E:T is increased to 10:1, 2/4 and 1/4 samples exhibited injured of erythroid and myeloid colony formation, respectively. The normalized result exhibited that CART123 enhances the erythroid colony formation but not myeloid as compared with NT. (Figure 7B)
Figure 7.
Effect of CART123 on nHSPCs in vitro. (A) The expression of CD123 and CD34 on MPB CD34+ cells was detected by flow cytometry. (B) CD34+ MPB cells were CD34-immunomagnetically selected and cultured in StemSpan for 24 hrs, then co-cultured with either NT or CART123 from the same healthy donor or media alone (control) for 12 hrs at E:T of 2:1 and 10:1. Subsequently, the cells were plated in a methylcellulose-based medium for 14–16 days and scored for the presence of erythroid (BFU-E/CFU-E) and myeloid (CFU-G/M/GM) colonies (mean±SEM of triplicates). Normalized data set NT 2:1 group as 100% and depicted as mean±SEM of four independent experiments; each point represents an average of triplicate. (C) The expression of CD123 on CD34+ cells was detected by flow cytometry after treated with cytokines for 36 hrs (mean±SEM, n=3). Data are depicted from three independent experiments; each point represented an average of triplicate. (D) Effector cells (CART123 or NT) and target cells (MOLM-13) were seeded in the upper compartment, and MPB CD34+ cells were seeded in the lower compartment. After co-culturing for 36 hrs, CD34+ cells were harvested and analyzed for the expression of CD123 by flow cytometry (mean±SEM, n=4). Data are depicted from four independent experiments; each point represented an average of triplicate. (E) CD123 MFI on CD34+ cells after co-culturing in the co-culture model with effector cells (CART123 or NT), target cells (MOLM-13), and neutralizing antibodies for 36 hrs (mean±SEM, n=3) (F) MPB CD34+ cells were treated with IFN-γ 1,000 U/mL and TNF-α 1,000 U/mL or media (control) for 24 hrs, and then co-cultured with either CART123 from the same healthy donor or media alone (control) for 12 hrs at E:T of 10:1. The colony numbers enumerated as described previously (mean±SEM of triplicates). Normalized data set of IFN-γ+TNF-α group as 100% and depicted as mean±SEM from four independent experiments; each point represented an average of triplicate. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Abbreviations: CAR, chimeric antigen receptor; NT, non-transduced T; MPB, mobilized peripheral blood; E:T, the effector to target cell ratio; IFN, interferon; TNF, tumor necrosis factor; CFU, colony forming unit; BFU, burst-forming unit-erythroid; G, granulocyte; M, macrophage, GM, granulocyte macrophage; E, erythroid.
Previous studies have mentioned that IFN-γ, TNF-α, and IL-1 could upregulate the expression of CD123 in CD34+ stem cells.37 Furthermore, we confirmed that the treatment with IFN-γ and TNF-α could regulate CD123 expression in MPB CD34+ cells, as well as incubation of CART123 with MOLM-13 cells in the co-culture model (Figure 7C and D). However, blocking IFN-γ and TNF-α when incubation of CART123 with MOLM-13 cells in the co-culture model cannot inhibit the upregulation of CD123 (Figure 7E). This result might be caused by other cytokines, such as IL-1 and granulocyte colony-stimulating factor, can also upregulate CD123 expression in CD34+ cells.11,37 To investigate whether the upregulation of CD123 results in increased damage to CD34+ cells by CART123, we treated CD34+ cells with IFN-γ and TNF-α for 24 hrs before incubation with CART123. Compared to CART123 group, 2/4 samples exhibited significantly injured erythroid colony formation by CART123 after treatment with IFN-γ and TNF-α; similar results were presented by 1/4 myeloid samples. The normalized result revealed that treatment with IFN-γ and TNF-α did not enhance the damage to erythroid and myeloid colony formation caused by CART123 (Figure 7F). Although IFN-γ and TNF-α upregulated the expression of CD123 on CD34+ cells, it could not enhance the damage of myeloid and erythroid colony formation caused by CART123.
Discussion
Based on the previous literature and clinical phenomena, we hypothesized that CART123 exacerbated the endothelial cell damage under CRS status that was preliminarily validated in vitro. Although human umbilical veins are only at certain stages of human life, HUVECs have been considered as a general model for endothelial cells in both normal and diseased conditions.38 In the current study, we confirmed that IFN-γ and TNF-α upregulate while IL-4 downregulates the expression of CD123 on HUVECs and HDMECs in vitro. AML cells and HUVECs challenged with CART123 exhibit specific killing and cytokine production. Thus, an in vitro co-culture model was designed to mimic the simplified CRS status that CART123 was stimulated by a large number of tumors and releases numerous cytokines. We found that incubation of CART123 with myeloid leukemia cells resulted in a significant upregulation of CD123 expression on HUVECs. The addition of IFN-γ and TNF-α neutralizing antibodies effectively reversed the upregulation of CD123 on the endothelial cells in the in vitro co-culture model. In the current study, IFN-γ- and TNF-α-pretreated HVUECs are vulnerable to CART123. Furthermore, we verified that the addition of IFN-γ and TNF-α neutralizing antibodies did not affect the killing effect of CART123 on AML cell lines.
The data from several clinical studies indicated that the level of IFN-γ and TNF-α was increased in CRS, especially in sCRS.19,24,26 Similarly, the large release of IFN-γ and TNF-α was validated in the current study and other preclinical studies of CART123.5,13,15 These results preliminary validated our hypothesis and provided a reasonable explanation for the emergence of CRS with CLS in CART123 clinical trials. Presently, the US FDA approved the blocking antibodies for both cytokines, Enbrel (etanercept) for TNF-α and Gamifant (emapalumab) for IFN-γ. Therefore, IFN-γ and TNF-α neutralizing antibodies could be reliable for protecting the endothelium in the treatment of CART123. Previous studies demonstrated that both IFN-γ and TNF-α exhibited an anti-tumor and a pro-tumor effect in previous studies.39,40 Whether blocking IFN-γ and TNF-α affects the efficacy of hematological malignancies requires further validation in animal models and clinical trials. The clinical research data revealed that the high level of IFN-γ and TNF-α in CAR T cell treatment is under the CRS status, which is remarkably low under normal circumstances.24,26 Therefore, timely or prophylactic administration of IFN-γ and TNF-α blocking antibodies at the time of CRS can effectively reduce endothelial cell damage in the treatment of CART123. In addition, reducing CRS, including the improvement in CAR design, decreasing the infusion dose, pretreatment intensity, and tumor burden, or controlling the CRS by drugs, might effectively reduce the occurrence of endothelial cell damage.19,26,41,42 Conversely, the use of neutralizing antibodies is simpler, more thorough, and more controllable. Although IL-6 is released in large quantities in CRS,43–46 it does not affect the expression of CD123 on HUVECs. mRNA microarray analysis showed that IL-6 was significantly upregulated after TNF-α treatment. These results indicated that endothelial cells could be the main source of IL-6 when directly or indirectly incubated with CAR T cell in the current study, as reported previously that endothelial cells are the key source of IL-6 in CART19 therapy.16 In the current study, the significant increase in the IL-6 level in the CART19 group could due to activated HUVECs caused by the adhesion or stimulated.47
Little was known about the expression of CD123 on T cells, until recently Kerstin Renner et al.48 found that CD123 was significantly upregulated in proliferating CD4+ and CD8+ T cells, and the upregulation of CD123 differs between various activators and can be further modulated by cytokines. This phenomenon was critical in the treatment of CART123. The current study proved that CART123 expresses a higher level of CD123 than CART19 and NT during in vitro culture, and the level of CD123 on T cells was upregulated after activation. Moreover, CART123, especially CAR123hi T cells were activated and marked by high PD-1, TIM-3, and CD25, resulting in a differentiated phenotype. During in vitro culture, CART123 exhibited a low expansion-fold and a high level of apoptosis. In summary, the activation and apoptosis of CART123 could be caused by recognition in trans or cis, putatively resulting in overactivation and fratricide effect. A fratricide effect was observed in CD7 CAR T cells.49 Besides, CAR19 might bind in cis to the CD19 epitope on the surface of leukemic cells.50 Thus, the underlying mechanism requires a detailed study. A high-density culture resulted in high expression of CD123 in CART123, which might be attributed to a low potency of CART123 and a low level of IFN-γ when CART123 indirectly incubated with HUVECs in the current study. Besides, the activation and apoptosis of CART123 caused some interference to in vitro experiments.
As a result, CD123 on HUVECs and CART123 changed in the in vitro culture. Also, CD123 is induced in endothelial cells, hematopoietic stem cells, T cells, and myoloid leukemia cells, and is dynamically altered during hematopoietic development, designating it as a variable marker.11,13,31,48,51,52 The high cytotoxicity to HUVECs might be related to the expression of CD123, and hence, the activation threshold necessitates further exploration.20 IL-3 might have critical roles in inflammation and anti-apoptosis.21,32–35 Therefore, the upregulation of CD123 (IL-3Rα) expression under inflammation or apoptosis is yet to be elucidated. Activated T cells are the primary source of IL-3.21 Thus, the upregulated level of CD123 is not only the target of the CART123 but may also exert an anti-apoptotic effect by receiving IL-3. Therefore, blocking both TNF-α and (or) IFN-γ could cause a significant decrease in CD123 and receive less IL-3. Additional studies are essential in animal models to verify the efficacy of TNF-α and IFN-γ antibodies.
Hematopoietic toxicity has been one of the most concerning side effects of CART123. Strikingly, recent clinical studies have shown that CART123 has an infusive effect without cytopenia.12 Our data revealed that CART123 enhanced the erythroid colony formation but not myeloid as compared to NT. These different outcomes in previous preclinical studies could be attributed to the about hematopoietic toxicity was that fetal liver-derived CD34+ cells and cord blood-derived CD34+ cells are different in CD123 expression.53,54 Although IFN-γ and TNF-α upregulated the expression of CD123 on CD34+ cells, pretreatment with IFN-γ and TNF-α could not enhance the injury by CART123. However, whether CART123 will aggravate the damage to the hematopoietic system under CRS status needs further evaluation using animal models, as well as clinical trials.
The limitation of this study was that no animal experiments were performed using NSG mice. However, it was difficult to mimic the effects of CART123 on human endothelial cells in animal models. In the current CRS animal model, endothelial cells are derived from mice, and CAR T cells and tumors are derived from humans.55–57 A cross-species reactivity chart of human and murine cytokines was detected in the mouse model of the study by Giavridis et al, and the results showed that human IFN-γ could not signal through the murine IFN-γ receptor, whereas human TNF-α can signal through the murine p55 TNF receptor but not the p75 TNF receptor.57 In the current study, the results of the cross-reactivity of the two core cytokines, IFN-γ and TNF-α, limited our experiments on this model. Therefore, new animal models need to be designed to evaluate the toxicity to endothelial cells. Non-human primates would be the ideal model.22
In conclusion, the current preliminary study indicated that the expression of CD123 on endothelial cells could be upregulated when co-cultured with CART123. Furthermore, IFN-γ and TNF-α could aggravate endothelial damage caused by CART123 in vitro. Also, we proved for the first time that CART123 expresses CD123 during in vitro culture, and the level of CD123 is upregulated after activation.
Acknowledgments
This work was supported by grants from the Science and Technology Planning Project of Beijing City (Z171100002217069).
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Videos S1; S2; S3; S4; S5; S6; S7; and S8 human umbilical vein endothelial cells (red) and CART123 or non-transduced T (green) were co-cultured for 24 hrs and recorded every 2.5 mins for each point selected by Nikon Ti-E Inverted Live Cell Imaging System Manuals at 5% CO2 and 37°C.
Heat map of mRNA expression on HUVECs. The heat map showed a distinguishable mRNA expression profiling between the groups treated with IFN-γ 500 U/mL, TNF-α 500 U/mL and IL-4 100 ng/mL or untreated. The main results are divided into four groups according to the Go database information (mean±SEM, n=3).
Abbreviations: IFN, interferon; TNF, tumor necrosis factor; HUVECs, human umbilical vein endothelial cells.
CD123 expression on CD3+, CD56+, CD14+, and CD11c+ cells. Effector cells (CART123 or NT) and target cells (MOLM-13, K562, and AML-3) were seed in the upper chamber and healthy donor-derived PBMC were seed in the lower chamber of in vitro co-culture model or treated PBMC with IFN-γ,TNF-α, IL-4, and IL-6. After cocultured for 24 hrs, PBMC was analyzed for CD3+, CD56+, CD14+, CD11c, and CD123 expression by flow cytometry (mean±SEM, n=3). Data show one representative experiment. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Abbreviations: IFN, interferon; TNF, tumor necrosis factor, CAR, chimeric antigen receptor; NT, non-transduced T; AML, acute myeloid leukemia; E:T, the effector to target cell ratio; PBMC, peripheral blood mononuclear cell.
References
- 1.Rebecca S, Deepa N, Ahmedin J. Cancer statistics, 2012. CA: a cancer journal for clinicians 2012;62(1):10–29. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum FR. Impact of allogeneic hematopoietic cell transplantation on the outcome of older patients with acute myeloid leukemia. Best practice & research Clinical haematology 2017;30(4):320–326. [DOI] [PubMed] [Google Scholar]
- 3.Pizzo PA, Poplack DG. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams & Wilkins; 2015. [Google Scholar]
- 4.Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184 [DOI] [PubMed] [Google Scholar]
- 5.Tettamanti S, Marin V, Pizzitola I, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol. 2013;161(3):389–401. doi: 10.1111/bjh.12282 [DOI] [PubMed] [Google Scholar]
- 6.Fan M, Li M, Gao L, et al. Chimeric antigen receptors for adoptive T cell therapy in acute myeloid leukemia. J Hematol Oncol. 2017;10(1):151. doi: 10.1186/s13045-017-0519-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan CT, Upchurch D, Szilvassy SJ, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14(10):1777–1784. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz L, Nomdedéu JF, López O, et al. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001;86(12):1261–1269. [PubMed] [Google Scholar]
- 9.Du W, Li X-E, Sipple J, Pang Q. Overexpression of IL-3Rα on CD34+ CD38− stem cells defines leukemia-initiating cells in Fanconi anemia AML. Blood. 2011;117(16):4243–4252. doi: 10.1182/blood-2010-09-309179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moretti S, Lanza F, Dabusti M, et al. CD123 (interleukin 3 receptor alpha chain). J Biol Regul Homeost Agents. 2001;15(1):98–100. [PubMed] [Google Scholar]
- 11.Sato N, Caux C, Kitamura T, et al. Expression and factor-dependent modulation of the interleukin-3 receptor subunits on human hematopoietic cells. Blood. 1993;82(3):752–761. [PubMed] [Google Scholar]
- 12.Budde L, Song JY, Kim Y, et al. Remissions of acute myeloid leukemia and blastic plasmacytoid dendritic cell neoplasm following treatment with CD123-specific CAR T Cells: a first-in-human clinical trial. Blood. 2017;130(Suppl 1):811. [Google Scholar]
- 13.Gill S, Tasian SK, Ruella M, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123(15):2343–2354. doi: 10.1182/blood-2013-09-529537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tasian SK, Kenderian SS, Shen F, et al. Optimized depletion of chimeric antigen receptor T cells in murine xenograft models of human acute myeloid leukemia. Blood. 2017;129(17):2395–2407. doi: 10.1182/blood-2016-08-736041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai T, Black KL, Naqvi A, et al. Abstract 2560: preclinical efficacy of allogeneic anti-CD123 CAR T-cells for the therapy of blastic plasmacytoid dendritic cell neoplasm (BPDCN). Cancer Res. 2018;78(13 Supplement):2560. doi: 10.1158/1538-7445.AM2018-2560 [DOI] [Google Scholar]
- 16.Obstfeld AE, Frey NV, Mansfield K, et al. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: clinicopathological insights. Blood. 2017;130(23):2569–2572. doi: 10.1182/blood-2017-08-802413 [DOI] [PubMed] [Google Scholar]
- 17.Maude SL. CAR emissions: cytokines tell the story. Blood. 2017;130(21):2238–2240. doi: 10.1182/blood-2017-10-808592 [DOI] [PubMed] [Google Scholar]
- 18.Mackall CL, Miklos DB. CNS endothelial cell activation emerges as a driver of CAR T cell-associated neurotoxicity. Cancer Discov. 2017;7(12):1371–1373. doi: 10.1158/2159-8290.CD-17-1084 [DOI] [PubMed] [Google Scholar]
- 19.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017;7(12):1404–1419. doi: 10.1158/2159-8290.CD-17-0698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arcangeli S, Rotiroti MC, Bardelli M, et al. Balance of anti-CD123 chimeric antigen receptor binding affinity and density for the targeting of acute myeloid leukemia. Mol Ther. 2017;25(8):1933–1945. doi: 10.1016/j.ymthe.2017.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korpelainen EI, Gamble JR, Vadas MA, Lopez AF. IL-3 receptor expression, regulation and function in cells of the vasculature. Immunol Cell Biol. 1996;74(1):1–7. doi: 10.1038/icb.1996.1 [DOI] [PubMed] [Google Scholar]
- 22.Dong L, Gill S, Bhoj V, et al. 399. Evaluation of CD123 targeting CART Cells in non-human primates. Mol Ther. 2016;24:S158. doi: 10.1016/S1525-0016(16)33208-7 [DOI] [Google Scholar]
- 23.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664–679. doi: 10.1158/2159-8290.CD-16-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Bio Res. 2018;6:4. doi: 10.1186/s40364-018-0116-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hay KA, Hanafi L-A, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor–modified T-cell therapy. Blood. 2017;130(21):2295–2306. doi: 10.1182/blood-2017-06-793141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engels B, Cam H, Schüler T, et al. Retroviral vectors for high-level transgene expression in T lymphocytes. Hum Gene Ther. 2003;14(12):1155. doi: 10.1089/104303403322167993 [DOI] [PubMed] [Google Scholar]
- 28.Cheng Z, Wei R, Ma Q, et al. In vivo expansion and antitumor activity of coinfused CD28-and 4-1BB-Engineered CAR-T cells in patients with B cell leukemia. Mol Ther. 2018;26(4):976–985. doi: 10.1016/j.ymthe.2018.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mardiros A, Dos Santos C, McDonald T, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122(18):3138–3148. doi: 10.1182/blood-2012-12-474056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fluckiger A, Dumont A, Derangère V, et al. Inhibition of colon cancer growth by docosahexaenoic acid involves autocrine production of TNFα. Oncogene. 2016;35:4611. doi: 10.1038/onc.2015.523 [DOI] [PubMed] [Google Scholar]
- 31.Korpelainen EI, Gamble JR, Smith WB, et al. The receptor for interleukin 3 is selectively induced in human endothelial cells by tumor necrosis factor alpha and potentiates interleukin 8 secretion and neutrophil transmigration. Proc Natl Acad Sci U S A. 1993;90(23):11137–11141. doi: 10.1073/pnas.90.23.11137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurata M, Yamazaki Y, Kanno Y, et al. Anti-apoptotic function of Xbp1 as an IL-3 signaling molecule in hematopoietic cells. Cell Death Dis. 2011;2(2):e118–e118. doi: 10.1038/cddis.2011.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy EP, Korapati A, Chaturvedi P, Rane S. IL-3 signaling and the role of Src kinases, JAKs and STATs: a covert liaison unveiled. Oncogene. 2000;19(21):2532–2547. doi: 10.1038/sj.onc.1203594 [DOI] [PubMed] [Google Scholar]
- 34.Ito T, Deng X, Carr B, May WS. Bcl-2 phosphorylation required for anti-apoptosis function. J Biol Chem. 1997;272(18):11671–11673. doi: 10.1074/jbc.272.18.11671 [DOI] [PubMed] [Google Scholar]
- 35.Lotem J, Cragoe EJ Jr., Sachs L. Rescue from programmed cell death in leukemic and normal myeloid cells. Blood. 1991;78(4):953–960. [PubMed] [Google Scholar]
- 36.Hiasa M, Abe M, Nakano A, et al. GM-CSF and IL-4 induce dendritic cell differentiation and disrupt osteoclastogenesis through M-CSF receptor shedding by up-regulation of TNF-alpha converting enzyme (TACE). Blood. 2009;114(20):4517–4526. doi: 10.1182/blood-2009-04-215020 [DOI] [PubMed] [Google Scholar]
- 37. Dembic Z. Cytokines of the immune system: interleukins. The cytokines of the immune system the role of cytokines in disease related to immune response. San Diego: Mica Haley. 2015:143–239. [Google Scholar]
- 38.Cao Y, Gong Y, Liu L, et al. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: a review. J Appl Toxicol. 2017;37(12):1359–1369. doi: 10.1002/jat.3470 [DOI] [PubMed] [Google Scholar]
- 39.Kursunel MA, Esendagli G. The untold story of IFN-gamma in cancer biology. Cytokine Growth Factor Rev. 2016;31:73–81. doi: 10.1016/j.cytogfr.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 40.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25(3):409–416. doi: 10.1007/s10555-006-9005-3 [DOI] [PubMed] [Google Scholar]
- 41.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JH, Riviere I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (Rituximab, IDEC-C2B8). Blood. 1999;94(7):2217. [PubMed] [Google Scholar]
- 44.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121(26):5154–5157. doi: 10.1182/blood-2013-02-485623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brentjens RJ. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970. doi: 10.2217/imt-2016-0020 [DOI] [PubMed] [Google Scholar]
- 47.Krishnaswamy G, Kelley J, Yerra L, Smith JK, Chi DS. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J Interferon Cytokine Res. 1999;19(2):91–104. doi: 10.1089/107999099314234 [DOI] [PubMed] [Google Scholar]
- 48.Renner K, Metz S, Metzger A-M, et al. Expression of IL-3 receptors and impact of IL-3 on human T and B cells. Cell Immunol. 2018;334:49–60. doi: 10.1016/j.cellimm.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 49.Gomes-Silva D, Srinivasan M, Sharma S, et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130(3):285–296. doi: 10.1182/blood-2017-01-761320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruella M, Xu J, Barrett DM, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. 2018;24(10):1499–1503. doi: 10.1038/s41591-018-0201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korpelainen EI, Gamble JR, Smith WB, Dottore M, Vadas MA, Lopez AF. Interferon-gamma upregulates interleukin-3 (IL-3) receptor expression in human endothelial cells and synergizes with IL-3 in stimulating major histocompatibility complex class II expression and cytokine production. Blood. 1995;86(1):176–182. [PubMed] [Google Scholar]
- 52.Chaoshao L, Jinping Z, Jianxin S, et al. Impact on the expression of CD123 on K562 Cells Induced by rhIFN-γ. J Kun Med Univ/Kun Yike Daxue Xuebao. 2016;37(8):5–8. [Google Scholar]
- 53.Mardiros A, Forman SJ, Budde LE. T cells expressing CD123 chimeric antigen receptors for treatment of acute myeloid leukemia. Curr Opin Hematol. 2015;22(6):484–488. doi: 10.1097/MOH.0000000000000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang S, Chen Z, Yu JF, et al. Correlation between IL-3 receptor expression and growth potential of human CD34+ hematopoietic cells from different tissues. Stem Cells. 1999;17(5):265–272. doi: 10.1002/stem.170265 [DOI] [PubMed] [Google Scholar]
- 55.Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133(7):697–709. doi: 10.1182/blood-2018-10-881722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739. doi: 10.1038/s41591-018-0036-4 [DOI] [PubMed] [Google Scholar]
- 57.Giavridis T, van der Stegen SJ, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731. doi: 10.1038/s41591-018-0041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heat map of mRNA expression on HUVECs. The heat map showed a distinguishable mRNA expression profiling between the groups treated with IFN-γ 500 U/mL, TNF-α 500 U/mL and IL-4 100 ng/mL or untreated. The main results are divided into four groups according to the Go database information (mean±SEM, n=3).
Abbreviations: IFN, interferon; TNF, tumor necrosis factor; HUVECs, human umbilical vein endothelial cells.

CD123 expression on CD3+, CD56+, CD14+, and CD11c+ cells. Effector cells (CART123 or NT) and target cells (MOLM-13, K562, and AML-3) were seed in the upper chamber and healthy donor-derived PBMC were seed in the lower chamber of in vitro co-culture model or treated PBMC with IFN-γ,TNF-α, IL-4, and IL-6. After cocultured for 24 hrs, PBMC was analyzed for CD3+, CD56+, CD14+, CD11c, and CD123 expression by flow cytometry (mean±SEM, n=3). Data show one representative experiment. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Abbreviations: IFN, interferon; TNF, tumor necrosis factor, CAR, chimeric antigen receptor; NT, non-transduced T; AML, acute myeloid leukemia; E:T, the effector to target cell ratio; PBMC, peripheral blood mononuclear cell.