Abstract
Many publications have described the potential cardioprotective action of different medicinal plants, relating this effect with blood lipid levels. However, these publications do not justify the right amount of plant administered, which can vary greatly. Sideritis hyssopifolia is a little woody plant endemic to western and southwestern Europe. We have quantified its antioxidant activity, which can be used as an indicator of its cardioprotective action. This study evaluates the antioxidant capacity of Sideritis hyssopifolia to design a feed whose hypolipidemic effects are proven in cholesterol-fed New Zealand rabbits. Antioxidant action was assessed in infusions, which were prepared with 1 or 3 g of plant in 200 mL of water by using an ABTS assay and expressed as Ascorbic acid Equivalent Antioxidant Capacity (AEAC). Aqueous infusions with infusion times of 10 min and prepared with 3 g plant exhibited the strongest antioxidant activity. Sideritis hyssopifolia showed an intermediate antioxidant capacity for the concentrations and times of the infusion tested. According to our results, we suggest incorporating 2.36 g of S. hyssopifolia every 150 g of rabbit feeding stuff (15.73 g/kg). This chow decreased cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides levels in cholesterol-fed rabbits, as well as the atherogenic index. This reduction was similar to that obtained with simvastatin.
Keywords: antioxidant activity, atherogenic index, cholesterol, rabbit, Sideritis hyssopifolia
1. Introduction
Atherosclerosis and its associated cardiovascular pathologies are the major causes of morbidity and mortality in Western countries, and represent one of the greatest worldwide economic, social and medical challenges that we are facing at the present time [1,2]. Hypercholesterolemia and its induced oxidative stress are considered one of the major contributors to the initiation and progression of atherosclerosis [3,4]. The initial arterial lesion, the fatty streaks formation and the development of atheroma plaques are closely linked with the accumulation of oxidized low-density lipoproteins (LDL) in macrophages. High lipid concentrations in plasma may alter lipoprotein metabolism, causing the accumulation of LDL in the subendothelial layer of the vessels, where these proteins undergo oxidative changes, resulting in oxidized LDL, a compound that is highly atherogenic [5,6,7,8]. Thus, oxidative modification of LDL is an important factor in atherosclerotic changes [6,7,8], whereas high density lipoproteins (HDL) play a key role in the protection against the oxidative damage of cells [9,10].
Even though there are several medications available to prevent the underlying causes of this disorder, adverse effects are consistently present. Thus, the development of additional therapies for controlling cholesterol levels is warranted, especially those with better safety profiles. In this way, in the last decades there has been a great interest in investigating the effectiveness and safety of lipid-lowering agents of natural origin obtained from medicinal plants.
Antioxidants obtained from these natural sources are increasingly being recognized as important health promoters in cardiovascular or neurodegenerative disorders [11,12,13,14]. Several authors have demonstrated that antioxidant intake is inversely related to the incidence of heart attacks and mortality from coronary heart disease [15,16,17].
Antioxidants suppress the development of hypercholesterolemic atherosclerosis, and induce its regression. This process is associated with decreases in oxidative stress and serum lipids [18,19]. Among antioxidants, polyphenols present in plants prevent LDL oxidation [20,21] and may significantly diminish the risk of mortality from cardiovascular diseases [22,23,24].
In recent years, the genus Sideritis L. (Lamiaceae family) has attracted the attention of the scientific community as it has shown a wide range of medicinal folk properties, such as being anti-infectious, anti-inflammatory, anti-rheumatic and anti-ulcer applications [25,26]. This genus includes at least 150 species of annual and perennial plants [27], widely distributed in the Mediterranean region, the Balkans or the Iberian Peninsula, but also in other regions. Sideritis hyssopifolia, L. is a little woody plant endemic to western and southwestern Europe, primarily in mountainous areas from northern Portugal through to western Switzerland and northern Italy. In Spain, this medicinal plant has been traditionally used since ancient times as tea due to its digestive properties and beneficial effects on the treatment of gastric ulcers. These properties have been attributed to the high polyphenolic content of these plants [28,29].
In this study, we evaluate the antioxidant activity of the medicinal plant S. hyssopifolia, and accordingly with this activity, we propose the preparation of a chow containing this medicinal plant at a certain amount, to carry out the experimental assessment of its cardioprotective action in a hypercholesterolemic rabbit model. In other studies, the authors have related the lipid-lowering action of different medicinal plants with their antioxidant activities, but they fail to justify the amounts used, which vary greatly in the different studies: 10 mg/kg [30] and 288 mg/kg [31] in the case of plant extracts, and 1000 mg/kg [32] and 13,000 mg/kg [33] for fresh plants or seeds.
Ascorbic acid is the most important vitamin present in fruits and vegetables, and it is involved in many physiological functions in human organisms, acting as the scavenger for oxidizing free radicals and harmful oxygen-derived species. As an antioxidant, it reduces the risk of suffering cardiovascular diseases, such as atherosclerosis, and some types of cancer [34]. In hypercholesterolemic patients, the administration of 2 g ascorbic acid decreased oxidative stress and improved endothelial dysfunction [35,36,37,38], probably by scavenging super-oxide anion and preventing inactivation of NO [36,39], as well as by the inhibition of LDL oxidation [40]. Therefore, a daily administration of 2 g ascorbic acid would have protective activity against cardiovascular diseases.
The purposes of this study were to evaluate the antioxidant activity of Sideritis hyssopifolia, and to determine the amount of this plant that should be added to a rabbit feed in order to achieve a protective action against cardiovascular disorders in hypercholesterolemic rabbits, taking into account those data reported by several authors on the effectiveness of ascorbic acid [35,36,37,38]. The proposed chow could serve as guidance for other medicinal plants. The lipid lowering properties and the atheroprotective effect of Sideritis hyssopifolia, L. in high cholesterol fed New Zealand rabbits was then proved by using this chow.
2. Results
2.1. Antioxidant Properties
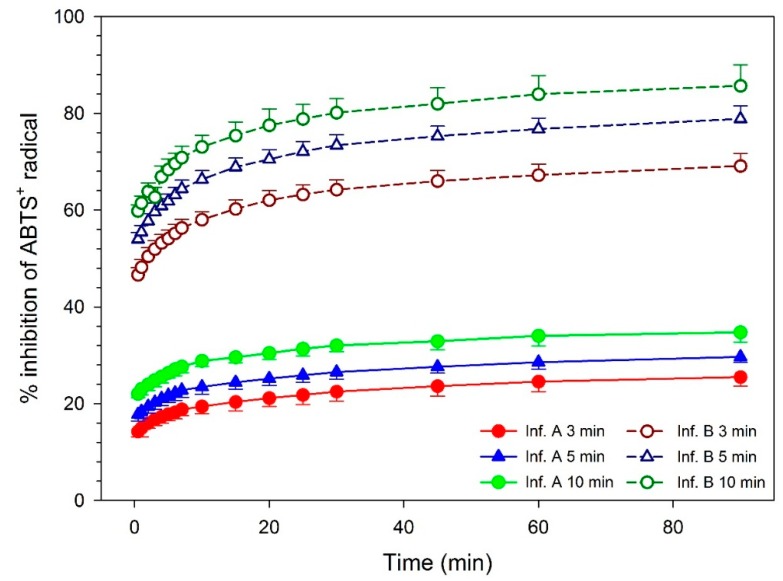
Inhibition percentages versus time for infusions A and B after 3, 5, and 10 min of contact plant-water contact are provided in Figure 1. As shown in the figure, the longer the time of the infusion, the higher inhibition capacity and, consequently, the higher the antioxidant activity. Accordingly, in infusion A samples, an initial inhibition percentage of 14.24% was obtained with 3 min of infusion; 17.76% after an infusion time of 5 min; and 22.04% with 10 min. With infusion B samples, an initial inhibition percentage of 46.62% was calculated for an infusion time of 3 min, 53.97% with 5 min, and 59.81% with 10 min.
Figure 1.
Antioxidant activity of Sideritis hyssopifolia infusions versus time. Antioxidant activity is expressed as inhibition percentage of ABTS•+ radical. Two different infusions (A: 5 × 10−5 g/mL; B: 1.5 × 10−4 g/mL), and three times of infusion were assayed (3, 5 and 10 min). Data are expressed as mean ± SD (n = 5).
Moreover, antioxidant activity increased with the amount of plant added. Thus, in infusion B samples (1.5 × 10−4 g/mL), an inhibition percentage of 70.82% was obtained with an infusion time of 10 min, while it dropped to 27.64% with infusion A samples (5 × 10−5 g/mL) in the same conditions.
IC50 values were then calculated for ascorbic acid and infusions A and B (Table 1). Calculations were made with absorbance obtained at 7 min of reaction for ascorbic acid, and at 45 min for infusions A and B, as values remained constant after this reading. Ascorbic acid Equivalent Antioxidant Capacity (AEAC) values were also calculated for the different infusion times and amount of plant used, ranging from 1.808 to 2.419 g/100 g.
Table 1.
IC50 and Ascorbic acid Equivalent Antioxidant Capacity (AEAC) values of Sideritis hyssopifolia.
| IC50 (µg/mL) |
AEAC (g/100 g) |
|
|---|---|---|
| Ascorbic acid | 2.025 ± 0.095 | - |
| Sideritis hyssopifolia | ||
| 3 min | 112.1 ± 4.0 | 1.808 ± 0.068 |
| 5 min | 96.9 ± 2.9 | 2.090 ± 0.063 |
| 10 min | 83.7 ± 2.5 | 2.419 ± 0.071 |
The antioxidant activity of 2 g ascorbic acid would be equivalent to 110.62 g of S. hyssopifolia after an infusion time of 3 min; 95.70 g of plant when an infusion time of 5 min was used, or 82.67 g with an infusion time of 10 min. Taking into account these results, if we add this plant to the feed of rabbits, the amount of antioxidant compounds available would be, at least, those found with the highest time of infusion (10 min). As the maximum extraction efficiency of antioxidant compounds was obtained with an infusion time of 10 min, we have used the value of 82.67 g (equivalent to 2 g ascorbic acid) to calculate the amount of S. hyssopifolia that should be added to the chow in order to achieve a protective action against cardiovascular disorders in hypercholesterolemic rabbits. A man (70 kg standard weight) would need 1.18 g/kg body weight [37]. Therefore, for a rabbit (2 kg mean weight), 2.36 g of plant should be included in its dairy feeding (150 g). Thus, we consider that 2.36 g of S. hyssopifolia should be added to every 150 g of feeding stuff (15.73 g/kg or 1.57% w/w) to assess its cholesterol-lowering effects in hypercholesterolemic rabbits.
2.2. Effects on Lipid Profile
No mortality and no clinical signs of toxicity were observed in the rabbits during the experimental period. All animals remained healthy and were suitable for the study.
Table 2 includes the mean values for the different parameters evaluated: total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, and atherogenic index at the beginning of the study and after 6 weeks of treatment with the different chows.
Table 2.
Lipid profile and atherogenic index (mean ± SD) of the different groups (n = 6) at the beginning and at the end of the treatments (6 weeks).
| 0 Weeks | 6 Weeks | |||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Control | Cholesterol | Sideritis | Simvastatin | Control | Cholesterol | Sideritis | Simvastatin |
| TC (mg/dl) | 89 ± 10 | 84 ± 12 | 86.1 ± 9.4 | 83 ± 11 | 81.8 ± 8.8 | 460 ± 67 | 366 ± 61 | 348 ± 62 |
| HDL-c (mg/dl) | 24.3 ± 3.1 | 23.8 ± 2.9 | 25.2 ± 3.7 | 25.8 ± 3.4 | 22.6 ± 3.9 | 58.2 ± 7.4 | 49.4 ± 9.6 | 49.1 ± 5.1 |
| LDL-c (mg/dl) | 47.9 ± 9.1 | 50.2 ± 8.6 | 5 ± 13 | 48.8 ± 9.4 | 46.4 ± 8.7 | 179 ± 23 | 148 ± 20 | 133 ± 22 |
| TG (mg/dl) | 101 ± 15 | 105 ± 13 | 99 ± 19 | 103± 16 | 97 ± 17 | 173 ± 19 | 146 ± 13 | 137 ± 15 |
| AI | 2.43 ± 0.14 | 2.61 ± 0.16 | 2.37 ± 0.15 | 2.54 ± 0.12 | 2.58 ± 0.14 | 7.06 ± 0.53 | 4.01 ± 0.32 | 3.37 ± 0.21 |
TC: total cholesterol; HDL-c: high density lipoprotein cholesterol; LDL-c: low density lipoprotein cholesterol; TG: triglycerides; AI: atherogenic index; SD: standard deviation.
Cholesterol rich chow caused hyperlipidemia in all the animals. After 6 weeks of treatment, total cholesterol levels increased significantly in cholesterol, Sideritis and Simvastatin groups (p ≤ 0.05). However, this increase was approximately 1.2-fold lower in Sideritis and Simvastatin groups than in cholesterol group, although significant differences only were only found between cholesterol and Simvastatin groups (p ≤ 0.05).
HDL-c values were also higher at the end of the treatment in cholesterol, Sideritis and Simvastatin groups (p ≤ 0.05). Differences between these three groups were lower than for the total cholesterol. The levels determined for LDL-c also increased from week 0 to week 6 in the cholesterol, Sideritis, and Simvastatin groups (p ≤ 0.05). This parameter showed significant differences between the cholesterol group and Sideritis and Simvastatin groups (p ≤ 0.05). The increase observed in triglycerides after receiving the diet was lower than that seen in cholesterol, HDL-c and LDL-c. The rise in triglyceride levels was also lower in the Sideritis and Simvastatin groups than in the cholesterol group, with no significant differences between them.
The highest value for the atherogenic index was obtained in the cholesterol group at the end of the treatment (significant differences between cholesterol group and Sideritis and Simvastatin groups; p ≤ 0.05).
3. Discussion
We have determined the antioxidant activity of this plant from infusions, which is the major way of incorporating this medicinal herb to a diet. To our knowledge, this is the first time that the antioxidant activity of a medicinal plant has been correlated to the amount of the plant that may be added to a feed or diet to demonstrate its hypocholesterolemic activity. According to the literature reviewed, other authors have used different medicinal plants to study their lipid-lowering effects in animal models [30,32,41,42,43,44], supplementing an animal diet with a certain amount of the plant or extracts of the plant, but these studies do not clearly associate the amount added with the antioxidant activity of the plant.
The intake of food with an antioxidant effect has been associated with a low risk of cardiovascular diseases [45]. In our study, the antioxidant activity of S. hyssopifolia was assessed after three different infusion times (3, 5 and 10 min). Our results showed that the longest infusion time (10 min) exhibited stronger AEAC values (and antioxidant activity) than the other two infusion times (3 and 5 min).
S. hyssopifolia exhibited an intermediate antioxidant capacity when comparing the AEAC values obtained in our study with those indicated by other authors. Thus, the AEAC values were higher in fresh plants of the Zingiberaceae family such as Alpinia zerumbet (3020 mg/100 g) or Etlingera elatior (2990 mg/100 g) [46], in several tropical and temperate herbal teas of lemon myrtle (Backhousia citriodora) (13,600 mg/100 g), guava (Psidium guajaba) (7430 mg/100 g), rosemary (Rosmarinus officinalis) (3090 mg/100 g) or mint (Mentha spicata) (4430 mg/100 g) [47], or in medicinal plants of Leguminosae family, which exhibited AEAC values for flowers and leaves, respectively, of 14,600 and 6410 mg/100 g for Bauhinia kockiana, 3350 and 7690 mg/100 g for Caesalpinia pulcherrima, and 4080 and 4130 mg/100 g for Cassia surattensis [48]. Nevertheless, other plants or teas clearly showed AEAC values clearly lower than those calculated by us, such as Curcuma longa (251 mg/100 g) or Kaempferia galangal (48 mg/100 g) [46], as well as teas of chamomila (Matricaria recutita) (966 mg/100 g) [47].
Flavonoids, ditherpenes and essential oils are present in the Sideritis species, which endow these plants with important antioxidant, anti-inflammatory, anti-ulcer, antimicrobial and analgesic properties [27]. Nine 7-ortho-alosil glycosides have been identified by spectroscopic methods from the methanol extract of the aerial part of Sideritis raeseri, conferring these components gastroprotective, anti-inflammatory and antioxidant characteristics [49]. Regarding S. hyssopifolia, it is known to contain four flavone glycosides, which are supposed to have antioxidant properties [50].
The hypolipidemic effect of the plant, included in the chow at the concentration determined after the evaluation of its antioxidant effect, was determined in cholesterol-fed New Zealand rabbits. Due to the high impact of atherosclerosis on health, several animal models have been used as tools to investigate dyslipidemia and the pathophysiology of this disease. Nowadays, rabbits are considered the main pre-clinical model to carry out these studies because this animal model is more sensitive to experimental atherosclerosis, which can be viewed after one week of exposure [51].
The anti-hypercholesterolemic effects of Sideritis hyssopifolia has been demonstrated in this study for the first time. Other authors have demonstrated the antioxidant properties of different Sideritis species [52,53] as well as their lowering effect on triglycerides [54]. The antioxidant activity found for these species was intermediate, as our results indicate for Sideritis hyssopifolia, L. This antioxidant activity has been related mainly to their content in polyphenols. Soluble fibers obtained from different plants have been also related to cholesterol lowering effects, but the amounts that need to be administered daily are much higher than those provided by the addition of Sideritis hyssopifolia to the chow.
In our research, the addition of 2% of cholesterol in the diet of rabbits caused an increase in all the parameters evaluated. The overall results of our study reveal that Sideritis hyssopifolia, L. in the dose added to the chow (2.36 g Sideritis hyssopifolia, L./150 g chow), has very similar hypolipidemic effects to simvastatin in the dose of 1,2 mg/kg/day, which may be of value in cardiovascular diseases. The total cholesterol, HDL-c, LDL-c and triglyceride values were lower in the Sideritis and Simvastatin groups than in cholesterol group, although only LDL-c showed significant differences. We think that, although Sideritis and Simvastatin showed a protective effect, the high levels of cholesterol reached with the diet, didn´t allow one to maintain the levels of the different parameters similar to those determined in the control group, even though these levels were smaller than those of cholesterol group.
The atherogenic index also showed significant differences between the cholesterol group and the Sideritis and Simvastatin groups. Further studies are required to determine the long-term effects of Sideritis hyssopifolia, L. on atherosclerosis and to evaluate the components responsible of this action.
4. Materials and Methods
4.1. Evaluation of the Antioxidant Properties
4.1.1. Reagents
ABTS (2,2’-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) di-ammonium salt) (Sigma-Aldrich, St. Louis, MO, USA), potassium persulfate (Sigma-Aldrich, St. Louis, MO, USA), L-ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA), methanol (Merck, Darmstadt, Germany), and distilled water were used. All reagents were of analytical grade.
4.1.2. Plant Material and Preparation of Sideritis Infusions
The aerial part of the plant was collected from the Picos de Europa, mountain chain that runs along Northern Spain. The plant was identified by Dr. Matilde Sierra. A sample of the plant was deposited in the herbarium at the University of Leon (LEB-203581). S. hyssopifolia infusions were obtained from the aerial part of the plant, following the instructions described previously [55]. Plants were cut into pieces that were homogenized to obtain a mixture with the same proportion of flowers and stalks. Later, portions of 1 g or 3 g of this plant material were weighed and stored until their use. Two-hundred milliliters of boiling water were added to 1 g (infusion A: 0.005 g/mL) or 3 g (infusion B: 0.015 g/mL) of plant material. Infusions were allowed to steep for 10 min with continuous swirling. Samples were then obtained at 3, 5, and 10 min.
4.1.3. Determination of Antioxidant Activity
Antioxidant activity of aqueous extracts was measured using the 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) radical cation (ABTS•+) decolorization assay [56].
Final concentrations measured in the assay were 5 × 10−5 g/mL in infusion A and 1.5 × 10−4 g/mL in infusion B. Absorbance measurements of the infusion samples (A sample) were made at 734 nm at 0.5, 1, 2, 3, 4, 5, 7, 10, 15, 20, 25, 30, 45 and 90 min. Samples were assayed in quintuplicate.
The inhibition percentage (p) of free radical formation was calculated as in equation:
| (1) |
Ascorbic acid was used to calculate a standard curve (0.5, 1, 1.5, 2, 2.5, 3, and 3.5 µg/mL). Absorbance measurements were made at 0.5, 1, 2, 3, 4, 5, 6, 7, 10, 15 and 20 min, and each sample was conducted in triplicate. Ascorbic acid inhibition percentages were plotted against concentration, and the calibration equation calculated was Y = −1.397 + 25.377X (R2 = 0.9936), where Y is the inhibition percentage and X is ascorbic acid concentration in µg/mL.
The radical scavenging activity of aqueous infusions was expressed as Ascorbic acid Equivalent Antioxidant Capacity (AEAC) in milligrams of ascorbic acid per 100 g of plant (mg/100 g) [42], which was calculated according to the Equation (2):
| (2) |
where IC50 is the concentration of ascorbic acid or infusion (IC50 infusion) that inhibits free radical formation by 50%.
4.2. Evaluation of the Effects on Lipid Profile
4.2.1. Animals
Twenty-four healthy New Zealand white rabbits weighing 1.75–2.35 kg from the Animal Housing of the University of Leon were used. The animals were housed in individual metal cages, which allowed the isolation of feces in a lower container to avoid coprophagia. The environmental conditions were: humidity (55 ± 10%), temperature (19 ± 2 °C) and 12 h light-12 h dark cycle. Rabbits were maintained under these conditions at least 1 week before the assay, with free access to water and standard laboratory chow.
The rabbits were randomly divided into four groups of 6 rabbits each. The first group, (Control group) received a standard laboratory chow, whose composition was: proteins 16.5%, fats 3.4%, fiber 15.5%, ashes 7.2%, carbohydrates 19.3%, sugars 4.5%, vitamin A 15,000 UI/kg, vitamin D3 1100 UI/kg, vitamin E 100 mg/kg, vitamin K 3.5 mg/kg and copper 5 mg/kg (Ssniff, Germany). The second one (Cholesterol group) was fed with the same standard laboratory chow but enriched with 0.2% of cholesterol.
The third group (Sideritis group) received the standard laboratory chow enriched with cholesterol 0.2% and the plant Sideritis hyssopifolia, L. (2.36 g Sideritis hyssopifolia, and L./150 g of standard chow, as calculated previously).
The fourth group (Simvastatin group) was fed with the standard laboratory chow enriched with 0.2% of cholesterol and 20 mg/kg of simvastatin (Ferrer Pharma, Spain).
Animals received 150 g of laboratory chow every day during 6 weeks. Before feeding the animals, it was assessed if they had eaten all the administered fraction of food. The study was approved by the Ethics Committee of the University of León (Spain) with the reference number ETICA-ULE-002-2016.
4.2.2. Blood Sampling
Blood samples (8–9 mL) were obtained from the marginal ear vein at the beginning (week 0), and at week 6 of the study. Immediately after collection, plasma was separated by centrifugation and stored at −80 °C until analyzed.
Total cholesterol (TC), high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c) and triglycerides (TG) in the plasma samples were determined by using specific kits (Chemicals diagnostic kits). All parameters were quantified by a spectrophotometer UV/VIS (Hitachi). The atherogenic index (IA) was calculated with the mathematic formula described by Hassan and Abdel-Wahhab [57].
4.2.3. Statistical Evaluation
All parameters measured were determined for each animal and presented as arithmetic mean ± standard deviation (mean ± SD). Data were analyzed using the Skewness test (to determine the normality) and Cochran test (to determine the uniformity of the variance).
When the data were normal and there was uniformity in the variance, analysis of variance (ANOVA) was carried out and the Duncan test was used to determine differences between data sets. When the data were not normal and/or there was not uniformity in the variance, the Friedman test was employed and the Wilcoxon test was used to determine differences between data sets. P ≤ 0.05 was used as the level of significance for all analyses.
5. Conclusions
We have demonstrated that Sideritis hyssopifolia exhibits a moderate antioxidant activity and could be used as an accessible source of natural antioxidants. We have also calculated the amount of plant that may exhibit a hypocholesterolemic effects in a rabbit experimental model, which will help to evaluate its potential activity against atherosclerosis. The study carried out using the experimental animal model showed that this chow decreased cholesterol, HDL-cholesterol, LDL-cholesterol and triglyceride levels in cholesterol-fed rabbits, as well as the atherogenic index. This reduction was similar to that obtained with simvastatin.
Author Contributions
M.S. and N.F. conceived and supervised the study; E.C., M.J.D., J.J.G. and A.M.S. took part in laboratory work and developed calculations; E.C., M.S., N.F. and J.J.G. analyzed the data; M.S. and N.F. wrote the paper, with the input of the other authors. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Herrington W., Lacey B., Sherliker P., Armitage J., Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ. Res. 2016;118:535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 2.Nordestgaard B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from Epidemiology, Genetics, and Biology. Circ. Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 3.Ouweneel A.B., Van Eck M. Lipoproteins as modulators of atherothrombosis: From endothelial function to primary and secondary coagulation. Vascul. Pharmacol. 2015;82:1–10. doi: 10.1016/j.vph.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Riaz M., Zia-Ul-Haq M., Saad B. Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects. Springer; Berlin, Germany: 2016. [Google Scholar]
- 5.Griendling K.K., Fitzgerald G.A. Oxidative stress and cardiovascular injury part I: Basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez-Tortosa M.C., Mesa M.D., Aguilera M.C., Quiles J.L., Baró L., Ramirez-Tortosa C.L., Mattinez-Victoria E., Gil A. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147:371–378. doi: 10.1016/S0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 7.Siti H.N., Kamisah Y., Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascul. Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 9.Meagher E.A. Addressing cardiovascular risk beyond low-density lipoprotein cholesterol: The high-density lipoprotein cholesterol story. Curr. Cardiol. Rep. 2004;6:457–463. doi: 10.1007/s11886-004-0055-2. [DOI] [PubMed] [Google Scholar]
- 10.Nofer J.R., Kehrel B., Fobker M., Levkau B., Assmann G., von Eckardstein A. HDL and arteriosclerosis: Beyond reverse cholesterol transport. Atheroesclerosis. 2002;161:1–16. doi: 10.1016/S0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 11.Di Matteo V., Esposito E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Curr. Drug Targets CNS Neurol. Disord. 2003;2:95–107. doi: 10.2174/1568007033482959. [DOI] [PubMed] [Google Scholar]
- 12.Gerber M., Boutron-Rault M.C., Hercberg S., Riboli E., Scalbert A., Siess M.H. Food and cancer: State of the art about the protective effect of fruits and vegetables. Bull. Cancer. 2002;89:293–312. [PubMed] [Google Scholar]
- 13.Zia-Ul-Haq M., Ahmad S., Bukhari S.A., Amarowicz R., Ercisli S., Jaafar H.Z. Compositional studies and biological activities of some mash bean (Vigna mungo (L.) Hepper) cultivars commonly consumed in Pakistan. Biol. Res. 2014;47:23. doi: 10.1186/0717-6287-47-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zia-Ul-Haq M., Riaz M., De Feo V., Haafar H.Z., Moga M. Rubus fruticosus L.: Constituents, biological activities and health related uses. Molecules. 2014;19:10998–11029. doi: 10.3390/molecules190810998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson J.V., Palombo R.D., Earl R. Position of the American Dietetic Association: The role of nutrition in health promotion and disease prevention programs. J. Am. Diet. Assoc. 1998;98:205–208. doi: 10.1016/s0002-8223(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 16.Hertog M.G., Feskens E.J., Hollman P.C., Katan M.B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- 17.Kris-Etherton P.M., Krummel D. Role of nutrition in the prevention and treatment of coronary heart disease in women. J. Am. Diet. Assoc. 1993;93:987–993. doi: 10.1016/0002-8223(93)92035-V. [DOI] [PubMed] [Google Scholar]
- 18.Amensour M., Sendra E., Abrini J., Bouhdid S. Total phenolic content and antioxidant activity of myrtle (Myrtus communis) extracts. Nat. Prod. Commun. 2009;4:819–824. doi: 10.1177/1934578X0900400616. [DOI] [PubMed] [Google Scholar]
- 19.Prasad K. A study on regression of hypercholesterolemic atherosclerosis in rabbits by flax lignan complex. J. Cardiovasc. Pharmacol. Ther. 2007;12:304–313. doi: 10.1177/1074248407307853. [DOI] [PubMed] [Google Scholar]
- 20.Gey K.F., Stahelin H.B., Eichholzer M. Poor plasma status of carotene and vitamin C is associated with higher mortality from ischemic heart disease and stroke. Basel prospective study. Clin. Investig. 1993;71:3–6. doi: 10.1007/BF00210955. [DOI] [PubMed] [Google Scholar]
- 21.Riemersma R.A., Wood D.A., Macintyre C.C., Elton R.A., Gey K.F., Oliver M.F. Risk of angina pectoris and plasma concentration of vitamins A, C and E and carotene. Lancet. 1993;337:1–5. doi: 10.1016/0140-6736(91)93327-6. [DOI] [PubMed] [Google Scholar]
- 22.Arts I.C., Hollman P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005;81:317–325. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y.S., Yang J.H., Bae M.J., Yoo W.K., Ye S., Xue C.C., Li C.G. Antioxidant and anti-hypercholesterolaemic activities of Wasabia japonica. Evid. Based Complement. Altern. Med. 2010;7:459–464. doi: 10.1093/ecam/nen038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozdemir B., Ekbul A., Topal N.B., Sarandöl E., Sağ S., Başer K.H., Cordan J., Güllülü S., Tuncel E., Baran I., et al. Effects of Origanum onites on endothelial function and serum biochemical markers in hyperlipidaemic patients. J. Int. Med. Res. 2008;36:1326–1334. doi: 10.1177/147323000803600621. [DOI] [PubMed] [Google Scholar]
- 25.Kitic D., Brankovic S., Radenkovic M., Savikin K., Zdunic G., Kocic B., Velickovic-Radovanovic R. Hypotensive, vasorelaxant and cardiodepressant activities of the ethanol extract of Sideritis raeseri spp. raeseri Boiss & Heldr. J. Physiol. Pharmacol. 2012;63:531–535. [PubMed] [Google Scholar]
- 26.Tadić V., Bojović D., Arsić I., Dorđević S., Aksentijevic K., Stamenić M., Janković S. Chemical and antimicrobial evaluation of supercritical and conventional Sideritis scardica Griseb., Lamiaceae extracts. Molecules. 2012;17:2683–2703. doi: 10.3390/molecules17032683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González-Burgos E., Carretero M.E., Gómez-Serranillos M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities- A review. J. Ethnopharmacol. 2011;135:209–225. doi: 10.1016/j.jep.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Dimitrios B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006;17:505–512. doi: 10.1016/j.tifs.2006.04.004. [DOI] [Google Scholar]
- 29.Samanidou V., Tsagiannidis A., Sarakatsianos I. Simultaneous determination of polyphenols and major purine alkaloids in Greek Sideritis species, herbal extracts, green tea, black tea, and coffee by high-performance liquid chromatography-diode array detection. J. Sep. Sci. 2012;35:608–615. doi: 10.1002/jssc.201100894. [DOI] [PubMed] [Google Scholar]
- 30.Barboza L.N., Lívero F.A., Prando T.B., Ribeiro R.C., Lourenço E.L., Budel J.M., de Souza L.M., Acco A., Dalsenter P.R., Gasparotto A. Atheroprotective effects of Cuphea carthagenensis (Jacq.) J.F. Macbr. in New Zealand rabbits fed with cholesterol-rich diet. J. Ethnopharmacol. 2016;187:134–145. doi: 10.1016/j.jep.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Liang R., Wang L., Yan R., Hou R., Gao S., Yanq B. Effects of an aqueous extract of Crataegus pinnatifida Bge. var. major N.E.Br. fruit on experimental atherosclerosis in rats. J. Ethnopharmacol. 2013;148:563–569. doi: 10.1016/j.jep.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 32.Al-Naqeep G., Al-Zubairi A.S., Ismail M., Amom Z.H., Esa N.M. Antiatherogenic potential of Nigella sativa seeds and oil in diet-induced hypercholesterolemia in rabbits. Evid. Based Complement. Altern. Med. 2011;2011:213628. doi: 10.1093/ecam/neq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghannadi A., Movahedian A., Jannesary Z. Hypocholesterolemic effects of Balangu (Lallemantia royleana) seeds in the rabbits fed on a cholesterol-containing diet. Avicenna J. Phytomed. 2015;5:167–173. [PMC free article] [PubMed] [Google Scholar]
- 34.Rekha C., Poornima G., Manasa M., Abhipsa V., Pavithra J., Vijay H.T., Prashith T.R. Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. Chem. Sci. Trans. 2012;1:303–310. doi: 10.7598/cst2012.182. [DOI] [Google Scholar]
- 35.Gokce N., Keaney J.F., Frei B., Holbrook M., Olesiak M., Zachariah B.J., Leeuwenburgh C., Heinecke J.W., Vita J.A. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999;99:3234–3240. doi: 10.1161/01.CIR.99.25.3234. [DOI] [PubMed] [Google Scholar]
- 36.Levine G.N., Frei B., Koulouris S.N., Gerhard M.D., Keaney J.F., Vita J.A. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996;93:1107–1113. doi: 10.1161/01.CIR.93.6.1107. [DOI] [PubMed] [Google Scholar]
- 37.Protogerou A.D., Lekakis J.P., Kontoyanni D.D., Stamatelopoulos K.S., Tsotsoros N.D., Papaioannou T.G., Tryfonopoulos D.J., Papamichael C.M., Stamatelopoulos S.F. Effect of ascorbic acid on forearm reactive hyperaemia in patients with hypercholesterolaemia. Eur. J. Cardiovasc. Prev. Rehabil. 2004;11:149–154. doi: 10.1097/01.hjr.0000095049.46631.b9. [DOI] [PubMed] [Google Scholar]
- 38.Uzun A., Yener U., Cicek O.F., Yener O., Yalcinkaya A., Diken A., Ozkan T., Turkvatan A., Ulas M. Does vitamin C or its combination with vitamin e improve radial artery endothelium-dependent vasodilatation in patients awaiting coronary artery bypass surgery? Cardiovasc. J. Afr. 2013;24:255–259. doi: 10.5830/CVJA-2013-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ting H.H., Timimi F.K., Boles K.S., Creager S.J., Ganz P., Creager M.A. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Clin. Investig. 1996;97:22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frei B., England L., Ames B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baskaran G., Salvamani S., Azlan A., Ahmad S.A., Yeap S.K., Shukor M.Y. Hypocholesterolemic and antiatherosclerotic potential of Basella alba leaf extract in hypercholesterolemia-induced rabbits. Evid. Based Complement. Altern. Med. 2015;2015:751714. doi: 10.1155/2015/751714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boban P.T., Nambisan B., Sudhakaran P.R. Dietary mucilage promotes regression of atheromatous lesions in hypercholesterolemic rabbits. Phytother. Res. 2009;23:725–730. doi: 10.1002/ptr.2728. [DOI] [PubMed] [Google Scholar]
- 43.Kabiri N., Asgary S., Setorki M. Lipid lowering by hydroalcoholic extracts of Amaranthus caudatus L. induces regression of rabbits atherosclerotic lesions. Lipids Health Dis. 2011;10:89. doi: 10.1186/1476-511X-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y., Yan F., Liu Y., Zhang C., Yu H., Zhang Y., Zhao Y. Aqueous extract of rhubarb stabilizes vulnerable atherosclerotic plaques due to depression of inflammation and lipid accumulation. Phytother. Res. 2008;22:935–942. doi: 10.1002/ptr.2429. [DOI] [PubMed] [Google Scholar]
- 45.Gey K.F. Ten-year retrospective on the antioxidant hypothesis of arteriosclerosis: Threshold plasma levels of antioxidant micronutrients related to minimum cardiovascular risk. J. Nutr. Biochem. 1995;6:206–236. doi: 10.1016/0955-2863(95)00032-U. [DOI] [Google Scholar]
- 46.Chan E.W.C., Lim Y.Y., Wong S.K., Lim K.K., Tan S.P., Lianto F.S., Yong M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2008;113:166–172. doi: 10.1016/j.foodchem.2008.07.090. [DOI] [Google Scholar]
- 47.Chan E.W.C., Lim Y.Y., Chong K.L., Tan J.B.L., Wong S.K. Antioxidant properties of tropical and temperate herbal teas. J. Food Comp. Anal. 2010;23:185–189. doi: 10.1016/j.jfca.2009.10.002. [DOI] [Google Scholar]
- 48.Chew Y.L., Goh J.K., Lim Y.Y. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in peninsular Malaysia. Food Chem. 2009;116:13–18. doi: 10.1016/j.foodchem.2009.01.091. [DOI] [Google Scholar]
- 49.Gabrieli C.N., Kelafas P.G., Kokkalou E.L. Antioxidant activity of flavonoids from Sideritis raeseri. J. Ethnopharmacol. 2005;96:423–428. doi: 10.1016/j.jep.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 50.Rodríguez-Lyon M.L., Díaz-Lanza A.M., Bernabé M., Villaescusa-Castillo L. Flavone glycosides containing acetylated sugars from Sideritis hyssopifolia. Magn. Reson. Chem. 2000;38:684–687. doi: 10.1002/1097-458X(200008)38:8<684::AID-MRC683>3.0.CO;2-G. [DOI] [Google Scholar]
- 51.Fan J., Kitajima S., Watanabe T., Xu J., Zhang J., Liu E., Chen Y.E. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 2015;146:104–119. doi: 10.1016/j.pharmthera.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Formisano C., Oliviero F., Rigano D., Arnold N.A., Senatore F. Comparative Chemical Composition and Antioxidant Properties of the Essential Oils of three Sideritis libanotica Subspecies. Nat. Prod. Commun. 2015;10:1075–1078. doi: 10.1177/1934578X1501000670. [DOI] [PubMed] [Google Scholar]
- 53.Georgakopoulou V., Dimou C., Karantonis H.C. In vitro antioxidant, antithrombotic, antiatherogenic and antidiabetic activities of Urtica dioica, Sideritis euboea and Cistus creticus water extracts and investigation of pasta fortification with the most bioactive one. Curr. Pharm. Biotechnol. 2019;20:1–14. doi: 10.2174/1389201020666190328114343. [DOI] [PubMed] [Google Scholar]
- 54.Jeremic I., Petricevic S., Tadic V., Petrovic D., Tosic J., Stanojevic Z., Petronijevic M., Vidicevic S., Trajkovic V., Isakovic A. Effects of Sideritis scardica Extract on Glucose Tolerance, Triglyceride Levels and Markers of Oxidative Stress in Ovariectomized Rats. Planta Med. 2019;85:465–472. doi: 10.1055/a-0835-6622. [DOI] [PubMed] [Google Scholar]
- 55.Ivanova D., Gerova D., Chervenkov T., Yankova T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol. 2005;96:145–150. doi: 10.1016/j.jep.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 56.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 57.Hassan H.A., Abdel-Wahhab M.A. Effect of soybean oil on atherogenic metabolic risks associated with estrogen deficiency in ovariectomized rats. J. Physiol. Biochem. 2012;68:247–253. doi: 10.1007/s13105-011-0137-8. [DOI] [PubMed] [Google Scholar]