Abstract
In this article the scientific activity carried out on stemarane diterpenes and diterpenoids, isolated over the world from various natural sources, was reviewed. The structure elucidation of stemarane diterpenes and diterpenoids was reported, in addition to their biogenesis and biosynthesis. Stemarane diterpenes and diterpenoids biotransformations and biological activity was also taken into account. Finally the work leading to the synthesis and enantiosynthesis of stemarane diterpenes and diterpenoids was described.
Keywords: stemarane, diterpenes, diterpenoids, isolation, structure, biogenesis, biosynthesis, synthesis, enantiosynthesis
1. Introduction
Stemarane diterpene and diterpenoids are tetracyclic compounds characterized by a unique hydrocarbon skeleton. They were named after (+)-stemarin 1 (Figure 1), isolated in 1975 by Manchand and Blount [1] at Hoffmann-La Roche Inc., Nutley, N.J. from Stemodia Maritima L. (Scrophulariaceae) (Figure 2), a rare West Indies littoral plant.
Figure 1.
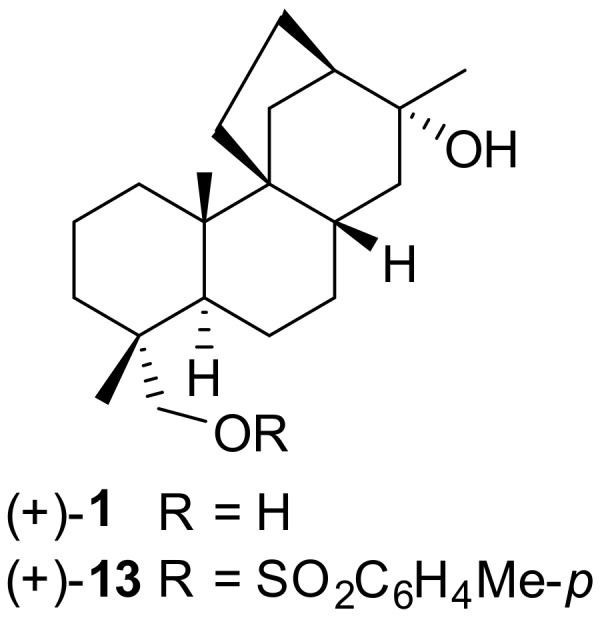
(+)-stemarin (1) and corresponding C(18) tosylate (13).
Figure 2.
Stemodia maritima L. (Scrophulariaceae). (From: Alex Popovkin, Bahia, Brazil, available online: https://flickr.com/photos/12589168@N00/15015148887.
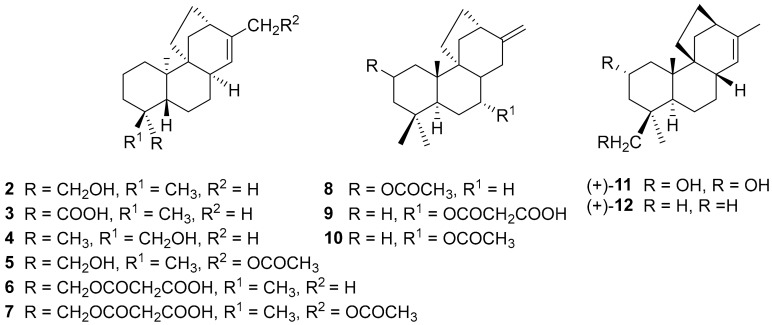
Some years later Garbarino and co-workers isolated—at Universidad Técnica Federico Santa Maria, Valparaiso—a number of stemarane diterpenes from Chilean plants of the Calceolaria genus (Figure 3), whose absolute configuration was opposite to that of (+)-stemarin (1). Thus, from the aerial parts of Calceolaria lepida Phil., a plant that grows in the coastal hills of central Chile, the Valparaiso group isolated ent-stemar-13(14)-en-19-ol 2 and ent-stemar-13(14)-en-19-oic acid 3 [2]. The former was also isolated, along with and (+)-ent-stemar-13(14)-en-18-ol 4, from Calceolaria latifolia Benth. [3], a species growing on hills of north and central Chile.
Figure 3.
Ent-stemarane diterpenoids isolated from Chilean plants of Calceolaria genus (2–10) and (+)-oryzalexin S (11) and (+)-13-stemarene (12).
The Valparaiso group also reported the isolation and structure elucidation from Calceolaria kingii Phil., which grows in north and central Chile, of ent-stemara-13(14)-en-17-acetoxy-18-ol 5 [4]; from Calceolaria polifolia Hook, a species which grows on the coastal hills of central Chile, of 19-malonyloxy-ent-stemar-13(14)-ene 6, 17-acetoxy-19-malonyloxy-ent-stemar-13(14)-ene 7, and already known ent-stemar-13(14)-en-19-ol 2 (vide supra) [5]; from the aerial parts of Calceolaria dentata Ruiz & Pav., a perennial plant which grows in the middle-South of Chile, of 2β-acetoxy-13-methylene-stemarane 8 and 7-malonyloxy-13-methylene-stemarane 9 [6] (In references [6,7], though a stemarane structure was attributed to compounds 8, 9 and 10, the stereochemistry of H-C(8) was not specified in the formulae, and the C(9)-C(11) two carbon bridge was drawn with stereochemistry opposite to the CH3-C(10) as in stemarane diterpenes and diterpenoids.); from Calceolaria glabrata Phil. var. glabrata, a shrub common in the southern part of Chile, of 13-methylene-7-acetoxy-stemarane 10 [7] and finally from Calceolaria paralia Hook of ent-stemar-13(14)-en-19-oic acid 3 [8].
In the following years Tamogami and co-workers at Central Research Laboratories, Idemitsu Kosan Co., Ltd., Chiba and at Ibaraki University, Ibara, isolated from Pyricularia oryzae Cav. attacked Oryza sativa L. (Poaceae) (Figure 4) (+)-oryzalexin S [9] 11, a stemarane diterpenoid which displays phytoalexin properties. The production by the plant of (+)-11 is also induced by UV irradiation [9]. The production of diterpenoid phytoalexins after induction by UV irradiation was also studied in five rice genotypes of different susceptibility to the rice blast fungus Pyricularia oryzae at Reading University by Harborne and his group [10].
Figure 4.
Oryza sativa. (From: https://www.pexels.com/photo/close-up-photo-of-rice-grains-during-daytime-162992/).
Finally in 2001 Oikawa, Sassa, and co-workers, at Hokkaido and Yamagata Universities respectively, reported the isolation of (+)-13-stemarene 12 [11] from the phytopathogenic fungus Phoma betae.
2. Structure
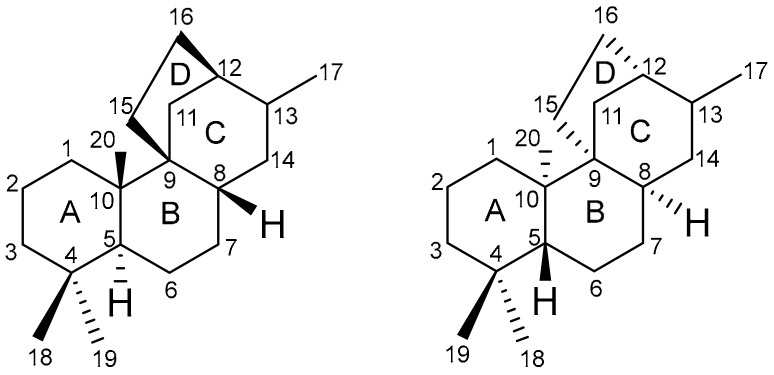
The stemarane diterpenes carbon skeleton is reported in Figure 5, and its main structural features are reported below: the bicyclic system C/D is constituted by a bicyclo[3.2.1]octane fused to the bicyclic A/B system in a different fashion with respect to other tetracyclic diterpenes possessing the bicyclo[3.2.1]octane system; two contiguous quaternary carbon atoms, the C(9) and C(10), are present, the former being a spirocyclic atom; oxygenated functions can be present at C(2), C(7), C(13), C(17), C(18), and C(19); the A/B ring junction is trans; the H-C(8) is syn to the CH3-C(10), and to the two carbon bridge connecting C-(9) and C-(12) (In this review only compounds with such features are reported.).
Figure 5.
Stemarane (left) and ent-stemarane (right) diterpenes carbon skeleta.
The structure and absolute stereochemistry of (+)-stemarin 1 was assigned by Manchand and Blount after a chemical study and an X-ray crystallographic analysis on tosylate (+)-13 [1].
The structure and relative configurations of stemarane diterpenoids 2–10 were assigned by Garbarino and co-workers by means of 1H- and 13C-NMR (Nuclear Magnetic Resonance), mass spectroscopy and by comparison with the spectral data of (+)-1. On the basis of biogenetic considerations, the ent absolute configuration was also assigned. No chemical correlations and/or X-ray structure determinations were ever made.
The structure proposed for (-)-2 [2,3,5] was not confirmed by the synthesis (vide infra) from (+)-podocarpic acid of (+)-2-deoxyoryzalexin S 2, reported by our group [12].
The structure and relative configuration of (+)-oryzalexin S 11 was established by a series of 2D-NMR experiments, which proved the relative configuration [13]. The structure and relative configuration of (+)-13-stemarene 12 was established by 1H-NMR [11]. The comparison with an authentic sample, obtained by our group from commercially available (+)-podocarpic acid, confirmed the proposed structure as well as its absolute configuration [14].
3. Biogenesis
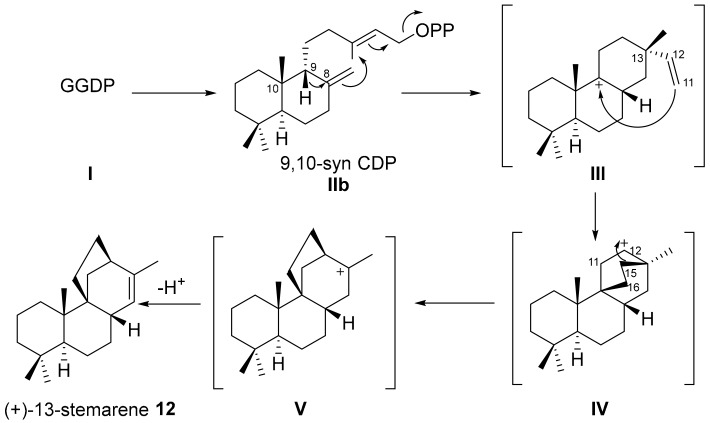
The biogenesis of stemarane diterpenes and diterpenoids, outlined in Scheme 1, was proposed by Tamogami and co-workers in 1993 [13]. The Authors proposed the biogenesis of this compound from GGPP (I) and compared it with the biogenesis of the other rice labdane-related diterpenoids. Since neither a proton nor a vinyl group is present at C(9) in (+)-oryzalexin S (11), the Authors assumed that its biogenesis begins with a hydride rearrangement from C(9) to C(8) by which II is converted into III in which the CH3-C(13) and the vinyl-C(13) group are α and β oriented, respectively. The attack to the C(9) carbocation by the vinyl group at C(13) gives then intermediate IV. A further rearrangement leads to intermediate V from which (+)-13-stemarene 12 is formed by the loss of H-. Oxidative processes at C(2) and C(19) give then (+)-oryzalexin S 11.
Scheme 1.
Biogenetic pathway to (+)-13-stemarene 12 as proposed by Tamogami and co-workers [13]. The black curved arrow represents the electrons’movement.
4. Biosynthesis
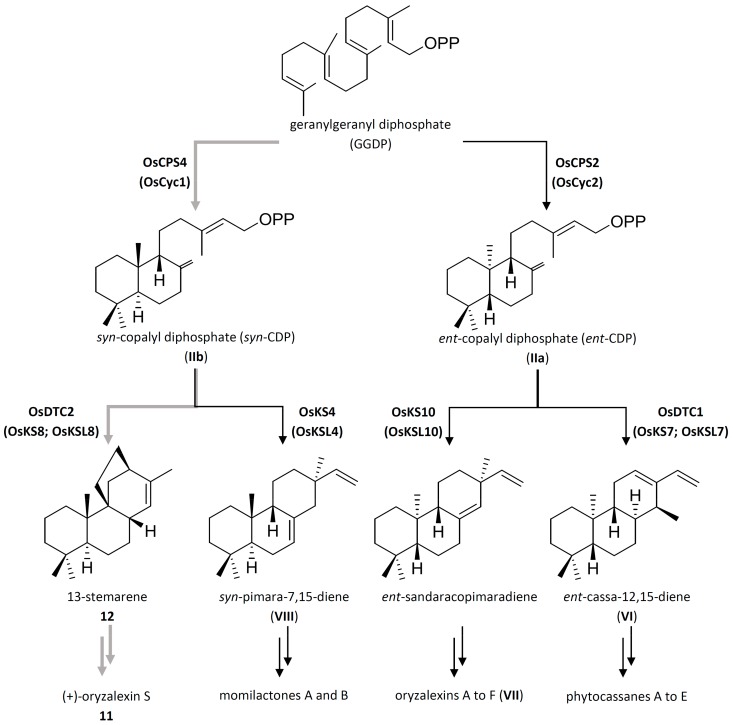
The few available studies on stemarane diterpene biosynthesis have been carried out on rice (Oryza spp.) plants and cell suspension cultures [15,16,17,18]. Rice diterpenes mainly belong to the class of labdane-related diterpenoids and most of them act as phytoalexins, i.e., molecules involved in plant defence whose biosynthesis is induced or enhanced by pathogen infection [19,20,21], treatment with signal molecules derived from pathogens (elicitors) [16], or exposure to UV radiation [9,16,22,23,24]. Gibberellins, a class of ubiquitous plant growth regulators, also belong to labdane-related diterpenoids [25]. On the basis of the structure of their hydrocarbon precursors, labdane-related diterpenoids are classified into four groups (Scheme 2): oryzalexins A to F [19,23,24], (–)-phytocassanes A to E [20,21,26,27], momilactones A and B [22,28], and (+)-oryzalexin S 11 [13].
Scheme 2.
Proposed pathway for the biosynthesis of diterpenoid phytoalexins in rice. Grey arrows indicate the branch that leads to (+)-oryzalexin S 11, while black arrows indicate the branches that leads to other labdane-related diterpenoids OsCPS2 (OsCyc2): ent-copalyl diphosphate synthase 2; OsCPS4 (OsCyc1) syn-copalyl diphosphate synthase; OsDTC1 (OsKSL7; OsKS7): ent-cassa-12,15-diene synthase; OsKS10 (OsKSL10): ent-sandaracopimaradiene synthase; OsKS4 (OsKSL4): syn-pimara-7,15-diene synthase; and OsDTC2 (OsKSL8; OsKS8) 13-stemarene synthase.
Labdane-related diterpenoids are biosynthesized from (E,E,E)-geranylgeranyl diphosphate (The molecule is also referred in the bibliographic sources as geranylgeranyl pyrophosphate) (GGPP), the universal diterpene precursor, which is cyclized to ent-copalyl diphosphate (ent-CDP) or syn-copalyl diphosphate (syn-CDP) by the labdane-related diterpene cyclases ent-copalyl diphosphate synthase 2 (OsCPS2) and syn-copalyl diphosphate synthase (OsCPS4), respectively. These cyclized diphosphate compounds can then be further cyclized and/or rearranged by more typical class I terpene synthases, which initiate catalysis via ionization of the allylic pyrophosphate group [29]. Ent-CDP is converted to ent-cassa-12,15-diene by ent-cassa-12,15-diene synthase (OsDTC1) or to ent-sandaracopimaradiene by ent-sandaracopimaradiene synthase (OsKS10), leading to phytocassanes A to E or oryzalexins A to F. Syn-CDP is converted to syn-pimara-7,15-diene by syn-pimara-7,15-diene synthase (OsKS4) or to (+)-13-stemarene 12 by 13-stemarene synthase (OsDTC2), leading to momilactones A and B and (+)-oryzalexin S 11.
In 1996, Mohan and co-workers from the University of Illinois reported the biosynthesis of cyclic diterpenes with enzyme extracts from rice cell suspension cultures to verify proposed pathways and intermediates in the production of momilactone and oryzalexin phytoalexins [15]. In the course of this work, the authors could confirm the role of 9,10-syn-copalyl diphosphate (syn-CDP) as a precursor of, inter alia, (+)-13-stemarene 12, from which (+)-oryzalexin S 11 is presumed to be formed.
In 2004, Yamane and co-workers from the University of Tokyo reported that two species of mRNA encoding OsDTC1 and OsDTC2, two putative diterpene cyclases, were expressed in rice cell suspensions in response to chitosan-elicitation [30]. The same authors obtained OsDTC2 cDNA from chitin-elicited suspension-cultured rice cells [16]. They overexpressed OsDTC2 cDNA in Escherichia coli as a fusion protein with glutathione S-transferase and demonstrated that the recombinant protein function as stemar-13-ene synthase, the enzyme that catalyse in the conversion of syn-CDP into (+)-13-stemarene 12, the putative precursor of (+)-oryzalexin S 11 (Scheme 2). They also observed (+)-13-stemarene 12 accumulation in both chitin-elicited suspension-cultured rice cells and in UV-irradiated rice leaves.
The carbocation reaction network for the formation of C(9)-ethano-bridged diterpenes, including stemarane diterpenes was described in 2002 by Oikawa and co-workers who integrated chemical and computational methods [31]. Finally, in 2018 Young and Tantillo from the University of California shed new light on the mechanisms of formation of the stemarene, stemodene, betaerdene, aphidicolene, and scopadulanol diterpenes from syn-CDP. The Authors demonstrated that the compounds of interest are interconnected by a complex network of reaction pathways, and that the interconnection of these paths leads to multiple routes for formation of each diterpene, which could lead to different origins for some carbon atoms in a given diterpenes under different conditions [32].
5. Biotransformations
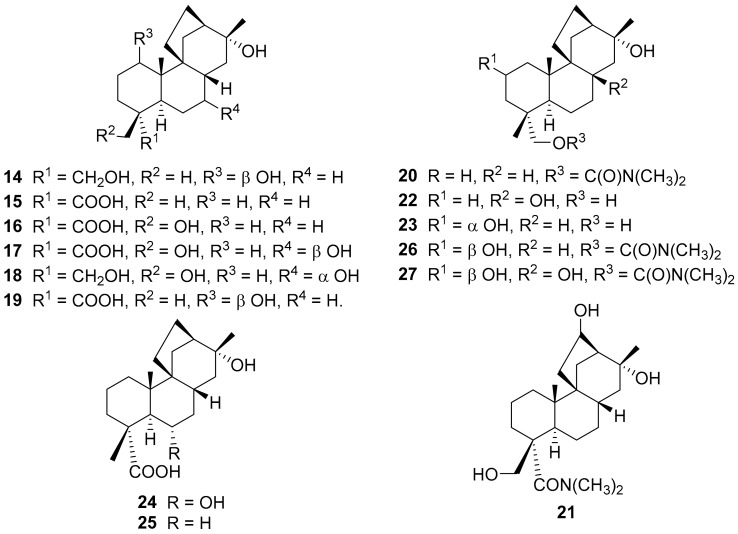
Extensive work in the area of biotransformations was carried out by Reese and his group at the University of West Indies, Mona. The Reese group reported the biotransformation by the action of Beauveria bassiana ATCC 7159 of (+)-stemarin 1 into 14 and 15 [33] (Figure 6). The same group reported that, by the action of Aspergillus niger ATCC 9142, (+)-1 is converted into four new metabolites (16, 17, 18, 19) while the (+)-dimethylcarbamate 20 gives 21 [34].
Figure 6.
Metabolites obtained by the action of several fungi on (+)-stemarin (1) and derivatives.
Later Reese and co-workers reported, inter alia, the hydroxylation of (+)-1 by Mucor plumbeus ATCC 4740 [35]. The incubation of (+)-1 with the fungus produced two new metabolites (+)-22 and (+)-23 to which on the basis of HRMS (High resolution mass spectrometry) data 13C and 2D NMR the structures of (+)-8,13,19-trihydroxystemarane and (+)-2α,13,19-trihydroxystemarane were attributed, respectively. On the contrary, the dimethylcarbamate derivative (+)-20 was not metabolised by the fungus. These biotransformations have been later reviewed [36]. In the following year, the Reese group described [37], inter alia, the bioconversion by Cunnighamella echinulata var. elegans of (+)-1 into (+)-6α,13-dihydroxystemaran-19-oic acid 24 while (+)-20 gave (+)-13-hydroxystemaran-19-oic acid 25 along with metabolites (+)-19-(N,N-Dimethylcarbamoxy)-2β,13-dihydroxystemarane 26 and (+)-19-(N,N-Dimethylcarbamoxy)-2β,8,13-trihydroxystemarane 27. No bioconversions were obtained from the fermentation of (+)-1 and (+)-20 with Phanerochaete chrysosporium.
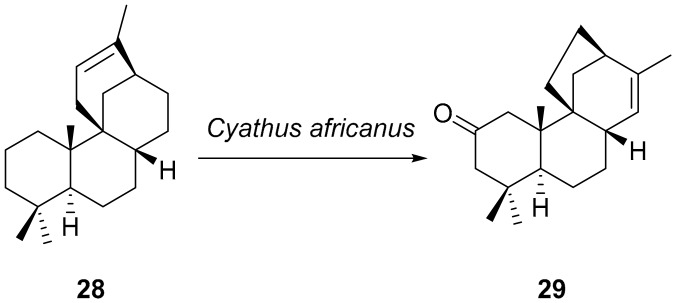
Finally, a very interesting observation was made by the Reese group; 12-stemodene 28, under the conditions the biotransformation by the fungus Cyathus africanus is carried out, not only is oxidized at C(2) but the skeleton is rearranged to the stemarene one giving 2-oxo-13-stemarene 29 (Scheme 3) [38]. In this respect, it should be recalled that stemodane and stemarane diterpenoids were both isolated from Stemodia maritima L. [1,39,40].
Scheme 3.
Conversion of 12-stemodene 28 into 2-oxo-13-stemarene 29 by the fungus Cyathus africanus.
6. Biological Activity
In the folk medicine of Dutch Antilles an infusion of leafy branches of sea lavander (Lavandula) and Stemodia maritima L., mixed with Epsom salts, is used by men against venereal diseases [41]. Plants of Calceolaria genus are used in Central and South America popular medicine as stomach tonics, bactericidal agents, and sweeteners [42]. Nevertheless, to our best knowledge, the biological activity of pure isolated stemarane diterpenes was not investigated with the exception (+)-oryzalexin S 11 which, as stated above, was found to possess phytoalexin activity [9].
Essential oils obtained by a Brazilian research group, leaded by Arriaga at Universidade Federal do Ceará, Fortaleza, from Stemodia maritima L. leaves and stems, collected in the state of Ceará, showed larvicidal properties against the larvae of the mosquito Aedes aegypti, responsible for the transmission of yellow fever in Central and South America and in west Africa and a vector of dengue hemorrhagic fever [43]. Nevertheless, the major components found in the leaf oil were β-caryophyllene and 14-hydroxy-9-epi-β-caryophyllene, while in the stem oil β-caryophyllene and caryophyllene oxide were the most abundant. This biological activity cannot therefore be attributed to stemarane diterpenes and diterpenoids.
Besides, the same research group evaluated the antioxidant and antibacterial activity of some Stemodia maritima L. isolated metabolites, but stemarane diterpenoids [44], and could also observe that Stemodia maritima L. extracts decrease inflammation, oxidative stress, and alveolar bone loss in an experimental periodontitis rat model [45].
It appears, therefore, that the biological activity of pure isolated stemarane diterpenes and diterpenoids, but (+)-oryzalexin S 11, has not been evaluated yet. The biological activity ascertained so far appears due to Stemodia maritima L. metabolites is different from that of stemarane diterpenes and diterpenoids. Comparing the content of stemarane diterpenes and diterpenoids among Stemodia maritima L. plants, collected in different geographical areas and extracted with the same procedure, seems a due task.
7. Synthesis
The C/D ring mojety constituted by a bicyclo[3.2.1]octane system fused in a novel way to the ring B, the presence of various stereocenters, two of which are the adjacent quaternary carbons (C-9 and C-10) and the interesting biological activity of some terms of this class of compounds, make stemarane diterpenoids a worthy synthetic challenge.
Kelly and co-workers at the University of New Brunswick (St. John N.B., Canada) and our group, both belonging to the Wiesner [46] school, got engaged with the synthesis of these very interesting compounds by the approach had been developed by their Mentor for the construction of the C/D ring system of diterpene alkaloids [47,48,49,50,51,52,53].
The approach adopted at first for obtaining this class of diterpenoids was based on the following steps:
-
(a)
Allene photoaddition to a suitably substituted α,β-unsaturated carbonyl intermediate [54,55,56,57,58,59,60];
-
(b)
Elaboration of the resulting photoadduct into a 1-methyl-6-hydroxybicyclo[2.2.2]octan-2-one [61,62];
-
(c)
Rearrangement of the 1-methyl-6-hydroxybicyclo[2.2.2]octane intermediate or suitable derivative into a bicyclo[3.2.1]octan-2-ene [63,64,65,66].
7.1. The (±)-Stemarin 1 Total Synthesis by Allene Photoaddition to a 9(11)-Podocarpen-12-one Intermediate
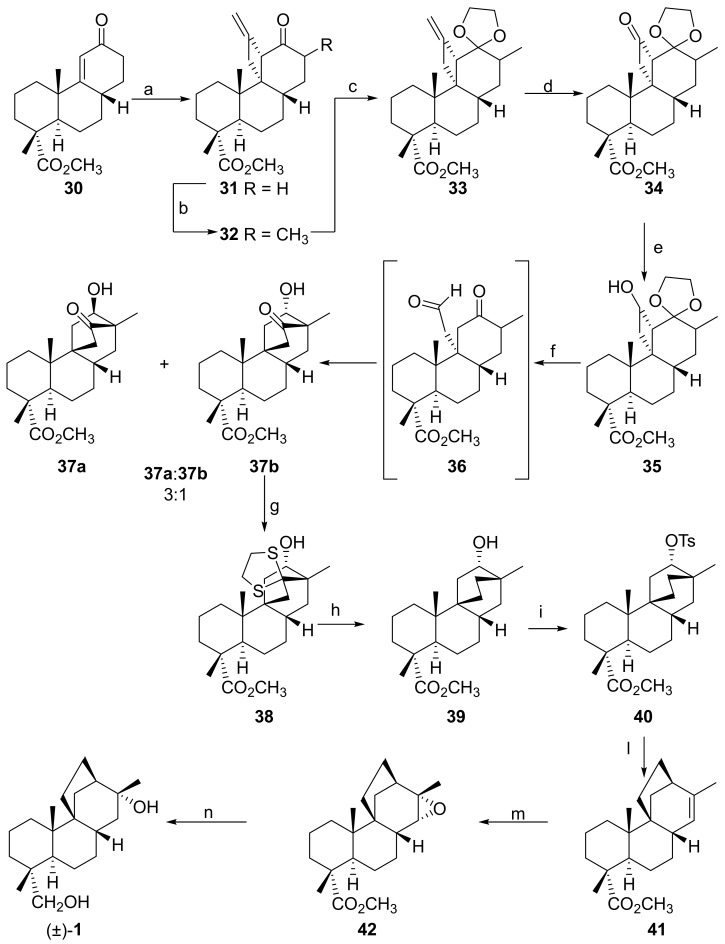
The first synthesis of a stemarane diterpene was disclosed in 1980 by Kelly and co-workers [67]. The St. John N.B. group obtained (±)-stemarin 1 from known racemic tricyclic intermediate 30 [68] (Scheme 4). Tricyclic enone 30, was submitted to allene photoaddition at −78 °C. The resulting photoadduct 31, whose stereochemistry followed from its conversion into the final compound, was methylated at C(13) to give 32. After protection of the carbonyl group (33), the exocyclic methylene was oxidatively cleaved to give 34. The latter was than reduced to the masked ketol 35. Thus, under acidic conditions the ketal group was removed and the resulting ketol underwent a retroaldol-aldol reaction to give ketols 37a and 37b in an about 3:1 ratio. Only the aldol 37b has the correct hydroxyl configuration for the subsequent rearrangement to the stemarin skeleton. The synthesis was therefore continued with 37b. Deoxygenation led to alcohol 39 which was converted into the tosylate 40. The latter upon rearrangement gave the olefinic ester 41. The conversion of this compound into (±)-stemarin 1 was then accomplished by stereoselective epoxidation to give 42 and hydride reduction of the latter. The synthetic material was found to be identical to an authentic sample by comparing the IR (Infrared radiation) and NMR spectra and by the identical behavior on TLC (Thin-layer chromatography) in a variety of solvents.
Scheme 4.
Schematic representation of (±)-stemarin 1 synthesis by Kelly and coworkers. Reagents and conditions: (a) allene, hν (Photochemical reaction), −78 °C; (b) THF (Tetrahydrofuran), −10 °C, lithium diisopropylamide, MeI; (c) (CH2OH)2, TsOH; (d) OsO4, NaIO4; (e) NaBH4; (f) HCl, THF; (g) (CH2SH)2, BF3·Et2O; (h) Raney-Ni, EtOH; (i) TsCl, Py; (l) NaCH2SOCH3, dimethyl sulfoxide; (m) meta-chloroperoxybenzoic acid; and (n) LiAlH4, Et2O.
7.2. Approaches for Diastereoselective Syntheses of (+)-13-Stemarene 12 and (+)-18-Deoxystemarin 2 and (±)-Stemarin 1 by Allene Photoaddition to 9(11)-Podocarpen-12-one and 8(9)-Podocarpen-14-one
While Kelly and his group were engaged in the synthesis of (±)-stemarin 1, our group was involved in the synthesis of stemodane [69,70,71] diterpenoids and aphidicolin [69,72,73]. After the successful conclusion of this work, a diastereoselective route to the key 6-hydroxy-1-methylbicyclo[2.2.2]octane intermediate (missing in the Kelly approach) appeared to us a worthwhile synthetic challenge. (+)-13-stemarene 12 and (+)-18-deoxystemarin 2, the simplest terms in the class were chosen as targets.
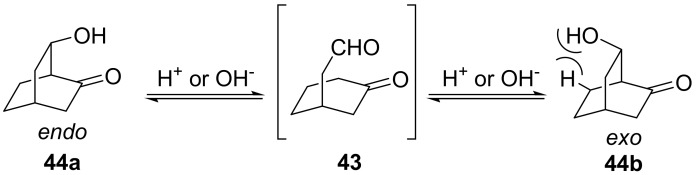
As mentioned before, under thermodynamically controlled conditions, 3-oxocyclohexaneethanals of type 43 give by intramolecular aldol reaction an about 85/15 endo/exo 6-hydroxybicyclo[2.2.2]octan-2-one 44 mixture (Scheme 5). It was shown by our group that this equilibrium distribution is due to an unfavourable 1,3 boat-axial interaction experimented in the exo epimer by the pseudo-axially oriented hydroxy group [74]. It follows that, in a substituted system, the location of the carbonyl influences the orientation of the hydroxyl group.
Scheme 5.
Endo/exo equilibration of 6-hydroxybicyclo[2.2.2]octan-2-ones under either basic or acid conditions.
We decided to solve the problem by the Wiesner two carbons annellation methodology. Two approaches were suggested by retrosynthetic analysis (Scheme 6): the first one (A), starting from a 9(11)-podocarpen-12-one, as in the Kelly approach, would have required the HO-C(12) configuration inversion; the second one (B) would have requested the photoaddition to be performed on a 8(9)-podocarpen-14-one, which, on the basis of the Wiesner empirical rule [57,58,59], should have ensured the same stereofacial selectivity and produced a 6-hydroxy-bicyclo[2.2.2]octan-2-one epimeric mixture whose major epimer should have the HO-(C12) group properly oriented (α) for the rearrangement to the stemarane system.
Scheme 6.
Retrosynthetic approaches to key intermediates of type VI.
In this approach, in the hydroxybiciclo[2.2.2]octanone intermediates XII the H-C(8) is adjacent to the C(14) carbonyl group. In principle, two epimers (XIIa and XIIb) at C(8) could be formed as result of the experimental conditions necessary for the intramolecular aldol reaction. Comparing the structures of XIIa and XIIb the epimer having the H–C(8) α configurated (XIIb) appears less stable because of the presence of a number of unfavourable steric interactions: while ring A is in a chair conformation ring B is in a boat conformation and the C(9)-C(10) and CH3-C(10) bonds are eclipsed (Figure 7, right). On the contrary in XIIa in which the H-C(8) is β configurated both rings A and B are in the chair conformation and the C(9)-C(10) and the CH3-C(10) bonds are staggered (Figure 7, left). After equilibration the C(8) stereogenic center should therefore materialize in the desired configuration in which the H-C(8) is β oriented.
Figure 7.
Three-dimesional structures of XIIa (left) and XIIb (right); red round indicates oxygen atom and grey one carbon atom.
7.2.1. Approach A: Diastereoselective Synthesis of (+)-13-Stemarene 12 and (+)-18-Deoxystemarin 2 by Allene Addition to 9(11)-Podocarpen-12-one
This approach—which has been recently reviewed [75]—has been successfully accomplished and resulted in the synthesis from (+)-podocarpic acid of (+)-13-stemarene 12 and (+)-18-deoxystemarin 2 (Scheme 7) [14,76,77]. It was based on the inversion of configuration of the HO-C(12). Thus the major ketol epimer 47a was converted into the corresponding tosylate 48 and the latter treated with Et4N(PhCOO) in acetone at reflux affording the exo-benzoate 49. This methodology, described in the past by Streitweiser and co-workers for the inversion of configuration of acyclic secondary alcohols [78], is quite convenient in that produces a locked exo-ketol which cannot therefore re-equilibrate to the more stable endo epimer.
Scheme 7.
Diastereoselective synthesis of (+)-12 and (+)-2 fom (+)-podocarpic acid. Reagents and conditions: (a) allene, THF, hν, −78 °C; (b) TsCl/Py; (c) NEt4(PhCOO); (d) Raney-Ni, EtOH, reflux; and (e) TsOH, benzene, reflux.
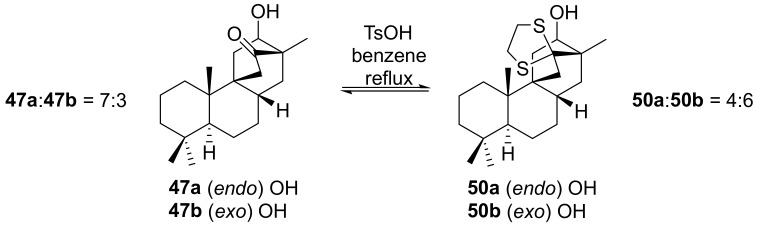
As can be observed in Scheme 8, the 6-exo-hydroxybicyclo[2.2.2]octan-2-one ethylene dithioacetal 50b is a key intermediate in the synthesis of 13-stemarene 12 and 18-deoxystemarin 2.
Scheme 8.
Ethylene dithioacetal equilibration.
Thus an expeditious preparation of 50b was also elaborated by equilibrating under acidic conditions the endo rich 6-hydroxybicyclo[2.2.2]octan-2-one ethylene dithioacetal mixture 50. It was found that, after equilibration, the exo epimer 50b is the major one [79].
7.2.2. Approach B: Attempted Diastereoselective Synthesis of (±)-Stemarin 1 by Allene Addition to a 8(9)-Podocarpen-14-one
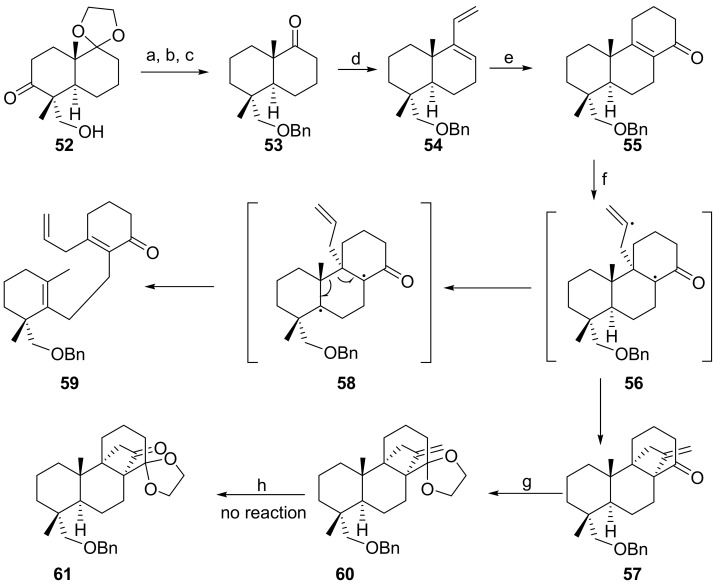
The approach to stemarane diterpenes via a 8(9)-podocarpen-14-one appeared quite attractive since the allene photoaddition was expected, on the basis of the Wiesner empirical rule (vide supra), to proceed from the α-side, as in the case of 9(11)-podocarpen-12-ones, thus ensuring the correct stereochemistry at C(9). Besides, the HO-C(12) and H-C(8) should have both emerged from the aldol reaction in the desired orientation (vide supra).
Surprisingly, after a successful preparation of the 8(9)podocarpen-14-one intermediate 46 [80] and a model work [81], the allene photoaddition to 8(9)-podocarpen-14-one 55, obtained from known 52 [82,83], gave, along the expected cyclo-photoadduct 57, compound 59 [84]. Compound 59 is the result of the H-C(5) abstraction by the initially side chain radical 56, to give the Cope rearrangement like intermediate 58, followed by homolytic cleavage of the C(9)-C(10) bond (Scheme 9). Photoadduct 57 was then acetalized with ethylene glycol to give 60. Unfortunately, we were unable to cleave the exocyclic double bond into 61 (Unpublished results by our group.). This approach was therefore discontinued.
Scheme 9.
Attempted approach via 8(9)-podocarpen-14-one 46. Reagents and conditions: (a) (1) TsNHNH2, THF, reflux, 3 h; (2) NaBH3CN, reflux, 8 h (b) (1) NaH, THF, reflux, 1 h; (2) BnBr, reflux, 3 h (c) 2N HCl, reflux, 30 min (d) (1) BrMgCHCH2, THF, 0 °C, N2; (2) PBr3, CH2Cl2, dimethylformamide, −10 °C, N2; 3 h 0 °C.; (e) α-acetoxycrilonitrile, Carius tube, 110 °C, N2, 3 d; (f) allene, hν, pyrex, −78 °C; (g) ethylene glycol, TsOH, benzene, Dean–Stark; and (h) OsO4/NaIO4.
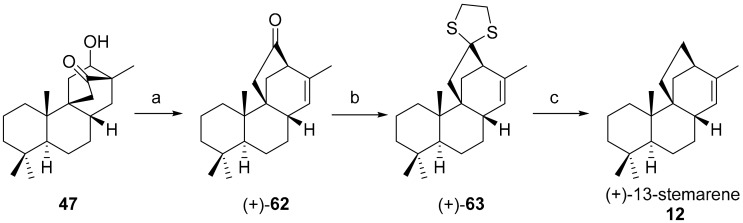
7.3. Regio- and Diastereoselective Synthesis of (+)-Stemar-13-ene 12 and (+)-18-Deoxystemarin 2 by the 6-Hydroxy-1-methylbicyclo[2.2.2]octan-2-one → 4-Methylbicyclo[3.2.1]oct-3-en-6-one Skeletal Rearrangement
This approach, which has been recently reviewed, was successfully accomplished by our group resulting in the synthesis of (+)-13-stemarene 12 and (+)-18-deoxystemarin 2. It was based on the 6-hydroxy-1-methyl-bicyclo-[2.2.2]octan-2-one → 4-methylbicyclo[3.2.1]oct-3-en-6-one skeletal rearrangement described in 2006 by Srikrishna and co-workers at the Indian Institute of Science, Bangalore, in the frame of a study on the reactivity of isotwistanes [85]. The remarkable feature of this approach [86] is that, owing to the stereospecificity of the rearrangement and to the 1-methyl-6-hydroxybicyclo[2.2.2]octan-2-ones endo/exo equilibrium, the whole 1-methyl-6-hydroxybicyclo[2.2.2]octan-2-ones endo/exo mixture 47 is converted into the rearrangement product in which is also present the C(13)-C(14) double bond (Scheme 10), a characteristic feature of some stemarane diterpenoids such as (+)-oryzalexin S 11 and a necessary tool for the introduction of the α configurated HO-C(13) if necessary.
Scheme 10.
Reagents and conditions: (a) TsOH, toluene, reflux; (b) 1,2-ethanedithiol, BF3·Et2O, 0 °C, 85%; and (c) Raney-Ni, EtOH, 60 °C, 76%.
7.4. Regio- and Diastereoselective Synthesis of (+)-2-Deoxyoryzalexin S 2 from (+)-Podocarpic Acid
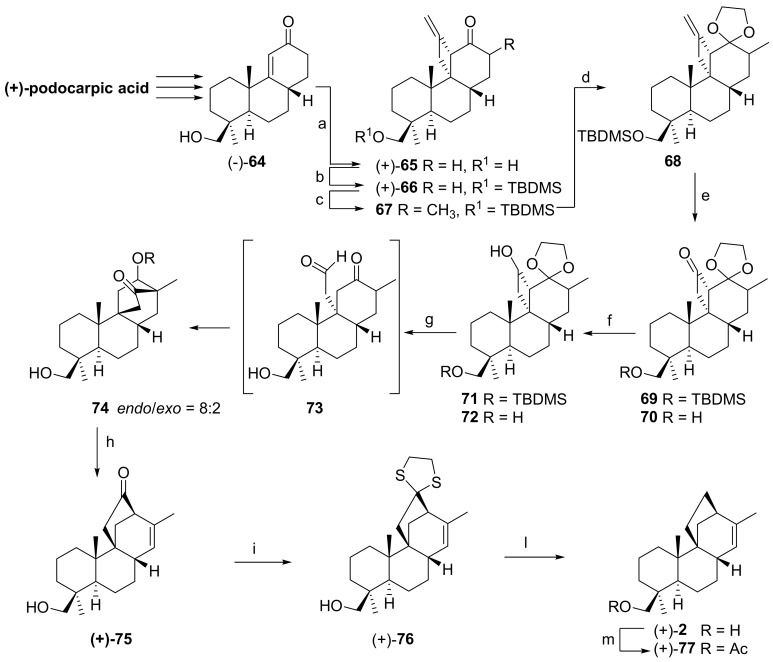
Finally, having in hand an efficient methodology for the construction of the C/D ring system of stemarane diterpenoids, we decided to apply it to the synthesis of (+)-2-deoxyoryzalexin S 2 the structure and absolute configuration of which had been established only on the basis of the 1H and 13C spectra [2,3,5]. The strategy adopted is described in Scheme 11. The starting material was (−)-19-hydroxypodocarp-9-en-12-one 64, available in four steps from (+)-podocarpic acid. Photoaddition of allene to (−)-64 in THF at 78 °C gave quantitatively the photoadduct (+)-65 the structure of which was established by 2D NMR experiments. Prior to methylation at C(13), the HO-C(19) in (+)-65 was protected to give (+)-66. Compound (+)-66 was then methylated to give 67. The latter was then converted into the acetal 68, and the exocyclic methylene cleaved with OsO4/NaIO4 to give the cyclobutanone 69 along with some unprotected keto-alcohol 70. Compound 69 was then reduced to the cyclobutanol 71. Treatment of 71 with a 2:1 THF/2N HCl mixture gave 74 as an about 80:20 endo/exo C(12) epimeric mixture. The latter dissolved in toluene was heated at 85 °C in the presence of TsOH, giving (+)-75. Thioacetalization of (+)-75 by standard methods followed by deoxygenation gave then (+)-2-deoxyoryzalexin S 2 which was then acetylated to (+)-77 [12]. Remarkably, in this work all chiral centers present in (+)-podocarpic acid are maintained.
Scheme 11.
Reagents and conditions: (a) allene, THF, −78 °C, hν, 81%; (b) TBDMSCl, imidazole, THF, 89%; (c) NaHDMS, CH3I, THF, −78 °C; (d) ethylene glycol, toluene, TsOH 78%; (e) OsO4/NaIO4, THF, H2O; (f) NaBH4, EtOH/MeOH, 0 °C; (g) THF/2N HCl, 88%; (h) TsOH, benzene, 85 °C, 66%; (i) 1,2-ethanedithiol, BF3·Et2O, 0 °C, 84%; (l) Raney-Ni, EtOH, 60 °C, 62%; and (m) Ac2O/Py, 99%.
The relative configuration of (+)-77 was confirmed by an X-Ray structure determination. This work allowed us to demonstrate that the structure of (+)-2-deoxyoryzalexin S 2 could not be attributed to a Chilean Calceolaria isolated diterpenoid to which this structure had been assigned.
7.5. Other Strategies
An approach to stemodane and stemarane diterpenoids was also described in 1985 by Ghatak and co-workers at the Indian Association for the Cultivation of Science, Jadavpur, Calcutta [87]. This approach (Scheme 12) was based on the formation of a bicyclo[2.2.2]octane intermediate by acid-catalyzed intramolecular C-alkylation followed by a [2.2.2] → [3.2.1] rearrangement (vide supra).
Scheme 12.
Approach by Ghatak and co-workers to stemarane and stemodane diterpenoids.
A short, expedient, though not diastereoselective route, inter alia, to a potentially key intermediate for their synthesis of stemarane diterpenoids was also realized by Subba Rao and Kaliappan at the Indian Institute of Science, Bangalore, India (Scheme 13) [88]. Also this approach, to our best knowledge, was not implemented with a total synthesis.
Scheme 13.
Subba Rao’s and Kaliappan’s approach to stemarane and aphidicolane diterpenoids: reagents and conditions: (a) NaBH4, EtOH; and (b) BF3.Et2O or MsCl/Et3N.
7.6. Enantioselective Synthesis of Stemarane Diterpenes and Diterpenoids via 9(11)-Podocarpen-12-ones
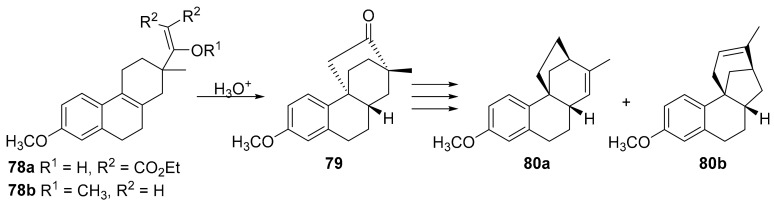
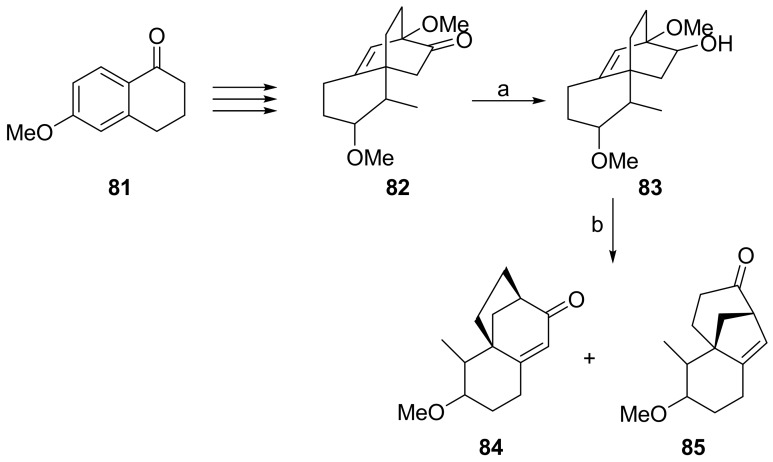
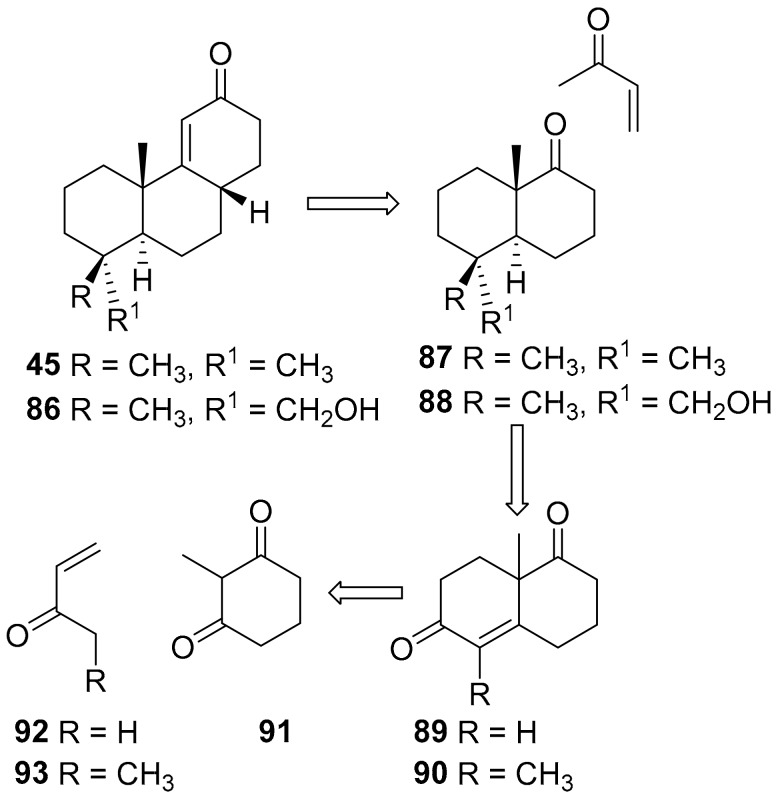
9(11)-podocarpen-12-ones such as 45 can be obtained, inter alia, by Robinson annulation of a substituted decalone with methyl vinyl ketone (Scheme 14). At the end of the annulation process, the vinilogous H-C(8) results installed in the more stable β configuration [70,72,89].
Scheme 14.
Retrosynthetic analysis for the obtaining of substituted 9(11)podocarpen-12-ones.
In turn the decalone intermediate such as 87 can be obtained from the Wieland–Miescher ketone. Since Wieland–Miescher ketone 89 and its C(4) homologue 90 can be obtained enantioselectively [90,91,92,93,94], this approach is quite convenient for the preparation of optically active 9(11)podocarpen-12-ones and hence of the target compounds.
Thus decalone 87 [95] was used to prepare (+)-9(11)podocarpen-12-one 45 and hence (+)-13-stemarene 12 and (+)-2-deoxystemarin 2. Decalone 88 [95] could be adopted in principle for the obtaining of (+)-stemarin 1.
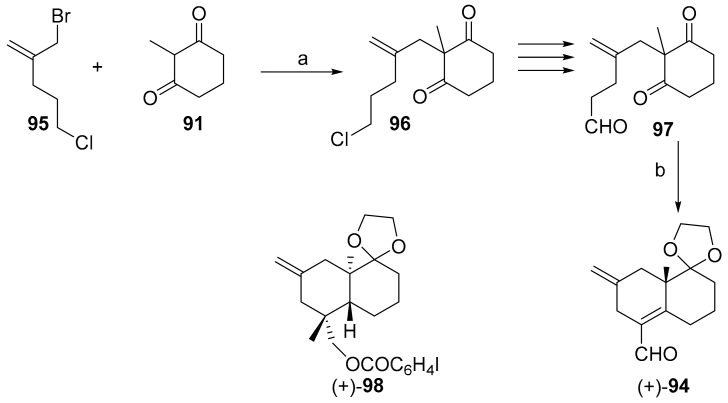
Recently, the synthesis of the bicyclic intermediate 94, necessary for the enantioselective obtaining of (+)-oryzalexin S 11 was described by our group (Scheme 15) [96].
Scheme 15.
Enantioselective obtaining of (+)-94. Reagents and conditions: (a) (1) NaH/DMF, 1 h, Ar; (2) then 95, 2 h; and (b) DMSO/1 M HClO4, d-tyrosine, 10 °C, 20 min.
To this end, it was necessary to elaborate the ad hoc side chain 95 to be used in the annulation process leading to ring A. The exocyclic double bond at C(2) would have easied the alkylation of 2-methyl-1,3-cyclohexanedione 91 and allowed its conversion at proper time into the α configurated HO-C(2).
The halide at the end of the side chain would have allowed the introduction of the formyl group necessary for the cyclization. Later the latter group could be converted in the HO-C(18).
Carrying out the aldol reaction in the presence of d-tyrosine at 10 °C the enantioselective cyclization of 97 to 94 was accomplished in very good yield and e.e. The obtaining of 94 followed an X-ray structure determination on 98. (Initially the work was carried out with the cheapest l-amino acid series.)
8. Conclusions
In this paper, the work by various research groups on stemarane diterpenes and diterpenoids, described in over forthy papers covering isolation, structure elucidation, biogenesis, biosynthesis, biotranformations, synthesis, and enantiosynthesis has been reviewed. From this review, it appears that further work is necessary to establish unambiguously the structure and absolute configuration of stemarane diterpenes and diterpenoids from the Chilean flora. In fact, while the structure and absolute configuration of (+)-stemarin 1 was established by X-ray diffraction, the structure and absolute configuration of stemarane diterpenes and diterpenoids from the Chilean flora was not confirmed by chemical correlation nor by a X-ray structure determination. The biogenesis proposed for this class of compounds was confirmed by several biosynthetic studies. Biotransformations under the activity of a number of fungi leading to interesting metabolites were also carried out. Besides, it appears that the biological activity of pure isolated stemarane diterpenes and diterpenoids, but (+)-oryzalexin S 11, has not been evaluated yet. The biological activity ascertained so far was attributed to Stemodia maritima L. metabolites, which differ from stemarane diterpenes and diterpenoids. Finally, a comparison about the content of stemarane diterpenes and diterpenoids among Stemodia maritima L. plants, collected in different geographical areas, seems also an interesting task. The extensive work towards the synthesis of this class of compounds resulted in a very efficient approach while recent studies allowed the enantiosynthesis of a key intermediate for the synthesis of (+)-oryzalexin S 11, and should pave the way to this interesting and bioactive compound. We hope this review will be useful to those who are interested in this field.
Funding
Financial support over the years to our work described in this review was received from the Italian National Research Council (CNR), the Public Education Ministry and the University of Rome “La Sapienza”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Manchand P.S., Blount J.F. X-ray Structure and Absolute Stereochemistry of Stemarin, a Diterpene with a New Skeleton. J. Chem. Soc. Chem. Commun. 1975:894–895. doi: 10.1039/c39750000894. [DOI] [Google Scholar]
- 2.Chamy M.C., Piovano M., Garbarino J.A., Miranda C., Gambaro V. Diterpenes from Calceolaria lepida. Phytochemistry. 1990;29:2943–2946. doi: 10.1016/0031-9422(90)87111-7. [DOI] [Google Scholar]
- 3.Garbarino J.A., Molinari A. Diterpenes from Calceolaria latifolia. Phytochemistry. 1990;29:3037–3039. [Google Scholar]
- 4.Garbarino J.A., Molinari A. Diterpenes from Calceolaria kingii. Phytochemistry. 1990;29:3040–3041. doi: 10.1016/0031-9422(90)87135-H. [DOI] [Google Scholar]
- 5.Chamy M.C., Piovano M., Garbarino J.A., Miranda C., Gambaro V. Diterpenes from Calceolaria polifolia. Phytochemistry. 1991;30:3365–3368. doi: 10.1016/0031-9422(91)83211-3. [DOI] [Google Scholar]
- 6.Chamy M.C., Piovano M., Garbarino J.A., Vargas C. Diterpenoids from Calceolaria dentata. Phytochemistry. 1995;40:1751–1754. doi: 10.1016/0031-9422(95)00476-N. [DOI] [Google Scholar]
- 7.Chamy M.C., Piovano M., Garbarino J.A., Mendoza J. Diterpenoids from Calceolaria glabrata. Bol. Soc. Chil. Quim. 2001;46:223–225. doi: 10.4067/S0366-16442001000200018. [DOI] [Google Scholar]
- 8.Chamy M.C., Piovano M., Garbarino J.A., Espinoza L. Diterpenoids from Calceolaria paralia. J. Chil. Chem. Soc. 2006;51:779–780. doi: 10.4067/S0717-97072006000100005. [DOI] [Google Scholar]
- 9.Kodama O., Li W.X., Tamogami S., Akatsuka T. Oryzalexin S a novel stemarane-type diterpene rice phytoalexin. Biosci. Biotech. Biochem. 1992;56:1002–1003. doi: 10.1271/bbb.56.1002. [DOI] [PubMed] [Google Scholar]
- 10.Dillon V.M., Overton J., Grayer R.J., Harborne J.B. Differences in Phytoalexin Response Among Rice Cultivars of Different Resistance to Blast. Phytochemistry. 1997;44:599–603. doi: 10.1016/S0031-9422(96)00619-X. [DOI] [Google Scholar]
- 11.Oikawa H., Ohashi S., König W.A., Kenmoku H., Sassa T. Diversity of diterpene hydrocarbons in fungus Phoma betae. Tetrahedron Lett. 2001;42:2329–2332. doi: 10.1016/S0040-4039(01)00165-4. [DOI] [Google Scholar]
- 12.Leonelli F., Latini V., Trombetta A., Bartoli G., Ceccacci F., La Bella A., Sferrazza A., Lamba D., Migneco L.M., Marini Bettolo R. Regio- and Diastereoselective Synthesis and X-ray Structure Determination of (+)-2-Deoxyoryzalexin S from (+)-Podocarpic Acid. Structural Non-identity with Its Nominal Natural Isolated Enantiomer. J. Nat. Prod. 2012;75:1944–1950. doi: 10.1021/np300518j. [DOI] [PubMed] [Google Scholar]
- 13.Tamogami S., Mitani M., Kodama O., Akatsuka T. Oryzalexin structure: A new stemarane-type diterpene rice phytoalexin and its biogenesis. Tetrahedron. 1993;49:2025–2032. doi: 10.1016/S0040-4020(01)86302-X. [DOI] [Google Scholar]
- 14.Berettoni M., Marini Bettolo R., Montanari V., Prencipe T., Romeo S. Studies for a Diastereoselective Synthesis of the Tetracyclic Diol Stemarin: A Model Study for a New Preparation of the Key Intermediate and the Synthesis of (+)-18-Deoxystemarin. Helv. Chim. Acta. 1991;74:1671–1678. doi: 10.1002/hlca.19910740807. [DOI] [Google Scholar]
- 15.Mohan R.S., Yee N.K.N., Coates R.M., Ren Y.-Y., Stamenkovic P., Mendez I., West C.A. Biosynthesis of Cyclic Diterpene Hydrocarbons in Rice Cell Suspensions: Conversion of 9,10-syn-Labda-8(17),13-dienyl Diphosphate to 9-Pimara-7,15-diene and Stemar-13-ene. Arch. Biochem. Biophys. 1996;330:33–47. doi: 10.1006/abbi.1996.0223. [DOI] [PubMed] [Google Scholar]
- 16.Nemoto T., Cho E.-M., Okada A., Okada K., Otomo K., Kanno Y., Toyomasu T., Mitsuhashi W., Sassa T., Minami E., Shibuya N., Nishiyama M., Nojiri H., Yamane H. Stemar-13-ene synthase, a diterpene cyclase involved in the biosynthesis of the phytoalexin oryzalexin S in rice. FEBS Lett. 2004;571:182–186. doi: 10.1016/j.febslet.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Nemoto T., Okada A., Okada K., Shibuya N., Toyomasu T., Nojiri H., Yamane H. Promoter analysis of the rice stemar-13-ene synthase gene OsDTC2, which is involved in the biosynthesis of the phytoalexin S. Biochim. Biophys. Acta Gene Struct. Expr. 2007;1769:678–683. doi: 10.1016/j.bbaexp.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Xu M., Wilderman P.R., Morrone D., Xu J., Roy A., Margis-Pinheiro M., Upadhyaya M., Coates R.M., Peters R.J. Functional characterization of the rice kaurene synthase-like gene family. Phytochemistry. 2007;68:312–326. doi: 10.1016/j.phytochem.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Akatsuka T., Kodama O., Sekido H., Kono Y., Takeuchi S. Novel phytoalexins (oryzalexins A, B and C) isolated from rice blast leaves infected with Pyricularia oryzae. Agric. Biol. Chem. 1985;49:1689–1694. doi: 10.1271/bbb1961.49.1689. Part I: Isolation, characterization and biological activities of oryzalexins. [DOI] [Google Scholar]
- 20.Koga J., Shimura M., Oshima K., Ogawa N., Yamauchi T., Ogasawara N. Phytocassanes A, B, C and D, novel diterpene phytoalexins from rice, Oryza sativa L. Tetrahedron. 1995;51:7907–7918. doi: 10.1016/0040-4020(95)00423-6. [DOI] [Google Scholar]
- 21.Koga J., Ogawa N., Yamauchi T., Kikuchi N., Ogasawara N., Shimura M. Functional moiety for the antifungal activity of phytocassane E, a diterpene phytoalexin from rice. Phytochemistry. 1997;44:249–253. doi: 10.1016/S0031-9422(96)00534-1. [DOI] [Google Scholar]
- 22.Cartwright D.W., Langcake P., Pryce R.J., Leworthy D.P., Ride J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry. 1981;20:535–537. doi: 10.1016/S0031-9422(00)84189-8. [DOI] [Google Scholar]
- 23.Kato H., Kodama O., Akatsuka T. Oryzalexin E, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry. 1993;33:79–81. [Google Scholar]
- 24.Kato H., Kodama O., Akatsuka T. Oryzalexin F, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry. 1994;36:299–301. [Google Scholar]
- 25.Zi J., Mafu S., Peters R.J. To gibberellins and beyond! Surveying the evolution of (di) terpenoid metabolism. Annu. Rev. Plant Biol. 2014;65:259–286. doi: 10.1146/annurev-arplant-050213-035705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yajima A., Mori K. Diterpenoid total synthesis, XXXII Synthesis and absolute configuration of (−)-phytocassane D, a diterpene phytoalexin isolated from the rice plant, Oryza sativa. Eur. J. Org. Chem. 2000;2000:4079–4091. [Google Scholar]
- 27.Yajima A., Mori K., Yabuta G. Total synthesis of ent-cassa-12,15-diene, a putative precursor of rice phytoalexins, phytocassanes A–E. Tetrahedron Lett. 2004;45:167–169. [Google Scholar]
- 28.Kato T., Kabuto C., Sasaki N., Tsunagawa M., Aizawa H., Fujita K., Kato Y., Kitahara Y., Takahashi N. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973;14:3861–3864. [Google Scholar]
- 29.Davis E.M., Croteau R. Biosynthesis. Springer; Berlin/Heidelberg, Germany: 2000. Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes; pp. 53–95. [Google Scholar]
- 30.Cho E.M., Okada A., Kenmoku H., Otomo K., Toyomasu T., Mitsuhashi W., Sassa T., Yajima A., Yabuta G., Mori K., et al. Molecular cloning and characterization of a cDNA encoding ent-cassa-12, 15-diene synthase, a putative diterpenoid phytoalexin biosynthetic enzyme, from suspension-cultured rice cells treated with a chitin elicitor. Plant J. 2004;37:1–8. doi: 10.1046/j.1365-313X.2003.01926.x. [DOI] [PubMed] [Google Scholar]
- 31.Oikawa H., Nakamura K., Toshima H., Toyomasu T., Sassa T. Proposed Mechanism for the Reaction Catalyzed by a Diterpene Cyclase, Aphidicolan-16â-ol Synthase: Experimental Results on Biomimetic Cyclization and Examination of the Cyclization Pathway by ab Initio Calculations. J. Am. Chem. Soc. 2002;124:9145–9153. doi: 10.1021/ja025830m. [DOI] [PubMed] [Google Scholar]
- 32.Hong Y.J., Tantillo D.J. A Maze of Dyotropic Rearrangements and Triple Shifts: Carbocation Rearrangements Connecting Stemarene, Stemodene, Betaerdene, Aphidicolene, and Scopadulanol. J. Org. Chem. 2018;83:3780–3793. doi: 10.1021/acs.joc.8b00138. [DOI] [PubMed] [Google Scholar]
- 33.Buchanan G.O., Reese P.B. Biotransformation of diterpenes and diterpene derivatives by Beauveria bassiana ATCC 7159. Phytochemistry. 2001;56:141–151. doi: 10.1016/S0031-9422(00)00403-9. [DOI] [PubMed] [Google Scholar]
- 34.Chen A.R.M., Reese P.B. Biotransformation of terpenes from Stemodia maritima by Aspergillus niger ATCC 9142. Phytochemistry. 2002;59:57–62. doi: 10.1016/S0031-9422(01)00355-7. [DOI] [PubMed] [Google Scholar]
- 35.Chen A.R.M., Ruddock P.L.D., Lamm A.S., Reynolds W.F., Reese P.B. Stemodane and stemarane diterpenoid hydroxylation by Mucor plumbeus and Whetzelinia sclerotiorum. Phytochemistry. 2005;66:1898–1902. doi: 10.1016/j.phytochem.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 36.De Oliveira Silva E., Furtado N.A.J.C., Aleu J., Gonzáles Collado I. Terpenoid biotransformations by Mucor species. Phytochem. Rev. 2013;12:857–876. doi: 10.1007/s11101-013-9313-5. [DOI] [Google Scholar]
- 37.Lamm A.S., Reynolds W.F., Reese P.B. Bioconversion of Stemodia maritima diterpenes and derivatives by Cunninghamella echinulata varelegans and Phanerochaete chrysosporium. Phytochemistry. 2006;66:1088–1093. doi: 10.1016/j.phytochem.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 38.McCook K.P., Chen A.R.M., Reynolds W.F., Reese P.B. The potential of Cyathus africanus for tranformation of terpenes substrates. Phytochemistry. 2012;82:61–66. doi: 10.1016/j.phytochem.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Manchand P.S., White J.D., Wright H., Clardy J. Structures of Stemodin and Stemodinone. J. Am. Chem. Soc. 1973;95:2705–2706. doi: 10.1021/ja00789a060. [DOI] [Google Scholar]
- 40.Hufford C.D., Guerrero R.O., Doorembos N.J. Two new diterpenes from Stemodia maritima L. J. Pharm. Sci. 1976;65:778–780. doi: 10.1002/jps.2600650550. [DOI] [PubMed] [Google Scholar]
- 41.Austin D.F. Florida Ethnobotany. CRC Press; Boca Raton, FL, USA: 2004. 162p [Google Scholar]
- 42.Garbarino J.A., Chamy M.C., Piovano M. Chemistry of the Calceolaria Genus. Structural and Biological Aspects. Molecules. 2000;5:302–303. doi: 10.3390/50300302. [DOI] [Google Scholar]
- 43.Arriaga A.M.C., Rodrigues F.E.A., Lemos T.L.G., de Oliveira M. da C.F., Lima J.Q., Santiago G.M.P., Braz-Filho R., Mafezoli J. Composition and larvicidal activity of essential oil from Stemodia maritima L. Nat. Prod. Commun. 2007;2:1237–1239. doi: 10.1177/1934578X0700201209. [DOI] [Google Scholar]
- 44.Da Silva F.R.L., Rodrigues F.E.A., Gomes A.R.S., Arriaga A.M.C., Mafezoli J., Lemos T.L.G., de Almeida M.C.S., Santiago G.M.P., Braz-Filho R., da Costa J.G.M., et al. Phytochemical study, antioxidant and antibacterial activities of Stemodia maritima. Quím. Nova. 2014;37:1474–1478. [Google Scholar]
- 45.Teixeira A.H., de Oliveira Freire J.M., de Sousa L.H.T., Parente A.T., de Sousa N.A., Arriaga A.M.C., Lopes da Silva F.R., Melo I.M., Castro da Silva I.I., Pereira K.M.A., et al. Stemodia maritima L. Extract Decreases Inflammation, Oxidative Stress, and Alveolar Bone Loss in an Experimental Periodontitis Rat Model. Front. Physiol. 2017;8:988. doi: 10.3389/fphys.2017.00988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider W.G., Valenta Z. Karel František Wiesner. 25 November 1919–28 November 1986. Biogr. Mem. Fellows Royal Soc. 1991 Nov 25;37:462–490. [Google Scholar]
- 47.Wiesner K., Tsai T.Y.R., Huber K., Bolton S.E., Vlahov R. Total synthesis of talatisamine, a delphinine type alkaloid. J. Am. Chem. Soc. 1974;96:4990–4992. doi: 10.1021/ja00822a048. [DOI] [Google Scholar]
- 48.Tsai T.Y.R., Tsai C.S.J., Sy W.W., Shanbag M.N., Liu W.C., Lee S.F., Wiesner K. A Stereospecific Total Synthesis of Chasmanine. Heterocycles. 1977;7:217–226. [Google Scholar]
- 49.Wiesner K. Centenary Lecture. Systematic Development of Strategy in the Synthesis of Polycyclic Polysubstituted Natural Products: The Aconite Alkaloids. Chem. Soc. Rev. 1977;6:413–430. [Google Scholar]
- 50.Wiesner K. Total Synthesis of Delphinine-Type Alkaloids by Simple, Fourth Generation Methods. Pure Appl. Chem. 1979;51:689–703. [Google Scholar]
- 51.Atwal K.S., Marini Bettolo R., Sanchez I.H., Tsai T.Y.R., Wiesner K. On the construction of the C/D ring systems of chasmanine and napelline by diene addition. Can. J. Chem. 1978;56:1102–1113. [Google Scholar]
- 52.Tsai T.Y.R., Nambiar K.P., Krikorian D., Botta M., Marini Bettolo R., Wiesner K. A new synthesis of chasmanine and 13-desoxydelphonine: A preferred route to the aromatic intermediate. Can. J. Chem. 1979;57:2124–2134. doi: 10.1139/v79-341. [DOI] [Google Scholar]
- 53.Sethi S.P., Atwal K.S., Marini Bettolo R., Tsai T.Y.R., Wiesner K. A stereospecific synthesis of napelline. Can. J. Chem. 1980;58:1889–1891. doi: 10.1139/v80-298. [DOI] [Google Scholar]
- 54.Ciamician G., Silber P. Chemische Lichtwirkungen. Ber. Dtsch. Chem. Ges. 1908;41:1928–1935. doi: 10.1002/cber.19080410272. [DOI] [Google Scholar]
- 55.Buchi G., Goldman I.M. Photochemical Reactions. VII.1 the Intramolecular Cyclization of Carvone to Carvonecamphor. J. Am. Chem. Soc. 1957;79:4741–4748. doi: 10.1021/ja01574a042. [DOI] [Google Scholar]
- 56.Corey E.J., Bass J., Dolf R., Mitra R.B. A Study of the Photochemical Reactions of 2-Cyclohexenones with Substituted Olefins. J. Am. Chem. Soc. 1964;86:5570–5583. doi: 10.1021/ja01078a034. [DOI] [Google Scholar]
- 57.Wiesner K. On the stereochemistry of photoaddition between α,β-unsaturated ketones and olefins. Tetrahedron. 1975;31:1658. doi: 10.1016/0040-4020(75)85082-4. [DOI] [Google Scholar]
- 58.Marini Bettolo G., Sahoo S.P., Poulton G.A., Tsai T.Y.R., Wiesner K. On the stereochemistry of photoaddition between α,β-unsaturated ketones and olefins—II. Tetrahedron. 1980;36:719–721. doi: 10.1016/S0040-4020(01)93683-X. [DOI] [Google Scholar]
- 59.Blount J.F., Gray G.D., Atwal K.S., Tsai T.Y.R., Wiesner K. On the stereochemistry of photoaddition between α,β-unsaturated ketones and olefins. III. Tetrahedron Lett. 1980;21:4413–4416. doi: 10.1016/S0040-4039(00)92187-7. [DOI] [Google Scholar]
- 60.Valenta K., Grein F. Excited states of acrolein: Ab initio model studies on α,β-unsaturated carbonyl compounds. Can. J. Chem. 1982;60:601–606. doi: 10.1139/v82-089. [DOI] [Google Scholar]
- 61.Bell R.A., Ireland R.E. The construction of the C/D ring system present in the diterpenoid alkaloids atisine and garryfoline. Tetrahedron Lett. 1963;4:269–273. doi: 10.1016/S0040-4039(01)90620-3. [DOI] [Google Scholar]
- 62.Migneco L.M., Leonelli F., Marini Bettolo R. The intramolecular aldol condensation of 3-oxocyclo-hexaneacetaldehydes: A useful tool in the synthesis of natural products. Arkivoc. 2004;7:253–265. [Google Scholar]
- 63.Walborsky H.M., Baum M.E., Youssef A.A. Acetolysis of Bicyclo[2.2.2]octyl-2 p-Bromobenzenesulfonate and the Absolute Configurations of Bicyclo[2.2.2]octanol-2 and cis- and trans-Bicyclo[3.2.1]octanol-2. J. Am. Chem. Soc. 1961;83:988–993. doi: 10.1021/ja01465a055. [DOI] [Google Scholar]
- 64.Goering H.L., Sloan M.F. Ionic Reactions in Bicyclic Systems. II. Carbonium Ion Reactions in Bicyclo[2.2.2]octane and Bicyclo[3.2.1]octane Derivatives. J. Am. Chem. Soc. 1961;83:1397–1401. doi: 10.1021/ja01467a033. [DOI] [Google Scholar]
- 65.Goering H.L., Sloan M.F. Ionic Reactions in Bicyclic Systems. III. Solvolysis of Bicycloöctanyl and Bicycloöctenyl p-Toluenesulfonates. J. Am. Chem. Soc. 1961;83:1992–1999. doi: 10.1021/ja01469a051. [DOI] [Google Scholar]
- 66.Kraus H.L., Chassin C., Chassin R., Schmutte P. Bicyclische Verbindungen, XVIII Solvolytische und reduktive Umlagerung von Bicyclo[2.2.2]octyltosylaten. Liebigs Ann. Chem. 1970;738:97–112. doi: 10.1002/jlac.19707380112. [DOI] [Google Scholar]
- 67.Kelly R.B., Harley M.L., Alward S.J. A total synthesis of (+)-stemarin, a diterpenoid with a unique bicyclic C/D ring system. Can. J. Chem. 1980;58:755–756. doi: 10.1139/v80-117. [DOI] [Google Scholar]
- 68.Spencer T.A., Weaver T.D., Villarica R.M., Friary R.J., Posler J., Schwartz M.A. Syntheses of methyl deisopropyldehydroabietate. Diterpenoid synthesis by the AB → ABC approach. J. Org. Chem. 1968;33:712–719. doi: 10.1021/jo01266a049. [DOI] [Google Scholar]
- 69.Bravetti D., Marini Bettolo R., Lupi A. On the Construction of the C/D-Ring Systems of Aphidicolin and Stemodin. A regio and Stereospecific Synthesis of 17-Noraphidicolan-16-one and 17-Norstemodan-16-one. Helv. Chim. Acta. 1982;65:371–376. doi: 10.1002/hlca.19820650139. [DOI] [Google Scholar]
- 70.Marini Bettolo R., Tagliatesta P., Lupi A., Bravetti D. A Stereoselective Total Synthesis of (±)-Maritimol, (±)-2-Deoxystemodinone, (±)-Stemodinone and (±)-Stemodin. Helv. Chim. Acta. 1983;66:760–770. doi: 10.1002/hlca.19830660307. [DOI] [Google Scholar]
- 71.Lupi A., Patamia M., Grgurina I., Marini Bettolo R., Di Leo O., Gioia P., Antonaroli S. Biogenetic-Type Total Synthesis of (+)-2-Deoxystemodinone. Helv. Chim. Acta. 1984;67:2261–2263. doi: 10.1002/hlca.19840670832. [DOI] [Google Scholar]
- 72.Marini Bettolo R., Tagliatesta P., Lupi A., Bravetti D. A Total Synthesis of Aphidicolin: Stereospecific Synthesis of (±)-3α,18-Dihydroxy-17-noraphidicolan-16-one. Helv. Chim. Acta. 1983;66:1922–1928. doi: 10.1002/hlca.19830660703. [DOI] [Google Scholar]
- 73.Lupi A., Patamia M., Marini Bettolo R. A Total Synthesis of (±)-Aphidicolin: Regio and Stereoselective Conversion of 3α,18-Di-O-benzyl-17-nor-14-aphidicolen-16-one into (±)-Aphidicolin. Helv. Chim. Acta. 1988;71:872–875. doi: 10.1002/hlca.19880710423. [DOI] [Google Scholar]
- 74.De Santis B., Iamiceli A.L., Marini Bettolo R., Migneco L.M., Scarpelli R., Cerichelli G., Fabrizi G., Lamba D. On the Diastereoselectivity of the Aqueous-Acid-Catalyzed Intramolecular Aldol Condensation of 3-Oxocyclohexaneacetaldehydes. Helv. Chim. Acta. 1998;81:2375–2387. doi: 10.1002/(SICI)1522-2675(19981216)81:12<2375::AID-HLCA2375>3.0.CO;2-0. [DOI] [Google Scholar]
- 75.La Bella A., Leonelli F., Migneco L.M., Marini Bettolo R. (+)-Podocarpic Acid as Chiral Template in the Synthesis of Aphidicolane, Stemodane and Stemarane Diterpenoids. Molecules. 2016;21:1197. doi: 10.3390/molecules21091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marini Bettolo R., Migneco L.M., Moretti P., Scarpelli R. Improved Conversion of 6-endo-Tosyloxybicyclo[2.2.2]octan-2-ones into 6-exo-Acetoxy and 6-exo-Benzoyloxy-bicyclo[2.2.2]octan-2-ones. J. Prakt. Chem. 1999;341:687–690. [Google Scholar]
- 77.Di Stefano S., Leonelli F., Garofalo B., Mandolini L., Marini Bettolo R., Migneco L.M. Elusive 6-exo-Hydroxybicyclo[2.2.2]octan-2-ones from the Corresponding Acetates by Methanolysis in the Presence of CH3ONa/La(OTf)3. Org. Lett. 2002;4:2783–2785. doi: 10.1021/ol026326p. [DOI] [PubMed] [Google Scholar]
- 78.Steiweiswer A., Jr., Walsh T.D., Wolfe J.R., Jr. Stereochemistry of Acetolysis of Alkyl Sulfonates. J. Am. Chem. Soc. 1965;87:3682–3685. [Google Scholar]
- 79.Leonelli F., Caschera B., Silvestri L., Prastaro A., Corso G., Ceccacci F., La Bella A., Migneco L.M., Marini Bettolo R. Synthesis of (+)-13-Stemarene and (+)-18-Deoxystemarin: Expeditious Preparation of the Key 6-exo-Hydroxybicyclo[2.2.2]octan-2-one Ethylene Dithioacetal. Helv. Chim. Acta. 2008;91:598–607. doi: 10.1002/hlca.200890063. [DOI] [Google Scholar]
- 80.Antonaroli S., Berettoni M., Cifarelli G., Lupi A., Marini Bettolo R., Romeo S. Studies for a Diastereoselective Synthesis of the Tetracyclic Diol Stemarin. An Improved Procedure to the Synthesis of 8(9)-Podocarpen-14-ones: The Total Synthesis of 18-benzyloxy-8(9)-Podocarpen-14-one and Related Studies. Gazz. Chim. Ital. 1992;122:55–59. doi: 10.1002/chin.199236242. [DOI] [Google Scholar]
- 81.Bartoletti A., Berettoni M., Catteruccia F., De Chiara G., Marini Bettolo R., Mastrangeli C., Scarpelli R., Tozzi C., Lamba D. Studies for a Diastereoselective Synthesis of the Tetracyclic Diterpenic Diol Stemarin. A Model Work for the Preparation, from an 8(9)podocarpen-14-one, of a Key Intermediate by the Wiesner Photochemical Method. Gazz. Chim Ital. 1996;126:223–226. doi: 10.1002/chin.199647185. [DOI] [Google Scholar]
- 82.Trost B.M., Nishimura Y., Yamamoto K., McElvain S.S. A Total Synthesis of Aphidicolin. J. Am. Chem. Soc. 1979;101:1328–1330. doi: 10.1021/ja00499a071. [DOI] [Google Scholar]
- 83.McMurry J.E., Andrus A., Ksander G.M., Musser J.H., Johnson M.A. Stereospecific Total Synthesis of Aphidicolin. J. Am. Chem. Soc. 1979;101:1330–1332. doi: 10.1021/ja00499a072. [DOI] [Google Scholar]
- 84.Berettoni M., Cocchi F., Marini Bettolo R., Montagnini di Mirabello L., Romeo S. Unprecedented Outcome of the Allene Photoaddition to a Fused α,β-Unsaturated Ketone. Tetrahedron Lett. 1993;34:715–716. doi: 10.1016/S0040-4039(00)61661-1. [DOI] [Google Scholar]
- 85.Srikrishna A., Satyanarayana G., Ravi Kumar P. Enantiospecific synthesis of tricyclo[5.2.1.04,8]decanes via acid catalysed rearrangement of isotwistanes. Tetrahedron Lett. 2006;47:363–366. doi: 10.1016/j.tetlet.2005.11.008. [DOI] [Google Scholar]
- 86.Leonelli F., Blesi F., Dirito P., Trombetta A., Ceccacci F., La Bella A., Migneco L.M., Marini Bettolo R. Diastereoselective Total Synthesis of (+)-13-Stemarene by Fourth Generation Methods: A Formal Total Synthesis of (+)-18-Deoxystemarin. J. Org. Chem. 2011;76:6871–6876. doi: 10.1021/jo200945s. [DOI] [PubMed] [Google Scholar]
- 87.Kanjilal P.R., Sarkar M., Patra S.K., Ghosh S., Ghatak U.R. Synthetic Studies toward Complex Diterpenoids. 16.1 A Novel SyntheticRoute to the Carbocyclic Skeleta of Stemodin and Stemarin byAcid-Catalyzed Intramolecular C-Alkylation and Rearrangement Reactions. J. Org. Chem. 1985;50:857–863. doi: 10.1021/jo00206a027. [DOI] [Google Scholar]
- 88.Kaliappan K., Subba Rao G.S.R. An Expedient Route to the Preparation of Key Intermediates for the Total Synthesis of Aphidicolin, Stemodin and Oryzalexin, S. Tetrahedron Lett. 1996;37:8429–8430. doi: 10.1016/0040-4039(96)01875-8. [DOI] [Google Scholar]
- 89.Leonelli F., Borocci S., Migneco L.M., Marini Bettolo R., Lamba D. The Formation of 8-Epipodocarp-9(11)-en-12-one in the Course of the Preparation of Podocarp-9(11)-en-12-one from O-Methylpodocarpane and Related Studies. Helv. Chim. Acta. 2002;85:2817–2826. doi: 10.1002/1522-2675(200209)85:9<2817::AID-HLCA2817>3.0.CO;2-7. [DOI] [Google Scholar]
- 90.Hajos Z.G., Parrish D.R. German Patent. DE 2102623. 1971 Jul 29
- 91.Hajos Z.G., Parrish D.R. Stereocontrolled synthesis of trans-hydrindan steroidal intermediates. J. Org. Chem. 1973;38:3239–3243. doi: 10.1021/jo00959a002. [DOI] [PubMed] [Google Scholar]
- 92.Hajos Z.G., Parrish D.R. Asymmetric synthesis of bicyclic intermediates of natural product chemistry. J. Org. Chem. 1974;39:1615–1621. doi: 10.1021/jo00925a003. [DOI] [Google Scholar]
- 93.Hagiwara H., Uda H.J. Optically pure (4aS)-(+)- or (4aR)-(-)-1,4a-dimethyl-4,4a,7,8-tetrahydronaphthalene-2,5(3H,6H)-dione and its use in the synthesis of an inhibitor of steroid biosynthesis. J. Org. Chem. 1988;53:2308–2311. doi: 10.1021/jo00245a033. [DOI] [Google Scholar]
- 94.Leonelli F., Garofalo B., Migneco L.M., Marini Bettolo R., Colais F., Sinibaldi M. Chiral HPLC Resolution of the Wieland-Miescher Ketone and Derivatives. J. Liq. Chromatogr. Relat. Technol. 2003;3:409–424. doi: 10.1081/JLC-120017179. [DOI] [Google Scholar]
- 95.Smith A.B., III, Mewshaw R. An Efficient Approach to Chiral, Nonracemic trans-Decahydro-5,8a-dimethyl-1,6-naphth alenedione Derivatives: Total Synthesis of (+)-Pallescensin, A. J. Org. Chem. 1984;49:3685–3689. doi: 10.1021/jo00194a003. [DOI] [Google Scholar]
- 96.Leonelli F., Trombetta A., La Bella A., Lucarelli G., Demitri N., Lamba D., Migneco L.M., Marini Bettolo R. Enantioselective Synthesis and X-ray Structure of (+)((4aS,5S,8aS)-5,8a-Dimethyl-7-methyleneoctahydro-2Hspiro[naphthalene-1,2′-[1,3]dioxolan]-5-yl)methyl-4-iodobenzoate. Eur. J. Org. Chem. 2019;2019:1594–1599. doi: 10.1002/ejoc.201801771. [DOI] [Google Scholar]