Abstract
To discover new antiproliferative agents with high efficacy and selectivity, a new series of 1-aryl-3-{4-[(pyridin-2-ylmethyl)thio]phenyl}urea derivatives (7a–7t) were designed, synthesized and evaluated for their antiproliferative activity against A549, HCT-116 and PC-3 cancer cell lines in vitro. Most of the target compounds demonstrated significant antiproliferative effects on all the selective cancer cell lines. Among them, the target compound, 1-[4-chloro-3-(trifluoromethyl)phenyl]-3-{4-{{[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methyl}thio}phenyl}urea (7i) was identified to be the most active one against three cell lines, which was more potent than the positive control with an IC50 value of 1.53 ± 0.46, 1.11 ± 0.34 and 1.98 ± 1.27 μM, respectively. Further cellular mechanism studies confirmed that compound 7i could induce the apoptosis of A549 cells in a concentration-dependent manner and elucidated compound 7i arrests cell cycle at G1 phase by flow cytometry analysis. Herein, the studies suggested that the 1-aryl-3-{4-[(pyridin-2-ylmethyl)thio]phenyl}urea skeleton might be regarded as new chemotypes for designing effective antiproliferative agents.
Keywords: antiproliferative agent, urea, synthesis, antiproliferative activity, apoptosis
1. Introduction
Cancer is a major public health problem in developed countries and will become the most serious life-threatening disease worldwide in the near future [1]. Some advances in cancer treatment by molecule-targeted drugs, such as imatinib, gefitinib, and trastuzumab, were expected to improve cancer cure rates and also to reduce severe adverse reactions because of the high specificity of the targeted molecules, which are expressed and have critical roles in cancer cells, but not in normal cells. However, the clinical effect was found to be limited and did not last for a long time period because of the acquired resistance of the tumor cells. Furthermore, these molecules often cause on-target and/or off-target severe toxicity [2]. Therefore, the development of more target-specific therapy, with minimum toxicity, is warranted to extend disease-free survival and improve the quality of life of cancer patients.
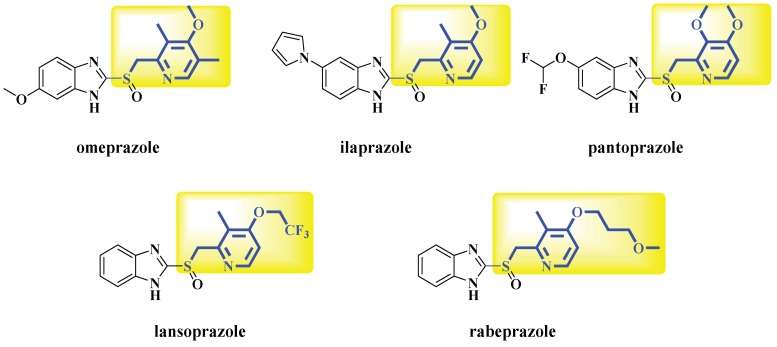
In recent years, proton pump inhibitors (PPIs) as potential anticancer agents were intensively studied in cancer treatment. Lugini et al. compared the anti-tumor efficacy of different PPIs, including omeprazole, esomeprazole, lansoprazole, rabeprazole and pantoprazole in vitro and in vivo. The result indicated that all the PPIs have shown different degrees of antitumor efficacy and lansoprazole showed a higher anti-tumor effect when compared to the other PPIs. [3]. Recently, the research by Zeng and Zheng et al. indicated that T-cell originated protein kinase (TOPK) activities were inhibited by pantoprazole and ilaprazole with high affinity and selectivity [4,5]. TOPK (also known as PBK or PDZ-binding kinase) was first reported by Abe et al. in 2000 [6], and it is a Ser/Thr protein kinase overexpressed in hematologic tumors, breast cancer, melanoma, colorectal cancer, prostate cancer, cervical cancer, bladder cancer and lung cancer [7,8,9,10,11,12,13,14]. The results of their studies demonstrated that pantoprazole can suppress the growth of colorectal cancer cells as a TOPK inhibitor both in vitro and in vivo, and also showed that the TOPK activities were inhibited by ilaprazole in HCT-116, ES-2, A549, SW1990 cancer cells in vitro [4,5]. As shown in Figure 1, all of the PPIs molecules contain thiomethylpyridine fragments. It can be predicted that these fragments should play an important role in the antiproliferative activity of proton pump inhibitors.
Figure 1.
Chemical structures of proton pump inhibitors (PPIs).
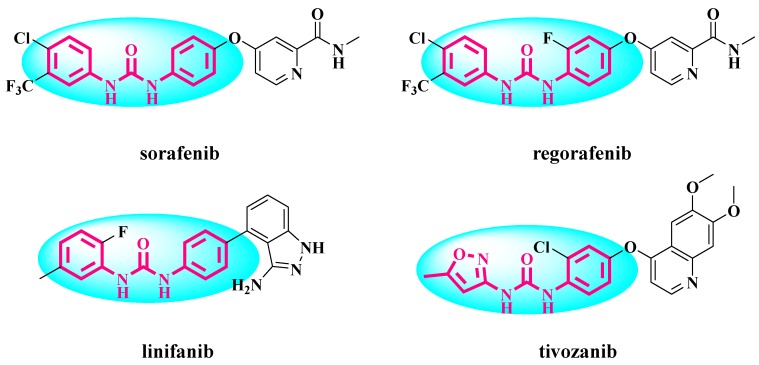
As known, the diaryl urea is a fragment of great importance in medicinal chemistry and can be used for the synthesis of numerous heterocyclic compounds with diversified biological activities, including antithrombotic [15], antimalarial [16], antibacterial [17,18] and anti-inflammatory [19] properties, and it is characterized by its ability to form hydrogen bond interactions with drug targets [20,21,22]. The carbonyl oxygen atom acts as a proton acceptor while the two amide nitrogen atoms are proton donors (Figure 2). This unique type of structure endows urea derivatives with the ability to bind a variety of enzymes and receptors in the biological systems. Remarkably, the diaryl urea moiety is widely used in the design of anticancer drugs, such as sorafenib, regorafenib, linifanib and tivozanib (Figure 3).
Figure 2.
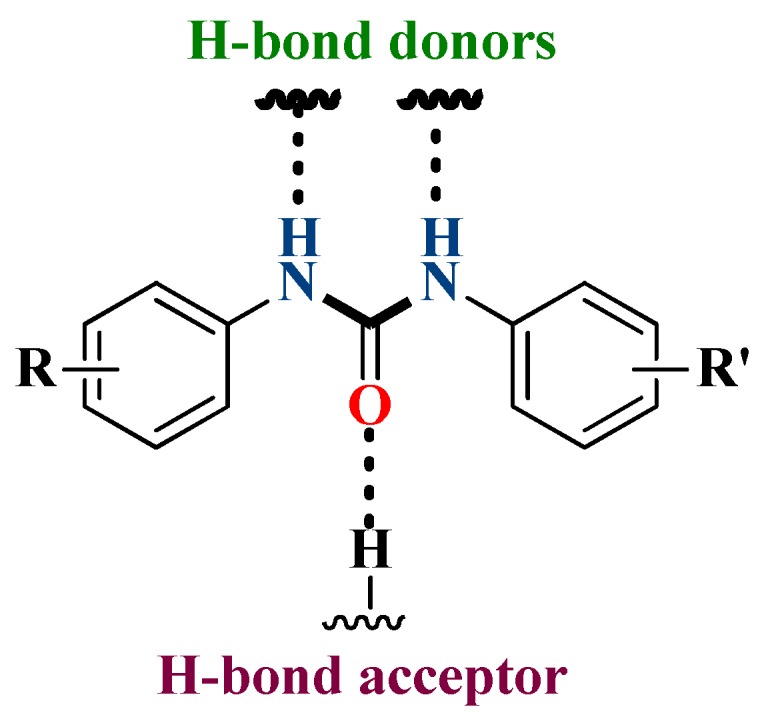
H-bond acceptor and donors within the diaryl urea scaffold.
Figure 3.
Some anticancer drugs of the diaryl urea moiety.
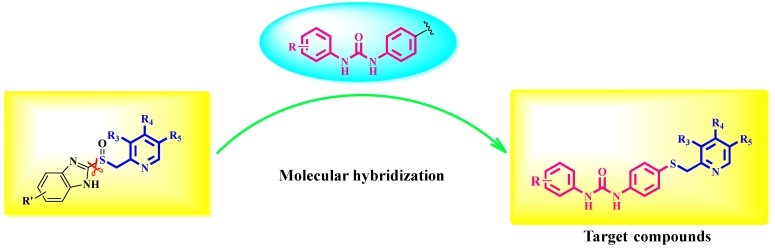
Molecular hybridization strategy is a useful concept in drug design and development based on the combination of pharmacophoric moieties of different bioactive substances to produce a new structure, the affinity and efficacy would be improved, when compared to the parent drugs [23]. These above interesting findings and our continuous quest to identify more potent antiproliferative agents led to the molecular hybridization of diaryl urea and thiomethylpyridine to integrate them in one molecular platform to generate a new hybrid, as shown in Figure 4, and expected that taking this way could get the antiproliferative agents with highly inhibitory activity.
Figure 4.
Rational design of the target compounds based on molecular hybridization strategy.
2. Results and Discussion
2.1. Chemistry
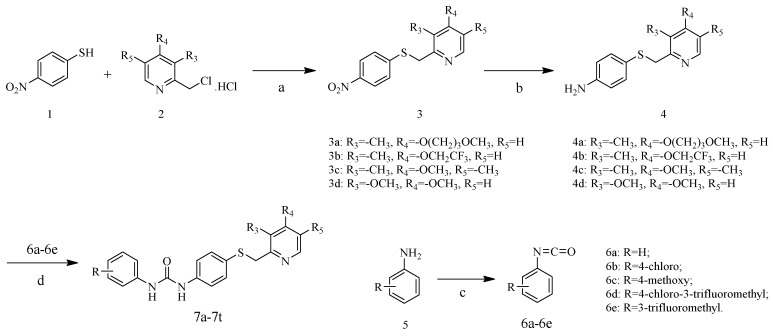
The general synthetic route is illustrated in Scheme 1. The reaction of the commercially available 4-nitrobenzenethiol (1) with 2-(chloromethyl)pyridine derivatives (2) in ethanol at r.t. (room temperature) obtained compounds 3a–3d [24], which converted to key intermediates 4a–4d via Pd-C catalytic hydrogenation reduction [25]. The aryl isocyanates 6a–6e were prepared by reaction between aromatic amines and bis(trichloromethyl)carbonate (BTC) [26]. Finally, treatment of 4a–4d with aryl isocyanates 6a–6e in methylene dichloride yielded 1-aryl-3-{4-[(pyridin-2-ylmethyl)thio]phenyl}urea derivatives (7a–7t) as the target compounds [26]. The structures of the target compounds were characterized by infraredspectra (IR), proton nuclear magnetic resonance spectra (1H-NMR), carbon nuclear magnetic resonance spectra (13C-NMR), electrospray ionization mass spectra (ESI-MS) and high-resolution mass spectra (HRMS).
Scheme 1.
Synthetic route of the target compounds 7a–7t. Reagents and conditions: (a) NaOH (aq. 2M), EtOH, r.t.; (b) H2, 1 atm, 10% Pd-C, MeOH, r.t.; (c) BTC, Et3N, CH2Cl2, r.t.; (d) intermediate 4, aryl isocyanates 6a–6e, CH2Cl2, r.t.
2.2. Biological Evaluation
2.2.1. Antiproliferative Activity
Using sorafenib as a positive control, all of the target compounds were evaluated for the antiproliferative activity in vitro against cancer cell lines, including A549 (lung cancer), HCT-116 (colorectal cancer), and PC-3 (prostate cancer) cell lines by MTT assay. The antiproliferative assay results evaluated as IC50 value (Table 1) and demonstrated that several target compounds have shown moderate to excellent potency against A549, HCT-116, and PC-3 cancer cell lines. Among the target compounds7i showed the more potent inhibitory effect against three cancer cell lines than positive control with IC50 values of 1.53 ± 0.46, 1.11 ± 0.34 and 1.98 ± 1.27 μM, respectively.
Table 1.
The chemical structures and inhibitory activities of the target compounds.
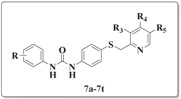
| Compound | R | R3 | R4 | R5 | IC50 (μM) a | ||
|---|---|---|---|---|---|---|---|
| A549 | HCT-116 | PC-3 | |||||
| 7a | H | -CH3 | -O(CH2)3OCH3 | H | 12.31 ± 1.90 | 29.26 ± 4.53 | 27.22 ± 3.36 |
| 7b | 4-chloro | -CH3 | -O(CH2)3OCH3 | H | 3.03 ± 2.79 | 4.80 ± 1.57 | 6.00 ± 0.22 |
| 7c | 4-methoxy | -CH3 | -O(CH2)3OCH3 | H | 6.35 ± 0.51 | 5.29 ± 0.22 | 6.95 ± 1.24 |
| 7d | 4-chloro-3-trifluoromethyl | -CH3 | -O(CH2)3OCH3 | H | 2.02 ± 2.20 | 1.94 ± 3.45 | 3.97 ± 1.02 |
| 7e | 3-trifluoromethyl | -CH3 | -O(CH2)3OCH3 | H | 2.90 ± 0.36 | 3.78 ± 1.41 | 11.48 ± 0.98 |
| 7f | H | -CH3 | -OCH2CF3 | H | 14.29 ± 1.77 | 19.31 ± 3.64 | 20.40 ± 2.89 |
| 7g | 4-chloro | -CH3 | -OCH2CF3 | H | 2.23 ± 1.27 | 3.04 ± 0.37 | 6.78 ± 0.23 |
| 7h | 4-methoxy | -CH3 | -OCH2CF3 | H | 4.71 ± 0.11 | 12.09 ± 1.46 | 15.65 ± 0.69 |
| 7i | 4-chloro-3-trifluoromethyl | -CH3 | -OCH2CF3 | H | 1.53 ± 0.46 | 1.11 ± 0.34 | 1.98 ± 1.27 |
| 7j | 3-trifluoromethyl | -CH3 | -OCH2CF3 | H | 4.22 ± 0.99 | 10.69 ± 0.87 | 14.33 ± 3.24 |
| 7k | H | -CH3 | -OCH3 | -CH3 | 13.37 ± 0.81 | >100 | >100 |
| 7l | 4-chloro | -CH3 | -OCH3 | -CH3 | 2.23 ± 1.38 | 3.20 ± 2.75 | 5.97 ± 0.55 |
| 7m | 4-methoxy | -CH3 | -OCH3 | -CH3 | 5.27 ± 1.01 | 3.49 ± 0.78 | 6.95 ± 0.35 |
| 7n | 4-chloro-3-trifluoromethyl | -CH3 | -OCH3 | -CH3 | 2.63 ± 1.25 | 2.51 ± 0.15 | 6.32 ± 1.68 |
| 7o | 3-trifluoromethyl | -CH3 | -OCH3 | -CH3 | 4.88 ± 1.02 | 3.50 ± 0.13 | 7.18 ± 1.58 |
| 7p | H | -OCH3 | -OCH3 | H | 26.98 ± 4.12 | 45.93 ± 6.65 | 30.95 ± 5.41 |
| 7q | 4-chloro | -OCH3 | -OCH3 | H | 14.52 ± 2.67 | 5.15 ± 1.01 | 18.57 ± 2.34 |
| 7r | 4-methoxy | -OCH3 | -OCH3 | H | 16.47 ± 4.51 | 33.10 ± 2.90 | 22.51 ± 3.07 |
| 7s | 4-chloro-3-trifluoromethyl | -OCH3 | -OCH3 | H | 6.23 ± 0.60 | 5.61 ± 0.66 | 10.05 ± 2.23 |
| 7t | 3-trifluoromethyl | -OCH3 | -OCH3 | H | 14.13 ± 1.82 | 17.37 ± 1.69 | 19.77 ± 0.74 |
| sorafenib | 2.12 ± 0.18 | 2.25 ± 0.71 | 3.60 ± 1.08 | ||||
a Inhibitory activity was assayed by exposure for 72 h to substance and expressed as the concentration required to inhibit tumor cell proliferation by 50% (IC50). Data are presented as the means ± SEMs of three independent experiments.
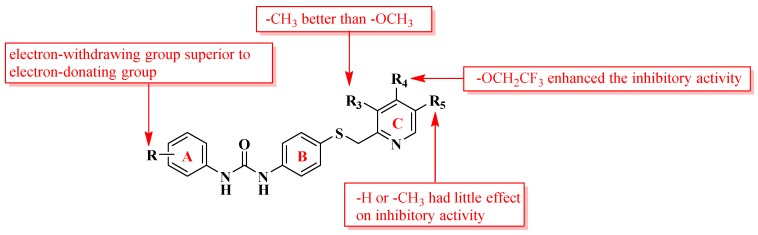
All the target compounds could be divided into four classes according to different substituents on the pyridine ring (Figure 5). The analyses of the structure-activity relationships (SARs) were summarized as follows: (1) The results of cytostatic activity assay showed that the substitutions of the 4 (R4) and 5 (R5) positions of the C ring had a weak effect on the inhibitory activity. However, if the 3-position (R3) hydrogen atom of the C ring was substituted by a methoxy group, the inhibitory activity was significantly decreased. At the same time, it could be seen that the inhibitory activity was better than other classes when the 4-position (R4) of the C ring was occupied by the trifluoroethoxy group. (2) The substituents on the A ring had a significant effect on the inhibitory activity of each class. When the substituents on C ring were the same, if there was no substituent on A ring, the inhibitory activity was worst in each class, such as compound 7a, 7f, 7k and 7p. Moreover, when the substituents in A ring were electron-withdrawing groups, such as 4-Cl or 3-CF3, the inhibitory activity was better than that substitution of the electron-donating groups, such as 4-OCH3 in each class. Furthermore, when the two electron-withdrawing groups coexist on the A ring, the target compounds displayed the strongest inhibitory activity, such as compound 7d, 7i, 7n and 7s.
Figure 5.
SARs summary for the target compounds.
2.2.2. Cell Apoptosis Assay
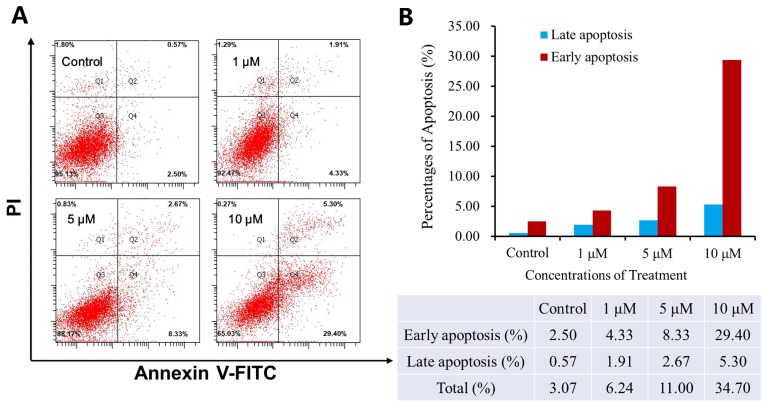
The acceptable antiproliferative activity of compound 7i promoted us to investigate its effect on cell apoptosis. To explore the effect of compound 7i on cell apoptosis, the apoptotic analysis was performed with Annexin V-FITC/PI double staining and analyzed with flow-cytometry calculation. Treatment of A549 cells with compound 7i resulted in a concentration-dependent apoptosis increase, as shown in Figure 6. Specifically, the percentage of early/primary apoptotic cells was about 4.33% for the low concentration (1 μM) of compound 7i. When treated with high concentration (10 μM) of compound 7i, around 29.47% of early/primary apoptosis rate was observed. While the late apoptosis rate of A549 cells was not changed significantly with increasing concentrations.
Figure 6.
Compound 7i induced apoptosis of A549 cells. (A) Apoptosis effect on A549 cell line induced by compound 7i for 24 h using Annexin V-FITC/PI double staining and flow-cytometry calculation. The lower left quadrant represents live cells, the lower right is for early/primary apoptotic cells, upper right is for late/secondary apoptotic cells, and the upper left represents cells damaged during the procedure; (B) Quantitative analysis of apoptotic cells. The experiments were performed three times, and a representative experiment is shown.
2.2.3. Cell Cycle Analysis
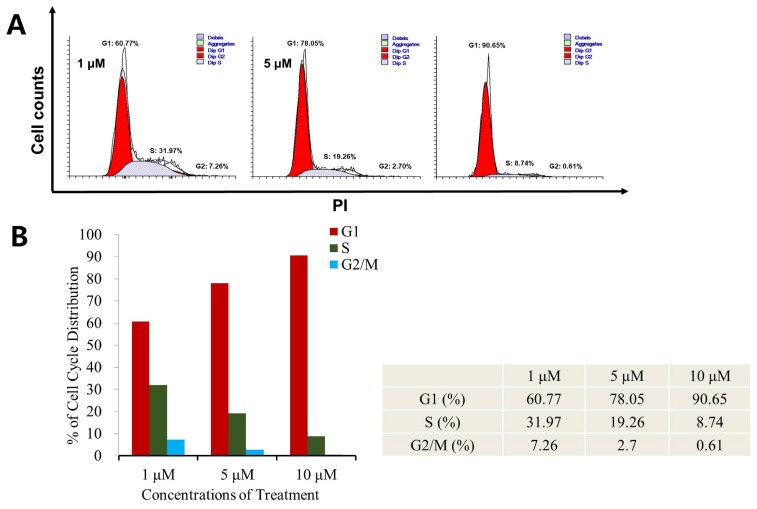
The effect of compound 7i on the cell cycle was also evaluated. After treatment of A549 cells with compound 7i for 24 h at indicated concentrations (1, 5, 10 μM), the percentage of cells in G1 phase were 60.77%, 78.05% and 90.65%, respectively (Figure 7), suggesting that compound 7i caused an obvious G1 arrest in a concentration-dependent manner with a concomitant decrease in terms of the number of cells in other phases of the cell cycle.
Figure 7.
Effects of compound 7i on A549 cell cycle progress for 24 h. (A) Treatment of A549 cells with compound 7i at different concentrations (1 μM, 5 μM, 10 μM) for 24 h. (B) Quantitative analysis of cell cycle. The experiments were performed three times, and a representative experiment is shown.
3. Materials and Methods
3.1. Synthesis
All reagents were obtained from commercial suppliers and used without further purification. Reaction progress was monitored by thin layer chromatography (TLC) on silica gel plates. The spots were visualized by ultraviolet (UV) light (254 nm). The column chromatography was performed using 200−300 mesh silica gel (Qingdao PUKE, Qingdao, China). Melting points were obtained by X-5 micro-melting point apparatus (Beijing Zhongyi Boteng Technology Co., Ltd., Beijing, China) and were uncorrected. 1H-NMR and 13C-NMR spectra were recorded on Bruker NMR spectrophotometers (Karlsruhe, Germany) using DMSO-d6 as the solvent and TMS as the internal standard. Mass spectra were measured with an electrospray (ESI-MS) on a Waters spectrometer (Waters Corporation, Milford, MA, USA). High resolution mass spectrometry (HRMS) analyses were performed on an Agilent Technologies 6530 Accurate-Mass Q-TOF Mass Spectrometer (Santa Clara, CA, USA). The purities were determined by high-performance liquid chromatography (HPLC) using an Agilent 1100 series HPLC (Santa Clara, CA, USA).
The original figures of 1H-NMR, 13C-NMR, MS and HRMS of all the target compounds as the Supplementary Materials are available online.
3.1.1. General Procedure for the Preparation of 2-{[(4-nitrophenyl)thio]methyl}pyridine Derivatives (3a–3d)
4-Nitrobenzenethiol 1 (1.55 g, 0.01 mol), and 2-(chloromethyl)pyridine hydrochloride derivatives 2 (0.01 mol) were dissolved in EtOH (100 mL), then aqueous NaOH (2M) was added dropwise. After the addition completed, the solution was stirred for 8 h at room temperature. Upon completion, the excess ethanol was evaporated to give the residue. A large number of white solids have been precipitated when 200 mL of water was added. The Precipitate was filtered off and washed with water to obtain the intermediates (3a–3d), which was used for next step without further purification.
4-(3-Methoxypropoxy)-3-methyl-2-{[(4-nitrophenyl)thio]methyl}pyridine (3a) by using compound 1 (1.55 g, 0.01 mol) and 2-(chloromethyl)-4-(3-methoxypropoxy)-3-methylpyridine hydrochloride (2.66 g, 0.01 mol), obtained a yellow solid (3.12 g) in 89.7% yield. ESI-MS (m/z): 349.3 ([M + H]+).
3-Methyl-2-{[(4-nitrophenyl)thio]methyl}-4-(2,2,2-trifluoroethoxy)pyridine (3b) by using compound 1 (1.55 g, 0.01 mol) and 2-(chloromethyl)-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine hydrochloride (2.76 g, 0.01 mol), obtained a yellow solid (3.26 g) in 91.2% yield. ESI-MS (m/z): 359.1 ([M + H]+).
4-Methoxy-3,5-dimethyl-2-{[(4-nitrophenyl)thio]methyl}pyridine (3c) by using compound 1 (1.55 g, 0.01 mol) and 2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloride (2.22 g, 0.01 mol), obtained a yellow solid (2.69 g) in 88.5% yield. ESI-MS (m/z): 305.0 ([M + H]+).
3,4-Dimethoxy-2-{[(4-nitrophenyl)thio]methyl}pyridine (3d) by using compound 1 (1.55 g, 0.01 mol) and 2-(chloromethyl)-3,4-dimethoxypyridine hydrochloride (2.24 g, 0.01 mol), obtained a yellow solid (2.86 g) in 93.5% yield. ESI-MS (m/z): 307.4 ([M + H]+).
3.1.2. General Procedure for the Preparation of 4-{[(pyridin-2-yl)methyl]thio}aniline Derivatives (4a–4d)
A mixture of 3a–3d (5 mmol) and 0.1 g of preequilibrated 10% palladium/carbon in MeOH (50 mL) was hydrogenated at room temperature and atmospheric pressure. The reaction was completely monitored by TLC. When the reaction has completed, the mixture was filtered, and the filtrate was evaporated to yield intermediate (4a–4d) as yellowish oil, which was used for the next step without further purification.
4-{{[4-(3-Methoxypropoxy)-3-methylpyridin-2-yl]methyl}thio}aniline (4a) by using compound 3a (1.74 g, 5 mmol), H2 and 10% Pd-C, obtained a yellowish oil (1.52 g) in 95.4% yield. ESI-MS (m/z): 319.3 ([M + H]+).
4-{{[3-Methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methyl}thio}aniline (4b) by using compound 3b (1.79 g, 5 mmol), H2 and 10% Pd-C, obtained a yellowish oil (1.58 g) in 96.1% yield. ESI-MS (m/z): 329.6 ([M + H]+).
4-{[(4-Methoxy-3,5-dimethylpyridin-2-yl)methyl]thio}aniline (4c) by using compound 3c (1.52 g, 5 mmol), H2 and 10% Pd-C, obtained a yellowish oil (1.30 g) in 94.8% yield. ESI-MS (m/z): 275.2 ([M + H]+).
4-{[(3,4-Dimethoxypyridin-2-yl)methyl]thio}aniline (4d) by using compound 3d (1.53 g, 5 mmol), H2 and 10% Pd-C, obtained a yellowish oil (1.30 g) in 94.2% yield. ESI-MS (m/z): 276.1 ([M + H]+).
3.1.3. General Procedure for the Preparation of the Target Compounds (7a–7t)
To a solution of BTC (1 mmol) in CH2Cl2 (20 mL) was added dropwise to primary aromatic amine 5 (1 mmol) in CH2Cl2 (20 mL) followed by the dropwise addition of triethylamine (1 mL) in CH2Cl2 (10 mL). The solvent was evaporated. The resulting residue was dissolved in CH2Cl2 (20 mL), and intermediates (4a–4d) (1 mmol) in CH2Cl2 (10 mL) was added dropwise. The mixture was stirred for about 3 h, monitored by TLC. After the reaction completed, the solvent was washed with water and brine, then dried over anhydrous magnesium sulfate. The mixture was filtered, the filtrate was evaporated and purified by silica gel chromatography (CH2Cl2/MeOH = 60/1, v/v) to obtain target compounds.
1-{4-{{[4-(3-Methoxypropoxy)-3-methylpyridin-2-yl]methyl}thio}phenyl}-3-phenylurea (7a)
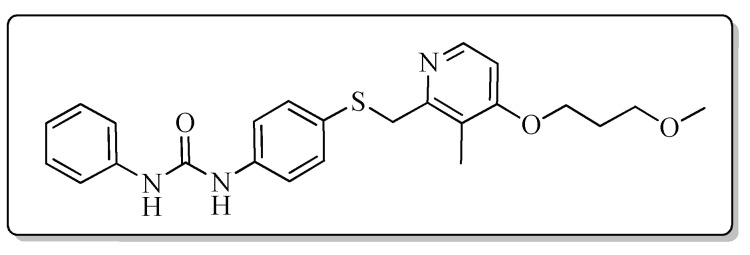
Compound 7a was prepared according to the general procedure by using compound 4a (0.32 g, 1 mmol) and aniline (0.10 g, 1 mmol), obtained a white solid (0.20 g) in 45.1% yield. m.p. 105.2–106.8 °C. IR (KBr, cm−1): υ 3421.1, 2922.7, 2852.4, 1596.1, 1545.1, 1492.4, 1460.8, 1440.3, 1398.2, 1385.2, 1309.3, 1231.3, 1174.2, 1092.1, 1006.7, 894.6, 832.0, 799.2, 751.9, 693.4, 617.3, 507.5. 1H-NMR (400 MHz, DMSO-d6) δ 8.74 (s, 1H), 8.69 (s, 1H), 8.17 (d, J = 5.6 Hz, 1H), 7.46 (s, 1H), 7.44 (s, 1H), 7.41 (s, 1H), 7.39 (s, 1H), 7.32 (s, 1H), 7.31–7.29 (m, 1H), 7.28 (s, 1H), 7.26 (s, 1H), 6.97 (t, J = 7.3 Hz, 1H), 6.90 (d, J = 5.7 Hz, 1H), 4.22 (s, 2H), 4.09 (t, J = 6.2 Hz, 2H), 3.48 (t, J = 6.2 Hz, 2H), 3.25 (s, 3H), 2.12 (s, 3H), 2.02–1.94 (m, 2H). 13C-NMR (101 MHz, DMSO-d6) δ 163.17, 156.31, 152.87, 147.91, 140.07, 131.77, 129.23, 128.07, 122.34, 120.30, 119.09, 118.69, 106.49, 68.79, 65.48, 58.44, 31.14, 29.16, 10.88. ESI-MS (m/z): 438.4 ([M + H]+), 460.2 ([M + Na]+). HRMS (ESI) (m/z): Calcd. for C24H27N3O3S, 438.1846 ([M + H]+), found: 438.1856 ([M + H]+). Purity (HPLC): 99.27%.
1-(4-Chlorophenyl)-3-{4-{{[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methyl}thio}phenyl}urea (7b)
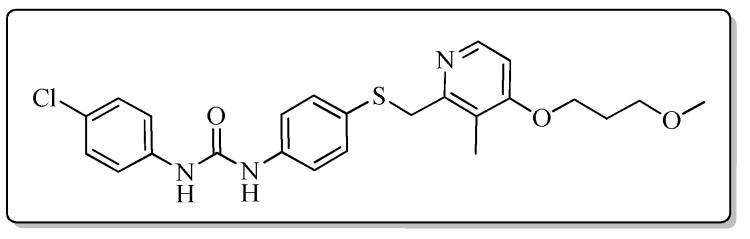
Compound 7b was prepared according to the general procedure by using compound 4a (0.32 g, 1 mmol) and 4-chloroaniline (0.13 g, 1 mmol), obtained a white solid (0.36 g) in 75.3% yield. m.p. 191.7–192.5 °C. IR (KBr, cm−1): υ 3428.1, 2923.1, 2852.9, 1631.8, 1490.8, 1398.9, 1384.8, 1298.9, 1273.5, 1237.0, 1174.2, 1121.4, 1086.2, 1008.0, 881.2, 832.1, 702.8, 619.7, 506.0. 1H-NMR (400 MHz, DMSO-d6) δ 8.83 (s, 1H), 8.77 (s, 1H), 8.17 (d, J = 5.6 Hz, 1H), 7.49 (d, J = 2.2 Hz, 1H), 7.47 (d, J = 2.1 Hz, 1H), 7.40 (d, J = 2.0 Hz, 1H), 7.39 (d, J = 2.1 Hz, 1H), 7.34 (s, 1H), 7.33 (s, 1H), 7.32 (d, J = 2.1 Hz, 1H), 7.31 (s, 1H), 6.90 (d, J = 5.7 Hz, 1H), 4.22 (s, 2H), 4.08 (t, J = 6.2 Hz, 2H), 3.48 (t, J = 6.2 Hz, 2H), 3.25 (s, 3H), 2.12 (s, 3H), 2.00–1.94 (m, 2H). 13C-NMR (101 MHz, DMSO-d6) δ 163.09, 156.35, 152.77, 147.99, 139.09, 138.88, 131.62, 129.07, 128.41, 125.86, 120.22, 119.24, 106.47, 68.80, 65.45, 58.43, 31.14, 29.16, 10.89. ESI-MS (m/z): 473.3 ([M + H]+), 495.2 ([M + Na]+). HRMS (ESI) (m/z): Calcd. for C24H26ClN3O3S, 472.1456 ([M + H]+), found: 472.1467 ([M + H]+). Purity (HPLC): 98.66%.
1-(4-Methoxyphenyl)-3-{4-{{[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methyl}thio}phenyl}urea (7c)
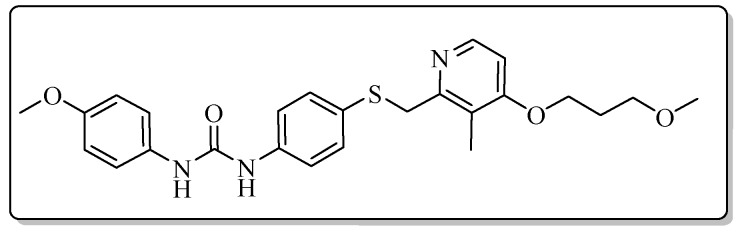
Compound 7c was prepared according to the general procedure by using compound 4a (0.32 g, 1 mmol) and 4-methoxyaniline (0.12 g, 1 mmol), obtained a white solid (0.22 g) in 46.3% yield. m.p. 144.8–146.6 °C. IR (KBr, cm−1): υ 3428.6, 2984.7, 2923.0, 2852.7, 1635.5, 1599.5, 1562.6, 1510.7, 1492.7, 1461.8, 1441.3, 1398.1, 1289.8, 1245.2, 1173.5, 1120.3, 1093.5, 1035.2, 1005.6, 800.0, 617.1, 548.4, 522.7. 1H-NMR (400 MHz, DMSO-d6) δ 8.64 (s, 1H), 8.47 (s, 1H), 8.16 (d, J = 5.7 Hz, 1H), 7.39 (d, J = 1.9 Hz, 1H), 7.37 (d, J = 2.2 Hz, 1H), 7.35 (d, J = 2.2 Hz, 1H), 7.33 (d, J = 2.3 Hz, 1H), 7.30 (d, J = 2.1 Hz, 1H), 7.29 (d, J = 2.0 Hz, 1H), 6.89 (d, J = 5.6 Hz, 1H), 6.87 (d, J = 2.2 Hz, 1H), 6.86 (d, J = 2.2 Hz, 1H), 4.21 (s, 2H), 4.08 (t, J = 6.2 Hz, 2H), 3.71 (s, 3H), 3.48 (t, J = 6.2 Hz, 2H), 3.25 (s, 3H), 2.12 (s, 3H), 2.00–1.94 (m, 2H). 13C-NMR (101 MHz, DMSO-d6) δ 163.06, 156.41, 154.97, 153.07, 148.01, 139.37, 133.09, 131.78, 127.82, 120.52, 120.22, 118.98, 114.44, 106.44, 68.79, 65.42, 58.43, 55.63, 31.14, 29.16, 10.88. ESI-MS (m/z): 468.4 ([M + H]+), 490.2 ([M + Na]+). HRMS (ESI) (m/z): Calcd. for C25H29N3O4S, 468.1952 ([M + H]+), found: 468.1959 ([M + H]+). Purity (HPLC): 98.91%.
1-[4-Chloro-3-(trifluoromethyl)phenyl]-3-{4-{{[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methyl}thio}phenyl}urea (7d)
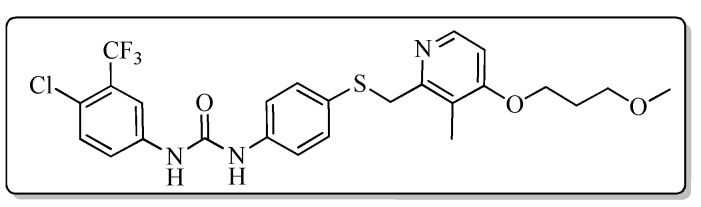
Compound 7d was prepared according to the general procedure by using compound 4a (0.32 g, 1 mmol) and 3-chloro-4-(trifluoromethyl)aniline (0.20 g, 1 mmol), obtained a white solid (0.24 g) in 43.8% yield. m.p. 136.0–137.2 °C. IR (KBr, cm−1): υ 3425.2, 2922.0, 2852.7, 1590.1, 1546.1, 1482.2, 1463.1, 1384.5, 1306.6, 1175.4, 1117.7, 1034.4, 820.6. 1H-NMR (400 MHz, DMSO-d6) δ 9.16 (s, 1H), 8.89 (s, 1H), 8.16 (d, J = 5.6 Hz, 1H), 8.10 (d, J = 2.2 Hz, 1H), 7.63 (d, J = 2.2 Hz, 1H), 7.62 (s, 1H), 7.42 (d, J = 2.0 Hz, 1H), 7.40 (d, J = 2.2 Hz, 1H), 7.33 (d, J = 2.1 Hz, 1H), 7.32 (d, J = 2.0 Hz, 1H), 6.89 (d, J = 5.7 Hz, 1H), 4.23 (s, 2H), 4.09 (t, J = 6.2 Hz, 2H), 3.48 (t, J = 6.2 Hz, 2H), 3.25 (s, 3H), 2.13 (s, 3H), 2.00–1.94 (m, 2H). 13C-NMR (101 MHz, DMSO-d6) δ 163.08, 156.34, 152.76, 148.01, 139.76, 138.48, 132.45, 131.46, 128.91, 123.55, 122.80, 120.24, 119.57, 117.23, 106.48, 68.80, 65.44, 58.43, 31.14, 29.16, 10.89. ESI-MS (m/z): 540.2 ([M + H]+), 562.0 ([M + Na]+). HRMS (ESI) (m/z): Calcd. for C25H25ClF3N3O3S, 540.1330 ([M + H]+), found: 540.1320 ([M + H]+). Purity (HPLC): 97.33%.
1-{4-{{[4-(3-Methoxypropoxy)-3-methylpyridin-2-yl]methyl}thio}phenyl}-3-[3-(trifluoromethyl)phenyl]urea (7e)
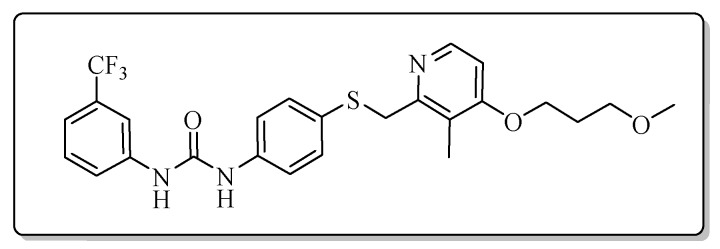
Compound 7e was prepared according to the general procedure by using compound 4a (0.32 g, 1 mmol) and 3-(trifluoromethyl)aniline (0.16 g, 1 mmol), obtained a white solid (0.27 g) in 53.1% yield. m.p. 136.1–137.9 °C. IR (KBr, cm−1): υ 3327.8, 2958.7, 2927.8, 2859.0, 2377.4, 2350.2, 2311.0, 1724.0, 1648.5, 1585.5, 1552.2, 1492.5, 1465.2, 1397.5, 1338.6, 1295.8, 1230.8, 1166.2, 1116.1, 1092.4, 1068.9, 1005.4, 890.0, 804.1, 732.9, 699.2, 602.3, 505.8. 1H-NMR (400 MHz, DMSO-d6) δ 9.05 (s, 1H), 8.84 (s, 1H), 8.17 (d, J = 5.6 Hz, 1H), 8.09 (s, 0H), 8.00 (d, J = 2.3 Hz, 1H), 7.57 (d, J = 8.4 Hz, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.42 (d, J = 2.0 Hz, 1H), 7.40 (s, 1H), 7.33 (d, J = 2.1 Hz, 1H), 7.32 (d, J = 2.1 Hz, 1H), 7.30 (s, 1H), 6.90 (d, J = 5.7 Hz, 1H), 4.23 (s, 2H), 4.09 (t, J = 6.2 Hz, 2H), 3.48 (t, J = 6.2 Hz, 2H), 3.25 (s, 3H), 2.13 (s, 3H), 2.01–1.94 (m, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 163.11, 156.32, 152.85, 147.97, 140.98, 138.68, 131.55, 130.34, 129.10, 128.64, 122.30, 120.25, 119.40, 118.53, 106.46, 68.79, 65.44, 58.42, 31.13, 29.16, 10.88. ESI-MS (m/z): 506.3 ([M + H]+). HRMS (ESI) (m/z): Calcd. for C25H26F3N3O3S, 506.1720 ([M + H]+), found: 506.1728 ([M + H]+). Purity (HPLC): 97.09%.
1-{4-{{[3-Methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methyl}thio}phenyl}-3-phenylurea (7f)
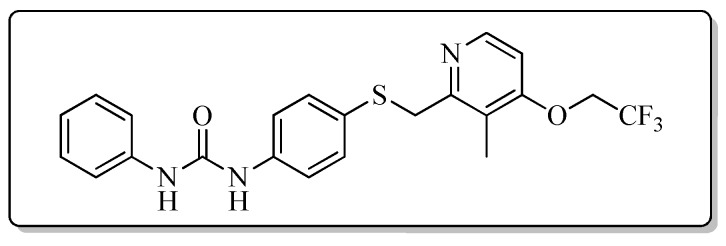
Compound 7f was prepared according to the general procedure by using compound 4b (0.33 g, 1 mmol) and aniline (0.10 g, 1 mmol), obtained a white solid (0.18 g) in 39.9% yield. m.p. 143.3–145.1 °C. IR (KBr, cm−1): υ 3424.1, 2923.9, 2852.6, 1687.8, 1639.9, 1600.0, 1548.5, 1495.9, 1441.2, 1399.4, 1384.7, 1307.8, 1284.4, 1266.4, 1232.1, 1176.6, 1112.2, 970.6, 915.9, 854.7, 836.1, 801.5, 783.1, 751.0, 696.2, 657.1, 618.8, 574.4. 1H-NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H), 8.67 (s, 1H), 8.24 (d, J = 5.7 Hz, 1H), 7.46 (s, 1H), 7.44 (s, 1H), 7.41 (s, 1H), 7.39 (s, 1H), 7.32 (s, 1H), 7.30 (s, 1H), 7.28 (s, 1H), 7.26 (s, 1H), 7.03 (d, J = 5.7 Hz, 1H), 6.97 (t, J = 7.3 Hz, 1H), 4.89 (q, J = 8.7 Hz, 2H), 4.25 (s, 2H), 2.16 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 161.62, 157.24, 152.87, 148.00, 140.05, 139.22, 131.90, 129.23, 127.85, 125.66, 122.90, 122.35, 120.44, 119.10, 118.71, 107.07, 64.92, 31.13, 10.74. ESI-MS (m/z): 448.4 ([M + H]+), 470.2 ([M + Na]+). HRMS (ESI) (m/z): Calcd. for C22H20F3N3O2S, 448.1301 ([M + H]+), found: 448.1295 ([M + H]+). Purity (HPLC): 97.04%.
1-(4-Chlorophenyl)-3-{4-{{[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methyl}thio}phenyl}urea (7g)
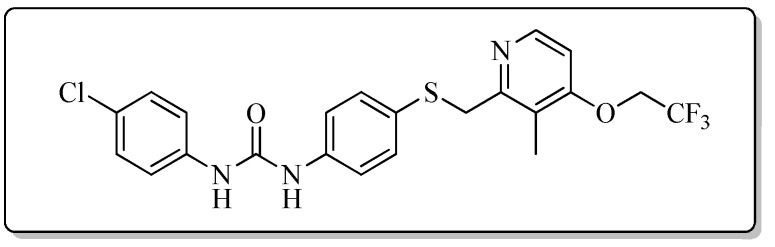
Compound 7g was prepared according to the general procedure by using compound 4b (0.33 g, 1 mmol) and 4-chloroaniline (0.13 g, 1 mmol), obtained a white solid (0.29 g) in 61.0% yield. m.p. 203.6–205.2 °C. IR (KBr, cm−1): υ 3424.1, 2984.9, 2923.1, 2851.9, 2350.0, 2311.0, 1611.4, 1548.6, 1491.9, 1440.3, 1399.6, 1384.9, 1370.1, 1311.1, 1268.3, 1172.6, 1111.7, 1051.4, 1004.4, 897.2, 798.3, 668.5, 615.4. 1H-NMR (400 MHz, DMSO-d6) δ 8.81 (s, 1H), 8.75 (s, 1H), 8.23 (d, J = 5.7 Hz, 1H), 7.48 (d, J = 2.1 Hz, 1H), 7.47 (d, J = 2.2 Hz, 1H), 7.40 (d, J = 2.0 Hz, 1H), 7.38 (s, 1H), 7.33 (d, J = 2.1 Hz, 1H), 7.32 (d, J = 2.2 Hz, 1H), 7.31 (s, 1H), 7.30 (d, J = 1.9 Hz, 1H), 7.03 (d, J = 5.7 Hz, 1H), 4.88 (q, J = 8.7 Hz, 2H), 4.25 (s, 2H), 2.16 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 161.62, 157.22, 152.77, 148.00, 139.08, 138.98, 131.80, 129.07, 128.11, 125.87, 122.90, 120.43, 120.23, 119.23, 107.08, 65.09, 31.14, 10.74. ESI-MS (m/z): 482.6 ([M + H]+), 504.3 ([M + Na]+). HRMS (ESI) (m/z): Calcd. for C22H19ClF3N3O2S, 482.0911 ([M + H]+), found: 482.0916 ([M + H]+). Purity (HPLC): 99.19%.
1-(4-Methoxyphenyl)-3-{4-{{[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methyl}thio}phenyl}urea (7h)
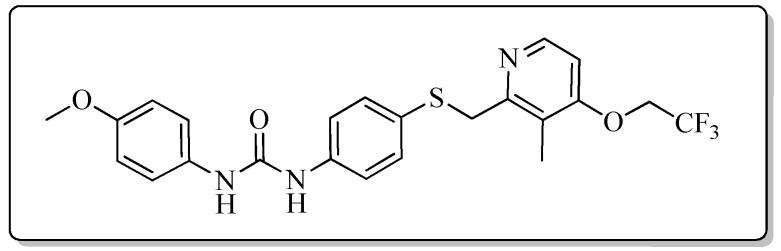
Compound 7h was prepared according to the general procedure by using compound 4b (0.33 g, 1 mmol) and 4-methoxyaniline (0.12 g, 1 mmol), obtained a white solid (0.22 g) in 45.9% yield. m.p. 171.3–172.1 °C. IR (KBr, cm−1): υ 3383.8, 2922.1, 2851.4, 2377.6, 2349.6, 1703.0, 1656.7, 1619.2, 1591.3, 1546.4, 1511.1, 1492.7, 1465.5, 1399.3, 1312.1, 1264.5, 1231.2, 1175.1, 1112.8, 1040.3, 1006.7, 970.2, 918.1, 897.2, 831.5, 799.7, 658.3. 1H-NMR (400 MHz, ) δ 8.64 (s, 1H), 8.47 (s, 1H), 8.16 (d, J = 5.7 Hz, 1H), 7.39 (d, J = 1.9 Hz, 1H), 7.37 (d, J = 2.2 Hz, 1H), 7.35 (d, J = 2.2 Hz, 1H), 7.33 (d, J = 2.3 Hz, 1H), 7.30 (d, J = 2.1 Hz, 1H), 7.29 (d, J = 2.0 Hz, 1H), 6.89 (d, J = 5.6 Hz, 1H), 6.87 (d, J = 2.2 Hz, 1H), 6.86 (d, J = 2.2 Hz, 1H), 4.91-4.85 (m, 2H), 4.21 (s, 2H), 3.71 (s, 3H), 2.12 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 161.84, 156.03, 154.81, 153.88, 153.39, 148.09, 133.72, 133.38, 125.65, 121.84, 120.37, 120.33, 115.40, 114.43, 108.04, 65.10, 55.63, 31.13, 10.44. ESI-MS (m/z): 478.3 ([M + H]+). HRMS (ESI) (m/z): Calcd. for C23H22F3N3O3S, 478.1407 ([M + H]+), found: 478.1408 ([M + H]+). Purity (HPLC): 99.66%.
1-[4-Chloro-3-(trifluoromethyl)phenyl]-3-{4-{{[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methyl}thio}phenyl}urea (7i)
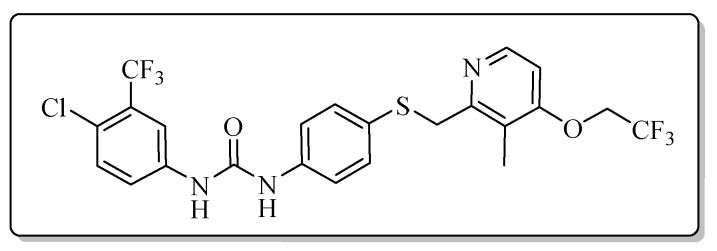
Compound 7i was prepared according to the general procedure by using compound 4b (0.33 g, 1 mmol) and 3-chloro-4-(trifluoromethyl)aniline (0.20 g, 1 mmol), obtained a white solid (0.27 g) in 49.5% yield. m.p. 142.0–143.0 °C. IR (KBr, cm−1): υ 3422.3, 2922.1, 2852.6, 1587.4, 1547.6, 1480.8, 1419.0, 1309.8, 1263.9, 1177.2, 1111.0, 1035.5, 974.7, 819.3, 618.3. 1H-NMR (400 MHz, DMSO-d6) δ 9.17 (s, 1H), 8.90 (s, 1H), 8.24 (d, J = 5.7 Hz, 1H), 8.10 (d, J = 2.2 Hz, 1H), 7.64 (dd, J = 8.9, 2.2 Hz, 1H), 7.61 (d, J = 8.7 Hz, 1H), 7.42 (d, J = 2.0 Hz, 1H), 7.40 (d, J = 2.2 Hz, 1H), 7.33 (d, J = 2.2 Hz, 1H), 7.32 (d, J = 2.1 Hz, 1H), 7.04 (d, J = 5.7 Hz, 1H), 4.89 (q, J = 8.7 Hz, 2H), 2.16 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 161.61, 157.18, 152.75, 148.00, 139.74, 138.60, 132.42, 131.64, 128.58, 127.33, 127.02, 125.65, 123.53, 122.80, 120.44, 119.55, 117.28, 117.22, 107.07, 64.79, 39.69, 10.72. ESI-MS (m/z): 550.1, 552.1, 553.1 ([M + H]+). HRMS (ESI) (m/z): Calcd. for C23H18ClF6N3O2S, 550.0785 ([M + H]+), found: 550.0769 ([M + H]+). Purity (HPLC): 99.97%.
1-{4-{{[3-Methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methyl}thio}phenyl}-3-[3-(trifluoromethyl)phenyl]urea (7j)
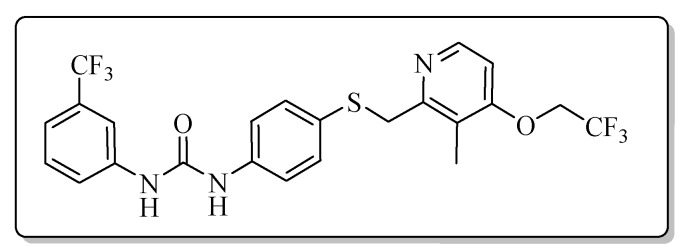
Compound 7j was prepared according to the general procedure C by using compound 4b (0.33 g, 1 mmol) and 3-(trifluoromethyl)aniline (0.16 g, 1 mmol), obtained a white solid (0.34 g) in 66.1% yield. m.p. 174.7–176.4 °C. IR (KBr, cm−1): υ 3421.4, 2985.6, 2924.1, 2852.9, 2349.2, 2311.0, 1614.9, 1491.8, 1445.1, 1399.1, 1339.8, 1313.1, 1288.0, 1264.6, 1231.2, 1173.2, 1114.6, 1071.2, 1006.3, 976.0, 832.4, 798.2, 700.6, 616.4. 1H-NMR (400 MHz, DMSO-d6) δ 9.07 (s, 1H), 8.87 (s, 1H), 8.24 (d, J = 5.7 Hz, 1H), 8.01 (d, J = 2.0 Hz, 1H), 7.60–7.54 (m, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.43 (d, J = 2.0 Hz, 1H), 7.41 (d, J = 2.1 Hz, 1H), 7.33 (d, J = 2.1 Hz, 1H), 7.33–7.31 (m, 1H), 7.30 (d, J = 1.7 Hz, 1H), 7.04 (d, J = 5.7 Hz, 1H), 4.89 (q, J = 8.7 Hz, 2H), 4.26 (s, 2H), 2.16 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 161.66, 157.16, 152.86, 147.96, 140.97, 138.80, 131.76, 130.35, 130.15, 129.84, 128.31, 122.30, 120.47, 119.40, 118.54, 114.59, 107.09, 65.09, 49.05, 31.13, 10.73. ESI-MS (m/z): 516.2 ([M + H]+). HRMS (ESI) (m/z): Calcd. for C23H19F6N3O2S, 516.1175 ([M + H]+), found: 516.1174 ([M + H]+). Purity (HPLC): 97.29%.
1-{4-{[(4-Methoxy-3,5-dimethylpyridin-2-yl)methyl]thio}phenyl}-3-phenylurea (7k)
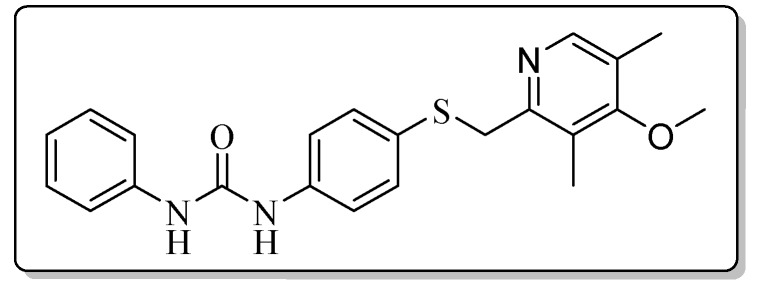
Compound 7k was prepared according to the general procedure by using compound 4c (0.27 g, 1 mmol) and aniline (0.10 g, 1 mmol), obtained a white solid (0.23 g) in 57.3% yield. m.p. 188.1–188.9 °C. IR (KBr, cm−1): υ 3422.4, 2923.8, 2852.4, 2351.0, 2321.9, 1644.0, 1597.4, 1553.7, 1494.2, 1441.5, 1398.0, 1312.9, 1270.7, 1237.5, 1173.3, 1127.7, 1073.5, 1002.5, 798.1, 755.7, 738.0, 694.0, 616.4. 1H-NMR (400 MHz, DMSO-d6) δ 8.72 (s, 1H), 8.67 (s, 1H), 8.12 (s, 1H), 7.45 (d, J = 1.3 Hz, 1H), 7.44-7.42 (m, 1H), 7.40 (d, J = 2.1 Hz, 1H), 7.39 (d, J = 2.1 Hz, 1H), 7.31 (d, J = 2.2 Hz, 1H), 7.29 (d, J = 2.1 Hz, 1H), 7.28 (s, 1H), 7.26 (d, J = 1.6 Hz, 1H), 6.97 (t, J = 7.4 Hz, 1H), 4.21 (s, 2H), 3.70 (s, 3H), 2.18 (s, 6H). 13C-NMR (101 MHz, DMSO-d6) δ 163.86, 155.80, 152.86, 148.97, 140.05, 139.16, 131.86, 129.24, 127.98, 125.19, 125.03, 122.35, 119.11, 118.70, 60.17, 31.15, 13.38, 11.34. ESI-MS (m/z): 394.6 ([M + H]+), 416.3 ([M + Na]+). HRMS (ESI) (m/z): Calcd. for C22H23N3O2S, 394.1584 ([M + H]+), found: 394.1586 ([M + H]+). Purity (HPLC): 99.89%.
1-(4-Chlorophenyl)-3-{4-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]thio}phenyl}urea (7l)
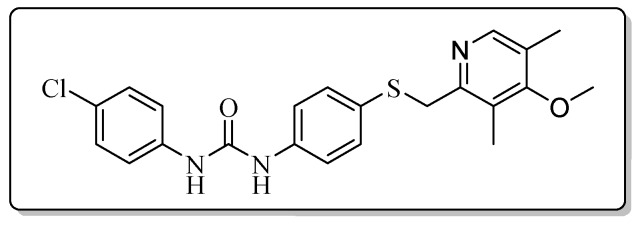
Compound 7l was prepared according to the general procedure by using compound 4c (0.27 g, 1 mmol) and 4-chloroaniline (0.13 g, 1 mmol), obtained a white solid (0.28 g) in 66.2% yield. m.p. 206.8–208.2 °C. IR (KBr, cm−1): υ 3422.5, 2923.0, 2852.0, 2377.1, 2349.6, 2310.8, 1630.4, 1547.7, 1491.7, 1439.7, 1399.2, 1385.1, 1309.9, 1270.9, 1235.5, 1173.0, 1124.1, 1051.3, 1004.6, 832.1, 798.2, 702.1, 668.3, 617.0. 1H-NMR (400 MHz, DMSO-d6) δ 8.81 (s, 1H), 8.75 (s, 1H), 8.11 (s, 1H), 7.48 (d, J = 2.1 Hz, 1H), 7.46 (d, J = 2.2 Hz, 1H), 7.40 (d, J = 2.0 Hz, 1H), 7.38 (d, J = 2.2 Hz, 1H), 7.33 (d, J = 2.1 Hz, 1H), 7.32 (s, 1H), 7.31 (s, 1H), 7.30 (d, J = 2.1 Hz, 1H), 4.21 (s, 2H), 3.70 (s, 3H), 2.18 (s, 3H), 2.18 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 163.86, 155.79, 152.77, 148.97, 139.08, 138.94, 131.76, 129.07, 127.98, 128.25, 125.87, 125.19, 125.03, 120.23, 119.25, 60.17, 31.14, 13.37, 11.33. ESI-MS (m/z): 428.7 ([M + H]+). HRMS (ESI) (m/z): Calcd. for C22H22ClN3O2S, 428.1194 ([M + H]+), found: 428.1199 ([M + H]+). Purity (HPLC): 99.53%.
1-{4-{[(4-Methoxy-3,5-dimethylpyridin-2-yl)methyl)]thio}phenyl}-3-(4-methoxyphenyl)urea (7m)
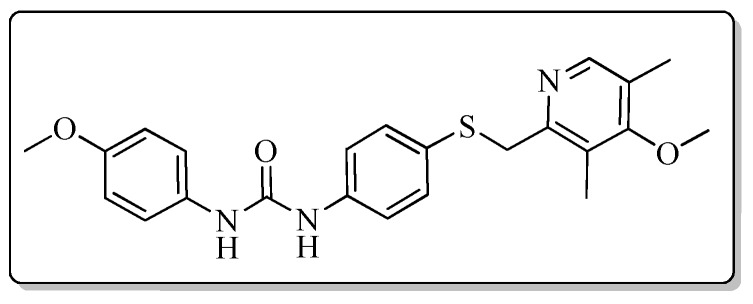
Compound 7m was prepared according to the general procedure by using compound 4c (0.27 g, 1 mmol) and 4-methoxyaniline (0.12 g, 1 mmol), obtained a white solid (0.21 g) in 49.2% yield. m.p. 171.4–172.6 °C. IR (KBr, cm−1): υ 3422.5, 2921.1, 2850.5, 1642.5, 1593.2, 1547.5, 1493.7, 1468.2, 1439.0, 1397.7, 1311.5, 1292.4, 1270.1, 1240.4, 1173.4, 1073.2, 1053.2, 1031.3, 1003.9, 828.2, 797.9, 616.3. 1H-NMR (400 MHz, DMSO-d6) δ 8.68 (s, 1H), 8.50 (s, 1H), 8.15 (s, 1H), 7.39 (d, J = 1.9 Hz, 1H), 7.37 (d, J = 2.1 Hz, 1H), 7.35 (d, J = 2.0 Hz, 1H), 7.33 (d, J = 2.2 Hz, 1H), 7.29 (d, J = 2.1 Hz, 1H), 7.27 (d, J = 1.9 Hz, 1H), 6.87 (d, J = 2.3 Hz, 1H), 6.86 (d, J = 2.2 Hz, 1H), 4.21 (s, 2H), 3.73 (s, 3H), 3.71 (s, 3H), 2.20 (s, 3H), 2.17 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 164.47, 155.40, 154.98, 153.08, 148.27, 139.64, 133.09, 132.28, 127.19, 125.70, 125.46, 125.41, 120.49, 118.95, 118.40, 114.46, 60.30, 55.65, 31.14, 13.45, 11.35. ESI-MS (m/z): 424.3 ([M + H]+). HRMS (ESI) (m/z): Calcd. for C23H25N3O3S, 424.1689 ([M + H]+), found: 424.1698 ([M + H]+). Purity (HPLC): 96.88%.
1-[4-Chloro-3-(trifluoromethyl)phenyl]-3-{4-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]thio}phenyl}urea (7n)
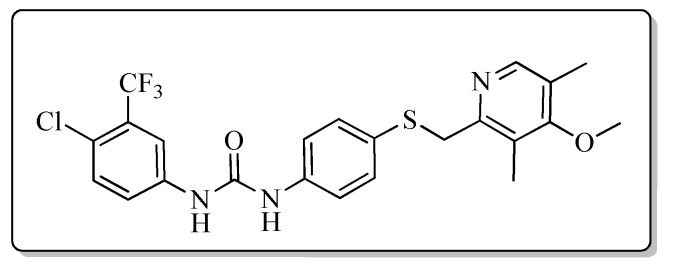
Compound 7n was prepared according to the general procedure by using compound 4c (0.27 g, 1 mmol) and 3-chloro-4-(trifluoromethyl)aniline (0.20 g, 1 mmol), obtained a white solid (0.35 g) in 70.1% yield. m.p. 152.3–153.1 °C. IR (KBr, cm−1): υ 3422.2, 2921.6, 2852.2, 1719.2, 1593.8, 1546.4, 1480.4, 1419.8, 1384.6, 1311.1, 1265.2, 1229.0, 1174.6, 1130.8, 1073.5, 1031.7, 823.3, 619.9. 1H-NMR (400 MHz, DMSO-d6) δ 9.18 (s, 1H), 8.91 (s, 1H), 8.12 (s, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.64 (d, J = 8.9 Hz, 1H), 7.62 (s, 1H), 7.42 (d, J = 2.1 Hz, 1H), 7.40 (d, J = 2.1 Hz, 1H), 7.33 (s, 1H), 7.31 (d, J = 1.9 Hz, 1H), 4.22 (s, 2H), 3.70 (s, 3H), 2.19 (s, 3H), 2.18 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 164.07, 154.69, 154.49, 153.03, 149.00, 140.01, 132.84, 132.38, 126.45, 126.36, 124.20, 123.34, 122.49, 121.03, 115.40, 60.18, 31.13, 13.43, 11.00. ESI-MS (m/z): 496.1; 497.1; 498.1; 499.1 ([M + H]+). HRMS (ESI) (m/z): Calcd. for C23H21ClF3N3O2S, 496.1068 ([M + H]+), found: 496.1066 ([M + H]+). Purity (HPLC): 98.56%.
1-{4-{[(4-Methoxy-3,5-dimethylpyridin-2-yl)methyl]thio}phenyl}-3-[3-(trifluoromethyl)pHenyl]urea (7o)
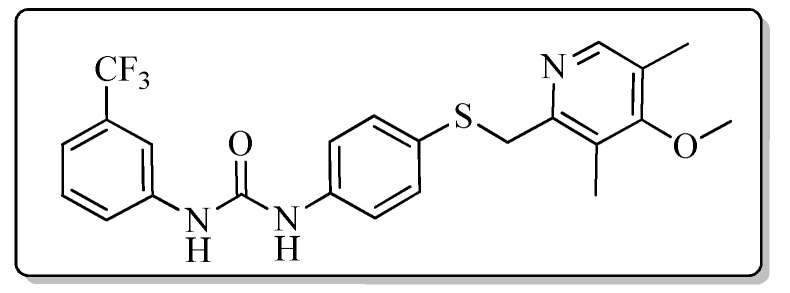
Compound 7o was prepared according to the general procedure by using compound 4c (0.27 g, 1 mmol) and 3-(trifluoromethyl)aniline (0.16 g, 1 mmol), obtained a white solid (0.30 g) in 64.7% yield. m.p. 156.9–158.1 °C. IR (KBr, cm−1): υ 3420.6, 2984.8, 2922.8, 2851.8, 2350.3, 2321.1, 1609.8, 1491.8, 1443.2, 1398.4, 1369.9, 1338.2, 1311.7, 1271.2, 1229.2, 1172.1, 1124.2, 1072.1, 1002.9, 797.9, 698.2, 616.0. 1H-NMR (400 MHz, DMSO-d6) δ 9.04 (s, 1H), 8.84 (s, 1H), 8.12 (s, 1H), 8.00 (d, J = 2.0 Hz, 1H), 7.57 (d, J = 8.8 Hz, 1H), 7.51 (t, J = 7.8 Hz, 1H), 7.42 (d, J = 1.9 Hz, 1H), 7.40 (d, J = 2.2 Hz, 1H), 7.33 (s, 1H), 7.32 (s, 1H), 7.30 (d, J = 2.6 Hz, 1H), 4.22 (s, 2H), 3.70 (s, 3H), 2.19 (s, 3H), 2.18 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 163.88, 155.76, 152.85, 148.95, 140.97, 138.73, 131.69, 130.35, 128.51, 125.21, 125.05, 123.31, 122.32, 119.43, 118.58, 114.64, 60.16, 31.13, 13.36, 11.33. ESI-MS (m/z): 462.3 ([M + H]+). HRMS (ESI) (m/z): Calcd. for C23H22F3N3O2S, 462.1458 ([M + H]+), found: 462.1469 ([M + H]+). Purity (HPLC): 99.79%.
1-{4-{[(3,4-Dimethoxypyridin-2-yl)methyl]thio}phenyl}-3-phenylurea (7p)
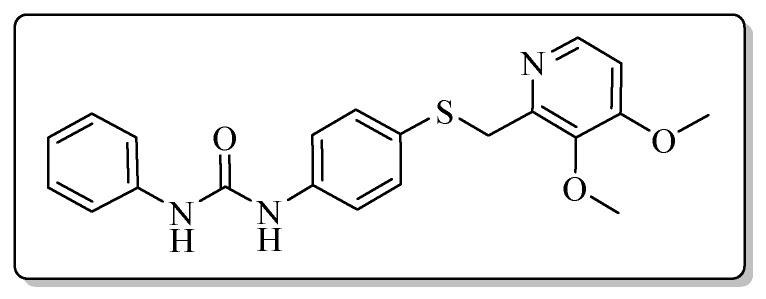
Compound 7p was prepared according to the general procedure by using compound 4d (0.28 g, 1 mmol) and aniline (0.10 g, 1 mmol), obtained a white solid (0.18 g) in 45.8% yield. m.p. 127.7–128.5 °C. IR (KBr, cm−1): υ 3287.2, 2937.3, 1654.0, 1593.7, 1548.0, 1487.0, 1442.7, 1421.2, 1379.4, 1297.9, 1270.4, 1231.6, 1175.0, 1071.7, 997.3, 932.8, 829.0, 782.9, 742.9, 692.4, 618.5, 516.5. 1H-NMR (400 MHz, DMSO-d6) δ 8.72 (s, 1H), 8.67 (s, 1H), 8.12 (d, J = 5.5 Hz, 1H), 7.49–7.45 (m, 1H), 7.44 (s, 1H), 7.41 (d, J = 2.0 Hz, 1H), 7.40 (d, J = 2.2 Hz, 1H), 7.33 (d, J = 2.0 Hz, 1H), 7.31 (d, J = 1.9 Hz, 1H), 7.29 (d, J = 7.7 Hz, 1H), 7.28–7.25 (m, 1H), 7.03 (d, J = 5.5 Hz, 1H), 6.97 (t, J = 7.3 Hz, 1H), 4.17 (s, 2H), 3.87 (s, 3H), 3.74 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 158.52, 152.88, 151.53, 145.72, 143.40, 138.98, 131.39, 129.24, 128.54, 122.33, 119.17, 118.69, 108.29, 60.99, 56.34, 36.37, 31.14. ESI-MS (m/z): 396.3 ([M + H]+), 418.2 ([M + Na]+). HRMS (ESI) (m/z): Calcd. for C21H21N3O3S, 396.1376 ([M + H]+), found: 396.1380 ([M + H]+). Purity (HPLC): 99.90%.
1-(4-Chlorophenyl)-3-{4-{[(3,4-dimethoxypyridin-2-yl)methyl]thio}phenyl}urea (7q)
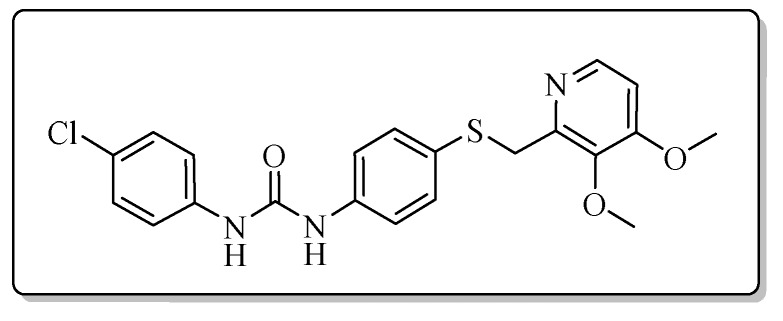
Compound 7q was prepared according to the general procedure by using compound 4d (0.28 g, 1 mmol) and 4-chloroaniline (0.13 g, 1 mmol), obtained a white solid (0.21 g) in 49.1% yield. m.p. 141.7–142.9 °C. IR (KBr, cm−1): υ 3345.3, 3096.8, 2924.2, 2852.2, 1711.6, 1631.2, 1590.8, 1535.1, 1490.0, 1449.2, 1427.9, 1399.4, 1300.6, 1284.5, 1237.1, 1195.2, 1174.0, 1087.1, 1067.2, 996.9, 828.3, 703.0, 509.1. 1H-NMR (400 MHz, DMSO-d6) δ 8.83 (s, 1H), 8.76 (s, 1H), 8.11 (d, J = 5.5 Hz, 1H), 7.49 (s, 1H), 7.47 (s, 1H), 7.41 (s, 1H), 7.39 (s, 1H), 7.34-7.31 (m, 2H), 7.03 (d, J = 5.5 Hz, 1H), 4.17 (s, 2H), 3.87 (s, 3H), 3.74 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 158.52, 152.78, 151.50, 145.71, 143.40, 139.10, 138.75, 131.29, 129.07, 128.79, 125.85, 120.21, 119.30, 108.30, 60.99, 56.34, 36.29, 31.14. ESI-MS (m/z): 430.6 ([M + H]+), 452.1 ([M + Na]+). HRMS (ESI) (m/z): Calcd. for C21H20ClN3O3S, 430.0987 ([M + H]+), found: 430.0993 ([M + H]+). Purity (HPLC): 99.33%.
1-{4-{[(3,4-Dimethoxypyridin-2-yl)methyl]thio}phenyl}-3-(4-methoxyphenyl)urea (7r)
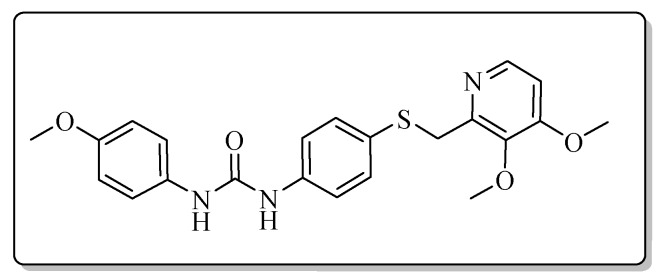
Compound 7r was prepared according to the general procedure by using compound 4d (0.28 g, 1 mmol) and 4-methoxyaniline (0.12 g, 1 mmol), obtained a white solid (0.20 g) in 47.9% yield. m.p. 179.0–180.6 °C. IR (KBr, cm−1): υ 3428.5, 2985.2, 2923.2, 2851.3, 1630.7, 1587.2, 1557.0, 1510.3, 1490.9, 1442.6, 1398.6, 1299.7, 1270.7, 1232.4, 1173.6, 1072.2, 1033.0, 1000.9, 934.0, 829.1, 799.5, 617.4, 549.0, 523.3. 1H-NMR (400 MHz, DMSO-d6) δ 8.62 (s, 1H), 8.46 (s, 1H), 8.11 (d, J = 5.5 Hz, 1H), 7.39 (d, J = 2.0 Hz, 1H), 7.38 (d, J = 2.2 Hz, 1H), 7.35 (d, J = 2.2 Hz, 1H), 7.33 (d, J = 2.2 Hz, 1H), 7.31 (d, J = 2.2 Hz, 1H), 7.30 (d, J = 2.0 Hz, 1H), 7.03 (d, J = 5.5 Hz, 1H), 6.87 (d, J = 2.2 Hz, 1H), 6.85 (d, J = 2.2 Hz, 1H), 4.15 (s, 2H), 3.87 (s, 3H), 3.74 (s, 3H), 3.71 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 158.51, 154.98, 153.07, 151.55, 145.73, 143.40, 139.22, 133.08, 131.45, 128.24, 120.54, 119.06, 114.46, 108.30, 60.99, 56.35, 55.65, 36.43, 31.15. ESI-MS (m/z): 426.3 ([M + H]+). HRMS (ESI) (m/z): Calcd. for C22H23N3O4S, 426.1482 ([M + H]+), found: 426.1489 ([M + H]+). Purity (HPLC): 98.84%.
1-[4-Chloro-3-(trifluoromethyl)phenyl]-3-{4-{[(3,4-dimethoxypyridin-2-yl)methyl]thio}pHenyl}urea (7s)
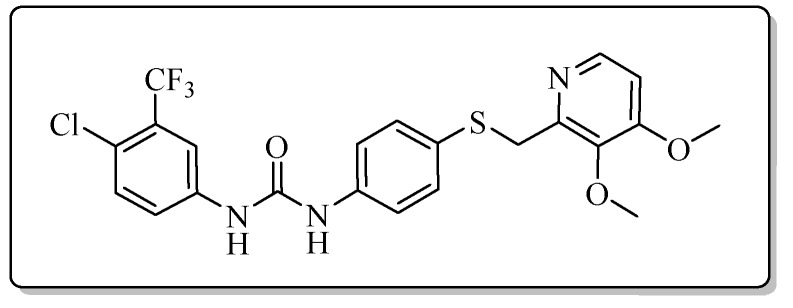
Compound 7s was prepared according to the general procedure by using compound 4d (0.28 g, 1 mmol) and 3-chloro-4-(trifluoromethyl)aniline (0.20 g, 1 mmol), obtained a white solid (0.32 g) in 64.5% yield. m.p. 188.1–189.2 °C. IR (KBr, cm−1): υ 3425.5, 2921.9, 2852.4, 1589.9, 1545.2, 1485.2, 1419.2, 1384.4, 1306.1, 1229.2, 1175.6, 1132.0, 1068.8, 1033.0, 824.9. 1H-NMR (400 MHz, DMSO-d6) δ 9.15 (s, 1H), 8.88 (s, 1H), 8.11 (d, J = 5.5 Hz, 1H), 8.10 (d, J = 2.2 Hz, 1H), 7.64 (d, J = 8.8 Hz, 1H), 7.62–7.58 (m, 1H), 7.42 (d, J = 2.0 Hz, 1H), 7.41 (d, J = 2.2 Hz, 1H), 7.34 (d, J = 2.2 Hz, 1H), 7.33 (d, J = 2.0 Hz, 1H), 7.03 (d, J = 5.5 Hz, 1H), 4.17 (s, 2H), 3.88 (s, 3H), 3.74 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 158.52, 152.76, 151.47, 145.73, 143.40, 139.76, 138.36, 132.44, 131.12, 129.28, 123.53, 122.78, 119.62, 117.24, 108.32, 61.00, 56.35, 36.17, 31.14. ESI-MS (m/z): 498.2 ([M + H]+). HRMS (ESI) (m/z): Calcd. for C22H19ClF3N3O3S, 498.0861 ([M + H]+), found: 498.0844 ([M + H]+). Purity (HPLC): 98.10%.
1-{4-{[(3,4-Dimethoxypyridin-2-yl)methyl]thio}phenyl}-3-[3-(trifluoromethyl)phenyl]urea (7t)
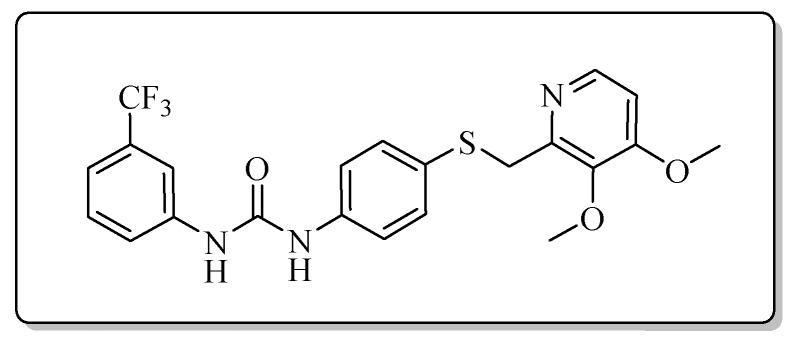
Compound 7t was prepared according to the general procedure by using compound 4d (0.28 g, 1 mmol) and 3-(trifluoromethyl)aniline (0.16 g, 1 mmol), obtained a white solid (0.21 g) in 44.3% yield. m.p. 198.4–199.8 °C. IR (KBr, cm−1): υ 3422.2, 2985.4, 2377.5, 2349.8, 2320.7, 2024.8, 1712.9, 1594.1, 1564.4, 1537.2, 1491.3, 1445.8, 1399.4, 1370.2, 1316.3, 1273.6, 1230.1, 1173.8, 1124.9, 1068.3, 1002.1, 932.4, 892.8, 828.2, 798.3, 743.3, 697.8, 615.6. 1H-NMR (400 MHz, DMSO-d6) δ 9.04 (s, 1H), 8.83 (s, 1H), 8.11 (d, J = 5.5 Hz, 1H), 8.01 (d, J = 2.0 Hz, 1H), 7.60–7.54 (m, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.43 (d, J = 2.0 Hz, 1H), 7.41 (d, J = 2.2 Hz, 1H), 7.34 (d, J = 2.2 Hz, 1H), 7.32 (d, J = 2.5 Hz, 1H), 7.30 (s, 1H), 7.03 (d, J = 5.6 Hz, 1H), 4.17 (s, 2H), 3.88 (s, 3H), 3.75 (s, 3H). 13C-NMR (101 MHz, DMSO-d6) δ 158.52, 152.87, 151.50, 145.72, 143.41, 140.98, 138.55, 131.22, 130.34, 129.08, 122.31, 119.49, 118.56, 114.65, 108.31, 60.99, 56.34, 36.24, 31.12. ESI-MS (m/z): 464.2 ([M + H]+). Purity (HPLC): 98.85%.
3.2. Biological Evaluation
3.2.1. Antiproliferative Activity Assays
The antiproliferative activities of target compounds were determined using a standard MTT assay [27,28,29,30]. Exponentially growing cells A549 (3 × 103 cells/well), HCT-116 (1 × 104 cells/well) and PC-3 (8 × 103 cells/well) were seeded into 96-well plates and incubated for 24 h to allow the cells to attach. After 24 h of incubation, the culture medium was removed and fresh medium containing various concentrations of the candidate compounds was added to each well. The cells were then incubated for 72 h, thereafter MTT assays were performed and cell viability was assessed at 570 nm by a microplate reader (ThermoFisher Scientific (Shanghai) Instrument Co., Ltd., Shanghai, China).
3.2.2. Cell Apoptosis Assay
A549 cells were seeded into a 6-well plate (2 × 105/well) and incubated for 24 h. Then the cells were treated with different concentrations of the tested compound 7i for 24 h. Thereafter, the cells were collected and the Annexin-V-FITC/PI apoptosis kit (Biovision, Milpitas, CA, USA) was used according to the manufacturer’s protocol. The cells were analyzed by Accuri C6 flow cytometric (Becton Dickinson, Franklin Lakes, NJ, USA) [31].
3.2.3. Cell Cycle Analysis
For flow cytometric analysis of DNA content, 5 × 105 A549 cells in exponential growth were treated with different concentrations of the compound 7i for 24 or 48 h. After an incubation period, the cells were collected, centrifuged and fixed with ice cold ethanol (70%). The cells were then treated with buffer containing RNAse A and 0.1% Triton X-100 and then stained with the propidium iodide (PI). The samples were analyzed on Accuri C6 flow cytometer (Becton Dickinson). [32].
4. Conclusions
In summary, a new series of 1-aryl-3-{4-[(pyridin-2-ylmethyl)thio]phenyl}urea derivatives were designed and synthesized based on molecular hybridization strategy. Majority of target compounds showed moderate to good growth inhibition against the tested cancer cells. Particularly, compound 7i exhibited more potent antiproliferative activity than well-known anticancer drug sorafenib against all three cancer cell lines (A549, HCT-116 and PC-3). The preliminary mechanism investigation showed that compound 7i could induce A549 cells to apoptosis, and halted cell cycle progression at the G1 phase. The SARs illustrated that these target compounds in this work might serve as bioactive fragments, and compound 7i could be used as a lead compound for the development of potent cancer chemotherapeutic agents in the drug discovery process.
Supplementary Materials
The following are available online. 1H-NMR, 13C-NMR, ESI-MS and HRMS of the target compounds, respectively.
Author Contributions
C.Z., X.T., J.F., Y.L., N.D. and Z.J. contributed to the synthetic work and the characterization of all target compounds. C.Z. and Z.J. the preparation of the manuscript. C.Z., X.T. and Q.M. performed the biological assays. X.L. and C.H. proposed the studies and contributed to their design, as well as to the writing of the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Science Foundation of China (Grant No. 21342006), the Program for Innovative Research Team of the Ministry of Education of China (Grant No. IRT_14R36), the Natural Science Foundation of Liaoning Province, China (Grant No. 201602695), and the Scientific Research Foundation of Department of Education of Liaoning Province, China (Grant No. L2015517).
Conflicts of Interest
The authors confirm that this article content has no conflict of interest.
Footnotes
Sample Availability: Samples of all the target compounds are available from the authors.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Keefe D.M., Bateman E.H. Tumor control versus adverse events with targeted anticancer therapies. Nat. Rev. Clin. Oncol. 2011;9:98–109. doi: 10.1038/nrclinonc.2011.192. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo Y., Park J.H., Miyamoto T., Yamamoto S., Hisada S., Alachkar H., Nakamura Y. TOPK inhibitor induces complete tumor regression in xenograft models of human cancer through inhibition of cytokinesis. Sci. Transl. Med. 2014;6:259. doi: 10.1126/scitranslmed.3010277. [DOI] [PubMed] [Google Scholar]
- 4.Zeng X.Y., Lin L., Zheng M.Z., Sun H.M., Xiao J.J., Lu T., Huang G.Q., Chen P.P., Zhang J.M., Zhu F., et al. Pantoprazole, an FDA-approved proton-pump inhibitor, suppresses colorectal cancer growth by targeting T-cell-originated protein kinase. Oncotarget. 2016;7:22460–22473. doi: 10.18632/oncotarget.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng M.Z., Luan S.S., Gao S.Y., Cheng L., Hao B., Li J.C., Chen Y., Hou X.M., Chen L.X., Li H. Proton pump inhibitor ilaprazole suppresses cancer growth by targeting T-cell-originated protein kinase. Oncotarget. 2017;8:39143–39153. doi: 10.18632/oncotarget.16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abe Y., Matsumoto S., Kito K., Ueda N. Cloning and Expression of a Novel MAPKK-like Protein Kinase, Lymphokine-activated Killer T-cell-originated Protein Kinase, Specifically Expressed in the Testis and Activated Lymphoid Cells. J. Biol. Chem. 2000;275:21525–21531. doi: 10.1074/jbc.M909629199. [DOI] [PubMed] [Google Scholar]
- 7.Park J.H., Lin M.L., Nishidate T., Nakamura Y., Katagiri T. PDZ-binding kinase/T-LAK cell-originated protein kinase, a putative cancer/testis antigen with anoncogenic activity in breast cancer. Cancer Res. 2006;66:9186–9195. doi: 10.1158/0008-5472.CAN-06-1601. [DOI] [PubMed] [Google Scholar]
- 8.Simons-Evelyn M., Bailey-Dell K., Toretsky J.A., Ross D.D., Fenton R., Kalvakolanu D., Rapoport A.P. PBK/TOPK is a novel mitotic kinase which is upregulated in Burkitt’s lymphoma and other highly proliferative malignant cells. Blood Cells, Mol., Dis. 2001;27:825–829. doi: 10.1006/bcmd.2001.0452. [DOI] [PubMed] [Google Scholar]
- 9.Nandi A., Tidwell M., Karp J., Rapoport A.P. Protein expression of PDZ-binding kinase is up-regulated in hematologic malignancies and strongly downregulated during terminal differentiation of HL-60 leukemic cells. Blood Cells Mol. Dis. 2004;32:240–245. doi: 10.1016/j.bcmd.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Zykova T.A., Zhu F., Lu C., Higgins L., Tatsumi Y., Abe Y., Bode A.M., Dong Z. Lymphokine-activated killer T-cell-originated protein kinase phosphorylation of histone H2AX prevents arsenite-induced apoptosis in RPMI7951 melanoma cells. Clin. Cancer Res. 2006;12:6884–6893. doi: 10.1158/1078-0432.CCR-06-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei D.C., Yeh Y.C., Hung J.J., Chou T.Y., Wu Y.C., Lu P.J., Cheng H.C., Hsu Y.L., Kuo Y.L., Chen K.Y., et al. Overexpression of T-LAK cell-originated protein kinase predicts poor prognosis in patients with stage I lung adenocarcinoma. Cancer Sci. 2012;103:731–738. doi: 10.1111/j.1349-7006.2011.02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shih M.C., Chen J.Y., Wu Y.C., Jan Y.H., Yang B.M., Lu P.J., Cheng H.C., Huang M.S., Yang C.J., Hsiao M., et al. TOPK/PBK promotes cell migration via modulation of the PI3K/PTEN/AKT pathway and is associated with poor prognosis in lung cancer. Oncogene. 2012;31:2389–2400. doi: 10.1038/onc.2011.419. [DOI] [PubMed] [Google Scholar]
- 13.Luo Q., Lei B., Liu S., Chen Y., Sheng W., Lin P., Li W., Zhu H., Shen H. Expression of PBK/TOPK in cervical cancer and cervical intraepithelial neoplasia. Int. J. Clin. Exp. Pathol. 2014;7:8059–8064. [PMC free article] [PubMed] [Google Scholar]
- 14.Dou X., Wei J., Sun A., Shao G., Childress C., Yang W., Lin Q. PBK/TOPK mediates geranylgeranylation signaling for breast cancer cell proliferation. Cancer Cell Int. 2015;15:27. doi: 10.1186/s12935-015-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiao J.X., Wang T.C., Ruel R., Thibeault C., L’Heureux A., Schumacher W.A., Spronk S.A., Hiebert S., Bouthillier G., Lloyd J., et al. Conformationally constrained orthoanilino diaryl ureas, discovery of 1-(2-(1′-neopentylspiro[indoline-3,4′-piperidine]-1-yl)phenyl)-3-(4-(trifluoro-methoxy) phenyl)urea, a potent, selective, and bioavailable P2Y1 antagonist. J. Med. Chem. 2013;56:9275–9295. doi: 10.1021/jm4013906. [DOI] [PubMed] [Google Scholar]
- 16.Anderson J.W., Sarantakis D., Terpinski J., Kumar T.R., Tsai H.C., Kuo M., Ager A.L., Jacobs W.R., Jr., Schiehser G.A., Ekins S., et al. Novel diaryl ureas with efficacy in a mouse model of malaria. Bioorg. Med. Chem. Lett. 2013;23:1022–1025. doi: 10.1016/j.bmcl.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keche A.P., Hatnapure G.D., Tale R.H., Rodge A.H., Kamble V.M. Synthesis, anti-inflammatory and antimicrobial evaluation of novel 1-acetyl-3,5-diaryl-4,5-dihydro (1H) pyrazole derivatives bearing urea, thiourea and sulfonamide moieties. Bioorg. Med. Chem. Lett. 2012;22:6611–6615. doi: 10.1016/j.bmcl.2012.08.118. [DOI] [PubMed] [Google Scholar]
- 18.Keche A.P., Hatnapure G.D., Tale R.H., Rodge A.H., Birajdar S.S., Kamble V.M. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties, synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg. Med. Chem. Lett. 2012;22:3445–3448. doi: 10.1016/j.bmcl.2012.03.092. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni R.G., Laufer S., Mangannavar C., Garlapati A. Design, synthesis and characterization of N’, N”-diaryl ureas as p38 kinase inhibitors. Med. Chem. 2013;9:213–221. doi: 10.2174/1573406411309020006. [DOI] [PubMed] [Google Scholar]
- 20.Xuan W., Ding W., Hui H.X. Synthesis and cytotoxic activity of diaryl urea derivatives with a 4-methylpiperazinylcarbonyl moiety. Med. Chem. Res. 2013;22:3857–3862. doi: 10.1007/s00044-012-0398-y. [DOI] [Google Scholar]
- 21.Lu C., Tang K., Li Y., Li P., Lin Z., Yin D., Chen X., Huang H. Design, synthesis and evaluation of novel diaryl urea derivative as potent antitumor agents. Eur. J. Med. Chem. 2014;77:351–360. doi: 10.1016/j.ejmech.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Kim H.J., Cho H.J., Kim H., El-Gamal M.I., Oh C.H., Lee S.H., Sim T., Hah J.M., Yoo K.H. New diarylureas and diarylamides possessing acet(benz)amidophenyl scaffold, design, synthesis, and antiproliferative activity against melanoma cell line. Bioorg. Med. Chem. Lett. 2012;22:3269–3273. doi: 10.1016/j.bmcl.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Claudio V.J., Amanda D., Vanderlan da Silva B., Eliezer J.B., Carlos alberto manssour F. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007;14:1829–1852. doi: 10.2174/092986707781058805. [DOI] [PubMed] [Google Scholar]
- 24.Seto M., Miyamoto N., Aikawa K., Aramaki Y., Kanzaki N., Iizawa Y., Babab M., Shiraishia M. Orally active CCR5 antagonists as anti-HIV-1 agents. Part 3: Synthesis and biological activities of 1-benzazepine derivatives containing a sulfoxide moiety. Bioorg. Med. Chem. 2005;13:363–386. doi: 10.1016/j.bmc.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen T., Yang T.M., Go M.L. Functionalized acridin-9-yl phenylamines protected neuronal HT22 cells from glutamate-induced cell death by reducing intracellular levels of free radical species. Bioorg. Med. Chem. Lett. 2014;24:1830–1838. doi: 10.1016/j.bmcl.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Liu D.Z., Tian Z., Yan Z.H., Wu L.X., Ma Y., Wang Q., Liu W., Zhou H.G., Yang C. Design, synthesis and evaluation of 1,2-benzisothiazol-3-one derivatives as potent caspase-3 inhibitors. Bioorg. Med. Chem. 2013;21:2960–2967. doi: 10.1016/j.bmc.2013.03.075. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immun. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Liu C.M., Zhang M., Zhang Z.H., Zhang S.B., Yang S.M., Zhang A., Yin L.J., Swarts S., Vidyasagar S., Zhang L.R., et al. Synthesis and anticancer potential of novel xanthone derivatives with 3,6-substituted chains. Bioorg. Med. Chem. 2016;24:4263–4271. doi: 10.1016/j.bmc.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L., Deng X.S., Zhang C., Meng G.P., Wu J.F., Li X.S., Zhao Q.C., Hu C. Design, synthesis and cytotoxic evaluation of a novel series of benzo[d]thiazole-2-carboxamide derivatives as potential EGFR inhibitors. Med. Chem. Res. 2017;26:2180–2189. doi: 10.1007/s00044-017-1925-7. [DOI] [Google Scholar]
- 30.Ke J., Lu Q., Wang X., Sun R., Jin Z., Zhan X., Hu J., Wan D.C., Hu C. Discovery of 4,5-Dihydro-1H-thieno[2 ′,3′:2,3]thiepino [4,5-c]pyrazole-3-carboxamide Derivatives as the Potential Epidermal Growth Factor Receptors for Tyrosine Kinase Inhibitors. Molecules. 2018;23:1980. doi: 10.3390/molecules23081980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Q., Li R.X., Xin A.Y., Han Y., Zhang Y.X., Liu J.X., Li W.G., Di D.L. Design, synthesis, and anticancer properties of isocorydine derivatives. Bioorg. Med. Chem. 2017;25:6542–6553. doi: 10.1016/j.bmc.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y.M., Jing D.W., Chen R., Rashid U.H., Jiang J., Liu X., Wang L.S., Xie P. Design, synthesis and evaluation of novel sophoridinic imine derivatives containing conjugated planar structure as potent anticancer agents. Bioorg. Med. Chem. 2018;26:4136–4144. doi: 10.1016/j.bmc.2018.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.