Abstract
Background
Positron emission tomography studies examining differences in D1-dopamine receptor binding between control subjects and patients with schizophrenia have been inconsistent, reporting higher, lower, and no difference in the frontal cortex. Exposure to antipsychotic medication has been suggested to be a likely source of this heterogeneity, and thus there is a need for studies of patients at early stages of the disorder who have not been exposed to such drugs.
Methods
Here, we compared 17 healthy control subjects and 18 first-episode neuroleptic naive patients with schizophrenia or schizophreniform psychosis using positron emission tomography and the D1-dopamine receptor radioligand [11C]SCH23390.
Results
We observed a statistically significant difference in the dorsolateral prefrontal cortex. Contrary to our expectations, patients had less D1-dopamine receptor availability with a moderate effect size. In a Bayesian analysis, we show that the data are over 50 times more likely to have occurred under the decrease as opposed to the increase hypothesis. This effect was not global, as our analysis showed that the null hypothesis was preferred over either hypothesis in the striatum.
Conclusions
This investigation represents the largest single sample of neuroleptic-naive patients examined for D1-dopamine receptor availability using PET and suggests a reduction of prefrontal D1-dopamine receptor density in the pathophysiology of schizophrenia. However, further work will be required to reach a consensus.
Keywords: schizophrenia, drug naïve, positron emission tomography, D1 dopamine receptor, dorsolateral prefrontal cortex
Significance Statement.
The present manuscript focuses on frontal D1-dopamine receptor (D1R) binding in patients with schizophrenia, an area of particular interest due to D1R linked to working memory impairment in schizophrenia and the discrepant literature on frontal D1R in schizophrenia.
The study represents the largest single sample of neuroleptic-naive patients (n = 18) examined for D1R availability using PET. Contrary to our hypothesis, the patients had significantly lower BPND values in the dorsolateral prefrontal cortex. This could have implications for interpreting previous clinical findings in studies of schizophrenia. The hypofrontality hypothesis of schizophrenia that was coined in the 1970s is based on reduced frontal blood flow and linked to negative symptoms and cognitive deficits. Moreover, it has been suggested that D1R agonists may have beneficial effects in schizophrenia. Indeed, recent studies with D1R agonists demonstrate reversal of antipsychotic-induced working memory deficits in nonhuman primates as well as improved working memory in humans.
Introduction
The dopamine system has been of central interest in the pathophysiology of schizophrenia for more than 50 years. Indeed, molecular imaging studies using positron emission tomography (PET) have provided a great deal of evidence for elevations in both presynaptic dopamine synthesis capacity and amphetamine-induced dopamine release in schizophrenia patients compared with controls (Howes and Kapur, 2009). Regarding dopamine receptor subtypes, the striatal D2-dopamine receptor (D2R) availability has been examined in numerous studies, providing some evidence for a small increase in patients compared with controls (Howes et al., 2012). In contrast, only a few PET studies have examined the D1-dopamine receptor (D1R) in schizophrenia. Compared with the D2R, there is a much higher concentration of D1R in the cortex (Hall et al., 1994), and the frontal cortex in particular is thought to be a crucial brain region for understanding the biological basis for schizophrenia (Selemon and Goldman-Rakic, 1999; Callicott et al., 2000; Wagstyl et al., 2016).
However, in vivo studies of the D1R in patients with schizophrenia have yielded mixed results (Cervenka, 2019). Initial studies using the radioligands [11C]SCH23390 or [11C]NNC112 found lower (Okubo et al., 1997), higher (Abi-Dargham et al., 2002), or no difference (Karlsson et al., 2002) in the availability of D1R in the frontal cortex compared with healthy control subjects. The former 2 research groups have both replicated their own respective results in chronic medicated patients (Kosaka et al., 2010) and in a subsample of drug-naive patients (Abi-Dargham et al., 2012), respectively. In another small sample of twin pairs discordant for schizophrenia, Hirvonen et al. (Hirvonen et al., 2006) reported lower D1R binding in chronic, medicated schizophrenia probands compared with controls. In contrast, higher levels were shown in monozygotic unaffected co-twins, that is, individuals at high genetic risk.
Importantly, in studies where both neuroleptic naive and either medicated or drug-free patients were examined, the latter group has consistently exhibited numerically lower D1R binding compared with the former (Okubo et al., 1997; Abi-Dargham et al., 2002, 2012; Poels et al., 2013; Cervenka, 2019). This may be explained by a reduction in D1R due to antipsychotic treatment as has been shown in experimental studies of nonhuman primates (NHPs) (Lidow and Goldman-Rakic, 1994; Lidow et al., 1997) although not confirmed in a human postmortem study Knable et al. (Knable et al., 1996). Moreover, in the case of ongoing medication (Hirvonen et al., 2006; Kosaka et al., 2010), caution must be exercised since a direct D1R occupancy has been shown for some antipsychotic drugs (Farde et al., 1992). To avoid this confounding factor, research to understand the role of the D1R in schizophrenia needs to focus on the early stages of the illness, that is, before antipsychotic treatment.
Though the PET studies of the D1R in schizophrenia patients have been conducted with small sample sizes, and therefore with low statistical power, a tentative interpretation of the results is that drug-naive patients with psychosis disorders, and potentially also unmedicated individuals at high genetic risk for schizophrenia, show higher D1R binding in frontal cortex (Cervenka, 2019).
To test this hypothesis, we used PET and the radioligand [11C]SCH23390 to examine 18 neuroleptic-naive, first-episode psychosis patients and 18 healthy controls and compared the availability of the D1R between the groups in the dorsolateral prefrontal cortex and striatum
Materials and Methods
Subjects
The study was conducted in compliance with the ethical principles originating in the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonization. The study was approved by the Ethics Committee and the Radiation Safety Committee at the Karolinska University Hospital (Stockholm, Sweden).
Eighteen patients, aged 19 to 51 years, and 18 healthy control subjects, aged 22 to 52 years, were enrolled after written informed consent at the Center for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet, and Stockholm County Council, Stockholm, Sweden. None of these patients were included in our previous study of D1R in first-episode psychosis (Karlsson et al., 2002).
Exclusion criteria for the healthy volunteers and the patients were nonpsychiatric brain disorder, other somatic disorder, history of head injury with loss of consciousness for more than 5 minutes, cranial fracture, history or presence of epilepsy, previous treatment with antipsychotic drugs, clinically significant abnormal laboratory test results, pregnancy, and history of alcohol or drug abuse according to DSM-III-R criteria or frequent nicotine use and brain injury. Further exclusion criteria for the healthy volunteers were history or presence of any psychiatric disorder and history of a psychiatric disorder in a first-degree relative.
All patients were recruited by P.S. from the psychiatry clinic at the Karolinska University Hospital, admitted for the first time to psychiatric services, and diagnosed with schizophrenia or schizophreniform psychosis according to DSM-III-R (Table 1). The recruitment phase lasted from 1994 to 2007. In addition, each patient was followed prospectively regarding diagnosis for 1 year after inclusion in the study.
Table 1.
Demographic Characteristics and BPRS Scores for 18 Patients With Schizophrenia or Schizoaffective Disorder
| Patient no | Age (y) | Sex | BPRS total score | BPRS positive symptoms score | BPRS negative symptoms score | Diagnosis at 1-year follow-up |
|---|---|---|---|---|---|---|
| 1 | 28 | F | 48 | 18 | 9 | Schizophrenia |
| 2 | 21 | M | 46 | 15 | 15 | Schizophrenia |
| 3 | 35 | F | 52 | 17 | 7 | Schizoaffective disorder |
| 4 | 19 | F | 37 | 15 | 10 | Schizophrenia |
| 5 | 22 | M | 26 | 11 | 4 | Schizophrenia |
| 6 | 32 | M | 50 | 19 | 12 | Schizoaffective disorder |
| 7 | 22 | M | 41 | 15 | 11 | Schizophrenia |
| 8 | 39 | M | 60 | 24 | 9 | Schizophrenia |
| 9 | 35 | M | 59 | 24 | 9 | Schizophrenia |
| 10 | 33 | F | 36 | 14 | 9 | Schizophrenia |
| 11 | 32 | M | 33 | 16 | 3 | Schizophrenia |
| 12 | 41 | M | 27 | 11 | 5 | Schizophrenia |
| 13 | 41 | M | 31 | 14 | 5 | Schizophrenia |
| 14 | 36 | F | 33 | 18 | 3 | Schizophrenia |
| 15 | 21 | M | 41 | 16 | 10 | Schizophrenia |
| 16 | 49 | M | 41 | 14 | 13 | Schizophrenia |
| 17 | 51 | F | 58 | 24 | 15 | Schizophrenia |
| 18 | 22 | F | 35 | 7 | 3 | Schizophrenia |
Clinical Ratings
Patients’ clinical symptoms were rated by using the 18-item Brief Psychiatric Rating Scale (BPRS) (each item rated on a 0–6 scale) (Overall and Gorham, 1962; Kolakowska, 1976). The overall total rating and scores on positive and negative symptom clusters were used (Bech et al., 1986). The positive symptom cluster consists of conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content (BPRS items 4, 11, 12, and 15). The negative symptom cluster consists of emotional withdrawal, motor retardation, and blunted affect (BPRS items 3, 13 and 16).
General Comments on MRI and PET Imaging
Due to the 14 years between examination of the first and the last subject, there were technical changes along the way in some of the experimental procedures. The changes comprised the use of different MRI protocols, the use of Neuroinsert (a PET gantry device in lead shielding radiation originating from the trunk), the PET acquisition time length, reconstruction parameters, and file formats. The experimental procedures and settings are for each individual listed in Supplementary Materials 2. As described below, the differences in experimental procedures were, when so required, included as confounders in the statistical analysis.
MRI Examination
All subjects underwent a T2-weighted MRI measurement to rule out any brain abnormality. In the beginning of data collection, only the T2 sequence was performed (n = 16) on a 1.5-T Signa unit (General Electric, Milwaukee, WI). A standard spin-echo sequence with a 256 × 256 matrix was used with a repetition time of 4 seconds. Echo times 85 msec with a total scanning time of about 10 minutes. The long echo time was to enhance the grey and white matter segmentation to allow the delineation of regions of interest (ROIs). The rationale for having only one MRI sequence was to reduce the risk of noncompliance with a longer examination including several sequences. In addition, the primary purpose of the MRI was a clinical examination to rule out any pathology.
Later in the data collection, software and coil upgrade with improved MR sequences allowed for shorter scanning time, and the remaining subjects (n = 19) had, in addition to the T2-weighted protocol, a T1-weighted sequence for improved grey and white matter segmentation with a total scanning time of about 10 minutes. This T2 protocol was based on the following sequence: repetition time/echo time = 6060/92.6 milliseconds, field of view 260 mm, image matrix 256 × 256, thickness/spacing 3/0,1 mm, flip angle 150°, slice thickness = 5 mm. The T1 protocol was based on a 3-dimensional axial Spoiled Gradient Recalled Acquisition with the following sequence: repetition time/echo time = 20/5 milliseconds, field of view 260 mm, image matrix 256 × 256, thickness/spacing 1/0 mm, flip angle 35°.
PET Examination
For each subject, PET examinations were performed on the Siemens ECAT EXACT HR PET system. Radioactivity in brain was measured with 2D data acquisition, except for 2 subjects who had 3D data acquisition. The spatial resolution in the reconstructed sections is 3.8 mm at the center of the field of view (Wienhard et al., 1994). A transmission scan was performed using 3 rotating 68Ge rod sources for about 5 minutes.
To minimize head movement during the PET measurement, a plaster helmet was made for each subject individually and used during the PET measurement (Bergstrom et al., 1981). At the start of the PET measurement, a sterile phosphate buffer (pH = 7.4) containing [11C]SCH23390 was injected as a bolus during several seconds into the cubital vein. The venous catheter was then immediately flushed with up to 10-mL saline solution.
[11C]SCH23390 was prepared as previously described (Halldin et al., 1986). The injected radioactivity was 317 ± 22 MBq (mean, SD). The molar activity was not analyzed for 2 of the healthy controls and 1 of the patients due to the small amount of product left in the vial after injection. The molar radioactivity for the remaining 32 subjects was 104 ± 133 MBq/nmol, which corresponded to an injected mass of 3.6 ± 5.7 μg (range 0.17–32 μg, median 2.62 μg). All subjects received 8 μg or less except for one patient who received 32 μg. This was due to delay of injection with subsequent decrease in molar radioactivity. Estimation of D1R occupancy by 32 μg SCH23390 based on published data is 6.0%, that is, the binding potential (BPND) was underestimated by 6%, which was corrected for in the statistical analysis by dividing this subject’s subjects BPND value by 0.94 (Farde, 1992; Fischer et al., 2010). The radioactivity and mass did not differ significantly between the patients and controls.
The PET protocol for each individual is listed in supplementary Materials 2. Following injection, emission data were collected in a sequence of time frames. The time frames of acquisition data were reconstructed and corrected for attenuation and scatter using 2D filtered-back projection into a series of 3D PET images of radioactivity concentration. The voxel size for the reconstructed volume was 2.030 × 2.030 × 3.125 mm.
Image Processing and Quantification
Despite the collection of data being conducted over several years, all image processing and quantification of the data were performed at the same time during 2018 using current analysis software. Three-dimensional PET images were for each time frame corrected for head motion using a postreconstruction frame-by-frame realignment algorithm, in which the dynamic PET image was first divided into blocks of frames of a minute or longer, that is, frames of less than a minute were summed together. Then all images were individually aligned to the first minute of acquisition using the SPM5 (Wellcome Department of Cognitive Neurology, University College London) (Friston et al., 1995). Integral PET images were created using ecatsum (v 1.4.3, Turku PET Centre). Finally, MR images were reoriented into the AC-PC plane and coregistered to integral PET images using SPM5.
Kinetic modelling was performed using the R package kinfitr (v 0.2.0) (https://github.com/mathesong/kinfitr). Regional BPND values were calculated using the simplified reference tissue model with the cerebellar grey matter as reference region (Lammertsma and Hume, 1996).
ROI Delineation
The MR images were used to delineate anatomical ROIs for the striatum (STR) (caudate and putamen), the dorsolateral prefrontal cortex (DLPFC) and the cerebellum (CBL). The STR and DLPFC were chosen since they are regions of central interest in schizophrenia research. For the DLPFC, several convergent findings relevant for schizophrenia and D1R transmission have been reported (Arnsten et al., 2017). In addition, we performed an exploratory analysis of additional cortical regions—anterior cingulate cortex (ACC), temporal cortex (TC), medial prefrontal cortex (MPFC), and orbito frontal cortex (OFC)—based on previous studies investigating D1-R in psychosis (Okubo et al., 1997; Abi-Dargham et al., 2002, 2012; Kosaka et al., 2010). The CBL was chosen as reference region for the concentration of free and nonspecifically bound [11C]SCH23390. Grey matter, white matter, and CSF were segmented using the SPM5 Unified Segmetation routine (Ashburner and Friston, 2005) on both T1 and T2 MR images. These maps were used to mask ROIs.
For the manual method, an in-house software, HBA (Roland et al., 1994), was used where the MR images were loaded into a 3-D volume for delineation of the ROIs on 1 of the 3 orthogonal projections with 1-mm slice thickness. The manual segmentation was performed by one investigator blinded to patient status (P.S.) who has more than 20 years of experience in manual ROI delineation. The caudate and putamen were delineated as described by (Mawlawi et al., 2001) with the modification that the sagittal planes were used instead of the coronal. The ventral striatum was not included. The DLPFC, MPFC, and OFC were traced on 20 coronal planes anterior to the genu of the corpus callosum. The anterior cingulate cortex and temporal cortex were traced on sagittal planes in their entirety. The cortical regions were masked by the GM map for gray-white matter segmentation. The cerebellum was drawn on the central 6 transaxial planes of the cerebellum and about 1 cm distant from the subarachnoidal space. The ROIs were translated into the respective PET image space using the inter-modality coregistration matrices.
Statistical Analysis
The aim was to compare BPND between healthy controls and first-episode psychosis patients. Inference was performed using both frequentist methods (i.e., P values) as well as Bayesian hypothesis testing methods. For both analyses, we included age in the regression model, as an age effect on [11C]SCH23390 BPND has consistently been reported (Suhara et al., 1991; Wang et al., 1998; Jucaite et al., 2010; Backman et al., 2011; de Boer et al., 2017).
For the frequentist models, age was entered as a covariate in the multiple linear regression models. For the Bayesian models, we defined informative priors for the relationship between age and [11C]SCH23390 BPND based on a weighted average of the results of previous studies examining this association. This procedure functions to essentially constrain the model to likely values of the parameter estimating the effect of age on [11C]SCH23390 BPND based on previous reports (Suhara et al., 1991; Wang et al., 1998; Jucaite et al., 2010; Backman et al., 2011; de Boer et al., 2017). This procedure limits the influence of uncertainty in the estimation of the association between age and binding estimated in this particular dataset, which might otherwise affect estimates of the effect of psychosis on binding estimates. This prior was parameterized as follows: for the DLPFC, mean = −1.56% per year, SD = 0.66%; for the STR, mean = −0.76% per year, SD = 0.04%.
For the assessment of the influence of patient status, we tailored our statistical models based on the mixed results in previous clinical PET studies assessing differences in D1R availability between schizophrenia patients and controls (Okubo et al., 1997; Abi-Dargham et al., 2002, 2012; Karlsson et al., 2002; Hirvonen et al., 2006; Kosaka et al., 2010). For the frequentist analysis, we made use of a 2-sided test, due to the mixed results, using an alpha of 0.05. For the Bayesian analysis, we made use of Bayesian hypothesis testing (i.e., Bayes Factors, BFs) to compare the relative plausibility of separate hypotheses: of higher and lower [11C]SCH23390 BPND, respectively, in patients compared wiith controls.
For the differences between patients and controls, we defined 1-sided half-normal distributions for the hypotheses of increased and decreased BPND in patients compared with controls, respectively, from previous studies using [11C]SCH23390 BPND (Hirvonen et al., 2006; Kosaka et al., 2010; Poels et al., 2013). Previous studies making use of [11C]NNC112 were not included, as the percentage change may not be directly applicable to [11C]SCH23390 BPND for several reasons. First, [11C]NNC112 has a significantly higher affinity for the D1R (Halldin et al., 1998). Second, for both radioligands 5-HT2A receptor binding contributes to a nonnegligible fraction of cortical binding, and differences in this fraction might cause systematic differences (Ekelund et al., 2007). Although this fraction has been shown to be similar in magnitude for both tracers, for [11C]SCH23390 this estimate was derived from a PET examination of only 2 baboons and not from humans (Ekelund et al., 2007). Lastly, in studies using [11C]NNC112, the main outcome parameter has been BPP, with BPND calculated in all cases but one (Kosaka et al., 2010) using arterial plasma measurements (Abi-Dargham et al., 2002; Abi-Dargham et al., 2012). To assure that these hypotheses were not differentially influenced by different sizes of previously reported effects, we opted to use the same scale for both the increase and decrease prior based on a weighted average of previous differences. We therefore used a SD of 31% for DLPFC and of 21% for the STR. More details regarding selection of priors are provided in Supplementary Materials 1.
We also tested whether other factors differing between measurements might have a substantial impact on the results and thereby act as a confounder. These factors relate to PET acquisition (2D or 3D PET acquisition, presence of absence of the Neuroinsert, measurement length, date of measurement, i.e., drift over time), PET reconstruction (R-Z filter resolution, R-Z filter cutoff), ROI delineation (unconscious biases during delineation, anatomical MR image modality - T1w vs T2w), data conversions (ecat6 or ecat7 as the original file format), and participant factors (sex and movement). For the assessment of whether BPND was affected by participant sex, we also made use of the outcome data from de Boer et al (de Boer et al., 2017), with gender provided through personal communication, as well as the raw data utilized in Bäckman et al. (Backman et al., 2011) but with image analysis and modelling performed in a manner identical to the present study.
To assess whether these factors impacted our outcomes, we tested whether a large specific effect was absent for each factor. Such conclusions cannot be drawn from an insignificant P value assessing the differences between groups but rather requires a test of groups’ similarity. For this reason, we made use of equivalence testing (Schuirmann, 1987; Lakens, 2017). This allows for testing of the equivalence of outcomes, rather than the difference, and requires the description of appropriate equivalence bounds within which the outcomes are assumed to be sufficiently similar. We performed a power analysis for the equivalence bound for 2-sample equivalence tests with a type I error rate of 0.05, a power of 0.8, and samples of 18 in each group. According to this analysis, there was sufficient power to assess equivalence within bounds of −1 < Cohen’s D < 1. Since Cohen’s D = 0.8 represents a large effect size (Cohen, 1988), we can thereby rule out large effects. While it would have been desirable to assess equivalence within more restrictive equivalence bounds, this approach is nonetheless superior to the statistically invalid approach of accepting (as opposed to failing to reject) the null hypothesis based on a nonsignificant P value in a test assessing differences.
Transparency Statement
We present the following transparency statement suggested by Simmons et al. (Simmons et al., 2012). All requirements are presented below.
We report how we determined our sample size, all data exclusions (if any), all manipulations, and all measures in the study.
Sample Size Determination
The final study sample size was determined by the number of participants included in the study when it was decided that data collection was to be concluded in 2008 and consisted of 18 patients and 18 healthy controls. No power analysis was performed before or during the study; however, a power analysis was performed prior to statistical analysis. For a 2 independent sample t test, with an alpha of 0.05, this study had 80% power to detect an effect size of Cohen’s D = 0.96. This corresponds to a Cohen’s U3 of 83% and a common language effect size of 75%. This effect is larger than we would expect, and we determined a priori that if significant, this result could likely represent a Type M error (Gelman and Carlin, 2014), and if insignificant, this result could likely represent a Type II error.
Exclusions
One PET measurement from a control subject was excluded from the analysis since it was only stored on an optical disc whose content could not be accessed. All other measurements were included in the analysis.
Measures and Analyses
The authors confirm that all ROI delineation was performed blind to the patient or control status of the subjects and that no regions other than the whole STR and the DLPFC were analyzed. All confounder checks were analyzed without testing whether they influenced the final outcome, and patient-control status was not included in any analysis other than that of ROI delineation bias, where it was the independent variable.
For transparency, we note that an exploratory SPM analysis was run on a subset of subjects during the data collection phase for which there were no significant differences between the groups using conventional statistical thresholds. Hence, there were no significant findings guiding the ROI analysis. However, due to the differences in MR modality, the fact that this analysis was not corrected for different lengths of measurement as well as the poor reliability of voxelwise estimates of cortical [11C]SCH23390 BPND (Matheson et al., 2017), we do not consider these results to be valid, and this is reported solely for the purpose of transparency. In the current analysis, we restricted the a priori ROIs to the DLPFC and STR, as we have in previous studies (REF here: https://www.biorxiv.org/content/10.1101/321646v2) to reduce the potential influence of multiple comparisons to test the D1R hypothesis.
Data and Code Availability
All analysis code is available at https://github.com/mathesong/D1DNPsychosis. Due to institutional restrictions, the data cannot be shared openly within this repository. These data are pseudonymized according to national (Swedish) and EU legislation and cannot be anonymized and published in an open repository. Metadata can be openly published, and the underlying data can instead be made available upon request on a case by case basis as allowed by the legislation and ethical permits. Requests for access can be made to the Karolinska Institutet’s Research Data Office at rdo@ki.se.
Results
Demographics and Sample Characteristics
At inclusion, all patients satisfied DSM-III-R criteria for schizophreniform disorder. After 1-year follow-up, 16 patients satisfied DSM-III-R criteria for schizophrenia and 2 patients for schizoaffective disorder (Table 1). All 18 healthy controls and 15 of the patients completed the PET examination for at least 51 minutes. Two of the patients were taken out of the PET system after 33 minutes and 1 patient after 39 minutes due to anxiety. One PET measurement from a control subject was excluded from the analysis since it was only stored on an optical disc whose content could no longer be accessed.
At clinical evaluation of the T2-weighted MRI images by a radiologist, 1 patient (number 14) had a relatively large right lateral ventricle. There were no other signs of a nonpsychiatric brain disorder in this individual, and therefore this finding was not considered to be a reason for exclusion. No brain abnormalities were reported for any other subjects.
Confounder Analysis
We performed a detailed analysis of all potential confounders as described in Supplementary Materials 2. BPND was negatively associated with age, corresponding to previous studies (Supplementary Materials 2; Figure 1), and we therefore opted to include age in the regression models.
The lengths of the PET measurements were 33 minutes (n = 2), 39 minutes (n = 1), 51 minutes (n = 23), and 63 minutes (n = 9). We found that [11C]SCH23390 BPND was not time stable and that longer measurements were associated with lower BPND values. We further observed that longer PET measurements were associated with lower variability, suggesting that measurement error is likely lower for longer measurements. We therefore corrected measurements to their 51-minute equivalent BPND values by removing later frames for the longer measurements and by multiplying BPND values by calibration factors calculated using the remainder of the sample for the shorter measurements. A detailed description is provided in Supplementary Materials 3.
Frequentist Analysis
In the frequentist analysis, we observed a statistically significant negative association between [11C]SCH23390 BPND and age in both the DLPFC (t = −4.66, P < .001) and STR (t = −3.90, P < .001).
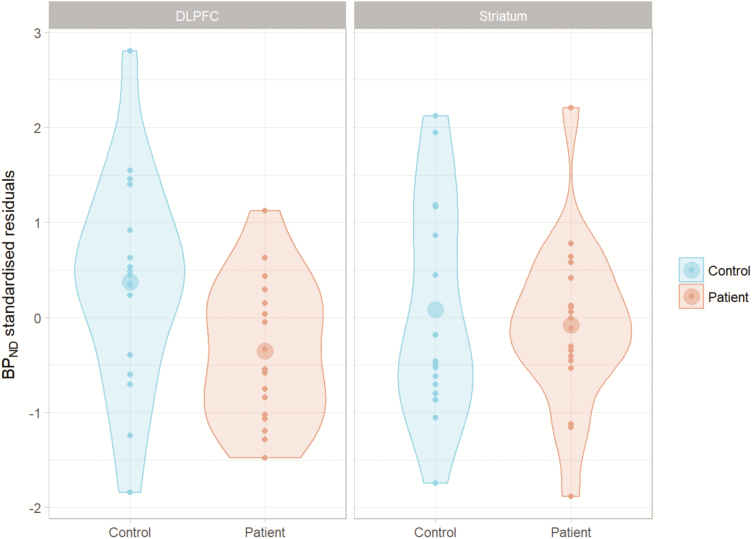
In the main analysis, psychosis patients had a 12.5% lower [11C]SCH23390 BPND in DLPFC compared with controls relative to the mean control BPND value (t = −2.30, P = .028; Figure 1). This difference corresponds with a moderate effect size (Cohen, 1988), although the 95% confidence interval (CI) for the effect size ranged between very small and very large (Hedges’ g = −0.579, 95% CI [−1.112, −0.046]) (Sawilowsky, 2009). For the STR, we failed to reject the null hypothesis of no difference in [11C]SCH23390 BPND between patients and controls (t = −0.47, P = .639, Figure 1), although the CI around the effect size ranged from a large negative effect to a moderate positive effect (Hedges’ g = −0.127, 95% CI [−0.676, 0.421]) (Cohen, 1988). Unstandardized regression coefficients are presented in Supplementary Materials 4.
Figure 1.
Standardized residuals representing the difference between healthy controls and psychosis patients after correction for the effect of age. Significant differences were obtained for the dorsolateral prefrontal cortex (DLPFC).
In the confounder analysis presented in Supplementary Materials 2, we concluded that the 2 patients whose PET measurements were conducted with 3D data acquisition instead of 2D may potentially have biased BPND values and that an additional analysis should be performed with these individuals removed. Removal of these 2 individuals resulted in similar effect sizes for both regions (DLPFC: Hedges’ g = −0.582, 95% CI [−1.132, −0.033]. STR: Hedges’ g = −0.131, 95% CI [−0.697, 0.434]), suggesting that any potential influence of 2D or 3D acquisition on the main results was negligible.
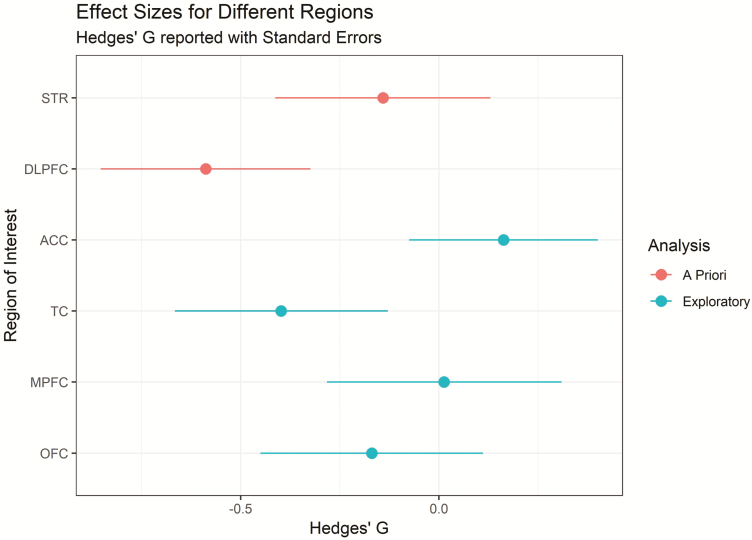
The above analysis was restricted to 2 a priori ROIs to minimize the potential for false positives (Matheson et al., 2017). As requested by reviewers, we have analyzed several other regions in an additional exploratory analysis. The effect sizes for the differences between patients and controls for these regions are presented in Figure 2. The mean and standard deviation of BPND values within each group for each region are presented in Table 2.
Figure 2.
Standardized effect sizes (Hedges’ G) representing the comparison between patients and controls after accounting for age for all a priori and exploratory analysis regions presented with error bars representing standard errors. ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; MPFC, medial prefrontal cortex; OFC, orbito frontal cortex; STR, striatum; TC, temporal cortex.
Table 2.
Group Means and SDs of BPND for All Presented Regions
| Region | Control | Patient | Analysis |
|---|---|---|---|
| STR | 1.61 (0.24) | 1.53 (0.21) | A Priori |
| DLPFC | 0.34 (0.075) | 0.28 (0.061) | A Priori |
| ACC | 0.40 (0.067) | 0.39 (0.089) | Exploratory |
| TC | 0.41 (0.078) | 0.36 (0.063) | Exploratory |
| MPFC | 0.37 (0.076) | 0.36 (0.098) | Exploratory |
| OFC | 0.40 (0.089) | 0.37 (0.090) | Exploratory |
These are the raw BPND values without any correction for age.
Bayesian Analysis
For the Bayesian analysis, we calculated BFs, which represent the relative probability of obtaining data supporting each of the testing hypotheses, instantiated as competing models, relative to one another. For the DLPFC, we observed medium evidence supporting the decrease hypothesis over the null and strong evidence for both the null and decrease hypotheses against the increase hypothesis. For the STR, however, there was moderate evidence supporting the null hypothesis over both the increase and decrease hypotheses. The BFs are shown in Table 3.
Table 3.
Bayes Factors Comparing Each Hypothesis (Rows) Against Each Other Hypothesis (Columns) for the Test of Differences in BPND Between Psychosis Patients and Controls
| Model | Increase | Decrease | Null |
|---|---|---|---|
| DLPFC | |||
| Increase | 1 | 0.02 | 0.07 |
| Decrease | 55.25 | 1 | 3.69 |
| Null | 14.97 | 0.27 | 1 |
| Striatum | |||
| Increase | 1 | 0.41 | 0.12 |
| Decrease | 2.46 | 1 | 0.3 |
| Null | 8.11 | 3.29 | 1 |
Discussion
The objective of the present PET study was to compare central D1R binding between healthy control subjects and neuroleptic naive first-episode patients with schizophrenia or schizophreniform psychosis. We hypothesized higher DLPFC D1R availability in patients compared with controls. In contrast, D1R BPND in the DLPFC was significantly lower in the patients. We conclude that this effect may be regional since it was not observed for the STR, where the data were most consistent with the null hypothesis. The difference between patients and controls in the DLPFC was 12.5%, which can be viewed in relation to the recently demonstrated test-retest repeatability of about 9.5% for this tracer in the DLPFC measured using the same PET system and methodology (Stenkrona et al., 2018). The Supplementary Materials has D1R BPND values for other cortical regions as well. The effect size (Hedges’ g = 0.579) is smaller than that reported in previous studies (Table 4). However, all PET studies of the D1 receptor in schizophrenia to date have been performed with small sample sizes, which do not individually allow for the effect sizes to be estimated with a high degree of precision. Even in this study, with the largest sample of drug-naive patients yet examined, the CI around the effect size spans from very large to negligible. Comparisons of effect size magnitudes between studies are therefore highly speculative.
Table 4.
PET Studies Comparing D1-R BPND Values in Patients With Schizophrenia or Schizophreniform Psychosis to that of Healthy Control Subjects
| Subjects | ||||
|---|---|---|---|---|
| Publication | SCZ (DN) / HC | Radioligand | Statistically significant differences | Hedges’ g Frontal Cortex |
| Okubo et al. 1997 (7) | 17 (10) / 18 | [11C]SCH23390 | PFC ↓ | -1.00 (DN) / -1.39 (DF) |
| Abi-Dargham et al. 2002 (8) | 16 (7) / 16 | [11C]NNC112 | DLPFC ↑ | 0.945 (DN) / 0.812 (DF) |
| Karlsson et al. 2002 (9) | 10 (10) / 10 | [11C]SCH23390 | no difference | 0.299 (DN) |
| Hirvonen et al. 2006 (12) | 9 (0) / 11 | [11C]SCH23390 | CAU, PUT, CX ↓ | -0.922 (M) |
| Kosaka et al. 2010 (10) | 6 (0) / 12 | [11C]SCH23390, [11C]NNC112 | FC, ACC, TC, STR ↓ | (SCH) -2.67 (M) (NNC) -2.80 (M) |
| Abi-Dargham et al. 2012 (11) | 25 (12) / 48 | [11C]NNC112 | DLPFC, MPFC, OFC ↑ in drug naive | 1.03 (DN) / -0.037 (DF) |
| Poels et al. 2013 (13) | 7 (4) / 11 | [11C]SCH23390 | no difference | 1.09 (DN) / 0.154 (DF) |
| Present study | 18 (18) / 17 | [11C]SCH23390 | DLPFC ↓ | -0.579 (DN) |
Abbreviations: ACC, anterior cingulate cortex; CAU, caudate nucleus; CX, cortical regions; DF, drug free; DLPFC, dorso lateral pre frontal cortex; DN, drug naïve; FC, frontal cotex; HC, healthy control subjects; M, medicated; MPFC, medial prefrontal cortex; OFC, orbito frontal cortex; PFC, prefrontal cortex; PUT, putamen; SCZ, patients with schizophrenia or schizophreniform psychosis; STR, striatum; TC, temporal cortex. Effect size Hedges’ g (Hedges,1981) calculated from the BPND values in frontal cortex or DLPFC published in each respective study (n, mean, and SD).
When reviewing the previous literature on D1R in psychosis, a pattern emerges of higher cortical D1R primarily in drug-naive patients and individuals at high risk in the majority of studies (Abi-Dargham et al., 2002, 2012; Hirvonen et al., 2006). In the only previous study finding lower levels in drug-naive patients (Okubo et al., 1997), binding potential was obtained using microparameters from 2TCM with plasma as input function. However, this method has shown low reliability for quantification of [11C]SCH23390 binding (Chan et al., 1998) due at least in part to the rapid metabolism of this tracer (Swahn et al., 1994). In the present largest sample of drug-naive patients hitherto reported in PET studies of D1R in psychosis, our analysis showed that the data were over 50 times more likely to have occurred under the decrease hypothesis model than they were under the increase hypothesis model (Table 2). It should be noted, however, that a meta-analysis of the results of the studies would likely result in no overall significant difference in frontal D1R due to the heterogeneity of the results, as well as the limited number of patients. In total there have only been 67 patients (42 drug naive) examined with [11C]SCH23390 and 47 patients (19 drug naive) examined with [11C]NNC112 (Cervenka, 2019). There is a need for more studies in larger samples of drug-naive patients to increase statistical power.
The lower D1R BPND found in the present sample may be due to either a lower density (Bmax) of D1R or a lower affinity (higher KD) or both. The KD reflects both the affinity and the endogenous dopamine levels. For D1R, ex vivo studies in rodents have shown no effect on [11C]SCH23390 binding by amphetamine-induced dopamine release or dopamine depletion (Thibaut et al., 1996), whereas other studies found a paradoxical decrease of [11C]SCH23390 binding in response to dopamine depletion (Guo et al., 2003). Moreover, PET studies employing amphetamine-induced release and reserpine-induced depletion of dopamine in NHPs have not shown any effect on the D1R Bmax or KD (Chou et al., 1999). Similarly, the D1 radiotracers [11C]NNC756 (Abi-Dargham et al., 1999) and [11C]SKF82957 (Laruelle et al., 1998) have been reported to be insensitive to amphetamine challenge in NHP and a study in humans did not show any effect on [11C]SCH23390 binding after DA depletion after a-methylparatyrosine (Verhoeff et al., 2002). Hence, the present finding of a lower D1R BPND in patients with schizophrenia is most likely due to a lower D1R density.
One caveat when interpreting [11C]SCH23390 binding in human cortical regions is that the values do not only represent D1R. Studies in NHPs have demonstrated a 5HT2A contribution to both [11C]SCH23390 and [11C]NNC112 binding in cortex of approximately one-quarter of the binding (Ekelund et al., 2007), the latter of which has been replicated in human studies (Slifstein et al., 2007). In addition, some studies suggest that 5-HT2A receptor availability may be lower in schizophrenia patients compared with controls (Ngan et al., 2000; Rasmussen et al., 2010, 2016) although other centers have reported no significant differences (Nordstrom et al., 1995; Trichard et al., 1998; Lewis et al., 1999; Okubo et al., 2000; Erritzoe et al., 2008). However, the magnitude of the current difference (12.5%) suggests that, if the present findings were to be entirely accounted for by differences in the availability of 5-HT2A receptors, they would correspond to a 50% reduction, which is clearly larger than the reported values. Hence, the presently observed decrease in DLPFC D1R can likely not be fully explained by a decrease in 5HT2A receptors. Similarly, SCH23390, like all currently available pharmacological agents, does not distinguish between D1R and D5R. However, the distribution of the D5 mRNA is rare and discrete with little overlap with the distribution pattern of the D1 mRNA (Beischlag et al., 1995). Hence, the signal attributable to the D5R in the DLPFC is unlikely to account for the present results.
If a reduction in frontal D1R density in psychosis can be confirmed, this could have implications for interpreting other clinical findings in studies of schizophrenia. Reduced frontal blood flow has been linked to negative symptoms and cognitive deficits (Ingvar and Franzen, 1974; Weinberger and Berman, 1988). Subsequent functional MRI studies have demonstrated reduced frontal activity and executive performance (Minzenberg et al., 2009). Brozoski et al. (Brozoski et al., 1979) showed in NHPs that dopamine in the PFC is essential for working memory functions and that depletion of dopamine from the DLPFC was as detrimental to cognition as removing the cortex itself. Similarly, Sawaguchi and Goldman-Rakic reported working memory deficits by specifically blocking D1R in the PFC (Sawaguchi and Goldman-Rakic, 1991, 1994). Interestingly, recent findings of blunted frontal dopamine release by amphetamine (Slifstein et al., 2015) and by cognitive tasks (Rao et al., 2018) in patients suggest that lower frontal dopamine transmission could be an underlying neurochemical mechanism for the observed hypofrontality. It can thus be speculated that reduced D1R density is either a contributing factor or a consequence of such a pathophysiological process.
All antipsychotic drugs are antagonists at the D2R subtype. However, occupancy at the D1R has also been reported in patients treated with some antipsychotic drugs such as clozapine (Farde and Nordstrom, 1992). The selective D1R antagonist SCH39166 has been tested as monotherapy in 3 open clinical trials in acutely ill psychotic patients with schizophrenia or schizophreniform psychosis (Debeaurepaire et al., 1995; Den Boer et al., 1995; Karlsson et al., 2002); however, there was no improvement in positive symptoms in any of the 3 studies and only a few patients improved negative symptoms (Den Boer et al., 1995). Neither was a placebo effect observed as is commonly seen in open trials and with acutely hospitalized patients. On the contrary, several patients deteriorated and were withdrawn prematurely. Assuming that patients do indeed have low frontal D1R density as we found in the present study, it is tempting to suggest that the lack of placebo effects and worsening of the symptoms after D1 antagonism may have been caused by lowering the dopamine D1 transmission even further with a D1R antagonist, and that instead, a D1R agonist may have beneficial effects (Sedvall and Farde, 1995). Preclinical studies show an inverted-U dose response to D1R agonists such that there is an optimal level of D1R mediated dopamine activity on cognitive behavior (Arnsten et al., 2017). Hence, the present finding of 12.5% reduced frontal D1R may be sufficient to induce cognitive deficits and negative symptoms. Thus, in patients with reduced D1R a D1R stimulation could improve cognitive function whereas D1R antagonism may worsen symptoms. Indeed, reversal of antipsychotic induced working memory deficits has been demonstrated in NHPs by the D1 agonist ABT 431 (Castner et al., 2000). However, an initial clinical trial failed to demonstrate improved cognition in patients with schizophrenia by the full selective D1R agonist DAR-0100A, which may have been due to low dosing and consequently also low D1R occupancy (Girgis et al., 2016). Recently, a combined haloperidol and levodopa administration, to achieve high selective D1R agonist effect, improved working memory-related brain activation in humans (van Ruitenbeek et al., 2018). Improved D1R agonists that achieve higher levels of D1R occupancy are needed to test the efficacy of this putative mechanism for cognitive enhancement in schizophrenia.
In summary, 17 healthy controls and 18 neuroleptic naïve patients with schizophrenia or schizophreniform psychosis each underwent one PET measurement with the D1R radioligand [11C]CH23390. Contrary to our hypothesis, the patients had significantly lower BPND values in the DLPFC. Although the changes in the settings of the PET acquisition and reconstruction throughout the data collection could be viewed as a limitation, our analyses show that the results are unlikely to be caused by any of the confounders. Furthermore, the magnitude of the differences suggests that they cannot be fully explained by potential decreases in 5-HT2A receptor availability.
Funding
The study was sponsored by the Swedish Science Council grant no. 2015–02398 to L.F.
P.S., L.F., and C.H. designed the study. P.S. did the screening and examination of the subjects. P.S., G.J.M., and L.F. analyzed the data. P.S., G.J.M., S.C., and L.F. interpreted the results and drafted the article. All authors critically revised the article and approved of the final version for publication.
Supplementary Material
Acknowledgments
The authors thank the Karolinska positron emission tomography group for excellent technical assistance and in particular Dr Zhisheng Jia and Guennadi Jogolev.
Statement of Interest
L.F. is a shareholder with AstraZeneca. S.C. has received grant support from AstraZeneca as a co-investigator and has served as a one-off speaker for Otsuka-Lundbeck and Roche. All other authors declare no conflict of interest.
References
- Abi-Dargham A, Simpson N, Kegeles L, Parsey R, Hwang DR, Anjilvel S, Zea-Ponce Y, Lombardo I, Van Heertum R, Mann JJ, Foged C, Halldin C, Laruelle M (1999) PET studies of binding competition between endogenous dopamine and the D1 radiotracer [11C]NNC 756. Synapse 32:93–109. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M (2002) Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 22:3708–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban N, Narendran R, Hwang DR, Laruelle M, Slifstein M (2012) Increased prefrontal cortical D1 receptors in drug naive patients with schizophrenia: a PET study with [¹¹C]NNC112. J Psychopharmacol 26:794–805. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Girgis RR, Gray DL, Mailman RB (2017) Novel dopamine therapeutics for cognitive deficits in schizophrenia. Biol Psychiatry 81:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Karlsson S, Fischer H, Karlsson P, Brehmer Y, Rieckmann A, MacDonald SW, Farde L, Nyberg L (2011) Dopamine D(1) receptors and age differences in brain activation during working memory. Neurobiol Aging 32:1849–1856. [DOI] [PubMed] [Google Scholar]
- Bech P, Kastrup M, Rafaelsen OJ (1986) Mini-compendium of rating scales for states of anxiety depression mania schizophrenia with corresponding DSM-III syndromes. Acta Psychiatr Scand Suppl 326:1–37. [PubMed] [Google Scholar]
- Beischlag TV, Marchese A, Meador-Woodruff JH, Damask SP, O’Dowd BF, Tyndale RF, van Tol HH, Seeman P, Niznik HB (1995) The human dopamine D5 receptor gene: cloning and characterization of the 5’-flanking and promoter region. Biochemistry 34:5960–5970. [DOI] [PubMed] [Google Scholar]
- Bergström M, Boëthius J, Eriksson L, Greitz T, Ribbe T, Widén L (1981) Head fixation device for reproducible position alignment in transmission CT and positron emission tomography. J Comput Assist Tomogr 5:136–141. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS (1979) Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205:929–932. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR (2000) Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 10:1078–1092. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV, Goldman-Rakic PS (2000) Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science 287:2020–2022. [DOI] [PubMed] [Google Scholar]
- Cervenka S. (2019) PET radioligands for the dopamine D1-receptor: application in psychiatric disorders. Neurosci Lett 691:26–34. [DOI] [PubMed] [Google Scholar]
- Chan GL, Holden JE, Stoessl AJ, Doudet DJ, Wang Y, Dobko T, Morrison KS, Huser JM, English C, Legg B, Schulzer M, Calne DB, Ruth TJ (1998) Reproducibility of the distribution of carbon-11-SCH 23390, a dopamine D1 receptor tracer, in normal subjects. J Nucl Med 39:792–797. [PubMed] [Google Scholar]
- Chou YH, Karlsson P, Halldin C, Olsson H, Farde L (1999) A PET study of D(1)-like dopamine receptor ligand binding during altered endogenous dopamine levels in the primate brain. Psychopharmacology (Berl) 146:220–227. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis for the behavioral sciences, 2nd ed. Hillsdale, New Jersey: Lawrence Erlbaum Ass. [Google Scholar]
- de Beaurepaire R, Labelle A, Naber D, Jones BD, Barnes TR (1995) An open trial of the D1 antagonist SCH 39166 in six cases of acute psychotic states. Psychopharmacology (Berl) 121:323–327. [DOI] [PubMed] [Google Scholar]
- de Boer L, Axelsson J, Riklund K, Nyberg L, Dayan P, Backman L, Guitart-Masip M (2017) Attenuation of dopamine-modulated prefrontal value signals underlies probabilistic reward learning deficits in old age. Elife 6 doi: 10.7554/eLife.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Boer JA, van Megen HJ, Fleischhacker WW, Louwerens JW, Slaap BR, Westenberg HG, Burrows GD, Srivastava ON (1995) Differential effects of the D1-DA receptor antagonist SCH39166 on positive and negative symptoms of schizophrenia. Psychopharmacology (Berl) 121:317–322. [DOI] [PubMed] [Google Scholar]
- Ekelund J, Slifstein M, Narendran R, Guillin O, Belani H, Guo NN, Hwang Y, Hwang DR, Abi-Dargham A, Laruelle M (2007) In vivo DA D(1) receptor selectivity of NNC 112 and SCH 23390. Mol Imaging Biol 9:117–125. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Rasmussen H, Kristiansen KT, Frokjaer VG, Haugbol S, Pinborg L, Baaré W, Svarer C, Madsen J, Lublin H, Knudsen GM, Glenthoj BY (2008) Cortical and subcortical 5-HT2A receptor binding in neuroleptic-naive first-episode schizophrenic patients. Neuropsychopharmacology 33:2435–2441. [DOI] [PubMed] [Google Scholar]
- Farde L. (1992) Selective D1- and D2-dopamine receptor blockade both induces akathisia in humans–a PET study with [11C]SCH 23390 and [11C]raclopride. Psychopharmacology (Berl) 107:23–29. [DOI] [PubMed] [Google Scholar]
- Farde L, Nordstrom AL (1992) PET analysis indicates atypical central dopamine receptor occupancy in clozapine-treated patients. Br J Psychiatry 17:30–33. [PubMed] [Google Scholar]
- Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G (1992) Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry 49:538–544. [DOI] [PubMed] [Google Scholar]
- Fischer H, Nyberg L, Karlsson S, Karlsson P, Brehmer Y, Rieckmann A, MacDonald SW, Farde L, Bäckman L (2010) Simulating neurocognitive aging: effects of a dopaminergic antagonist on brain activity during working memory. Biol Psychiatry 67:575–580. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ (1995) Spatial registration and normalization of images. Human Brain Mapping 3:165–189. [Google Scholar]
- Gelman A, Carlin J (2014) Beyond power calculations: assessing type S (sign) and type M (magnitude) errors. Perspect Psychol Sci 9:641–651. [DOI] [PubMed] [Google Scholar]
- Girgis RR, Van Snellenberg JX, Glass A, Kegeles LS, Thompson JL, Wall M, Cho RY, Carter CS, Slifstein M, Abi-Dargham A, Lieberman JA (2016) A proof-of-concept, randomized controlled trial of DAR-0100A, a dopamine-1 receptor agonist, for cognitive enhancement in schizophrenia. J Psychopharmacol 30:428–435. [DOI] [PubMed] [Google Scholar]
- Guo N, Hwang DR, Lo ES, Huang YY, Laruelle M, Abi-Dargham A (2003) Dopamine depletion and in vivo binding of PET D1 receptor radioligands: implications for imaging studies in schizophrenia. Neuropsychopharmacology 28:1703–1711. [DOI] [PubMed] [Google Scholar]
- Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L (1994) Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology 11:245–256. [DOI] [PubMed] [Google Scholar]
- Halldin C, Stone-Elander S, Farde L, Ehrin E, Fasth KJ, Långström B, Sedvall G (1986) Preparation of 11C-labelled SCH 23390 for the in vivo study of dopamine D-1 receptors using positron emission tomography. Int J Rad Appl Instrum A 37:1039–1043. [DOI] [PubMed] [Google Scholar]
- Halldin C, Foged C, Chou YH, Karlsson P, Swahn CG, Sandell J, Sedvall G, Farde L (1998) Carbon-11-NNC 112: a radioligand for PET examination of striatal and neocortical D1-dopamine receptors. J Nucl Med 39:2061–2068. [PubMed] [Google Scholar]
- Hirvonen J, van Erp TG, Huttunen J, Aalto S, Någren K, Huttunen M, Lönnqvist J, Kaprio J, Cannon TD, Hietala J (2006) Brain dopamine d1 receptors in twins discordant for schizophrenia. Am J Psychiatry 163:1747–1753. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S (2012) The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry 69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S (2009) The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull 35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar DH, Franzén G (1974) Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta Psychiatr Scand 50:425–462. [DOI] [PubMed] [Google Scholar]
- Jucaite A, Forssberg H, Karlsson P, Halldin C, Farde L (2010) Age-related reduction in dopamine D1 receptors in the human brain: from late childhood to adulthood, a positron emission tomography study. Neuroscience 167:104–110. [DOI] [PubMed] [Google Scholar]
- Karlsson P, Farde L, Halldin C, Sedvall G (2002) PET study of D(1) dopamine receptor binding in neuroleptic-naive patients with schizophrenia. Am J Psychiatry 159:761–767. [DOI] [PubMed] [Google Scholar]
- Knable MB, Hyde TM, Murray AM, Herman MM, Kleinman JE (1996) A postmortem study of frontal cortical dopamine D1 receptors in schizophrenics, psychiatric controls, and normal controls. Biol Psychiatry 40:1191–1199. [DOI] [PubMed] [Google Scholar]
- Kolakowska T. (1976) Brief psychiatric rating scale: glossary and rating instructions. Oxford: Oxford University Press. [Google Scholar]
- Kosaka J, Takahashi H, Ito H, Takano A, Fujimura Y, Matsumoto R, Nozaki S, Yasuno F, Okubo Y, Kishimoto T, Suhara T (2010) Decreased binding of [11C]NNC112 and [11C]SCH23390 in patients with chronic schizophrenia. Life Sci 86:814–818. [DOI] [PubMed] [Google Scholar]
- Lakens D. (2017) Equivalence tests: A practical primer for t tests, correlations, and meta-analyses. Soc Psychol Personal Sci 8:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP (1996) Simplified reference tissue model for PET receptor studies. Neuroimage 4:153–158. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Simpson S, Kegeles LS, Parsey RV, Hwang DR (1998) PET studies of binding competition between endogenous dopamine and D1 antagonists and agonists. Soc Neurosc Abst 24:93–109. [DOI] [PubMed] [Google Scholar]
- Lewis R, Kapur S, Jones C, DaSilva J, Brown GM, Wilson AA, Houle S, Zipursky RB (1999) Serotonin 5-HT2 receptors in schizophrenia: a PET study using [18F]setoperone in neuroleptic-naive patients and normal subjects. Am J Psychiatry 156:72–78. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Elsworth JD, Goldman-Rakic PS (1997) Down-regulation of the D1 and D5 dopamine receptors in the primate prefrontal cortex by chronic treatment with antipsychotic drugs. J Pharmacol Exp Ther 281:597–603. [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS (1994) A common action of clozapine, haloperidol, and remoxipride on D1- and D2-dopaminergic receptors in the primate cerebral cortex. Proc Natl Acad Sci USA 91:4353–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson GJ, Stenkrona P, Cselényi Z, Plavén-Sigray P, Halldin C, Farde L, Cervenka S (2017) Reliability of volumetric and surface-based normalisation and smoothing techniques for PET analysis of the cortex: A test-retest analysis using [11C]SCH-23390. Neuroimage 155:344–353. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M (2001) Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21:1034–1057. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009) Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngan ET, Yatham LN, Ruth TJ, Liddle PF (2000) Decreased serotonin 2A receptor densities in neuroleptic-naive patients with schizophrenia: a PET study using [(18)F]setoperone. Am J Psychiatry 157:1016–1018. [DOI] [PubMed] [Google Scholar]
- Nordström AL, Farde L, Eriksson L, Halldin C (1995) No elevated D2 dopamine receptors in neuroleptic-naive schizophrenic patients revealed by positron emission tomography and [11C]N-methylspiperone. Psychiatry Res 61:67–83. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M (1997) Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature 385:634–636. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M (2000) Serotonin 5-HT2 receptors in schizophrenic patients studied by positron emission tomography. Life Sci 66:2455–2464. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Report 10:799–812. [Google Scholar]
- Poels EM, Girgis RR, Thompson JL, Slifstein M, Abi-Dargham A (2013) In vivo binding of the dopamine-1 receptor PET tracers [¹¹C]NNC112 and [¹¹C]SCH23390: a comparison study in individuals with schizophrenia. Psychopharmacology (Berl) 228:167–174. [DOI] [PubMed] [Google Scholar]
- Rao N, Northoff G, Tagore A, Rusjan P, Kenk M, Wilson A, Houle S, Strafella A, Remington G, Mizrahi R (2018) Impaired prefrontal cortical dopamine release in schizophrenia during a cognitive task: a [11C]FLB 457 positron emission tomography study. Schizophr Bull. doi: 10.1093/schbul/sby076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H, Erritzoe D, Andersen R, Ebdrup BH, Aggernaes B, Oranje B, Kalbitzer J, Madsen J, Pinborg LH, Baaré W, Svarer C, Lublin H, Knudsen GM, Glenthoj B (2010) Decreased frontal serotonin2a receptor binding in antipsychotic-naive patients with first-episode schizophrenia. Arch Gen Psychiatry 67:9–16. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Frokjaer VG, Hilker RW, Madsen J, Anhøj S, Oranje B, Pinborg LH, Glenthøj B, Knudsen GM (2016) Low frontal serotonin 2A receptor binding is a state marker for schizophrenia? Eur Neuropsychopharmacol 26:1248–1250. [DOI] [PubMed] [Google Scholar]
- Roland PE, Graufelds CJ, W Hlin J, Ingelman L, Andersson M, Ledberg A, Pedersen J, Akerman S, Dabringhaus A, Zilles K (1994) Human brain atlas: for high-resolution functional and anatomical mapping. Hum Brain Mapp 1:173–184. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS (1991) D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251:947–950. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS (1994) The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol 71:515–528. [DOI] [PubMed] [Google Scholar]
- Sawilowsky SS. (2009) New effect size rules of thumb. J Mod Appl Stat Methods 8:597–599. [Google Scholar]
- Schuirmann DJ. (1987) A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 15:657–680. [DOI] [PubMed] [Google Scholar]
- Sedvall G, Farde L (1995) Chemical brain anatomy in schizophrenia. Lancet 346:743–749. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS (1999) The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 45:17–25. [DOI] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U (2012) A 21 Word Solution https://ssrn.com/abstract=2160588: SSRN.
- Slifstein M, Kegeles LS, Gonzales R, Frankle WG, Xu X, Laruelle M, Abi-Dargham A (2007) [11C]NNC 112 selectivity for dopamine D1 and serotonin 5-HT(2A) receptors: a PET study in healthy human subjects. J Cereb Blood Flow Metab 27:1733–1741. [DOI] [PubMed] [Google Scholar]
- Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, Hackett E, Girgis R, Ojeil N, Moore H, D’Souza D, Malison RT, Huang Y, Lim K, Nabulsi N, Carson RE, Lieberman JA, Abi-Dargham A (2015) Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry 72:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkrona P, Matheson GJ, Cervenka S, Sigray PP, Halldin C, Farde L (2018) [11C]SCH23390 binding to the D1-dopamine receptor in the human brain-a comparison of manual and automated methods for image analysis. EJNMMI Res 8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara T, Fukuda H, Inoue O, Itoh T, Suzuki K, Yamasaki T, Tateno Y (1991) Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology (Berl) 103:41–45. [DOI] [PubMed] [Google Scholar]
- Swahn CG, Halldin C, Farde L, Sedvall G (1994) Metabolism of the PET ligand [11C]SCH 23390. Identification of two radiolabelled metabolites with HPLC. Human Psychopharmacology 9:25–31. [Google Scholar]
- Thibaut F, Vaugeois JM, Bonnet JJ, Costentin J (1996) In vivo striatal binding of the D1 antagonist SCH 23390 is not modified by changes in dopaminergic transmission. Neuropharmacology 35:267–272. [DOI] [PubMed] [Google Scholar]
- Trichard C, Paillère-Martinot ML, Attar-Levy D, Blin J, Feline A, Martinot JL (1998) No serotonin 5-HT2A receptor density abnormality in the cortex of schizophrenic patients studied with PET. Schizophr Res 31:13–17. [DOI] [PubMed] [Google Scholar]
- van Ruitenbeek P, Hernaus D, Mehta MA (2018) A proof-of-principle study of the effect of combined haloperidol and levodopa administration on working memory-related brain activation in humans. Hum Psychopharmacol 33:e2675. [DOI] [PubMed] [Google Scholar]
- Verhoeff NP, Hussey D, Lee M, Tauscher J, Papatheodorou G, Wilson AA, Houle S, Kapur S (2002) Dopamine depletion results in increased neostriatal D(2), but not D(1), receptor binding in humans. Mol Psychiatry 7:233, 322–233, 328. [DOI] [PubMed] [Google Scholar]
- Wagstyl K, Ronan L, Whitaker KJ, Goodyer IM, Roberts N, Crow TJ, Fletcher PC (2016) Multiple markers of cortical morphology reveal evidence of supragranular thinning in schizophrenia. Transl Psychiatry 6:e780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, Stoessl AJ (1998) Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse 30:56–61. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF (1988) Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophr Bull 14:157–168. [DOI] [PubMed] [Google Scholar]
- Wienhard K, Dahlbom M, Eriksson L, Michel C, Bruckbauer T, Pietrzyk U, Heiss WD (1994) The ECAT EXACT HR: performance of a new high resolution positron scanner. J Comput Assist Tomogr 18:110–118. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All analysis code is available at https://github.com/mathesong/D1DNPsychosis. Due to institutional restrictions, the data cannot be shared openly within this repository. These data are pseudonymized according to national (Swedish) and EU legislation and cannot be anonymized and published in an open repository. Metadata can be openly published, and the underlying data can instead be made available upon request on a case by case basis as allowed by the legislation and ethical permits. Requests for access can be made to the Karolinska Institutet’s Research Data Office at rdo@ki.se.