Abstract
Background
The goal of this study was to examine baseline transcranial magnetic stimulation measures of cortical inhibition and excitability in depressed patients and characterize their longitudinal posttreatment changes.
Methods
Fifteen adolescents (age 13–17 years) with moderate to severe major depressive disorder and 22 healthy controls (age 9–17) underwent single- and paired-pulse transcranial magnetic stimulation and clinical assessments. Transcranial magnetic stimulation measures included short-interval intracortical inhibition (2 and 4 milliseconds), long-interval intracortical inhibition (100, 150, and 200 milliseconds), cortical silent period, and intracortical facilitation (10, 15, and 20 milliseconds). Ten participants with major depressive disorder initiated antidepressant treatment or had dose adjustments. These participants were reassessed after treatment. Depression symptom severity was measured with the Children’s Depression Rating Scale, Revised. Robust regression modeling compared healthy and depressed adolescents at baseline. Relationships between changes in cortical inhibition and changes in depressive symptom severity were assessed in the depressed adolescents receiving antidepressant treatment.
Results
Our results revealed that at baseline, short-interval intracortical inhibition-2 was significantly reduced (Padj = .01) in depressed participants, suggesting impaired cortical inhibition compared with healthy controls. At follow-up, improvement in Children’s Depression Rating Scale, Revised scores correlated with improvement in short-interval intracortical inhibition-4 amplitude (greater inhibition) after antidepressant treatment (R2 = 0.63; P = .01).
Conclusions
These results suggest that cortical inhibition measures may have promise as biomarkers in adolescents treated for depression.
Keywords: adolescent, cortical inhibition, mood disorders, paired-pulse transcranial magnetic stimulation, short-interval intracortical inhibition
Significance Statement.
This is the first study, to our knowledge, in adolescents showing that improvement in depression severity after antidepressant treatment is associated with restored cortical inhibition, as indexed by short-interval cortical inhibition (SICI) measured by transcranial magnetic stimulation (TMS). These findings provide important preliminary data that will inform future studies investigating the neurobiology of adolescent depression and enable the development of better diagnostic methods and individualized, biomarker-guided therapeutic approaches in this population.
Introduction
Major depressive disorder (MDD) is one of the most common psychiatric illnesses for adolescents, with estimated 1-year and lifetime prevalence rates of 8% and 11%, respectively. Depression in youth is associated with increased psychiatric comorbidities, suicide risk, and considerable negative impact on quality of life and morbidity (Birmaher et al., 1996; Perou et al., 2013; Avenevoli et al., 2015). Despite available treatments, only approximately 60% of patients respond to standard treatments, and remission rates are 30% to 40% (March et al., 2004; Cheung et al., 2005; Avenevoli et al., 2015).
The neurobiology of depression in children and adolescents has several potential mechanisms, including aberrant neurocircuitry in the amygdala, hypothalamus, and subgenual anterior cingulate cortex; genetic and epigenetic factors; and hypothalamic-pituitary-adrenal hyperactivity (Zalsman et al., 2006; Henje Blom et al., 2016). Current evidence also suggests an altered excitatory-inhibitory balance of cortical networks in depression (Levinson et al., 2010; Croarkin et al., 2011; Anticevic and Murray, 2017). For example, studies using proton magnetic resonance spectroscopy have shown that levels of γ-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain, are reduced in adults and adolescents with depression (Hasler et al., 2007; Gabbay et al., 2012, 2017) and that treatment with antidepressants can restore GABAergic inhibition (Manganotti et al., 2001; Sanacora et al., 2002; Robol et al., 2004; Minelli et al., 2010). In addition to reduced GABAergic inhibition, elevated glutamate levels (Sanacora et al., 2012) and excitatory activity have also been associated with depression. Indeed, glutamatergic antagonism with ketamine has been associated with antidepressant effects (Dutta et al., 2015). Despite these promising results in understanding the potential role of altered excitatory-inhibitory balance in the pathophysiology of depression, its implications in children and adolescents require further investigation because of the incomplete maturation of the young brain and developmental shifts in excitatory-inhibitory balance in children and adolescents (Croarkin et al., 2014b; Hameed et al., 2017).
Single- and paired-pulse transcranial magnetic stimulation (TMS) offers a noninvasive way to measure inhibitory and excitatory activity associated with GABAergic and glutamatergic pathways, which are impaired in depression (Levinson et al., 2010; Croarkin et al., 2011; Radhu et al., 2013). When coupled with electromyography, TMS can quantify the stimulus effect in the motor cortex by measuring motor-evoked potentials (MEPs). TMS-induced effects on MEPs are measured with various paradigms, including intracortical inhibition (ICI), interhemispheric inhibition, cortical silent period (CSP), intracortical facilitation (ICF), and motor threshold (Anand and Hotson, 2002). ICF is a measure of cortical excitability (Kujirai et al., 1993; Hanajima et al., 1996; Ziemann et al., 1996) and is most likely mediated through N-methyl-d-aspartate glutamatergic receptor activity (Liepert et al., 1997; Ziemann et al., 1998; Schwenkreis et al., 1999, 2000), whereas ICI and CSP (Day et al., 1989a, 1989b; Cantello et al., 1992; Siebner et al., 1998; Stetkarova and Kofler, 2013) are measures of cortical inhibition and are mediated through GABAergic function (Ziemann, 1999, 2003). Depending on the duration of the interstimulus interval (ISI) and the intensity of the conditioning stimulus, ICI can be further categorized as SICI (ISIs of 2–4 milliseconds) (Hanajima et al., 1996; Ziemann et al., 1996; Di Lazzaro et al., 1998; Fisher et al., 2002) and long-interval intracortical inhibition (LICI; ISIs of 100–200 milliseconds) (Valls-Solé et al., 1992; Inghilleri et al., 1993; Nakamura et al., 1997; Chen et al., 1999; Di Lazzaro et al., 2002), which are thought to reflect GABAA and GABAB receptor functioning, respectively (Hanajima et al., 1998; Werhahn et al., 1999; Di Lazzaro et al., 2000, 2002; Ilić et al., 2002; Hanajima et al., 2003; Pierantozzi et al., 2004; McDonnell et al., 2006).
A meta-analysis of TMS studies (Radhu et al., 2013) showed impaired CSP (a measure of GABAB activity) and SICI (a measure of GABAA activity) in adults with MDD compared with healthy controls. Other studies of adults treated for MDD found a relationship between the restoration of GABAergic activity measured by TMS and antidepressant treatment (Manganotti et al., 2001; Sanacora et al., 2002; Robol et al., 2004; Minelli et al., 2010). The few studies that investigated TMS correlates of depression in children and adolescents have reported mixed results. Our group previously reported elevated ICF in medication-naïve children and adolescents with depression (Croarkin et al., 2013), an association between pretreatment LICI deficits and poor treatment response to fluoxetine (Croarkin et al., 2014a), and an inverse relationship between depression severity and CSP duration (Lewis et al., 2016). However, the adolescent TMS literature to date has consisted of cross-sectional studies.
In this study, we aimed to investigate TMS correlates of longitudinal treatment outcomes in children and adolescents with MDD. We assessed TMS measures of cortical inhibition and excitability in (1) a sample of adolescents with depression who received antidepressant pharmacotherapy, and (2) a healthy control comparator group. Among adolescents with MDD who were receiving antidepressants, we further explored pretreatment and posttreatment changes in TMS measures of cortical inhibition and excitability. On the basis of prior evidence in adults and our previous work, we hypothesized that adolescents with depression would have impaired cortical inhibition and that the improvement in depression severity would be associated with enhanced cortical inhibition.
Materials and Methods
All study procedures were approved by the institutional review boards of Mayo Clinic and University of Texas Southwestern Medical Center. Informed consent was obtained from the participants’ legal guardians or parents, and assent was obtained from adolescent participants.
Participants
The sample consisted of treatment-seeking participants with MDD (age 13–17 years ) who were a subset of a sample of adolescents recruited from clinical practices as part of a larger study (ClinicalTrials.gov identifier: NCT02307617). Patients were recruited at Mayo Clinic (Rochester, MN). Healthy controls (age 9–17 years) were recruited as part of a previous trial with identical TMS methodology (Croarkin et al., 2013); their data were included in the current analysis. Healthy participants were recruited at UT Southwestern Medical Center (Dallas, Texas).
Inclusion criteria for depressed participants were as follows: (1) MDD that was diagnosed by a clinical and research interview with the Schedule for Affective Disorders and Schizophrenia for School Age Children–Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997); (2) a minimum score of 40 on the Children’s Depression Rating Scale, Revised (CDRS-R) (Poznanski et al., 1984; Poznanski and Mokros, 1996) and a score of 4 or more on the Clinical Global Impression–Severity (CGI-S) (Guy, 1976) consistent with at least moderate depressive symptom severity; and (3) no use of stimulant or benzodiazepine medications on the day of TMS assessments. A board-certified child and adolescent psychiatrist (P.E.C.) completed all clinical interviews, K-SADS-PL interviews, and clinical rating scales.
Potential patients and controls were ineligible for the study if they had a contraindication to TMS, as assessed by the TMS Adult Safety Screen (Keel et al., 2001). Contraindications were a history of unprovoked seizures, seizure disorder, history of febrile seizures, family history of epilepsy, prior neurosurgery, or brain tumor. Potential controls were excluded if they had a personal history of psychiatric treatment or psychotropic medications, or a family history (first- or second-degree relatives) of psychiatric illness.
Study Overview and Clinical Measures
The CDRS-R, K-SADS-PL, CGI-S, Edinburgh Handedness Inventory (Oldfield, 1971), and Columbia Suicide Severity Rating Scale (Posner et al., 2011) were administered at baseline. Single-pulse and paired-pulse TMS measures were obtained from each participant. Depressed participants receiving pharmacotherapy were asked to return for a follow-up visit in about 8 weeks. At follow-up, they again underwent assessment with single- and paired-pulse TMS and clinical measures (CDRS-R, CGI-S, Clinical Global Impression–Improvement, and Columbia Suicide Severity Rating Scale).
The CDRS-R was used to measure baseline and follow-up depression severity. The CDRS-R is a clinician-rated 17-item scale that incorporates scores from a parent and the adolescent to determine a composite score. All study participants completed the CDRS-R. For the depressed patients who returned for follow-up, the difference between baseline and follow-up scores was calculated to quantify change in depressive symptoms that occurred during the treatment period.
TMS
Magnetic pulses were generated by Magstim 200 magnetic stimulators connected via a BiStim Module (Magstim Co. Ltd). TMS measures were obtained from the left primary motor cortex, which was stimulated with a 70-mm figure-of-8 electromagnetic coil placed tangentially over the scalp. Participants remained seated throughout the procedure and wore earplugs. MEPs were recorded via surface electromyography of the contralateral abductor pollicis brevis (APB). The optimal scalp location for APB stimulation was determined according to a previously published method (Daskalakis et al., 2002). The APB location was found by manual movement of the TMS coil while administering single pulses, followed by titration of the magnetic pulse intensity to determine the motor threshold. This was conducted in as few pulses as possible (maximum of 144, typically fewer than 72), with a rest period of 5 minutes or longer to reduce the impact of these preliminary single pulses on subsequent measures of excitability and inhibition.
Single-pulse paradigms included determination of the resting motor threshold (RMT), defined as the pulse intensity to elicit a minimum 50-μV peak-to-peak MEP response on electromyography in 5 of 10 trials. The CSP was obtained via the delivery of a single TMS pulse at 140% of RMT while participants contracted their contralateral APB at 20% of maximum contraction strength, as measured by a hand-held dynamometer. CSP duration was measured from the TMS pulse to the spontaneous resumption of motor activity on electromyography. Mean CSP duration was calculated from 10 trials.
For the paired-pulse SICI and ICF paradigms, the conditioning stimulus was set to 80% of each participant’s RMT, while the subsequent test stimulus was delivered at the intensity that resulted in a peak-to-peak MEP amplitude of 1 mV. SICI was tested using ISIs of 2 or 4 milliseconds, and ICF was tested using ISIs of 10, 15, or 20 milliseconds. LICI was tested using 2 identical suprathreshold stimuli (both calibrated to evoke 1-mV peak-to-peak amplitude MEPs) separated by ISIs of 100, 150, or 200 milliseconds. SICI, LICI, and ICF were recorded as the ratio of the conditioned MEP amplitude (elicited by the test [second] stimulus) to the mean unconditioned MEP amplitude. A conditioned/unconditioned MEP amplitude ratio <1.0 indicated net inhibition, whereas a ratio >1.0 indicated net facilitation. Twelve trials at each ISI were performed (10 trials for LICI) and averaged.
Statistical Analysis
Statistical analyses were performed with Stata/MP 14.1 (StataCorp, LLC). Continuous variables were described with means and standard deviations. Categorical variables were described with frequencies and percentages. Demographic and clinical variables were compared with χ2 tests for categorical variables and t tests for continuous variables.
Healthy participants and depressed participants were compared by using a robust regression model for each TMS measure in which the TMS measure was the dependent variable and “group” was the independent variable, with age and sex included as covariates. In all of our regression models, we used a “robust regression” analysis (Stata/MP 14.1, StataCorp, LLC) that allows weighted analysis for outliers based on the residuals. Posthoc correction for multiple comparisons was completed by the Bonferroni method for a total of 9 comparisons.
For the subgroup of depressed participants who returned for follow-up assessments after treatment with antidepressant medication, the associations between the change in depression severity (ΔCDRS-R) and the change in TMS measures between baseline and follow-up visits was tested in a robust regression model. The change in CDRS-R score was the dependent variable and the change in each TMS measure was the independent variable, with age and sex included as covariates.
Given the limited number of covariates we could add in our regression models, we also ran 2 exploratory sensitivity analyses to test the possible effects of medications on measures of cortical excitability that had significant results in the main analyses.
Results
Participant Characteristics
Table 1 shows the demographic characteristics of the participants. The depressed group consisted of 15 participants (9 female; mean [SD] age, 15.4 [1.2] years; age range, 13–17 years). The mean (SD) length of illness was 1.3 (1.8) years. The healthy control group consisted of 22 participants (11 female; mean [SD] age, 13.8 [2.2] years; age range, 9–17 years). Among the depressed participants, 11 participants were experiencing their first episode of depression, and the remaining 4 had recurrent depression (range 2–3 episodes). Two of the patients were previously treated with sertraline. Thirty-three percent of the depressed participants had co-morbid attention-deficit/hyperactivity disorder, 13% had generalized anxiety disorder, 13% had posttraumatic stress disorder, 13% had dysthymia, and 13% had substance use (cannabis, alcohol) that did not meet the threshold for a diagnosis of a substance use disorder. We noted significant baseline differences between groups for age (t = −2.63; P = .01), race/ethnicity (χ2 = 9.9; P = .02), and CDRS-R scores (t = −19.88; P < .001).
Table 1.
Characteristics of Depressed Participants and Healthy Controls (N = 37)
| Characteristic | Healthy Controls (n = 22) | Depressed Participants (n = 15) | P Valuea |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 13.8 (2.2) | 15.4 (1.2) | .01 |
| Range | 9–17 | 13–17 | |
| Male sex, No. (%) | 11 (50) | 6 (40) | .55 |
| Race/ethnicity, No. (%) | .02 | ||
| White | 8 (36) | 13 (87) | |
| Black | 11 (50) | 1 (7) | |
| Hispanic | 1 (5) | 0 (0) | |
| Other | 2 (9) | 1 (7) | |
| Right handedness, No. (%) | 20 (91) | 13 (87) | .68 |
| Comorbid psychiatric diagnoses, No. (%) | — | — | |
| ADHD | 5 (33) | ||
| GAD | 2 (13) | ||
| PTSD | 2 (13) | ||
| Dysthymia | 2 (13) | ||
| Substance useb | 2 (13) | ||
| Otherc | 3 (20) | ||
| Family history of psychiatric illness, No. (%) | 0 (0) | 14 (93) | |
| CDRS-R | <.001 | ||
| Mean (SD) | 19.6 (1.6) | 53.7 (7.9) | |
| Range | 17–24 | 42–66 | |
| CGI-S | — | — | |
| Mean (SD) | 5.0 (0.8) | ||
| Range | 4–6 | ||
| Current mood episode duration, y | — | — | |
| Mean (SD) | 1.3 (1.8) | ||
| Range | 0.1–7.0 | ||
| Previous mood episode(s), No. (%) | — | 4 (27) | — |
| Medications after initial assessments (n = 10) | — | — | |
| Fluoxetine | 8 (80) | ||
| Bupropion | 1 (10) | ||
| Escitalopram | 1 (10) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CDRS-R, Children’s Depression Rating Scale, Revised; CGI-S, Clinical Global Impression–Severity; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision; GAD, generalized anxiety disorder; K-SADS-PL, Schedule for Affective Disorders and Schizophrenia for School Age Children−Present and Lifetime Version; PTSD, posttraumatic stress disorder.
a χ2 or t test.
b Cannabis use, not meeting DSM-IV-TR diagnostic criteria for cannabis abuse or dependence on K-SADS-PL.
c Tic disorder (n = 1), prenatal alcohol exposure (n = 1), history of concussions (n = 1).
Due to group differences in age and sex, we added these as covariates in our regression models. For race/ethnicity, given limited sample size, we did not include it as a covariate but ran secondary analyses comparing baseline TMS measures between black and white participants within the healthy group, as the majority of black participants were in this group (11 of 12). No differences were found between black and white participants on any of the TMS excitability measures (all P > .05).
With regard to medication use, one of the depressed participants was taking fluoxetine at the time of the baseline visit. Four patients were being treated with stimulant medications, which were withheld on the day of TMS assessments. Of the 10 participants who later returned for follow-up assessment, 9 were started on antidepressant medications after the baseline visit, and 1 patient who was taking fluoxetine at baseline had a dose increase after baseline TMS and clinical measures were obtained. Of these 10 patients, 8 were prescribed fluoxetine (maximum daily dose, 20–60 mg), 1 started treatment with bupropion (maximum daily dose, 150 mg), and 1 started treatment with escitalopram (maximum daily dose, 10 mg).
Baseline Comparison of TMS Measures Between Groups
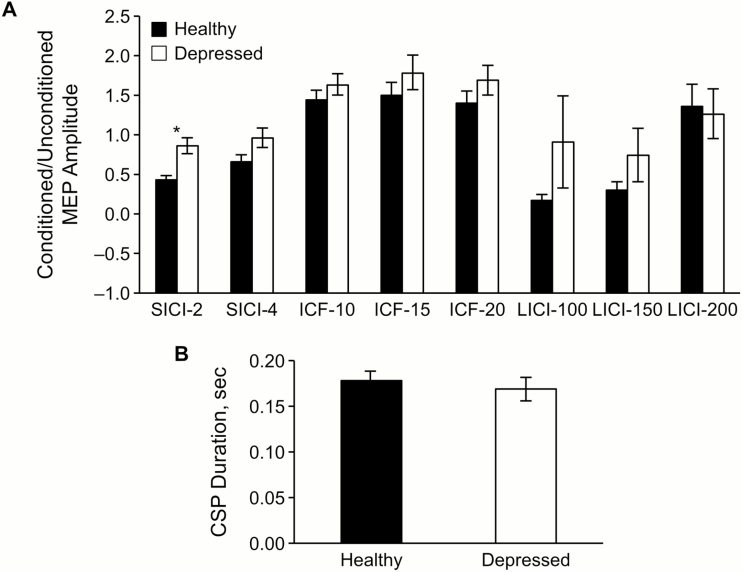
In the healthy group, SICI data was missing for 1 patient, ICF was missing for 1, LICI was missing for 3, and CSP was missing for 4 participants. There were no missing data for the patient group. Baseline comparison of groups with robust regression controlled for age and sex showed significant differences between healthy and depressed adolescents in SICI-2 (P = .001, R2 = 0.30) and SICI-4 (P = .027, R2 = .15). There were no significant differences between the healthy control and depressed groups for other TMS measures, including CSP (P = .62), LICI-100 (P = .83), LICI-150 (P = .16), LICI-200 (P = .80), ICF-10 (P = .13), ICF-15 (P = .10), and ICF-20 (P = .44). When corrected for multiple comparisons, only SICI-2 remained significant (Padj = .01), while SICI-4 was not (Padj = .24). Figure 1 and Table 2 show mean values of each measurement for both groups.
Figure 1.
Baseline transcranial magnetic stimulation (TMS) measures for depressed participants and healthy controls. Error bars represent standard error of means. (A) Comparison of short-interval intracortical inhibition (SICI), intracortical facilitation (ICF), and long-interval intracortical inhibition (LICI). Numbers (e.g., SICI-2) represent the interstimulus intervals in milliseconds. The asterisk indicates a significant group difference after Bonferroni correction for multiple comparisons (P = .001, Padj = .01). (B) Comparison of cortical silent period (CSP) duration. MEP, motor-evoked potential.
Table 2.
Baseline and Follow-up Transcranial Magnetic Stimulation Measures
| Depressed Participants | ||||
|---|---|---|---|---|
| Measure | Healthy Controls, Baseline (n = 22) | Baseline (n = 15) | Follow-up (n = 10)a | P adj Valueb |
| SICI | ||||
| 2 ms | 0.45 (0.23) | 0.88 (0.39) | 1.06 (0.53) | .01 |
| 4 ms | 0.69 (0.35) | 0.98 (0.48) | 1.15 (0.62)c | .24 |
| ICF | ||||
| 10 ms | 1.47 (0.55) | 1.66 (0.53) | 1.58 (0.67) | >.99 |
| 15 ms | 1.53 (0.71) | 1.81 (0.84) | 1.49 (0.51) | .87 |
| 20 ms | 1.43 (0.66) | 1.71 (0.74) | 1.34 (0.53) | >.99 |
| LICI | ||||
| 100 ms | 0.20 (0.28) | 0.93 (2.27) | 0.31 (0.29) | >.99 |
| 150 ms | 0.32 (0.48) | 0.76 (1.30) | 0.44 (0.42) | >.99 |
| 200 ms | 1.39 (1.18) | 1.29 (1.23) | 0.60 (0.46) | >.99 |
| CSP, s | 0.18 (0.04) | 0.17 (0.05) | 0.16 (0.03) | >.99 |
Abbreviations: CDRS-R, Children’s Depression Rating Scale, Revised; CSP, cortical silent period; ICF, intracortical facilitation; LICI, long-interval intracortical inhibition; SICI, short-interval intracortical inhibition.
a Follow-up assessment timeframe: mean [SD] follow-up time, 7.9 [5.6] weeks; range, 2–20 weeks) for a subset of depressed participants treated with antidepressant medication.
b Comparison of healthy vs depressed participants at baseline. Bonferroni-corrected P values (Padj).
c For adolescents in the follow-up group, improvement in CDRS-R positively correlated with improvement in SICI-4 (P = .01; R2 = 0.63).
Follow-up Assessment of Depressed Participants
Ten of the depressed participants who were treated with antidepressant medications returned for a follow-up visit about 8 weeks later (mean [SD] follow-up time, 7.9 [5.6] weeks; range, 2–20 weeks). Four continued to have clinically significant depressive symptoms based on their CDRS-R scores (scores remained ≥40). Mean (SD) changes in CDRS-R and CGI-S scores were −21.9 (9.26) and −2.2 (1.14), respectively.
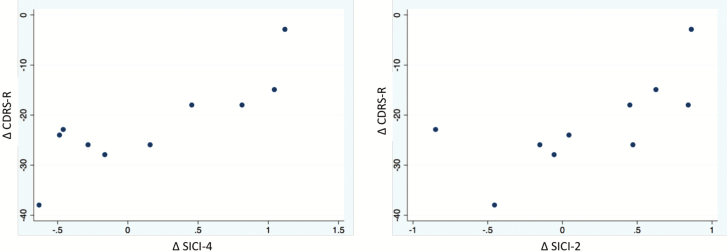
We further tested the association between the change in CDRS-R scores (the difference between follow-up and baseline scores, ΔCDRS-R) and change in SICI-2 and SICI-4 (ΔSICI-2; ΔSICI-4) in robust regression models controlled for age and sex. Larger negative numbers for ΔCDRS-R indicated greater improvement in depressive symptoms, whereas larger negative numbers for ΔSICI-2 and ΔSICI-4 indicated greater improvement in cortical inhibition (ie, greater reduction of the conditioned/unconditioned MEP amplitude ratio). Improvement in CDRS-R positively correlated with improvement in ΔSICI-4 (P = .01; R2 = 0.63). There was no significant relationship with ΔSICI-2 (P = .10; R2 = 0.33) (Figure 2). Given the variability in follow-up times among participants (2–20 weeks), we ran a secondary analysis looking at the relationship between ΔSICI and time elapsed between visits using additional regression models (not age and sex controlled). We found no association between follow-up time and either ΔSICI-2 or ΔSICI-4 (both P > .05)
Figure 2.
Scatter plot graphs showing the association between change in Children’s Depression Rating Scale, Revised (CDRS-R) (ΔCDRS-R) and change in short-interval intracortical inhibition (SICI)-2 (ΔSICI-2) and SICI-4 (ΔSICI-4). Greater improvement in CDRS-R (more negative difference) corresponded to greater improvement in cortical inhibition (more negative difference). DIFF, difference.
Sensitivity Analyses
We performed an exploratory sensitivity analysis comparing baseline excitability measures between healthy and depressed groups, excluding participants on stimulants. Results of the robust regression analysis were similar and remained significant (SICI-2: P = .007; SICI-4: P = .051), suggesting that stimulant use did not account for the baseline differences between the groups. Of note, stimulants were held on the day of assessments with the goal of preventing the possible impact on cortical excitability measures. We also conducted a sensitivity analysis for patients who were treated with fluoxetine (excluding the patients who were on bupropion and escitalopram) to test the possible effects of different medications on cortical excitability. Robust regression analysis of the correlation between the change in CDRS-R scores (ΔCDRS-R) and change in cortical inhibition (ΔSICI-2 and ΔSICI-4), controlled for age and sex, showed similar results (SICI-2: P = .127; SICI-4: P < .001) even when excluding the patients who were taking medications other than fluoxetine.
Discussion
To our knowledge, this is the first longitudinal study of TMS measures of cortical inhibition and excitability in adolescents with depression. Adolescents with moderate to severe symptoms of MDD had impaired cortical inhibition compared with healthy controls. Improvement in depressive symptom severity with antidepressant treatment was associated with restored cortical inhibition, as indexed by improved SICI.
Our results are consistent with prior TMS studies of adults that showed impaired SICI for patients with depressive disorders, further supporting the role of impaired GABAA-mediated cortical inhibition in depression (Croarkin et al., 2011). In a meta-analysis (Radhu et al., 2013) of 3 studies (115 adult patients with MDD and 130 healthy controls), (Bajbouj et al., 2006; Lefaucheur et al., 2008; Levinson et al., 2010) SICI was markedly impaired in the MDD group. Results of that meta-analysis also showed shortened CSP in MDD, suggesting impaired GABAB-mediated cortical inhibition. However, we did not find any significant differences in CSP in the current study. In contrast to adult studies, prior TMS studies with depressed adolescents have reported mixed results. Croarkin and colleagues (Croarkin et al., 2013) previously found that medication-naïve children and adolescents with MDD had increased ICF compared with healthy controls, but no significant differences were observed for cortical inhibition measures, including SICI and CSP. However, in a separate posthoc analysis, depression severity negatively correlated with ICF and CSP, despite the absence of group differences (Lewis et al., 2016). In the current study, we did not find any difference in ICF or identify correlations between baseline depression severity and TMS measures. However, it is important to note that the group with depression in the aforementioned adolescent studies (Croarkin et al., 2013; Lewis et al., 2016) differed from the sample in the current study with regard to previous antidepressant medication history, lower mean age (13.9 vs 15.4 years), shorter disease duration (0.9 vs 1.3 years), and exclusion of other comorbid psychiatric disorders (in the previous study).
Overall, the results of the current study suggest that older, previously treated adolescents with MDD and a longer duration of illness might have cortical excitability patterns that are similar to those of depressed adults, even though adolescents may not have adult levels of cortical maturation. An intermediate level of maturation in our sample of adolescents with depression could explain some of the differences between our results and those of previous adult studies (e.g., shortened CSP, a marker of GABAB-mediated cortical inhibition, which was not present in our depressed group) while supporting other similarities (e.g., impaired SICI). A known difference between adults and children is that children have less cortical inhibition (Mall et al., 2004), which might have contributed to the lack of CSP findings in our sample. CSP also can show variability in children and adolescents (Garvey and Mall, 2008) and is affected by interhemispheric differences; these potential confounders were not assessed in this study. Moreover, different TMS indices may have differing patterns of age- or maturation-related changes along different time courses, and little is known about the developmental trajectories of TMS measures in the setting of psychiatric illness (Croarkin et al., 2014b). For example, prior work suggests that depressed children and adolescents might have delayed maturation of cortical excitability when compared with age-matched controls (Croarkin et al., 2014b). Additionally, several other psychiatric disorders in adults (Radhu et al., 2013), as well as attention-deficit/hyperactivity disorder in children (Gilbert et al., 2011), have been associated with impaired excitatory-inhibitory balance. In contrast to previous adolescent studies, we did not exclude participants with comorbid psychiatric disorders; thus, the sample from our present study may be more generalizable to adolescents encountered in typical clinical settings.
The most notable finding of this study was the positive association between the improvement in depression severity and improvement in SICI after antidepressant therapy. Given that SICI indirectly reflects GABAA activity (Hanajima et al., 1998; Di Lazzaro et al., 2000, 2005; Ilić et al., 2002; Hanajima et al., 2003), this result suggests that restored GABAergic inhibition is associated with improved depression severity in the setting of antidepressant treatment. Prior research in adults has shown restored GABAergic activity with therapeutic interventions such as electroconvulsive therapy (Sanacora et al., 2003), repetitive TMS (rTMS) (Daskalakis et al., 2006), and selective serotonin reuptake inhibitors (SSRIs) (Sanacora et al., 2002). Daskalakis and colleagues (Daskalakis et al., 2006) found state-dependent effects of rTMS on SICI in healthy participants and showed that those with less cortical inhibition at baseline (higher SICI ratios) showed more improvement in SICI after rTMS. They also showed potentiation of CSP with high- and low-frequency rTMS. In a proton magnetic resonance spectroscopy study of patients with MDD, Sanacora and colleagues (Sanacora et al., 2002) showed that occipital GABA levels increased after treatment with SSRIs. Other studies also showed increased SICI (greater inhibition) in patients with MDD and healthy controls after administration of SSRIs (Manganotti et al., 2001; Robol et al., 2004). Even though no study has assessed longitudinal changes in TMS measures in adolescents, another study of depressed adolescents from our group has shown an association between pretreatment LICI impairment (which reflects GABAB-mediated activity) and poor treatment response to fluoxetine, suggesting that deficits in GABAB functioning may be associated with treatment nonresponse (Croarkin et al., 2014a). In our study, almost all patients had some degree of improvement in their total CDRS-R scores (range, −3 to −38), which may explain the lack of differences in these GABAB measures. It is possible that adolescent samples with greater degrees of treatment resistance would show different relationships between the change in depressive symptoms and changes in LICI and CSP.
Several mechanisms potentially explain how serotonergic (5-HT) activity and GABAergic activity interact. First, GABAergic neurons inhibit 5-HT neurons in midbrain raphe nuclei (Tao and Auerbach, 2000). Also, previous research has shown that the serotonergic innervation of the cortex is mediated through the excitatory effects of 5-HT2A and 5-HT3 receptors on GABAergic interneurons in the cortex, hippocampus, and amygdala (Freund et al., 1990; Jakab and Goldman-Rakic, 2000). Indeed, 5-HT2 and 5-HT3 agonists increase the activity of GABAergic interneurons in these areas (Gellman and Aghajanian, 1993; Abi-Saab et al., 1999). These findings are consistent with aforementioned studies showing temporary increases in cortical inhibition, as measured by TMS, after administration of single doses of clomipramine (Manganotti et al., 2001) and citalopram (Robol et al., 2004). When administered regularly, SSRIs can induce plasticity within these neural networks (Normann et al., 2007; Liu et al., 2017). Although it is difficult to ascertain whether the modulation of GABAergic activity with SSRIs leads to improvement of depressive symptoms or whether improvement in depressive symptoms through other mechanisms leads to changes in inhibitory and excitatory networks, our results suggest that SICI is a potential treatment target in depression because of its potential to index deficits in GABAergic activity.
There are several limitations of this study. First, our sample size for the follow-up group was small and did not allow us to control for all potential covariates of interest. We also had a relatively heterogeneous group with regard to age, race/ethnicity, current medications, and history of prior antidepressant use. With regard to the potential impact of race or ethnicity, we are not aware of any previous studies comparing cortical excitability measures between black and white participant groups; however, one small study showed cortical excitability differences between Han Chinese and Caucasians (Yi et al., 2014). On the other hand, comparisons of TMS measures, including our main findings (SICI-2, SICI-4), between black and white participants within the healthy group did not show any significant differences between these groups. In terms of medication use, our sensitivity analysis showed similar results when we excluded participants taking medications other than fluoxetine. Although the mean age of the healthy participants was lower than that of the depressed participants, we did control for age in the statistical analyses. Further, younger participants would be expected to have greater SICI-2 impairment (Mall et al., 2004). Despite this age difference in our study, the older depressed sample had greater SICI-2 impairment. Finally, TMS testing of the motor cortex employed manual localization of the APB “hotspot.” This could introduce bias into MEP findings based on the variability of pulses among subjects and between sessions, with potential resultant changes in cortical inhibition. While our methodology attempted to mitigate the potential bias that this could have introduced among MEP measures, future studies should utilize position tracking devices, conventional measurement (e.g., 10–20 EEG system), or neuro-navigation (Ahdab et al., 2010). The major strength of our study was being the first study, to our knowledge, to compare longitudinal changes in TMS measures associated with adolescent depression before and after treatment. This study adds important preliminary data to the existing literature toward developing target biomarkers of depression and antidepressant treatment.
Conclusion
In this naturalistic, longitudinal study investigating TMS markers in adolescents with MDD, we showed that cortical inhibition was impaired, with patterns somewhat similar to those reported for adults with depression. Moreover, we showed that improvement in depression severity after antidepressant treatment is associated with restored cortical inhibition, as indexed by SICI. This was the first study to our knowledge to investigate longitudinal changes in TMS measures of cortical inhibition associated with antidepressant treatment in an adolescent population. These findings provide important preliminary data that will inform future studies investigating the neurobiology of adolescent depression and enable the development of better diagnostic methods and individualized, biomarker-guided therapeutic approaches in this population.
Funding
This research was supported by grants from the Brain and Behavior Research Foundation (Young Investigator Award 20883), the National Institute of Mental Health (K23 MH100266 and R01MH113700). This publication was also made possible by the Mayo Clinic Clinical Translational Science Award (CTSA) through grant number UL1TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
The sponsors had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Editing, proofreading, and reference verification were provided by Scientific Publications, Mayo Clinic.
Statement of Interest
D.D.C. receives research support from the Mayo Clinic Foundation Departmental Small Grant Program. C.P.L. receives research support from the Brain and Behavior Research Foundation and the Mayo Clinic Foundation Departmental Small Grant Program and is a site investigator for a multicenter studies funded by Neuronetics, Inc and NeoSync, Inc.
P.E.C. has received research grant support from Pfizer, Inc, NIMH (K23 MH100266 and R01MH113700), the Brain and Behavior Research Foundation, and the Mayo Clinic Foundation. He has served as a site subprincipal or principal investigator (without additional compensation) for Eli Lilly and Co, Forest Laboratories, Inc, Merck & Co, Inc, and Pfizer, Inc; has received equipment support from Neuronetics, Inc; and receives supplies and genotyping services from Assurex Health, Inc for an investigator-initiated study. He is the primary investigator for a multicenter study funded by Neuronetics, Inc. He is a site primary investigator for a study funded by NeoSync, Inc. He has consulted for Procter and Gamble. In the last 5 years, Z.J.D. has received research and equipment in-kind support for an investigator-initiated study through Brainsway, Inc. and Magventure, Inc. His work was supported by the Ontario Mental Health Foundation (OMHF), the Canadian Institutes of Health Research (CIHR), the National Institutes of Mental Health (NIMH), and the Temerty Family and Grant Family and through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute. A.I.S., A.L.N., and M.A.G. have no financial disclosures.
References
- Abi-Saab WM, Bubser M, Roth RH, Deutch AY (1999) 5-HT2 receptor regulation of extracellular GABA levels in the prefrontal cortex. Neuropsychopharmacology 20:92–96. [DOI] [PubMed] [Google Scholar]
- Ahdab R, Ayache SS, Brugières P, Goujon C, Lefaucheur JP (2010) Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol Clin 40:27–36. [DOI] [PubMed] [Google Scholar]
- Anand S, Hotson J (2002) Transcranial magnetic stimulation: neurophysiological applications and safety. Brain Cogn 50:366–386. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Murray JD (2017) Rebalancing altered computations: considering the role of neural excitation and inhibition balance across the psychiatric spectrum. Biol Psychiatry 81:816–817. [DOI] [PubMed] [Google Scholar]
- Avenevoli S, Swendsen J, He J-P, Burstein M, Merikangas KR (2015) Major depression in the National Comorbidity Survey–Adolescent Supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry 54:37–44.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajbouj M, Lisanby SH, Lang UE, Danker-Hopfe H, Heuser I, Neu P (2006) Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol Psychiatry 59:395–400. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, Perel J, Nelson B (1996) Childhood and adolescent depression: a review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry 35:1427–1439. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Civardi C, Mutani R (1992) Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology 42:1951–1959. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P (1999) Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res 128:539–542. [DOI] [PubMed] [Google Scholar]
- Cheung AH, Emslie GJ, Mayes TL (2005) Review of the efficacy and safety of antidepressants in youth depression. J Child Psychol Psychiatry 46:735–754. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Levinson AJ, Daskalakis ZJ (2011) Evidence for gabaergic inhibitory deficits in major depressive disorder. Neurosci Biobehav Rev 35:818–825. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Nakonezny PA, Husain MM, Melton T, Buyukdura JS, Kennard BD, Emslie GJ, Kozel FA, Daskalakis ZJ (2013) Evidence for increased glutamatergic cortical facilitation in children and adolescents with major depressive disorder. JAMA Psychiatry 70:291–299. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Nakonezny PA, Husain MM, Port JD, Melton T, Kennard BD, Emslie GJ, Kozel FA, Daskalakis ZJ (2014a) Evidence for pretreatment LICI deficits among depressed children and adolescents with nonresponse to fluoxetine. Brain Stimul 7:243–251. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Nakonezny PA, Lewis CP, Zaccariello MJ, Huxsahl JE, Husain MM, Kennard BD, Emslie GJ, Daskalakis ZJ (2014b) Developmental aspects of cortical excitability and inhibition in depressed and healthy youth: an exploratory study. Front Hum Neurosci 8:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S (2002) Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry 59:347–354. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Möller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R (2006) The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res 174:403–412. [DOI] [PubMed] [Google Scholar]
- Day BL, Marsden CD, Rothwell JC, Thompson PD, Ugawa Y (1989a) An investigation of the EMG silent period following stimulation of the brain in normal man. J Physiol 414:14P. [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Maertens de Noordhout A, Nakashima K, Shannon K, Marsden CD (1989b) Delay in the execution of voluntary movement by electrical or magnetic brain stimulation in intact man. Evidence for the storage of motor programs in the brain. Brain 112:649–663. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC (1998) Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 119:265–268. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC (2000) Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol 111:794–799. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, Visocchi M, Colosimo C, Tonali PA, Rothwell JC (2002) Direct demonstration of long latency cortico-cortical inhibition in normal subjects and in a patient with vascular parkinsonism. Clin Neurophysiol 113:1673–1679. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone R, Ranieri F, Musumeci G, Fiorilla T, Tonali P (2005) Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol 564:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, McKie S, Deakin JFW (2015) Ketamine and other potential glutamate antidepressants. Psychiatry Res 225:1–13. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H (2002) Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res 143:240–248. [DOI] [PubMed] [Google Scholar]
- Freund T, Gulyás A, Acsády L, Görcs T, Tóth K (1990) Serotonergic control of the hippocampus via local inhibitory interneurons. 87:8501–8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, Alonso CM, Shungu DC (2012) Anterior cingulate cortex γ-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry 69:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Bradley KA, Mao X, Ostrover R, Kang G, Shungu DC (2017) Anterior cingulate cortex γ-aminobutyric acid deficits in youth with depression. Transl Psychiatry 7:e1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey MA, Mall V (2008) Transcranial magnetic stimulation in children. Clin Neurophysiol 119:973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellman RL, Aghajanian GK (1993) Pyramidal cells in piriform cortex receive a convergence of inputs from monoamine activated gabaergic interneurons. Brain Res 600:63–73. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Isaacs KM, Augusta M, Macneil LK, Mostofsky SH (2011) Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology 76:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. (1976) Clinical global impressions. In: ECDEU assessment manual for psychopharmacology, Revised, pp 217–222. Rockville, MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs. [Google Scholar]
- Hameed MQ, Dhamne SC, Gersner R, Kaye HL, Oberman LM, Pascual-Leone A, Rotenberg A (2017) Transcranial magnetic and direct current stimulation in children. Curr Neurol Neurosci Rep 17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Ogata K, Kanazawa I (1996) Ipsilateral cortico-cortical inhibition of the motor cortex in various neurological disorders. J Neurol Sci 140:109–116. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I (1998) Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol 509:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Furubayashi T, Iwata NK, Shiio Y, Okabe S, Kanazawa I, Ugawa Y (2003) Further evidence to support different mechanisms underlying intracortical inhibition of the motor cortex. Exp Brain Res 151:427–434. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC (2007) Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64:193–200. [DOI] [PubMed] [Google Scholar]
- Henje Blom E, Ho TC, Connolly CG, LeWinn KZ, Sacchet MD, Tymofiyeva O, Weng HY, Yang TT (2016) The neuroscience and context of adolescent depression. Acta Paediatr 105:358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U (2002) Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol 545:153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M (1993) Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol 466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS (2000) Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol 417:337–348. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997) Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988. [DOI] [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM (2001) A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol 112:720. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Lucas B, Andraud F, Hogrel JY, Bellivier F, Del Cul A, Rousseva A, Leboyer M, Paillère-Martinot ML (2008) Inter-hemispheric asymmetry of motor corticospinal excitability in major depression studied by transcranial magnetic stimulation. J Psychiatr Res 42:389–398. [DOI] [PubMed] [Google Scholar]
- Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ (2010) Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry 67:458–464. [DOI] [PubMed] [Google Scholar]
- Lewis CP, Nakonezny PA, Ameis SH, Vande Voort JL, Husain MM, Emslie GJ, Daskalakis ZJ, Croarkin PE (2016) Cortical inhibitory and excitatory correlates of depression severity in children and adolescents. J Affect Disord 190:566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Schwenkreis P, Tegenthoff M, Malin JP (1997) The glutamate antagonist riluzole suppresses intracortical facilitation. J Neural Transm (Vienna) 104:1207–1214. [DOI] [PubMed] [Google Scholar]
- Liu B, Liu J, Wang M, Zhang Y, Li L (2017) From serotonin to neuroplasticity: evolvement of theories for major depressive disorder. Front Cell Neurosci 11:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall V, Berweck S, Fietzek UM, Glocker FX, Oberhuber U, Walther M, Schessl J, Schulte-Mönting J, Korinthenberg R, Heinen F (2004) Low level of intracortical inhibition in children shown by transcranial magnetic stimulation. Neuropediatrics 35:120–125. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Bortolomasi M, Zanette G, Pawelzik T, Giacopuzzi M, Fiaschi A (2001) Intravenous clomipramine decreases excitability of human motor cortex. A study with paired magnetic stimulation. J Neurol Sci 184:27–32. [DOI] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J, Treatment for Adolescents With Depression Study (TADS) Team (2004) Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: treatment for adolescents with depression study (TADS) randomized controlled trial. Jama 292:807–820. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U (2006) The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res 173:86–93. [DOI] [PubMed] [Google Scholar]
- Minelli A, Bortolomasi M, Scassellati C, Salvoro B, Avesani M, Manganotti P (2010) Effects of intravenous antidepressant drugs on the excitability of human motor cortex: a study with paired magnetic stimulation on depressed patients. Brain Stimul 3:15–21. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H (1997) Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol 498:817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann C, Schmitz D, Fürmaier A, Döing C, Bach M (2007) Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry 62:373–380. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. (1971) The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Perou R, Bitsko RH, Blumberg SJ, Pastor P, Ghandour RM, Gfroerer JC, Hedden SL, Crosby AE, Visser SN, Schieve LA, Parks SE, Hall JE, Brody D, Simile CM, Thompson WW, Baio J, Avenevoli S, Kogan MD, Huang LN, Centers for Disease Control and Prevention (CDC) (2013) Mental health surveillance among children–united states, 2005-2011. MMWR Suppl 62:1–35. [PubMed] [Google Scholar]
- Pierantozzi M, Marciani MG, Palmieri MG, Brusa L, Galati S, Caramia MD, Bernardi G, Stanzione P (2004) Effect of vigabatrin on motor responses to transcranial magnetic stimulation: an effective tool to investigate in vivo gabaergic cortical inhibition in humans. Brain Res 1028:1–8. [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ (2011) The columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R (1984) Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Psychiatry 23:191–197. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB (1996) Children’s Depression Rating Scale, Revised (CDRS-R). Los Angeles: Western Psychological Services. [Google Scholar]
- Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ (2013) A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol 124:1309–1320. [DOI] [PubMed] [Google Scholar]
- Robol E, Fiaschi A, Manganotti P (2004) Effects of citalopram on the excitability of the human motor cortex: a paired magnetic stimulation study. J Neurol Sci 221:41–46. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Krystal JH (2002) Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry 159:663–665. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, Berman RM, Krystal JH (2003) Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry 160:577–579. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M (2012) Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenkreis P, Witscher K, Janssen F, Addo A, Dertwinkel R, Zenz M, Malin JP, Tegenthoff M (1999) Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neurosci Lett 270:137–140. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Liepert J, Witscher K, Fischer W, Weiller C, Malin JP, Tegenthoff M (2000) Riluzole suppresses motor cortex facilitation in correlation to its plasma level. A study using transcranial magnetic stimulation. Exp Brain Res 135:293–299. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B (1998) Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve 21:1209–1212. [DOI] [PubMed] [Google Scholar]
- Stetkarova I, Kofler M (2013) Differential effect of baclofen on cortical and spinal inhibitory circuits. Clin Neurophysiol 124:339–345. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB (2000) Regulation of serotonin release by GABA and excitatory amino acids. J Psychopharmacol 14:100–113. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M (1992) Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85:355–364. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J (1999) Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol 517:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X, Fisher KM, Lai M, Mansoor K, Bicker R, Baker SN (2014) Differences between han chinese and caucasians in transcranial magnetic stimulation parameters. Exp Brain Res 232:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G, Oquendo MA, Greenhill L, Goldberg PH, Kamali M, Martin A, Mann JJ (2006) Neurobiology of depression in children and adolescents. Child Adolesc Psychiatr Clin N Am 15:843–68, VII. [DOI] [PubMed] [Google Scholar]
- Ziemann U. (1999) Intracortical inhibition and facilitation in the conventional paired TMS paradigm. Electroencephalogr Clin Neurophysiol Suppl 51:127–136. [PubMed] [Google Scholar]
- Ziemann U. (2003) Pharmacology of TMS. In: Supplements to Clinical Neurophysiology (Paulus W, Tergau F, Nitsche MA, Rothwell JG, Ziemann U, Hallett M, eds), pp 226–231. Amsterdam: Elsevier. [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M (1998) Dextromethorphan decreases the excitability of the human motor cortex. Neurology 51:1320–1324. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC (1996) Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol 496:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]