Abstract
The adult brain, even though largely postmitotic, is now known to have dividing cells that can make both glia and neurons. Of these, the precursor cells that have the potential to make new neurons in the adult brain have attracted great attention from researchers, anticipating their therapeutic potential for neurodegenerative conditions. In this review, I will focus on adult neurogenesis, from the perspective of the overall neurogenic potential in the adult brain, current understanding of the ‘adult neural stem cell’, and the importance of niche as a decisive factor for neurogenesis under homeostasis and pathologic conditions.
Keywords: astrocyte, dentate gyrus, glia, hippocampus, neurogenesis, neural stem cell, niche, fate potential, regeneration, transplantation, radial glia
Introduction
The tantalizing possibility that non-differentiated precursors in the adult brain could differentiate to make new neurons was proposed first in 1962 by Joseph Altman.1 This hypothesis arose from a side observation of an experiment designed to measure kinetics of glial proliferation in rats after brain trauma through incorporation of radio-labelled thymidine, in which Altman had observed some radiolabel retaining neuronal cells. This was quickly followed up in 1963, when Altman2 described for the first time, a proliferative region in the dentate gyrus (DG) in the hippocampus. Finally, in 1965, Altman and Das3 published a detailed study of hippocampal neurogenesis, where they reported germinal regions in the subventricular zone (SVZ) and subgranular zone (SGZ) of the adult rat brain, and also noted a rapid decline with age in the germinal pool of cells. It has been 56 years since, and it is now widely accepted that adult neurogenesis, the process of new neuron generation happens in a variety of species including birds, fish, reptiles and mammals. The frequency, location and function of adult neurogenesis in humans continues to be contentious and an intense area of research. However, the debate of whether the adult brain is completely post-mitotic or not, has largely been rested with the verdict that the adult brain indeed retains a variety of mitotic progenitors that retain both glial and neuronal potential.
In this review, the latest findings in the field will be discussed, describing the potential origin of the neural stem cells (NSCs) of the adult brain, the differences between the two bona fide adult neurogenic niches and the generative potential of the adult brain beyond the bona fide neurogenic niches. The primary focus of this review will be hippocampal adult neurogenesis. In addition, it will also review studies that shed light on the de novo potential of the adult brain parenchyma in general, for neurogenesis under homeostatic and pathologic conditions. We hope to provide insights by integrating studies that are important for uncovering the molecular process of adult neurogenesis with those that are geared towards exploring its potential therapeutic usability.
Neurogenic Regions in the Adult Brain
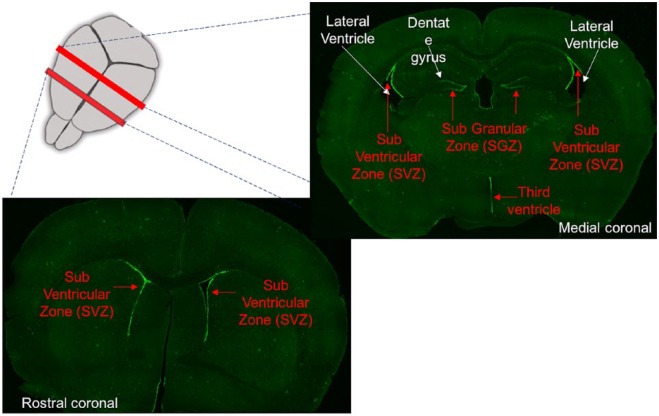
The two prominent locations for adult neurogenesis are the DG in the hippocampus and the lateral ventricles. Adult neurogenesis in the DG occurs on the hilar side of the granule cell (GC) layer, in a two- to three-cell-layer thick region called the subgranular zone or SGZ (Figure 1). In the lateral ventricles, a similar germinal region runs along the ventricles, called the subventricular zone or SVZ.
Figure 1.
Rostro-caudal sections showing the adult neurogenic regions of the murine brain as green lining of GFP expression driven by Nestin promoter, marking the adult neural stem cells.
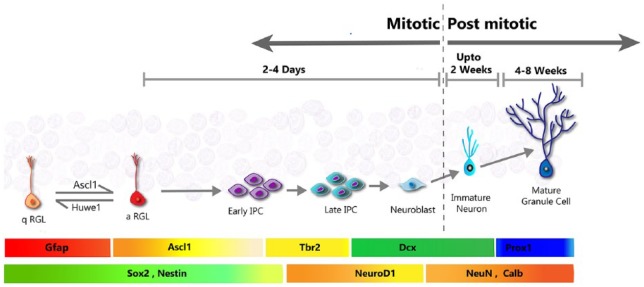
The process of adult neurogenesis in the hippocampus starts with the quiescent NSCs, which are also called the radial glia-like (RGL) cells because of their morphology and ontogeny. The quiescent radial glia-like (qRGL) cell when activated (aRGL) can divide to self-renew and/or make intermediate proliferating progenitors (IPCs), which proliferate multiple rounds before differentiating into neuroblasts. About 25% of these neuroblasts are able to survive and mature to become granule neurons of the DG.4 The timeline, course of developmental progression, and the specific cellular markers which identify each of the specific cell-stages of this process, are very well characterized (Figure 2). Many regulators, both cell-intrinsic and cell-extrinsic, have been identified for the process of adult neurogenesis. However, the fate-determining factor(s) that might be responsible for making a choice between neuronal or astrocytic fate in adult hippocampal neural progenitors, still remain to be identified. The details of the molecular process involved in hippocampal adult neurogenesis5–8 and adult neurogenesis in the olfactory bulb (OB) or SVZ has been reviewed in detail elsewhere.9
Figure 2.
Hippocampal adult (SGZ) neurogenesis. The quiescent Radial Glial-like (RGL) cells, also called the neural stem cell (NSC) upon activation can divide to make intermediate proliferating cells (IPC), thereby initiating adult neurogenesis. The IPC proliferate to expand themselves and differentiate to neuroblasts. A fraction of these neuroblasts go on to mature and integrate as mature granule neurons of the Dentate Gyrus. The approximate timeline of the steps, and the stage-specific protein expression are shown in the coloured horizontal panels adjacent to the specific cell-stages.
Although adult neurogenesis in the SGZ and SVZ have many similarities, the fate potential and ultimate outcome of the neurogenic process in these two regions are distinct. The neurons produced during adult neurogenesis in SVZ are mostly interneurons of the OB, which are inhibitory in nature, whereas SGZ neurogenesis gives rise to excitatory granule neurons of the DG. While SVZ neurogenesis involves migratory maturation, SGZ new-born neurons are largely restricted to the GC layer and do not need much migration. The neural progenitors in SVZ are fate-restricted and diverse, making specific neurons or oligodendrocyte precursor cells (OPCs). This was demonstrated first by in vivo lineage tracing by Buylla and colleagues.10,11 In contrast to the wide variety of spatially oriented fate-restricted progenitors in SVZ, the SGZ neural progenitors are thought to be rather homogeneous in their bipotential fate throughout the SGZ.12,13
Notably, the inherent organization of the SVZ-OB region and the DG present distinct tissue architectures with respect to the cellular composition, function, density of cells, and the existing circuitry into which the new neurons need to integrate (Figure 3). While in the OB the SVZ-derived new-born neurons get integrated into a laminar structure, in the DG, the SGZ-derived new-born neurons are in a thickly packed cell layer. Thus, the integration process for new-born neurons generated in the densely packed region of DG is expected to be different from that in the OB. Despite these distinctions, the continual generation of new neurons, of which about 50-70% die during the first few days of birth, is common to the two neurogenic niches. While the death of majority of the new-born neurons in the neurogenic niche could just be a matter of stringent limitations for making it to maturation and integration into existing circuitry, it is thought that the short-living new-born neurons, which have different electrical properties than their mature counterparts, may have a functional importance of their own.
Figure 3.
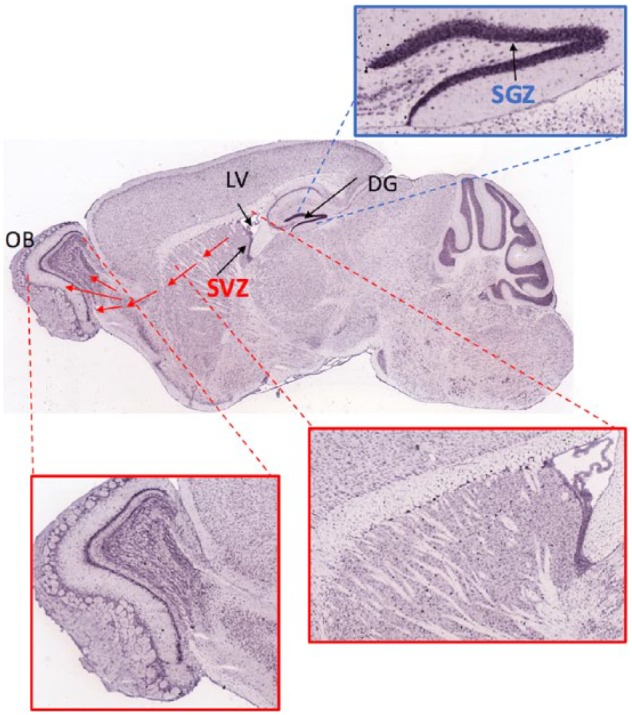
Tissue architecture of SVZ and SGZ neurogenic niches.
Map2 mRNA of mouse sagittal brain sections obtained from Allen Brain Atlas illustrates the tissue architecture. Location of the neurogenic niches SVZ, along the Lateral ventricles (LV), and SGZ along the Dentate Gyrus (DG) is shown with arrows. The cellular architecture of the regions where new-born neurons from the respective niches need to migrate (or not, for SGZ) and integrate, is shown in insets for depicted areas. As can be seen by Map2 staining the region of migration and integration of new-born neurons in SVZ is less dense compared to the thickly packed cellular region of DG where new-born neurons from the SGZ are born and reside.
Hippocampal adult neurogenesis is known to play a role in pattern separation, a specialized function of the DG, which allows similar contexts and memories to be distinguished and coded distinctly. More recently, studies have suggested that adult-born GCs may be more functional in the contextual and sensory aspect of hippocampal/DG function, rather than spatial encoding.14,15 In tune with this, the activity of new-born neurons, specifically in the ventral hippocampus was shown to be important for stress resilience.16 Using in vivo calcium imaging to record activity from a large number of GCs in the DG, this study showed that adult born neurons of the ventral DG supress the activity of mature GCs that preferentially respond to attacks or anxiogenic stimuli, thereby conferring resilience to chronic stress.
Other than the two active neurogenic niches in the brain, adult neurogenesis has also been reported in other parts of the brain such as in the hypothalamus along the third ventricle and in the striatum. While hypothalamic adult neurogenesis has been reported in rodent models, the striatal neurogenesis has been shown only in humans. This will be discussed more in later section of this review.
Origins of Adult NSCs
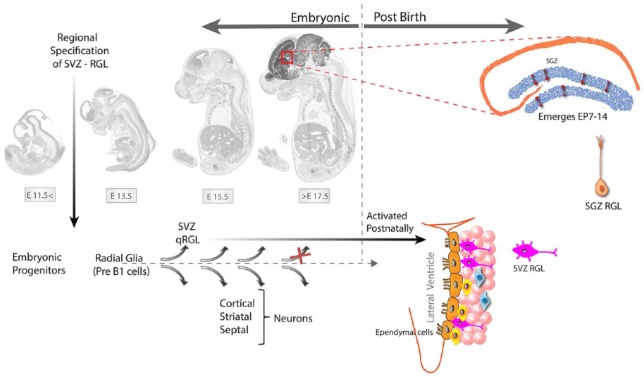
Lineage tracing and single-cell transcriptomic studies suggest that the lineage association and diversification of embryonic NSCs from adult NSC in SVZ and SGZ follow distinct timelines (Figure 4). While adult NSC (also called radial glia like, RGL) of both SVZ and SGZ are derived from embryonic radial glial (RG) cells; in the case of SVZ, the adult NSC abruptly bifurcate at embryonic day 14 (E14) when the RGs destined to become SVZ NSC upregulate p57kip2 to enter quiescence and divide only later in SVZ.11,17 In contrast, the SGZ RGL seem to take their distinct identity only in the postnatal weeks 2 to 3 (P14-P18), where their transcriptomic identity becomes distinct from that of their precursors, the embryonic radial glia cells.18
Figure 4.
Origin of the adult neural stem cells or radial glia-like cells (NSC/RGL) of the SVZ and SGZ.
The SVZ adult neural stem cells are made during embryogenesis between E13.5 and 15.5 from the same progenitors that make neurons of the embryonic brain. The qRGLs get activated after birth to participate in adult neurogenesis in the SVZ. The RGL of the adult hippocampus arise from the ventral part of the late embryonic lateral ventricle around postnatal day 7-14. Hopx + precursors arising at E11.5 dentate neuroepithelium adopt quiescent RGL properties in adult DG,
Retroviral lineage tracing of proliferating progenitors from embryonic day 12.5 (E12.5) to postnatal day 53 (P53) revealed that the precursors at E13.5 to E15.5 give rise to both adult NSC of SVZ and the embryonic NSC that make neurons of the cortex, striatum and septum.11 Interestingly, this lineage sharing between embryonic and adult neurogenesis was lost after E17.5, after which the embryonic proliferating progenitors do not contribute to the adult SVZ NSC. Furthermore, this study demonstrated that the embryonic precursors already had spatial restrictions such that NSC from a specific region of the lateral ventricle gave rise to neurons only of a certain region of the brain.
The precursors of adult SGZ NSC are not as well identified as that of SVZ NSC. Using Emx1-Cre-driven inducible deletion of Smoothened and Sonic hedgehog, it was demonstrated that the Sonic hedgehog responding cells from the ventricular zone of amygdalo-hippocampal area, during late gestation, contributes towards SGZ formation in perinatal stages.19 Another study used a suite of stem and progenitor cell markers to demonstrate that a condensed germinal zone in SGZ appears first during postnatal days 7-14, before which neither the bona fide RGLs (Blbp + Sox2 + Nestin+ cells) nor a defined region of DCX-positive cells are found.20 The concentrated expression of DCX and Tbr2, proteins expressed in IPC, neuroblasts and immature neurons, in SGZ appears only after P7. Given the distinct temporal dynamics of the neurogenic niche formation in SVZ and SGZ, this study argued that the later emergence of SGZ could be because of its dependence on neural activity for adult neurogenesis. A recent report suggests that a dentate-specific neural progenitor, arising in mice at ~E11.5 and marked by Hopx positivity, persists through embryonic development to adulthood. These progenitors give rise to primitive (E18.5) and postnatal (P7) dentate region and then transition to quiescence early postnatally, only to contribute to neurogenesis during adult lifespan. However, this study suggested that the Hopx + RGLs have limited capacity for self-renewal, are skewed towards neurogenic differentiation, and rarely make astrocytes.21 Apart from ontogeny, another difference between SVZ and SGZ RGL is there morphology. While RGL of both SGZ and SVZ have a long radial process, the SGZ RGL show further branching only at the end of the radial process, much distal to the cell body. However, the RGLs in SVZ show processes proximal to some as well. This could potentially be attributed to the differential tissue architecture in which they emerge and function.
Fate Potential of Adult NSC
Even though the term ‘neural’ stem cell indicates that the differentiated fate of these cells is ‘neuron’, the neural stem cells have multiple fate potential (Figure 5). In addition, the mode of differentiation between SGZ and SVZ RGL/NSC are different. Clonal analysis based on genetic tracking of RGL and recent unbiased single-cell transcriptome-based approaches, as well as whole mount time-lapse imaging have provided great insights into the molecular nature of adult brain NSC during their progression from quiescence to activation and differentiation.
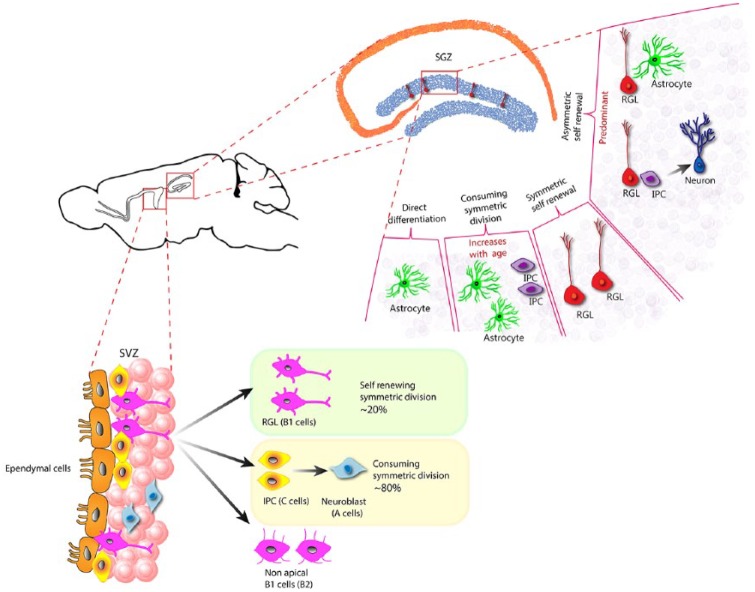
Figure 5.
Fate potential of adult neural stem cells in SGZ and SVZ.
The quiescent radial glial-like (RGL) cells, in the SGZ upon activation can undergo four distinct routes: they can asymetrically divide to self-renew and make an astrocyte or an intermediate proliferating cell (IPC). An RGL can also undergo symmetric division to make two RGLs resulting in only self-renewal without making a differentiated progeny. An RGL could also undergo consuming symmetric division making two astrocytes or two IPC, without self-renewing itself, and finally, an RGL could undergo direct differentiation without cell division to become an astrocyte. The mode of asymmetric self-renewing division is the more common outcome in an adult healthy brain, whereas the consuming symmetric division is thought to increase with age, resulting in reduction in NSC in aged brains. In the SVZ, the RGL mostly undergo symmetric cell division that could be either self-renewing or consuming. The SVZ RGL symmetric self-renewal could occasionally also result in another type of RGL that lack the apical process, named as the non-apical B1 cells or B2 cells.
Clonal analysis, genetic lineage tracing and BrdU pulse-chase experiments revealed that RGL cells of the DG/SGZ are capable of both symmetric and asymmetric cell division to undergo self-renewal and bi-lineage differentiation. Bonaguidi et al12 used genetic reporter-based tracing to perform clonal analysis of individual RGL over a long time period. Their data demonstrated that self-renewal through symmetric division represents the fate of a minority of RGLs, whereas most of RGLs undergo asymmetric cell division resulting in self-renewal and differentiation to form either an astrocyte or neuron but never an oligodendrocyte under normal conditions. Interestingly, this study found that the frequency of clones of RGL containing a differentiated astrocyte or neuroblast were nearly comparable with only a small skew towards neuronal fate, suggesting the relatively unbiased bipotent nature of the SGZ RGL. Although, other studies have shown a latent potential for RGL to make oligodendrocyte under induced conditions.22,23
Long-term tracing of RGLs further revealed that the frequency of qRGL stabilizes during aging after initial sharp decline in the first few months of adulthood. However, the number of RGL clones without an RGL, but only differentiated progeny, increased with age. Notably, a significant number of clones contained RGL and a differentiated progeny at 12 months after induction, suggesting that RGL are maintained through self-renewal for a long term. The observation that an activated RGL could be found at a much later time point has been supported by a recent study which demonstrated that activated RGL could return back to quiescence owing to the protein Huwe1, an ubiquitin ligase, which destabilizes Ascl1 (an activation inducing transcription factor) in RGLs to allow them to return to quiescence.24
Another study using genetic reporter and single and double nucleotide labelled tracing methods argued that RGLs in SGZ only undergo asymmetric cell division,13 in which an activated RGL undergoes 3 to 4 rounds of divisions, each time making an RGL and an IPC, and then exits the cell cycle to differentiate into an astrocyte. This study argued that the ultimate differentiation of RGL into astrocytes underlies the age-related reduction of NSC in SGZ. In contrast to the observations in Bonaguidi et al’s study, this study noted that approximately three-fourth of the NSC became neurons, whereas only one-fourth of them took an astrocytic fate. The different observations in these two studies could be a result of different tracing methods and use of different Nestin-based reporter lines, as the possible heterogeneity in SGZ RGL based on differential expression of Nestin has also been reported.25
In the SVZ, symmetric cell division has been demonstrated to be the mode of differentiation and self-renewal.26 Buylla et al performed genetic lineage tracing along with ex vivo whole mount time-lapse imaging, demonstrating that about 20% to 30% of SVZ RGL divide symmetrically making two RGLs, whereas most RGLs undergo differentiative symmetric divisions making type C cells, the IPC equivalents of SGZ. Their data also demonstrate that the minor percentage of NSC undergoing self-renewing symmetric division underlies the sustained pool of NSC through aging in SVZ.
What Have We Learned From Single-Cell Transcriptome Data?
Discerning continual progressive developmental stages from inherent cellular heterogeneity of a given tissue sample has been a daunting task for decades. Single-cell transcriptomics has made it possible to approach this issue in an unbiased way, through transcriptomic profiling of single cells which could be analysed for a dynamic process, such as development, using sophisticated algorithms that use known information to plot cells in a pseudo-temporal progression.
The pioneering study by Shin et al used single-cell transcriptomics to investigate the dynamics of NSC progression from quiescence to activation during hippocampal adult neurogenesis.27 In this study, Nestin-driven genetic reporter was used to manually pick single cells that expressed Nestin-driven fluorescent protein or their immediate progeny. Given that quiescent RGL in SGZ are Nestin positive, the isolated cells consisted of quiescent and activated RGL and their immediate progeny, the early intermediate progenitor cells (eIPC). Single-cell transcriptome profiling of about 142 cells from the SGZ demonstrated that the adult NSCs follow similar dynamics as other somatic stem-cell systems, whereby induction of translation and metabolic and niche signalling genes, mark the activation of stem cells to pursue the process of differentiation. Using a novel algorithm to address the dynamic process of neurogenesis, this study was able to capture a continuum of gene expression dynamics, thereby discerning stochastic heterogeneity from developmental trajectory. This single-cell transcriptome analysis confirmed known observations and established that quiescence in NSC is marked by high expression of signalling genes, whereas increasing the capacity for translation is the first hallmark of NSC activation. An important observation was the switch in expression of genes encoding membrane-targeted to nuclear-targeted proteins which suggested that while quiescent NSC closely follow extrinsic or niche signals, the activated NSC and early IPC are instructed mainly by cell-intrinsic regulations.
Later studies used single-cell transcriptomics to extend their investigations beyond the NSC/RGL populations in the hippocampus. Artegiani et al28 combined data sets from Nestin-GFP cells isolated based on known markers, and cells from unbiased selection (after negatively selecting for mature GCs in the DG), resulting in isolation of the DG neurogenic niche that consisted of neuronal, glial, endothelial, and hematopoietic cell. An important aspect of this study was the single-cell transcriptome comparison of aged neurogenic niche with the young, which suggested that the relative abundance of the specific cell types, rather than the molecular nature of each cell type differs between the young and old neurogenic niche in DG. Briefly, this analysis demonstrated that the aged DG had much higher abundance of microglia, OPCs, but much fewer NSC and lower ratio of NSC to IPC. This supports earlier findings by Encinas’s and Bonaguidi’s studies, which also demonstrated a decrease in the number of RGL due to terminal differentiation and/or lower frequency of symmetric self-renewal with age.
In a recent study, the single-cell information was further elaborated with the largest data set so far, consisting of more than 24 000 cells from the DG across different developmental time points.18 This included cells from embryonic, perinatal, juvenile, and adult mouse DG, providing the first molecular evidence of distinctions and commonality in the molecular trajectory between embryonic and postnatal neurogenesis in DG. Confirming previous observations, this study provided molecular evidence for the divergence of adult NSC, the RGL, from the embryonic stem cell, RG, demonstrating that RG to RGL transition happens sharply in the second postnatal week in mice. Interestingly, while the molecular identity of RGL and GCs change from postnatal to adult brain, the neuroblasts and immature neuron remain indistinguishable at all stages. The common molecular nature of neuroblast and immature neurons demonstrated the commonality of the developmental progression during embryonic and adult neurogenesis, while highlighting the inherent distinction in the nature (and therefore potentially the responses) of the NSC between the embryonic and adult brain. Furthermore, the study also suggests that the critical decision of fate choice happens early on when RGL begin to divide after activation because the IPC transcriptome already shows neurogenic programme. This is consistent with the clonal analysis by Bonaguidi et al demonstrating that RGL is bipotential, whereas IPC are fate-restricted to be neurons.
The fate potential for RGL of the adult SVZ has been described through genetic labelling of adult stem cells in SVZ and tracking their fate in vivo.10 This study showed that while adult SVZ RGL retain multipotentiality of fate,29–31 they were fate-restricted for making only a certain kind of neuron which was regionally specified, similar to embryonic NSC. A recent study that performed single-cell analysis of NSC from adult SVZ, demonstrated distinct populations of qNSC and aNSC, expressing distinct combination of lineage-specific transcription factors.32 They posited that the fate restrictions for different neuronal and glial subtypes could be observed both for qNSC and aNSC in the SVZ niche. The existence of multiple populations of aNSC was further supported by another single-cell study,33 which also observed a transcriptionally distinct continuum of activated NSC in SVZ.
In contrast to the heterogeneity of NSC in SVZ, unbiased clustering from single-cell sequencing data from the SGZ niche show a rather tight single population of NSC.18,28 However, data from Shin et al demonstrated that the transition from qRGL to early IPC in SGZ involve at least five different transcriptome states demonstrating a continuum of heterogeneity during RGL to IPC transition. These data, although in line with SVZ NSC heterogeneity, however, do not suggest lineage restriction in the case of SGZ RGL. Strikingly, a recent study that prospectively isolated qNSC and aNSC from the adult rodent SVZ, demonstrated that qNSC in the adult SVZ do not express Nestin.34 This observation is critical because a lot of studies use Nestin as a marker for NSC/RGL in general. The absence of Nestin expression in SVZ NSC could be yet another distinction between SGZ and SVZ NSC, since single-cell transcriptome in SGZ does not suggest that qNSC in SGZ are Nestin negative.
The General Neurogenic Potential of the Adult Brain
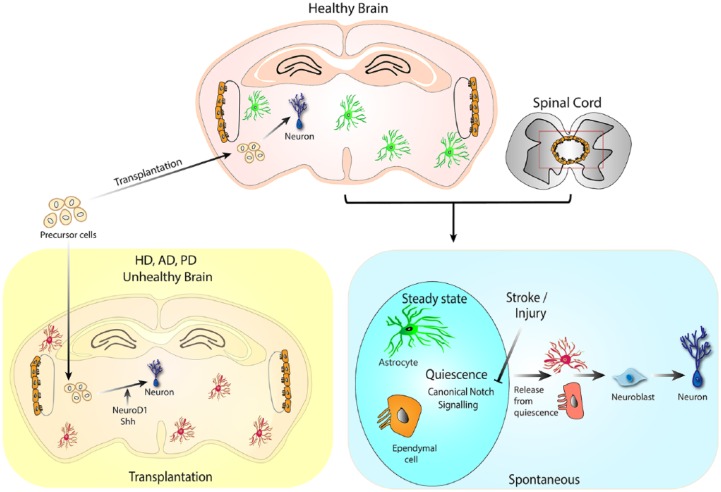
While it is clear that the adult brain is not completely postmitotic, as both neuronal and glial cells can be generated in the adult brain, an important question to ponder over is: does the entire adult brain parenchyma have neurogenic potential in general? Various studies have indicated neurogenesis in brain regions outside of the two bona fide niches, SGZ and SVZ. Although, other studies have demonstrated the capacity for regeneration using transplantations of stem or pluripotent cells. I will discuss these studies under two categories: ‘the spontaneous potential’ and ‘ the transplantation potential’ for new neuron generation and integration in the adult brain (Figure 6).
Figure 6.
Generative potential of adult brain parenchyma.
Parenchymal astrocytes and ependymal cells have been shown to have neurogenic potential. In the steady-state brain canonical notch signalling keeps these cells in quiescence. Insults, such as stroke can block notch signalling to activate the neurogenic potential of the parenchymal astrocyte and ependymal cells. Transplantation of neural precursors grown in vitro or derived from embryonic brain can undergo neurogenic differentiation in healthy and pathogenic adult brain. HD: Huntington’s Disease, AD: Alzheimers Disease, PD: Parkinson’s Disease
Spontaneous neurogenic potential
The neurogenic potential of astrocytes has been highlighted in multiple studies. Given that the brain consists of a large number of astrocytes, the limited potential of neurogenesis in the adult brain is intriguing. A reasonable hypothesis for limited potential for neurogenesis would be the difficulty in integration of new neurons into existing circuit in a mature healthy brain, as opposed to in a developing brain where the neuronal populations and circuits are still emerging. However, upon injury or neuronal loss, the mechanism and possibility of new neuron generation and integration could be expected to dramatically change. SGZ and SVZ are examples of two differently organized circuits, into which new-born neurons are remarkably capable of integrating in the steady state. This suggests that new neuron integration could happen quite naturally for different types of neurons, and into densely packed cell layer of homogeneous cell type as well as into regions of relative cellular diversity. Notably, studies have shown that other regions of the brain, such as the striatum are capable of new neuron generation and integration in the context of injury.35,36
The latent neurogenic potential of parenchymal astrocytes has been demonstrated in a model of stroke in rodents.36 It was shown that continual notch signalling keeps the striatal astrocytes in a non-NSC status under homeostatic conditions. Stroke suppresses notch signalling in astrocytes, thereby unleashing its latent neurogenic programme giving rise to neuroblasts (DCX + PSA-NCAM+) in the striatum and medial cortex. Some of these neuroblasts also expressed NeuN and nNOS (a marker for striatal interneurons) 7 weeks after stroke. Notably, stroke-induced neuroblasts were contributed both by Nestin-expressing cells from SVZ as well as resident striatal astrocytes, with a higher percentage contribution from striatal astrocytes. Spontaneous striatal neurogenesis was also shown in a lesion mouse model of Huntington’s disease.37
Interestingly, continual striatal adult neurogenesis has been shown in humans and seems to be a unique feature of the adult human brain.38 Neurogenesis in striatum was shown through approaches of carbon birth dating and lipofuscin quantitation (a pigment seen in old cells with auto fluorescent matrix and lipid droplets). It was also shown that not all striatal neurons, but only striatal interneurons, undergo postnatal turnover. Furthermore, this study established a correlation between striatal neuronal turnover and Huntington’s disease by showing that patients with Huntington’s disease have significantly lower turnover rates of striatal neurons and oligodendrocytes.
Similar to striatal astrocytes, cerebral cortical astrocytes have been shown to elicit a neurogenic programme instructed by sonic hedgehog signalling under acute injury context, but not chronic neurodegenerative contexts.39 In addition to the spontaneous neurogenesis during injury/degeneration, several studies have reported the neurogenic potential of parenchymal astrocytes by in vivo reprogramming through overexpression of specific transcription factors such as Sox2.40–42 Apart from astrocytes, ependymal cells that are also maintained in quiescence by canonical notch signalling, have been shown as a natural source for neurogenesis in the adult brain and spinal cord under conditions of injury.43,44 However, it was shown that ependymal cells that could act as a source of neurons in injury, do not qualify as NSCs, as they fail to self-renew sufficiently and exhaust themselves during stroke.
In an ischemic brain injury model,45 regeneration of hippocampal pyramidal neurons was shown to take place from in situ proliferating parenchymal precursors. BrdU labelling of proliferating progenitors in the CA1 region showed that these cells differentiate into mature neurons by 28 days after injury and exhibit normal electrophysiological properties. Intraventricular infusion of growth factors was shown to augment the regenerative responses.
Adult neurogenesis has been also reported in rodent hypothalamus, with a link to diet.46 The RGL ependymal cells at the base of the third ventricle of median eminence, also known as tanycytes, were shown to express the typical NSC markers, Nestin, Sox2, and Vimentin. BrdU labelling and genetic fate tracking of tanycytes showed expression of early and late neuronal markers such as Hu, NeuN, and other hypothalamic mature neuronal markers. It was also shown that hypothalamic neurogenesis increases under high-fat diet, and ablation of neurogenesis specifically from this region results in higher oxygen consumption and energy expenditure. Two subsequent studies that used different promoters for progenitor cells, such as Fgf10-CreER47 and GlastCreER,48 to fate-track tanycytes, demonstrated that specifically alpha-tanycytes are capable of self-renewal and can give rise to both astrocytes and neurons. Notably, not all, but only glial fibrillary acidic protein (GFAP)-expressing dorsal alpha2-tanycytes possess neural progenitor characteristics.
Transplantation neurogenic potential
Given its therapeutic usefulness, the neurogenic possibility for transplanted neural stem or progenitor cells has been studied in animal models of degeneration such as Huntington’s, Alzheimer’s, and Parkinson’s disease (PD).
In a mouse model of dementia that used nucleus basalis of Meynert lesion for frontal cortex, it was shown that transplanted mouse-NSC derived neurosphere grew choline acetyl transferase (ChAT) and serotonin-positive neurons at the lesion site resulting in improved performance in radial maze test.49 Notably, it was shown that these neurospheres when transplanted into healthy brains did not make neurons, but rather had detrimental effects. The ability of transplanted human NSC to migrate to lesion sites and mitigate ongoing atrophy has been shown in Hungtinton’s disease model of quinolinic-acid-injected striatal lesion.50 It was noted that the NSC makes both neurons and astrocytes at the lesion site, but not in undamaged brain. Intravenously transplanted NSC migration to damaged areas of brain has also been described in a stroke model of rats.51
In a primate model for PD, implanted undifferentiated human NSCs were shown to migrate to the lesion site, survive and improve functioning of the nigrostriatal system. Some of these NSC showed tyrosine hydroxylase and dopamine transporter (DAT) immunopositivity, but no other neuronal markers. However, the transplanted brains showed restoration of neuronal numbers and size, dopamine levels and reduction of alpha-synuclein aggregation.52 In another study, in a mouse model of PD, engraftment of NSC from mouse embryonic fibroblast was shown to differentiate into dopaminergic neurons that migrated to the subsantia niagra.53
In a postnatal, leptin-receptor-deficient mouse model of obesity, transplantation of embryonic leptin-responsive hypothalamic cells were shown to differentiate into four major types of hypothalamic neurons, forming functional synapses and partially rescuing leptin-deficient phenotype of hyperglycemia and obesity.54 In this study, the fate and functionality of the transplanted cells were tracked up to 20 weeks after transplantation, demonstrating the electrophysiological properties of newly generated hypothalamic neurons and their response to energy signals such as glucose, insulin, and leptin.
An important point to consider in most of the transplantation studies mentioned above is that these demonstrate the migration and survival of NSC into the damaged location, along with cognitive and behavioural improvement. However, the detailed tracking of whether the cognitive improvement actually happened due to generation and integration of functional new neurons remains to be done. It is possible that NSC migration to the damaged sites allows for further signalling and trophic factor action allowing survival of neurons in the regions, to improve or adapt for the loss, resulting in cognitive improvements. Nevertheless, these examples demonstrate the receptive environment of adult brain for neuronal generation and/or integration.
In a recent study by Falkner et al,55 the question regarding functional integration of new neurons from transplanted cells has been addressed. In this study, genetically labelled embryonic precursors were transplanted into mouse visual cortex about a week after ablation of Layer 2/3 neurons. Laminar localization, structural maturation, and functional connection of transplanted neurons were compared with endogenous neurons. Chronic in vivo imaging and monosynaptic tracing methods showed that dendritic, spine, and axonal development as well as brain-wide synaptic connections of transplanted neurons was functional and similar to endogenous neurons.
The Role of the Niche as a Decisive Factor for Neurogenesis
The stem-cell field has long recognized the importance of the microenvironment in which a stem cell sits, for its function and fate. This microenvironment is termed the niche. Adult neurogenesis, while known to be influenced by systemic or macroenvironment, such as enriched environment or stressful experience, is also greatly dependent on cues from the niche. The SVZ demonstrates an instance for how the origin of adult NSC might determine its specific neuronal cell fate. Complimentary to that concept, it has been shown that progenitor cells residing in non-neurogenic locations in the central nervous system (CNS), could produce neurons when placed in the bona fide neurogenic niche.56,57 Lie et al showed that transplanted progenitors from Substantia Nigra that are glia fate-restricted in situ, could make neurons when transplanted to SGZ niche. However, complimentarily when the progenitors from hippocampus are transplanted into S Nigra, these could not differentiate to neurons. Notably these in situ glia-restricted progenitors, derived from S Nigra, could give rise to both neurons and glia in vitro. This suggests that the niche is capable of providing both ‘restrictive’ or ‘instructive’ cues to a neural progenitor for a certain fate, based on its location and perhaps requirements.
The niche for stem cells consists of both cellular and non-cellular components, such as secreted factors, vascular components, and the extracellular matrix in the microenvironment. Among the cellular components, the role of astrocytes through paracrine signalling is well recognized. In SGZ, astrocytes express high fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor A (VEGF), which are required for stem cell survival and proliferation.58,59 In addition, astrocytes also instruct NSC for neurogenic fate through Wnt and EphrinB2 signalling.60–63 In the context of cellular microenvironment as part of the neurogenic niche, the activity of mature neurons should also be considered as an important cell-extrinsic factor from the niche that influences adult neurogenesis. It has been shown that higher neuronal activity is sensed by NSC through their N-methyl-D-aspartate (NMDA) receptors and L-type calcium channels, resulting in increased expression of pro-neuronal genes within NSC.64 In addition, increased activity also results in increased production of VEGF and brain-derived neurotrophic factor (BDNF) by mature neurons, further promoting neurogenesis.65,66 Indeed, increased neuronal activity during the acute phase of epilepsy has been shown to increase adult neurogenesis,67 which is followed by a phase of reduction in adult neurogenesis with a concomitant increase in microglial activation, gliogenesis, and neurodegeneration.68,69
The role of vasculature and systemic factors on adult neurogenesis have been best demonstrated through parabiosis experiments. In these studies, the blood circulations of a young and old mice were surgically connected through attachment of their skins on the lateral torso. This leads to connection of vasculature in the joined region within a few days, such that the blood circulation from one mouse can be traced in the other through congenic genetic markers. Heterochronic parabiosis experiments showed that the presence of young mouse blood in the circulatory system of an older animal resulted in increased proliferation of neural progenitors and increased adult neurogenesis in the neurogenic niches. In corollary, adult neurogenesis in the younger animal was affected as a result of systemic influences from the circulated old-animal blood resulting in reduced adult neurogenesis.70 In this study, a systemic chemokine CCL11, which is increased in older circulation milieu, was shown to have a detrimental effect on adult hippocampal neurogenesis. In another study, also using heterochronic parabiosis, it was shown that circulating factors from young mice improved SVZ neurogenesis and olfactory discrimination.71 This study additionally demonstrated the vascular remodelling that ensues in old heterochronic parabiont, leading to increased vessel volume and cerebral blood flow comparable to young mice.
The choroid plexus (CP), residing in the ventricles, is a highly vascular structure responsible for production of the cerebrospinal fluid (CSF) which acts as an interface between the brain and the periphery. Aging causes changes in the CP transcriptome and secretome. The aging-related changes in the blood that are reflected in CP include elevated expression of interferon (IFN)-1-dependent genes and a reduction in IFN-II expression profiles.72–74 In addition, several other factors such as interleukin (IL)-6 and CCL11, BMP5, and insulin-like growth factor 1 (IGF-1) are also affected in the aged CP. Many of these factors, as well as the CP conditioned media have been shown to influence SVZ neurogenesis by affecting the activated neural precursor cells.74
While NSC in the SVZ directly contact the CSF of CP, the NSC in the SGZ do not have such access to CP. Despite this, changes in CP seem to also influence hippocampal neurogenesis. Baruch et al72 showed that blocking IFN-1 programme at the CP of old mice increased progenitor proliferation and number of immature neurons in the DG. Interestingly, while increased IFN-1 and decreased IFN-II programme, both are detrimental for adult neurogenesis, heterochronic parabiosis experiment between old and young mice showed that the IFN-1 changes in CP are not blood borne but perhaps regulated by factors within the CSF, whereas the IFN-II expression profile is influenced by circulating blood. While not close to the CP, the DG NSC and niche cells have ample scope of interaction with the DG vasculature, which starts from the arterioles of the hippocampal fissure, passing as capillaries through the GC layer along the rostro-caudal axis. In fact, the physical proximity of RGL and other proliferating cells in DG to blood vessels has been demonstrated by microscopy.75
Support for a role of systemic factors from the blood on adult neurogenesis has also been demonstrated by multiple studies. In a transplantation study, a single injection of human umbilical cord blood cells (hUCBMC), but not young adult blood cells, was shown to enhance proliferation and neuronal differentiation in DG of aged rats.76 A later study showed that the CD4 T cells, derived from hUCBMC, are critical for the positive effect on adult neurogenesis.77
The role of the hematopoietic system or cells from the peripheral blood on adult neurogenesis has also been shown. In a surprising revelation, it was shown that SCID and nude mice, which lack T and B cells, have lower levels of adult neurogenesis. Adoptive transfer of T cells, but not other splenocytes, could rescue the reduction in neurogenesis.78 It was further shown that CNS-specificity of T cell helps in boosting adult neurogenesis, wherein possible interaction of T cells with activated microglia in the DG was suggested to play a role. However, the presence of T cells in the DG, or their direct interaction with microglia was not demonstrated in this study, thereby leaving the mechanism of potential T cell’s function in adult neurogenesis unresolved.
In a subsequent study, Wolf et al79 showed that specific depletion of CD4 T cells, and not CD8 T cells, affected adult neurogenesis and cognitive functions in Morris Water Maze. However, they showed that antigen specificity was not required for the positive effect of T cells on adult neurogenesis, and that exercise could overcome the negative effect of CD4 T cell depletion on adult neurogenesis. They also noted that CD4 T cells were not present in the parenchyma, and the effect possibly does not require direct cell-cell interaction between the brain cells and immune cells but could be through factors produced by the hematopoietic cells that access the parenchyma. Notably, they compared the T-cell-deficient mice with Rag2-deficient mice, which lack all the adaptive immune cells, the T, B and NK cells. In this comparison, reduced adult neurogenesis in Rag2-deficient mice could not be rescued with exercise, indicating the existence of a compensatory mechanism from the other cells of the adaptive immune system, which potentially makes up for CD4 T cell loss in the context of adult neurogenesis.
A role of the immune system in regulating adult neurogenesis was further suggested by another study that showed that even innate immune cells, namely monocytes, are important for adult neurogenesis.80 In this study, it was shown that antibiotic treatment is detrimental to adult neurogenesis and cognition. The innate immune cell type, Ly6c hi monocytes, were shown to mediate the effect of gut flora on adult neurogenesis. Antibiotic treatment resulted in depletion of Ly6c hi monocyte population in the brain, whereas replenishment of Ly6c hi cells could rescue reduced neurogenesis. Importantly, they showed that physical exercise and probiotic treatment, two regimes that boosted adult neurogenesis, also resulted in an increase of the Ly6c hi monocyte populations in the brain. In addition, this was the only study, that showed the presence of the specific immune cell type in the brain parenchyma. However, since this detection was done through flow cytometry, the in situ location of the Ly6chi monocyte in the brain parenchyma or their interaction with brain cells remained elusive.
Could Adult Neurogenic Potential be Harnessed for Regeneration and Repair?
Multiple degenerative diseases show reduced adult neurogenesis. Despite differences in the specific neuron type that degenerates in a particular neurodegenerative disease, all of them show alterations in adult neurogenesis, indicating that the degenerative environment itself may influence the process of neurogenesis. It is thus hypothesized that the common non-motor, non-cognitive symptoms, such as depression and anxiety that is observed in early presymptomatic stages of neurodegenerative diseases could be a result of suppressed hippocampal neurogenesis that drives the consequent mood-disorder. Adult neurogenesis in the context of neurodegenerative disorders has been reviewed elsewhere.81 While the human data related to functional adult neurogenesis is only correlative at this point,82 the potential of adult NSCs always presents a promising option to harness for regeneration. However, the response of adult neurogenesis to neurodegeneration cannot be expected to be simply compensatory because (1) adult neurogenesis under homeostatic conditions is limited to a couple of neurogenic locations, not necessarily coinciding with the regions where neurodegeneration occur (with an exception of Alzheimer’s disease where the hippocampus is affected) and (2) the continually generated new neurons of the neurogenic regions seem to have a specific bona fide function relevant for the circuit where the niche is located. For instance, the DG adult-born neurons are important for enhanced contextual and sensory coding for pattern separation, whereas OB new-born neuron generation has been shown to be dependent on new odour experience.15,83–85 Thus, perhaps a more relevant proposition for regenerative contexts would be the latent neurogenic precursors, that presumably lie outside the neurogenic niches, such as the striatum. An understanding of the scope of cell-replacement as a potential therapy beyond adult NSC can be found in a recent review.86
Another important factor to consider in the neurodegenerative context, would be the niche signals. A neurodegenerative microenvironment will be substantially different from the steady-state niche of adult neurogenesis. A neurodegenerative microenvironment will consist of not only dead and dying neurons but will also include glial and vascular responses to degeneration and, possibly to aging. Thus, the signals from a degenerative niche, which play a decisive role for NSC fate, will be very different from the normal adult neurogenic niche signals. Under steady state, the quiescent NSC of the neurogenic niche proactively convey, and respond to, the niche signals for maintenance of quiescence, thereby primarily limiting neurogenesis. The cues which might signal an NSC towards differentiation in the neurogenic niche under steady state would possibly be guided by the continual dynamic functional needs of the circuitry they are involved or required in. In contrast, in the face of neuronal loss, due to degeneration or injury, the niche signals are unlikely to be uniform, since a degenerative microenvironment could be expected to include a variety of inflammatory, anti-inflammatory, and, presumably, cumulative cellular stress (from aging) signals. Therefore, the NSC cellular response and the mechanism of cell-fate decisions and maturation, could be expected to be different between the steady-state NSC of the neurogenic niches and the precursors in the parenchyma which are induced only upon degeneration or injury.
It is clear that the brain parenchyma harbours multipotent cells capable of making both neurons and glia, in regions other than the bona fide neurogenic niches of the adult brain. The limitation to regenerative usability of these cells is probably a function of their microenvironment, which could either support or avert their neurogenic differentiation potential. In neurodegenerative disorders, the causative insults that lead to degeneration of neurons seem to often accompany a niche response that opposes neurogenesis. Thus, there seems to be two parallel approaches that would be required to use the potential of parenchymal resident precursors for regeneration; (1) to manipulate the niche response so as to make it neurogenesis conducive and (2) to manipulate NSCs so that their responsiveness to a degenerative microenvironment protects them against senescence, quiescence, death, or alternate cell-fate acquisition, such as gliogenesis. To achieve this, further insights into the mechanisms by which both the niche and the NSC are regulated would be necessary.
A recent study has again stirred up the controversy regarding the relevance and existence of adult neurogenesis in humans.87 This study was quickly followed up by two other detailed reports that demonstrated ongoing adult neurogenesis in adult and aged humans, with one of them reporting decline in adult neurogenesis with Alzheimer Disease progression.88 While more subjects, better characterization of data and more sensitive methods would hopefully continue to bring more answers, all existing studies on human adult neurogenesis have been discussed in detail, in a recent review.89 Whether or how much adult neurogenesis really occurs in humans is a question of great importance to understand its functional role in humans. However, the physiologic process of adult neurogenesis in another organism gives us an invaluable model system to gain in vivo insights about the nature of NSC, its niche responses and ultimately to understand what it takes for new neurons to be generated and integrated in an already established network of the adult brain parenchyma under steady state and in pathogenesis. Thus, further studies focused on molecular details of NSC regulation and niche responses would help us take the therapeutic promise of adult NSC closer to its potential.
Acknowledgments
The author thanks Jean Hébert for his insightful suggestions and critical reading of the manuscript, and Megha Murthy for the graphic diagrams. The research in the Ghosh Laboratory is supported by Ramanujan Fellowship, NCBS-TIFR, and DST-SERB funding.
Footnotes
Funding:The author received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contribution: HSG reviewed literature and wrote the manuscript.
ORCID iD: Hiyaa S Ghosh  https://orcid.org/0000-0001-6224-1043
https://orcid.org/0000-0001-6224-1043
References
- 1. Altman J. Are new neurons formed in the brains of adult mammals. Science. 1962;135:1127–1128. [DOI] [PubMed] [Google Scholar]
- 2. Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963;145:573–591. [DOI] [PubMed] [Google Scholar]
- 3. Altman J, Das GD. Autoradiographic and histologicalevidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. [DOI] [PubMed] [Google Scholar]
- 4. Kempermann G, Gast D, Kronenberg G, et al. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 2013;130:391–399. [DOI] [PubMed] [Google Scholar]
- 5. Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsieh J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev. 2012;26:1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Urbán N, Guillemot F. Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci. 2014;8:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beckervordersandforth R, Zhang C, Lie DC. Transcription-factor-dependent control of adult hippocampal neurogenesis. Cold Spring Harb Perspect Biol. 2015;7:a018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim DA, Alvarez-Buylla A. The adult ventricular – subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb Perspect Biol. 2016;8:a018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. [DOI] [PubMed] [Google Scholar]
- 11. Fuentealba LC, Rompani SB, Parraguez JI, et al. Embryonic origin of postnatal neural stem cells. Cell. 2015;161:1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonaguidi MA, Wheeler MA, Shapiro JS, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Encinas JM, Michurina TV, Peunova N, et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vivar C, Potter MC, Choi J, et al. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun. 2012;3:1107–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danielson NBB, Kaifosh P, Zaremba JD, et al. Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron. 2016;90:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anacker C, Luna VM, Stevens GS, et al. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 2018;559:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Furutachi S, Miya H, Watanabe T, et al. Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat Neurosci. 2015;18:657. [DOI] [PubMed] [Google Scholar]
- 18. Hochgerner H, Zeisel A, Lonnerberg P, Linnarsson S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat Neurosci. 2018;21:290–299. [DOI] [PubMed] [Google Scholar]
- 19. Li G, Fang L, Fernandez G, Pleasure SJ. The ventral hippocampus is the embryonic origin for adult neural stem cells in the dentate gyrus. Neuron. 2013;78:658–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nicola Z, Fabel K, Kempermann G. Development of the adult neurogenic niche in the hippocampus of mice. Front Neuroanat. 2015;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berg DA, Su Y, Jimenez-Cyrus D, et al. A common embryonic origin of stem cells drives developmental and adult neurogenesis. Cell. 2019;177:654–668.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun GJ, Zhou Y, Ito S, et al. Latent tri-lineage potential of adult hippocampal neural stem cells revealed by Nf1 inactivation. Nat Neurosci. 2015;18:1722–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rolando C, Erni A, Grison A, et al. Multipotency of adult hippocampal NSCs in vivo is restricted by Drosha/NFIB. Cell Stem Cell. 2016;19:653–662. [DOI] [PubMed] [Google Scholar]
- 24. Urban N, van den Berg DL, Forget A, et al. Return to quiescence of mouse neural stem cells by degradation of a proactivation protein. Science. 2016;353:292–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeCarolis NA, Mechanic M, Petrik D, et al. In vivo contribution of nestin- and GLAST-lineage cells to adult hippocampal neurogenesis. Hippocampus. 2013;23:708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Obernier K, Cebrian-Silla A, Thomson M, et al. Adult neurogenesis is sustained by symmetric self-renewal and differentiation. Cell Stem Cell. 2018;22:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shin J, Berg DA, Zhu Y, et al. Single – cell RNA – seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell. 2015;17:360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Artegiani B, Lyubimova A, Muraro M, van Es JH, van Oudenaarden A, Clevers H. A single-cell RNA sequencing study reveals cellular and molecular dynamics of the hippocampal neurogenic niche. Cell Rep. 2017;21:3271–3284. [DOI] [PubMed] [Google Scholar]
- 29. Marshall CAG, Goldman JE. Subpallial Dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. J Neurosci. 2002;22:9821–9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marshall CAG, Novitch BG, Goldman JE. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci. 2005;25:7289–7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roybon L, Hjalt T, Stott S, Guillemot F, Li JY, Brundin P. Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS ONE. 2009;4:e4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A. Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell. 2015;17:329–340. [DOI] [PubMed] [Google Scholar]
- 33. Dulken BW, Leeman DS, Boutet SC, Hebestreit K, Brunet A. Single-cell transcriptomic analysis defines heterogeneity and transcriptional dynamics in the adult neural stem cell lineage. Cell Rep. 2017;18:777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Codega P, Silva-Vargas V, Paul A, et al. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82:545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. [DOI] [PubMed] [Google Scholar]
- 36. Magnusson JP, Goritz C, Tatarishvili J, et al. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346:237–241. [DOI] [PubMed] [Google Scholar]
- 37. Nato G, Caramello A, Trova S, et al. Striatal astrocytes produce neuroblasts in an excitotoxic model of Huntington’s disease. Development. 2015;142:840–845. [DOI] [PubMed] [Google Scholar]
- 38. Ernst A, Alkass K, Bernard S, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. [DOI] [PubMed] [Google Scholar]
- 39. Sirko S, Behrendt G, Johansson PA, et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog glia. Cell Stem Cell. 2013;12:426–439. [DOI] [PubMed] [Google Scholar]
- 40. Niu W, Zang T, Zou Y, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15:1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brulet R, Matsuda T, Zhang L, et al. NEUROD1 instructs neuronal conversion in non-reactive astrocytes. Stem Cell Reports. 2017;8:1506–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14:188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carlen M, Meletis K, Goritz C, et al. Forebrain ependymal cells are notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. [DOI] [PubMed] [Google Scholar]
- 44. Barnabe-Heider F, Goritz C, Sabelstrom H, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. [DOI] [PubMed] [Google Scholar]
- 45. Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. [DOI] [PubMed] [Google Scholar]
- 46. Lee DA, Bedont JL, Pak T, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15:700–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haan N, Goodman T, Najdi-Samiei A, et al. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci. 2013;33:6170–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Robins SC, Stewart I, McNay DE, et al. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun. 2013;4:2049. [DOI] [PubMed] [Google Scholar]
- 49. Wang Q, Matsumoto Y, Shindo T, et al. Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Invest. 2006;53:61–69. [DOI] [PubMed] [Google Scholar]
- 50. Lee ST, Chu K, Park JE, et al. Intravenous administration of human neural stem cells induces functional recovery in Huntington’s disease rat model. Neurosci Res. 2005;52:243–249. [DOI] [PubMed] [Google Scholar]
- 51. Chu K, Kim M, Chae SH, et al. Distribution and in situ proliferation patterns of intravenously injected immortalized human neural stem-like cells in rats with focal cerebral ischemia. Neurosci Res. 2004;50:459–465. [DOI] [PubMed] [Google Scholar]
- 52. Redmond DE, Jr, Bjugstad KB, Teng YD, et al. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci U S A. 2007;104:12175–12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Choi D-H, Kim JH, Kim SM, Kang K, Han DW, Lee J. Therapeutic potential of induced neural stem cells for Parkinson’s disease. Int J Mol Sci. 2017;18:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Czupryn A, Zhou YD, Chen X, et al. Transplanted hypothalamic neurons restore leptin signaling and ameliorate obesity in db/db mice. Science. 2011;334:1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Falkner S, Grade S, Dimou L, et al. Transplanted embryonic neurons integrate into adult neocortical circuits. Nature. 2016;539:248–253. [DOI] [PubMed] [Google Scholar]
- 56. Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult Substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. [DOI] [PubMed] [Google Scholar]
- 59. Cao L, Jiao X, Zuzga DS, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. [DOI] [PubMed] [Google Scholar]
- 60. Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. [DOI] [PubMed] [Google Scholar]
- 61. Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. [DOI] [PubMed] [Google Scholar]
- 62. Ashton RS, Conway A, Pangarkar C, et al. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat Neurosci. 2012;15:1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barkho BZ, Song H, Aimone JB, et al. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. [DOI] [PubMed] [Google Scholar]
- 65. Ma DK, Jang MH, Guo JU, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Segi-Nishida E, Warner-Schmidt JL, Duman RS. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci U S A. 2008;105:11352–11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ekdahl CT, Claasen Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Borges K, Gearing M, McDermott DL, et al. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. [DOI] [PubMed] [Google Scholar]
- 70. Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Baruch K, Deczkowska A, David E, et al. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014;346:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Baruch K, Ron-Harel N, Gal H, et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci U S A. 2013;110:2264–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, Doetsch F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell. 2016;19:643–652. [DOI] [PubMed] [Google Scholar]
- 75. Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal. J Comp Neurol. 2000;425:479–494. [DOI] [PubMed] [Google Scholar]
- 76. Bachstetter AD, Pabon MM, Cole MJ, et al. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008;9:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shahaduzzaman M, Golden JE, Green S, et al. A single administration of human umbilical cord blood T cells produces long-lasting effects in the aging hippocampus. Age (Dordr). 2013;35:2071–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ziv Y, Ron N, Butovsky O, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. [DOI] [PubMed] [Google Scholar]
- 79. Wolf SA, Steiner B, Akpinarli A, et al. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol. 2009;182:3979. [DOI] [PubMed] [Google Scholar]
- 80. Mohle L, Mattei D, Heimesaat MM, et al. Ly6Chi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15:1945–1956. [DOI] [PubMed] [Google Scholar]
- 81. Winner B, Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2015;7:a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nature Medicine. 2019;25:554–560. [DOI] [PubMed] [Google Scholar]
- 83. Lazarini F, Lledo PM. Is adult neurogenesis essential for olfaction? Trends Neurosci. 2011;34:20–30. [DOI] [PubMed] [Google Scholar]
- 84. Imayoshi I, Sakamoto M, Ohtsuka T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. [DOI] [PubMed] [Google Scholar]
- 85. Magavi SSP, Mitchell BD, Szentirmai O, Carter BS, Macklis JD. Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J Neurosci. 2005;25:10729–10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hebert JM, Vijg J. Cell replacement to reverse brain aging: challenges, pitfalls, and opportunities. Trends Neurosci. 2018;41:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sorrells SF, Paredes MF, Cebrian-Silla A, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Boldrini M, Fulmore CA, Tartt AN, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kempermann G, Gage FH, Aigner L, et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]