Abstract
The aim of this study was to evaluate 1-year follow-up results in an all “comers” population treated with a new cobalt chromium bare-metal stent (BMS) design. Since August 2016 to March 2017, 201 (9.7% of screening population) consecutive patients undergoing coronary stent implantation in 11 centers in Argentina were prospectively included in our registry. The inclusion criteria were multiple-vessel disease and/or unprotected left main disease, acute coronary syndromes (ACS) with at least one severe (⩾70%) stenosis in any of major epicardial vessel. In-stent restenosis, protected left main stenosis, or impossibility to receive dual-antiplatelet therapy was an exclusion criterion. Major adverse cardiac events (MACE) were the primary endpoint and included cardiac death, myocardial infarction (MI), and target lesion revascularization (TLR); also, all components of the primary endpoint were separately analyzed. Completeness of revascularization was analyzed as post hoc data using residual SYNTAX or ERACI risk scores. Demographic characteristics showed that 6.5% of patients were very elderly, 22.5% have diabetes, 47% have multiple-vessel disease, 67% have ACS, and 32% have ST elevation MI. At a mean of 376 ± 18.1 days of follow-up, MACE was observed in 10.4% of patients: death + MI + cardiovascular accident (CVA) in 3% (6 of 201) and cardiac death + MI + CVA in 1.5% (3 of 201). Residual ERACI score ⩽5 was associated with 98% of event-free survival (P < .04). In conclusion, this prospective, multicenter, and observational all-comers registry with this novel BMS design showed a low incidence of adverse events at 1 year mainly due to coronary restenosis.
Keywords: stents, bare metal stents, drug eluting stents, completeness of revascularization, SYNTAX score, multiple-vessel disease, risk scores
Introduction
Since the introduction of drug eluting stent (DES) deployment in clinical practice during percutaneous coronary interventions (PCI), DES became default PCI strategy with safety and efficacy shown in almost all clinical and angiographic scenarios.1–4
Nevertheless, the role of bare-metal stents (BMS) in some circumstances still prevailed. Concerns about a higher risk of bleeding in elderly patients or noncompliance with the mandatory dual-antiplatelet therapy (DAPT) are still a limitation for DES use.5–7
On this account, BMS have not been discarded, and their use during PCI procedures in very elderly patients and even in patients with ST elevation myocardial infarction (STEMI) has been currently reported in our country in 70% of all cases.6,7
In addition, recent randomized clinical trials (RCT) such as NORSTENT or ISAR-CABG demonstrated a similar incidence of adverse events and quality of life between BMS and DES at 5 years of follow-up, suggesting that the use of BMS in clinical practice should not be discharged and still holds its place, clearly not only because of socioeconomic reasons.8,9 In fact, the late loss of the initial benefit of DES compared either to coronary artery bypass grafting (CABG) or BMS has been observed10 in previously published studies such as MAIN COMPARE,11 who reported a late loss of the initial benefit with DES compared with either BMS or coronary bypass surgery (CABG) at 10 years of follow-up in patients with left main coronary artery (LMCA). Finally, a recent meta-analysis from all RCT compared mortality rates in stents vs CABG at late follow-up and benefits in favor to CABG were only seen in the DES group of patients.12
The purpose of the WALTZ registry was to perform a multicenter, single-arm, observational study with a novel cobalt chrome stent design in a wide clinical spectrum of patients with coronary artery disease (CAD), including left main, multiple-vessel disease and evolving acute myocardial infarction (MI). Hereby, we report for the first time 1-year follow-up results of this registry.
Methods
Study design
Protocol and study design were already previously mentioned.13,14 All investigators reviewed and approved final version of the manuscript. A complete list of investigators responsible for patient inclusion appears in Appendix 1.
Briefly, the WALTZ registry was a prospective, observational, single-arm, multicentre, all-comers registry that enrolled subjects with a severe (⩾70%) coronary artery lesion in a native artery with reference diameter by visual estimation ⩾2.50 mm to ⩽4.00 mm in a consecutive population.
The study will be considered complete (regarding the primary endpoint) after all subjects have completed the 12-month primary endpoint. Between August 2016 and February 2017, 2068 patients were screened in 11 sites in Argentina. Patient screening was described in Figure 1; 1867 patients were excluded because they did not meet the inclusion criteria, thus 201 patients (9.7%) were included in the registry and are the subject of the study.
Figure 1.
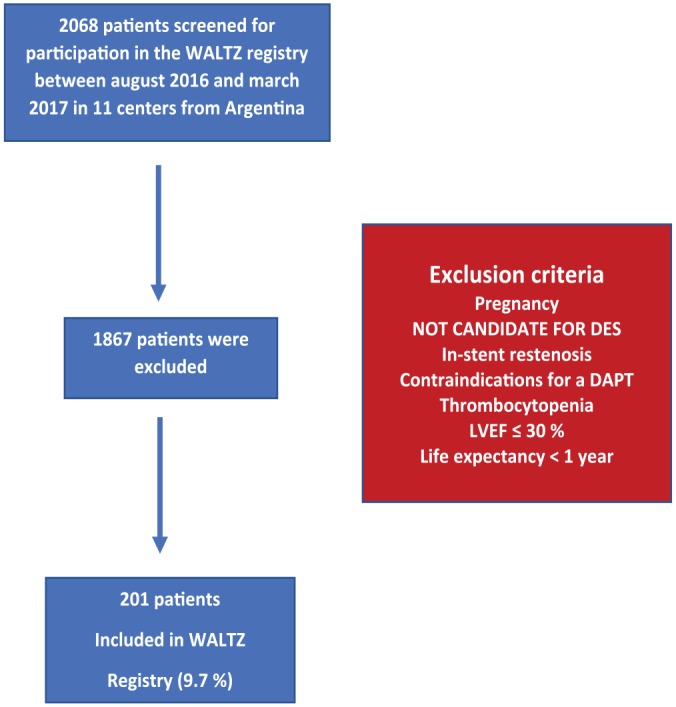
Study design from WALTZ registry. DAPT indicates dual-antiplatelet therapy; DES, drug eluting stent; LVEF, left ventricular ejection fraction.
Reasons for exclusion were as follows: refused to sign informed consent, better candidate for DES implantation according to site investigator’s assessment, unable to take long-term DAPT, short life expectancy, major bleeding within the last 6 months, pregnancy, in-stent restenosis, thrombocytopenia or leukopenia, poor left ventricular ejection fraction (LVEF < 30%), a known allergy, and previously planned staged PCI (Figure 1).
Stent design
Stent design allowed in this study was a cobalt chromium alloy stent (WALTZ™; MicroPort Corp., Shanghai, China). Design of the stent includes uniform sine wave and “S” links that offer excellent balance between supporting and enhanced radial strength, flexibility, trackability, and pushability. Strut thickness of the stent was 86.3 µm with a crossing profile of 0.093 mm. The open cell design of the struts also allows an easy side branch access. Metal covered area between 11.6% and 14.3%. Design and characteristics of this stent were previously described in detail.13,14
Endpoints
Primary
Primary endpoint was the incidence of major adverse cardiac events (MACE) at 1-year follow-up. The MACE was defined as a composite of cardiac death (if the event could not be determined with certainty, it would be assumed to be cardiac), MI (both ST and non-ST elevation), and any ischemic-driven target lesion revascularization (TLR). However, endpoints were also measured at 30 days and 6 months of follow-up.
Secondary
Secondary endpoints included the composite of cardiac death, MI, and cerebrovascular accident (CVA); TLR and target vessel revascularization (TVR); stent thrombosis15,16; and any individual components of MACE. The TVR refers to an ischemic-driven revascularization of the treated coronary artery.
As a “post hoc” analysis, we also correlated freedom from adverse events at 1 year with residual angiographic risk scores after PCI. In the WALTZ registry, original SYNTAX score (SS)17 was calculated; however, we also used a modification of the original SS, the ERACI risk score (ES), excluding from the analysis all intermediate (⩾50 to <70 visual estimation) lesions and/or severe stenosis (⩾70%) in vessels <2.0 mm. This new score was in agreement with the PCI strategy used in the study and was reported in detail elsewhere.18,19
Eligibility Criteria for the Study
Inclusion criteria
The study enrolled “real-world, all-comers” patients eligible for PCI with lesions suitable for stent implantation. Inclusion criteria were selected to reflect patients who would be eligible in routine clinical practice (“real-world, all-comers” patients).
Patients had to meet all of the following inclusion criteria: be at least 18 years old, need a treatment with WALTZ BMS, have the presence of one or more coronary artery stenosis in a native coronary artery from 2.50 to 4.0 mm in diameter that could be covered with one or multiple stents, with LMCA, and no limitation on the number of treated lesions, vessels, and lesion length.
Exclusion criteria
Subjects were excluded from the study if they have had any of the following:
Previous coronary intravascular brachytherapy treatment at any time.
PCI of a nontarget vessel or side branch within 1 day prior to the index procedure.
Planned-stage PCI procedure previous to inclusion.
PCI of the target vessel or side branch within 12 months prior to the index procedure.
PCI within 10 mm proximal or distal to the target lesion (by visual estimate) at any time prior to the index procedure.
In-stent restenosis.
Left ventricular ejection fraction <30%.
Life expectancy less than 1 year.
Contraindication for DAPT therapy.
The full description of inclusion and exclusion criteria is shown in Table 1.
Table 1.
Inclusion and exclusion criteria.
|
Inclusion criteria: clinical and
angiographic
CI1. Subject must be at least 18 years of age. CI2. Subject (or legal guardian) indicates understanding of the trial requirements and the treatment procedures and provides written informed consent before procedures are performed. CI3. Subject is eligible for percutaneous coronary intervention (PCI). CI4. Subject has symptomatic coronary artery disease or silent ischemia with objective evidence of ischemia or acute coronary syndromes and qualifies for PCI. CI5. Subject is an acceptable candidate for coronary artery bypass grafting (CABG). CI6. Subject has a left ventricular ejection fraction (LVEF) >34% as measured within 60 days prior to enrollment. CI7. Subject is willing to comply with all protocol-required follow-up evaluations. AI1. Subject has one or more coronary artery stenosis of ⩾50% in a native coronary artery with visually estimated reference vessel diameter (RVD) ⩾2.50 mm and ⩽4.0 mm. AI2. Coronary anatomy is likely to allow delivery of a study stent to the target lesions(s). Exclusion criteria: clinical and angiographic CE1. Subject has a known allergy to contrast (that cannot be adequately premedicated) and/or the trial stent system or protocol-required concomitant medications (eg, cobalt chromium alloy, stainless steel, all P2Y12 inhibitors, or aspirin). CE2. Planned surgery within 30 days after the index procedure. CE3. Subject has one of the following (as assessed prior to the index procedure): • Other serious medical illness (eg, cancer, congestive heart failure) with estimated life expectancy of less than 12 months. • Current problems with substance abuse (eg, alcohol, cocaine, heroin, etc). • Planned procedure that may cause noncompliance with the protocol or confound data interpretation. CE4. Subject has a history of bleeding diathesis or coagulopathy or will refuse blood transfusions. CE5. Subject is participating in another investigational drug or device clinical trial that has not reached its primary endpoint, or that, in the opinion of the investigator, may cause noncompliance with the protocol or confound data interpretation. CE6. Subject intends to participate in another investigational drug or device clinical trial within 12 months after the index procedure. CE7. Subject with known intention to procreate within 12 months after the index procedure (women of child-bearing potential who are sexually active must agree to use a reliable method of contraception from the time of screening through 12 months after the index procedure). CE8. Subject is a woman who is pregnant or nursing (a pregnancy test must be performed within 7 days prior to the index procedure in women of child-bearing potential). Note: No restrictions are placed on the total number of treated lesions, treated vessels, lesion length, or number of stents implanted. AE1. Target lesion meets any of the following criteria: • Restenosis from previous intervention. AE3. Subject has protected left main coronary artery disease. AE4. Subject has an additional clinically significant lesion(s) in the target vessel for which an intervention within 12 months after the index procedure may be required. |
DAPT was required for all included patients. Aspirin ⩾300 mg was administrated orally at least 1 hour prior to catheterization and an oral loading dose of thienopyridines: either clopidogrel (300-600 mg), prasugrel (60 mg) or ticagrelor (180 mg), preferably, but not mandatory, ⩾6 hours prior to procedure. During PCI, unfractionated heparin was recommended as necessary to maintain an activated clotting time as current guidelines suggested. Alternatively, enoxaparin, bivalirudin, or other antithrombotic agents could be administrated per standard of care and according to operator’s discretion. Dual-antiplatelet therapy was maintained for at least 1 month after stent deployment, followed by acetylsalicylic acid (ASA) monotherapy indefinitely. In patients with ACS, DAPT was recommended for 1 year after PCI. A maintenance dose per day of 75 mg of clopidogrel, 10 mg of prasugrel, or 90 mg of ticagrelor was recommended.
The revascularization strategy was planned prior to the procedure, and the aim was to achieve complete functional (coronary flow reserve [CFR]) or “reasonable” incomplete revascularization (IR) arbitrarily defined when residual SS or ES was ⩽5.
Percutaneous revascularization was considered functionally complete if no residual severe stenosis (70% or more) remained in any major epicardial vessel and all severe stenosis had been successfully treated with stents. On the contrary, any residual scores above those numbers after PCI were classified as IR.
Staged procedure strategy was not allowed either in target or nontarget vessels and was an exclusion criterion. According to our previous PCI and stent deployment strategy, mild or intermediate stenosis was not treated and stents were indicated in severe stenosis only. Stents in small vessels (⩽2.0 mm) were usually not recommended to treat and were not to be included as part of the revascularization strategy.17
The statistical package of SPSS v.17.0.1® (IBM, Armonk, NY, USA) was used to perform statistical analysis. Continuous variables were measured using the analysis of variance (ANOVA) test with Bonferroni correction and categorical variables using chi-square or Fisher exact test.
The continuous variables were expressed as average and standard deviation and the categorical variables MACE, MI, TLR, CVA, and cardiac death were expressed as percentages. Kaplan-Meier curves were used to compare outcomes with residual risk scores. Log rank test P < .05 was considered significant.
An independent data monitoring and clinical events committee adjudicated all reported events of MACE and other clinical events, including stent thrombosis.9 They were responsible for controlling all reported adverse events and evaluating safety data. All the required patient’s information needed to fulfill the research were incorporated to the database by each site’s researchers, trained for that purpose, using a password-protected electronic case report form (CRF). The Cardiovascular Research Center (CECI) was responsible for the development of the protocol registry, database, e-CRF, and statistical analyses. Also, 25% of patients had a random onsite monitoring. The informed consent form (ICF) was approved by Argentina Department of Justice (Inspección General de Justicia [IGJ]). Database was also approved by this national bureau, following personal data protection law (Casefile Number SO4:0032164/16).
This registry has received exemption from ethics approval, given the fact that it is a postmarketing PHASE IV Registry by Health Regulatory Authorities (Administración Nacional de Medicamentos, Alimentos y Tecnología Médica [ANMAT]); although the ethical board committee of the Cardiovascular Research Center approved it. Protocol for the registry was presented before the National Argentine Administration of Food, Drug and Medical Technology (ANMAT), and they authorized us in writing to recruit patients. Each involved hospital authority also approved the protocol. The stent used was approved for routine PCI by ANMAT on February 19, 2016 (case file number 1-47-3110-3045/15-6). The registry followed Good Clinical Practice (GCP) and the Declaration of Helsinki for human research. All patients signed an ICF. As there is no case report in this registry, a statement on informed consent for publication was not required. The ANMAT authorities were aware of recruitment process and adverse events rate during the entire follow-up of the study.
Results
In-hospital and 30-day results were published in detail elsewhere previously.13,14 Patient population and study group (201 patients) are described in Figure 1; 9.7% of the screened population from 11 sites in Argentina were selected and included in the registry.
A list of hospital and operators is given in Appendix 1. Baseline demographics and clinical and angiographic characteristics of the population are described in Table 2.
Table 2.
Clinical demographic and angiographic baseline characteristics.
| Variable | |
|---|---|
| Age | 61.5 ± 12.4 years |
| Male | 80.1% |
| Age > 80 years | 6.5% |
| Dyslipidemia | 67.1% |
| High blood pressure | 66.7% |
| Diabetes mellitus | 22.4% |
| Current smoker | 34.3% |
| Body mass index | 27.9 ± 4.2 |
| Previous acute myocardial infarction | 24.4% |
| Previous revascularization | 22.9% |
| Percutaneous coronary intervention | 21.9% |
| Bypass surgery | 3.0% |
| Peripheral vascular disease | 4.5% |
| Cerebrovascular accident | 7.5% |
| Chronic obstructive pulmonary disease | 6.0% |
| At admission | |
| Acute coronary syndrome | 67.2% |
| ST elevation myocardial infarction | 31.8% |
| Non-ST elevation myocardial infarction | 30.7% |
| Unstable angina | 47.4% |
| Diameter stenosis >70% | 1.55 ± 0.71 per patient |
| Lesion length average | 16.15 ± 8.9 |
| Lesion diameter average | 2.95 ± 0.49 |
| Multiple-vessel disease | 46.8% |
| No. of stent implanted per patient (median) | 1.49 |
| No. of lesions treated per patient (median) | 1.34 |
| Basal SYNTAX score | 11.8 ± 6.8 |
| Basal ERACI score | 7.8 ± 5.3 |
| Thienopyridines at discharge | |
| Clopidogrel | 52.3% |
| Prasugrel | 11.4% |
| Ticagrelor | 36.3% |
Demographic characteristics reflected the all-comers study population of the registry: 6.5% were 80-year-old or older patients, 22.5% were diabetics, 23% had previous revascularization, 6% had chronic obstructive pulmonary disease, 7.5% had a previous CVA, and 67.2% had ACS (32% of them with STEMI). Angiographic characteristics were described in Table 2.
A total of 1.5 stent were implanted and 1.34 lesions treated per patient. Dual-antiplatelet therapy was selected according to operator’s discretion; a loading dose of clopidogrel was taken by 52.3% of patients, whereas a loading dose of prasugrel and ticagrelor was taken in 11.4% and 36.3%, respectively.
Baseline initial SYNTAX score was ±11.8, but dropped to ±7.8 when ERACI score was measured (P = .0016 for comparison) as described in Table 3.
Table 3.
Comparison between SYNTAX versus ERACI residual risk scores.
| Variable | SYNTAX score | ERACI score | P value |
|---|---|---|---|
| Patient number | 201 | 201 | |
| Basal score | 11.8 ± 6.8 | 7.8 ± 5.3 | .001 |
| Residual score | 5.4 ± 5.6 | 1.3 ± 2.9 | <.001 |
| Completeness of revascularization with score ⩽5a | 58% | 90% | <.001 |
Residual risk score: score ⩽5 post procedure, “reasonable” incomplete revascularization.
In-hospital and 30-day results
There was no procedural related death in the overall cohort of 201 patients. One patient with STEMI and cardiogenic shock developed severe ventricular tachycardia and died 21 days after PCI while on a waiting list for a cardiac defibrillator implantation (0.5%). Two patients had MI, one STEMI and one non-STEMI; one of them had vessel closure due to stent thrombosis. The incidence of death + MI was 1.5% (3 of 201). Overall, MACE at 30 days was 1.5% (3 of 201). In-hospital and 30-day outcome are described in Table 4.
Table 4.
In-hospital, 30 days, and 6 and 12 months of follow-up and cumulative adverse cardiac events at 376 ± 18.1 days.
| Variable (n = 201) | N (%) | |||
|---|---|---|---|---|
| In-hospital and 30 days | 6 months | 12 months | Cumulative | |
| Any cause of death | 1 (0.5) | 2 (1.0) | 2 (1.0) | 5 (2.5) |
| Cardiovascular death | 1 (0.5) | 2 (1.0) | 0 (0.0) | 3 (1.5) |
| Acute myocardial infarction (MI) | 1 (0.5) | 1 (0.5) | 0 (0.0) | 2 (1.0) |
| Cerebrovascular accident (CVA) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Death/MI/CVA | 1 (0.5) | 3 (1.5) | 2 (1.0) | 6 (3.0) |
| Target lesion revascularization (TLR) | 2 (1.0) | 6 (3.0) | 9 (4.5) | 17 (8.5) |
| Unplanned revascularization | 2 (1.0) | 8 (4.0) | 9 (4.5) | 19 (9.5) |
| MACE (cardiovascular death, MI, TLR) | 3 (1.5) | 9 (4.5) | 9 (4.5) | 21 (10.5) |
| Stent thrombosis (definitive, probable) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
Abbreviations: MACE, major adverse cardiac events.
After PCI, residual SYNTAX and ERACI scores were 5.4 ± 5.6 and 1.3 ± 2.9, respectively (P < .001). Despite average of SYNTAX score being low, degree of IR when using such score risk was high; 42% of patients had lesions or vessels without treatment. In contrast, IR was only 10% (P < .001 for differences) if we used ERACI risk score. Meaning that 90% and 58% achieved CFR according to ERACI and SYNTAX risk scores, respectively (P < .001), as shown in Table 3.
One-year follow-up results
Table 3 describes 30-day, 6- and 12-month, and cumulative follow-up results. One-year follow-up (mean 376 ± 18.1 days) was obtained in 100% of patients either by personal or phone contact.
At 1 year, the primary endpoint of MACE was observed in 10.4% (21 of 201) of patients: death + MI + CVA in 3% (6 of 201) and cardiac death + MI + CVA in 1.5% (3 of 201).
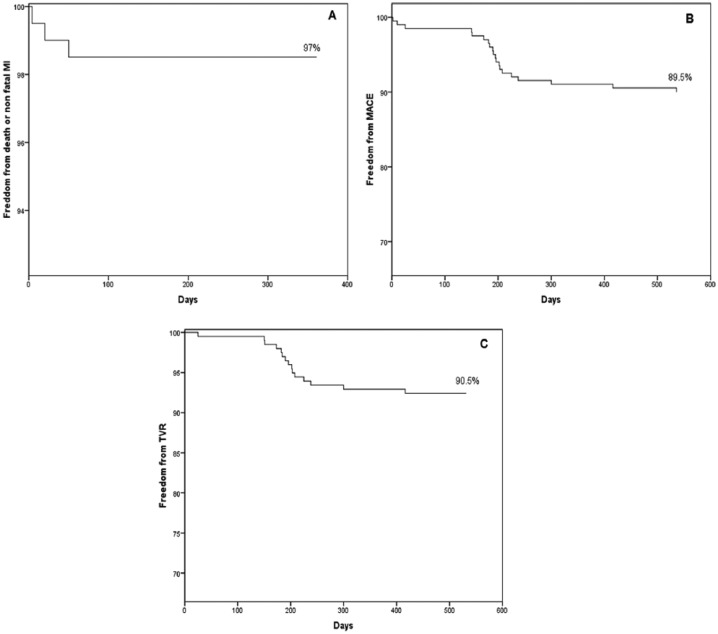
Repeat unplanned revascularization in 9.4% (19 of 201) but TLR/TVR in 17 of 201 (8.4%). Only two patients had repeat revascularization in a non-TLR lesion (1%), which was not included in the initial PCI strategy. Definitive and probable late stent thrombosis was not seen. Cumulative follow-up events are described in Table 4. Freedom from overall death + MI, MACE, and TVR was described in Figure 2A to C, respectively.
Figure 2.
Survival curves. (A) Freedom from death or nonfatal myocardial infarction (MI). (B) Freedom from MACE (cardiac death, nonfatal MI, or TLR). (C) Freedom from unplanned revascularization. MACE indicates major adverse cardiac events; TLR, target lesion revascularization.
We made a comparison of survival events, MACE, using residual risk scores ⩽ or >5, with both risk scores SS and ES, and a Kaplan-Meir curve (Figure 3A and B) was used for comparisons.
Figure 3.
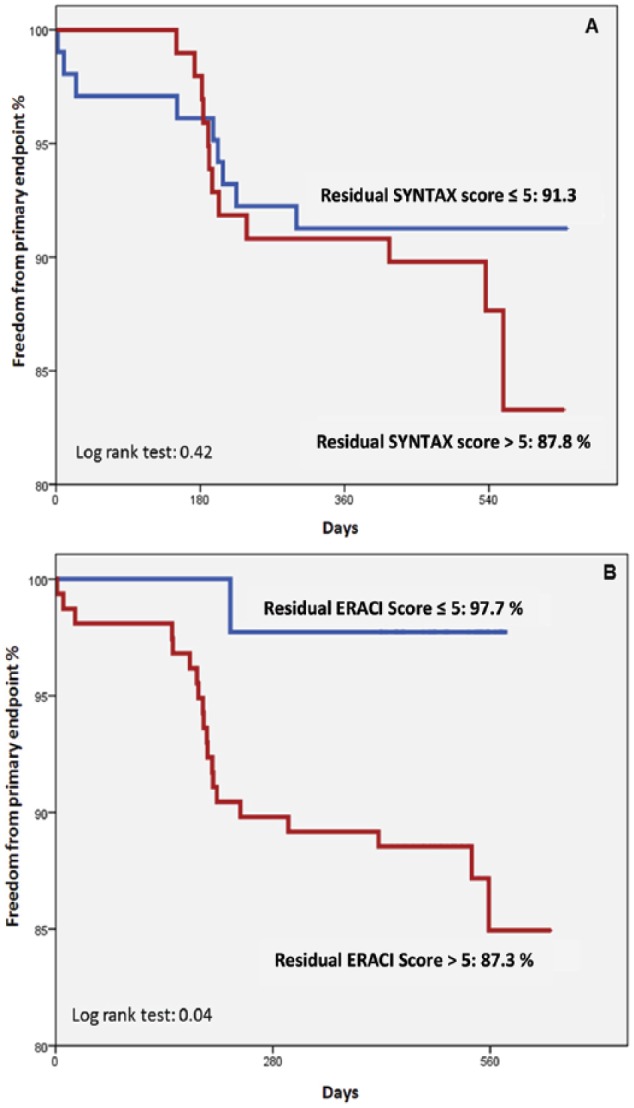
Freedom from primary endpoint at 376 ± 18.1 days of follow-up (MACE: cardiovascular death, acute myocardial infarction, target lesion revascularization) according to residual scores (>5 and ⩽5), SYNTAX, and ERACI score. (A) Residual SYNTAX score. (B) Residual ERACI score. MACE indicates major adverse cardiac events.
At 376 days of follow-up, residual SS ⩽5 or >5 was not predictive for freedom from MACE differences, 91.3% and 87.8%, respectively (P = .42); in contrast, residual ES ⩽5 or >5 was associated with significant differences of freedom from MACE, 97.7% and 87.3%, respectively (P < .04 log rank test), as shown in Figure 3.
During follow-up, 56.4% of patients received clopidogrel, 18.8% prasugrel, and 24.8% ticagrelor; 4% of patients later switched to other thienopyridines. At 1-year follow-up, no statistical differences were seen between DAPT groups in MACE (P = .32). Clopidogrel was the most frequent thienopyridine used in patients with STEMI (P = .01).
Discussion
One-year results (mean 376 ± 18.1 days) of this real-world cohort of patients treated with PCI with a novel cobalt chromium alloy BMS showed a low incidence of cumulative adverse events.
The low adverse events were observed during the entire follow-up period with an incidence of the primary composite endpoint of cardiac death, MI, and TLR of 10.4% (Figure 2B and Table 4). These figures of adverse events were mainly due to the presence of TLR in 8.4% (90.5% of the overall MACE). Unplanned new revascularization was observed in 9.4%, meaning that only 2 patients (1%) required new revascularization in a lesion not included in the initial PCI strategy. This suggested that our conservative PCI strategy was correct.
Incidence of death, MI, and CVA of 3% at 1 year was very low taking into consideration the baseline characteristics of the population included in the registry. The 100% compliance rate at 1 year of follow-up obtained in this registry is remarkably high and related to the close monitoring of the study (on site 25%), and also, the scientific quality of the investigators from each of the 11 sites involved in the registry.
Patients included in this study represent everyday PCI practice in Argentina with wide range of inclusion criteria such as multiple-vessel disease, diabetics, ACS including STEMI, and no age limit restriction (Table 2).
As we described previously, the high numbers of IR in patients with successful PCI was mainly explained by the presence of either severe stenosis in small vessels (<2.0 mm) or intermediate stenosis (<70) in large vessel. Often, WALTZ investigators did not include them in the revascularization strategy, in agreement with policies described by PCI operators using functional flow reserve.20,21
In a previous study, we reported 1-, 2-, and 3-year follow-up patients with complex multiple-vessel disease and unprotected LMCA, and we also found very low MACE rate, using the same conservative PCI revascularization strategy.22–24
However, in that study,22 ERACI IV, a second-generation DES was used and we did not know whether we would be able to achieve similar results with a BMS design such as the one used here. As expected, in the WALTZ registry at 1 year, a higher number of MACE compared with ERACI IV was seen. Still, 90% of these adverse events were caused by ischemic TLR, meaning BMS restenosis. Incidence of cardiac death + MI + CVA was similar between both studies (1.5% in WALTZ and 0.9% in ERACI; P = .44).22 In the WALTZ registry, whether patients had single- or multiple-vessel CAD, TLR (and not new revascularization of non-TLR) was the main reason for failure at follow-up.
Finally, the findings of clopidogrel as the most frequent thienopyridine used in WALTZ registry, including patients with STEMI, are out of current guideline indication25,26 for DAPT therapy. This could be related to the low-income rate of the vast majority of patients included in the registry, and although none of the thienopyridines have a relationship to the differences in the late outcome, discussion of this finding is out of the scope of this presentation.
Study limitations
This study has limitations. First, it is a nonrandomized and only an observational study. Sample size is small although it represents the daily PCI practice in many sites of Argentina where the use of BMS reaches 50% of procedures and included all-comers patient population. Furthermore, 25% of patients had a random “on-site” monitoring design, which is unusual for this sort of registry. That is the reason we called it “controlled.”
In addition, the use of BMS instead of DES could be another limitation and we understand that DES today is the default stent strategy during PCI. However, the lack of mortality benefit of DES over BMS when compared with coronary artery bypass surgery shown by large meta-analysis from RCT12 and registries suggested that the place of BMS in the armamentarium of PCI is not gone yet.
Finally, visual assessment of coronary lesions or vessels diameter is not the most accurate method to evaluate functional revascularization. However, fractional flow reserve (FFR) also has well-known anatomical limitations, and measuring FFR could be challenging,27,28 plus it is not always available in many catheterization laboratories around the world at the time of PCI. Finally, 2 recent RCT reported a lack of clinical benefit with FFR use.29,30
In conclusion, this prospective, multicenter, and observational all-comers registry with this novel BMS design showed a low incidence of adverse events at 1 year mainly due to coronary restenosis.
At 1 year, the incidence of cardiac death, MI, and CVA was extremely low, 1.5%, suggesting the important role of BMS during PCI, especially in selected populations with socioeconomic restrictions such as the ones reported here.
Appendix 1
WALTZ registry study organization
Principal Investigator (PI) Alfredo E Rodriguez, MD, PhD
Co PI: William Pan MD, Zheng Ming;
Data monitoring committee
Clinical events committee:
David Antoniucci, MD (CEC Chairperson), Florence, Italy;
Eduardo Gabe, MD (Sanatorio Otamendi, Buenos Aires, Argentina);
Pablo Stutzbach, MD (Sanatorio Las Lomas, San Isidro, Argentina).
Angio Core Laboratory:
Santiago Burda, Bs, and Yasmin Navarro, Bs As (Centro de Estudios en Cardiología Intervencionista, Buenos Aires, Argentina).
Clinical Project Management:
Centro de Estudios en Cardiología Intervencionista (Alfredo M. Rodriguez-Granillo, MD, and Graciela Romero, MD Project Manager; Secretary: Claudia Masclef).
Biostatistical Analysis: Centro de Estudios en Cardiología Intervencionista (Alfredo M. Rodriguez-Granillo, MD).
Participating centers and study sites investigators
Sanatorio Otamendi, CABA (Carlos Fernández-Pereira MD, PhD)
Clínica IMA, Adrogué, Buenos Aires (Juan Mieres MD)
Sanatorio Las Lomas, San Isidro, Buenos Aires (Omar Santaera, MD)
Sanatorio de la Trinidad, Quilmes, Buenos Aires (Carlos Haiek, MD)
Sanatorio San Miguel, San Miguel, Buenos Aires (Juan Lloberas, MD)
Hospital Español, Mendoza (Miguel Larribau, MD)
Clínica Cuyo, Mendoza (Miguel Larribau, MD)
Clínica Privada Angiocor, La Plata, Buenos Aires (Elías Sisu, MD)
Sanatorio Plaza, Rosario (Menéndez Marcelo, MD)
Clínica 25 de Mayo, Mar del Plata (Iravedra Jorge, MD)
Clinica Sagrada Familia, CABA (Montoya Mario, MD)
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Zheng Ming, MD and William Pan, MD are employees of MicroPort Inc., and Alfredo E. Rodriguez, MD, PhD, perceived modest grants and speaker’s fee from MicroPort Inc. (stent manufacturer).
Author Contributions: All authors who signed the manuscript made major contributions in the preparation, either with protocol design (ZM and WP), coordination (AER and ZM), data analysis (AER, GR, and DA), statistics (ARG), preparation and writing of the final version (AER and CFP), or patient inclusion (HP, JM, CFP, ML, OS, JL, CH, ES, and MM).
ORCID iD: Carlos Fernandez-Pereira  https://orcid.org/0000-0003-0795-0335
https://orcid.org/0000-0003-0795-0335
References
- 1. Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus eluting stents with bare-metal stents. N Engl J Med. 2007;356:1030–1039. [DOI] [PubMed] [Google Scholar]
- 2. Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897–1907. [DOI] [PubMed] [Google Scholar]
- 3. Amin AP, Spertus JA, Cohen DJ, et al. Use of drug-eluting stents as a function of predicted benefit: clinical and economic implications of current practice. Arch Intern Med. 2012;172:1145–1152. [DOI] [PubMed] [Google Scholar]
- 4. Schömig A, Mehilli J, de Waha A, Seyfarth M, Pache J, Kastrati A. A meta-analysis of 17 randomized trials of a percutaneous coronary intervention-based strategy in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52:894–904. [DOI] [PubMed] [Google Scholar]
- 5. Cassese S, De Luca G, Ribichini F, et al. ORAl iMmunosuppressive therapy to prevent in-Stent rEstenosiS (RAMSES) cooperation: a patient-level meta-analysis of randomized trials. Atherosclerosis. 2014;237:410–417. [DOI] [PubMed] [Google Scholar]
- 6. Fernández Pereira C, Descalzo A, Cherro A, et al. ; en representación del grupo RAdAC. Resultados intrahospitalarios en pacientes con infarto agudo de miocardio tratados con angioplastia dentro del Registro Argentino de Angioplastia Coronaria (RadAC). Rev Argent Cardioangiol. 2012;3:28–36. [Google Scholar]
- 7. Rubilar B, Martin R, Coroleu S, et al. Resultados intrahospitalarios de la angioplastia coronaria en octogenarios. Subestudio del Registro Argentino de Angioplastia Coronaria (RAdAC). Rev Argent Cardioangiol. 2015;4:180–186. [Google Scholar]
- 8. Bønaa KH, Mannsverk J, Wiseth R, et al. ; for the NORSTENT Investigators. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. 2016;375:1242–1252. [DOI] [PubMed] [Google Scholar]
- 9. Colleran R, Kufner S, Mehilli J, et al. Efficacy over time with drug-eluting stents in saphenous vein graft lesions. J Am Coll Cardiol. 2018;71:1973–1982. [DOI] [PubMed] [Google Scholar]
- 10. Rodriguez AE, Maree AO, Mieres J, et al. Late loss of early benefit from DES when compared with BMS and CABG: 3 years follow-up of the ERACI III registry. Eur Heart J. 2007;28:2118–2125. [DOI] [PubMed] [Google Scholar]
- 11. Park DW, Ahn JM, Yun SC, et al. Ten-year outcomes of stents versus coronary-artery bypass grafting for left main coronary artery disease. J Am Coll Cardiol. 2018;72:2813–2822. [DOI] [PubMed] [Google Scholar]
- 12. Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. 2018;391:939–948. [DOI] [PubMed] [Google Scholar]
- 13. Mieres J, Lloberas J, Haeik C, et al. ; on behalf of WALTZ investigators. New cobalt-chromium stent design in the treatment of real world coronary artery disease: rationality and study design of the all comers observational, multicenter WALTZ Registry. Rev Argent Cardioangiol. 2017;1:12–17. [Google Scholar]
- 14. Lloberas J, Iravedra J, Haeik C, et al. ; on behalf of WALTZ investigators. In hospital and 30 days results of the prospective, observational, multicenter and controlled “real world” WALTZ registry. Rev Argent Cardioangiol Interv. 2017;8:131–136. [Google Scholar]
- 15. Rodriguez AE, Mieres J, Fernández-Pereira C, et al. Coronary stent thrombosis in the current drug-eluting stent era: insights from the ERACI III trial. J Am Coll Cardiol. 2006;47:205–207. [DOI] [PubMed] [Google Scholar]
- 16. Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 17. Rodriguez AE, Fernández-Pereira C, Mieres J, Santaera O, Antoniucci D; ERACI IV Investigators. Modifying angiographic syntax score according to PCI strategy: lessons learnt from ERACI IV study. Cardiovasc Revasc Med. 2015;16:418–420. [DOI] [PubMed] [Google Scholar]
- 18. Rodríguez AE, Fernández-Pereira C, Mieres J, et al. Lowering risk score profile during PCI in multiple vessel disease is associated with low adverse events: the ERACI risk score. Cardiovasc Revasc Med. 2018;19:792–794. [DOI] [PubMed] [Google Scholar]
- 19. Rodriguez AE, Fernandez-Pereira C, Mieres J, Mendoza J, Sartori F. Can we improve the outcomes of multivessel disease using modified SYNTAX and residual SYNTAX scores? Curr Cardiol Rep. 2017:20. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi Y, Nam CW, Tonino PA, et al. ; FAME Study Investigators. The prognostic value of residual coronary stenoses after functionally complete revascularization. J Am Coll Cardiol. 2016;67:1701–1711. [DOI] [PubMed] [Google Scholar]
- 21. Escaned J, Collet C, Ryan N, et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-yearresults of the SYNTAX II study. European Heart Journal. 2017;38:3124–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haiek C, Fernández-Pereira C, Santaera O, et al. Second vs. first generation drug eluting stents in multiple vessel disease and left main stenosis: two-year follow-up of the observational, prospective, controlled, and multicenter ERACI IV registry. Catheter Cardiovasc Interv. 2017;89:37–46. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez AE, Santaera O, Larribau M, et al. ; ERACI IV Investigators. Second versus first drug eluting stents in complex lesions subsets: 3 years follow up of ERACI IV Study. Minerva Cardioangiol. 2017;65:81–90. [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez AE, Pavlovsky H, Del Pozo JF. Understanding the outcome of randomized trials with drug-eluting stents and coronary artery bypass graft in patients with multivessel disease: a review of a 25-year journey. Clin Med Insights Cardiol. 2016;10:195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 26. Rodriguez AE, Rodriguez-Granillo AM, Ascarrunz SD, Peralta-Bazan F, Cho MY. Did prasugrel and ticagrelor offer the same benefit in patients with acute coronary syndromes after percutaneous coronary interventions compared to clopidogrel? Insights from randomized clinical trials, registries and meta-analysis. Curr Pharm Des. 2018;24:465–477. [DOI] [PubMed] [Google Scholar]
- 27. Toth G, De Bruyne B, Casselman F, et al. Fractional flow reserve-guided versus angiography-guided coronary artery bypass graft surgery. Circulation. 2013;128:1405–1411. [DOI] [PubMed] [Google Scholar]
- 28. Shah T, Geleris JD, Zhong M, Swaminathan RV, Kim LK, Feldman DN. Fractional flow reserve to guide surgical coronary revascularization. J Thorac Dis. 2017;9:S317–S326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rioufol G, Roubille F, Perret T, et al. FUnctional testing underlying revascularization: the FUTURE trial. Paper presented at: European Society of Cardiology Congress (Late Breaking Trials Session); August, 2018; Munich, Germany http://www.clinicaltrialresults.org/Slides/ESC2018/FUTURE_Rioufol.pdf. [Google Scholar]
- 30. Thuesen AL, Riber LP, Veien KT, et al. Fractional flow reserve versus angiographically-guided coronary artery bypass grafting. J Am Coll Cardiol. 2018;72:2732–2743. [DOI] [PubMed] [Google Scholar]