Abstract
Context:
Amid extensive debate, evidence surrounding the use of platelet-rich plasma (PRP) for musculoskeletal injuries has rapidly proliferated, and an overall assessment of efficacy of PRP across orthopaedic indications is required.
Objectives:
(1) Does PRP improve patient-reported pain in musculoskeletal conditions? and (2) Do PRP characteristics influence its treatment effect?
Data Sources:
MEDLINE, EMBASE, Cochrane, CINAHL, SPORTDiscus, and Web of Science libraries were searched through February 8, 2017. Additional studies were identified from reviews, trial registries, and recent conferences.
Study Selection:
All English-language randomized trials comparing platelet-rich therapy with a control in patients 18 years or older with musculoskeletal bone, cartilage, or soft tissue injuries treated either conservatively or surgically were included. Substudies of previously reported trials or abstracts and conference proceedings that lacked sufficient information to generate estimates of effect for the primary outcome were excluded.
Study Design:
Systematic review and meta-analysis.
Level of Evidence:
Level 1.
Data Extraction:
All data were reviewed and extracted independently by 3 reviewers. Agreement was high between reviewers with regard to included studies.
Results:
A total of 78 randomized controlled trials (5308 patients) were included. A standardized mean difference (SMD) of 0.5 was established as the minimum for a clinically significant reduction in pain. A reduction in pain was associated with PRP at 3 months (SMD, –0.34; 95% CI, –0.48 to –0.20) and sustained until 1 year (SMD, –0.60; 95% CI, –0.81 to –0.39). Low- to moderate-quality evidence supports a reduction in pain for lateral epicondylitis (SMD, –0.69; 95% CI, –1.15 to –0.23) and knee osteoarthritis (SMD, –0.91; 95% CI, –1.41 to –0.41) at 1 year. PRP characteristics did not influence results.
Conclusion:
PRP leads to a reduction in pain; however, evidence for clinically significant efficacy is limited. Available evidence supports the use of PRP in the management of lateral epicondylitis as well as knee osteoarthritis.
Keywords: platelet-rich plasma, growth factor, orthopaedics, sports medicine, regenerative medicine, arthritis
Platelet-rich plasma (PRP), defined as autologous blood with supraphysiological concentrations of platelets, has become a popular minimally invasive biological and injectable treatment for musculoskeletal injuries. Despite a lack of evidence-based recommendations for its efficacy, the global market for PRP is projected to increase to $451 million in the next decade.45 Support for PRP is fueled by evidence that bioactive proteins and growth factors promote healing and recovery.4,6,32,74,76,113,129 Optimal preparation is the subject of considerable controversy and debate.4,32,76,129 Multiple PRP preparation systems and techniques are available, each differing in final platelet concentration, presence of leukocytes and noncellular plasma clotting factors, and use of exogenous activating agents.4,32,74,82
Many prior systematic reviews on the use of PRP in orthopaedics have been conducted, but have been limited in their method and scope, leading to conflicting interpretations of the evidence (see Appendix Table A1, available in the online version of this article).8,17,22,25,30,33,38,41,42,58,63,71,72,75,77,86,91,104,112,113,119,124,129 More than one-third of prior reviews were unable to perform a quantitative analysis,30,33,38,41,58,77,119,129 and more than 60% include lower-level evidence, such as nonrandomized cohort studies or case series.22,30,33,38,41,58,63,72,75,86,104,113,119,129 Less than half of prior reviews explored reasons for substantial variability between studies, leading to misleading effect estimates with high heterogeneity, and only 2 of the meta-analyses summarized the overall confidence in the evidence. A substantial amount of new evidence is currently available, with more than 30% of all randomized controlled trials (RCTs) being published within the past 2 years, and 60 additional RCTs being published since the last broad systematic reviews were completed.113,129
Despite an exponential increase in publications associated with PRP use, the evidence is far from conclusive. Trials vary in population, sample size, and methodological quality, further vindicating the need for a comprehensive updated review. Given the widespread applicability of an evidence-based recommendation, we conducted a systematic review and updated meta-analysis of RCTs to provide the current best estimate of whether PRP reduces patient-reported pain in musculoskeletal conditions, and whether particular PRP characteristics influence its treatment effect.
Methods
Search Strategy and Criteria
A systematic review and meta-analysis was performed according to the methods of the Cochrane Handbook for Systematic Reviews and is reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.59,111 A protocol for our review was registered online with Prospero (No. 42017057900; https://www.crd.york.ac.uk/PROSPERO/).
The following electronic databases were systematically searched: MEDLINE (from 1946), EMBASE (from 1974), Cochrane (from 2005), CINAHL (from 1981), SPORTDiscus (from 1800), and Web of Science (from 1976). The last updated search was conducted on February 8, 2017. Search strategies combined keywords and database-specific subject heading terms for the patient population and interventions of interest, and the highly sensitive Cochrane search strategy filter for identifying RCTs was used (see Appendix Table A2, available online).59 Abstracts from recent (3 years) relevant annual meetings were reviewed for unpublished literature, and international trial registries were used to identify ongoing studies (see Appendix Table A3, available online). Attempts were made to contact the primary investigators of ongoing studies to collect further information. Nonindexed electronic ahead-of-print publications were identified using PubMed search filters. Additional studies were identified by reviewing the reference lists of eligible studies. After removing duplicates, 3 reviewers (HJ, NE, MK) independently screened all titles, abstracts, and full texts of potentially eligible studies for final inclusion. For full-text articles deemed ineligible, the reason(s) for exclusion were recorded. Disagreement was resolved through discussion.
Inclusion and Exclusion
We identified all English-language randomized trials comparing platelet-rich therapy with a control in patients 18 years or older with musculoskeletal bone, cartilage, or soft tissue injuries treated either conservatively or surgically, including (1) injuries to tendons or ligaments such as ruptures, tears, and sprains; (2) traumatic meniscal and labral lesions; (3) acute fractures or fractures with delayed or nonunion; (4) acute or chronic tendinopathies and fasciopathies; and (5) articular cartilage pathology, such as osteochondral defects and degenerative osteoarthritis. Treatments considered for the control groups included standard operative or nonoperative treatment, placebo, hyaluronic acid (HA), corticosteroid, local anesthetic, or whole blood injection. Platelet-rich therapies may have been used as the sole treatment or as an adjunct to surgical treatment provided to all participants in a given trial. The outcome of interest was the difference in patient-reported pain at different time intervals posttreatment. Substudies of previously reported trials or abstracts and conference proceedings that lacked sufficient information to generate estimates of effect for the primary outcome were excluded.
Assessment of Study Quality and Overall Estimate of Effect
Two independent reviewers (HJ and NE ) assessed the risk of bias of the included RCTs using the Cochrane Collaboration risk of bias tool.60 Trials were scored as low risk, moderate risk, or high risk of bias based on methodologic considerations. Differences were settled by discussion and involvement of a third reviewer as necessary.
The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach was used to evaluate confidence in the pooled effect estimates.10,50 According to GRADE, data from RCTs are considered high-quality evidence but can be rated down due to risk of bias, imprecision, inconsistency, indirectness, or publication bias.10 The quality of the evidence was graded as high, moderate, low, or very low and applied to each outcome of interest separately, organized by indication.51
Data Collection and Abstraction
Two teams of reviewers (HJ, MK, SE, NH) independently collected and confirmed all relevant information in duplicate using piloted data forms. Any disagreements were resolved by discussion. Collected data included study design, first author, journal, year of publication, patient characteristics, indication, target tissue, PRP preparation details/characteristics, control intervention details, surgical co-interventions, sample size and losses in each group, duration of follow-up, and patient outcomes. Several classification systems exist that define PRP products based on characteristics that may affect performance.29,42,83 Although no single system has been validated in the literature, there is overlap among them with regard to the inclusion of leukocyte concentration, platelet concentration, and use of an exogenous activating substance. However, there is disagreement as to the contributions of each of these factors to overall efficacy.32 To assess this, we abstracted leukocyte concentration (increased vs decreased over baseline), platelet activation (exogenous activation vs no exogenous activation), and platelet concentration (less than or greater than 5-fold over baseline) based on features and cutoffs common to previously described classification systems.29,83 Manufacturers of the preparation systems described in the studies were contacted if PRP product characteristics were not readily available. Based on clinical significance and previous literature, pain intensity was the primary outcome of interest.1,5,12,18,22,41,58,63,64,71,86,113 If data were provided on more than 1 scale for pain, only the most commonly reported scales across included studies (for which complete data were available) were combined. To be consistent with analyses of other reported injectable interventions, and to best capture the data presented in all studies, the time points of outcomes were grouped into 3-month, 6-month, and 1-year intervals.11,107
Statistical Analysis and Assessment of Heterogeneity
Continuous outcomes were calculated and expressed as the standardized mean difference (SMD), along with 95% CI. SMD was defined as the between-group differences in mean values reported at each follow-up divided by the SD. Cohen effect size criteria were used as a guide for the clinical interpretation of the SMDs.24 An SMD of 0.2 or less represented a small effect, 0.2 to 0.8 represented a moderate effect, and greater than 0.8 represented a large effect. Based on estimates from previous authors, an SMD of 0.5 (half an SD) was used to approximate a clinically significant reduction in pain.88
Heterogeneity was assessed visually with inspection of the forest plots, and objectively with the χ2 (P < 0.10 indicates heterogeneity) and the I2 statistic (I2 < 40%, low heterogeneity; I2 ≥ 75%, substantial heterogeneity). A random effects model was used to pool data with moderate or substantial heterogeneity (I2 > 40%); otherwise, a fixed effects model was used.
Subgroup and meta-regression analyses were carried out if significant heterogeneity was identified (I2 > 40%) to assess the influence of study characteristics on effect estimates.
A priori, we hypothesized that heterogeneity may be due to differences in population (clinical indication), PRP properties (platelet concentration, leukocyte concentration, exogenous activation), and comparator characteristics (type of control). Tests for significance were 2-tailed, and P < 0.05 was considered significant. Sensitivity analyses were performed to investigate heterogeneity within subgroups and the importance of study quality by omitting studies with high risk of bias.
The Cohen kappa statistic (κ) was used to evaluate agreement between reviewers with regard to study eligibility and reviewer assessments. This was interpreted using accepted cutoffs for levels of agreement.114 All analyses were performed using STATA 14.0 software (STATA Corp) and Review Manager 5.3 (The Nordic Cochrane Center; The Cochrane Collaboration).
Results
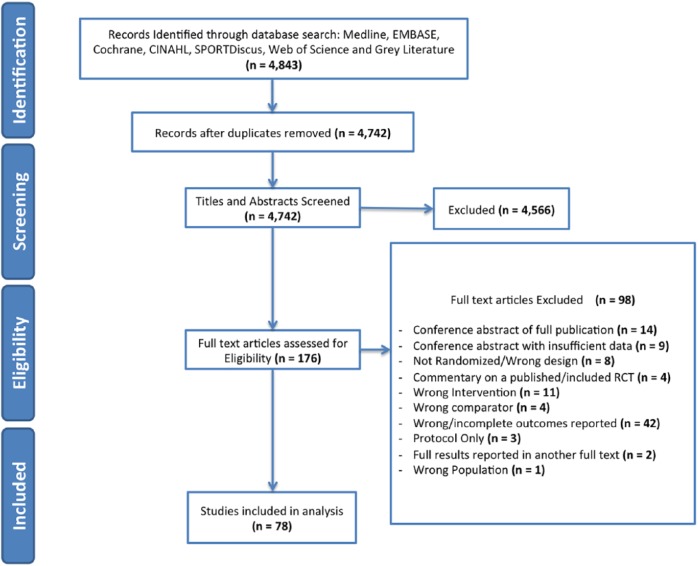
After removing duplicates, we identified 4843 potential articles, 176 of which were reviewed as full texts (Figure 1). Ninety-eight articles were excluded, and 78 RCTs that compared a platelet-rich product with a control in patients with an orthopaedic injury were included for analysis (n = 5308 randomized patients).2,3,7,9,13-16,19-21,23,26-28,31,34-37,39,40,43,44,46-49,56,57,61,62,65,67-70,73,78,79, 81,84,85,87,89,90,92-103,105,108-110,115-118,120-123,125-128,130,131,133 Interobserver agreement was substantial for screening of titles and abstracts (κ, 0.75; 95% CI, 0.72-0.78) and almost perfect for review of full texts (κ, 0.95; 95% CI, 0.92-0.98).
Figure 1.
Study flow diagram depicting the number of studies at each stage of the systematic review, with reasons for exclusion. In the end, 78 RCTs that compared a platelet-rich product to a control in patients with an orthopaedic injury were included for analysis (n = 5308 randomized patients).
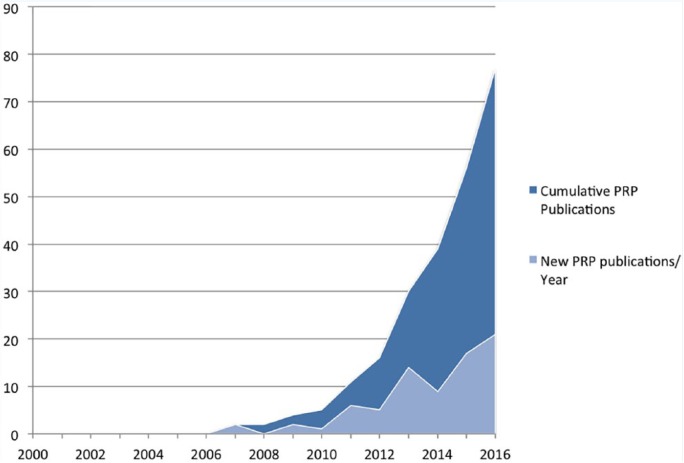
Figure 2 graphically depicts the cumulative number of RCTs relative to the year of publication and the number of RCTs published annually. All 78 included RCTs were published in the past decade, with 60 (73%) of them published since the last overarching systematic review on the topic in 2012.113
Figure 2.
Graphic depiction of the number of randomized controlled trials (RCTs) published annually (light shade) as well as the cumulative number of RCTs (dark shade) relative to the year of publication since the year 2000. The number of RCTs is on the y-axis, with year of publication along the x-axis. Note the rapid rise in RCTs examining this emerging therapy over the past decade. PRP, platelet-rich plasma.
Participants averaged 47.9 years of age (range, 22.9-76 years) (Table 1). Sample sizes ranged from 9 to 380 patients, with the duration of follow-up ranging from 5 days to 72 months. The efficacy of PRP was examined across a wide range of orthopaedic indications and target tissues, and 44% of studies used PRP as an adjunct during surgical treatment (Table 1). Details of the platelet-based product preparation used in each study can be found in Appendix Tables A4 and A5 (available online) and are summarized in Table 1.
Table 1.
Study characteristics
| Total number of trials | 78 |
| Age, y, mean (range) | 47.9 (22.9-76.0) |
| Sample size, mean (range) | 62 (9-380) |
| Duration of follow-up, mean (range) | 11.7 mo (5 d to 72 mo) |
| PRP type | |
| Type 1 | 33 |
| Type 2 | 11 |
| Type 3 | 13 |
| Type 3 | 19 |
| Not reported/unable to be determined | 28 |
| Orthopaedic indications | |
| Rotator cuff pathology | 21 |
| Lateral epicondylitis | 13 |
| Hip osteoarthritis | 3 |
| Knee osteoarthritis | 19 |
| ACL reconstruction | 7 |
| Plantar fasciitis | 10 |
| Achilles tendinopathy/rupture | 5 |
| Other | 26 |
| Target tissue | |
| Tendon | 42 |
| Cartilage | 26 |
| Fascia | 10 |
| Ligament | 8 |
| Bone | 15 |
| Muscle | 3 |
| Outcome measures | |
| Pain | 78 |
| Clinical outcome scores | 95 |
| Radiographic/MRI healing | 34 |
| Physical examination | 20 |
| PRP manufacture | |
| Biomet | 19 |
| Arthrex | 13 |
| Medtronic | 3 |
| Biotechnology Institute | 3 |
| Not reported | 20 |
| Other | 46 |
| PRP concentration | |
| ≥5-fold | 20 |
| Less than 5-fold | 29 |
| Not reported | 55 |
ACL, anterior cruciate ligament; MRI, magnetic resonance imaging; PRP, platelet-rich plasma.
Thirty-two manufacturers were used by 64 trials that reported the PRP preparation system used, with nearly half of trials using 6 main manufacturers. Seventeen studies reported receiving funding from the manufacturer, and an additional 34 studies did not report funding source or specify conflicts of interest.
PRP Impact on Pain
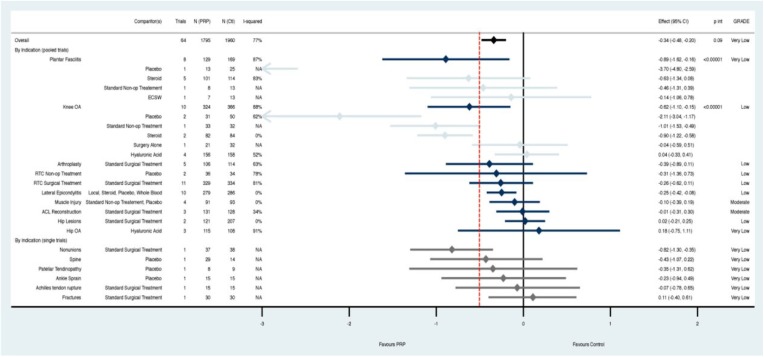
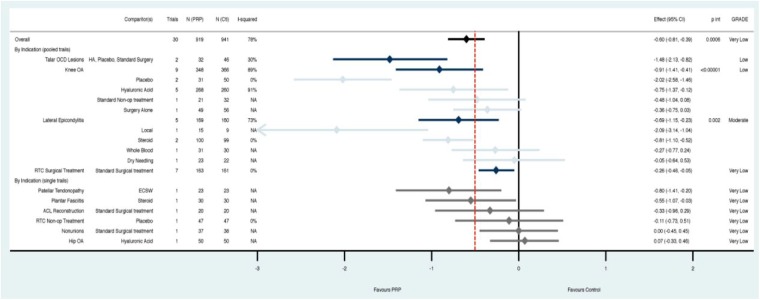
The overall analysis across all indications demonstrated a statically but not clinically significant reduction in pain with PRP compared with controls at 3 months (SMD, –0.34; 95% CI, –0.48 to –0.20; P ≤ 0.00001) (Figure 3). This effect grew at 6 months (SMD, –0.55; 95% CI, –0.73 to –0.36; P ≤ 0.00001) (Appendix Figure A1, available online) and 1 year (SMD, –0.60; 95% CI, –0.81 to –0.39; P ≤ 0.00001) (Figure 4). However, the CIs overlapped the 0.5 effect size threshold for a clinically important difference.
Figure 3.
At 3 months, platelet-rich plasma (PRP) demonstrated a statistically but not clinically significant reduction in pain compared with controls across all indications. Subgroup analysis by clinical indication, and comparator where there were multiple trials, revealed that PRP outperformed placebo and steroid both statistically and clinically for knee osteoarthritis only. The remaining subgroups either had CIs that overlapped thresholds for minimally important difference (red line = standardized mean difference [SMD] 0.5) or statistical significance (black line = SMD 0); or were composed of only a single study. Pooled estimates by indication are in navy, with comparator subgroups in light blue. Estimates for indications with only single trials (gray) are found at the bottom; p int, p interaction for subgroup effects.
Figure 4.
At 12 months, platelet-rich plasma (PRP) demonstrated a statistically but not clinically significant reduction in pain compared with controls across all indications. Subgroup analysis by clinical indication, and comparator where there were multiple trials, revealed that PRP clinically and statistically significantly improved pain compared with placebo in patients with knee osteoarthritis, compared with steroid in patients with lateral epicondylitis, and compared with hyaluronic acid, placebo, or surgery combined for patients with talar osteochondral lesions (I2 = 30%). The remaining subgroups either had CIs that overlapped thresholds for minimally important difference (red line = standardized mean difference [SMD] 0.5) or statistical significance (black line = SMD 0); or were composed of only a single study. Pooled estimates by indication are in navy, with comparator subgroups in light blue. Estimates for indications with only single trials (gray) are found at the bottom; p int, p interaction for subgroup effects.
Heterogeneity was substantial (range, 77%-82%) and was explored further through subgroup analysis at each time point initially by clinical indication, and subsequently by comparator if there was substantial heterogeneity (I2 > 40%) (Figures 3 and 4; Appendix A1, available online). When assessing by indication, low to moderate quality evidence indicated that there was a clinically important treatment effect when PRP was used for lateral epicondylitis (compared with steroid; I2 = 0%) as well as knee osteoarthritis (compared with placebo or steroid; I2 = 0%) and talar osteochondral lesions (compared with HA, placebo, or surgery combined; I2 = 30%). The remaining indications and time points did not convincingly demonstrate an effect with use of PRP and are based on low- or very low–quality evidence due to high residual heterogeneity, high risk of bias, or inclusion of data from only a single trial (Figures 3 and 4; Appendix A1, available online). Sensitivity results were similar when assessing first by comparator, and subsequently by indication; however, the evidence for effectiveness of PRP in patients with talar osteochondral lesions came only from single studies or was no longer significant at 12 months (Appendix Figures A2-A4, available online).
PRP Characteristics and Treatment Effect
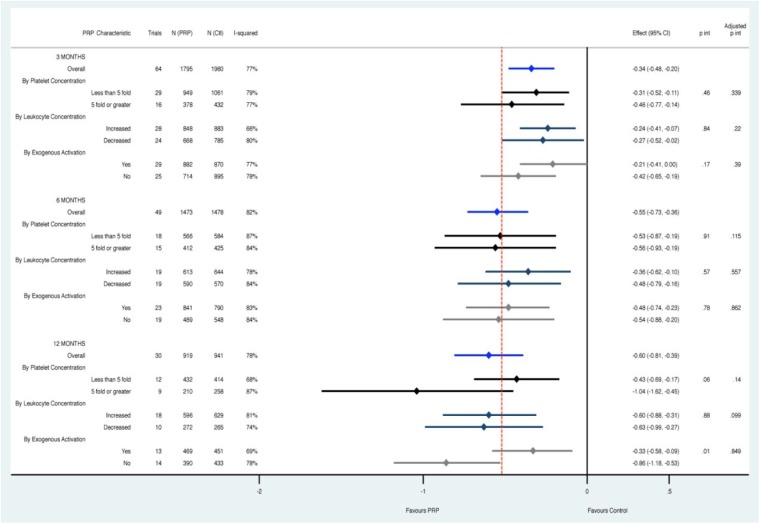
Assessment of PRP characteristics through subgroup analysis and multivariate metaregression did not reveal either leukocyte concentration, platelet concentration, or use of an exogenous activator to be associated with increased effectiveness when controlling for the indication and comparator used (Figure 5).
Figure 5.
Assessment of platelet-rich plasma (PRP) characteristics through subgroup analysis and multivariate metaregression did not reveal any characteristic to be consistently associated with increased effectiveness when controlling for the indication and comparator used across all time points. Pooled estimates at each time point are in blue, with subanalysis below based on platelet concentration (black), leukocyte concentration (navy), and exogenous activation (gray). p int, p interaction for subgroup effects; adjusted p int, p interaction from metaregression controlling for other characteristics.
Risk of Bias and Summary of Evidence
Using the Cochrane risk of bias tool, 14 studies were judged to be of low risk of bias, and the remaining 64 (82%) studies were judged to be of moderate to high risk of bias, with substantial agreement between reviewers (κ = 0.80; 95% CI, 0.63-0.96).
GRADE assessments of the overall quality of evidence were low at 3 months, 6 months, and 1 year (see Appendix Tables A6, A7, and A8, respectively, available online).52-55
Discussion
This systematic review and meta-analysis of 78 trials finds PRP results in a statistically significant reduction in pain. However, evidence for clinically significant efficacy is limited. Evidence supports a statistically significant reduction in pain for lateral epicondylitis as well as for knee osteoarthritis as primary indications. The confidence in efficacy for the remaining interventions was either very low or no efficacy was noted. This includes the use of PRP in the treatment of muscle injuries, anterior cruciate ligament reconstruction, and rotator cuff tears. Leukocyte concentration, platelet concentration, or use of an activator did not influence results.
Our findings are consistent with 2 previous network meta-analyses that assessed lateral epicondylitis.8,71 We provide an updated analysis of the lateral epicondylitis literature, with an additional 3 trials, demonstrating that 1-year PRP results suggest a clinically significant effect when compared with local anesthetic and corticosteroid injections and a similar effect to autologous whole blood and dry needling. The findings with respect to autologous whole blood and dry needling are interesting, as both present a simpler and less costly alternative for the patient. The findings may be due to 1 of 2 reasons: (1) the simple act of introducing whole blood or inciting local bleeding and inflammation to the damaged tissue may result in enough healing factors, albeit fewer than PRP, to result in a clinical improvement in pain; or (2) some component of the placebo effect may be present. Further studies with large, high-quality trials are required to delineate differences between these treatment options.
The majority of the RCTs in this analysis investigated PRP for knee osteoarthritis. Overall, moderate-quality evidence supports an early effect sustained to 1 year. Substantial heterogeneity remained, largely among trials comparing the effectiveness of PRP with HA. This is consistent with Dai et al,25 who also noted significant heterogeneity with respect to studies comparing PRP with HA. As both interventions continue to evolve, comparison between the 2 must be done with caution. HA characteristics such as molecular weight and cross-linkage have been shown to influence this treatment’s overall effectiveness in the conservative management of knee osteoarthritis,66 and these traits must be considered when using it as a control therapy in trials. Further trials comparing PRP with HA in the nonsurgical management of knee osteoarthritis should focus on highly crosslinked, high–molecular weight HA formulations that have been shown to be the most effective.66
Our analysis provides insight into the role of PRP characteristics that are thought to influence effectiveness. These include leukocyte concentration, platelet concentration, and use of an exogenous activating agent, all of which can be modified using PRP preparation methods such as speed of centrifugation, number of centrifugation cycles, order of pellet and supernatant separation, and addition of products such a thrombin prior to PRP use. In separate laboratory investigations, Rubio-Azpeitia et al106 and Zhang et al132 each found that leukocyte-rich formulations were more proinflammatory, and the formulations with higher platelet concentrations exhibited stronger chemotactic and proliferative qualities. A further laboratory investigation showing that leukocyte-rich PRP may result in significant synoviocyte cell death and increase proinflammatory mediators has since been contradicted by an in vivo investigation of 36 patients with osteoarthritic knees who received intra-articular injections with either leukocyte-rich PRP or HA, neither of which resulted in a difference in pro- or anti-inflammatory markers compared with baseline results.80 A recent network meta-analysis found no impact of leukocyte concentration on the overall effectiveness of PRP injections for the treatment of osteoarthritis,104 with findings confirmed by our results for the same patient population at 1 year. Looking across other indications and at the overall cohort of 78 RCTs, the present study found no evidence that leukocyte concentration, platelet concentration, or use of an exogenous activating agent affects the overall effectiveness of PRP.
Strengths
Our review focused specifically on evidence exclusively from RCTs to limit the influence of confounding. It included substantially more trials in each indication subgroup than any other systematic review published in this area to date, many of which included evidence from nonrandomized trials, retrospective cohorts, and case series.8,17,22,25,30,33,38,41,42,58,63,71,72,75,77,86,91,104,112,113,119,124,129 This maximized the power of our pooled analysis to detect an overall effect and allowed us to explore further where heterogeneity was a concern. Where heterogeneity was noted, a priori subgroup and metaregression analysis were conducted to assess important differences attributable to indication, PRP characteristics, comparator used, and duration of follow-up. A particular strength of this study was the assessment of PRP product characteristics across the large body of clinical evidence regarding its use. Although postulated to be a source of considerable heterogeneity, PRP characteristics have received limited attention as an effect modifier in previous meta-analysis. We abstracted PRP characteristics as reported and derived characteristics based on PRP preparation methods described and systems used, and contacted manufacturers to confirm details as needed. Risk of bias was assessed for each study individually using a widely accepted and standardized framework,60 and confidence in both overall evidence and specific indications was summarized using the GRADE approach.
Limitations
Our review has limitations. Between-study heterogeneity remained high and unexplained across many indications; however, we set several a priori hypotheses to explain heterogeneity and were able to identify indications where there was certainty of effect and lack of effect. Study quality was not uniformly high, and variability in comparators and design limitations were identified in nearly 50% of trials. Additionally, study sample sizes were small, further limiting inferences from individual trials.
Conclusion
This meta-analysis demonstrates available evidence supports the clinical efficacy of PRP in patients with lateral epicondylitis and knee osteoarthritis. PRP leads to a reduction in pain across indications; however, evidence for clinically significant efficacy is limited. Future investment of resources in high-quality research should focus on those indications with promise and aim to resolve definitively the utility of PRP in such indications.
Supplemental Material
Supplemental material, 30072_Appendix for Impact of Platelet-Rich Plasma Use on Pain in Orthopaedic Surgery: A Systematic Review and Meta-analysis by Herman Johal, Moin Khan, Shu-hang Patrick Yung, Mandeep S. Dhillon, Freddie F. Fu, Asheesh Bedi and Mohit Bhandari in Sports Health: A Multidisciplinary Approach
Acknowledgments
We thank Nathan Evaniew, MD, PhD, Seper Ekhtiari, MD, Shu-hang Patrick Yung, MBChB, FRCS, FCSHK, FHKAM, Mandeep S. Dhillon, MBBS, MS, and Nolan Horner, MD (McMaster University, Canada) for helping with our study screening, data abstraction, and input for the final manuscript.
Footnotes
The following authors declared potential conflicts of interest: Asheesh Bedi, MD, is a consultant for Arthrex and had stock/stock options in A3 Surgical. Mohit Bhandari, MD, PhD, FRCSC, is a consultant for Smith & Nephew, DePuy, Eli Lily, Bioventus, Stryker, Zimmer, and Amgen and has grants/grants pending from Smith & Nephew, Stryker, Amgen, Zimmer, Moximed, Bioventus, Merck, Eli Lily, Sanofi, Ferring, and ConMed.
References
- 1. Abrams GD, Frank RM, Fortier LA, Cole BJ. Platelet-rich plasma for articular cartilage repair. Sports Med Arthrosc. 2013;21:213-219. [DOI] [PubMed] [Google Scholar]
- 2. Acosta-Olivo C, Elizondo-Rodriguez J, Lopez-Cavazos R, Vilchez-Cavazos F, Simental-Mendia M, Mendoza-Lemus O. Plantar fasciitis—a comparison of treatment with intralesional steroids versus platelet-rich plasma. A randomized, blinded study. J Am Podiatr Med Assoc. 2017;107:490-496. [DOI] [PubMed] [Google Scholar]
- 3. Aggarwal AK, Shashikanth VS, Marwaha N. Platelet-rich plasma prevents blood loss and pain and enhances early functional outcome after total knee arthroplasty: a prospective randomised controlled study. Int Orthop. 2014;38:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alsousou J, Thompson M, Hulley P. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91:987-996. [DOI] [PubMed] [Google Scholar]
- 5. Andia I, Maffulli N. Platelet-rich plasma for muscle injury and tendinopathy. Sports Med Arthrosc. 2013;21:191-198. [DOI] [PubMed] [Google Scholar]
- 6. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4-15. [DOI] [PubMed] [Google Scholar]
- 7. Antuna S, Barco R, Diez JM. Platelet-rich fibrin in arthroscopic repair of massive rotator cuff tears: a prospective randomized pilot clinical trial. Acta Orthop Belg. 2013;79:25-30. [PubMed] [Google Scholar]
- 8. Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthopaed Traumatol. 2016;17:101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azcárate AV, Lamo-Espinosa J, Aquerreta Beola JD, Hernandez Gonzalez M, Mora Gasque G, Valentí Nin JR. Comparison between two different platelet-rich plasma preparations and control applied during anterior cruciate ligament reconstruction. Is there any evidence to support their use? Injury. 2014;45(suppl 4):S36-S41. [DOI] [PubMed] [Google Scholar]
- 10. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401-406. [DOI] [PubMed] [Google Scholar]
- 11. Bannuru RR, Vaysbrot EE, Sullivan MC, McAlindon TE. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;43:593-599. [DOI] [PubMed] [Google Scholar]
- 12. Barber FA. Platelet-rich plasma for rotator cuff repair. Sports Med Arthrosc. 2013;21:199-205. [DOI] [PubMed] [Google Scholar]
- 13. Battaglia M, Guaraldi F, Vannini F, et al. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics. 2013;36:e1501-e1508. [DOI] [PubMed] [Google Scholar]
- 14. Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23:6-10. [DOI] [PubMed] [Google Scholar]
- 15. Bubnov R, Yevseenko V, Semeniv I. Ultrasound guided injections of platelets rich plasma for muscle injury in professional athletes. Comparative study. Med Ultrason. 2013;15:101-105. [DOI] [PubMed] [Google Scholar]
- 16. Bui B, Nguyen L. Efficacy of PRP injection in treatment of primary knee OA. Int J Rheum Dis. 2014;17(suppl 1):35. [Google Scholar]
- 17. Cai Y-Z, Zhang C, Lin X-J. Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: a meta-analysis. J Shoulder Elbow Surg. 2015;24:1852-1859. [DOI] [PubMed] [Google Scholar]
- 18. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:2213-2221. [DOI] [PubMed] [Google Scholar]
- 19. Carr AJ, Murphy R, Dakin SG, et al. Platelet-rich plasma injection with arthroscopic acromioplasty for chronic rotator cuff tendinopathy: a randomized controlled trial. Am J Sports Med. 2015;43:2891-2897. [DOI] [PubMed] [Google Scholar]
- 20. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39:258-265. [DOI] [PubMed] [Google Scholar]
- 21. Cervellin M, de Girolamo L, Bait C, Denti M, Volpi P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: a randomized, controlled clinical study. Knee Surg Sports Traumatol Arthrosc. 2012;20:114-120. [DOI] [PubMed] [Google Scholar]
- 22. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28:1718-1727. [DOI] [PubMed] [Google Scholar]
- 23. Chew KTL, Leong D, Lin CY, Lim KK, Tan B. Comparison of autologous conditioned plasma injection, extracorporeal shockwave therapy, and conventional treatment for plantar fasciitis: a randomized trial. PM R. 2013;5:1035-1043. [DOI] [PubMed] [Google Scholar]
- 24. Cohen J. Statistical Power Analysis for the Behavioral Sciences (Rev. ed.). New York, NY: Academic Press; 1977. [Google Scholar]
- 25. Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. [DOI] [PubMed] [Google Scholar]
- 26. Dallari D, Savarino L, Stagni C, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg Am. 2007;89:2413-2420. [DOI] [PubMed] [Google Scholar]
- 27. de Almeida AM, Demange MK, Sobrado MF, Rodrigues MB, Pedrinelli A, Hernandez AJ. Patellar tendon healing with platelet-rich plasma: a prospective randomized controlled trial. Am J Sports Med. 2012;40:1282-1288. [DOI] [PubMed] [Google Scholar]
- 28. De Carli A, Lanzetti RM, Ciompi A, et al. Can platelet-rich plasma have a role in Achilles tendon surgical repair? Knee Surg Sports Traumatol Arthrosc. 2016;24:2231-2237. [DOI] [PubMed] [Google Scholar]
- 29. DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28:998-1009. [DOI] [PubMed] [Google Scholar]
- 30. Di Matteo B, Filardo G, Kon E, Marcacci M. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy—a systematic review. Musculoskelet Surg. 2015;99:1-9. [DOI] [PubMed] [Google Scholar]
- 31. Di Sante L, Villani C, Santilli V, et al. Intra-articular hyaluronic acid vs platelet-rich plasma in the treatment of hip osteoarthritis. Med Ultrason. 2016;18:463-468. [DOI] [PubMed] [Google Scholar]
- 32. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27:158-167. [DOI] [PubMed] [Google Scholar]
- 33. Dold AP, Zywiel MG, Taylor DW, Dwyer T, Theodoropoulos J. Platelet-rich plasma in the management of articular cartilage pathology: a systematic review. Clin J Sport Med. 2014;24:31-43. [DOI] [PubMed] [Google Scholar]
- 34. Dragoo JL, Wasterlain AS, Braun HJ, Nead KT. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. [DOI] [PubMed] [Google Scholar]
- 35. Duif C, Vogel T, Topcuoglu F, Spyrou G, Pellengahr CS, Lahner M. Does intraoperative application of leukocyte-poor platelet-rich plasma during arthroscopy for knee degeneration affect postoperative pain, function and quality of life? A 12-month randomized controlled double-blind trial. Arch Orthop Trauma Surg. 2015;135:971-977. [DOI] [PubMed] [Google Scholar]
- 36. Duymus TM, Mutlu S, Dernek B, Komur B, Aydogmus S, Kesiktas FN. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc. 2017;25:485-492. [DOI] [PubMed] [Google Scholar]
- 37. Everts PA, Devilee RJJ, Brown Mahoney C, et al. Exogenous application of platelet-leukocyte gel during open subacromial decompression contributes to improved patient outcome. A prospective randomized double-blind study. Eur Surg Res. 2008;40:203-210. [DOI] [PubMed] [Google Scholar]
- 38. Figueroa D, Figueroa F, Calvo R, Vaisman A, Ahumada X, Arellano S. Platelet-rich plasma use in anterior cruciate ligament surgery: systematic review of the literature. Arthroscopy. 2015;31:981-988. [DOI] [PubMed] [Google Scholar]
- 39. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582. [DOI] [PubMed] [Google Scholar]
- 40. Forogh B, Mianehsaz E, Shoaee S, Ahadi T, Raissi GR, Sajadi S. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. 2016;56:901-908. [PubMed] [Google Scholar]
- 41. Franceschi F, Papalia R, Franceschetti E, Paciotti M, Maffulli N, Denaro V. Platelet-rich plasma injections for chronic plantar fasciopathy: a systematic review. Br Med Bull. 2014;112:83-95. [DOI] [PubMed] [Google Scholar]
- 42. Fu C-J, Sun J-B, Bi Z-G, Wang X-M, Yang C-L. Evaluation of platelet-rich plasma and fibrin matrix to assist in healing and repair of rotator cuff injuries: a systematic review and meta-analysis. Clin Rehabil. 2017;31:158-172. [DOI] [PubMed] [Google Scholar]
- 43. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23:1-5. [DOI] [PubMed] [Google Scholar]
- 44. Ghaffarpasand F, Shahrezaei M, Dehghankhalili M. Effects of platelet rich plasma on healing rate of long bone non-union fractures: a randomized double-blind placebo controlled clinical trial. Bull Emerg Trauma. 2016;4:134-140. [PMC free article] [PubMed] [Google Scholar]
- 45. GlobalData. Platelet Rich Plasma (PRP) Market—Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2016-2024. London, England: GlobalData; 2016:1-5. [Google Scholar]
- 46. Gogna P, Gaba S, Mukhopadhyay R, Gupta R, Rohilla R, Yadav L. Plantar fasciitis: a randomized comparative study of platelet rich plasma and low dose radiation in sportspersons. Foot (Edinb). 2016;28:16-19. [DOI] [PubMed] [Google Scholar]
- 47. Gormeli G, Karakaplan M, Gormeli CA, Sarkaya B, Elmal N, Ersoy Y. Clinical effects of platelet-rich plasma and hyaluronic acid as an additional therapy for talar osteochondral lesions treated with microfracture surgery: a prospective randomized clinical trial. Foot Ankle Int. 2015;36:891-900. [DOI] [PubMed] [Google Scholar]
- 48. Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39:1200-1208. [DOI] [PubMed] [Google Scholar]
- 49. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23:2384-2389. [DOI] [PubMed] [Google Scholar]
- 50. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction. J Clin Epidemiol. 2011;64:383-394. [DOI] [PubMed] [Google Scholar]
- 51. Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66:151-157. [DOI] [PubMed] [Google Scholar]
- 52. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283-1293. [DOI] [PubMed] [Google Scholar]
- 53. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence. J Clin Epidemiol. 2011;64:1303-1310. [DOI] [PubMed] [Google Scholar]
- 54. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence. J Clin Epidemiol. 2011;64:1294-1302. [DOI] [PubMed] [Google Scholar]
- 55. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence. J Clin Epidemiol. 2011;64:407-415. [DOI] [PubMed] [Google Scholar]
- 56. Hak A, Rajaratnam K, Ayeni OR, et al. A Double-blinded placebo randomized controlled trial evaluating short-term efficacy of platelet-rich plasma in reducing postoperative pain after arthroscopic rotator cuff repair: a pilot study. Sports Health. 2014;7:58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hamid MS, Mohamed Ali MR, Yusof A, George J, Lee LPC. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42:2410-2418. [DOI] [PubMed] [Google Scholar]
- 58. Hamid MS, Yusof A, Mohamed Ali MR. Platelet-rich plasma (PRP) for acute muscle injury: a systematic review. PLoS One. 2014;9:e90538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011). London, England: The Cochrane Collaboration; 2011. [Google Scholar]
- 60. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:D5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Holtby R, Christakis M, Maman E, et al. Impact of platelet-rich plasma on arthroscopic repair of small- to medium-sized rotator cuff tears. Orthop J Sports Med. 2016;4:232596711666559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Horstmann WG, Slappendel R, Van Hellemondt GG, Wymenga AW, Jack N, Everts PAM. Autologous platelet gel in total knee arthroplasty: a prospective randomized study. Knee Surg Sports Traumatol Arthrosc. 2010;19:115-121. [DOI] [PubMed] [Google Scholar]
- 63. Hsiao M-Y, Hung C-Y, Chang K-V, Chien K-L, Tu Y-K, Wang T-G. Comparative effectiveness of autologous blood-derived products, shock-wave therapy and corticosteroids for treatment of plantar fasciitis: a network meta-analysis. Rheumatology. 2015;54:1735-1743. [DOI] [PubMed] [Google Scholar]
- 64. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739-748. [DOI] [PubMed] [Google Scholar]
- 65. Jain K, Murphy PN, Clough TM. Platelet rich plasma versus corticosteroid injection for plantar fasciitis: a comparative study. Foot. 2015;25:235-237. [DOI] [PubMed] [Google Scholar]
- 66. Johal H, Devji T, Schemitsch EH, Bhandari M. Viscosupplementation in knee osteoarthritis: evidence revisited. JBJS Rev. 2016;4:e11-e111. [DOI] [PubMed] [Google Scholar]
- 67. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blind, parallel-group trial. Am J Sports Med. 2013;41:2240-2248. [DOI] [PubMed] [Google Scholar]
- 68. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43:2102-2110. [DOI] [PubMed] [Google Scholar]
- 69. Kesikburun S, Tan AK, Yılmaz B, Yaşar E, Yazıcıoǧlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013;41:2609-2616. [DOI] [PubMed] [Google Scholar]
- 70. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625-635. [DOI] [PubMed] [Google Scholar]
- 71. Krogh TP, Bartels EM, Ellingsen T, et al. Comparative effectiveness of injection therapies in lateral epicondylitis: a systematic review and network meta-analysis of randomized controlled trials. Am J Sports Med. 2013;41:1435-1446. [DOI] [PubMed] [Google Scholar]
- 72. Laudy ABM, Bakker EWP, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:1-17. [DOI] [PubMed] [Google Scholar]
- 73. Lee GW, Son J-H, Kim J-D, Jung G-H. Is platelet-rich plasma able to enhance the results of arthroscopic microfracture in early osteoarthritis and cartilage lesion over 40 years of age? Eur J Orthop Surg Traumatol. 2013;23:581-587. [DOI] [PubMed] [Google Scholar]
- 74. Leitner GC, Gruber R, Neumuller J, et al. Platelet content and growth factor release in platelet-rich plasma: a comparison of four different systems. Vox Sang. 2006;91:135-139. [DOI] [PubMed] [Google Scholar]
- 75. Liddle AD, Rodriguez-Merchan EC. Platelet-rich plasma in the treatment of patellar tendinopathy. Am J Sports Med. 2015;43:2583-2590. [DOI] [PubMed] [Google Scholar]
- 76. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26:269-278. [DOI] [PubMed] [Google Scholar]
- 77. Maffulli N, Papalia R, D’Adamio S, Diaz Balzani L, Denaro V. Pharmacological interventions for the treatment of Achilles tendinopathy: a systematic review of randomized controlled trials. Br Med Bull. 2015;113:101-115. [DOI] [PubMed] [Google Scholar]
- 78. Mahindra P, Yamin M, Selhi HS, Singla S, Soni A. Chronic plantar fasciitis: effect of platelet-rich plasma, corticosteroid, and placebo. Orthopedics. 2016;39:e285-e289. [DOI] [PubMed] [Google Scholar]
- 79. Malavolta EA, Gracitelli MEC, Neto AAF, Assunção JH, Bordalo-Rodrigues M, de Camargo OP. Platelet-rich plasma in rotator cuff repair: a prospective randomized study. Am J Sports Med. 2014;42:2446-2454. [DOI] [PubMed] [Google Scholar]
- 80. Mariani E, Canella V, Cattini L, et al. Leukocyte-rich platelet-rich plasma injections do not up-modulate intra-articular pro-inflammatory cytokines in the osteoarthritic knee. PLoS One. 2016;11:e0156137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martínez-Zapata MJ, Orozco L, Balius R, et al. Efficacy of autologous platelet-rich plasma for the treatment of muscle rupture with haematoma: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Blood Transfus. 2016;14:245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94:308-316. [DOI] [PubMed] [Google Scholar]
- 83. Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13:1185-1195. [DOI] [PubMed] [Google Scholar]
- 84. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42:463-471. [DOI] [PubMed] [Google Scholar]
- 85. Montalvan B, Le Goux P, Klouche S, Borgel D, Hardy P, Breban M. Inefficacy of ultrasound-guided local injections of autologous conditioned plasma for recent epicondylitis: results of a double-blind placebo-controlled randomized clinical trial with one-year follow-up. Rheumatology. 2016;55:279-285. [DOI] [PubMed] [Google Scholar]
- 86. Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2016;4:CD010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nin JRV, Gasque GM, Azcárate AV, Beola JDA, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009;25:1206-1213. [DOI] [PubMed] [Google Scholar]
- 88. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582-592. [DOI] [PubMed] [Google Scholar]
- 89. Omar AS, Ibrahim ME, Ahmed AS, Said M. Local injection of autologous platelet rich plasma and corticosteroid in treatment of lateral epicondylitis and plantar fasciitis: randomized clinical trial. Egypt Rheumatol. 2012;34:43-49. [Google Scholar]
- 90. Pandey V, Bandi A, Madi S, et al. Does application of moderately concentrated platelet-rich plasma improve clinical and structural outcome after arthroscopic repair of medium-sized to large rotator cuff tear? A randomized controlled trial. J Shoulder Elbow Surg. 2016;25:1312-1322. [DOI] [PubMed] [Google Scholar]
- 91. Pas HI, Reurink G, Tol JL, Weir A, Winters M, Moen MH. Efficacy of rehabilitation (lengthening) exercises, platelet-rich plasma injections, and other conservative interventions in acute hamstring injuries: an updated systematic review and meta-analysis. Br J Sports Med. 2015;49:1197-1205. [DOI] [PubMed] [Google Scholar]
- 92. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364. [DOI] [PubMed] [Google Scholar]
- 93. Paterson KL, Nicholls M, Bennell KL, Bates D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Musculoskelet Disord. 2016;17:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Peerbooms JC, de Wolf GS, Colaris JW, Bruijn DJ, Verhaar JAN. No positive effect of autologous platelet gel after total knee arthroplasty. Acta Orthop. 2009;80:557-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens Taco Y. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38:255-262. [DOI] [PubMed] [Google Scholar]
- 96. Raeissadat S, Rayegani S, Hassanabadi H, Rahimi R, Sedighipour L, Rostami K. Is platelet-rich plasma superior to whole blood in the management of chronic tennis elbow: one year randomized clinical trial. BMC Sports Sci Med Rehabil. 2014;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rafols C, Monckeberg JE, Numair J, Botello J, Rosales J. Platelet-rich plasma augmentation of arthroscopic hip surgery for femoroacetabular impingement: a prospective study with 24-month follow-up. Arthroscopy. 2015;31:1886-1892. [DOI] [PubMed] [Google Scholar]
- 99. Randelli PS, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20:518-528. [DOI] [PubMed] [Google Scholar]
- 100. Rayegani SM, Raeissadat SA, Sanei Taheri M, et al. Does intra articular platelet rich plasma injection improve function, pain and quality of life in patients with osteoarthritis of the knee? A randomized clinical trial. Orthop Rev (Pavia). 2014;6:5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Redmond JM, Gupta A, Stake CE, Hammarstedt JE, Finch NA, Domb BG. Clinical results of hip arthroscopy for labral tears: a comparison between intraoperative platelet-rich plasma and bupivacaine injection. Arthroscopy. 2015;31:445-453. [DOI] [PubMed] [Google Scholar]
- 102. Reurink G, Goudswaard GJ, Moen MH, et al. Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370:2546-2547. [DOI] [PubMed] [Google Scholar]
- 103. Rha DW, Park GY, Kim YK, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013;27:113-122. [DOI] [PubMed] [Google Scholar]
- 104. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44:792-800. [DOI] [PubMed] [Google Scholar]
- 105. Rowden A, Dominici P, D’Orazio J, et al. Double-blind, randomized, placebo-controlled study evaluating the use of platelet-rich plasma therapy (PRP) for acute ankle sprains in the emergency department. J Emerg Med. 2015;49:546-551. [DOI] [PubMed] [Google Scholar]
- 106. Rubio-Azpeitia E, Bilbao AM, Sánchez P, Delgado D, Andia I. The properties of 3 different plasma formulations and their effects on tendinopathic cells. Am J Sports Med. 2016;44:1952-1961. [DOI] [PubMed] [Google Scholar]
- 107. Rutjes AWS, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157:180-191. [DOI] [PubMed] [Google Scholar]
- 108. Samy AM. The role of platelet rich plasma in management of fracture neck femur: new insights. Int Orthop. 2016;40:1019-1024. [DOI] [PubMed] [Google Scholar]
- 109. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28:1070-1078. [DOI] [PubMed] [Google Scholar]
- 110. Shams A, El-Sayed M, Gamal O, Ewes W. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837-842. [DOI] [PubMed] [Google Scholar]
- 111. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:G7647. [DOI] [PubMed] [Google Scholar]
- 112. Shen L, Yuan T, Chen S, Xie X, Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94:298-307. [DOI] [PubMed] [Google Scholar]
- 114. Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85:257-268. [PubMed] [Google Scholar]
- 115. Simental-Mendia M, Vílchez-Cavazos JF, Peña-Martínez VM, Said-Fernández S, Lara-Arias J, Martínez-Rodríguez HG. Leukocyte-poor platelet-rich plasma is more effective than the conventional therapy with acetaminophen for the treatment of early knee osteoarthritis. Arch Orthop Trauma Surg. 2016;136:1723-1732. [DOI] [PubMed] [Google Scholar]
- 116. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891. [DOI] [PubMed] [Google Scholar]
- 117. Spaková T, Rosocha J, Lacko M, Harvanová D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91:411-417. [DOI] [PubMed] [Google Scholar]
- 118. Sys J, Weyler J, Van Der Zijden T, Parizel P, Michielsen J. Platelet-rich plasma in mono-segmental posterior lumbar interbody fusion. Eur Spine J. 2011;20:1650-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21:344-352. [DOI] [PubMed] [Google Scholar]
- 120. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130-2134. [DOI] [PubMed] [Google Scholar]
- 121. Tiwari M, Bhargava R. Platelet rich plasma therapy: a comparative effective therapy with promising results in plantar fasciitis. J Clin Orthop Trauma. 2013;4:31-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, et al. Lumbar intradiskal platelet-rich plasma (PRP) injections: a prospective, double-blind, randomized controlled study. PM R. 2016;8:1-10. [DOI] [PubMed] [Google Scholar]
- 123. Vaquerizo V, Plasencia MÁ, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy. 2013;29:1635-1643. [DOI] [PubMed] [Google Scholar]
- 124. Vavken P, Sadoghi P, Palmer M, et al. Platelet-rich plasma reduces retear rates after arthroscopic repair of small- and medium-sized rotator cuff tears but is not cost-effective. Am J Sports Med. 2015;43:3071-3076. [DOI] [PubMed] [Google Scholar]
- 125. Vetrano M, Castorina A, Vulpiani MC, Baldini R, Pavan A, Ferretti A. Platelet-rich plasma versus focused shock waves in the treatment of jumper’s knee in athletes. Am J Sports Med. 2013;41:795-803. [DOI] [PubMed] [Google Scholar]
- 126. Wang A, McCann P, Colliver J, et al. Do postoperative platelet-rich plasma injections accelerate early tendon healing and functional recovery after arthroscopic supraspinatus repair? A randomized controlled trial. Am J Sports Med. 2015;43:1430-1437. [DOI] [PubMed] [Google Scholar]
- 127. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41:263-270. [DOI] [PubMed] [Google Scholar]
- 128. Wesner M, Defreitas T, Bredy H, et al. A pilot study evaluating the effectiveness of platelet-rich plasma therapy for treating degenerative tendinopathies: a randomized control trial with synchronous observational cohort. PLoS One. 2016;11:e0147842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Willits K, Kaniki N, Bryant D. The use of platelet-rich plasma in orthopedic injuries. Sports Med Arthrosc. 2013;21:225-230. [DOI] [PubMed] [Google Scholar]
- 130. Zavadil DP, Satterlee CC, Costigan JM, Holt DW, Shostrom VK. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39:177-182. [PMC free article] [PubMed] [Google Scholar]
- 131. Zedde P, Cudoni S, Lisai P, Fadda M. Effect of platelet-rich plasma and microfracture reparative technique combined in chondral lesions treatment. J Biol Res. 2015;88:5229. [Google Scholar]
- 132. Zhang L, Chen S, Chang P, et al. Harmful effects of leukocyte-rich platelet-rich plasma on rabbit tendon stem cells in vitro. Am J Sports Med. 2016;44:1941-1951. [DOI] [PubMed] [Google Scholar]
- 133. Zumstein MA, Rumian A, Thélu CÉ, et al. SECEC Research Grant 2008 II. Use of platelet- and leucocyte-rich fibrin (L-PRF) does not affect late rotator cuff tendon healing: a prospective randomized controlled study. J Shoulder Elbow Surg. 2016;25:2-11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 30072_Appendix for Impact of Platelet-Rich Plasma Use on Pain in Orthopaedic Surgery: A Systematic Review and Meta-analysis by Herman Johal, Moin Khan, Shu-hang Patrick Yung, Mandeep S. Dhillon, Freddie F. Fu, Asheesh Bedi and Mohit Bhandari in Sports Health: A Multidisciplinary Approach