Abstract
Background:
The impact of maturation on lower extremity strength and function after anterior cruciate ligament reconstruction (ACLR) may help guide future studies of age-specific rehabilitation.
Hypothesis
Pediatric ACLR patients would demonstrate higher thigh strength symmetry and knee-related function at return to sport (RTS) compared with adolescent and young adult participants who underwent traditional ACLR.
Study Design:
Prospective cohort study.
Level of Evidence:
Level 2.
Methods:
A total of 144 young athletes at the time of RTS clearance post-ACLR were classified into 3 maturational groups (pediatric, n = 16 with physeal-sparing ACLR [mean age = 12.3 years; range = 9.2-14.6 years]; adolescent, n = 113 [mean age = 16.5 years; range = 14.1-19.8 years]; young adult, n = 15 [mean age = 22.0 years; range = 20.5-24.9 years]). Quadriceps and hamstring strength were measured using an electromechanical dynamometer. Knee-related function was measured using the International Knee Documentation Committee (IKDC) subjective form and single-leg hop tests. The Limb symmetry Index (LSI) was used in calculations for hop and strength tests. Group differences were compared with Kruskal-Wallis tests and Mann-Whitney U post hoc tests. Proportions of participants meeting literature-recommended RTS criterion cutoffs were compared among the groups using chi-square tests.
Results:
The pediatric group demonstrated higher quadriceps LSI (P = 0.01), IKDC scores (P < 0.01), single-hop LSI (P < 0.01), and crossover-hop LSI (P = 0.02) compared with the young adult group. In addition, the pediatric group demonstrated higher IKDC scores (P < 0.01) and single-hop LSI (P = 0.02) compared with the adolescent group. The adolescent group demonstrated higher IKDC scores (P < 0.01), single-hop LSI (P = 0.02), and crossover-hop LSI (P = 0.03) compared with the young adult group. The proportions of participants meeting all RTS criterion cutoffs were highest in the pediatric group and lowest in the young adult group (P = 0.03).
Conclusion:
Young athletes at RTS clearance after pediatric ACLR demonstrated higher quadriceps strength symmetry and knee-related function than adolescents and young adults after traditional ACLR.
Clinical Relevance:
These findings demonstrate the need for further study regarding the impact of these group differences on longitudinal outcomes after ACLR, including successful RTS and risk of second ACL injury.
Keywords: ACL reconstruction, outcomes, maturation
Participation in organized sports is a primary risk factor for anterior cruciate ligament (ACL) injuries in young athletes.3,31,39,41,54 With youth participation in organized sports at an all-time high, pediatric and adolescent individuals are currently at high risk for ACL injuries.3 The skeletally immature population presents a challenge for ACL reconstruction (ACLR) due to open growth plates. Historically, fear of damage to the open growth plates limited ACLR procedures primarily to adults.13 Typically, younger athletes with open growth plates were treated conservatively, including bracing and activity modification, and allowed to reach skeletal maturity before undergoing traditional, transphyseal ACLR.2,53 However, recent studies have shown that left untreated, ACL injuries in young individuals with open growth plates lead to significantly worse function and greater risk of secondary knee injuries, including intra-articular damage, medial meniscal tears, medial collateral ligament tears, and recurrent knee instability.31,37,39 The rising rate of ACL injuries in young athletes combined with the poor results from nonoperative management have led to the development of modern pediatric ACLR procedures.13,39 By avoiding the open growth plates, pediatric ACLR procedures mitigate the traditional surgical risks for the skeletally immature population, including growth arrest and deformity.13,39
Young athletes undergoing ACLR do not consistently recover adequate strength and function at the time of return-to-sport (RTS) clearance.9,48 Traditionally, RTS decisions after ACLR were often based on postoperative time, with 6 to 9 months used as a common benchmark for return to participating in sport activities.5,38 However, previous work demonstrated that despite being cleared for return to participation in sports activity, quadriceps femoris (QF) strength deficits and functional deficits are commonly observed in young athletes of all maturity levels at RTS.11,40,48 Because of the increased risk of second ACL injury and suboptimal function for young athletes after RTS participation post-ACLR,24,42,43 appropriate postoperative rehabilitation and objective RTS criteria along with time post-ACLR and subjective readiness for RTS are critical.13,20 While individuals undergoing pediatric, physeal-sparing ACLR procedures or individuals of varying maturational status after ACLR may require different rehabilitation approaches or different objective criteria for RTS decision making, information is lacking in the literature currently.
Recent work indicates that young patients undergoing pediatric ACLR demonstrate excellent functional outcomes between 3 and 4 years postsurgery44,53 and that younger age is a key factor associated with higher levels of recovery of muscle strength and function after traditional ACLR.28 As a first step toward the development of age-appropriate, objective rehabilitation guidelines and RTS criteria, it is important to understand differences in strength and function between maturity groups in young athletes at the time of RTS clearance after ACLR. Therefore, the purpose of this study was to examine thigh strength symmetry and knee-related function across maturational levels in young athletes after ACLR at the time of RTS. It was hypothesized that participants who underwent pediatric, physeal-sparing ACLR would demonstrate higher thigh strength symmetry and knee-related function at RTS after ACLR compared with adolescent and young adult participants who underwent traditional ACLR. It was also hypothesized that a higher proportion of those with pediatric, physeal-sparing ACLR would meet literature-recommended RTS criterion cutoffs compared with adolescents and young adults.
Methods
The institutional review board approved this study prior to recruitment, and all participants (and parents/guardians when appropriate) provided signed informed consent before participation.
Participants
Participants were between the ages of 9 and 25 years and were included from the ACL REconstruction Long-term outcomes in Adolescents and Young adults (ACL-RELAY) study between 2007 and 2016. The ACL-RELAY study is an ongoing, prospective study of outcomes in young, active individuals after ACLR. All participants are recruited from orthopaedic surgeon practices and physical therapy clinics in the greater Cincinnati and northern Kentucky areas and are enrolled in the study at the time of RTS clearance after a primary, unilateral ACLR. Participants are included if they have completed their rehabilitation program, are cleared to return to high-level athletic competition by their surgeon and treating rehabilitation specialist, and plan to participate in high-level sports on a regular basis (more than 50 hours per year). Participants are excluded if they have a history of back injury or surgery, a previous lower extremity injury (excluding their primary ACL injury), or a concomitant ligament injury along with their ACL injury (excluding medial collateral ligament grade 1 sprain). Participants with or without a meniscal tear are included in the ACL-RELAY study. In addition, the ACL-RELAY study does not control rehabilitation or RTS clearance decision making, or whether any objective criteria are used in this decision. All data at the initial testing visit are collected within 4 weeks of each participant’s RTS clearance.
For these analyses, participants were classified into 3 maturational groups. The pediatric group (n = 16, age range = 9.2-14.6 years) consisted of individuals who underwent an all-epiphyseal physeal-sparing ACLR procedure. The adolescent group (n = 113, age range = 14.1-19.8 years) consisted of individuals who underwent a conventional, transphyseal ACLR and were defined as adolescents using the World Health Organization (WHO) definition (14-19 years old).55 The young adult group (n = 15, age range = 20.5-24.9 years) consisted of individuals who underwent conventional, transphyseal ACLR and were older than the WHO definition of adolescence (>19 years old).55 A total of 161 potential participants post-ACLR completed the initial RTS testing visit. However, 8 participants were excluded from the current analyses because of a history of bilateral ACL injuries. Additionally, 9 participants were excluded from the current analyses due to not completing the entire RTS testing battery described below (muscle strength assessment; self-reported function; functional performance testing).
Muscle Strength Assessment at RTS
QF and hamstring (HS) muscle strength were both assessed using an electromechanical dynamometer (Biodex Medical Systems). QF strength and HS strength were assessed isokinetically, at 180 deg/s, from 90° of flexion to full available knee extension. Strength testing using similar methods has shown good reliability in healthy individuals and individuals after ACL injury/ACLR and is able to differentiate strength asymmetries between limbs.14,16,26,27,36,46 QF and HS peak torques were calculated from the 3 trials for each limb, normalized to body mass (Nm/kg), and used to calculate a Limb Symmetry Index (LSI) for QF and HS strength as follows:
| (1) |
The LSI is the most frequently reported criterion used to quantify limb symmetry with measures of strength and hop tests after ACL injury and ACLR.4,15,33,48 An LSI < 100% indicates deficits of the involved limb, and an LSI ≤ 90% after ACLR is considered unsatisfactory.30,33,35,48
Self-Reported Knee Function Assessment at RTS
Self-reported knee function was measured using the International Knee Documentation Committee subjective knee form (IKDC). The IKDC is a knee-specific measure of symptoms, function, and sports activity. The IKDC has been found to be valid and reliable in individuals after ACL injury and ACLR.23 Additionally, the IKDC has been validated for use in a pediatric population after knee injury or surgery.49 The IKDC is scored from 0 to 100, with 100 indicating no knee-related limitations.18
Knee-Related Functional Performance at RTS
Four single-leg hop tests were used as measures of knee functional performance. These hop tests are commonly performed in the clinical setting and have been shown to have good reliability in patients after ACLR.8,45 The single-leg hop tests were performed in the following order: single hop for distance (SH; cm), triple hop for distance (TH; cm), crossover hop for distance (CH; cm), and the 6-m timed hop (6m-TH; seconds). After a practice trial, participants completed 2 trials on both the involved and the uninvolved limbs, in random order. The 2 measurement trials were averaged and used to calculate an LSI for distance single-leg hops (SH, TH, CH) using Equation (1) and an LSI for the 6m-TH as follows:
| (2) |
Literature-Recommended RTS Criteria
Criterion-based objective measures to determine RTS readiness have been advocated. Based on published recommendations, the objective criterion cutoffs evaluated in this study included thigh muscle strength, the IKDC score, and single-leg hop test performance (SH, TH, CH, and 6m-TH).1,7,12,21,30,34,35 Meeting literature-recommended RTS criterion cutoffs were defined as demonstrating (1) an LSI ≥ 90% for QF and HS strength, (2) an IKDC score ≥ 90, and (3) an LSI ≥ 90% for all hop tests.17,30,32,33,35,48,51
Statistical Analyses
Prior to performing statistical analyses, equal group variance was confirmed using Levene tests.10 The data were assessed for normality using histograms and quantile-quantile plots within each maturational group, as well as across the entire cohort (n = 144). The independent variable was group assignment (pediatric, adolescent, young adult), and the dependent variables were participant demographic information, QF and HS strength data, knee functional performance data, and self-reported function data at the time of RTS clearance. Because of strength and function data nonnormality and unsuccessful data transformations using natural log and square root transformations, along with disparate group sizes, differences in strength and knee-related function data were compared among the maturational groups using Kruskal-Wallis tests (α = 0.05). If significant group differences were identified, post hoc testing was performed using Mann-Whitney U tests between individual groups (α = 0.05). Categorical demographic data were compared among the groups with chi-square tests for independence (or Fisher exact tests, when minimum expected cell frequencies were not maintained). Similarly, proportions of participants meeting literature-recommended RTS criterion cutoffs were compared among the groups with chi-square tests for independence (or Fisher exact tests) (α = 0.05). IBM SPSS (version 24.0) was used for all statistical analyses.
Results
Participant Characteristics
Significant group differences were identified in age, height, weight, and preinjury Tegner Activity Scale scores among the pediatric, adolescent, and young adult groups. No group differences were identified for time from ACLR to baseline testing at the time of RTS clearance. Additionally, significant group differences were identified for sex and graft type distribution among the groups (Table 1).
Table 1.
Participant characteristics by maturational group a
| Pediatric | Adolescent | Young Adult | P | |
|---|---|---|---|---|
| Number of participants | 16 | 113 | 15 | |
| Age, y | 12.3 ± 1.8 | 16.5 ± 1.5 | 22.0 ± 1.5 | <0.01 b , c , d |
| Height, cm | 155.9 ± 16.3 | 168.3 ± 8.3 | 172.6 ± 12.9 | <0.01 b , c |
| Weight, kg | 52.5 ± 23.7 | 67.3 ± 12.7 | 79.8 ± 22.9 | <0.01 b , c |
| Time from ACLR to RTS, mo | 8.5 ± 2.3 | 8.3 ± 2.4 | 8.3 ± 2.4 | 0.74 |
| Preinjury Tegner Activity Scale score | 7.4 ± 1.2 | 8.8 ± 0.7 | 8.7 ± 1.4 | <0.01 b , c |
| Sex distribution, n | <0.01 b , c , e | |||
| Females | 2 | 83 | 8 | |
| Males | 14 | 30 | 7 | |
| Graft type distribution, n | <0.01 b , c , d , e | |||
| Hamstring autograft | 16 f | 61 | 4 | |
| BPTB autograft | — | 46 | 6 | |
| Allograft | — | 6 | 5 |
ACLR, anterior cruciate ligament reconstruction; BPTB, bone–patellar tendon–bone; RTS, return to sport.
Data are mean ± SD unless otherwise indicated.
Indicates significant differences between the pediatric and adolescent groups.
Indicates significant differences between the pediatric and young adult groups.
Indicates significant differences between the adolescent and young adult groups.
Distribution compared among groups using chi-square tests.
All-epiphyseal physeal-sparing procedure.
QF and HS Strength
Significant group differences were identified for QF LSI among the groups (P = 0.03). Post hoc testing revealed that the pediatric group (mean ± SD; 94.5% ± 9.1%) demonstrated significantly higher QF LSI than the young adult groups (83.3% ± 12.0%; P = 0.01) (Figure 1). However, no statistically significant differences were found for QF LSI between the adolescent (89.8% ± 11.1%) and young adult groups (P = 0.05) or between the pediatric and adolescent groups (P = 0.09). Additionally, no significant group differences were identified for HS LSI among the groups (P = 0.22) (Figure 1).
Figure 1.
Quadriceps and hamstring LSI and IKDC by maturational groups.a
HS, hamstring; IKDC, International Knee Documentation Committee subjective knee form; LSI, Limb Symmetry Index; QF, quadriceps femoris.
**P < 0.05 for post hoc comparisons; solid gray—pediatric group; lined gray—adolescent group; dotted gray—young adult group.
aValues are LSI (%) for QF LSI and HS LSI and score (range 0-100) for IKDC.
Self-Reported Knee Function and Knee Functional Performance
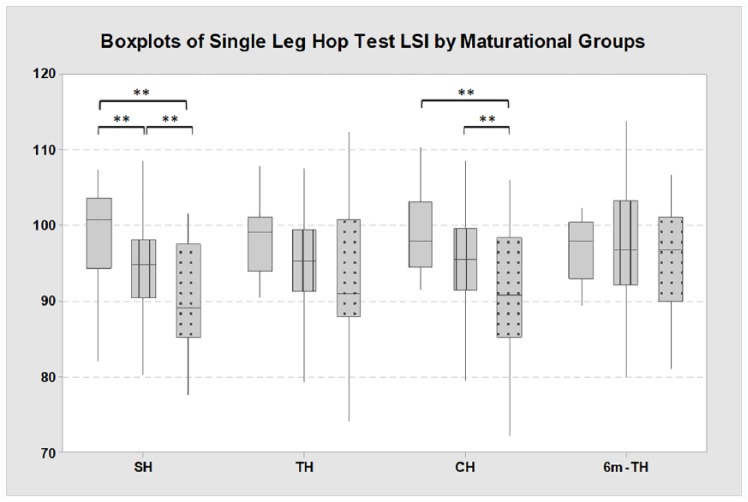
For self-reported function, significant group differences were identified for the IKDC among the groups (P < 0.01). Post hoc testing revealed that the pediatric group (96.1 ± 4.2) demonstrated higher scores than both the adolescent group (89.4 ± 10.0; P < 0.01) and the young adult group (80.3 ± 11.1; P < 0.01) (Figure 1). Additionally, the adolescent group demonstrated higher IKDC scores than the young adult group (P < 0.01) (Figure 1). For knee functional performance, significant group differences were identified for the SH LSI among the groups (P < 0.01). Post hoc testing revealed that the pediatric group (98.5% ± 6.9%) demonstrated significantly higher SH LSI compared with both the adolescent group (94.5% ± 6.0%; P = 0.02) and the young adult group (89.7% ± 7.3%; P < 0.01) (Figure 2). Additionally, the adolescent group demonstrated higher SH LSI compared with the young adult group (P = 0.02) (Figure 2). Significant group differences were also identified for the CH LSI among the groups (P = 0.03). Post hoc testing revealed that the pediatric group (97.8% ± 7.1%) demonstrated significantly higher CH LSI compared with the young adult group (88.3% ± 14.0%; P = 0.02) and that the adolescent group (95.3% ± 7.0%) demonstrated significant higher CH LSI compared with the young adult group (P = 0.03). No significant group differences were identified among the groups for the TH LSI (P = 0.05) or the 6m-TH LSI (P = 0.83) (Figure 2).
Figure 2.
Single-leg hop test LSI by maturational groups.
6m-TH, 6-meter timed hop; CH, crossover-hop for distance; LSI, Limb Symmetry Index; SH, single-hop for distance; TH, triple-hop for distance.
**P < 0.05 for post hoc comparisons; solid gray—Pediatric group; lined gray—Adolescent group; dotted gray—Young Adult group.
Proportions Meeting Literature-Recommended RTS Criterion Cutoffs
The proportions of participants who demonstrated QF or HS LSI of 90% or greater did not statistically differ among the maturational groups (P = 0.182 and P = 0.082, respectively) (Table 2). For self-reported knee function, the highest proportion who reported an IKDC score of 90 or greater was the pediatric group, with the lowest proportion who met the cutoff being in the young adult group (P < 0.01) (Table 2). For the SH, TH, and CH tests, the pediatric group had a higher proportion who demonstrated an LSI of 90% or greater compared with the young adult group (P < 0.01, P = 0.02, and P = 0.03, respectively; Table 2). When the proportion of participants who met all the criteria cutoffs was evaluated, nearly one-third (31%) of the pediatric group met all criterion cutoffs, which was higher than the adolescent group (12% met all criterion cutoffs) and the young adult group (0% met all criterion cutoffs) (P = 0.030) (Table 2).
Table 2.
Proportion of participants meeting return-to-sport criteria by maturational group a
| Pediatric | Adolescent | Young Adult | P | |
|---|---|---|---|---|
| Thigh muscle strength | ||||
| QF LSI ≥ 90% | 69 | 46 | 40 | 0.18 |
| HS LSI ≥ 90% | 38 | 66 | 53 | 0.08 |
| Self-reported knee function | ||||
| IKDC score ≥ 90 | 88 | 55 | 13 | <0.01 b , c , d |
| Knee functional performance | ||||
| SH LSI ≥ 90% | 88 | 79 | 40 | <0.01 c , d |
| TH LSI ≥ 90% | 100 | 82 | 60 | 0.02 c , d |
| CH LSI ≥ 90% | 94 | 78 | 53 | 0.03 c , d |
| 6m-TH LSI ≥ 90% | 81 | 83 | 73 | 0.63 |
| All criteria cutoffs | 31 | 12 | 0 | 0.03 b , c , e |
6m-TH, 6-meter timed hop; CH, crossover-hop for distance; HS, hamstring; IKDC, International Knee Documentation Committee subjective knee form; LSI, Limb Symmetry Index; QF, quadriceps femoris; SH, single-hop for distance; TH, triple-hop for distance.
Data are percentage (%) of the maturational group that met criterion cutoff.
Indicates significant differences between the pediatric and adolescent groups.
Indicates significant differences between the pediatric and young adult groups.
Indicates significant differences between the adolescent and young adult groups.
Fisher exact tests performed.
Discussion
The findings from the current study supported the first hypothesis, as participants after pediatric, physeal-sparing ACLR demonstrated generally higher isometric QF strength symmetry, higher single-leg hop test symmetry, and higher self-reported function on the IKDC at the time of RTS compared with adolescent and young adult participants that underwent traditional ACLR. In addition, for the IKDC and single-leg hop tests, the adolescent group also demonstrated higher function than the young adult group. A higher proportion of the pediatric group also met RTS criterion cutoffs on self-reported and physical performance measures of knee function compared with the adolescent and young adult groups. Importantly, the groups also did not differ in the amount of time between ACLR and the initial testing visit at the time of RTS clearance, which indicated that no group took part in rehabilitation for a longer period than another prior to the testing session. Overall, the differences between maturity groups identified in the current study appear to demonstrate that there may be a need to develop maturity and procedure-specific rehabilitation guidelines and RTS criteria in young athletes after ACLR.
Younger individuals and those with pediatric ACLR may demonstrate higher strength and function than their older counterparts. Krych et al28 reported that 6 months after traditional ACLR, younger age was a primary factor associated with excellent strength and functional recovery (defined as strength LSI > 85% and hop test LSI > 90%). Previous work examining specific pediatric ACLR outcomes demonstrated that skeletally immature participants demonstrated excellent functional outcomes at approximately 3 years53 (mean IKDC score of 96.5) and at 3.5 years44 (mean IKDC score of 92.4) after physeal-sparing ACLR. In contrast, in a retrospective study by Greenberg et al19 at a mean of 7.1 months after physeal-sparing ACLR, relatively low proportions of participants demonstrated strength and hop test symmetry >90%. Specifically, only 56% of participants met the 90% LSI cutoff for QF strength, while 94% met the 90% LSI cutoff for HS strength.19 The current study found that a higher proportion of participants with pediatric ACLR met the 90% LSI cutoff for QF strength (69%) than the 90% LSI cutoff for HS strength (38%). A recent study by Toole et al52 evaluated the proportions of young athletes meeting recommended criterion cutoffs after previous RTS clearance (excluding those with pediatric, physeal-sparing procedures). This study found that a much higher proportion of participants met the cutoff for HS strength versus QF strength.51 While it may be hypothesized that less of the pediatric participants that underwent physeal-sparing ACLR in the current study met the HS cutoff due to graft type (all HS autograft), the study by Greenberg et al19 also reported that all participants underwent physeal-sparing ACLR with HS autograft. These contrasting findings indicate that further research regarding the interactions between muscle strength recovery and graft type in those with pediatric ACLR is warranted.
Regardless of maturational status, individuals after traditional ACLR demonstrate deficits in strength and function at the time of RTS. Previous studies have recommended achievement of self-reported function values greater than 90 (out of 100) and muscle strength and single-leg hop test symmetry greater than 90% as desired outcomes for individuals after ACLR.30,35,50 However, young athletes at the time of RTS after ACLR commonly demonstrate deficits in QF strength, self-reported function, and measures of functional performance.11,25,29,40,48,56,57 In the current study, both the adolescent and the young adult groups demonstrated group mean values below literature-recommended RTS criterion cutoffs (90 for self-reported measures; 90% LSI for strength) for QF strength symmetry and the IKDC. Additionally, less than 50% of both the adolescent and young adult groups met the 90% LSI cutoff for QF strength, and a very small proportion of each of these groups met the cutoffs for all recommended criteria (12% and 0%, respectively). Importantly, deficits in self-reported knee function appear to persist over time and are commonly reported in longitudinal studies of individuals years after ACLR.6,22 Additionally, deficits in QF strength at the time of RTS have been linked with altered landing mechanics as well as increased risk for re-injury over time after RTS post-ACLR.20,25,47 Thus, development and implementation of more effective rehabilitation interventions for individuals of all ages and maturity levels after ACLR to restore function and QF strength remains a critical need in this patient population.
There are many significant limitations to this study. First, the role of graft type was not considered in the current analyses. Specifically, the pediatric group all underwent all-epiphyseal physeal-sparing ACLR procedures with HS autografts, while the adolescent and young adult groups included a mix of HS autografts, patellar tendon autografts, and allografts. Thus, the differences between the maturational groups may be driven by the differences in graft type distribution among the groups. In addition, surgical technique data (tourniquets or nerve blocks) were not collected. Second, the group sizes varied significantly, as there were many more participants in the adolescent group (n = 113) compared with the pediatric group (n = 16) and the young adult group (n = 15). Because of the relatively small samples in the pediatric and young adult groups (n = 16 and n = 15, respectively), this study is underpowered to identify all differences among the groups in strength and function at the time of RTS. Third, there were significant differences in sex distribution among the groups; the current analyses did not control for sex distribution. Fourth, the young adult group was relatively young (mean age of 22 years) and active (mean Tegner score of 8.1). Thus, the findings of the current study may not be generalizable to middle-aged adults after ACLR. Fifth, the effect of objective sexual maturation (ie, Tanner stage) or specific growth plate status (open vs closed) were not collected or evaluated by the current study. Individuals in the adolescent group may have had open growth plates, but still underwent transphyseal ACLR. Furthermore, while all individuals in the pediatric group underwent physeal-sparing ACLR, the pediatric and adolescent group participants did overlap in age (oldest in pediatric group, 14.6 years; youngest in adolescent group, 14.1 years). Last, the current study did not control the overall rehabilitation experience (ie, total number of rehabilitation visits, rehabilitation focus, or guidelines used) or factors that contributed to allowing clearance for RTS (ie, whether objective criteria were used). While the time from ACLR to RTS clearance was very similar among the groups, differences in specific factors related to rehabilitation or RTS decision making may have contributed to the differences in strength and function among the groups.
Conclusion
Young athletes at the time of RTS clearance after all-epiphyseal physeal-sparing ACLR demonstrated higher QF strength symmetry, higher knee functional performance, and higher self-reported knee function than adolescents and young adults after traditional ACLR. Additionally, young athletes after physeal-sparing ACLR met literature-recommended RTS criterion cutoffs established for adults, at higher proportions than adolescents and young adults after traditional ACLR. However, study limitations clearly limit the generalizability of these conclusions.
Acknowledgments
The authors thank the staff at the Sports Medicine Biodynamics Center and the Sports and Orthopaedic Team in the Division of Occupational and Physical Therapy at Cincinnati Children’s Hospital Medical Center for their contribution to this work.
Footnotes
The following authors declared potential conflicts of interest: Matthew P. Ithurburn, PT, DPT, PhD, received a graduate studies fellowship from the Foundation for Physical Therapy. Mark V. Paterno’s, PT, PhD, institution (Cincinnati Children’s Hospital Medical Center) and Laura C. Schmitt’s, PT, PhD, institution (The Ohio State University) received a grant from the National Institutes of Health and the National Football League Charities Medical Research Foundation.
References
- 1. Abrams GD, Harris JD, Gupta AK, et al. Functional performance testing after anterior cruciate ligament reconstruction: a systematic review. Orthop J Sport Med. 2014;2:2325967113518305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aichroth PM, Patel DV, Zorrilla P. The natural history and treatment of rupture of the anterior cruciate ligament in children and adolescents. A prospective review. J Bone Joint Surg Br. 2002;84:38-41. [DOI] [PubMed] [Google Scholar]
- 3. Albright JC, Crepeau AE. Functional bracing and return to play after anterior cruciate ligament reconstruction in the pediatric and adolescent patient. Clin Sports Med. 2011;30:811-815. [DOI] [PubMed] [Google Scholar]
- 4. Barber SD, Noyes FR, Mangine RE, McCloskey JW, Hartman W. Quantitative assessment of functional limitations in normal and anterior cruciate ligament-deficient knees. Clin Orthop Relat Res. 1990;255:204-214. [PubMed] [Google Scholar]
- 5. Barber-Westin SD, Noyes FR. Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:1697-1705. [DOI] [PubMed] [Google Scholar]
- 6. Barenius B, Forssblad M, Engstrom B, Eriksson K. Functional recovery after anterior cruciate ligament reconstruction, a study of health-related quality of life based on the Swedish National Knee Ligament Register. Knee Surg Sport Traumatol Arthrosc. 2013;21:914-927. [DOI] [PubMed] [Google Scholar]
- 7. Beynnon BD, Johnson RJ, Fleming BC, et al. Anterior cruciate ligament replacement: comparison of bone-patellar tendon-bone grafts with two-strand hamstring grafts. A prospective, randomized study. J Bone Joint Surg Am. 2002;84-A:1503-1513. [DOI] [PubMed] [Google Scholar]
- 8. Bolgla LA, Keskula DR. Reliability of lower extremity functional performance tests. J Orthop Sport Phys Ther. 1997;26:138-142. [DOI] [PubMed] [Google Scholar]
- 9. Boyle MJ, Butler RJ, Queen RM. Functional movement competency and dynamic balance after anterior cruciate ligament reconstruction in adolescent patients. J Pediatr Orthop. 2016;36:36-41. [DOI] [PubMed] [Google Scholar]
- 10. Brown MB, Forsythe AB. Robust tests for equality of variances. J Am Stat Assoc. 1974;69:364-367. [Google Scholar]
- 11. Clagg S, Paterno MV, Hewett TE, Schmitt LC. Performance on the modified star excursion balance test at the time of return to sport following anterior cruciate ligament reconstruction. J Orthop Sport Phys Ther. 2015;45:444-452. [DOI] [PubMed] [Google Scholar]
- 12. Czuppon S, Racette BA, Klein SE, Harris-Hayes M. Variables associated with return to sport following anterior cruciate ligament reconstruction: a systematic review. Br J Sports Med. 2014;48:356-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fabricant PD, Jones KJ, Delos D, et al. Reconstruction of the anterior cruciate ligament in the skeletally immature athlete: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2013;95:e28. [DOI] [PubMed] [Google Scholar]
- 14. Feiring DC, Ellenbecker TS, Derscheid GL. Test-retest reliability of the biodex isokinetic dynamometer. J Orthop Sport Phys Ther. 1990;11:298-300. [DOI] [PubMed] [Google Scholar]
- 15. Fitzgerald GK, Lephart SM, Hwang JH, Wainner RS. Hop tests as predictors of dynamic knee stability. J Orthop Sport Phys Ther. 2001;31:588-597. [DOI] [PubMed] [Google Scholar]
- 16. Fitzgerald GK, Piva SR, Irrgang JJ. A modified neuromuscular electrical stimulation protocol for quadriceps strength training following anterior cruciate ligament reconstruction. J Orthop Sport Phys Ther. 2003;33:492-501. [DOI] [PubMed] [Google Scholar]
- 17. Gokeler A, Welling W, Zaffagnini S, Seil R, Padua D. Development of a test battery to enhance safe return to sports after anterior cruciate ligament reconstruction. Knee Surg Sport Traumatol Arthrosc. 2017;25:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee subjective knee form in comparison to the Western Ontario and Mcmaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal art. Am J Sports Med. 2010;38:891-902. [DOI] [PubMed] [Google Scholar]
- 19. Greenberg EM, Greenberg ET, Ganley TJ, Lawrence JT. Strength and functional performance recovery after anterior cruciate ligament reconstruction in preadolescent athletes. Sports Health. 2014;6:309-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50:804-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hartigan EH, Axe MJ, Snyder-Mackler L. Time line for noncopers to pass return-to-sports criteria after anterior cruciate ligament reconstruction. J Orthop Sport Phys Ther. 2010;40:141-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ingelsrud LH, Granan LP, Terwee CB, Engebretsen L, Roos EM. Proportion of patients reporting acceptable symptoms or treatment failure and their associated KOOS values at 6 to 24 months after anterior cruciate ligament reconstruction: a study from the Norwegian Knee Ligament Registry. Am J Sports Med. 2015;43:1902-1907. [DOI] [PubMed] [Google Scholar]
- 23. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29:600-613. [DOI] [PubMed] [Google Scholar]
- 24. Ithurburn MP, Paterno MV, Ford KR, Hewett TE, Schmitt LC. Young athletes after anterior cruciate ligament reconstruction with single-leg landing asymmetries at the time of return to sport demonstrate decreased knee function 2 years later. Am J Sports Med. 2017;45:2604-2613. [DOI] [PubMed] [Google Scholar]
- 25. Ithurburn MP, Paterno MV, Ford KR, Hewett TE, Schmitt LC. Young athletes with quadriceps femoris strength asymmetry at return to sport after anterior cruciate ligament reconstruction demonstrate asymmetric single-leg drop-landing mechanics. Am J Sports Med. 2015;43:2727-2737. [DOI] [PubMed] [Google Scholar]
- 26. Keskula DR, Dowling JS, Davis VL, Finley PW, Dell’omo DL. Interrater reliability of isokinetic measures of knee flexion and extension. J Athl Train. 1995;30:167-170. [PMC free article] [PubMed] [Google Scholar]
- 27. Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011;29:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krych AJ, Woodcock JA, Morgan JA, Levy BA, Stuart MJ, Dahm DL. Factors associated with excellent 6-month functional and isokinetic test results following ACL reconstruction. Knee Surg Sport Traumatol Arthrosc. 2015;23:1053-1059. [DOI] [PubMed] [Google Scholar]
- 29. Kuenze C, Hertel J, Saliba S, Diduch DR, Weltman A, Hart JM. Clinical thresholds for quadriceps assessment after anterior cruciate ligament reconstruction. J Sport Rehabil. 2015;24:36-46. [DOI] [PubMed] [Google Scholar]
- 30. Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sport Med. 2004;34:269-280. [DOI] [PubMed] [Google Scholar]
- 31. Lawrence JTR, Argawal N, Ganley TJ. Degeneration of the knee joint in skeletally immature patients with a diagnosis of an anterior cruciate ligament tear: is there harm in delay of treatment? Am J Sports Med. 2011;39:2582-2587. [DOI] [PubMed] [Google Scholar]
- 32. Lentz TA, Zeppieri G, Tillman SM, et al. Return to preinjury sports participation following anterior cruciate ligament reconstruction: contributions of demographic, knee impairment, and self-report measures. J Orthop Sport Phys Ther. 2012;42:893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Logerstedt D, Lynch A, Axe MJ, Snyder-Mackler L. Symmetry restoration and functional recovery before and after anterior cruciate ligament reconstruction. Knee Surg Sport Traumatol Arthrosc. 2013;21:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Logerstedt DS, Scalzitti DA, Bennell KL, et al. Knee pain and mobility impairments: meniscal and articular cartilage lesions revision 2018. J Orthop Sport Phys Ther. 2018;48:A1-A50. [DOI] [PubMed] [Google Scholar]
- 35. Lynch AD, Logerstedt DS, Grindem H, et al. Consensus criteria for defining “successful outcome” after ACL injury and reconstruction: a Delaware-Oslo ACL cohort investigation. Br J Sports Med. 2015;49:335-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCleary RW, Andersen JC. Test-retest reliability of reciprocal isokinetic knee extension and flexion peak torque measurements. J Athl Train. 1992;27:362-365. [PMC free article] [PubMed] [Google Scholar]
- 37. Millett PJ, Willis AA, Warren RF. Associated injuries in pediatric and adolescent anterior cruciate ligament tears: does a delay in treatment increase the risk of meniscal tear? Arthroscopy. 2002;18:955-959. [DOI] [PubMed] [Google Scholar]
- 38. Myer GD, Martin L, Jr, Ford KR, et al. No association of time from surgery with functional deficits in athletes after anterior cruciate ligament reconstruction: evidence for objective return-to-sport criteria. Am J Sports Med. 2012;40:2256-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Newman JT, Carry PM, Terhune EB, et al. Factors predictive of concomitant injuries among children and adolescents undergoing anterior cruciate ligament surgery. Am J Sports Med. 2015;43:282-288. [DOI] [PubMed] [Google Scholar]
- 40. Palmieri-Smith RM, Lepley LK. Quadriceps strength asymmetry after anterior cruciate ligament reconstruction alters knee joint biomechanics and functional performance at time of return to activity. Am J Sports Med. 2015;43:1662-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parkkari J, Pasanen K, Mattila VM, Kannus P, Rimpelä A. The risk for a cruciate ligament injury of the knee in adolescents and young adults: a population-based cohort study of 46 500 people with a 9 year follow-up. Br J Sports Med. 2008;42:422-426. [DOI] [PubMed] [Google Scholar]
- 42. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22:116-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42:1567-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Redler LH, Brafman RT, Trentacosta N, Ahmad CS. Anterior cruciate ligament reconstruction in skeletally immature patients with transphyseal tunnels. Arthroscopy. 2012;28:1710-1717. [DOI] [PubMed] [Google Scholar]
- 45. Reid A, Birmingham TB, Stratford PW, Alcock GK, Giffin JR. Hop testing provides a reliable and valid outcome measure during rehabilitation after anterior cruciate ligament reconstruction. Phys Ther. 2007;87:337-349. [DOI] [PubMed] [Google Scholar]
- 46. Rudolph KS, Axe MJ, Snyder-Mackler L. Dynamic stability after ACL injury: who can hop? Knee Surg Sport Traumatol Arthrosc. 2000;8:262-269. [DOI] [PubMed] [Google Scholar]
- 47. Schmitt LC, Paterno MV, Ford KR, Myer GD, Hewett TE. Strength asymmetry and landing mechanics at return to sport after anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2015;47:1426-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmitt LC, Paterno MV, Hewett TE. The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sport Phys Ther. 2012;42:750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmitt LC, Paterno MV, Huang S. Validity and internal consistency of the International Knee Documentation Committee Subjective Knee Evaluation Form in children and adolescents. Am J Sports Med. 2010;38:2443-2447. [DOI] [PubMed] [Google Scholar]
- 50. Thomee R, Kaplan Y, Kvist J, et al. Muscle strength and hop performance criteria prior to return to sports after ACL reconstruction. Knee Surg Sport Traumatol Arthrosc. 2011;19:1798-1805. [DOI] [PubMed] [Google Scholar]
- 51. Thomee R, Neeter C, Gustavsson A, et al. Variability in leg muscle power and hop performance after anterior cruciate ligament reconstruction. Knee Surg Sport Traumatol Arthrosc. 2012;20:1143-1151. [DOI] [PubMed] [Google Scholar]
- 52. Toole AR, Ithurburn MP, Rauh MJ, Hewett TE, Paterno MV, Schmitt LC. Young athletes after anterior cruciate ligament reconstruction cleared for sports participation: how many actually meet recommended return-to-sport criteria cutoffs? J Orthop Sport Phys Ther. 2017;47:1-27. [DOI] [PubMed] [Google Scholar]
- 53. Willimon SC, Jones CR, Herzog MM, May KH, Leake MJ, Busch MT. Micheli anterior cruciate ligament reconstruction in skeletally immature youths. Am J Sports Med. 2015;43:2974-2981. [DOI] [PubMed] [Google Scholar]
- 54. Wojtys EM, Brower AM. Anterior cruciate ligament injuries in the prepubescent and adolescent athlete: clinical and research considerations. J Athl Train. 2010;45:509-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. World Health Organization. Adolescent health. http://www.who.int/topics/adolescent_health/en/. Accessed June 2, 2015.
- 56. Xergia SA, McClelland JA, Kvist J, Vasiliadis HS, Georgoulis AD. The influence of graft choice on isokinetic muscle strength 4-24 months after anterior cruciate ligament reconstruction. Knee Surg Sport Traumatol Arthrosc. 2011;19:768-780. [DOI] [PubMed] [Google Scholar]
- 57. Zwolski C, Schmitt LC, Quatman-Yates C, Thomas S, Hewett TE, Paterno MV. The influence of quadriceps strength asymmetry on patient-reported function at time of return to sport after anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43:2242-2249. [DOI] [PubMed] [Google Scholar]