Abstract
Advanced glycation end products (AGEs) are a family of compounds of diverse chemical nature that are the products of nonenzymatic reactions between reducing sugars and proteins, lipids, or nucleic acids. AGEs bind to one or more of their multiple receptors (RAGE) found on a variety of cell types and elicit an array of biologic responses. In this review, we have summarized the data on the nature of AGEs and issues associated with their measurements, their receptors, and changes in their expression under different physiologic and disease states. Last, we have used this information to prescribe lifestyle choices to modulate AGE-RAGE cycle for better health.
Keywords: advanced glycation end products, AGEs, diet, inflammation, lifestyle
‘Exogenous AGEs [advanced glycation end products], for the most part, are derived from food that we consume.’
Advanced Glycation End Products: Origin and Metabolism
Advanced glycation end products (AGEs) are a family of compounds that are the products of nonenzymatic reactions between reducing sugars and proteins, lipids, or nucleic acids.1-4 Roasting and broiling food at high temperatures is a common practice in cooking. These high temperatures facilitate chemical reactions between primary and secondary amino groups of amino acids in proteins and carbonyl groups of reducing sugars, resulting in formation of AGEs; this reaction is commonly referred to as Maillard reaction.5 While there are at least over 3 dozen known AGEs, only about half of these have been identified in foods. Some typical AGEs in food include, Nϵ-carboxymethyl-lysine (CML), Nϵ-carboxyethyl-lysine (CEL), pyrraline, crossline, pentosidine, imidazolium cross-link derived from glyoxal and lysine-lysine (GOLD), and imidazolium cross-link derived from methylglyoxal and lysine-lysine (MOLD).6 These compounds play a very important role by giving special aroma, color, and taste to different foods.7-9
AGEs are also produced in vivo as a result of normal metabolic processes, or can come from diet. Methylglyoxal (MG) is the most common endogenous mediator of AGEs synthesis that is present ubiquitously in all cells. MG is largely derived as a result of carbohydrate, lipid, or amino acid metabolism involving both enzymatic and nonenzymatic reactions.10-13 MG synthesis is catalyzed by MG synthase, cytochrome P450 2E1, myeloperoxidase, and amino oxidase, participating in glycolytic bypass, acetone metabolism, and amino acid breakdown, respectively.12 The nonenzymatic pathways of MG synthesis include the spontaneous decomposition of dihydroxyacetone phosphate, the Maillard reaction, the oxidation of acetol, and lipid peroxidation.12 Exogenous AGEs, for the most part, are derived from food that we consume. The total body AGEs burden is the sum total of AGEs from dietary sources and endogenous synthesis. However, the relative contribution of endogenous versus exogenous AGEs in determining total body AGEs burden and its physiological relevance is difficult to assess. This is due to the multiplicity of AGE molecules, their differing biopotencies, and lack of reliable data on their metabolism, absorption, and distribution in body compartments.14-24
AGEs in Health and Disease
Interest in the role of AGEs in health and disease was sparked by initial reports of progressive rise of in vivo AGEs with normal aging,25-27 by their ability to cross-link proteins in an irreversible fashion,28-30 and by their modulation of extracellular-signal-regulated kinases (ERK) signaling.31-36 A series of reports demonstrating rise in circulating AGEs in people with diabetes37-41 and chronic kidney disease42,43 stimulated further interest in health implications of AGEs. This followed a plethora of empirical studies exhibiting an association between AGEs and a variety of conditions such as decline in memory with age,44-46 pathophysiology of eye diseases,47-49 polycystic ovary syndrome,50-55 wound healing,56-61 cardiovascular complications,62-68 bone health,69-71 periodontitis,58,72 erectile dysfunction,73-76 anemia in older community-dwelling women,77 slow walking speed in older adults,78 peripheral neuropathy,79,80 peripheral artery disease,81-85 obstructive sleep apnea,86-89 islet β-cell dysfunction,90-96 cancer,97-99 elevated cellular oxidative and inflammatory state,100-105 schizophrenia,106 Alzheimer’s disease,107-113 and risk for metabolic syndrome in adults and children.114,115
In a recent study, Foster et al examined racial (European ancestry vs African American) differences in serum level of AGE by ELISA and prostate tissue expression of RAGE (receptor for AGE) by immunocytochemistry in subjects who underwent surgery for prostate cancer.97 The results of this study showed higher expression of both AGE and RAGE in African Americans compared with those of European ancestry, suggesting the possibility that AGE-RAGE axis may be a marker for cancer health disparity.97 The relationship between AGE and its receptors is described in detail under “The AGE-RAGE Cycle” section. While most of these empirical studies do not address a cause-and-effect relationship between AGEs and a disease condition, there are observations supporting altered physiologic responses following administration of exogenous AGEs, foods rich in AGEs, or perturbation of their endogenous levels.116-128 Some examples of such observations from animal and human studies include reproductive abnormalities and prostatic disorders in mice,116 induction of inflammatory mediators,116,117 promotion of insulin resistance in mice following oral administration of AGEs,119 acute state of impaired endothelial function,120 acute impairment of vascular function after a high AGEs meal,121 enhancement of low-density lipoprotein (LDL)–induced vascular toxicity by high AGEs diet,122 increased proteinuria,123,124 and increase in lung level of high mobility group box protein 1 (HMGB1). HMGB1 is a nuclear protein that acts like an agonist for RAGE.125 In a recent pilot study, we examined whether the effect of dietary AGE on circulating AGE may be controlled by fat content (low-fat vs high-fat) of the diet.126 In this study, CML was measured as a surrogate marker of AGE.126 Results of this study show that while there was no change in serum CML following consumption of a low-fat, high-AGE breakfast, there was a small but significant rise in CML after the high-fat, high-AGE breakfast. These data are suggestive that perhaps high dietary fat may increase health risk associated with AGE. A recent human study shows a very weak reduction in circulating AGE following 3 months of low-calorie Mediterranean diet.127 In a double-blind, randomized, crossover human trial, a diet low in AGE resulted in improved insulin sensitivity and decreased urinary AGE excretion.128
As described above, while there is a large volume of published data implicating the role of AGEs in a variety of chronic diseases, there are also publications that contradict many of the above observations.129-131 Data from the cross-sectional Reykjavik Study did not show any significant association between AGEs and age-related macular degeneration.132 While evaluating the efficacy of Irbesartan in 450 patients with type 2 diabetes and nephropathy, Busch et al observed a lack of any role for AGEs in predicting cardiovascular events and renal outcomes in these patients,133 an observation contrary to earlier reports.42,43,62-68 In another study, Somoza and colleagues observed a diet high in AGEs enhanced antioxidant capacity as well as chemopreventive enzymes—glutathione-S-transferase and UDP-glucuronyl-transferase,129 again an observation contradicting a prooxidant role for AGEs.100-103 Another example is the observation that intake of bread crust rich in AGEs did not negatively affect calcium bioavailability and bone metabolism,130 an observation unlikely to explain positive correlation between bone fracture and AGEs.69-71 A large randomized controlled trial that demonstrates no effect of dietary AGEs on endothelial function and inflammation131 contradicts earlier reports associating AGEs with inflammation.104,105
While reasons underlying these apparent contradictory observations are not clear, few appear very apparent. AGEs refer to a collection of structurally different molecules that may have different affinity for receptors for AGE. Hence, there are possibilities for different physiological responses. Yet we almost always measure single chemical entities (eg, CML, CEL, GOLD, or pentosidine), or administer a single chemical entity and assume it to represent all AGEs. The measurements of AGEs employ multiple methods utilizing physicochemical (high-performance liquid chromatography [HPLC]) and immunological (enzyme-linked immunosorbent assay [ELISA]) on extracted as well as unextracted samples. The use of different methods will most certainly yield different values for AGE. The specificity and affinity of the antibody used as well as exposure of antigenic epitopes in samples affect the outcome of ELISA assay. These are some of the very obvious measurement-associated variables that may explain the observed differences in association studies. Additionally, choice of AGEs (eg, CML vs pentosidine vs CEL) for in situ, in vivo, or in vitro studies may yield different results. In many association studies, skin autofluorescence has been used as a surrogate measure of AGEs39,42,70,81; however, almost none of these studies has ever ascertained whether skin autofluorescence correlates with circulating AGEs. Furthermore, it is well known that only select AGEs produce autofluorescence.7 The observation of lack of correlation between skin autofluorescence readings and circulating AGEs in patients with systemic lupus erythematous134 further emphasizes the need for validation. This line of thinking is supported by the observation from Stirban and colleagues where they reported postprandial increases in skin autofluorescence in both diabetic and healthy subjects.135 Our lack of understanding of how AGEs interact with their receptors vis-è-vis how AGEs regulate their receptors further complicates the interpretation of such studies.
Since the ultimate goal is to manage levels and actions of AGEs for better health, the following section summarizes the processes and mediators involved in action(s) of AGEs. This would enable investigators to plan how AGEs or downstream mediators of AGEs and their actions could be managed.
The AGE-RAGE Cycle
There are 4 known receptors for AGEs: full-length RAGE, N-truncated RAGE (Nt-RAGE), and C-truncated RAGE, which has 2 isoforms, secretory RAGE (sRAGE) and endogenous secretory RAGE (esRAGE). RAGEs belong to a member of the immunoglobulin superfamily of receptors.136 RAGE, an approximately 45-kDa protein, has an extracellular component consisting of a variable (V) immunoglobulin-like domain followed by 2 constant domains (types C and C′).137 It has a single transmembrane domain followed by a cytosolic tail.138 The N-terminus of the V domain is the ligand-binding site, and the cytosolic tail is essential for RAGE-induced intracellular signaling.138 Nt-RAGE resides in the plasma membrane, but its function is poorly understood. However, expression of cellular Nt-RAGE, like other RAGE variants, is regulated differentially by different RAGE ligands.139-142 For example, receptor engagement by distinct AGEs (CML-HSA, MG-HSA, diabetic RBC) differentially enhances expression of RAGE isoforms (RAGE, Nt-RAGE) in HUVEC cells.143,144 C-truncated isoforms lack cytosolic and transmembrane domain and circulate in the blood. There are 2 isoforms of C-truncated RAGE: total sRAGE and esRAGE. sRAGE is formed by ectodomain shedding of RAGE catalyzed by membrane-bound proteases including matrix metalloproteases and the closely related ADAM (a disintegrin and metalloprotease domain).141-143 esRAGE, a variant of RAGE lacking transmembrane domain, is formed from alternative splicing of native membrane receptor.138 Serum levels of sRAGE are 5 times higher than esRAGE in healthy subjects.145 Both sRAGE and esRAGE act as decoy for RAGE ligands by sequestering RAGE ligands or competing with full RAGE for ligand binding and thus have cytoprotective effects against AGEs-RAGE interaction.146
RAGE is widely expressed in a variety of tissues (heart, lung, skeletal muscle, and vessel wall) and cell types (neurons, microglia, astrocytes, cerebral endothelial cells, pericytes, smooth muscle cells, monocytes/macrophages, and lymphocytes).138,147 It is a pattern recognition receptor.146 and it has a large repertoire of ligands, enabling it to participate in the etiology of chronic diseases associated with cellular stress and inflammation. Some of these ligands include AGEs; amyloid-β peptide (which accumulates in Alzheimer’s disease); amyloid A (which accumulates in systemic amyloidosis); S100/calgranulins, a family of closely related calcium-binding polypeptides that accumulate extracellularly at sites of chronic inflammation; DNA-binding protein HMGB1 (amphoterin), which is released by cells undergoing apoptosis; and surface molecules on bacteria and leukocytes (macrophage-1 antigen or Mac-1).138-140 Binding of AGEs to RAGE regulates transcription factors, such as nuclear factor kappa B (NF-kB), activator protein 1 (a Jun-Jun homodimer or a Jun-Fos heterodimer), and forkhead box protein O4 via various signal transduction cascades, such as mitogen-activated protein kinases (MAPK), c-Jun N-terminal kinases, extracellular signal-regulated protein kinases 1 and 2, and Janus kinase/signal transducers and activators of transcription (JAK-STAT).148-153 It is noteworthy to mention that the common cellular response associated with all of these signal transduction cascades is inflammation. As mentioned earlier, 2 isoforms of RAGE—sRAGE and esRAGE—bind to AGEs but fail to initiate intracellular signal transduction cascade and, therefore, elicit a protective anti-inflammatory response.154,155
Like sRAGE and esRAGE, there are other receptors that bind AGEs but do not transduce cellular signals; thus, physiologically they serve as antagonists of AGEs action.155 Such receptors include macrophage scavenger receptor types I and II (SR-A),155 oligosaccharyl transferase-4 (OST-48 or AGE-R1),156 galactin-3 (AGE-R3),157 protein kinase C substrate (AGE-R2),158 and lectin-like oxidized LDL receptor-1 (LOX-1).159-162 AGE-R1 or OST belongs to a family of proteins complexes (commonly known as translocon) responsible for the translocation of polypeptides across membranes in eukaryotes. It is a single transmembrane protein that has a small extracellular N-terminal domain and a cytoplasmic C-terminal domain.163 AGE-R2, an 80 to 90 kD protein containing a tyrosine-phosphorylated section anchored in the plasma membrane of the cell, participates in the intracellular signaling of various receptors, like the fibroblast growth factor receptor.158 AGE ligands bind at the C-terminus of AGE-R3 with high affinity.157 AGEs also bind to the class E scavenger receptor, LOX-1, and have been shown to increase LOX-1 expression in diabetic rats.159-162
The Lifestyle Choices Affecting AGE-RAGE Cycle
Lifestyle choices have a significant effect on total body AGEs load, expression/action of RAGE and its isoforms, and resulting metabolic consequences. Figure 1 summarizes effects of lifestyle on AGE-RAGE cycle and plurality of pathophysiologic consequences. Summarized below are the role of lifestyle choices such as smoking, exercise, diet, and dietary supplements on the status of AGE-RAGE cycle and its consequences.
Figure 1.
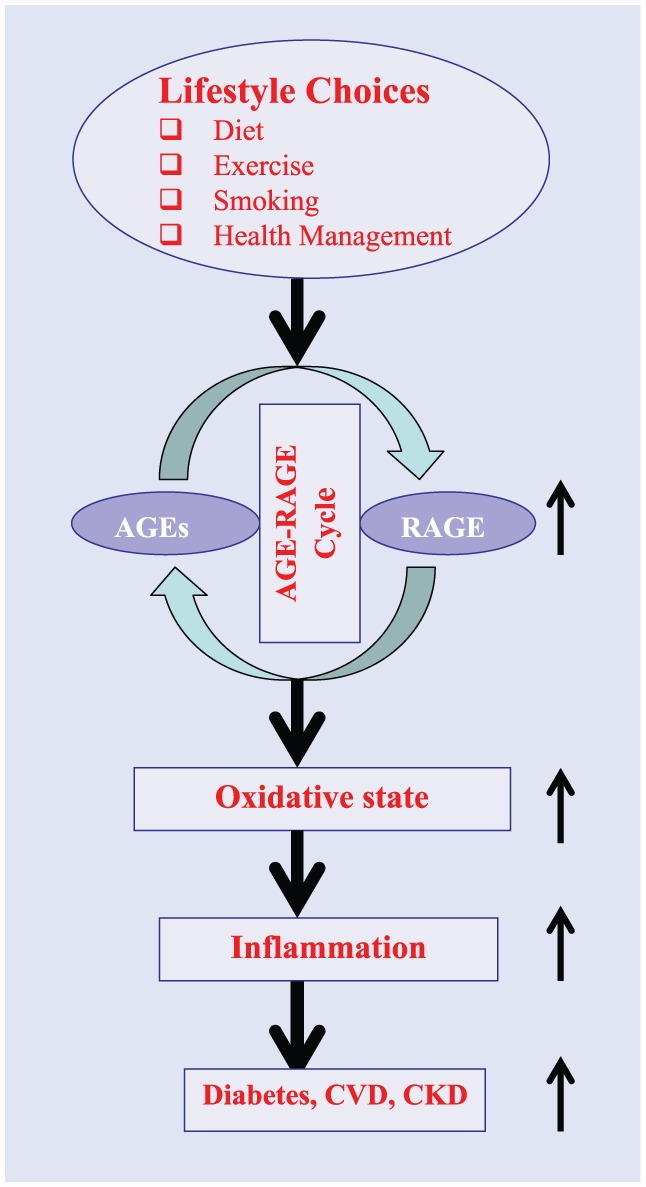
A schematic representation of possible steps between lifestyle choices and emergence of chronic diseases.
Smoking
Certain components of cigarette smoke can react with plasma and extracellular matrix proteins to form covalent adducts with many properties similar to AGEs.164,165 This has been suggested as a possible explanation for higher incidence of cardiovascular disease and cataracts in smokers than in nonsmokers.165 This is further supported by observations that in smokers, tobacco-derived AGEs accumulate in plasma LDL, structural proteins present within the vascular wall, the lens proteins of the eye, and the collagen in the skin.165,166
Exercise
The data on the regulation of AGE-RAGE cycle by exercise in human remains controversial. Aging rats on an exercise regimen have shown a decrease in circulating AGEs with concomitant attenuation of cardiac fibrosis.167 These observations were further supported by another animal study in which Steppan et al suggested that exercise combined with Alagebrium (an AGEs breaker) prevented formation of new AGEs as well as breakdown of already formed AGEs.168 This may represent a therapeutic strategy for age-related ventricular and vascular stiffness.168
In a recent human study, 17 middle-aged sedentary nonsmoking healthy females (35-70 years) free of overt metabolic, cardiovascular, and renal disease underwent a 3-month-long lifestyle modification program that included educational sessions for healthy eating and exercise. At the end of the study, there was a significant decrease in serum levels of CML and pentosidine.169 However, other human studies where measured outcomes were sRAGE or esRAGE, known to attenuate action of AGEs, have yielded conflicting results.170 For example, Choi et al reported aerobic exercise to increase sRAGE levels along with improvement of various cardiometabolic risk factors in patients with type 2 diabetes.171 These observations were further supported by Santilli et al, who reported a significant increase in plasma esRAGE following an 8-week standardized aerobic high-amount–high-intensity training program in 22 sedentary human subjects.170 In contrast, Kotania et al found a decrease in plasma sRAGE with increase in physical activity in an elderly population.169 It is conceivable that these results might have suffered from the fact that the sample size was very small and there was no adequate description of subjects recruited for this study.169 This observation warrants further studies on the clinical relevance of sRAGE changes with physical activity.
Diet
Western diet is high in AGEs. Two large studies have attempted to quantify AGEs in a variety of foods.4,172 These studies were performed by the same group using the same method (ELISA with anti-CML antibody). Another smaller study by Hull et al used HPLC-mass spectrophotometry (MS) to determine CML concentrations in foods.173 In most studies, CML has been the surrogate marker to estimate AGE content of foods. This is based on earlier studies indicating that CML levels directly correlate with levels of other protein- or lipid-derived AGEs.174 Reliability of measures of AGEs in foods is complicated by lack of specificity of measurements and methods used to calculate their concentrations.4,172
Many recent dietary intervention studies have used the tables developed by Goldberg et al to calculate AGE in their test diets rather than measuring AGE in the test foods.4 To develop this table, Goldberg et al obtained a variety of foods from convenience stores and fast food restaurants and also prepared foods using standard cooking times with variation in cooking methods: boiled, broiled, deep fried, oven fried, and roasted. All foods were analyzed for AGE using ELISA that quantifies CML. However, this study did not report whether multiple samples/trials were performed in order to determine AGE. By using this method, concentrations of AGEs were lowest in carbohydrate foods with the lowest levels within this group being found in milk, followed by vegetables and fruits. Broiled beef, chicken, oils heated at high temperature, and roasted nuts were among the highest CML foods. Using these charts the researchers analyzed 3-day food records from healthy participants and found mean daily AGE intake to be about 16 000 ± 5000 kU AGE.4
A follow-up study by the same group repeated the study using greater variations in cooking techniques—marinating, various temperatures, use of AGE inhibitors—and reported that fats have higher levels of AGEs per gram.172 Beef and cheese were found to have the highest levels of AGEs followed by poultry, pork, fish, and eggs. In addition, higher fat and aged cheeses were found to have more AGEs than lower fat cheeses.172
The study by Hull et al, which used HPLC-MS to determine CML in foods, found that AGEs were highest in cereal and lowest in fruits and vegetables expressed as mg/100 g of food. CML remained highest for meat products when expressed as milligram per serving. However, levels of CML in oils were very low.173
An analysis of European liquid infant formulas found elevated concentrations of various AGEs including CML with 3- to 8-fold higher concentrations in liquid infant formula compared with cow’s milk. In the same study, the level of CML in powder infant formula was 2.5 to 5 times higher in concentration than standard powdered milk.175 Also, the levels of CML in infant formula have been found to be 70 times higher compared with breast milk. Plasma levels of CML in infants were also consistently higher in the formula-fed than those who were breast-fed.176
A study of CML levels in a variety of common foods published in 2009 found it to vary from as low as 0.3 mg/kg of raw milk and 0.35 mg/kg of skim milk to as high as 46.1 mg/kg of whole meal bread crust (compared to just 4.45 mg/kg of bread crumb).177 Commercial breakfast cereals, ice cream, and barbecue sauces also appear to be sources of AGE.178-180 An evaluation of a variety of processing methods for nuts and seeds found that CML levels were increased by roasting.181 Thus, consumption of cooked foods compared to raw foods increases AGE ingestion.181 While low-fat vegan diets are lower in AGEs, the research indicates that plasma AGE is actually higher in vegans.182
Intermediate glycation metabolites which may or may not form AGEs such as MG and glyoxal have also been evaluated in various foods. In general, products containing high fructose corn syrup were found to be higher in MG in comparison to diet drinks.183 In contrast, a study by Uribarri et al found high quantities of MG in diet coke and low MG in regular Coke and low quantities of CML in both.172 For Pepsi, the reverse was true.172 Drinks with caramel additives such as Coke Classic or Diet Coke were found to contain 8500 and 9500 units/cup compared to 475 units per cup in Sprite, 600 in orange juice, and around 2000 in coffee and tea.20 The problem with these studies is that they vary in the method of quantifying AGE—some quantify CML versus other AGE products. There are multiple concerns with the reported values of AGEs. These include the following: what is measured, how is it measured, and how much is absorbed.4,172,173,177-180,184
Dietary Supplements
Steps associated with synthesis and breakdown of AGEs is summarized in Figure 2. The major goals of research in the use of dietary supplements including herbals in regulating AGEs are to either block/delay its synthesis or enhance degradation of existing AGEs. These studies can generally be divided into 3 types in order of preponderance in published literature: (1) in vitro and cell culture studies, (2) animal studies, and (3) human studies. In in vitro screening, glycation reaction is initiated by incubating high concentrations of a reducing sugar (hexose or pentose) with a model protein (eg, lysozyme, bovine serum albumin, collagen type 1). The reaction is slow and it takes days to weeks to plateau. Test substances (supplements or plant extracts) are added to the incubation mixture and anti-glycation activity is evaluated by assessing nature of model protein used by sodium dodecyl polyacrylamide gel electrophoresis (SDS-PAGE), ELISA, and HPLC.
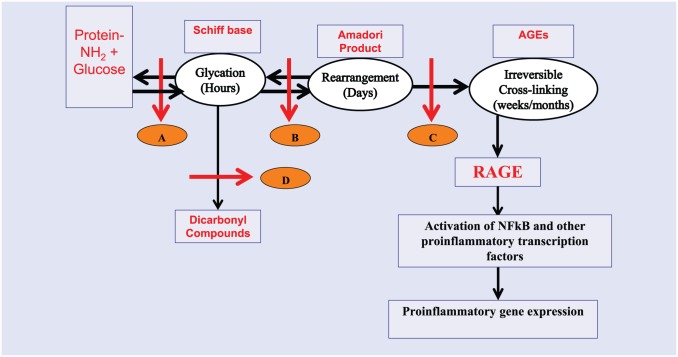
Figure 2.
A diagrammatic representation of steps in the formation of irreversible advanced glycation end products. Letters A to D denote sites where AGEs formation could be intervened through lifestyle changes or use of pharmacologic agents. A, Schiff’s base formation leading to glycation; B, Rearrangement of glycated proteins into Amadori adducts; C, Cross-linking of Amadori adducts into irreversible AGEs; D, Conversion of glycated products into reactive dicrbonyl compounds.
Multiple investigators have tested a variety of dietary supplements and aqueous or organic extracts of medicinal and food plants for their ability to inhibit protein glycation in in vitro or in animal studies (Table 1). While these observations are interesting, establishing their relevance to health outcomes would require standardization of the extraction method, stability of bioactivity, evaluation of toxicity, and finally human studies to document safety and efficacy. Many of these extracts come from commonly consumed foods, and thus may have a lower possibility of toxicity. Unfortunately, most of these initial observations have not progressed to the next step in the process. In contrast to in vitro and animal studies, there are few cell culture studies.200 Encouraging results from the tests using crude preparations led many investigators to examine the effects of purified/semipurified plant components, bioactive food components, and their synthetic counterparts on protein glycation resulting in several candidate agents for possible human study (Table 2). Unfortunately, human studies to assess safety and efficacy of such preparations are far fewer.223 Of the agents tested so far in human studies, pyridoxamine and thiamin—2 B-vitamins—have provided clinical data of uncertain value.215-219 Pyridoxamine, for example, has no established safe human dosage, and at high dosage, it produces profound sensory loss and sensory neuropathy with axonal degeneration.224 Benfotiamine, a synthetic S-acyl derivative of thiamin (vitamin B1) widely used in Germany for sciatica and other painful nerve conditions, has shown inconclusive results in every study when glycation-related outcomes were evaluated.225-228 There have been a series of human studies examining role of aged garlic in managing coronary artery calcification.229-231 The results of these studies show aged garlic alone or in various combinations with B vitamins, folic acid, coenzyme Q10, and l-arginine to retard the progression of subclinical atherosclerosis.229-231 There are multiple other potential bioactive compounds that deserve possible evaluation in human studies. Some of these include curcumin,208 carnosine,207 G-rutin,209-211 genistein,213 and resveratrol.221
Table 1.
A Select List of Plant Extracts Known to Inhibit Protein Glycation In Vitro.
| Common Name | Use | Preparation | Source |
|---|---|---|---|
| Grains of paradise | Spice | Whole fruit extract | Aframomum melegueta 188 |
| Melegueta pepper | |||
| Alligator pepper | |||
| Guinea pepper | |||
| Shallot | Spice | Whole bulb extract | Allium cepa 188 |
| Garlic | Spice | Whole bulb extract | Allium sativum 185 |
| Japanese Angelica-tree | Herbal medicine | Triterpenoid saponins | Aralia taibaiensis 189 |
| Annatto | Colorant and condiment | Whole fruit extract | Bixa orellana 197 |
| Tea | Drink | Leaves | Camellia sinensis 195 |
| Caraway | Spice | Seeds | Carum carvi 187 |
| Cinnamon | Spice | Bark | Cinnamomum verum 187 |
| Spiral ginger | Herbal medicine | Costus pictus 190 | |
| Chipilín | Vegetable | Leaves | Crotolaria longirostrata 187 |
| Turmeric | Spice | Rhizome | Curcuma longa 187 |
| Orchid | Herbal medicine | Dendrobium aqueum 191 | |
| Ornamental plant | Eulophia ochreata 192 | ||
| Yerba mate | Coffee-like drink | Ilex paraguariensis 196 | |
| Bay laurel | Spice | Laurus nobilis 187 | |
| Mint | Spice | Mentha arvensis 187 | |
| Nutmeg and mace | Spice | Myristica fragrans 186 | |
| Marjoram | Spice | Origanum majorana 187 | |
| Root beer plant | Spice | Piper auritum 187 | |
| Thyme | Spice | Thymus vulgaris 187 | |
| Red grape | Fruit, skin, seed | Skin extract | Vitis vinifera 199 |
| Rosemary | Spice | Leaf extract | Rosmarinus officinalis 187 |
| Winter savory | Medicinal food | Whole plant extract | Satureja macrostema 187 |
| Milk thistle | Weed | Silymarin | Silybum marianum 198 |
| Rice bean | Food protein | Whole bean extract | Vigna umbellata L193 |
| Ginger | Spice | Whole rhizome extract | Zingiber officinale 186 |
| Luobuma tea | Herbal tea | Leaf extract | Apocynum venetum 194 |
| Poacynum hendersonii 194 |
Table 2.
A Select List of Phytochemicals Known to Inhibit Protein Glycation in In Vitro or in Animal Studies.
| Phytochemical or Bioactive Food Components | Common Name | Source/Nature | Reference |
|---|---|---|---|
| Anthraquinones | Sickle senna | Cassia tora | 201 |
| Apigenin | A flavone common to many plants | 202 | |
| Arbutin | Alpine bearberry | Arctostaphylos alpine | 203 |
| Berberine | Berberies | Berberis aquifolium (Oregon grape) | 204, 205 |
| Berberis vulgaris (barberry) | |||
| Berberis aristata (tree turmeric) | |||
| Boldine | Boldo tree (Peumus boldus) | 206 | |
| Japanese evergreen spicebush (Lindera aggregata) | |||
| Carnosine | Synthetic, natural metabolite | 207 | |
| Curcumin | Turmeric or haldi | Curcuma longa | 208 |
| G-Rutin | Glycoside common to many edible plant species (eg, citrus peel, buckwheat, asparagus and berries) | 209-211 | |
| Garcinol | Kokum | Garcinia indica | 212 |
| Genistein | An isoflavone rich in soybeans; first isolated from Genista tinctoria | 213 | |
| Hyperoside | A 3-O-galactoside of quercetin common to many plants | 214 | |
| Pyridoxamine (B6 family) | Synthetic | 215-219 | |
| Quercetin | A flavonol common to many plants | 220 | |
| Resveratrol | 221 | ||
| Triterpenoid saponins | Japanese Angelica-tree | Aralia taibaiensis | 222 |
Pharmacologic Interventions
Although a discussion on the development and use of pharmacological agents for improving glycation-related outcomes is beyond the scope of this review, a brief overview of our experience with a few compounds studied so far is worth mention. Aminoguanidine (trade name: pimagedine) is a diamine oxidase and nitric oxide synthetase inhibitor that reduces circulating AGEs.232 A human trial of pimagedine was terminated in 2000 due to an unfavorable risk-to-benefit ratio.233,234 Alagebrium (trade name: ALT-711) was another compound that underwent clinical trial for glycation-related outcomes. While there were some interesting clinical outcomes,235-237 again this trial was terminated in 2007 due to unfavorable risk-to-benefit ratio. Metformin (biguanidine) is a first line agent for suppressing glucose production by liver in obese-overweight insulin-resistant patients that also decreases AGEs production perhaps secondary to decrease in plasma glucose.238,239
Conclusion: Where Do We Go From Here?
AGEs include a diverse group of a family of compounds (exceeding over 3 dozens) that are products of nonenzymatic reactions between reducing sugars and proteins, lipids, or nucleic acids. Except for methylglyoxal10,12,13—a product of normal metabolism—all other AGEs are exogenous in nature derived from food. Although there is a progressive rise of in vivo AGEs with normal aging,25-27 in healthy individuals, the in vivo homeostasis of circulating AGEs is maintained through regulation of renal clearance of AGE-peptides.240
Results of almost all of the human studies examining role of AGEs in health and disease are limited by the fact that they are cross-sectional in nature with great variances in ethnicity, gender, age, sample size, and end point measurements (see Table 3 and 4). The interventional studies are limited by exposure duration of hours to 6 weeks at the most. It is difficult to imagine how a short duration may have a meaningful consequence to a chronic health condition such as cardiovascular disease or diabetes. However, with few exceptions, the results are qualitatively similar. These results are summarized as follows:
Table 3.
A Summary of Human Studies Examining Role of Advanced Glycation End Products in Noncommunicable Diseases.
| Type of Study | Purpose of the Study | Duration of Intervention or Follow-up | Study Population Characteristics | Key Findings | Reference |
|---|---|---|---|---|---|
| Population-based prospective cohort study | To evaluate role of AGE in risk of all-cause and CVD mortality | 6 Years | N = 1013; age: ≥65 years | • Older adults with high plasma AGEs are at higher risk of all-cause and CVD mortality | 25 |
| Longitudinal study | To evaluate role of AGE in CKD and eGFR | 6 Years | N = 750; men and women, aged 26-93 years | • Elevated AGE is independently associated with
CKD • Elevated AGE is independently associated with eGFR |
43 |
| Cross-sectional study | To evaluate role of AGE in memory decline in aged | 1.25-7 Years | 71.0 Years ± 8.1 SD | • AGEs are associated with cognitive decline • High levels of dietary AGEs are associated with faster decline in memory • High serum methylgluoxal are associated with faster decline in attention • Modifying AGEs in the diet may be a strategy to diminish cognitive compromise |
44 |
| Intervention study | To evaluate effect of dietary AGE intake on hormonal and metabolic profile in women with PCOS | 2-Month dietary intervention | 23 Women with PCOS; age: 23.4 ± 5.7 years | • Modifications of dietary AGEs intake are associated with parallel changes in serum AGEs, metabolic, hormonal, and oxidative stress biomarkers in women with PCOS | 50 |
| Cross-sectional study | Is serum level of AGE altered in women with PCOS? | — | 29 Women with PCOS; 22 healthy control women; age: 23.4 ± 5.7 years | • PCOS women without overt hyperglycemia have increased AGE levels and elevated RAGE expression when compared with controls | 54 |
| Cross-sectional study | Can AGEs predict CAD in diabetics? | — | 145 Diabetic and nondiabetic subjects; 63 ± 9 years, 58% men | • Serum AGEs independently predict obstructive CAD and the severity of coronary atherosclerosis irrespective of arterial stiffness in diabetics | 66 |
| Cross-sectional study | Is high serum pentosidine concentration associated with increased arterial stiffness and thickness in patients with type 2 diabetes | — | 159 Diabetic and nondiabetic subjects; 63 ± 9 years, 58% men | • Serum pentosidine is positively associated with both arterial stiffness and thickness and CVD in patients with type 2 diabetes | 67 |
| Cross-sectional study | To examine the role of circulating levels of CML as a biomarker of hip fracture risk | 9.22 Years | 3373 Subjects; 78 years, 39.8% men | • Increasing levels of CML are associated with hip fracture risk in older adults, independent of hip BMD | 71 |
| Cross-sectional study | To examine the role of penile level of AGE in erectile dysfunction | — | N = 38, 62 ± 4 years | • AGES are elevated in diabetic human penile tissue, but not in serum, and are localized to the collagen of the penile tunica and corpus cavernosum | 74 |
| Cross-sectional study | To determine whether serum AGE, and circulating total receptor for AGEs (sRAGE) and endogenous secretory receptor for AGEs (esRAGE) are associated with anemia | — | N = 159 women, ≥65 years | • AGEs and circulating RAGE are independently associated with hemoglobin and anemia in older women | 77 |
| Cross-sectional study | To examine the relationship between AGE and slow walking speed in older adults | — | N = 944 adults, aged ≥65 years | • In older community-dwelling adults, elevated plasma AGE is independently associated with slow walking speed | 78 |
| Cross-sectional study | To examine the role of AGE in peripheral arterial disease | — | N = 170 adults, aged 55 ± 9 years | • Serum AGE (pentosidine) was an independent determinant of ankle-brachial index (ABI), a measure of peripheral arterial disease, in healthy men; Subjects with an ABI less than 1.10 showed higher AGE concentrations | 85 |
| Cross-sectional study | To examine the relationship between OSA and AGE | — | N = 190 OSA patents, N = 234 healthy controls | • Serum AGEs were increased in OSA subjects, as compared with controls | 86 |
| Cross-sectional study | To examine the relationship between increased serum levels of AGE and severity of sleep disordered breathing | — | N = 105 adults, aged 43.5 ± 9.2 years | • Serum AGE levels correlate with AHI in nondiabetic adult males | 87 |
| Cross-sectional study | To examine the relationship between pro-insulin to insulin ratio and plasma AGE level | — | N = 64 patients with type 2 diabetes, aged 62 ± 7 years | • A disproportionate elevation of pro-insulin to insulin ratio, a predictor of beta-cell dysfunction, is positively correlated with plasma AGE level | 90 |
| Cross-sectional study | To examine the relationship between AGE level in prostate cancer tissue sample and severity of disease | — | N = 26 | • AGE levels are elevated in prostate cancer
tissue • Higher the grade of cancer, higher the level of AGE • Subjects of African American decent had higher level of AGE than those from European decent |
97 |
| Cross-sectional study | To examine diagnostic value of AGE level in diagnosis of schizophrenia | — | N = 45 | • Level of AGE is elevated in a subpopulation of schizophrenic patients | 106 |
| Cross-sectional study | To examine role of AGE as a risk factor for metabolic syndrome | — | N = 5848, aged 19-70 years | • Subjects in the highest compared to the lowest quartile category of AGEs intake had higher risk of abdominal obesity and hypertriglyceridemia, 2 markers of metabolic syndrome | 114 |
| Crossover interventional study | To examine role of dietary AGEs on inflammatory molecules in diabetic subjects | 3-6 Weeks | N = 24, diabetics, aged 52 ± 5 years | • Dietary AGEs are significant contributors to serum AGEs in humans; Sustained reduction in dietary AGEs intake led to reduction in serum AGEs and suppression of inflammatory molecules in diabetic subjects | 117 |
| Experimental | To examine role of dietary AGEs on markers of endothelial function in diabetic and nondiabetic subjects | Single exposure | N = 44 stable diabetic subjects and 10 healthy subjects | • There was a significant increase in serum AGEs with altered clinical measures of endothelial function in diabetic and nondiabetic subjects after a single modest AGE-rich beverage | 120 |
| Experimental | To examine role of dietary AGEs on vascular function in diabetic subjects | Single exposure | N = 20, diabetic subjects, aged 41-71 years | • In diabetic patients, a high AGE meal induces a more pronounced acute impairment of vascular function than does an otherwise identical low AGE meal | 121 |
| Experimental | To examine role of dietary AGEs on vascular function in diabetic subjects | 6 Weeks | N = 24, diabetic subjects | • Daily exposure to a diet high in AGE enhances LDL-induced vascular toxicity via redox-sensitive mitogen-activated protein kinase activation | 122 |
| Crossover interventional study | To examine role of dietary AGEs on serum AGE and markers of inflammation | Single exposure | N = 19 | • In healthy subjects, a high AGE meal does not alter serum AGE or CRP | 126 |
| Double-blind, randomized, crossover trial | To examine whether changing dietary AGE intake could modulate insulin sensitivity and secretion in healthy, overweight individuals | 2 Weeks | 20 participants (6 women and 14 men; mean ± SD body mass index [in kg/m2]: 29.8 ± 3.7) | • A diet that is low in AGEs may reduce the risk of type 2 diabetes by increasing insulin sensitivity | 128 |
| Randomized, parallel-arm, controlled dietary intervention | The examine the effects of a diet high or low in AGEs on endothelial function, circulating AGEs, inflammatory mediators, and circulating receptors for AGEs in healthy adults | 6 Weeks | N = 24, healthy adults, aged 50-69 years | • In healthy middle-aged to older adults, consumption of a diet high or low in AGEs for 6 weeks had no impact on endothelial function and inflammatory mediators, 2 precursors of cardiovascular disease | 131 |
| Cross-sectional study | To examine the role of AGEs on AMD in healthy aged adults | — | N = 4907, healthy adults, aged ≥66 years | • Higher serum AGEs had no significant cross-sectional association with prevalent AMD in healthy older adults in Iceland | 132 |
| Longitudinal | To examine the role of AGEs as a predictor of cardiovascular events and renal outcomes in patients with type 2 diabetic kidney disease and hypertension | 2.6 Years | N = 450 (137 women, 313 men); subjects with type 2 diabetes and nephropathy, aged 62 ± 7 years | • Serum AGEs could not be identified as an independent risk factor for cardiovascular or renal outcomes in the examined population | 133 |
Abbreviations: AGE, advanced glycation end products; AHI, apnea-hypopnea index; AMD, age-related macular degeneration; BMD, bone mineral density; CAD, coronary artery disease; CKD, chronic kidney disease; CRP, C-reactive protein; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; OSA, obstructive sleep apnea; PCOS, polycystic ovary syndrome; RAGE, receptor for AGE.
Table 4.
Summary of Animal Studies on the Impact of Advanced Glycation End Products in Noncommunicable Diseases.
| Type of Study | Purpose of the Study | Duration of Intervention or Follow-up | Study Population Characteristics | Key Findings | Reference |
|---|---|---|---|---|---|
| Experimental study | To investigate role of feeding AGE to fruit fly on health and life span | 30 Days | N = 6-120; Drosophila melanogaster | • Decreased life span • Increased age-related functional decline • Decreased proteasome • Increased lysosomal cathepsins |
26 |
| Experimental study | To investigate effect of dietary AGE load on life span | 140 Weeks | N = 84; C57BL/6 mice | • Decreased life span on high AGE diet | 27 |
| Experimental study | To investigate role of AGE in gestational DM | 18 Days | N = 6-10; rabbits | • Maternal DM initiates AGE formation in preimplantation
embryos • AGE accumulates in blastocysts if the maternal DM is poorly controlled |
38 |
| Experimental (in vitro) study | To investigate the effects of exogenous AGE on different eye compartments | — | — | • Diabetic keratopathy and endothelial cell loss in cornea
leading to cataract formation • Promotion of microvascular damage to retina |
47-49 |
| Experimental (in vivo) study | To investigate the effects of anti-AGE agents on soft wound healing | 28 Days | N = 72, male Sprague-Dawley rats | • Anti-AGE agents aminoguanidine and N-phenacylthiazolium bromide facilitated the healing of palatal wounds via the inhibition of the AGE-RAGE axis | 58 |
| Experimental (in vivo) study | To investigate the effects of anti-AGE agents (sRAGE) on wound healing | 21 Days | N = 4-7; C57BLKS+/+Leprdb mice | Administration of RAGE antagonist sRAGE promotes wound healing | 61 |
| Experimental (in vivo) study | To investigate the effects of anti-AGE agents (aminoguanidine) on wound healing | 28 Days | N = 6; Sprague-Dawley rats | Anti-AGE agents appeared to facilitate palatal wound healing by reducing AGE-associated inflammation and promoting the recovery process | 58 |
| Experimental (in vivo) study | To investigate transgenerational effect of feeding isocaloric diets with or without AGEs on insulin resistance and diabetes | 4 Generations | N = 12; C57BL6 mice per observation group | By generation 3 mice manifested increased adiposity and premature insulin resistance compared to mice on AGE-free diet | 119 |
| Experimental (in vivo) study | To examine the effect of chronic consumption of AGEs on renal function | 6 Weeks | N = 12; male Wistar rats | Long-term consumption of a diet rich in AGEs may lead to damage of the kidneys | 123, 124 |
Abbreviations: AGE, advanced glycation end products; DM, diabetes mellitus; RAGE, receptor for AGE; sRAGE, soluble RAGE.
Administration of exogenous AGEs to healthy adults does not lead to increased inflammation or endothelial dysfunction.126,131,132
Elevated AGEs are associated with higher risk of all-cause mortality, severity of coronary atherosclerosis, and cardiovascular disease mortality as well as chronic kidney disease.25,43,66,67
Elevated AGEs are associated with cognitive decline in elderly.44
Elevated AGEs are associated with metabolic abnormalities in polycystic ovarian syndrome.50,54
Exogenously administered AGEs have deleterious metabolic consequences in diabetic as well as patients with compromised renal function.117,120-122
A diet that is low in AGEs may reduce the risk of type 2 diabetes by increasing insulin sensitivity.128
Irrespective of the role of AGE in initiation or progression of metabolic disorders, reduction in its level is certainly of health benefit. This has sparked great interest in defining ways to reduce circulating AGE levels. Approaches to reduction in deleterious effects of AGE include reduction in its formation, inhibition of its action, or increase in its metabolism by pharmacologic and dietary means. Pharmacologic agents, no matter how effective, generally, if not always, tend to carry undesired side effects. Therefore, approaches that may use dietary supplements or dietary modification in achieving a reduction in level or effect of AGEs would be preferable. In this review, we have summarized data on biology and chemistry of AGEs as well as surveyed available data on modification of its formation and action by lifestyle changes. Finally, we have made some lifestyle change recommendations to manage glycation load (Table 5).
Table 5.
Lifestyle Modification Recommendations to Lower Glycation Overload.
| Recommended lifestyle behaviors |
| • Keep fasting blood sugar close to normal (<90-100 mg/dL) |
| • Do not smoke |
| • Protect from excessive sun exposure |
| • Plant trees |
| • Hydrate your skin |
| • Practice moderate regular exercise |
| Recommendations for food preparation and consumption |
| • Eat vegetables and fruits raw, boiled, or steamed |
| • Avoid processed carbohydrates, high fructose corn syrup, and browned and fried foods |
| • Cook meats slow at low temperatures; do not fry or use high heat |
| Avoid supplements lacking research on outcomes |
Acknowledgments
We thank Ms Amanda Bertucci (MS student) for help with literature search.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by The Human Nutrition Research Fund, the State of Texas.
References
- 1. Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci. 2005;1043:461-466. [DOI] [PubMed] [Google Scholar]
- 2. Fu MX, Requena J, Jenkins A, Lyons T, Baynes J, Thorpe S. The advanced glycation end product, ϵN-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982-9986. [DOI] [PubMed] [Google Scholar]
- 3. Estevez M. Protein carbonyl in meat systems: a review. Meat Sci. 2011;89:259-279. [DOI] [PubMed] [Google Scholar]
- 4. Goldberg T, Cai W, Peppa M, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287-1291. [DOI] [PubMed] [Google Scholar]
- 5. Maillard LC. Action des acides aminés sur les sucres: formation des mélanoïdines par voie méthodique. C R Acad Sci. 1912;154:66-68. [Google Scholar]
- 6. Van Nguyen C. Toxicity of the AGEs generated from the Maillard reaction: on the relationship of food-AGEs and biological-AGEs. Mol Nutr Food Res. 2006;50:1140-1149. [DOI] [PubMed] [Google Scholar]
- 7. Poulsen MW, Hedegaard RV, Andersen JM, et al. Advanced glycation end products in food and their effects on health. Food Chem Toxicol. 2013;60:10-37. [DOI] [PubMed] [Google Scholar]
- 8. Hofmann T. Acetylformoin—a chemical switch in the formation of colored Maillard reaction products from hexoses and primary and secondary amino acids. J Agric Food Chem. 1998;46:3918-3928. [Google Scholar]
- 9. Yaylayan VA, Haffenden L, Chu FL, Wnorowski A. Oxidative pyrolysis and postpyrolytic derivatization techniques for the total analysis of Maillard model systems—investigation of control parameters of Maillard reaction pathways. Ann N Y Acad Sci. 2005;1043:41-54. [DOI] [PubMed] [Google Scholar]
- 10. Kalapos MP. Methylglyoxal in living organisms—chemistry, biochemistry, toxicology and biological implications. Toxicol Lett. 1999;110:145-175. [DOI] [PubMed] [Google Scholar]
- 11. Beisswenger PJ, Howell SK, Nelson RG, Mauer M, Szwergold BS. α-Oxoaldehyde metabolism and diabetic complications. Biochem Soc Trans. 2003;31:1358-1363. [DOI] [PubMed] [Google Scholar]
- 12. Kalapos MP. Methylglyoxal and glucose metabolism: a historical perspective and future avenues for research. Drug Metab Drug Interact. 2008;23:69-91. [DOI] [PubMed] [Google Scholar]
- 13. Kalapos MP. The tandem of free radicals and methylglyoxal. Chem Biol Interact. 2008;171:251-271. [DOI] [PubMed] [Google Scholar]
- 14. Finot PA, Furniss DE. Metabolic transit and toxicity of Maillard reaction products. Prog Clin Biol Res. 1989;304:343-358. [PubMed] [Google Scholar]
- 15. Finot PA, Magnenat E. Metabolic transit of early and advanced Maillard products. Prog Food Nutr Sci. 1981;5:193-198. [PubMed] [Google Scholar]
- 16. Kimiagar M, Lee TC, Chichester CO. Long term feeding effects of browned egg albumin to rats. J Agric Food Chem. 1980;28:150-155. [DOI] [PubMed] [Google Scholar]
- 17. Oste RE, Dahlqvist A, Sjostrom H, Noren O, Miller R. Effect of Maillard reaction products on protein digestion: in vitro studies. J Agric Food Chem. 1986;34:355-358. [Google Scholar]
- 18. Chuyen NV, Utsunomiya N, Kato H. Nutritional and physiological effects of casein modified by glucose under various conditions on growing and adults rats. Agric Biol Chem. 1991;55:657-664. [Google Scholar]
- 19. Utsunomiya N, Chuyen NV, Kato H. Nutritional and physiological effects of casein modified by glucose, diacetyl, or hexanal. J Nutr Sci Vitaminol. 1990;36:387-397. [DOI] [PubMed] [Google Scholar]
- 20. Koschinsky T, He CJ, Mitsuhashi T, et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A. 1997;94:6474-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Forster A, Kuhne Y, Henle T. Studies on absorption and elimination of dietary Maillard reaction products. Ann N Y Acad Sci. 2005;1043:474-481. [DOI] [PubMed] [Google Scholar]
- 22. Vlassara H. Advanced glycation in health and disease: role of the modern environment. Ann N Y Acad Sci. 2005;1043:452-460. [DOI] [PubMed] [Google Scholar]
- 23. Somoza V, Lindenmeier M, Hofmann T, et al. Dietary bread crust advanced glycation end products bind to the receptor for AGEs in HEK-293 kidney cells but are rapidly excreted after oral administration to healthy and subtotally nephrectomized rats. Ann N Y Acad Sci. 2005;1043:492-500. [DOI] [PubMed] [Google Scholar]
- 24. Roncero-Ramos I, Delgado-Andrade C, Tessier FJ, et al. Metabolic transit of N(ϵ)-carboxymethyl-lysine after consumption of AGEs from bread crust. Food Funct. 2013;4:1032-1039. [DOI] [PubMed] [Google Scholar]
- 25. Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L. Plasma carboxymethyl-lysine, and advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J Am Geriatr Soc. 2009;57:1874-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsakiri EN, Iliaki KK, Höhn A, et al. Diet-derived advanced glycation end products or lipofuscin disrupts proteostasis and reduces life span in Drosophila melanogaster. Free Radic Biol Med. 2013;65:1155-1163. [DOI] [PubMed] [Google Scholar]
- 27. Peppa M, Uribarri J, Vlassara H. Aging and glycoxidant stress. Hormones (Athens). 2008;7:123-132. [DOI] [PubMed] [Google Scholar]
- 28. Shamsi FA, Partal A, Sady C, Glomb MA, Nagaraj RH. Immunological evidence for methylglyoxal-derived modifications in vivo. Determination of antigenic epitopes. J Biol Chem. 1998;273:6928-6936. [DOI] [PubMed] [Google Scholar]
- 29. Bourajjaj M, Stehouwer CD, Van Hinsbergh VW, Schalkwijk CG. Role of methylglyoxal adducts in the development of vascular complications in diabetes mellitus. Biochem Soc Trans. 2003;31:1400-1402. [DOI] [PubMed] [Google Scholar]
- 30. Kim J, Jeong IH, Kim CS, Lee YM, Kim JM, Kim JS. Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross-linking. Arch Pharm Res. 2011;34:495-500. [DOI] [PubMed] [Google Scholar]
- 31. Piperi C, Adamopoulos C, Dalagiorgou G, Diamanti-Kandarakis E, Papavassiliou AG. Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab. 2012;97:2231-2242. [DOI] [PubMed] [Google Scholar]
- 32. Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002;45:309-326. [DOI] [PubMed] [Google Scholar]
- 33. Poitout V, Hagman D, Stein R, Artner I, Robertson RP, Harmon JS. Regulation of the insulin gene by glucose and fatty acids. J Nutr. 2006;136:873-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Y, Liu CP, Xu KF, et al. Effect of taurine-conjugated ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am J Nephrol. 2008;28:1014-1022. [DOI] [PubMed] [Google Scholar]
- 35. Rasheed Z, Akhtar N, Haqqi TM. Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes. Rheumatology. 2011;50:838-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loughlin DT, Artlett CM. Precursor of advanced glycation end products mediates ER-stress-induced caspase-3 activation of human dermal fibroblasts through NAD(P)H oxidase 4. PLoS One. 2010;5:e11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223-234. [DOI] [PubMed] [Google Scholar]
- 38. Haucke E, Navarrete Santos A, Simm A, et al. Accumulation of advanced glycation end products in the rabbit blastocyst under maternal diabetes. Reproduction. 2014;148:169-178. [DOI] [PubMed] [Google Scholar]
- 39. Araszkiewicz A, Naskret D, Niedzwiecki P, Samborski P, Wierusz-Wysocka B, Zozulinska-Ziolkiewicz D. Increased accumulation of skin advanced glycation end products is associated with microvascular complications in type 1 diabetes. Diabetes Technol Ther. 2011;13:837-842. [DOI] [PubMed] [Google Scholar]
- 40. Sugiyama T, Okuno T, Fukuhara M, et al. Angiotensin II receptor blocker inhibits abnormal accumulation of advanced glycation end products and retinal damage in a rat model of type 2 diabetes. Exp Eye Res. 2007;85:406-412. [DOI] [PubMed] [Google Scholar]
- 41. Forbes JM, Thomas MC, Thorpe SR, Alderson NL, Cooper ME. The effects of valsartan on the accumulation of circulating and renal advanced glycation end products in experimental diabetes. Kidney Int Suppl. 2004;92:S105-S107. [DOI] [PubMed] [Google Scholar]
- 42. Oleniuc M, Schiller A, Secara I, et al. Evaluation of advanced glycation end products accumulation, using skin autofluorescence, in CKD and dialysis patients. Int Urol Nephrol. 2012;44:1441-1449. [DOI] [PubMed] [Google Scholar]
- 43. Semba RD, Fink JC, Sun K, Windham BG, Ferrucci L. Serum carboxymethyl-lysine, a dominant advanced glycation end product, is associated with chronic kidney disease: the Baltimore longitudinal study of aging. J Ren Nutr. 2010;20:74-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. West R, Moshier E, Lubitz I, et al. Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech Ageing Dev. 2014;140:10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carnevale D, Mascio G, D’Andrea I, et al. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60:188-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mazarati A, Maroso M, Iori V, Vezzani A, Carli M. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and Receptor for advanced glycation end products. Exp Neurol. 2011;232:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kandarakis SA, Piperi C, Topouzis F, Papavassiliou AG. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog Retin Eye Res. 2014;42:85-102. [DOI] [PubMed] [Google Scholar]
- 48. Tezel G, Luo C, Yang X. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci. 2007;48:1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ni J, Yuan X, Gu J, et al. Plasma protein pentosidine and carboxymethyllysine, biomarkers for age-related macular degeneration. Mol Cell Proteomics. 2009;8:1921-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tantalaki E, Piperi C, Livadas S, et al. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS). Hormones (Athens). 2014;13:65-73. [DOI] [PubMed] [Google Scholar]
- 51. Kandaraki E, Chatzigeorgiou A, Piperi C, et al. Reduced ovarian glyoxalase-I activity by dietary glycotoxins and androgen excess: a causative link to polycystic ovarian syndrome. Mol Med. 2012;18:1183-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Christakou C, Economou F, Livadas S, et al. Strong and positive association of endothelin-1 with AGEs in PCOS: a causal relationship or a bystander? Hormones (Athens). 2011;10:292-297. [DOI] [PubMed] [Google Scholar]
- 53. Diamanti-Kandarakis E, Christakou C, Marinakis E. Phenotypes and environmental factors: their influence in PCOS. Curr Pharm Des. 2012;18:270-282. [DOI] [PubMed] [Google Scholar]
- 54. Diamanti-Kandarakis E, Piperi C, Kalofoutis A, Creatsas G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2005;62:37-43. [DOI] [PubMed] [Google Scholar]
- 55. Merhi Z. Advanced glycation end products and their relevance in female reproduction. Hum Reprod. 2014;29:135-145. [DOI] [PubMed] [Google Scholar]
- 56. Peppa M, Raptis SA. Glycoxidation and wound healing in diabetes: an interesting relationship. Curr Diabetes Rev. 2011;7:416-425. [DOI] [PubMed] [Google Scholar]
- 57. Peppa M, Stavroulakis P, Raptis SA. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009;17:461-472. [DOI] [PubMed] [Google Scholar]
- 58. Chang PC, Tsai SC, Jheng YH, Lin YF, Chen CC. Soft-tissue wound healing by anti-advanced glycation end-products agents. J Dent Res. 2014;93:388-393. [DOI] [PubMed] [Google Scholar]
- 59. Shi L, Chen H, Yu X, Wu X. Advanced glycation end products delay corneal epithelial wound healing through reactive oxygen species generation. Mol Cell Biochem. 2013;383:253-259. [DOI] [PubMed] [Google Scholar]
- 60. Zhu Y, Lan F, Wei J, et al. Influence of dietary advanced glycation end products on wound healing in nondiabetic mice. J Food Sci. 2011;76:5-10. [DOI] [PubMed] [Google Scholar]
- 61. Goova MT, Li J, Kislinger T, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159:513-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Peppa M, Raptis SA. Advanced glycation end products and cardiovascular disease. Curr Diabetes Rev. 2008;4:92-100. [DOI] [PubMed] [Google Scholar]
- 63. Geronikaki A, Gavalas A, Dislian V, Giannoglou G. Inhibition of renin-angiotensin system and advanced glycation end products formation: a promising therapeutic approach targeting on cardiovascular diseases. Cardiovasc Hematol Agents Med Chem. 2007;5:249-264. [DOI] [PubMed] [Google Scholar]
- 64. Yamagishi S, Matsui T, Ueda S, Nakamura K, Imaizumi T. Advanced glycation end products (AGEs) and cardiovascular disease (CVD) in diabetes. Cardiovasc Hematol Agents Med Chem. 2007;5:236-240. [DOI] [PubMed] [Google Scholar]
- 65. Kerkeni M, Weiss IS, Jaisson S, et al. Increased serum concentrations of pentosidine are related to presence and severity of coronary artery disease. Thromb Res. 2014;134:633-638. [DOI] [PubMed] [Google Scholar]
- 66. Won KB, Chang HJ, Park SH, Hong SY, Jang Y, Chung N. High serum advanced glycation end-products predict coronary artery disease irrespective of arterial stiffness in diabetic patients. Korean Circ J. 2012;42:335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yoshida N, Okumura K, Aso Y. High serum pentosidine concentrations are associated with increased arterial stiffness and thickness in patients with type 2 diabetes. Metabolism. 2005;54:345-350. [DOI] [PubMed] [Google Scholar]
- 68. Prasad A, Bekker P, Tsimikas S. Advanced glycation end products and diabetic cardiovascular disease. Cardiol Rev. 2012;20:177-183. [DOI] [PubMed] [Google Scholar]
- 69. Neumann T, Lodes S, Kästner B, et al. High serum pentosidine but not esRAGE is associated with prevalent fractures in type 1 diabetes independent of bone mineral density and glycaemic control. Osteoporos Int. 2014;25:1527-1533. [DOI] [PubMed] [Google Scholar]
- 70. Momma H, Niu K, Kobayashi Y, et al. Skin advanced glycation end-product accumulation is negatively associated with calcaneal osteo-sono assessment index among non-diabetic adult Japanese men. Osteoporos Int. 2012;23:1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barzilay JI, Bůžková P, Zieman SJ, et al. Circulating levels of carboxy-methyl-lysine (CML) are associated with hip fracture risk: the Cardiovascular Health Study. J Bone Miner Res. 2014;29:1061-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zizzi A, Tirabassi G, Aspriello SD, Piemontese M, Rubini C, Lucarini G. Gingival advanced glycation end-products in diabetes mellitus-associated chronic periodontitis: an immunohistochemical study. J Periodontal Res. 2013;48:293-301. [DOI] [PubMed] [Google Scholar]
- 73. Cellek S, Cameron NE, Cotter MA, Muneer A. Pathophysiology of diabetic erectile dysfunction: potential contribution of vasa nervorum and advanced glycation end products. Int J Impot Res. 2013;25:1-6. [DOI] [PubMed] [Google Scholar]
- 74. Seftel AD, Vaziri ND, Ni Z, et al. Advanced glycation end products in human penis: elevation in diabetic tissue, site of deposition, and possible effect through iNOS or eNOS. Urology. 1997;50:1016-1026. [DOI] [PubMed] [Google Scholar]
- 75. Cameron NE, Cotter MA. Erectile dysfunction and diabetes mellitus: mechanistic considerations from studies in experimental models Curr Diabetes Rev. 2007;3:149-158. [DOI] [PubMed] [Google Scholar]
- 76. Jiaan DB, Seftel AD, Fogarty J, et al. Age-related increase in an advanced glycation end product in penile tissue. World J Urol. 1995;13:369-375. [DOI] [PubMed] [Google Scholar]
- 77. Semba RD, Ferrucci L, Sun K, Patel KV, Guralnik JM, Fried LP. Elevated serum advanced glycation end products and their circulating receptors are associated with anaemia in older community-dwelling women. Age Ageing. 2009;38:283-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L. Relationship of an advanced glycation end product, plasma carboxymethyl-lysine, with slow walking speed in older adults: the InCHIANTI study. Eur J Appl Physiol. 2010;108:191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aubert CE, Michel PL, Gillery P, et al. Association of peripheral neuropathy with circulating advanced glycation end products, soluble receptor for advanced glycation end products and other risk factors in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014;30:679-685. [DOI] [PubMed] [Google Scholar]
- 80. Meerwaldt R, Links TP, Graaff R, et al. Increased accumulation of skin advanced glycation end-products precedes and correlates with clinical manifestation of diabetic neuropathy. Diabetologia. 2005;48:1637-1644. [DOI] [PubMed] [Google Scholar]
- 81. Noordzij MJ, Lefrandt JD, Loeffen EAH, et al. Skin autofluorescence is increased in patients with carotid artery stenosis and peripheral artery disease. Int J Cardiovasc Imaging. 2012;28:431-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schmidt AM. Skin autofluorescence, 5-year mortality, and cardiovascular events in peripheral arterial disease: all that glitters is surely not gold. Arterioscler Thromb Vasc Biol. 2014;34:697-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. de Vos LC, Mulder DJ, Smit AJ, et al. Skin autofluorescence is associated with 5-year mortality and cardiovascular events in patients with peripheral artery disease. Arterioscler Thromb Vasc Biol. 2014;34:933-938. [DOI] [PubMed] [Google Scholar]
- 84. de Vos LC, Noordzij MJ, Mulder DJ, et al. Skin autofluorescence as a measure of advanced glycation end products deposition is elevated in peripheral artery disease. Arterioscler Thromb Vasc Biol. 2013;33:131-138. [DOI] [PubMed] [Google Scholar]
- 85. Takahashi R, Imamura A, Yoshikane M, et al. High serum concentrations of pentosidine, an advanced glycation end product, are associated with low normal value of ankle-brachial index in apparently healthy men. Metabolism. 2011;60:649-654. [DOI] [PubMed] [Google Scholar]
- 86. Tan KC, Chow WS, Lam JC, et al. Advanced glycation endproducts in nondiabetic patients with obstructive sleep apnea. Sleep. 2006;29:329-333. [DOI] [PubMed] [Google Scholar]
- 87. Lam JC, Tan KC, Lai AY, Lam DC, Ip MS. Increased serum levels of advanced glycation end-products is associated with severity of sleep disordered breathing but not insulin sensitivity in non-diabetic men with obstructive sleep apnoea. Sleep Med. 2012;13:15-20. [DOI] [PubMed] [Google Scholar]
- 88. Mokhlesi B, Gozal D. In the fight against advanced glycation end-products (AGEs), you should treat OSA, shouldn’t you? Sleep Med. 2012;13:5-6. [DOI] [PubMed] [Google Scholar]
- 89. Kotani K, Kimura S, Komada I, Sakane N, Gugliucci A. Continuous positive air pressure treatment reduces serum advanced glycation end products in patients with obstructive sleep apnoea syndrome: a pilot study. Prim Care Respir J. 2011;20:336-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Saisho Y, Maruyama T, Hirose H, Saruta T. Relationship between proinsulin-to-insulin ratio and advanced glycation endproducts in Japanese type 2 diabetic subjects. Diabetes Res Clin Pract. 2007;78:182-188. [DOI] [PubMed] [Google Scholar]
- 91. Puddu A, Sanguineti R, Durante A, et al. Glucagon-like peptide-1 triggers protective pathways in pancreatic beta-cells exposed to glycated serum. Mediators Inflamm. 2013;2013:317120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Song YM, Song SO, You YH, et al. Glycated albumin causes pancreatic β-cells dysfunction through autophagy dysfunction. Endocrinology. 2013;154:2626-2639. [DOI] [PubMed] [Google Scholar]
- 93. Han D, Yamamoto Y, Munesue S, et al. Induction of receptor for advanced glycation end products by insufficient leptin action triggers pancreatic β-cell failure in type 2 diabetes. Genes Cells. 2013;18:302-314. [DOI] [PubMed] [Google Scholar]
- 94. Pejnovic NN, Pantic JM, Jovanovic IP, et al. Galectin-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets. Diabetes. 2013;62:1932-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Costal F, Oliveira E, Raposo A, et al. Dual effect of advanced glycation end products in pancreatic islet apoptosis. Diabetes Metab Res Rev. 2013;29:296-307. [DOI] [PubMed] [Google Scholar]
- 96. Puddu A, Sanguineti R, Durante A, Viviani GL. Pioglitazone attenuates the detrimental effects of advanced glycation end-products in the pancreatic beta cell line HIT-T15. Regul Pept. 2012;177:79-84. [DOI] [PubMed] [Google Scholar]
- 97. Foster D, Spruill L, Walter KR, et al. AGE metabolites: a biomarker linked to cancer disparity. Cancer Epidemiol Biomarkers Prev. 2014;23:2186-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yamagishi S, Nakamura K, Inoue H, Kikuchi S, Takeuchi M. Possible participation of advanced glycation end products in the pathogenesis of colorectal cancer in diabetic patients. Med Hypotheses. 2005;64:1208-1210. [DOI] [PubMed] [Google Scholar]
- 99. Jiao L, Taylor PR, Weinstein SJ, et al. Advanced glycation end products, soluble receptor for advanced glycation end products, and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1430-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wong RK, Pettit AI, Davies JE, Ng LL. Augmentation of the neutrophil respiratory burst through the action of advanced glycation end products: a potential contributor to vascular oxidant stress. Diabetes. 2002;51:2846-2853. [DOI] [PubMed] [Google Scholar]
- 101. Chappey O, Dosquet C, Wautier MP, Wautier JL. Advanced glycation end products, oxidant stress and vascular lesions. Eur J Clin Invest. 1997;27:97-108. [DOI] [PubMed] [Google Scholar]
- 102. Wautier JL, Wautier MP, Schmidt AM, et al. Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: a link between surface-associated AGEs and diabetic complications. Proc Natl Acad Sci U S A. 1994;91:7742-7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yan SD, Schmidt AM, Anderson GM, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889-9897. [PubMed] [Google Scholar]
- 104. Nakajima Y, Inagaki Y, Kido JI, Nagata T. Advanced glycation end-products increase expression of S100A8 and A9 via RAGE-MAPK in rat dental pulp cells. Oral Dis. 2015;21:328-334. [DOI] [PubMed] [Google Scholar]
- 105. Ehlermann P, Eggers K, Bierhaus A, et al. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Arai M, Miyashita M, Kobori A, Toriumi K, Horiuchi Y, Itokawa M. Carbonyl stress and schizophrenia. Psychiatry Clin Neurosci. 2014;68:655-665. [DOI] [PubMed] [Google Scholar]
- 107. Yamagishi S, Nakamura K, Inoue H, Kikuchi S, Takeuchi M. Serum or cerebrospinal fluid levels of glyceraldehyde-derived advanced glycation end products (AGEs) may be a promising biomarker for early detection of Alzheimer’s disease. Med Hypotheses. 2005;64:1205-1207. [DOI] [PubMed] [Google Scholar]
- 108. Angeloni C, Zambonin L, Hrelia S. Role of methylglyoxal in Alzheimer’s disease. Biomed Res Int. 2014;2014:238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sasaki N, Fukatsu R, Tsuzuki K, et al. Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am J Pathol. 1998;153:1149-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Krautwald M, Münch G. Advanced glycation end products as biomarkers and gerontotoxins—a basis to explore methylglyoxal-lowering agents for Alzheimer’s disease? Exp Gerontol. 2010;45:744-751. [DOI] [PubMed] [Google Scholar]
- 111. Münch G, Kuhla B, Lüth HJ, Arendt T, Robinson SR. Anti-AGEing defences against Alzheimer’s disease. Biochem Soc Trans. 2003;31:1397-1399. [DOI] [PubMed] [Google Scholar]
- 112. Colaco CA, Ledesma MD, Harrington CR, Avila J. The role of the Maillard reaction in other pathologies: Alzheimer’s disease. Nephrol Dial Transplant. 1996;11:7-12. [DOI] [PubMed] [Google Scholar]
- 113. Münch G, Taneli Y, Schraven E, et al. The cognition-enhancing drug tenilsetam is an inhibitor of protein crosslinking by advanced glycosylation. J Neural Transm Park Dis Dement Sect. 1994;8:193-208. [DOI] [PubMed] [Google Scholar]
- 114. Angoorania P, Ejtahedbc HS, Mirmiranad P, Mirzaeia S, Azizi F. Dietary consumption of advanced glycation end products and risk of metabolic syndrome. Int J Food Sci Nutr. 2016;67:170-176. [DOI] [PubMed] [Google Scholar]
- 115. Gupta A, Uribarri J. Dietary advanced glycation end products and their potential role in cardiometabolic disease in children. Horm Res Paediatr. 2016;85:291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Moschonas DP, Piperi C, Korkolopoulou P, et al. Impact of diet-induced obesity in male mouse reproductive system: the role of advanced glycation end product-receptor for advanced glycation end product axis. Exp Biol Med (Maywood). 2014;239:937-947. [DOI] [PubMed] [Google Scholar]
- 117. Vlassara H, Cai W, Crandall J, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A. 2002;99:15596-15601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zheng F, He C, Cai W, Hattori M, Steffes M, Vlassara H. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Metab Res Rev. 2002:18:224-237. [DOI] [PubMed] [Google Scholar]
- 119. Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Nat Acad Sci U S A. 2012;109:15888-15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Uribarri J, Stirban A, Sander D, et al. Single oral challenge by advanced glycation end products acutely impairs endothelial function in diabetic and nondiabetic subjects. Diabetes Care. 2007;30:2579-2582. [DOI] [PubMed] [Google Scholar]
- 121. Negrean M, Stirban A, Stratmann B, et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85:1236-1243. [DOI] [PubMed] [Google Scholar]
- 122. Cai W, He JC, Zhu L, et al. High levels of dietary advanced glycation end products transform low-density lipoprotein into a potent redox-sensitive mitogen-activated protein kinase stimulant in diabetic patients. Circulation. 2004;110:285-291. [DOI] [PubMed] [Google Scholar]
- 123. Sebeková K, Hofmann T, Boor P, et al. Renal effects of oral Maillard reaction product load in the form of bread crusts in healthy and subtotally nephrectomized rats. Ann N Y Acad Sci. 2005;1043:482-491. [DOI] [PubMed] [Google Scholar]
- 124. Sebeková K, Faist V, Hofmann T, Schinzel R, Heidland A. Effects of a diet rich in advanced glycation end products in the rat remnant kidney model. Am J Kidney Dis. 2003;41:S48-S51. [DOI] [PubMed] [Google Scholar]
- 125. Bartling B, Fuchs C, Somoza V, Niemann B, Silber RE, Simm A. Lung level of HMBG1 is elevated in response to advanced glycation end product-enriched food in vivo. Mol Nutr Food Res. 2007;51:479-487. [DOI] [PubMed] [Google Scholar]
- 126. Davis KE, Prasad C, Vijayagopal P, Juma S, Adams-Huet B, Imrhan V. Contribution of dietary advanced glycation end products (AGE) to circulating AGE: role of dietary fat. Br J Nutr. 2015;114:1797-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rodŕguez JM, Balich LV, Concha MJ, et al. Reduction of serum advanced glycation end-products with a low calorie Mediterranean diet. Nutr Hosp. 2015;31:2511-2517. [DOI] [PubMed] [Google Scholar]
- 128. de Courten B, de Courten MP, Soldatos G, et al. Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: a double-blind, randomized, crossover trial. Am J Clin Nutr. 2016;103:1426-1433. [DOI] [PubMed] [Google Scholar]
- 129. Somoza V, Wenzel E, Lindenmeier M, Grothe D, Erbersdobler HF, Hofmann T. Influence of feeding malt, bread crust, and a pronylated protein on the activity of chemopreventive enzymes and antioxidative defense parameters in vivo. J Agric Food Chem. 2005;53:8176-8182. [DOI] [PubMed] [Google Scholar]
- 130. Roncero-Ramos I, Delgado-Andrade C, Haro A, Ruiz-Roca B, Morales FJ, Navarro MP. Effects of dietary bread crust Maillard reaction products on calcium and bone metabolism in rats. Amino Acids. 2013;44:1409-1418. [DOI] [PubMed] [Google Scholar]
- 131. Semba RD, Gebauer SK, Baer DJ, et al. Dietary intake of advanced glycation end products did not affect endothelial function and inflammation in healthy adults in a randomized controlled trial. J Nutr. 2014;144:1037-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Semba RD, Cotch MF, Gudnason V, et al. Serum carboxymethyllysine, an advanced glycation end product, and age-related macular degeneration: the Age, Gene/Environment Susceptibility-Reykjavik Study. JAMA Ophthalmol. 2014;132:464-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Busch M, Franke S, Wolf G, et al. The advanced glycation end product N(epsilon)-carboxymethyllysine is not a predictor of cardiovascular events and renal outcomes in patients with type 2 diabetic kidney disease and hypertension. Am J Kidney Dis. 2006;48:571-579. [DOI] [PubMed] [Google Scholar]
- 134. Nienhuis HL, de Leeuw K, Bijzet J, et al. Skin autofluorescence is increased in systemic lupus erythematosus but is not reflected by elevated plasma levels of advanced glycation end products. Rheumatology (Oxford). 2008;47:1554-1558. [DOI] [PubMed] [Google Scholar]
- 135. Stirban A, Nandrean S, Negrean M, Koschinsky T, Tschoepe D. Skin autofluorescence increases postprandially in human subjects. Diabetes Technol Ther. 2008;10:200-205. [DOI] [PubMed] [Google Scholar]
- 136. Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R-28R. [DOI] [PubMed] [Google Scholar]
- 137. Raucci A, Cugusi S, Antonelli A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008;22:3716-3727. [DOI] [PubMed] [Google Scholar]
- 138. Kalea AZ, Schmidt AM, Hudson BI. Alternative splicing of RAGE: roles in biology and disease. Front Biosci (Landmark Ed). 2011;16:2756-2770. [DOI] [PubMed] [Google Scholar]
- 139. López-Díez R, Rastrojo A, Villate O, Aguado B. Complex tissue-specific patterns and distribution of multiple RAGE splice variants in different mammals. Genome Biol Evol. 2013;5:2420-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Drinda S, Franke S, Eidner T, et al. Decreased RAGE expression in peripheral blood mononuclear cells of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2009;27:483-490. [PubMed] [Google Scholar]
- 141. Lieuw-a-Fa ML, Schalkwijk CG, Engelse M, van Hinsbergh VW. Interaction of Nepsilon(carboxymethyl)lysine- and methylglyoxal-modified albumin with endothelial cells and macrophages. Splice variants of RAGE may limit the responsiveness of human endothelial cells to AGEs. Thromb Haemost. 2006;95:320-328. [DOI] [PubMed] [Google Scholar]
- 142. Yonekura H, Yamamoto Y, Sakurai S, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Grossin N, Wautier MP, Picot J, Stern DM, Wautier JL. Differential effect of plasma or erythrocyte AGE-ligands of RAGE on expression of transcripts for receptor isoforms. Diabetes Metab. 2009;35:410-417. [DOI] [PubMed] [Google Scholar]
- 144. Quade-Lyssy P, Kanarek AM, Baiersdörfer M, Postina R, Kojro E. Statins stimulate the production of a soluble form of the receptor for advanced glycation end products. J Lipid Res. 2013;54:3052-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Koyama H, Shoji T, Yokoyama H, et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:2587-2593. [DOI] [PubMed] [Google Scholar]
- 146. Fritz G. RAGE a single receptor fits multiple ligands. Trends Biochem Sci. 2011;36:625-632. [DOI] [PubMed] [Google Scholar]
- 147. Ramasamy R, Yan SF, Schmidt AM. Advanced glycation endproducts: from precursors to RAGE: round and round we go. Amino Acids. 2012;42:1151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem. 2001;81:102-113. [DOI] [PubMed] [Google Scholar]
- 149. Huttunen HJ, Fages C, Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999;274:19919-19924. [DOI] [PubMed] [Google Scholar]
- 150. Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein. J Biol Chem. 1997;272:17810-17814. [DOI] [PubMed] [Google Scholar]
- 151. Mahali S, Raviprakash N, Raghavendra PB, Manna SK. Advanced glycation end products (AGEs) induce apoptosis via a novel pathway: involvement of Ca2+ mediated by interleukin-8 protein. J Biol Chem. 2011;286:34903-34913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685-E694. [DOI] [PubMed] [Google Scholar]
- 153. Yeh CH, Sturgis L, Haidacher J., et al. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes. 2001;50:1495-1504. [DOI] [PubMed] [Google Scholar]
- 154. Bucciarelli LG, Wendt T, Rong L, et al. RAGE is a multiligand receptor of the immunoglobulin superfamily: implications for homeostasis and chronic disease. Cell Mol Life Sci. 2002;59:1117-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Yang Z, Makita Z, Horii Y, et al. Two novel rat liver membrane proteins that bind advanced glycosylation end products: relationship to macrophage receptor for glucose modified proteins. J Exp Med. 1991;174:515-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kelleher DJ, Kreibich G, Gilmore R. Oligosaccharyltransferase activity is associated with a protein complex composed of ribophorins I and II and a 48 kd protein. Cell. 1992;69:55-65. [DOI] [PubMed] [Google Scholar]
- 157. Vlassara H, Li YM, Imani F, et al. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med. 1995;1:634-646. [PMC free article] [PubMed] [Google Scholar]
- 158. Horiuchi S, Sakamoto Y, Sakai M. Scavenger receptors for oxidized and glycated proteins. Amino Acids. 2003;25:283-292. [DOI] [PubMed] [Google Scholar]
- 159. Jono T, Miyazaki A, Nagai R, Sawamura T, Kitamura T, Horiuchi S. Lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) serves as an endothelial receptor for advanced glycation end products (AGE). FEBS Lett. 2002;511:170-174. [DOI] [PubMed] [Google Scholar]
- 160. Chen M, Nagase M, Fujita T, Narumiya S, Masaki T, Sawamura T. Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: possible role of LOX-1 ligand and AGE. Biochem Biophys Res Commun. 2001;287:962-968. [DOI] [PubMed] [Google Scholar]
- 161. Hegab Z, Gibbons S, Neyses L, Mamas MA. Role of advanced glycation end products in cardiovascular disease. World J Cardiol. 2012;4:90-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Franke S, Dawczynski J, Strobel J, Niwa T, Stahl P, Stein G. Increased levels of advanced glycation end products in human cataractous lenses. J Cataract Refract Surg. 2003;29:998-1004. [DOI] [PubMed] [Google Scholar]
- 163. Krieg UC, Johnson AE, Walter P. Protein translocation across the endoplasmic reticulum membrane: identification by photocross-linking of a 39-kD integral membrane glycoprotein as part of a putative translocation tunnel. J Cell Biol. 1989;109:2033-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Nicholl ID, Bucala R. Advanced glycation endproducts and cigarette smoking. Cell Mol Biol. 1998;44:1025-1033. [PubMed] [Google Scholar]
- 165. Nicholl D, Stitt AW, Moore JE, Ritchie AJ, Archer DB, Bucala R. Increased levels of advanced glycation endproducts in the lenses and blood vessels of cigarette smokers. Mol Med. 1998;4:594-601. [PMC free article] [PubMed] [Google Scholar]
- 166. Pokupec R, Kalauz M, Turk N, Turk Z. Advanced glycation endproducts in human diabetic and non-diabetic cataractous lenses. Graefes Arch Clin Exp Ophthalmol. 2003;241:378-384. [DOI] [PubMed] [Google Scholar]
- 167. Wright KJ, Thomas MM, Betik AC, Belke D, Hepple RT. Exercise training initiated in late middle age attenuates cardiac fibrosis and advanced glycation end-product accumulation in senescent rats. Exp Gerontol. 2014;50:9-18. [DOI] [PubMed] [Google Scholar]
- 168. Steppan J, Tran H, Benjo AM, et al. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol. 2012;47:565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kotania K, Caccavello R, Sakane N, Yamada T, Taniguchi N, Gugliucci A. Influence of physical activity intervention on circulating soluble receptor for advanced glycation end products in elderly subjects. J Clin Med Res. 2011;3:252-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Santilli F, Vazzana N, Iodice P, et al. Effects of high-amount-high-intensity exercise on in vivo platelet activation: modulation by lipid peroxidation and AGE/RAGE axis. Thromb Haemost. 2013;110:1232-1240. [DOI] [PubMed] [Google Scholar]
- 171. Choi KM, Han KA, Ahn HJ, et al. Effects of exercise on sRAGE levels and cardiometabolic risk factors in patients with type 2 diabetes: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97:3751-3758. [DOI] [PubMed] [Google Scholar]
- 172. Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hull GL, Woodside JV, Ames JM, Cuskelly GJ. N-carboyxmethyl lysine content of foods commonly consumed in a Western-style diet. Food Chem. 2012;131:170-174. [Google Scholar]
- 174. Requena JR, Ahmed MU, Fountain CW, Degenhardt TP, Reddy S, Perez C. Carboxyethylethanolamine, a biomarker of phospholipids modification during the Maillard reaction in vivo. J Biol Chem. 1997;272:17453-17479. [DOI] [PubMed] [Google Scholar]
- 175. Birlouez-Aragon I, Locquet N, de St Louvent E, Bouveresse DJ, Stahl P. Evaluation of the Maillard reaction in infant formulas by means of front-face fluorescence. Ann N Y Acad Sci. 2005;1043:308-318. [DOI] [PubMed] [Google Scholar]
- 176. Sebeková K, Saavedra G, Zumpe C, Somoza V, Klenovicsová K, Birlouez-Aragon I. Plasma concentration and urinary excretion of N-epsilon-(carboxymethyl)lysine in breast milk- and formula-fed infants. Ann N Y Acad Sci. 2008;1126:177-180. [DOI] [PubMed] [Google Scholar]
- 177. Assar SH, Moloney C, Lima M, Magee R, Ames JM. Determination of N-(carboxymethyl) lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino Acids. 2009;36:317-326. [DOI] [PubMed] [Google Scholar]
- 178. Delgado-Andrade C, Rufian-Henares JA, Morales FJ. Study on fluorescence of Maillard reaction components in breakfast cereals. Mol Nutr Food Res. 2006;50:799-804. [DOI] [PubMed] [Google Scholar]
- 179. Delgado-Andrade C, Seiquer I, Navarro MP, Morales FJ. Maillard reaction indicators in diets usually consumed by the adolescent population. Mol Nutr Food Res. 2007;51:341-351. [DOI] [PubMed] [Google Scholar]
- 180. Drusch S, Faist V, Erbersdobler HF. Determination of N-carboxymethyllysine in milk products by a modified reversed phase HPLC method. Food Chem. 1998;65:547-553. [Google Scholar]
- 181. Yaacoub R, Saliba R, Nsouli B, Khalaf G, Birlouez-Aragon I. Formation of lipid oxidation and isomerization products during processing of nuts and sesame seeds. J Agri Food Chem. 2008;56:7082-7090. [DOI] [PubMed] [Google Scholar]
- 182. McCarty MF. The low-AGE content of low-fat vegan diets could benefit diabetics—though concurrent taurine supplementation may be needed to minimize endogenous AGE production. Med Hypotheses. 2005;64:394-398. [DOI] [PubMed] [Google Scholar]
- 183. Tan Y, Wang CY, Lo S, Sang CT. Methylglyoxal: its presence in beverages and potential scavengers. N Y Acad Sci. 2008;1126:72-75. [DOI] [PubMed] [Google Scholar]
- 184. Cai W, Gao QD, Zhu L, Peppa M, He C, Vlassara H. Oxidative stress-inducing carbonyl compounds from common foods: novel mediators of cellular dysfunction. Mol Med. 2002;8:337-346. [PMC free article] [PubMed] [Google Scholar]
- 185. Ahmed MS, Ahmed N. Antiglycation properties of aged garlic extract: possible role in prevention of diabetic complications. J Nutr. 2006;136:796S-799S. [DOI] [PubMed] [Google Scholar]
- 186. Kazeem MI, Akanji MA, Hafizur RM, Choudhary MI. Antiglycation, antioxidant and toxicological potential of polyphenol extracts of alligator pepper, ginger and nutmeg from Nigeria. Asian Pac J Trop Biomed. 2012;2:727-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Gutírrez RMP, Diaz SL, Reyes IC, Gonzalez AMN. Anti-glycation effect of spices and chilies uses in traditional Mexican cuisine. J Nat Prod. 2010;3:95-102. [Google Scholar]
- 188. Kousar S, Sheikh MA, Asghar M, Rashid R. Effect of onion (Allium cepa L.) extract on Maillard reaction under in vitro conditions. Pak J Agric Sci. 2008;45:103-106. [Google Scholar]
- 189. Xi M, Hai C, Tang H, et al. Antioxidant and antiglycation properties of triterpenoid saponins from Aralia taibaiensis traditionally used for treating diabetes mellitus. Redox Rep. 2010;15:20-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Majumdar M, Parihar PS. Antibacterial, antioxidant and antiglycation potential of Costus pictus from Southern region, India. Asian J Plant Sci Res. 2012;2:95-101. [Google Scholar]
- 191. Mukherjee S, Phatak D, Parikh J, Jagtap S, Shaikh S, Tupe R. Antiglycation and antioxidant activity of a rare medicinal orchid Dendrobium aqueum Lindl. Med Chem Drug Dis. 2012;2:46-54. [Google Scholar]
- 192. Harsulkar A, Jagtap S, Narkhede A, Nirmal P, Tupe R, Kulkarni O. In vitro antioxidant, Antiglycation and a-amylase inhibitory potential of Eulophiaochreata L. J Pharm Res. 2012;5:2532-2537. [Google Scholar]
- 193. Yao Y, Cheng XZ, Wang LX, Wang SH, Ren G. Major phenolic compounds, antioxidant capacity and antidiabetic potential of rice bean (Vigna umbellata L.) in China. Int J Mol Sci. 2012;13:2707-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Ma M, Hong CL, An SQ, Li B. Seasonal, spatial, and interspecific variation in quercetin in Apocynum venetum and Poacynum hendersonii, Chinese traditional herbal teas. J Agric Food Chem. 2003;51:2390-2393. [DOI] [PubMed] [Google Scholar]
- 195. Yan SJ, Wang L, Li Z, et al. Inhibition of advanced glycation end product formation by Puerh tea ameliorates progression of experimental diabetic nephropathy. J Agric Food Chem. 2012;60:4102-4110. [DOI] [PubMed] [Google Scholar]
- 196. Lunceford N, Gugliucci A. Ilex paraguariensis extracts inhibit AGE formation more efficiently than green tea. Fitoterapia. 2005;76:419-427. [DOI] [PubMed] [Google Scholar]
- 197. Roehrs M, Figueiredo CG, Zanchi MM, et al. Bixin and Norbixin have opposite effects on glycemia, lipidemia, and oxidative stress in streptozotocin-induced diabetic rats. Int J Endocrinol. 2014;2014:839095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Awasthia S, Saraswathi NT. Silybin, a flavonolignan from milk thistle seeds, restrains the early and advanced glycation end product modification of albumin. RSC Adv. 2015;5:87660-87666. [Google Scholar]
- 199. Jariyapamornkoon N, Yibchok-Anun S, Adisakwattana S. Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement Altern Med. 2013;13:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Hui YY, McAmis WC, Baynes JW, Schaeffer RC, Jr, Wolf MB. Effect of advanced glycation end products on oxidative stress in endothelial cells in culture: a warning on the use of cells studied in serum-free media. Diabetologia. 2001;44:1310-1317. [DOI] [PubMed] [Google Scholar]
- 201. Jang DS, Lee GY, Kim YS, et al. Anthraquinones from the seeds of Cassia tora with inhibitory activity on protein glycation and aldose reductase. Biol Pharm Bull. 2007;30;2207-2210. [DOI] [PubMed] [Google Scholar]
- 202. Chandler D, Woldu A, Rahmadi A, et al. Effects of plant-derived polyphenols on TNF-alpha and nitric oxide production induced by advanced glycation endproducts. Mol Nutr Food Res. 2010;54:S141-S150. [DOI] [PubMed] [Google Scholar]
- 203. Jedsadayanmata A. In vitro antiglycation activity of arbutin. Nasresuan Univ J. 2005;13:35-41. [Google Scholar]
- 204. Wu D, Wen W, Qi CL, et al. Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine. 2012;19:712-718. [DOI] [PubMed] [Google Scholar]
- 205. Hao M, Li SY, Sun CK, et al. Amelioration effects of berberine on diabetic microendothelial injury model by the combination of high glucose and advanced glycation end products in vitro. Eur J Pharmacol. 2011;654:320-325. [DOI] [PubMed] [Google Scholar]
- 206. O’Brien P, Carrasco-Pozo C, Speisky H. Boldine and its antioxidant or health-promoting properties. Chem Biol Interact. 2006;159:1-17. [DOI] [PubMed] [Google Scholar]
- 207. Budzeń S, Rymaszewska J. The biological role of carnosine and its possible applications in medicine. Adv Clin Exp Med. 2013;22:739-744. [PubMed] [Google Scholar]
- 208. Sajithlal GB, Chithra P, Chandrakasan G. Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochem Pharmacol. 1998;56:1607-1614. [DOI] [PubMed] [Google Scholar]
- 209. Cervantes-Laurean D, Schramm DD, Jacobson EL, Halaweish I, Bruckner GG, Boissonneault GA. Inhibition of advanced glycation end product formation on collagen by rutin and its metabolites. J Nutr Biochem. 2006;17:531-540. [DOI] [PubMed] [Google Scholar]
- 210. Nagasawa T, Tabata N, Ito Y, Nishizawa N, Aiba Y, Kitts DD. Inhibition of glycation reaction in tissue protein incubations by water soluble rutin derivative. Mol Cell Biochem. 2003;249:3-10. [PubMed] [Google Scholar]
- 211. Nagasawa T, Tabata N, Ito Y, Aiba Y, Nishizawa N, Kitts DD. Dietary G-rutin suppresses glycation in tissue proteins of streptozotocin-induced diabetic rats. Mol Cell Biochem. 2003;252:141-147. [DOI] [PubMed] [Google Scholar]
- 212. Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa H. Antioxidative and antiglycation activity of garcinol from Garcinia indica fruit rind. J Agri Food Chem. 2000;48:180-185. [DOI] [PubMed] [Google Scholar]
- 213. Lv L, Shao X, Chen H, Ho CT, Sang S. Genistein inhibits advanced glycation end product formation by trapping methylglyoxal. Chem Res Toxicol. 2011;24:579-586. [DOI] [PubMed] [Google Scholar]
- 214. Zhang Z, Sethiel MS, Shen W, Liao S, Zou Y. Hyperoside downregulates the receptor for advanced glycation end products (RAGE) and promotes proliferation in ECV304 cells via the c-Jun N-terminal kinases (JNK) pathway following stimulation by advanced glycation end-products in vitro. Int J Mol Sci. 2013;14:22697-22707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Alderson NL, Chachich ME, Youssef NN, et al. The AGE inhibitor pyridoxamine inhibits lipemia and development of renal and vascular disease in Zucker obese rats. Kidney Int. 2003;63:2123-2133. [DOI] [PubMed] [Google Scholar]
- 216. Almeida F, Santos-Silva D, Rodrigues T, et al. Pyridoxamine reverts methylglyoxal-induced impairment of survival pathways during heart ischemia. Cardiovasc Ther. 2013;31:e79-e85. [DOI] [PubMed] [Google Scholar]
- 217. Chetyrkin SV, Zhang W, Hudson BG, Serianni AS, Voziyan PA. Pyridoxamine protects proteins from functional damage by 3-deoxyglucosone: mechanism of action of pyridoxamine. Biochemistry. 2008;7:997-1006. [DOI] [PubMed] [Google Scholar]
- 218. Kang Z, Li H, Li G, Yin D. Reaction of pyridoxamine with malondialdehyde: mechanism of inhibition of formation of advanced lipoxidation end-products. Amino Acids. 2006;30:55-61. [DOI] [PubMed] [Google Scholar]
- 219. Nagaraj RH, Sarkar P, Mally A, Biemel KM, Lederer MO, Padayatti PS. Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Arch Biochem Biophys. 2002;402:110-119. [DOI] [PubMed] [Google Scholar]
- 220. Li X, Zheng T, Sang S, Lv L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J Agric Food Chem. 2014;62:12152-12158. [DOI] [PubMed] [Google Scholar]
- 221. Khazaei M, Karimi J, Sheikh N, et al. Effects of resveratrol on receptor for advanced glycation end products (RAGE) expression and oxidative stress in the liver of rats with type 2 diabetes. Phytother Res. 2016;3:66-71. [DOI] [PubMed] [Google Scholar]
- 222. Xi M, Hai C, Tang H, et al. Antioxidant and antiglycation properties of triterpenoid saponins from Aralia taibaiensis traditionally used for treating diabetes mellitus. Redox Rep. 2010;15:20-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Chauhan B, Kumar G, Kalam N, Ansari SH. Current concepts and prospects of herbal nutraceutical: a review. J Adv Pharm Technol Res. 2013;4:4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Schaumburg H, Kaplan J, Windebank A, et al. Sensory neuropathy from pyridoxine abuse—a new megavitamin syndrome. N Engl J Med. 1983;309:445-448. [DOI] [PubMed] [Google Scholar]
- 225. Alkhalaf A, Kleefstra N, Groenier KH, et al. Effect of benfotiamine on advanced glycation endproducts and markers of endothelial dysfunction and inflammation in diabetic nephropathy. PLoS One. 2012;7:e40427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Schupp N, Dette EM, Schmid U, et al. Benfotiamine reduces genomic damage in peripheral lymphocytes of hemodialysis patients. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:283-291. [DOI] [PubMed] [Google Scholar]
- 227. Stirban A, Negrean M, Stratmann B, et al. Benfotiamine prevents macro and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care. 2006;29:2064-2071. [DOI] [PubMed] [Google Scholar]
- 228. Du X, Edelstein D, Brownlee M. Oral benfotiamine plus alpha-lipoicacid normalises complication-causing pathways in type 1 diabetes. Diabetologia. 2008;51:1930-1932. [DOI] [PubMed] [Google Scholar]
- 229. Budoff M. Aged garlic extract retards progression of coronary artery calcification. J Nutr. 2006;136:741S-744S. [DOI] [PubMed] [Google Scholar]
- 230. Budoff MJ, Ahmadi N, Gul KM, et al. Aged garlic extract supplemented with B vitamins, folic acid and l-arginine retards the progression of subclinical atherosclerosis: a randomized clinical trial. Prev Med. 2009;49:101-107. [DOI] [PubMed] [Google Scholar]
- 231. Zeb I, Ahmadi N, Nasir K, et al. Aged garlic extract and coenzyme Q10 have favorable effect on inflammatory markers and coronary atherosclerosis progression: a randomized clinical trial. J Cardiovasc Dis Res. 2012;3:185-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Freedman BI, Wuerth JP, Cartwright K, et al. Design and baseline characteristics for the Aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Control Clin Trials. 1999;20:493-510. [DOI] [PubMed] [Google Scholar]
- 233. Bolton WK, Cattran DC, Williams ME, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24:32-40. [DOI] [PubMed] [Google Scholar]
- 234. Kass DA, Shapiro EP, Kawaguchi M, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464-1470. [DOI] [PubMed] [Google Scholar]
- 235. Hartog JW, Willemsen S, van Veldhuisen DJ, et al. Effects of alagebrium, an advanced glycation endproduct breaker, on exercise tolerance and cardiac function in patients with chronic heart failure. Eur J Heart Fail. 2011;13:899-908. [DOI] [PubMed] [Google Scholar]
- 236. Zieman SJ, Melenovsky V, Clattenburg L, et al. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. J Hypertens. 2007;25:577-583. [DOI] [PubMed] [Google Scholar]
- 237. Little WC, Zile MR, Kitzman DW, Hundley WG, O’Brien TX, Degroof RC. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail. 2005;11:191-195. [DOI] [PubMed] [Google Scholar]
- 238. Ahmad S, Shahab U, Baig MH, et al. Inhibitory effect of metformin and pyridoxamine in the formation of early, intermediate and advanced glycation end-products. PLoS One. 2013;8:e72128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Diamanti-Kandarakis E, Alexandraki K, Piperi C, et al. Effect of metformin administration on plasma advanced glycation end product levels in women with polycystic ovary syndrome. Metabolism. 2007;56:129-134. [DOI] [PubMed] [Google Scholar]
- 240. Gugliucci A, Bendayan M. Renal fate of circulating advanced glycated end products (AGE): evidence for reabsorption and catabolism of AGE-peptides by renal proximal tubular cells. Diabetologia. 1996;39:149-160. [DOI] [PubMed] [Google Scholar]