Abstract
Intestinal cancer is a disease with high morbidity and high mortality in China. Previous studies have shown that Codonopsis foetens can inhibit cellular autophagy and promote the apoptosis of intestine cancer cells. Based on metabolomics method coupled with liquid chromatography-mass spectrometry (LC-MS) technology, we aimed to analyze intestinal small molecule metabolites in the intestinal cancer model group and the Codonopsis foetens treated group. Principal component analysis (PCA) and Partial Least Squares (PLS-DA) were used to identify the pattern of the data. And the metabolic characteristics of the cancer model group were explored based on the metabolic differences between the groups. Multivariate statistical analysis revealed that metabolites presented with differences included: Acetamide, Phosphoric acid, Hydrogen sulfite, Pyruvic acid, Cytosine, 2-Hydroxypyridine, Phosphoric acid, Uracil, Gamma-Aminobutyric acid, Glycerol alpha-monochlorohydrin, Thiosulfic acid, L-Valine, Cysteamine, Taurine, Creatine, Homocysteine, Hypoxanthine, Se-Methylselenocysteine, 5-Hydroxymethyluracil, Oxoglutaric acid, LysoPC(20:0), LysoPC(22:4(7Z,10Z,13Z,16Z)), LysoPC(18:2(9Z,12Z)), LysoPC(16:1(9Z)), LysoPE(0:0/16:0), LysoPE(0:0/18:2(9Z,12Z)), LysoPE(18:0/0:0), LysoPE(20:1(11Z)/0:0), etc. Combined with metabolic pathway analysis, pathways presented with differences included: Citrate cycle (TCA cycle), ABC transporters, 2-Oxocarboxylic acid metabolism, Taurine and hypotaurine metabolism, Butanoate metabolism), Phenylalanine, tyrosine and tryptophan biosynthesis, Biosynthesis of amino acids, Protein digestion and absorption, Aminoacyl-tRNA biosynthesis, C5-Branched dibasic acid metabolism, GABAergic synapse, Proximal tubule bicarbonate reclamation, Mineral absorption, Phenylalanine metabolism. The results showed that the proliferation of intestinal cancer cells caused cell metabolism disorders, manifesting as changes in metabolic pathways and resulting in changes in metabolites.
Keywords: Codonopsis foetens, Intestinal cancer, Metabonomics, Metabolites, Metabolic pathway
1. Introduction
According to data from disease center, intestine cancer is a disease with high morbidity and high mortality. Rectum is the predominant predilection site of intestine cancer, followed by sigmoid and others (Yu et al., 2016, Jemal et al., 2011, Nordin et al., 2018). Originated in the 1970s, metabonomics technology provides a new perspective for tumor research. In recent years, diagnosis and treatment of tumor have become the focus of research in life science. Metabolomics refers to the overall change of metabolites during a certain period, directly reflecting the final state of biological systems, which is particularly suitable for analyzing the changes of metabolic substances in the body caused by the synthesis and release of tumor cells in tumorigenesis and proliferation processes (Zhang and Wu, 2017, Sun et al., 2017, Qiao, 2018).
Preliminary research had demonstrated that long-term constipation could lead to intestinal cancer (Luan et al., 2016, Xiao and Liu, 2018). A group researchers performed a research on the effects of Codonopsis foetens on human colon cancer HCT116 and SW480 cell lines, of which results showed that Codonopsis foetens inhibited cell autophagy and induced apoptosis of colon cancer cells via activating the NF-κB pathway and promoting nuclear transportation of P65 (Luan et al., 2018, Zhao and Li, 2018).
In the early stage, models of intestinal cancer and Codonopsis foetens treatment model were established. Long-term constipation-induced intestinal cancer is a long-term accumulation process. This study focuses on long-term constipation induced intestinal cancer (CCa), long-term constipation induced intestinal cancer with treatment group (CCaT) and blank control group(B) and perform metabonomic analysis.
Therefore, the present study was based on liquid chromatography-mass spectrometry (LC-MS) coupled with metabolomics. Combined with bioinformatics analysis technology, we aimed to identify potential biomarkers and analyze their metabolic pathways, laying a certain foundation for revealing the mechanisms underlying long-term constipation induced cancer and the pharmacological action of Codonopsis foetens.
2. Materials and methods
2.1. The construction of long-term constipation model, intestinal cancer model and Codonopsis foetens treatment model in mice
21 healthy Kunming mice were routinely fed in laboratory for 3 days and later designated into 7 groups, including blank group (B), 1,2-dimethylhydrazine induced intestinal cancer group (CaD), long-term constipation group (C), long-term constipation induced intestinal cancer group (CCa), 1,2-dimethylhydrazine induced intestinal cancer with treatment group (CaDT), long-term constipation with treatment group (CT), long-term constipation induced intestinal cancer with treatment group (CCaT). According to methods mentioned in references, 2.5 mg/(Kg d) of loperamide hydrochloride were administered orally by gavage to mice in all groups except for blank group, constructing constipation mice model, while mice in blank group were gavaged with equal amounts of saline. Gastrogavage was performed for 2 consecutive weeks, and successful construction of constipation model was confirmed with intestine propelling rates and defecation rates. 1,2-dimethylhydrazine were injected intraperitoneally to mice of CaD group and successful construction of intestinal cancer model was confirmed by pathomorphology 6 weeks later.
To construct 3 Codonopsis foetens treatment groups, namely the 1,2-dimethylhydrazine induced intestinal cancer with treatment group (CaDT), long-term constipation with treatment group (CT) and long-term constipation induced intestinal cancer with treatment group (CCaT), total extract of Codonopsis foetens were given by gavage to mice of 1,2-dimethylhydrazine induced intestinal cancer group (CaD), long-term constipation group (C) and long-term constipation induced intestinal cancer group (CCa).
2.2. Metabolite extraction
500 μL of bacteria solution was obtained and volatilized to dryness. 300 μL of methanol-water = 4:1 (V/V) was added to reconstitute, then solution was eddied for 30 s, sonicated for 3 min in an ice-water bath and centrifuged for 10 min at low temperature (14,000 rpm, 4 °C). 180 μL of supernatant was loaded into an LC-MS vial with liner and analyzed by LC-MS.
2.3. Metabolite detection
The instrument platform for LC-MS analysis was Ultra-High-Performance Liquid Chromatography Tandem Time-of-Flight Mass Spectrometry UPLC-Q-TOF/MS from Waters. The chromatographic conditions were listed as follows: column BEH C18; mobile phase A was water (containing 0.1% formic acid) and mobile phase B was acetonitrile (containing 0.1% formic acid).
The flow rate was 0.40 mL/min, the injection volume was 3 μL, and the column temperature was 50 °C. The mass spectrometry conditions were as follows: positive and negative ion scan modes was adopted for mass spectrometry signal acquisition of the sample. The electrospray capillary voltage, injection voltage and collision voltage were 1.0 kV, 40 V and 6 eV, respectively. The ion source temperature and solvent temperature were 120 °C and 500 °C; carrier gas flow rate: 900L/h; mass spectrum scanning range: 50–1000 m/z; scan time and interval time: 0.1 s and 0.02 s, respectively.
2.4. Data analysis
Baseline filtering, peak identification, integration, retention time correction, peak alignment, and normalization were performed on the raw data obtained after mass spectrometry analysis to finally obtain a data matrix of retention time, mass-to-charge ratio, and peak intensity. According to the characteristics of biomarkers under the liquid phase chromatography and mass spectrometry conditions, it was finally confirmed by comparing with the standard and database. Multivariate statistical analysis (PCA analysis, PLS-DA analysis, OPLS-DA) was performed using the normalized data matrix. By using a combination of multivariate statistical analysis of OPLS-DA and univariate statistical analysis, differential metabolites were screened.
3. Results and analysis
3.1. Preliminary research showed that long-term constipation could induce intestine tumor.
Preliminary research was based on long-term constipation group (C) induced by loperamide hydrochloride and blank group (B). Successful construction of intestinal cancer model was confirmed by pathomorphology (showed at Fig. 1, Fig. 2 and Fig. 3) (see Table 1).
Fig. 1.

Typical picture of constipation model (fecal was solid and aggregated in mass in intestinal tract).
Fig. 2.

Typical picture of intestinal tumor morphology (arrow showed macroscopic sarcoma in intestinal tract).
Fig. 3.

Intestinal tumor section under 40X magnification (pathological section of intestinal tumor cells, with varied cellular size and morphology, irregular nuclear, denser coloration, revealing different characteristics from maternal cells).
Table 1.
Intestinal propulsion rates of mice in constipation model group (C) and blank group (B).
| Total length of small intestine | The length of carbon particle propelling | Propulsion rate (%) | |
|---|---|---|---|
| C | 57 | 29 | 50.88 |
| B | 59 | 55 | 93.22 |
3.2. Base peak chromatogram under typical positive and negative ion modes of intestinal cancer group and Codonopsis foetens treatment group
Base Peak Chromatogram (BPC) was first visually examined for all samples. Fig. 4, Fig. 5 show base peak chromatogram of quality control samples under typical positive and negative ion modes. The results showed that all samples had strong signals, large peak capacity and reproducible retention time.
Fig. 4.
Base peak chromatogram under positive ion mode.
Fig. 5.
Base peak chromatogram under negative ion mode.
3.3. Principal component analysis of intestinal cancer group and Codonopsis foetens treatment group
PCA analysis is an unsupervised multidimensional statistical analysis method that can reflect the overall metabolic difference between samples and the variability between samples within the group, as shown in Fig. 6. From Fig. 6, we can see that samples from control group, intestinal cancer group and Codonopsis foetens treatment group have a better separation, indicating significant changes of metabolic profiles in intestinal cancer group and Codonopsis foetens treatment group. All the metabolites were normalized and then analyzed by cluster heat map, as shown in Fig. 7. From Fig. 7, the metabolite expression patterns in all samples are visually displayed.
Fig. 6.
PCA analysis scores plot of different model groups.
Fig. 7.

Cluster heat map of all metabolites in different model groups.
3.4. Screen for different metabolites between intestinal cancer group and Codonopsis foetens treatment group
Metabolites of intestinal cancer group and Codonopsis foetens treatment group were distinguished by PCA method. Then loading plots of samples from different groups were obtained, which contributes to identifying the most influential metabolites that would change the metabolites model between groups. Combining the VIP values from multivariate statistical analysis of OPLS-DA and the P values from univariate statistical analysis T-test, we could screen for significant differences in metabolites between different groups, and the number of metabolites presenting with either up-regulation or down-regulation is shown in Fig. 8. Results from multivariate statistical analysis showed that metabolites presented with differences included: Acetamide, Phosphoric acid, Hydrogen sulfite, Pyruvic acid, Cytosine, 2-Hydroxypyridine, Phosphoric acid, Uracil, Gamma-Aminobutyric acid, Glycerol alpha-monochlorohydrin, Thiosulfic acid, L-Valine, Cysteamine, Taurine, Creatine, Homocysteine, Hypoxanthine, O-Phosphoethanolamine, Hydroxidodioxidosulfidosulfate, L-Methionine, 2-Vinylthiophene, 3-hydroxy-cis,cis-muconic acid, (±)-2-Heptanol, DL-2-Aminooctanoic acid, Taurine, Phenol sulphate, Se-Methylselenocysteine, 5-Hydroxymethyluracil, Oxoglutaric acid, LysoPC(20:0), LysoPC(22:4(7Z,10Z,13Z,16Z)), LysoPC(18:2(9Z,12Z)), LysoPC(16:1(9Z)), LysoPE(0:0/16:0), LysoPE(0:0/18:2(9Z,12Z)), LysoPE(18:0/0:0), LysoPE(20:1(11Z)/0:0).
Fig. 8.
Statistical graph of different metabolites in different model groups.
3.5. Enrichment analysis of metabolic pathways in intestinal cancer group and Codonopsis foetens treatment group
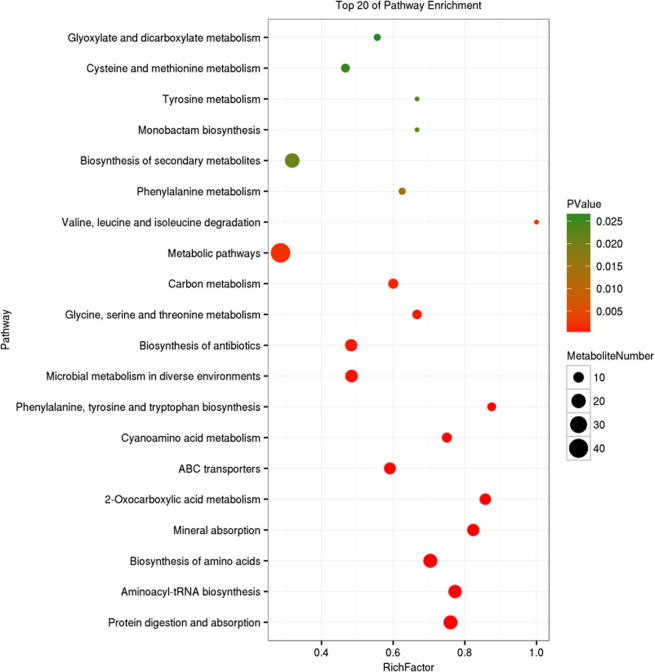
Different metabolites were mapped in the KEGG database in search for metabolic pathways. KO enrichment analysis bubble chart of metabolic pathways of Group B and Group CCa were shown in Fig. 9. KO enrichment analysis bubble chart of metabolic pathways of Group CCa and Group CCaT were shown in Fig. 10. Metabolic pathways presented with differences shown in Figs. 9 and 10 includes: Citrate cycle (TCA cycle), ABC transporters, 2-Oxocarboxylic acid metabolism, Taurine and hypotaurine metabolism, Butanoate metabolism, Phenylalanine, tyrosine and tryptophan biosynthesis, Biosynthesis of amino acids, Protein digestion and absorption, Aminoacyl-tRNA biosynthesis, C5-Branched dibasic acid metabolism, GABAergic synapse, Proximal tubule bicarbonate reclamation, Mineral absorption, Phenylalanine metabolism, Glucagon signaling pathway, Arginine and proline metabolism, Glucagon signaling pathway, Alanine, aspartate and glutamate metabolism, Biosynthesis of antibiotics, D-Glutamine and D-glutamate metabolism, Protein digestion and absorption, Aminoacyl-tRNA biosynthesis, Mineral absorption, Cyanoamino acid metabolism, Microbial metabolism in diverse environments, Biosynthesis of antibiotics, Glycine, serine and threonine metabolism, Carbon metabolism, Metabolic pathways, Valine, leucine and isoleucine degradation, Biosynthesis of secondary metabolites, Tyrosine metabolism, Monobactam biosynthesis, Cysteine and methionine metabolism, Glyoxylate and dicarboxylate metabolism, Valine, leucine and isoleucine biosynthesis, Thyroid hormone synthesis, Glutathione metabolism, Dopaminergic synapse, Melanogenesis, Prolactin signaling pathway.
Fig. 9.
KO enrichment analysis bubble chart of metabolic pathways of Group B and Group CCa.
Fig. 10.
KO Enrichment analysis bubble chart of metabolic pathways of Group CCa and Group CCaT.
4. Discussion
In this research, by comparing different intestinal cancer groups, intestinal cancer treatment groups and control group, we showed that metabolites presented with differences included: Acetamide, Phosphoric acid, Hydrogen sulfite, Pyruvic acid, Cytosine, 2-Hydroxypyridine, Phosphoric acid, Uracil, Gamma-Aminobutyric acid, Glycerol alpha-monochlorohydrin, Thiosulfic acid, L-Valine, Cysteamine, Taurine, Creatine, Homocysteine, Hypoxanthine, O-Phosphoethanolamine, Hydroxidodioxidosulfidosulfate, L-Methionine, 2-Vinylthiophene, 3-hydroxy-cis,cis-muconic acid, (±)-2-Heptanol, DL-2-Aminooctanoic acid, Taurine, Phenol sulphate, Se-Methylselenocysteine, 5-Hydroxymethyluracil, Oxoglutaric acid, LysoPC(20:0), LysoPC(22:4(7Z,10Z,13Z,16Z)), LysoPC(18:2(9Z,12Z)), LysoPC(16:1(9Z)), LysoPE(0:0/16:0), LysoPE(0:0/18:2(9Z,12Z)), LysoPE(18:0/0:0), LysoPE(20:1(11Z)/0:0). These metabolites belong to different metabolic pathways: glucose metabolism, amino acid metabolism, lipid metabolism and mitochondrial biosynthesis, etc.
4.1. Glucose metabolism of Codonopsis foetens induced apoptosis of intestinal cancer cells
The rapid proliferation of tumor cells is energy-consuming. It has been found that the transformation of normal cells into tumor cells is accompanied by the remodeling of energy metabolism pathways. Most typically, energy supply of tumor cells is mainly dependent on the glycolytic pathway even in the presence of oxygen. These kind of remodeling is called aerobic glycolysis or the Warburg effect. Such change could provide abundant energy for the rapid growth of tumor cells, which is also an adaptive changes of tumor cells to the living environment under stress conditions. That is, to establish a foundation for tumor cells to adapt to changes in the microenvironment (Vander Heiden et al., 2009, Hu et al., 2018, Reddy and Aqueel, 2018).
4.2. Amino acid metabolic pathways
The up-regulation of a series of amino acids suggests a perturbation of the amino acid transportability during metabolism, which may be to meet the large amount of energy required for tumor growth, or to meet the demand for substances during the rapid growth of tumor cells (Hu et al., 2018, Zeeshan et al., 2018a, Zeeshan et al., 2018b).
The level of amino acids (especially His, Lys, Arg) was significantly increased in the intestinal cancer model group compared with that of control group, indicating disorder in the amino acid metabolism in cancer model group. Then cell lesion occurred, causing diseases. Compared with the intestinal cancer model group, the level of amino acid in the treatment group decreased, indicating that Codonopsis foetens can affect the metabolites by regulating the amino acid metabolism pathway, displaying therapeutic effects.
4.3. Lipid metabolic pathways
Piles of basic researches, clinical and interventional studies, and epidemiological investigations have shown that abnormal lipid metabolism is an important factor in the development of tumors (Sa and Wu, 2016, Shareef and Akhtar, 2018). LysoPC (20:0), LysoPC (22:4 (7Z, 10Z, 13Z, 16Z)), LysoPC (18:2 (9Z, 12Z)) and LysoPC (16:1 (9Z)) are lysophosphatidylcholine, LysoPE (22:0/0:0) and LysoPE (0:0/20:0) are lysophosphatidylethanolamine. Both lysophosphatidylcholine and lysophosphatidylethanolamine showed an increasing tendency in the intestinal cancer model group compared with the control group, indicating that their metabolic disorders could lead to the occurrence of cell lesions, which in turn would cause intestinal tumors. After treatment of Codonopsis foetens, the level of lipids were down-regulated, which were close to those of the control group. Such tendency indicated that the Codonopsis foetens regulates the lipid metabolic pathway, displaying significant therapeutic effects on cancer.
Changes of cellular metabolism is an important feature of tumors, and it interacts as both cause and effect of tumor occurrence and development. The occurrence and development of tumor will cause disorders in various metabolic pathways, such as the glycometabolism pathway, mitochondrial biosynthesis, amino acid metabolism, lipid metabolism and others (Zhou et al., 2016, Luan et al., 2017, Zeeshan et al., 2018a, Zeeshan et al., 2018b). In this study, the metabolites of the cancer model group and the control group also changed correspondingly. And such changes in the treatment group were close to those of the control group. Given that previous study had demonstrated Codonopsis foetens could promote apoptosis of colon cancer cells, subsequent studies are need to investigate transcriptomics, proteomics and metabolomics and further explore its mechanisms of action.
5. Conclusions
Through metabonomics analysis of intestinal cancer group, control group and Codonopsis foetens treatment group, we searched for different metabolites and mapped them on different metabolic pathways. Of those, metabolites presented with differences included: Acetamide, Phosphoric acid, Hydrogen sulfite, Pyruvic acid, Cytosine, 2-Hydroxypyridine, Phosphoric acid, Uracil, Gamma-Aminobutyric acid, Glycerol alpha-monochlorohydrin, Thiosulfic acid, L-Valine, Cysteamine, Taurine, Creatine, Homocysteine, Hypoxanthine, LysoPC(20:0), LysoPC(22:4(7Z,10Z,13Z,16Z)), LysoPC(18:2(9Z,12Z)), LysoPC(16:1(9Z)), LysoPE(0:0/16:0), LysoPE(0:0/18:2(9Z,12Z)), LysoPE(18:0/0:0), LysoPE(20:1(11Z)/0:0). These metabolites belong to different metabolic pathways, such as glucose metabolism, amino acid metabolism, lipid metabolism and mitochondrial biosynthesis. In the level of metabolic pathways, Metabolic pathways presented with differences shown in Fig. 9 and Fig. 10 includes: Citrate cycle (TCA cycle), ABC transporters, 2-Oxocarboxylic acid metabolism, Taurine and hypotaurine metabolism, Butanoate metabolism, Phenylalanine, tyrosine and tryptophan biosynthesis, Biosynthesis of amino acids, Protein digestion and absorption, Aminoacyl-tRNA biosynthesis, Phenylalanine metabolism.
In conclusion, we demonstrated that the proliferation of intestinal cancer cells would cause metabolic disorders, manifesting as changes in metabolic pathways, and thus leading to changes in metabolites.
Acknowledgements
This study was funded by National Natural Science Foundation of China (NSFC) (61363061/31660029) and Advanced and Characteristic Key Biological Disciplines of Yunnan Province (project serial code: 50097505). The thesis of “Scientific and Technological Innovation Team Construction Project for Protection and Utilization of Under-forest Biological Resources (project serial code: 51400605)” was completed through joint support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Hu Y.L., Hao F.H., Wang Y.L. NMR-Based metabomic analyses on spleen tissues of 4T1 Tumor-bearing mice subjected to chemotherapies with different drug delivery strategies. Chinese J. Magn. Reson. 2018;35(1):8–21. [Google Scholar]
- Jemal A., Bray F., Center M.M. Global cancer statistics. CA Cancer J. Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Luan Y., Cao Y., Mao D., Li Y., Yue X., Zhao Y., Xiong F., Rong J., He C. RNA sequencing reveals the differences between chemical and chronic constipation induction of intestinal tumor. Biomed. Res. 2017;28(22):10053–10061. (SCI: IF=0.219) [Google Scholar]
- Luan Y., Li Y., Zhu L., Zheng S., Mao D., Chen Z., Cao Y. Codonopis bulleynana Forest ex Diels inhibits autophagy and induces apoptosis of colon cancer cells by activating the NF-κB signaling pathway. Int. J. Mol. Med. 2018;41:1305–1314. doi: 10.3892/ijmm.2017.3337. (SCI: IF=2.341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y., Mao D., Li Y., Wu P., Ao C. Effect of chronic constipation on Dimethylhydrazine-Induced intestinal cancer in Kunming Mice. J. Balkan Tribol. Assoc. – Bio Tribol. 2016;22(3A-Ⅱ):3383–3390. [Google Scholar]
- Nordin N.F.H., Ibrahim N.H.S., Chowdhury A.J.K. Physicochemical parameters and bacterial composition in sungau pusu gombak. Sci. Heritage J. 2018;2(1):10–12. [Google Scholar]
- Qiao F. Research on design principles of visual identity in campus environment. Sci. Heritage J. 2018;2(2):01–03. [Google Scholar]
- Reddy B.N., Aqueel M.A. Atypical fracture femur following long term prednisolone and alendron ate medication: a case report. Matrix Sci. Pharma. 2018;2(2):04–05. [Google Scholar]
- Sa R.N., Wu H.J. Pathways of lipid metabolism and their associated disorder in cancer. Cancer Res. Prevent. Treat. 2016;43(10):907–912. [Google Scholar]
- Shareef M., Akhtar M.S. Neem (Azadirachtaindica) and its potential for safeguarding health, prevention and treatment of diseases. Matrix Sci. Med. 2018;2(1):04–08. [Google Scholar]
- Sun S.K., Cong L.X., Li M.J., Chen Z., Guo H.Y. Metabonomics investigation of Lung Adenocarcinoma in male patients based on liquid chromatography-mass spectrometry. Cancer Res. Prevent. Treat. 2017;44(9):607–611. [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D., Liu Y. The evaluation indexes of scientific and technological achievements in Jiangxi province, China. Acta Sci. Malay. 2018;2(2):21–22. [Google Scholar]
- Yu F., Liu S.Z., Zhong M.W., Huang X., Jiao J., Hu S.Y., Yu W.B. Metabolomics analysis of colorectal cancer based on GC-TOF-MS. J. Shandong Univ. (Health Sci.) 2016;54(7):60–68. [Google Scholar]
- Zeeshan U., Barkat M.Q., Mahmood H.K. Phytochemical and antioxidant screening of Cassia Angustifolia, Curcuma Zedoaria, Embelia Ribes, Piper Nigrum, Rosa Damascena, Terminalia Belerica, Terminalia Chebula, Zingiber Officinale and their effect on stomach and liver. Matrix Sci. Pharma. 2018;2(2):15–20. [Google Scholar]
- Zeeshan U., Barkat M.Q., Mahmood H.K. Phytochemical And Antioxidant Screening Of Cassia Angustifolia, Curcuma Zedoaria, Embelia Ribes, Piper Nigrum, Rosa Damascena, Terminalia Belerica, Terminalia Chebula, Zingiber Officinale and their effect on stomach and liver. Matrix Sci. Med. 2018;2(2):21–27. [Google Scholar]
- Zhang S.S., Wu H.X. Application of metabolomics in the diagnosis of colorectal cancer. J. Pract. Oncol. 2017;31(6):573–576. [Google Scholar]
- Zhao J., Li S.M. The Impact of Tourism Development On The Environment In China. Acta Sci. Malay. 2018;2(1):01–04. [Google Scholar]
- Zhou X., Wang Y.P., Xu Y.Y., Lei Q.Y. Metabolic dysfunction and cancer development. Chem. Life. 2016;36(6):739–744. [Google Scholar]