Abstract
Currently, there is increasing interest in assessing the potential of bacterial laccases for industrial and environmental applications especially in harsh conditions. The environmental impact of the textile industry requires novel and effective technologies to mitigate the presence of dyes in wastewaters before discharging into the environment. Dyes usually remain stable in the presence of a variety of chemicals, light and are recalcitrant to microbial degradation. Among available technologies the biological treatments offer environmentally friendly strategies for decolorizing and detoxifying these compounds. The recent discovery of versatile laccases in streptomycetes opens up new opportunities for their commercial application. The aim of this study is to assess the potential of a novel bacterial laccase SilA produced by Streptomyces ipomoeae CECT 3341 active over wide temperature and pH ranges for use as an eco-friendly, biological treatment for the degradation of textile dyes. Insights into the enhancement of the oxidative action of this enzyme through the use of natural redox mediators are presented together with an assessment of the potential toxicity of the degradation products. Our results confirm that the combination of the laccase and natural mediators such as acetosyringone and methyl syringate enhanced the decolorization and detoxification of a variety of textile dyes up to sixfold and 20-fold, respectively. Mediator concentration was found to have a significant effect (p < 0.05) on dye decolorization at 60 °C; thus, the decolorization of Acid Orange 63 increased from 6 to 70-fold when the mediator concentration was increased from 0.1 to 0.5 mM. Further, the toxicity of tartrazine decreased 36-fold when the SilA-MeS system was used to decolorize the dye. The thermal properties of the SilA coupled with the stability of SilA at high pH suggest a potential commercial application for use in the decolorization of textile wastewaters which generally are performed at high temperature (>55 °C) and salinity and neutral pH, conditions which are unfavourable for conventional fungal laccases.
Keywords: Laccase, Dyes decolorization, Streptomyces, Natural mediators, Detoxification
1. Introduction
Laccases (EC 1.10.3.2) are a part of a larger group of enzymes called multi-copper enzymes which contain four copper atoms in the catalytic centre, structured in three different cupredoxine-type domains. Laccases are widely distributed in nature and have been described in fungi, plants, insects, bacteria and archaea (Uthandi et al., 2010). The biological role of fungal and plant laccases has been exhaustively studied and shown to be related to the degradation and synthesis of lignin, respectively (Riva, 2006). It has been suggested that bacterial laccases are involved in morphogenesis, biosynthesis of spore pigments, copper homeostasis and lignin solubilisation (Roberts et al., 2002, Arias et al., 2016).
Laccases cause a mono-electronic oxidation of a broad group of phenolic compounds coupled to the 4e− reduction of O2 to H2O. In some cases, the capacity of laccases to oxidize a substrate is hindered by the non-phenolic nature of the compounds or their high redox potential. The redox potential of laccases spans widely, from 400 mV for plant and bacterial laccases to 790 mV estimated for fungal laccases (Gutiérrez et al., 2006, Mikolasch and Schauer, 2009). The low substrate specificity, the lack of cofactors and the ready availability of oxygen as an electron acceptor, makes laccases promising and versatile enzymes with application in a number of biotechnological processes (Riva, 2006). The list of potential applications include biobleaching and pulp delignification, decolourisation and detoxification of dyes (Arias et al., 2003, Molina-Guijarro et al., 2009, Moya et al., 2010), food processing (Minussi et al., 2002), bioremediation and biosensor and biofuel cell construction (Amir et al., 2009, Brijwani et al., 2010).
In addition, there is the possibility of expanding laccases oxidation range through the use of redox mediators; this extending the biotechnological potential of the enzymes. The redox mediators act as electron shuttles and provide an indirect oxidation step. The oxidized radical form of the mediator is able to oxidize a wide range of large molecules and non-phenolic substrates. ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) was the first artificial mediator used in the laccase-mediator systems (LMS) (Bourbonnais and Paice, 1990). Other synthetic mediators such as 1-hydroxybenzotriazole (HBT) and the oxime violuric acid (VLA) have also demonstrated their ability to oxidize recalcitrant aromatic compounds (Cañas and Camarero, 2010). However, because of the toxicity and cost associated with the use of these synthetic mediator compounds there is an urgent need to find more suitable natural compounds, capable of acting as mediators to laccases. Furthermore, currently this is a key limitation to the commercial application of laccases. Previous research has reported that phenolic compounds related to lignin have shown their capability and efficiency to act as natural laccase mediators (Camarero et al., 2005). However, additional work is required to further assess the potential commercial use of mediators to laccases.
The textile and paper industries manufacture thousands of different dyes which must remain stable in the presence of a variety of chemicals, light exposure and microbial degradation. Currently there are over 10,000 synthetic dyes being used worldwide. Because of this inherent recalcitrance, 90% of reactive textile dyes remain in the sewage plants and ultimately discharged into rivers. A number of specific combined oxic and anoxic treatment processes have been developed capable of degrading recalcitrant dyes. However, the generation of carcinogenic amines from azo dyes in anoxic processes poses a serious health hazard. The application of laccases to the textile and paper industries is therefore particularly important. Fungal and bacterial laccases have also been shown to effectively decolorize and degrade synthetic dyes (Abadulla et al., 2000, Molina-Guijarro et al., 2009, Pereira et al., 2009, Moya et al., 2010, Mendes et al., 2011, Saratale et al., 2011, Forootanfar et al., 2012, Asgher et al., 2013) as well as flexographic inks (Fillat et al., 2012). However, currently the commercial application of fungal laccases for the removal of dyes from wastewaters is limited mainly due to the relatively slow growth of the fungi and the requirement for a controlled source of nitrogen (Anastasi et al., 2011). Bacterial laccases have a greater commercial potential compared with fungal systems mainly in extreme environments (Sharma et al., 2007).
In this work we screen the potential of bacterial laccases for the decolorization and degradation of textile dyes. Molecular and physicochemical characterization of the recombinant laccase (SilA) from Streptomyces ipomoeae CECT 3341 is presented, together with an assessment of its effectiveness in decolorizing and detoxifying commercial dyes. The aim of this study is to assess the commercial potential of this novel bacterial laccase as an eco-friendly, biological treatment for the degradation of industrial textile dyes. Insights into the mechanism of action of this enzyme are also presented together with an assessment of the toxicity of the degradation products.
2. Materials and methods
2.1. Enzyme production and purification
A recombinant laccase (SilA) of Streptomyces ipomoeae CECT 3341 was obtained as previously described (Molina-Guijarro et al., 2009). For the production of SilA, 2 litres of Luria-Bertani (LB) liquid medium containing 50 µg mL−1 kanamycin was inoculated with 40 mL of a pre-inoculum of E. coli BL21 (DE3) transformed with a pET28 plasmid containing the codifying gene of SilA. After growth, cells were harvested by centrifugation at 4 °C for 10 min at 12,000g, and the pellet washed twice with phosphate buffered saline (PBS). To obtain the recombinant protein, cells were suspended in chilled 10 mM phosphate buffer (pH 7) and completely disrupted with a French press (Sim-Aminco) after three passes at 1500 psi. The insoluble fraction was discarded after centrifugation (10 min at 15,000g at 4 °C), and the soluble fraction containing the protein was collected. To activate the enzyme, this fraction was incubated for 3 h on ice with 1 mM CuSO4, and then dialyzed at 4 °C overnight against 5 litres of 10 mM phosphate buffer (pH 7). The active SilA was stored at −20 °C until its purification.
The recombinant laccase was purified chromatographically using a HisTrap HP column (GE Healthcare) due to the presence of His-tag residue from the pET plasmid. The column was equilibrated with 20 mM phosphate buffer, 0.5 M NaCl, and 10 mM imidazole (pH 7.4). Prior to loading on the column, the samples were mixed with an equal volume of the mobile phase (2-fold concentrated). The protein was eluted with the same buffer amended with 750 mM imidazole. Active fractions were dialyzed against PBS buffer in a PD-10 desalting column (GE Healthcare) to remove imidazole (Molina-Guijarro et al., 2009)
Laccase activity was routinely determined at room temperature by measuring the oxidation of 5 mM 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) in 50 mM acetate buffer (pH 4.5) and 5 mM 2,6-dimethoxyphenol (DMP) in 100 mM phosphate buffer (pH 8). The increase in the absorbance at 436 nm for ABTS and 469 nm for 2,6-DMP were monitored using a Hitachi 2001 spectrophotometer, using a molar extinction coefficient of 29,300 M−1 cm−1 for oxidized ABTS and 27,500 M−1 cm−1 for oxidized 2,6-DMP (Arias et al., 2003).
2.2. Enzyme stability assays
Thermodynamic stability was assessed by steady-state fluorescence measured with a Varian Cary Eclipse spectrofluorometer using wavelengths of 280 nm and 296 nm for excitation and 340 nm for emission. Samples containing protein (Abs296 = 0.1) in phosphate buffer, pH 7 were placed onto a thermostatically controlled thermal block and heated at a rate of 1 °C/min up to 100 °C. For chemical stability studies, increased guanidinium hydrochloride (GdnHCl) concentrations were used to induce protein unfolding, monitored through a combination of fluorescence intensity and emission maximum as previously described (Durão et al., 2006).
Kinetic stability studies were performed by incubating the enzyme at 95 °C in 10 mM phosphate buffer, pH 7. At fixed time intervals sample aliquots were taken for activity at 25 °C. All assays were performed in triplicate. Enzyme activity was assayed using the ABTS substrate solution and the protein content was determined by the Bradford assay using bovine serum albumin as a standard.
2.3. Redox titration
Redox titration was performed at 25 °C and pH 7, in anaerobic conditions under an argon atmosphere. The reaction mixture contained 35 µM enzyme in phosphate buffer pH 7 and the following mediators: 10 µM final concentration of dimethyl-p-phenylenediamine (+344 mV), sodium ferrocyanide (+433 mV), monocarboxylic acid ferrocene (+530 mV), 1,1′-dicarboxylic acid ferrocene (+644 mV) and Fe (II/III) tris-(1,10-phenantroline) (+1070 mV). Potassium hexachloroiridate (IV) was used as oxidant and sodium dithionite as reductant. The redox potential was monitored by visible spectroscopy (300–700 nm) using a Shimadzu UV-1603 spectrophotometer with a combined silver/silver chloride electrode and calibrated with a quinhydrone saturated solution at pH 7.0. The redox potentials are quoted against the standard hydrogen electrode.
2.4. Dye decolorization with laccase and laccase-mediator system
All dyes were purchased from Sigma-Aldrich with the highest grade available. Table 1 lists the dyes used in this study, together with their chemical structure, their molar extinction coefficient at pH 8 and the maximal absorption wavelength.
Table 1.
Maximal absorption wavelength, molar extinction coefficient at pH 8 and chemical structure of the selected dyes (Sigma-Aldrich).
| Wavelength (nm) | Molar Coefficient (M−1 cm−1) | Chemical structure | |
|---|---|---|---|
| Acid Black 48 (AB48) | 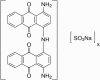 |
||
| Antraquinone type (Ref. 194247) | 657 | 14,759 | |
| Acid Orange 63 (AO63) | 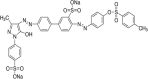 |
||
| Diazo type (Ref. 201979) | 418 | 40,165 | |
| Reactive Black 5 (RB5) | 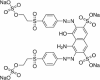 |
||
| Diazo type (Ref. 306452) | 596 | 44,226 | |
| Orange II (OII) | 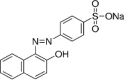 |
||
| Azo type (Ref. 195235) | 485 | 23,352 | |
| Tartrazine (Tart) | 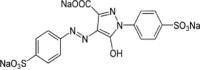 |
||
| Azo type (Ref. T0388) | 425 | 28,568 | |
| Azure B (AB) | 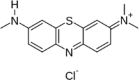 |
||
| Heterocyclic type (Ref. A4043) | 645 | 50,359 | |
| Indigo carmine (IC) | 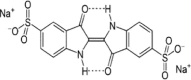 |
||
| Indigo type (Ref. 73436) | 609 | 22,711 | |
| Cresol red (CR) | 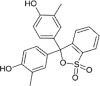 |
||
| Triarylmethane type (Ref. 114472) | 434 | 22,133 |
The natural compounds used as mediators were 3,5-dimethoxy-4-hydroxyacetophenone (acetosyringone, AS), 3,5-dimethoxy-4-hydroxybenzaldehyde (syringaldehyde, SA) purchased from Sigma–Aldrich and methyl 4-hydroxy-3,5-dimethoxybenzoate (methyl syringate, MeS) purchased from Novozymes.
To evaluate the ability of purified recombinant laccase SilA to decolorize the selected dyes reaction mixtures containing 0.4 U laccase mL−1 and 50 µM of each dye were incubated in 50 mM phosphate buffer pH 8 for 24 h at 35 °C. Decolorization was monitored by measuring the absorbance at the maximum absorption visible wavelength of each dye in a Shimadzu UV-160A spectrophotometer. To increase the oxidative potential of the enzyme, the phenolic compounds derived from lignin structure acetosyringone, syringaldehyde and methyl syringate were assayed as mediators together with laccase in decolorization experiments. For this purpose, different conditions were used. Two different concentrations of each mediator (0.1 and 0.5 mM) were tested together with 0.4 U laccase mL−1 and 50 µM of Acid Orange 63 and tartrazine at two different temperatures (35 and 60 °C). Decolorization was determined by the decrease in the absorbance at 418 and 425 nm for Acid Orange 63 and tartrazine respectively, compared with controls. Experiments were carried out in triplicate and heat inactivated laccase was used in control reactions.
2.5. Assessment of toxicity of dyes, degradation intermediates and products
Toxicity was carried out using a Pseudokirchneriella subcapitata test, performed according to OECD Guideline No. 201 (OECD, 1984b), using the Algaltoxkit FTM (Microbiotests, BE). Algal inocula were prepared by incubation of stock cells at 24 ± 1 °C in uniform illumination conditions (10,000 lux for sideway illumination or minimum 3000–4000 lux for bottom illumination) until an algal density of 1 × 106 cells mL−1 was reached. A dilution series of each dye as well as of the samples obtained after 24 h enzyme treatment from 50 to 3.125 µM were prepared and then each dilution was inoculated with the algal suspension to obtain 1 × 104 cells mL−1. Tests were performed with the same previous temperature and illumination conditions for 72 h, in 25 mL flasks, with daily determination of algal growth using absorbance values related to a standard curve supplied by Microbiotests BE.
The EC50 (72 h) values for the algal reference test were calculated according to the procedure outlined in OECD Guideline 201 (ISO 8692) for the estimation of growth rates. All toxicity assays were carried out in triplicate and toxicity degree was calculated as (1/EC50)x100.
2.6. Statistical analysis
Decolorization by the different enzymatic treatments over time was statistically analysed using repeated measure ANOVA implemented in Statistica v8.0 software. Mediators (AS, SA and MeS), mediator concentration (0.1 and 0.5 mM) and temperature (35 and 60 °C) were included as fixed factors.
3. Results
3.1. Decolorization studies and toxicity determination
All assays carried out with SilA in the absence of mediator showed a low decolorization rate (15–30%) (Table 2). Further, SilA was ineffective in the decolorization of Acid Black 48, Acid Orange 63, Reactive Black 5 and Azure B dyes. However, the addition of the redox mediators resulted in a significant (p < 0.05) increase in activity. The addition of acetosyringone (AS) resulted in an increase (average 7-fold) in decolorization after 24 h of incubation of all the dyes compared to SilA degradation treatments without the addition of AS as mediator. Similarly, the addition of syringaldehyde (SA) and methyl syringate (MeS) as mediators resulted in a 4–6-fold increase respectively in decolorization compared to those observed with the enzyme. In all three treatments the dyes showing the highest decolorization were Reactive Black 5 (RB5), Orange II (OII) and Indigo carmine (IC).
Table 2.
Decolorization percentages of the dyes Acid Black 48 (AB48), Acid Orange 63 (AO63), Reactive Black 5 (RB5), Orange II (OII), Tartrazine (TART), Azure B (AB), Indigo Carmine (IC) and Cresol Red (CR) by the laccase SilA in the presence or absence of the three mediators (0.1 mM), AS (acetosyringone), SA (syringaldehyde) and MeS (methyl syringate) after 24 h at 35 °C pH 8. Results are the means of triplicates with standard errors presented.
| Decolorization of dye (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| AB48 | AO63 | RB5 | OII | TART | AB | IC | CR | |
| SilA | 0 | 0 | 29.34 ± 4.89 | 14.2 ± 3.18 | 9.24 ± 0.09 | 0 | 27.32 ± 1.97 | 12.05 ± 0.33 |
| SilA + AS | 18.5 ± 1.04 | 3.76 ± 0.71 | 90.82 ± 0.18 | 90.91 ± 0.31 | 19.22 ± 0.20 | 13.53 ± 3.17 | 96.26 ± 0.93 | 8.24 ± 1.27 |
| SilA + SA | 1.67 ± 1.01 | 0 | 35.86 ± 0.51 | 86.83 ± 0.63 | 10.4 ± 0.80 | 12.4 ± 2.17 | 93.78 ± 1.38 | 8.34 ± 1.72 |
| SilA + MeS | 21.97 ± 4.43 | 13.96 ± 0.96 | 94.11 ± 1.35 | 88.86 ± 3.95 | 20.97 ± 1.38 | 9.32 ± 1.78 | 98.4 ± 0.61 | 12.36 ± 0.30 |
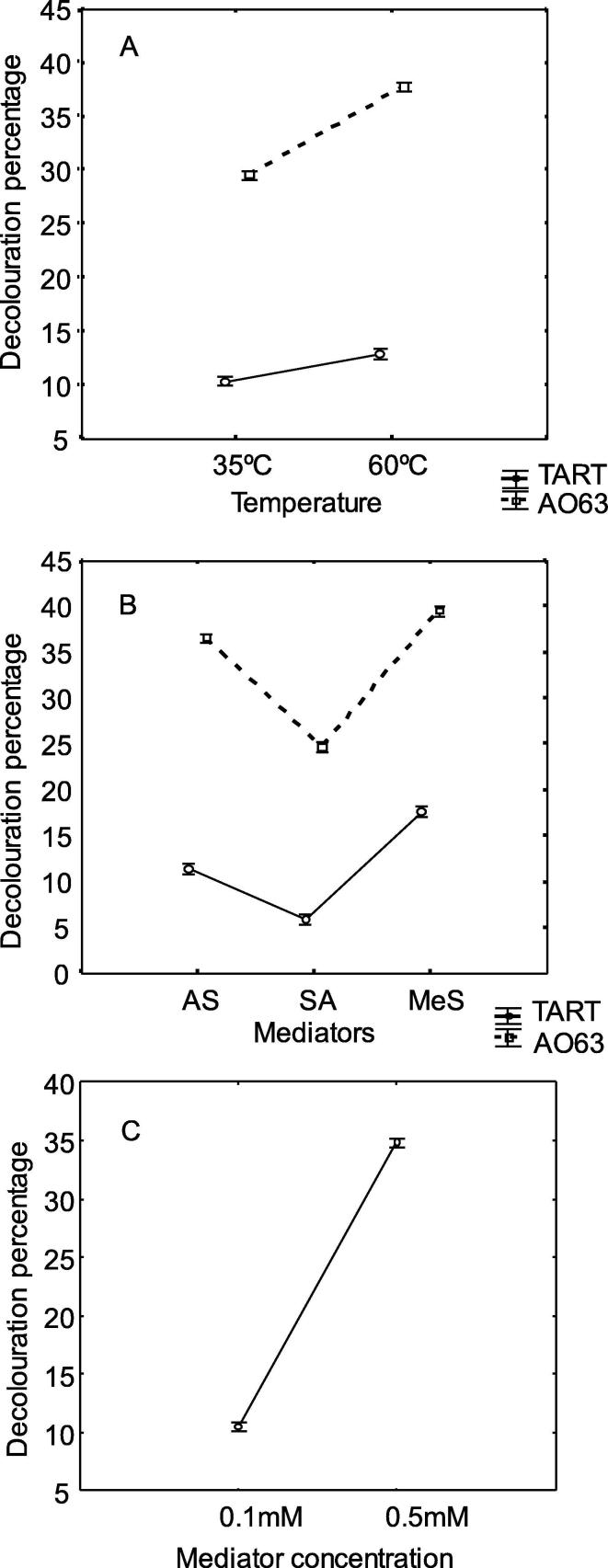
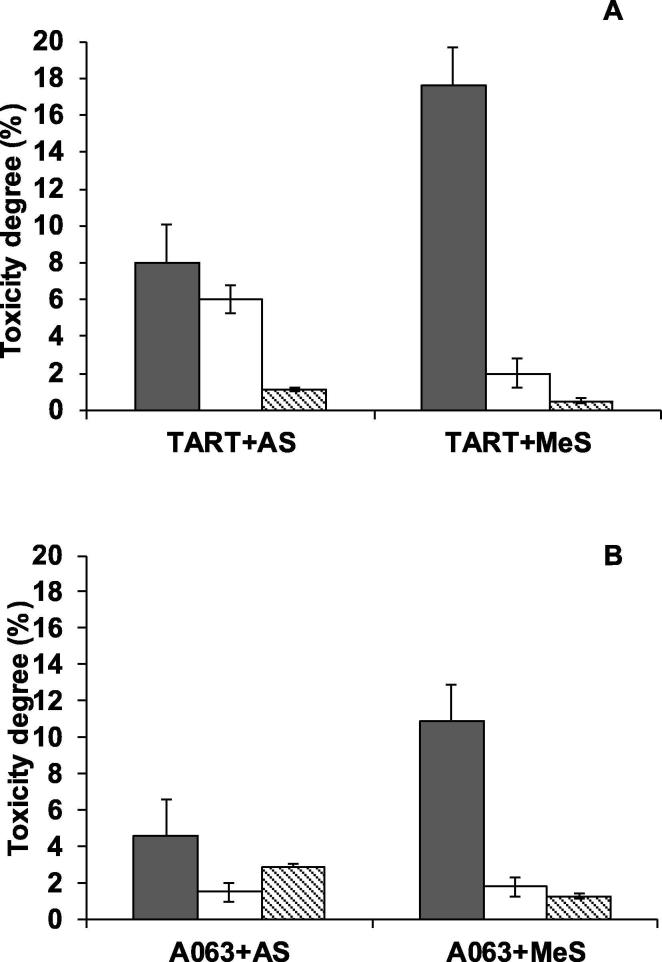
In previous studies SilA was shown to exhibit moderate thermostability retaining more than 50% activity at 60 °C after 24 h. As many textile dye wastewaters usually have a high temperature (>55 °C), the ability of the enzyme and mediator to degrade dyes at higher temperatures was assessed. For this purpose, two of the previous dyes, tartrazine (TART) and Acid Orange 63 (AO63) were incubated under the same conditions but at 60 °C, the optimum temperature of SilA. Incubation at this temperature resulted in a significant increase in dye decolorization [F(1,48) = 44.447, p < 0.05, Fig. 1A] regardless the mediator used. In the Fig. 1B is shown that MeS was the most efficient mediator [F(2, 48) = 17.851, p < 0.05] at both temperatures tested (Fig. 1B). In order to optimize the degradation yields of the SilA-mediator system, higher concentration of the mediators (0.5 mM) was tested with the two selected dyes, AO63 and TART, at 35 and 60 °C. The mediator concentration was found to have a significant effect [F(1, 48) = 3137.2, p < 0.05] on dye decolorization (Fig. 1C) resulting in a 3.5-fold increase in decolorization for both dyes at any temperature. The interaction of mediator concentration-temperature resulted in a significant effect [F(1, 48) = 66.119, p < 0.05]. The loss of colour in a reaction indicates breakdown of the parent compound. However, it is possible that the breakdown products, although colourless, may still remain hazardous in terms of ecological impact. This point is often overlooked in dye degradation studies. Therefore, ecotoxicity assays were performed with Acid Orange 63 and tartrazine reaction mixtures before and after SilA-mediator treatments. This was assessed using Pseudokirchneriella subcapitata as a bioindicator. The toxicity degree of AO63 and TART at an initial concentration of 50 µM with 0.1 mM AS or MeS, prior to the enzyme treatment was between 5 and 18% (Fig. 2, black columns). The toxicity of the dyes was higher in the presence of methyl syringate as mediator, presumably due to the toxicity of the mediator. The toxicity degree following enzyme treatment at 35 °C decreased 10-fold, when MeS was used as mediator; however, the toxicity in the treatments with AS at the same temperature decreased 3-fold (Fig. 2, white columns). At the higher temperature, 60 °C, a 36-fold decrease in the toxicity degree of TART was observed with MeS (Fig. 2, striped column). The toxicity degree for TART with AS and for AO63 with AS and MeS at 60 °C decreased around 1.5–10-fold (Fig. 2, striped columns).
Fig. 1.
Statistical analysis of decolorization of tartrazine (TART) and Acid Orange 63 (AO63) in relation to temperature (35 and 60 °C), type of mediators (acetosyringone, AS; syringaldehyde, SA; methyl syringate, MeS) and mediator concentration (0.1 and 0.5 mM). A: Effect of the temperature on TART and AO63 decolorization (%) obtained with the mean of three mediators. B: Effect of the mediators on TART and AO63 decolorization (%) obtained by using the mean of both temperatures. C: Effect of the concentration of the three mediators on decolorization of both dyes (%) regardless mediators and dyes. Results are the means of triplicate measurements with standard errors shown.
Fig. 2.
Toxicity of the degradation products of AO63 (A) and TART (B) before (grey bars) and after 24 h of SilA-mediator (0.1 mM acetosyringone, AS or methyl syringate, MeS) system treatment at 35 °C (white bars) and 60 °C (striped bars).
3.2. SilA stability assays
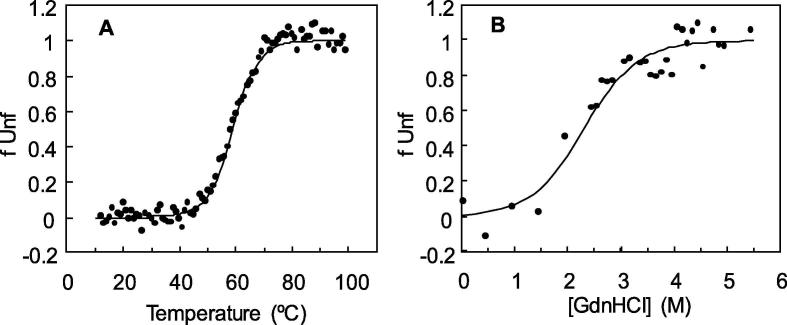
The thermodynamic and kinetic stability of the laccase represents important properties for any commercial process but particularly for those involving exposure to dye-containing wastewaters at elevated temperatures. The thermodynamic stability of the laccase, studied by probing the tertiary structure (fluorescence intensity) at increasing temperature or in the presence of the chemical denaturant GdnHCl is shown in Fig. 3. The unfolding of the enzyme induced by either temperature or GdnHCl is apparently described by two-state process where the folded and unfolded states seem to be the only states that accumulate in significant amounts. The laccase represents a moderately thermostable enzyme with a melting temperature (Tm, where 50% of the protein molecules were denaturated) of 60 ± 6 °C, a ΔHTm of 44 ± 4 kcal/mol and ΔSTm of 0.13 ± 0.01 kcal/mol K. During chemical unfolding the tertiary structure of the laccase unfolded with a mid-point (GdnHCl concentration where 50% of the protein molecules were unfolded) of around 2.2 M. Further, the native state was more stable than the unfolded state by 2.8 kcal/mol at 25 °C.
Fig. 3.
Unfolded fraction (fUnf) of protein by (A) temperature and (B) GdnHCl as measured by fluorescence emission. The solid curve is a model that fits well with the equation fU = e[(−ΔG°/RT)/(1 + e (−ΔG°/RT)].
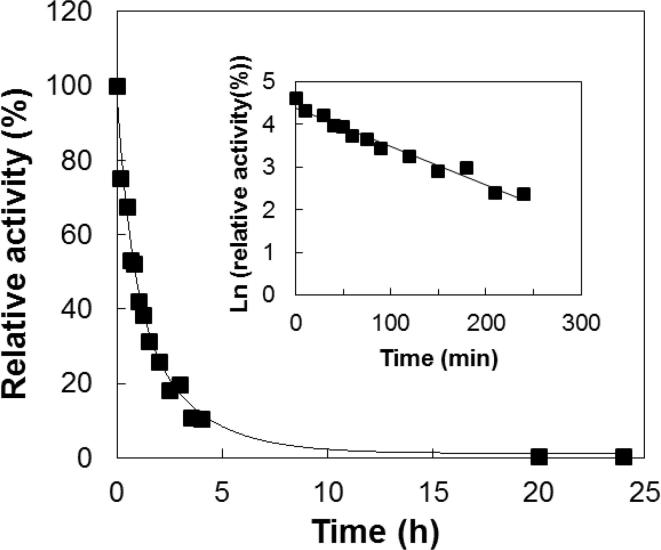
In order to quantify the irreversible loss of laccase activity during incubation at a certain temperature, caused by aggregation or precipitation processes, a kinetic or long-term stability assay was carried out. As seen in Fig. 4 the SilA laccase was found to be thermostable showing a half-life value at 95 °C of 1.3 h.
Fig. 4.
SilA thermal inactivation after incubation at 95 °C. The inset represents the first-order kinetics of deactivation (ln activity = ln activity(t = 0) – kdt, where kd is the rate constant of deactivation). The calculated half-life of inactivation (t1/2 = ln 2/kd), of SilA at 95 °C was 1.3 h.
3.3. Redox titration
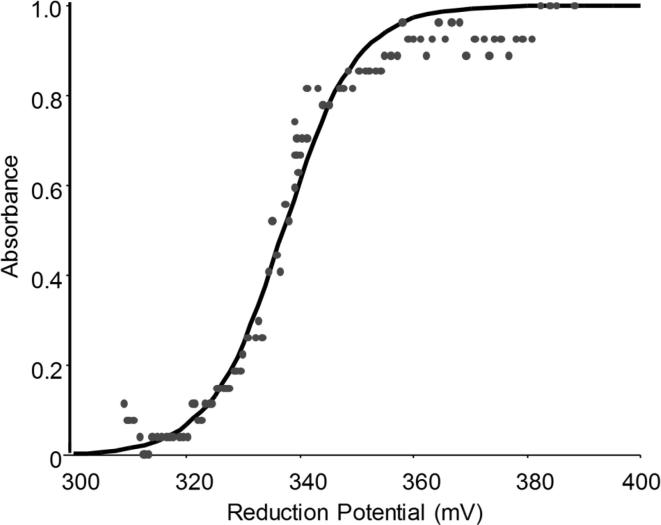
One of the putative problems in the degradation processes using bacterial lacasses is the low redox potential of these enzymes as compared to their fungal counterparts. In order to assess the commercial potential application of the SilA laccase in the decolorization of textile dyes the midpoint reduction potential (E′0) at the T1 site of SilA was determined. The reduction of the T1 copper ion was monitored spectroscopically from 300 to 700 nm. The midpoint potential obtained by the application of the Nernst equation was estimated at 337 mV (Fig. 5).
Fig. 5.
Redox titration of SilA. The solid curve represents the best fit to the potential-dependent normalized absorption (Abs normal) at 600 nm given by a one-electron Nernst equation.
4. Discussion
Each year, around 3 × 105 tonnes of textile dyes are discharged into wastewaters worldwide (Ogugbue and Sawidis, 2011). These wastewaters have negative impacts on the environment, not only by tanning waters but also by direct toxicity and mutagenic capacity (Singh et al., 2015). A wide range of different physico-chemical processes has been used for the degradation and decolorization of these dyes. However, these approaches are expensive and produce high amounts of waste sludge, which also represents a serious environmental hazard. In contrast sustainable, biological treatments are promising alternative strategies due to their low cost and eco-friendly nature (Chen, 2006).
Laccase SilA produced by Streptomyces ipomoeae shows activity even at high concentrations of sodium chloride and over a broad pH range (Molina-Guijarro et al., 2009). As the pH of most textile wastewaters is neutral to alkaline pH, the stability of SilA at high pH could be advantageous; fungal laccases are not generally active in alkaline conditions.
When examining the potential of SilA to degrade and detoxify several textile dyes belonging to different groups (i.e. anthraquinone, diazo, azo, heterocyclic, triarylmethane and indigo), SilA was not able to directly oxidize Acid Black 48, Acid Orange 63 and Azure B dyes. This finding could be attributed to the low redox potential of the enzyme or to the chemical structure of the dyes, which is also considered an important point when assessing the efficiency of decolorization. The structural heterogeneity of the dyes affects the charge density distribution resulting in a lower or higher redox potential (Ciullini et al., 2008). In general, textile dyes with simpler structures and low molecular weight presented higher decolorization yields. The other key factor was the relationship between the redox potential of the laccase and the energy required to withdraw an electron from a reduced substrate. Laccases with high redox potentials are able to readily oxidize a wide range of substrates; bacterial laccases possess low and medium redox potentials (SilA 337 mV) restricting their oxidative ability to a limited group of compounds, as demonstrated in this study.
Molina-Guijarro et al. (2009) previously reported the inability of SilA to decolorize the azo-dye Orange II. Similar results were obtained with purified laccase from Trametes versicolor (Forootanfar et al., 2012) and a recombinant laccase from Streptomyces cyaneus (Moya et al., 2010) in the decolorization of synthetic dyes; neither processes were shown to be effective, highlighting the difficulty in finding effective biological treatments. The use of phenolic lignin-related compounds, to act as an electron shuttle between the enzyme and the dyes, opens up new possibilities for the developing cost-effective biological treatment for textile dyes. The presence of an oxidized phenolic redox mediator can extend the range of substrates capable of being degraded by SilA, including compounds with a high redox and/or non-phenolic compounds. Previous studies have reported that acetosyringone is an effective redox mediator in the decolorization of textile dyes (Camarero et al., 2005). In this study higher decolorization was observed in the presence of, not only acetosyringone, but also methyl syringate. In fact, methyl syringate was the most efficient natural mediator in all conditions tested. These results are in line with a previous study where methyl syringate was observed to be the most efficient mediator for oxidation of non-phenolic lignin unit’s (Rosado et al., 2012). This was explained by the stability of the phenoxy radicals generated by the three phenolic compounds oxidation (MeS > AS > SA), related to the presence of electron donor groups at the para-position. The oxidation of MeS bearing the weakest acceptor group at the para-position gives origin to the most stable phenoxy radical. This fact could explain the efficiency of methyl syringate and acetosyringone as redox mediators to degrade and decolorize all the textile dyes studied in the present work. It was also observed that high decolorization was achieved with higher mediator concentrations, indicating that the reactive species originated by the mediator’s oxidation are involved in competitive routes, i.e. coupling reactions that lead to the formation of dimeric and trimeric structures (Rosado et al., 2012).
It is also important to consider that degradation of textile dyes lead not only to decolorization but also to detoxification. Many of the dyes used in the textile industry can be toxic and/or mutagenic (Dos Santos et al., 2007). More than 90% of the 4000 dyes studied by ETAD (Ecological and Toxicological Association of the Dyestuffs Manufacturing Industry) showed high toxicity rates (Robinson et al., 2001). Most reported degradation studies fail to evaluate the toxicity of either the dyes and/or the reaction products. In fact, the degradation of azo-dyes may result in the production of compounds of increased toxicity (Zaharia and Suteu, 2012). In this work the SilA-acetosyringone system was able not only to degrade the azo dyes under study but also to detoxify them at alkaline pH. It is also remarkable that this laccase-natural mediator system is able to decolorize and detoxify a wide range of dyes.
The high levels of decolorization observed from this SilA laccase-mediator system at neutral to alkaline pH range and at high temperatures (60 °C) is advantageous for dye-containing wastewater treatments, since most fungal laccases are only active in acid conditions and at mesophilic temperatures (Nyanhongo et al., 2002, Kokol et al., 2007). When proteins are exposed to increasing temperature or denaturing compounds, loss of solubility or enzymatic activity occurs. The thermal stability of an enzyme is a key factor when assessing the commercial feasibility of an emerging biotechnology developed to treat high temperature wastes. Long-term stability or kinetic stability, generally expressed as the enzyme’s half-life at a particular temperature, depends on the energy barrier to irreversible inactivation. Previous studies carried out with recombinant laccase SilA showed its capability to retain more than 50% activity after 24 h at 50 and 60 °C (Molina-Guijarro et al., 2009). SilA remains active at 95 °C with a half-life of 1.3 h. Other thermostable bacterial laccases, such as the CotA laccase have been reported to show a similar thermal stability, with a half-life of 2.9 h at 80 °C (Durão et al., 2006). These values contrast with others for some fungal laccases from Myceliophthora thermophila and Scytalidium thermophilum which were unable to withstand 1 h at 80 °C (Chefetz et al., 1998). Among fungal laccases, Pycnoporus sanguineus produces a thermostable laccase with a half-life of 2.8 h at 75 °C (Litthauer et al., 2007); however, this laccase is only effective under acidic conditions, not under the conditions common in dye wastewaters.
5. Conclusions
One of the main bottlenecks that must be overcome in order to use laccases for biotechnological and environmental applications is their loss of effectiveness under extreme conditions. The heterologous production of SilA using E. coli as host, its stability to high temperatures, alkaline pH and high salt-resistance, together with the possibility to enhance its oxidative action through the use of natural mediators, makes this enzyme a good candidate for bioremediation applications. Removal of dyes from wastewaters using laccases and laccase-mediator systems represent a potentially commercially viable, effective and environmentally friendly alternative to traditional physicochemical methods which require a high energy and investment.
Acknowledgments
Acknowledgement
We thank Dr. Javier Martínez (University of Alcalá) for the statistical analysis.
Funding
This study was funded in part by project CTQ2014-56038-C3-2-R from the Spanish Ministry for Economy and Competitiveness. A. Blánquez was awarded a grant from the same project
Footnotes
Peer review under responsibility of King Saud University.
References
- Abadulla E., Tzanov T., Costa S., Robra K.H., Cavaco-Paulo A., Gubitz G.M. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl. Environ. Microbio. 2000;66:3357–3362. doi: 10.1128/aem.66.8.3357-3362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir L., Tam T.K., Pita M., Meijler M.M., Alfonta L., Katz E. Biofuel cell controlled by enzyme logic systems. J. Am. Chem. Soc. 2009;131:826–832. doi: 10.1021/ja8076704. [DOI] [PubMed] [Google Scholar]
- Anastasi A., Parato B., Spina F., Tigini V., Prigione V., Varese G.C. Decolourisation and detoxification in the fungal treatment of textile wastewaters from dyeing processes. New Biotechnol. 2011;29:38–45. doi: 10.1016/j.nbt.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Arias M.E., Arenas M., Rodríguez J., Soliveri J., Ball A.S., Hernández M. Kraft pulp biobleaching and mediated oxidation of a nonphenolic substrate by laccase from Streptomyces cyaneus CECT 3335. Appl. Environ. Microb. 2003;69:1953–1958. doi: 10.1128/AEM.69.4.1953-1958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias M.E., Blánquez A., Hernández M., Rodríguez J., Ball A.S., Jiménez-Morillo N.T., González-Vila F.J., González-Pérez J.A. Role of a thermostable laccase produced by Streptomyces ipomoeae in the degradation of wheat straw lignin in solid state fermentation. J. Anal. Appl. Pyrol. 2016;122:202–208. [Google Scholar]
- Asgher M., Yasmeen Q., Iqbal H.M.N. Enhanced decolorization of Solar brilliant red 80 textile dye by an indigenous white rot fungus Schizophyllum commune IBL-06. Saudi J. Biol. Sci. 2013;20(4):347–352. doi: 10.1016/j.sjbs.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbonnais R., Paice M.G. Oxidation of Nonphenolic Substrates – an expanded role for laccase in lignin biodegradation. Febs Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- Brijwani K., Rigdon A., Vadlani V.P. Fungal laccases: production, function, and applications in food processing. Enzyme Res. 2010 doi: 10.4061/2010/149748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero S., Ibarra D., Martínez M.J., Martínez A.T. Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl. Environ. Microb. 2005;71:1775–1784. doi: 10.1128/AEM.71.4.1775-1784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañas A.I., Camarero S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010;28:694–705. doi: 10.1016/j.biotechadv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Chefetz B., Kerem Z., Chen Y., Hadar Y. Isolation and partial characterization of laccase from a thermophilic composted municipal solid waste. Soil Biol. Biochem. 1998;30:1091–1098. [Google Scholar]
- Chen H. Recent advances in azo dyes degrading enzyme research. Curr. Protein Pept. Sc. 2006;7:101–111. doi: 10.2174/138920306776359786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciullini I., Tilli S., Scozzafava A., Briganti F. Fungal laccase, cellobiose dehydrogenase, and chemical mediators: combined actions for the decolorization of different classes of textile dyes. Bioresource Technol. 2008;99:7003–7010. doi: 10.1016/j.biortech.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Dos Santos A.B., Cervantes F.J., Van Lier J.B. Review paper on current technologies for decolourisation of textile wastewaters: perspectives for anaerobic biotechnology. Bioresource Technol. 2007;98:2369–2385. doi: 10.1016/j.biortech.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Durão P., Bento I., Fernandes A.T., Melo E.P., Lindley P.F., Martins L.O. Perturbations of the T1 copper site in the CotA laccase from Bacillus subtilis: structural, biochemical, enzymatic and stability studies. J. Biol. Inorg. Chem. 2006;11:514–526. doi: 10.1007/s00775-006-0102-0. [DOI] [PubMed] [Google Scholar]
- Fillat U., Prieto A., Camarero S., Martínez A.T., Martínez M.J. Biodeinking of flexographic inks by fungal laccases using synthetic and natural mediators. Biochem. Eng. J. 2012;67:97–103. [Google Scholar]
- Forootanfar H., Moezzi A., Ghaie-Khozani M., Mahmoudjanlou Y., Ameri A., Niknejad F., Faramarzi M.A. Synthetic dye decolorization by three sources of fungal laccase. Iran. J. Environ. Healt. 2012;9 doi: 10.1186/1735-2746-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez A., Del Río J.C., Ibarra D., Rencoret J., Romero J., Speranza M., Camarero S., Martínez M.J., Martínez A.T. Enzymatic removal of free and conjugated sterols forming pitch deposits in environmentally sound bleaching of eucalypt paper pulp. Environ. Sci. Technol. 2006;40(10):3416–3422. doi: 10.1021/es052547p. [DOI] [PubMed] [Google Scholar]
- Kokol V., Doliška A., Eichlerová I., Baldrian P., Nerud F. Decolorization of textile dyes by whole cultures of Ischnoderma resinosum and by purified laccase and Mn-peroxidase. Enzyme Microbiol. Technol. 2007;40:1673–1677. [Google Scholar]
- Litthauer D., van Vuuren M.J., van Tonder A., Wolfaardt F.W. Purification and kinetics of a thermostable laccase from Pycnoporus sanguineus (SCC 108) Enzyme Microb. Tech. 2007;40:563–568. [Google Scholar]
- Mendes S., Farinha A., Ramos C.G., Leitao J.H., Viegas C.A., Martins L.O. Synergistic action of azoreductase and laccase leads to maximal decolourization and detoxification of model dye-containing wastewaters. Bioresource Technol. 2011;102:9852–9859. doi: 10.1016/j.biortech.2011.07.108. [DOI] [PubMed] [Google Scholar]
- Mikolasch A., Schauer F. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl. Microbiol. Biot. 2009;82(4):605–624. doi: 10.1007/s00253-009-1869-z. [DOI] [PubMed] [Google Scholar]
- Minussi R.C., Pastore G.M., Duran N. Potential applications of laccase in the food industry. Trends Food Sci. Tech. 2002;13:205–216. [Google Scholar]
- Molina-Guijarro J.M., Pérez J., Muñoz-Dorado J., Guillén F., Moya R., Hernández M., Arias M.E. Detoxification of azo dyes by a novel pH-versatile, salt-resistant laccase from Streptomyces ipomoea. Int. Microbiol. 2009;12:13–21. [PubMed] [Google Scholar]
- Moya R., Hernández M., García-Martín A.B., Ball A.S., Arias M.E. Contributions to a better comprehension of redox-mediated decolouration and detoxification of azo dyes by a laccase produced by Streptomyces cyaneus CECT 3335. Bioresource Technol. 2010;101:2224–2229. doi: 10.1016/j.biortech.2009.11.061. [DOI] [PubMed] [Google Scholar]
- Nyanhongo G.S., Gomes J., Gübitz G.M., Zvauya R., Read J., Steiner W. Decolorization of textile dyes by laccases from a newly isolated strain of Trametes modesta. Water Res. 2002;36:1449–1456. doi: 10.1016/s0043-1354(01)00365-7. [DOI] [PubMed] [Google Scholar]
- OECD Guideline for Testing of Chemicals, 1984b. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test. “Alga, Growth Inhibition Test”. 7 June 1984.
- Ogugbue C.J., Sawidis T. Bioremediation and Detoxification of Synthetic Wastewater Containing Triarylmethane Dyes by Aeromonas hydrophila. Isolated from Industrial Effluent. Biotechnol. Res. Int. 2011 doi: 10.4061/2011/967925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Coelho A.V., Viegas C.A., dos Santos M.M.C., Robalo M.P., Martins L.O. Enzymatic biotransformation of the azo dye Sudan Orange G with bacterial CotA-laccase. J. Biotechnol. 2009;139:68–77. doi: 10.1016/j.jbiotec.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Riva S. Laccases: blue enzymes for green chemistry. Trends Biotechnol. 2006;24:219–226. doi: 10.1016/j.tibtech.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Roberts S.A., Weichsel A., Grass G., Thakali K., Hazzard J.T., Tollin G., Rensing C., Montfort W.R. Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. P. Natl. Acad. Sci. USA. 2002;99:2766–2771. doi: 10.1073/pnas.052710499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T., McMullan G., Marchant R., Nigam P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresource Technol. 2001;77:247–255. doi: 10.1016/s0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- Rosado T., Bernardo P., Koci K., Coelho A.V., Robalo M.P., Martins L.O. Methyl syringate: an efficient phenolic mediator for bacterial and fungal laccases. Bioresource Technol. 2012;124:371–378. doi: 10.1016/j.biortech.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Saratale R.G., Saratale G.D., Chang J.S., Govindwar S.P. Bacterial decolorization and degradation of azo dyes: a review. J. Taiwan Inst. Chem. E. 2011;42:138–157. [Google Scholar]
- Sharma P., Goel R., Capalash N. Bacterial laccases. World J. Microb. Biot. 2007;23:823–832. [Google Scholar]
- Singh N.K., Kazmi A.A., Starkl M. A review on full-scale decentralized wastewater treatment systems: techno-economical approach. Water Res. Technol. 2015;71(4):468–478. doi: 10.2166/wst.2014.413. [DOI] [PubMed] [Google Scholar]
- Uthandi S., Saad B., Humbard M.A., Maupin-Furlow J.A. LccA, an archaeal laccase secreted as a highly stable glycoprotein into the extracellular medium by Haloferax volcanii. Appl. Environ. Microb. 2010;76:733–743. doi: 10.1128/AEM.01757-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharia, C., Suteu, D., 2012. Textile Organic Dyes. Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents. In: Puzyn, Tomasz (Ed.), A Critical Overview, Organic Pollutants Ten Years After the Stockholm Convention. Environmental and Analytical Update, ISBN: 978-953-307-917-2.