Abstract
Lepidium sativum (garden cress) seed oil was examined for its antimicrobial, antioxidant, and anti-inflammatory activities. The oil was obtained by hydrodistillation, where gas chromatography coupled with mass spectrometry that utilized to study its chemical composition. Microdilution method was used to test the antimicrobial effect of oil against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica, Klebsiella pneumoniae, and Candida albicans. The antioxidant activity was assessed by radical scavenging activity assay using 2,2-diphenyl-1-picrylhydrazyl radical. The major constituents found in the oil were 7,10-hexadecadienoic acid, 11-octadecenoic acid, 7,10,13-hexadecatrienoic acid, and behenic acid. The minimum inhibitory concentration (MIC) against all pathogens was 47.5 mg/ml, except for Salmonella enterica, which showed MIC of 90 mg/ml. The oil demonstrated antioxidant activity in a dose dependent pattern, with a half maximal inhibitory concentration (IC50) value of 40 mg/ml, and exerted anti-inflammatory activity, wherein 21% protection was shown at a concentration of 300 μg/ml. Thus, L. sativum seed oil shows antimicrobial, antioxidant, and anti-inflammatory properties.
Keywords: Lepidium sativum oil, Chemical composition, Antioxidant activity, Anti-inflammatory activity, Antimicrobial activity
1. Introduction
Utilization of natural products for the synthesis of bioactive constituents, particularly medicine, has been described. Among world’s population, 80% uses traditional medicines, mainly herbs, for primary healthcare.
Lepidium sativum, popularly named as Garden cress, member of the Brassicaceae family. It is edible herb which is growing fast and has been cultivated as a culinary vegetable in North America, Europe, and all through Asia. In Saudi Arabia, L. sativum is grown in several regions of the country and is known as rashad or thufa (Ageel et al., 1987, Rahman et al., 2004). It is an erect and herbaceous annual plant that grows from 15 to 45 cm in height. Long racemes of L. sativum has small white flowers, with broad or obovate pods which emarginated at the apex and winged (Diwakar et al., 2010).
In folk medicine, L. sativum is used as a therapy for inflammatory diseases including diabetes mellitus, arthritis, and hepatitis (Bigoniya and Shukla, 2014, Sakran et al., 2014). Several studies have revealed that the extract of L. sativum possesses antioxidant, antidiarrheal, antispasmodic, antimicrobial, anti-inflammatory and hepatoprotective effects against oxidative damage (Doke, 2014, Al-Sheddi et al., 2016, Raish et al., 2016).
L. sativum seeds contain 24% oil which composed mainly of α-linolenic acid (ALA) (32%) and linolenic acid (LA) (12%). This oil is reactively stable owing to its high content of antioxidants and phytosterols (Moser et al., 2009, Diwakar et al., 2010). L. sativum oil (LSO) reported to show synergistic effects of inhibition in platelet aggregation and thromboxane B2 levels in the spleen and lung tissues of Wistar rats (Raghavendra and Naidu, 2011). In other study conducted in rat, LSO found to reduce lymphocyte proliferation and production of inflammatory mediators from peritoneal macrophages (Diwakar et al., 2011). A different research found that the feeding of Wistar rats on a diet with LSO during 60 days increased tocopherol levels and antioxidant enzymes activity (Umesha and Naidu, 2015).
The effects of L. sativum have largely been studied using its extracts; however, few studies have reported the antimicrobial and antioxidant activities of LSO in Saudi Arabia. Therefore, this study designed to assess the chemical constituents and antioxidant, antimicrobial, and anti-inflammatory characteristics of oil extracted from the seeds of L. sativum.
2. Materials and methods
2.1. Plant material and oil extracts
We obtained L. sativum seeds from a local herbal medicine supplier in Riyadh, Saudi Arabia and authenticated by Dr. Mohammad Atiqur Rahman, a taxonomist at King Saud University. We manually screened the seeds and good ones are chosen and ground using electrical grinder. Oil from ground seeds extracted with petroleum ether (60–80 °C) for 12 h in a Soxhlet apparatus, in accordance with a method described by AOCS (William, 1980). Then dried the obtained oil over anhydrous sodium sulfate, and kept at 4 °C.
2.2. GC–MS analysis of LSO
Analysis of LSO was achieved using gas chromatography-mass spectrometry. A Shimadzu GC–MS-QP2010 Ultra instrument with an RTX-5MS column (30 m long; 0.25 mm in diameter; 0.25 μm thick) was used, as Helium (purity: 99.99%) was the carrier gas. The components were identified using MS library (NIST) and further confirmed using the observed fragmentation pattern.
2.3. Antimicrobial activity
Microorganisms including four gram-negative bacteria [Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Salmonella enterica (ATCC 25566), and Klebsiella pneumoniae (ATCC13883)] and two gram-positive bacteria [Staphylococcus aureus (ATCC 25923) and Bacillus subtilis], and fungus Candida albicans were used to assess the antimicrobial activity of LSO. Tested microorganisms were cultured on Mueller–Hinton broth (MHB) for 18 h at 37 °C.
2.3.1. Broth dilution method
Microdilution method was utilized to determine antimicrobial properties of LSO in compliance with a modified version of a well-established procedures, known as the Institutional Protocol of Clinical and Laboratory Standards (Institute, 2007). The test microorganisms were grown on MHB for 18 h at 37 °C and adjusted to 0.5 on the McFarland standard. Dilution of oil was carried out in 50% dimethyl sulfoxide (DMSO) at a concentration of 190 mg/ml. The prepared oil solution was filter-sterilized and then serially diluted from a concentration of 190 to 6.4 mg/ml in sterile NaCl in 96-well plates. Then, 50 μl of microorganism suspensions was added at final concentrations of 5 × 105 colony-forming units/ml. Experimental positive control is defined as the cultured broth in absence of oil. In contrast, MHB supplemented with oil diluted in 30% DMSO were left uninoculated, without inoculation (negative control) and incubated at 37 °C for 24 h. Both, minimum inhibitory concentration (MIC) was determined and the minimum bactericidal concentration (MBC) was measured based on MIC results (carried out in triplicate).
2.4. Antioxidant activity
Antioxidant properties of LSO was studied using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay in 96-microwell plates via a modified version of a previously described method (Lopes-Lutz et al., 2008). Stock solution of DPPH in methanol was prepared at a 40 μg/ml and kept in the dark. Methanol was used to dilute the oils at concentrations of 40, 32, 24, 16, 8, and 5 mg/ml. Designated wells were filled with 50 μl of different concentrations of oil in methanolic solution. Then, 50 μl of the prepared DPPH stock solution was added to each well. The test was conducted (n = 3), and the prepared plate was kept in the dark for 30 min; then, the absorbance was read using an Elx 800 microplate reader at a wavelength of 520 nm. The following formula was used to calculate the percentage of inhibition:
2.5. Anti-inflammatory activity
LSO was screened for in vitro anti-inflammatory activity. Five ml freshly drawn human blood was mixed with sterilized Alsever’s solution of equal volumes (2% dextrose, 0.8% sodium citrate, 0.05% citric acid, 0.42% sodium chloride, and 100 ml of distilled water). Then, solution was centrifuged at 3,000 rpm for 10 min and subsequently washed three times with an equivalent volume of normal saline, followed by reconstitution with normal saline at 10% v/v concentration. Typically, reaction mixture composed of 1 ml of test sample prepared at different concentrations (300, 250, 200, 150, and 100 µg/ml) in normal saline and 0.5 ml of 10% human red blood cells (HRBC) suspension, 1 ml of 0.2 M phosphate buffer, and 1 ml of hyposaline which was incubated for 30 min at 37 °C, then centrifuged at 3,000 rpm for 30 min. The content of hemoglobin in the supernatant solution was spectrophotometrically estimated at 560 nm. Diclofenac sodium was utilized as a standard and distilled water as control. Where the blood control represents HRBCs 100% lysis or 0% stability, the inhibition percentage of HRBC hemolysis was calculated using the following formula:
3. Results and discussion
3.1. Identification of chemical compounds in LSO by GC–MS
GC–MS was used to determine the chemical composition of LSO as presented in Table 1. The chemical constituents detected in the seed oil were as follows: 1.14% 9,12-hexadecadienoic acid, 1.33% heneicosanoic acid, 1.39% 10-octadecenoic acid, 5.68% 15-tetracosenoic acid, 4.31% hexadecanoic acid, 3.81% steric acid, 9.93% 7,10,13-hexadecatrienoic acid, 9.67% behenic acid, 15.5% 11-octadecenoic acid, and 44.37% 7,10-hexadecadienoic acid (Table 1). 7,10-Hexadecadienoic acid was the most abundant omega-6 fatty acid (44.37%), whereas 7,10,13-hexadecatrienoic acid was the most abundant omega-3 fatty acid (9.93%).
Table 1.
Chemical composition of Lepidium sativum seed oil.
| Peak No. | R. Time | Area | Percentage (%) | Compounds |
|---|---|---|---|---|
| 1 | 12.715 | 1,256,314 | 0.05 | 6-Heptenoic acid, methyl ester |
| 2 | 16.105 | 2,578,368 | 0.11 | Capric acid methyl ester |
| 3 | 17.990 | 1,838,829 | 0.08 | 8-Nonynoic acid, methyl ester |
| 4 | 18.040 | 4,987,601 | 0.21 | Cyclopropanepentanoic acid, 2-undecyl-, methyl ester, trans- |
| 5 | 18.330 | 231,404,984 | 9.67 | Behenic acid, methyl ester |
| 6 | 19.215 | 2,188,114 | 0.09 | Eicosanoic acid, methyl ester |
| 7 | 19.915 | 27,366,302 | 1.14 | 9,12-Hexadecadienoic acid, methyl ester |
| 8 | 20.150 | 1,062,259,533 | 44.37 | 7,10-Hexadecadienoic acid, methyl ester |
| 9 | 20.245 | 237,629,754 | 9.93 | 7,10,13-Hexadecatrienoic acid, methyl ester |
| 10 | 20.320 | 91,278,589 | 3.81 | Stearic acid, methyl ester |
| 11 | 20.41 | 3,125,744 | 0.13 | 4-Tridecen-6-yne, (Z)- |
| 12 | 20.470 | 10,389,319 | 0.43 | 9,12-Octadecadienoic acid, methyl ester |
| 13 | 20.585 | 4,419,646 | 0.18 | 13,16-Octadecadienoic acid, methyl ester |
| 14 | 21.080 | 1,716,986 | 0.07 | Tetradecanoic acid, 12-methyl-, methyl ester |
| 15 | 21.860 | 371,013,227 | 15.50 | 11-Octadecenoic acid, methyl ester |
| 16 | 22.015 | 103,093,532 | 4.31 | Hexadecanoic acid, 15-methyl-, methyl ester |
| 17 | 23.160 | 3,717,773 | 0.16 | 7-Octadecynoic acid, methyl ester |
| 18 | 23.450 | 135,982,486 | 5.68 | 15-Tetracosenoic acid, methyl ester |
| 19 | 23.585 | 31,833,165 | 1.33 | Heneicosanoic acid, methyl ester |
| 20 | 24.155 | 2,642,571 | 0.11 | 13-Docosenoic acid, methyl ester |
| 21 | 24.320 | 2,147,984 | 0.09 | Triacontanoic acid, methyl ester |
| 22 | 24.930 | 33,219,152 | 1.39 | 10-Octadecenoic acid, methyl ester |
| 23 | 25.070 | 20,624,212 | 0.86 | Tetracosanoic acid, methyl ester |
| 24 | 26.335 | 2,371,814 | 0.10 | Oleic acid, methyl ester |
A recent study in Saudi Arabia demonstrated that the main constituents of LSO are β-amyrin (31.33%), 9,12,15-octadecatrienoic acid methyl ester (15.97%), 9-octadecenoic acid methyl ester (11.93%), α-amyrin (9.32%), 11-eicosenoic acid methyl ester (6.64%), 9,12-octadecadienoic acid (6.03%), and hexadecanoic acid methyl ester (5.24%) (Abo El-Maati et al., 2016). This slight variation in the chemical composition of LSO between the two studies may be due to environmental factors, including edaphic factors of soil and weather, geographical location, climate conditions, method of extraction, and season of collection. It is well known that LSO is an abundant source of omega-3 and omega-6, which makes it suitable for use as a food supplement and for medicinal purposes (Diwakar et al., 2010).
3.2. Microbiological activity of LSO
The investigation of new natural products is considered to be a promising approach to discover new sources of antimicrobial activity. This is particularly important due to the global threat of bacterial resistance to currently used antibiotics, which affect people worldwide (Nothias et al., 2016).
The results of antimicrobial activity of LSO against different bacteria and fungi are presented in Table 2. The results clearly show that bacteria and fungi tested were susceptible to LSO, for all of which the MIC was 47.5 mg/ml, except of S. enterica, which showed a higher MIC of 90 mg/ml. The MBC of LSO was found to be equivalent to 100 mg/ml for inhibiting the growth of all bacteria and fungi. This comparable antimicrobial activity against the tested gram-negative and gram-positive bacteria and the fungus reveals that LOS exhibits broad-spectrum antimicrobial action. A recent study conducted in another province of Saudi Arabia (Abo El-Maati et al., 2016) showed comparable antimicrobial activity of LSO, in terms of its spectrum, against tested microorganisms, except for S. aureus, which was resistant to the LSO used. The MIC in our study was lower than that observed in the study by Abdel Karim et al. (2017), which may be due to the differences in the method of antimicrobial testing used and the method of oil extraction.
Table 2.
Antimicrobial activity of Lepidium sativum seed oil.
| Microorganism | MIC (mg/ml) | MBC (mg/ml) |
|---|---|---|
| Staphylococcus aureus | 47.5 | 190 |
| Bacillus subtilis | 47.5 | 190 |
| Escherichia coli | 47.5 | 190 |
| Pseudomonas aeruginosa | 47.5 | 190 |
| Salmonella enterica | 95 | 190 |
| Klebsiella pneumoniae | 47.5 | 190 |
| Candida albicans | 47.5 | 190 |
| DMSO | ND | ND |
ND denotes: Not detected.
A previous study conducted by Adam et al. (2011) showed that petroleum ether, aqueous, and methanolic extracts of L. sativum seed obtained from Sudan exhibit antimicrobial activity against six opportunistic microrganisms: S. aureus, E. coli, K. pneumoniae, Proteus vulgaris, P. aeruginosa, and the fungus C. albicans. In this previous study, petroleum ether at different concentrations (2.5%, 5%, and 10%) was found to be a better solvent for extracting antimicrobial substances from L. sativum seeds than methanol and water (Adam et al., 2011). Recently, another study conducted in Egypt showed that L. sativum extract exhibits antimicrobial activity against different gram-negative and gram-positive bacteria, and in accordance with our study, there were no significant differences between the gram-positive and gram-negative bacteria in their sensitivity to the tested extracts (Abo El-Maati et al., 2016). In a different study, the crude extract from Ethiopian L. sativum seeds exhibited antimicrobial properties against tested fungi (A. niger, F. oxysporum, and F. solani) and bacteria (E. coli, S. typhi, B. subtilis, and S. aureus) (Berehe and Boru, 2014). However, in all of these studies (Adam et al., 2011; Berehe and Boru, 2014, Abo El-Maati et al., 2016), the values of MIC were not calculated for L. sativum extracts; thus, we could not compare the antimicrobial activities of LSO observed in our study and extracts in terms of concentrations.
3.3. Free radical scavenging activity of LSO using DPPH assay
Antioxidant activity of oil was determined using free radical scavenging activity (DPPH) by adding different concentrations of oil to DPPH. DPPH remaining amount of was assessed at 30 min with λ level at 520 nm, then the% of inhibition was calculated. LSO showed dose-dependent scavenging of DPPH, with an IC50 value of 40 mg/ml (Table 3). Compared with the finding of a previous study by Umesh et al. in India, wherein the IC50 was 25 mg/ml (Umesha and Naidu, 2015), the IC50 obtained in our study was higher. Other studies conducted in India and Morocco to assess the antioxidant activity of the methanolic extract of L. sativum found that the IC50 values are 62 µg/ml (Ahamad et al., 2015) and 925 ppm (Chatoui et al., 2016), respectively.
Table 3.
Antioxidant activity of Lepidium sativum seed oil.
| Concentrations of L. sativum seed oil (mg/ml) | % Inhibition |
|---|---|
| 40 | 50 ± 0.7 |
| 32 | 40 ± 0.6 |
| 24 | 32.02 ± 0.11 |
| 16 | 31.2 ± 0.4 |
| 8 | 28.7 ± 0.3 |
| 5 | 22.15 ± 0.2 |
Values are expressed as mean ± SD (n = 3).
3.4. Anti-inflammatory activity of LSO
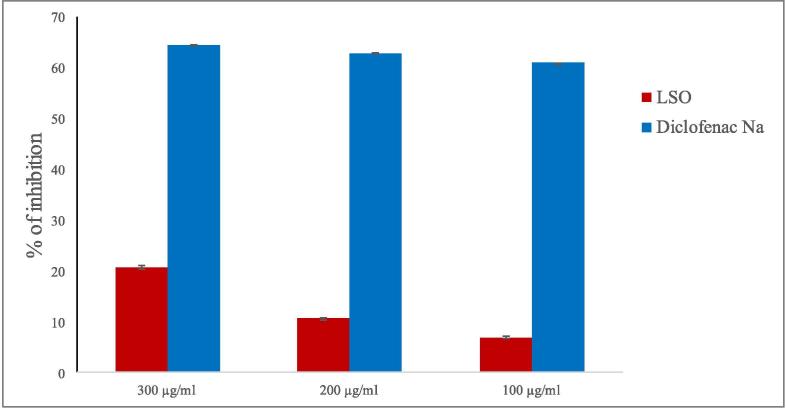
The analysis showed concentration-dependent protection of the cell membrane. The levels of protection of LSO observed were 21%, 11%, and 7% for the concentrations of 300, 200, and 100 μg/ml, respectively (Fig. 1). The standard drug, diclofenac Na, showed 65%, 63%, and 61% membrane stabilization at concentrations of 300, 200, and 100 μg/ml, respectively.
Fig. 1.
Anti-inflammatory activity of Lepidium sativum seed oil.
4. Conclusions
In summary, extracted seeds' oil of L. sativum was evaluated for its antimicrobial, antioxidant, and anti-inflammatory activities. Upon chemical analysis, 7,10-hexadecadienoic acid, 11-octadecenoic acid, 7,10,13-hexadecatrienoic acid, and behenic acid was identified as the major compounds. LSO were active against tested bacteria and fungus, suggesting its broad-spectrum antimicrobial activity. The oil also showed dose-dependent antioxidant and anti-inflammatory activities. The results reveal that extracted seeds' oil of L. sativum chemical products could be valuable sources of bioactive compounds with substantial biological activities.
Acknowledgments
The authors are grateful to the generous support and endless cooperation of the Deanship of Scientific Research, King Saud University (Research Group Grant Number RGP-1438-003).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel Karim M., Sufian A., Kamal M.S., Inas O. GC–MS analysis and antimicrobial activity of fixed oil from Saudi Lepidium sativum (crusifereae) seeds. Int. J. Adv. Res. 2017;5(3):1662–1670. [Google Scholar]
- Abo El-Maati M.F., Labib S., Al-Gaby A.M.A., Ramadan M.F. Antioxidant and antibacterial properties of different extracts of garden cress (Lepidium sativum L.) Zagazig J. Agric. Biochem. Appl. 2016;43(5):1685–1697. [Google Scholar]
- Adam S., Salih S., Abdelgadir W. In vitro antimicrobial assessment of Lepidium sativum L. seeds extracts. Asian J. Med. Sci. 2011;3(6):261–266. [Google Scholar]
- Ageel A.M., Tariq M., Mossa J.S., Al-Yahya M.A. King Saud University Press; Riyadh, Saudi Arabia: 1987. Plant Used in Saudi Folk Medicine: Experimental Report Submitted to the King Abdul Aziz City for Science and Technology. [Google Scholar]
- Ahamad R., Mujeeb M., Anwar F., Ahmad A. Phytochemical analysis and evaluation of anti-oxidant activity of methanolic extract of Lepidium sativum L. seeds. Der. Pharm. Lett. 2015;7(7):427–434. [Google Scholar]
- Al-Sheddi E.S., Farshori N.N., Al-Oqail M.M., Musarrat J., Al-Khedhairy A.A., Siddiqui M.A. Protective effect of Lepidium sativum seed extract against hydrogen peroxide-induced cytotoxicity and oxidative stress in human liver cells (HepG2) Pharm. Biol. 2016;54(2):314–321. doi: 10.3109/13880209.2015.1035795. [DOI] [PubMed] [Google Scholar]
- Berehe S.G., Boru A.D. Phytochemical screening and antimicrobial activities of crude extract of Lepidium sativum seeds grown in Ethiopia. Int. J. Pharm. Sci. Res. 2014;5(10):4182–4187. [Google Scholar]
- Bigoniya P., Shukla A. Phytopharmacological screening of Lepidium sativum seeds total alkaloid: hepatoprotective, antidiabetic and in vitro antioxidant activity along with identification by LC/MS/MS. Pharm. Nutrit. 2014;2(3):90. [Google Scholar]
- Chatoui K., Talbaoui A., Aneb M., Bakri B., Harhar H., Tabyaoui M. Phytochemical screening, antioxidant and antibacterial activity of Lepidium sativum seeds from Morocco. J. Mater. Environ. Sci. 2016;7(8):2938–2946. [Google Scholar]
- Clinical and Laboratory Standards Institute, 2007. Performance Standards for Antimicrobial Susceptibility Testing. Seventh Informational Supplement CLSI document M100-S17, Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania, USA.
- Diwakar B.T., Dutta P.K., Lokesh B.R., Naidu K.A. Physicochemical properties of garden cress (Lepidiumsativum L.) seed oil. J. Am. Oil Chem. Soc. 2010;87:539–548. [Google Scholar]
- Diwakar B.T., Lokesh B.R., Naidu K.A. Modulatory effect of alpha-linolenic acid-rich garden cress (Lepidium sativum L.) seed oil on inflammatory mediators in adult albino rats. Br. J. Nutr. 2011;106(4):530–539. doi: 10.1017/S0007114511000663. [DOI] [PubMed] [Google Scholar]
- Doke S.G.M. Garden cress (Lepidium sativum L.) seed-an important medicinal source: a review. J. Nat. Prod. Plant Resour. 2014;4:69–80. [Google Scholar]
- Lopes-Lutz D., Alviano D.S., Alviano C.S., Kolodziejczyk P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008;69(8):1732–1738. doi: 10.1016/j.phytochem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Moser B.R., Shah S.N., Winkler-Moser J.K., Vaughn S.F., Evangelista R.L. Composition and physical properties of cress (Lepidiumsativum L.) and field pennycress (Thlaspiarvense L) oils. Ind. Crop Prod. 2009;30:199–205. [Google Scholar]
- Nothias L.F., Knight R., Dorrestein P.C. Antibiotic discovery is a walk in the park. Proc. Natl. Acad. Sci. U.S.A. 2016;113(51):14477–14479. doi: 10.1073/pnas.1618221114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra R.H., Naidu K.A. Eugenol and n-3 rich garden cress seed oil as modulators of platelet aggregation and eicosanoids in Wistar albino rats. Open Nutraceut. J. 2011;4:144–150. [Google Scholar]
- Rahman M.A., Mossa J.S., Al-Said M.S., Al-Yahya M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: a report on seven plant families. Fitoterapia. 2004;75(2):149–161. doi: 10.1016/j.fitote.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Raish M., Ahmad A., Alkharfy K.M., Ahamad S.R., Mohsin K., Al-Jenoobi F., Al-Mohizea A.M., Ansari M.A. Hepatoprotective activity of Lepidium sativum seeds against D-galactosamine/lipopolysaccharide induced hepatotoxicity in animal model. BMC Complem. Altern. Med. 2016;16(1):501. doi: 10.1186/s12906-016-1483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakran M., Selim Y., Zidan N. A new isoflavonoid from seeds of Lepidium sativum L. and its protective effect on hepatotoxicity induced by paracetamol in male rats. Molecules. 2014;19(10):15440–15451. doi: 10.3390/molecules191015440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesha S.S., Naidu K.A. Antioxidants and antioxidant enzymes status of rats fed on n-3 PUFA rich Garden cress (Lepidium sativum L.) seed oil and its blended oils. J. Food Sci. Technol. 2015;52(4):1993–2002. doi: 10.1007/s13197-013-1196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William, H., 1980. AOCS, Official Methods of Analysis of the Association of Official Analytical Chemist, Washington DC, USA.