Abstract
The field of dental implantology has made progress in recent years, allowing safer and predictable oral rehabilitations. Surely the rehabilitation times have also been reduced, thanks to the advent of the new implant surfaces, which favour the osseointegration phases and allow the clinician to rehabilitate their patients earlier. To carry out this study, a search was conducted in the Pubmed, Embase and Elsevier databases; the articles initially obtained according to the keywords used numbered 283, and then subsequently reduced to 10 once the inclusion and exclusion criteria were applied. The review that has been carried out on this type of surface allows us to fully understand the features and above all to evaluate all the advantages or not related. The study materials also are supported by a manufacturing company, which provided all the indications regarding surface treatment and confocal microscopy scans. In conclusion, we can say that, thanks to these new surfaces, it has been possible to shorten the time necessary to obtain osseointegration and, therefore, secondary stability on the part of implants. The surfaces, therefore, guarantee an improved cellular adhesion and thanks to the excellent wettability all the biological processes that derive from it, such as increases in the exposed implant surface, resulting in an increase in bone-implant contact (BIC).
Keywords: dental implants, surface properties, osseointegration, bone-implant interface
1. Introduction
1.1. Rationale
Currently, implants are almost all made of titanium. The most used are the endosseous screw-type, in most cases left submerged under the gingiva for a reasonable period depending on the site. Dental implantology is, therefore, subdivided into endosseous and juxta-osseous, the latter using only non-submerged fixed-grid systems and, therefore, for non-osseointegrable seat and loading modalities, if made of chrome-cobalt-molybdenum, or even osseointegratable if made in titanium and inserted with special surgical techniques favouring the new bone formation above their structure.
The endosseous implantology is currently the most widespread, and uses cylindrical/conical implants (the actual implant body) threaded on the outside and with an internal connection of varying conformation for the emerging part (stump) and rarely cylinders or cones without external threads, but with similar internal connection systems for the abutment, screws full of a single body (implant body and abutment made from solid and, therefore, without any connection), blades and needles. According to the surgical protocol we will, therefore, have submerged and non-submerged implantology (transmucosal); based on the timing of use (functionalization) we will have immediate, anticipated, deferred loading. The most used material for the production of implants is titanium, in a commercially pure form or in its alloys for dental use, a biocompatible material that does not involve reactions from the organism (popularly, but erroneously, known as rejection). The implants, positioned in the patient’s bone will be strongly incorporated into it by the physiological mechanisms of bone regeneration, i.e., osteointegration will take place both in the event of delayed loading and in the case of immediate loading. The trend of recent years has been to accelerate dental rehabilitation as much as possible, also undertaking immediate rehabilitation protocols. This has been possible thanks to the improvement of the geometries of the dental implants, but also to the improvement of the surfaces, thanks to surface treatments that allow a better bone-implant interaction. We have evaluated these surfaces and we will analyse them in detail in the following sections, once we have also made a quick reference to titanium surface treatments.
1.2. Objectives
The objective of this study is to evaluate the characteristics of sandblasted and acid etched (SA) implant surfaces, their treatment methods and surface interactions [1,2,3,4,5,6,7,8,9,10]. The authors then carry out a systematic review of the results in the literature on the study, the characteristics of this surface. Furthermore, to support the literature, a confocal microscopic analysis of a surface SA [11,12,13,14,15,16]. Implantology (dental) means that set of surgical techniques designed to functionally rehabilitate a patient suffering from total or partial edentulism through the use of dental implants or devices, metallic or not, surgically inserted into the mandibular or maxillary bone, or above it but under the gingiva, acting in turn to allow the connection of fixed or mobile prostheses, for the restitution of the masticatory function. These implants can be of different shapes, inserted in different locations with different techniques and then connected to the prosthesis at different times.
2. Material and Methods
2.1. Protocol and Registration
This review is registered at PROSPERO with ID number 136038. PROSPERO is an international database of prospectively registered systematic reviews in health and social care.
2.2. Eligibility Criteria
The following focus question was developed according to the population, intervention, comparison, and outcome (PICO) study design:
What are the surface characteristics of sandblasted and acid etched dental implants?
2.3. Information Sources
The search strategy incorporated examinations of electronic databases, supplemented by hand searches. A search of PubMed, Dentistry, and Oral Sciences Source, for relevant studies published in the English language. A hand search of the reference lists in the articles retrieved was carried out to source additional relevant publications and to improve the sensitivity of the search.
2.4. Search
The keywords used in the search of the selected electronic databases included the following:
(“sandblasted” OR “acid etched”) AND (“dental implant” OR “implantology”)
The choice of keywords was intended to collect and to record as much relevant data as possible without relying on electronic means alone to refine the search results.
2.5. Selection of Studies
Two independent reviewers singularly analysed the obtained papers in order to select inclusion and exclusion criteria. For the stage of reviewing full-text articles, a complete independent dual revision was performed.
2.6. Study Selection
After the first literature analysis, all article titles were screened to exclude irrelevant publications, case reports and non-English language publications. Then, studies were not selected based on data obtained from screening the abstracts. The final stage of screening involved reading the full texts to confirm each study’s eligibility, based on the inclusion and exclusion criteria.
The full text of all studies of possible relevance was obtained for assessment against the following inclusion criteria:
All randomized clinical trials about the use of SA implant surfaces on humans;
Clinical follow up about SA implant surface use on humans; and
All confocal studies on SA surfaces.
The applied exclusion criteria for studies were as follows:
Studies involving patients with other specific diseases, immunologic disorders, or other oral risk-related systemic conditions;
Not enough information regarding the selected topic; or
No access to the title and abstract in the English language.
The review included studies on humans and animal published in the English language. Letters, editorials, and PhD theses were excluded. The review included all human prospective and retrospective follow-up studies and clinical trials, cohort studies, case–control studies, and case series studies, animal studies and literature review published, on sandblasted and acid etched dental implants uses for rehabilitation and implantology. The data were independently extracted from studies in the form of variables, according to the aims and themes of the present review, as listed onwards.
2.7. Data Collection Process
Data were collected from the included articles and arranged in the following fields (Table 1):
Author (Year)—Authors and year of publication;
Sample Size—Size of sample evaluated;
Torque—Implant positioning torque nm (Newton/meter);
Follow up—Implant follow up period (maximum value);
Statistic—Statistical results; and
Type of Parameters evaluated—Evaluated parameters about the implant.
Table 1.
Selected studies evaluated parameters. (✔ = histologic examination done).
| Author (Year) | Sample Size | Torque | Follow up | Statistic | Type of Parameters Evaluated | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| RFA Evaluation | Histologic | Histomophometric | Prothestic Failures | Implant Failures | In Vitro | |||||
| Novellino et al. (2017) [56] | 21 | 35.125 ± 4.498 | 1 y | p < 0.01 | CG: 42–81 SA |
|||||
| TG: 32.5–82.5 MSA | ||||||||||
| Mangano et al. (2017) [57] | 10 | Not significant | ✔ | TG: BIC 35.9% BD 31.8 |
||||||
| CG: BIC 29.9% BD 32.5% | ||||||||||
| Schmitt et al. (2015) [55] | 10 | BIC 0.002; | ✔ | Machined | ||||||
| SA | ||||||||||
| Hydroxyapatite surface | ||||||||||
| Cannizzaro et al. (2016) [58] | 50 | >50 | 6 m | Not significant | ✔ | ✔ | ||||
| Schwarz et al. (2013) [59] | 30 | 8 w | p < 0.05 | ✔ | ||||||
| Corvino et al. (2012) [60] | 15 | 2 m | BIC p = 0.028 | ✔ | ✔ | ✔ | ||||
| Karabuda et al. (2010) [61] | 22 | CG: 25.48 | 6 w | p < 0.05 | CG: 58.21 | ✔ | ✔ | |||
| TG: 23.75 | TG: 58.15 | |||||||||
| D’Avila et al. (2010) [62] | 7 | 2 m | BIC significant | Machined: BIC 10.40% | ✔ | |||||
| Sandblasted: BIC 22.19% | ||||||||||
| Shibli et al. (2010) [63] | 10 | 2 m | BIC p < 0.05;BA not significant | ✔ | ✔ | ✔ | ||||
| Khang et al. (2001) [64] | 97 | 6 m | ✔ | ✔ | ||||||
2.8. Data Items
PICO has been used to conduct the review, and of any assumptions or simplifications made.
2.9. Risk of Bias Assessment
Assessment of risk of bias was undertaken by two authors during data extraction process (G.C. and L.F.). For the included studies, this was conducted using the Cochrane Collaboration’s two-part tool for assessing risk of bias [17,18,19,20,21,22]. An overall risk of bias was then assigned to each trial according to Higgins et al. [19]. The levels of bias were classified as follows: low risk, if all the criteria were met; moderate risk, when only one criterion was missing; high risk, if two or more criteria were missing; and unclear risk, if too few details were make a judgement of certain risk assessment.
2.10. Implantology and Different Surfaces
A dental implant is an alloplastic structure inserted by means of appropriate surgery, in the structure of the jaw bones to rehabilitate a patient who has a condition of edentulism. The implants have characteristics related to the materials with which they are made and to their geometry, macroscopic and microscopic. The material normally used is commercially pure titanium, this material has characteristics of biocompatibility, low density, electrochemical stability, high mechanical strength and sufficient rigidity [23,24,25,26,27,28,29]. The macroscopic implant geometries evaluate macroscopic differences in the shape of dental implants. In this case the turns of the dental implant with different dimensions, number and pitch may be affected. Coils may be present or not [30,31,32,33,34,35,36,37,38,39,40]. The function of the loops is to efficiently distribute the masticatory load and to guarantee stability in the early post-surgical phases of the implant [41,42,43]. On the other hand, secondary or surface geometries are the basis of the osseointegration potential of the implant. The methods adopted for surface treatment are different. We certainly distinguish additive and subtractive techniques. Subtractive techniques include:
Sandblasting: the bombardment of titanium surfaces with granules of variable diameter of oxides (titanium dioxide, aluminum oxide, zirconium dioxide and silicon carbide)
Acid etching: carried out with sulfuric, hydrofluoric or hydrochloric acid according to different protocols.
Combination of sandblasting and acid etching (SA)
Oxidation in a galvanic bath
Elettroerosion (EDM)
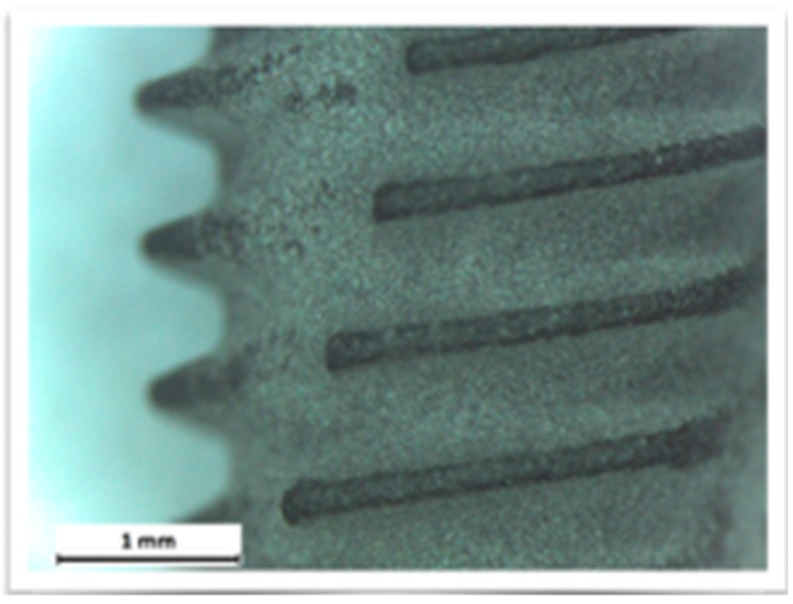
The additive techniques use a flow of plasma to deposit on the implant surface different materials, such as titanium powder, hydroxyapatite powder, or titanium microspheres. The degree of bacterial proliferation on these surfaces is also influenced by the type of surface [44,45,46,47,48,49,50,51,52] (Figure 1, Figure 2 and Figure 3).
Figure 1.
Competitor 1.
Figure 2.
Test group surface.
Figure 3.
Competitor 2.
2.11. 3D Confocal Microscopy
The confocal microscope is an optical microscope, a scientific instrument that is based on a technology aimed at significantly increasing the spatial resolution of the sample, eliminating the halos due to the light diffused by the out-of-focus planes of the preparation.
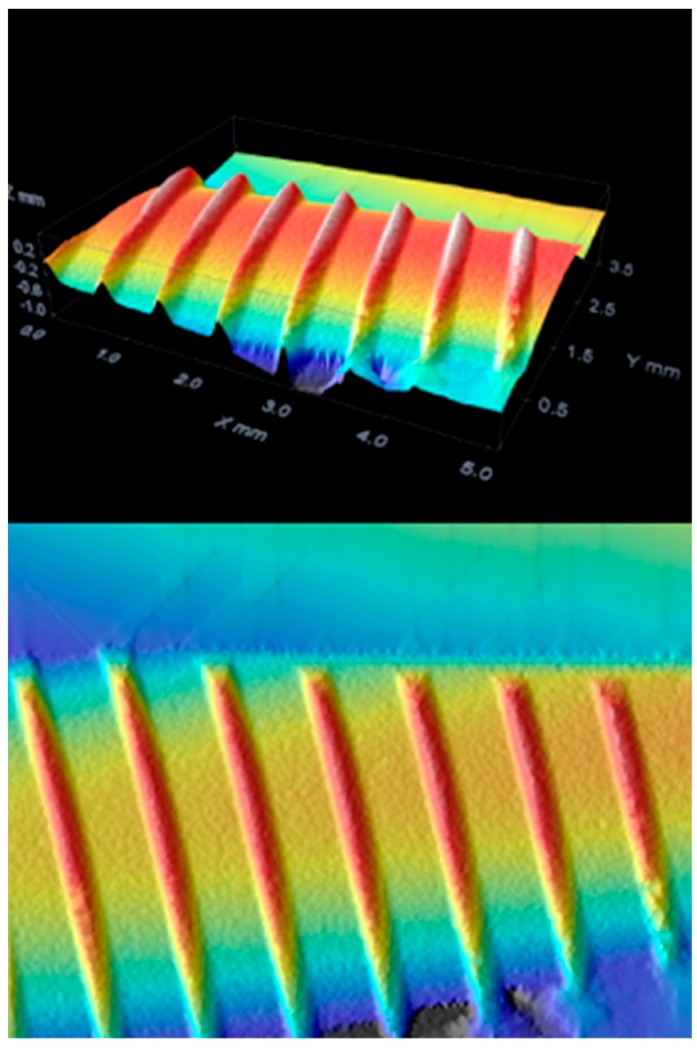
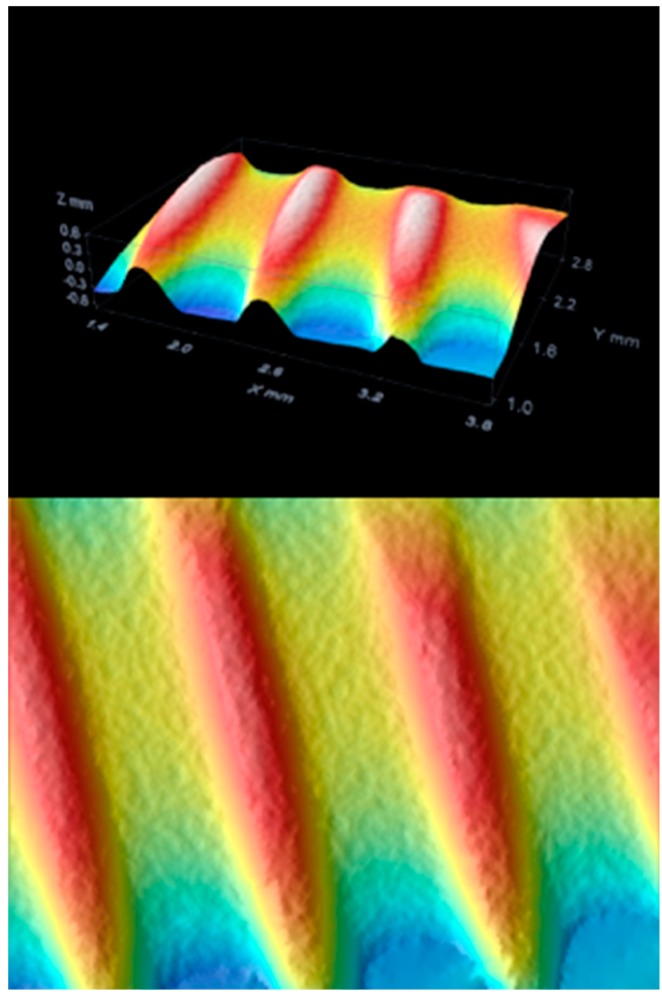
The instrument operates in the conventional field of the magnifications of normal optical microscopy, and is schematically constituted by a normal transmission microscope to which an apparatus that deals with illuminating and detecting the image of an illuminated sample with a point-to-point scan is superimposed. There are different techniques to achieve this: rotating disk (Nipkow disk), programmable array microscopes (PAM), and laser. The latter type, the most widespread and called CLSM (confocal laser scanning microscope), is an advanced fluorescence microscope that allows to focus with extreme precision a laser on the preparation, greatly increasing the resolution and depth of field. Its light source consists of one or more lasers, generally semiconductor, for each different frequency of excitation required. The light beam direction mechanism is managed by computerized systems. The images obtained, by synchronizing the detection device with the excitation beam, are particularly defined and spectacular, and can allow the different molecules present in the preparation to be highlighted in different colors, making it possible to appreciate their three-dimensionality [53,54] (Figure 4, Figure 5, Figure 6 and Figure 7).
Figure 4.
Competitor 1 confocal microscopy.
Figure 5.
Test group confocal microscopy.
Figure 6.
Competitor 2 confocal microscopy.
Figure 7.
Roughness differences.
2.12. Risk of Bias across Studies
There were several limitations present in the current review. The current review includes studies written in English only, which could introduce a publication bias. There were various degrees of heterogeneity in each study design, case selection, and treatment provided among studies.
3. Results
The results were collected from all the articles taken into consideration, articles that discuss dental materials and their use in the field of rehabilitative and restorative dentistry. In this article we have taken into consideration therapeutic planning, aesthetic and functional rehabilitation articles, as well.
3.1. Inclusion Study Flow
Article review and data extraction were performed according to PRISMA flow diagram (Figure 8). The initial electronic and hand search retrieved 283 articles. After titles and abstracts were reviewed, only 10 articles were included.
Figure 8.
PRISMA flow chart.
3.2. Study Characteristics and Summary Measures
During the selection of the studies the individual characteristics of each one were evaluated. The characteristics assessed mainly concern the clinical signs and follow up period as described in Section 2.12 (Table 2)
Bone to implant contact (BIC)
Bone Density
Implant or prosthetic failures
Soft tissue signs
Histological or histomorphological evaluation
RFA
Region of interest (ROI) percentage
Table 2.
Topics of SA surface studies.
| Osstem SA® (Seoul, South Korea) Surface Field of Study | References |
|---|---|
| Implant survival rate | [66,67] |
| Implant surface | [11,12,13,14,15,16] |
| Implant loading time | [68,69] |
| Prosthetic study | [70,71,72,73,74,75,76,77,78] |
| Maxillary sinus lifts and implant | [79,80,81,82] |
| Bone augmentation and implant | [83,84,85,86,87] |
| Implant stability | [88,89,90,91,92] |
| FEM on implant components | [93] |
| Microbial flora on implant | [94] |
| Implant studies on animals | [95,96,97,98,99] |
3.3. Risk of Bias within Studies
Many studies have been evaluated with an unclear risk of bias, as there is not enough information to establish the risk. Only one study presents assessments that allows a risk of low bias [55].
3.4. Results of Individual Studies and Synthesis of Results
The results of the individual studies were collected accordingly to the data obtained after the research as follows in Table 1.
3.5. Additional Analysis
In addition to reviewing and supporting the literature, a study was conducted on the surface of a test dental implants. The surface was evaluated from different points of view, first with optical microscopy and subsequently with confocal microscopy to evaluate those that are the characteristics related to surface roughness. The tested fixture has a macroscopic geometry, for details see Section 2.10 with loops at intervals of 0.8 mm and a depth of 0.25 mm. The geometry of the loops is progressive, and reach a depth of 0.5 mm in the apical direction. The surface is an SA surface. Three different implant fixtures were evaluated: A test dental implant and two other competitors, named as Competitor 1 and Competitor 2.
The images obtained with optical microscopy are shown in Figure 1, Figure 2 and Figure 3.
On examination with confocal optical microscopy, the differences between the test surface and the competitor implants are evident, as shown in the graph.
Optical microscopy does not show statistical differences between implants using surface analysis software, while confocal microscopy analysis shows a clear difference in surface roughness. This characteristic is closely linked to cell proliferation, healing of the fixture in the bone, then osseointegration and BIC [65].
4. Discussion
4.1. Summary of Evidence
There are many studies about SA surface implants and, more specifically, about tested surface implants. These articles take into consideration different topics related to implant surgery and can be summarized as follows:
The SA surfaces are, therefore, used for the construction of dental implants. After this surface treatment the implants have been subjected to strict quality controls and studies that evaluate their reliability. The studies present in the literature, as shown in Table 2, are numerous and all go to evaluate different aspects: First of all, the implant survival, hence, the clinical conditions of the implant subjected to function; the loading protocols of the dental implant, where many studies concern the prosthesis itself; the differences between screwed or cemented prostheses on implants, use of bars, built with different materials and made with different methods, and the very important studies carried out with the Finite Element Method (FEM) [93,100,101,102]; articles concerning pre-implant surgical techniques, including bone grafts, maxillary sinus augmentations or bone regeneration [103,104,105,106,107,108,109,110,111]; and, finally, the study of the bacterial flora on the surface of the dental implants. With attention we will evaluate these last works. There is talk of success of a dental implant when it meets a series of criteria once it is placed in the oral cavity and prosthesized. These criteria are related to certain clinical parameters, to the symptoms and to the functionality of implant-prosthetic rehabilitation [66,67].
According to Novellino et al. [56] implants with hydrophilic sandblasted and acid etched (SAE) surfaces osseointegrate faster than implants with SAE surfaces. SA surfaces and SAE surfaces have only a different nomenclature, but both regard sandblasted acid etched surfaces. In this study, Novellino et al. used a modified SAE surface (Acqua Neodent®, Crawley, West Sussex, UK) with improved hydrophilic properties. The titanium oxide layer on the surface of the implants is generally electronegative. The consequence of this particular characteristic is the reduction of the contact between the implant surface and the blood, this also being electronegative. Implants with a hydrophilic surface are characterized by a layer of electropositive titanium oxide. The physical-chemical activation of the water surface modifies the negatively-charged surface in positive, attracting ions from the blood, improving the contact as proven by in vitro studies [112]. The stability gain is faster according to radiofrequency analysis (RFA) measurement. According to Mangano et al.’s study [57] on BioMed Research International, their histomorphometric study shows the healing phases of a calcium-incorporated surface implant and an SA surface implant. According to this RCT the first type of surface increased the peri-implant endosseous healing properties. According to Schmitt et al. [55] surfaces with a biomimetic layer can improve the healing process. This study revealed a long-term effect to the biofunctionalization with a peri-implant bone formation in regions of poor bone quantity. According to Cannizzaro et al. [58] in an up to six-month loading machined and roughened flapless-placed implant, similar results have been provided. An article by Schwarz et al. in 2013 [59] says that a modified SA surface may have the potential to enhance soft tissue adhesion. Corvino et al. [60] underline that implant surface topography entails on cells proliferation. According to Karabuda et al. [61] modified sandblasted and acid etched surface demonstrated a better stability and reduced healing time, having a positive success and survival rate at the end of a 15-month follow up. D’Avila et al. [62] demonstrated how SA surfaces present better results than the machined surfaces. They evaluated BIC, and reactive oxygen species concentration on smokers [113]. According to Shibli et al. [63] bioceramic molecular impregnated surfaces heal positively. According to Khang et al. [64] SA surfaces show better success rates in the conditions of poor quality or soft bone.
The articles taken into consideration often present different surgical techniques for the positioning of dental implants. These techniques may first of all take into account the clinical conditions of the patients, which may represent problems related to healing or, in some cases, even to the risk of the patients [114,115,116,117]. It is very difficult to manage patients with congenital bleeding disorders that require careful attention. It is necessary to emphasize the perioperative management of the patient with bleeding disorders [118]. Cases of rare bleeding disorders, such as congenital afibrinogenemia, have been reported in the literature. The bleeding manifestations with gingival bleeding were repeated in this patient. An individual approach is needed if the patient needs maxilofacial surgery [119]. Venous thromboembolism is the second most common medical complication, the second most common cause of increased length of hospital stay, the third most common cause of mortality and a significant increase in financial cost [117,120,121]. Surgical risk may also be related to local factors related to the patient, such as the presence of anatomical abnormalities, oral lesions or oral neoformations [122]. Certainly, these situations can make the placement of an implant difficult or secondary to other surgeries that may also be reconstructive [103]. Implant surgery should certainly be avoided in the case of biomolecular markers positive for potentially malignant lesions or the presence of acute or chronic inflammatory processes in progress [113,123,124]. Furthermore, before talking about the surgical phase, it is necessary to set patients with a correct pharmacological protocol and correct antibiotic prophylaxis [125,126,127]. Implant surgery, therefore, requires, after a correct anaesthesia, a mucous incision in order to set up and elevate a surgical flap, so as to highlight the underlying bone tissue and proceed with the implant preparation. This phase is not necessary in flapless techniques, which do not include incisions and flaps. Implant preparation can be done with rotating burs, osteotomes or piezo surgical instruments [128,129]. Once the preparation is complete, implant placement is performed, followed by suturing. Returning to the bioengineering discourse, rather than the surgical phase, the SA surfaces show excellent osseointegration results in agreement with all the examined works, and some works show results with modern, modified or improved surfaces. During the writing of this study it was certainly necessary to pay attention also to other implant surfaces, the trend of recent years, in fact, is to improve the surface technologies of the plants so as to guarantee better performance, performances that sometimes test the biological principles of the organism [123]. The micro- and nanostructured surfaces that have been made available on the market today [10], and the SA surfaces are excellent compromises, and thanks to the presence of long-term follow-up the literature reassures us about this. Improved dental materials technologies, and efficient and conservative surgical techniques have enabled us to rehabilitate patients in an increasingly predictable manner. Dental care tends to be more conservative than in the past, above all, thanks to advances in medicine [10,93,103,113,114,117,124,130,131,132,133,134,135].
4.2. Limitations
Efforts were concentrated in the study of the SA surface, although the other surfaces have been evaluated, there are excellent studies that express favourable opinions on nanotexturized surfaces that are not the subject of this review. SA surfaces, however, show excellent clinical results.
5. Conclusions
In conclusion, we can say that the SA surfaces obtain good results highlighted by the works both from a clinical, biomechanical and histological point of view. The healing phases are stimulated by this type of implant surface, therefore, sandblasting and acid etching appears to be a safe method that produces reliable and predictable surfaces to what emerges from the results. Furthermore, the experimental study carried out by the University of Messina provided important information regarding the SA surface of dental implants. The roughness of this implant has emerged to be greater than that of competitors, guaranteeing a whole series of clinical aspects already dealt with in the manuscript. The literature also offers good news regarding the interaction by eukaryotic and prokaryotic cells with this surface, showing also excellent characteristics in case of contamination by a biofilm and, therefore, in the progress of the peri-implant pathology. Implants built with these characteristics are reliable in accordance with the results and have good integration with hard and soft tissues.
Abbreviations
| BIC | Bone to implant contact; |
| BD | Bone area; |
| ROI | Reactive oxygen species; |
| CG | Control group; |
| TG | Test group; |
| SA | Sandblasted acid etched surface; |
| MSA | Modified SA; |
| Ti | Titanium; |
| EDM | Electroerosion; |
| RFA | Radiofrequency analysis; |
| FEM | Finite Element Method. |
Author Contributions
L.F. is the author responsible for the paper’s writing. G.C. is the chief reviewer for the collected data, responsible for the language proofing and revision. G.C. and L.F. were responsible for collecting data and creating the tables. G.C., G.I., D.S. and L.F. were responsible for funding acquisition data. M.C. and L.F. were responsible for editing, original data and text preparation. M.R. is responsible for supervision. M.C. was responsible for supervision, and is the corresponding author.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wirsching K., Lehle K., Jacob P., Gleich O., Strutz J., Kwok P. Influence of Surface Processing on the Biocompatibility of Titanium. Materials. 2011;4:1238–1248. doi: 10.3390/ma4071238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traini T., Murmura G., Sinjari B., Perfetti G., Scarano A., D’Arcangelo C., Caputi S. The Surface Anodization of Titanium Dental Implants Improves Blood Clot Formation Followed by Osseointegration. Coatings. 2018;8:252. doi: 10.3390/coatings8070252. [DOI] [Google Scholar]
- 3.Terada C., Komasa S., Kusumoto T., Kawazoe T., Okazaki J. Effect of Amelogenin Coating of a Nano-Modified Titanium Surface on Bioactivity. Int. J. Mol. Sci. 2018;19:1274. doi: 10.3390/ijms19051274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Salle A., Spagnuolo G., Conte R., Procino A., Peluso G., Rengo C. Effects of various prophylactic procedures on titanium surfaces and biofilm formation. J. Periodontal Implant Sci. 2018;48:373–382. doi: 10.5051/jpis.2018.48.6.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi X., Xu L., Wang Q., Sunarso, Xu L. Hydrothermal Sterilization Improves Initial Osteoblast Responses on Sandpaper-Polished Titanium. Materials. 2017;10:812. doi: 10.3390/ma10070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandracci P., Mussano F., Rivolo P., Carossa S. Surface Treatments and Functional Coatings for Biocompatibility Improvement and Bacterial Adhesion Reduction in Dental Implantology. Coatings. 2016;6:7. doi: 10.3390/coatings6010007. [DOI] [Google Scholar]
- 7.Lan T.H., Pan C.Y., Liu P.H., Chou M. Fracture Resistance of Monolithic Zirconia Crowns in Implant Prostheses in Patients with Bruxism. Materials. 2019;12:1623. doi: 10.3390/ma12101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Lima Cavalcanti J.H., Matos P.C., Depes de Gouvêa C.V., Carvalho W., Calvo-Guirado J.L., Aragoneses J.M., Pérez-Díaz L., Gehrke S.A. In Vitro Assessment of the Functional Dynamics of Titanium with Surface Coating of Hydroxyapatite Nanoparticles. Materials. 2019;12:840. doi: 10.3390/ma12050840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang H.-J., Chou H.-H., Ou K.-L., Sugiatno E., Ruslin M., Waris R.A., Huang C.-F., Liu C.-M., Peng P.-W. Evaluation of Surface Characteristics and Hemocompatibility on the Oxygen Plasma-Modified Biomedical Titanium. Metals. 2018;8:513. doi: 10.3390/met8070513. [DOI] [Google Scholar]
- 10.Cicciu M., Fiorillo L., Herford A.S., Crimi S., Bianchi A., D’Amico C., Laino L., Cervino G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines. 2019;7:12. doi: 10.3390/biomedicines7010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung J.H., Kim S.Y., Yi Y.J., Lee B.K., Kim Y.K. Hydroxyapatite-coated implant: Clinical prognosis assessment via a retrospective follow-up study for the average of 3 years. J. Adv. Prosthodont. 2018;10:85–92. doi: 10.4047/jap.2018.10.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaluderovic M.R., Schreckenbach J.P., Graf H.L. Titanium dental implant surfaces obtained by anodic spark deposition—From the past to the future. Mater. Sci Eng. C Mater. Biol. Appl. 2016;69:1429–1441. doi: 10.1016/j.msec.2016.07.068. [DOI] [PubMed] [Google Scholar]
- 13.Kim S.B., Kim Y.K., Kim S.G., Oh J.S., Kim B.H. Comparative Study of the Early Loading of Resorbable Blasting Media and Sandblasting with Large-grit and Acid-etching Surface Implants: A Retrospective Cohort Study. Maxillofac. Plast Reconstr. Surg. 2014;36:247–252. doi: 10.14402/jkamprs.2014.36.6.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S.G., Yun P.Y., Park H.S., Shim J.S., Hwang J.W., Kim Y.K. Effect of loading time on the survival rate of anodic oxidized implants: Prospective multicenter study. J. Adv. Prosthodont. 2012;4:18–23. doi: 10.4047/jap.2012.4.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pae A., Kim S.S., Kim H.S., Woo Y.H. Osteoblast-like cell attachment and proliferation on turned, blasted, and anodized titanium surfaces. Int. J. Oral Maxillofac. Implants. 2011;26:475–481. [PubMed] [Google Scholar]
- 16.Jung S.W., Son M.K., Chung C.H., Kim H.J. Abrasion of abutment screw coated with TiN. J. Adv. Prosthodont. 2009;1:102–106. doi: 10.4047/jap.2009.1.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whiting P., Savovic J., Higgins J.P.T., Caldwell D.M., Reeves B.C., Shea B., Davies P., Kleijnen J., Churchill R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. Recenti Prog. Med. 2018;109:421–431. doi: 10.1016/j.jclinepi.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Coburn K.M., Vevea J.L. Publication bias as a function of study characteristics. Psychol. Methods. 2015;20:310–330. doi: 10.1037/met0000046. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansournia M.A., Higgins J.P., Sterne J.A., Hernan M.A. Biases in Randomized Trials: A Conversation Between Trialists and Epidemiologists. Epidemiology. 2017;28:54–59. doi: 10.1097/EDE.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savovic J., Turner R.M., Mawdsley D., Jones H.E., Beynon R., Higgins J.P.T., Sterne J.A.C. Association Between Risk-of-Bias Assessments and Results of Randomized Trials in Cochrane Reviews: The ROBES Meta-Epidemiologic Study. Am. J. Epidemiol. 2018;187:1113–1122. doi: 10.1093/aje/kwx344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachelet V.C., Pardo-Hernandez H. Quality of reporting and risk of bias of randomized clinical trials published in Spanish and Latin American journals. Medwave. 2019;19:7573. doi: 10.5867/medwave.2019.01.7573. [DOI] [PubMed] [Google Scholar]
- 23.Zielinski R., Kozakiewicz M., Swiniarski J. Comparison of Titanium and Bioresorbable Plates in "A" Shape Plate Properties-Finite Element Analysis. Materials. 2019;12:1110. doi: 10.3390/ma12071110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szafranska A., Antolak-Dudka A., Baranowski P., Bogusz P., Zasada D., Malachowski J., Czujko T. Identification of Mechanical Properties for Titanium Alloy Ti-6Al-4V Produced Using LENS Technology. Materials. 2019;12:886. doi: 10.3390/ma12060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh G., Pruncu C.I., Gupta M.K., Mia M., Khan A.M., Jamil M., Pimenov D.Y., Sen B., Sharma V.S. Investigations of Machining Characteristics in the Upgraded MQL-Assisted Turning of Pure Titanium Alloys Using Evolutionary Algorithms. Materials. 2019;12:999. doi: 10.3390/ma12060999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T., Ding D., Li N. Anodic Fabrication of Ti-Ni-Si-O Nanostructures on Ti10Ni5Si Alloy. Materials. 2019;12:1315. doi: 10.3390/ma12081315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gehrke S.A., Cavalcanti de Lima J.H., Rodriguez F., Calvo-Guirado J.L., Aramburu Junior J., Perez-Diaz L., Mazon P., Aragoneses J.M., De Aza P.N. Microgrooves and Microrugosities in Titanium Implant Surfaces: An In Vitro and In Vivo Evaluation. Materials. 2019;12:1287. doi: 10.3390/ma12081287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Bagoury N., Ahmed S.I., Ahmed Abu Ali O., El-Hadad S., Fallatah A.M., Mersal G.A.M., A Amin M. The Influence of Microstructure on the Passive Layer Chemistry and Corrosion Resistance for Some Titanium-Based Alloys. Materials. 2019;12:1233. doi: 10.3390/ma12081233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohseni E., Tang W., Wang S. Investigation of the Role of Nano-Titanium on Corrosion and Thermal Performance of Structural Concrete with Macro-Encapsulated PCM. Molecules. 2019;24:1360. doi: 10.3390/molecules24071360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soto-Penaloza D., Caneva M., Vina-Almunia J., Martin-de-Llano J.J., Penarrocha-Oltra D., Penarrocha-Diago M. Bone-Healing Pattern on the Surface of Titanium Implants at Cortical and Marrow Compartments in Two Topographic Sites: An Experimental Study in Rabbits. Materials. 2018;12:85. doi: 10.3390/ma12010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinjari B., D’Addazio G., Bozzi M., Celletti R., Traini T., Mavriqi L., Caputi S. Comparison of a Novel Ultrasonic Scaler Tip vs. Conventional Design on a Titanium Surface. Materials. 2018;11:2345. doi: 10.3390/ma11122345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marenzi G., Impero F., Scherillo F., Sammartino J.C., Squillace A., Spagnuolo G. Effect of Different Surface Treatments on Titanium Dental Implant Micro-Morphology. Materials. 2019;12:733. doi: 10.3390/ma12050733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim W.H., Song E.S., Ju K.W., Lee J.H., Kim M.Y., Lim D., Kim B. Finite Element Analysis of Novel Separable Fixture for Easy Retrievement in Case with Peri-Implantitis. Materials. 2019;12:235. doi: 10.3390/ma12020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganbold B., Kim S.K., Heo S.J., Koak J.Y., Lee Z.H., Cho J. Osteoclastogenesis Behavior of Zirconia for Dental Implant. Materials. 2019;12:732. doi: 10.3390/ma12050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cicciu M., Cervino G., Milone D., Risitano G. FEM Analysis of Dental Implant-Abutment Interface Overdenture Components and Parametric Evaluation of Equator((R)) and Locator((R)) Prosthodontics Attachments. Materials. 2019;12:592. doi: 10.3390/ma12040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cicciu M., Cervino G., Milone D., Risitano G. FEM Investigation of the Stress Distribution over Mandibular Bone Due to Screwed Overdenture Positioned on Dental Implants. Materials. 2018;11:1512. doi: 10.3390/ma11091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang J.Z., Tsai P.I., Kuo M.Y., Sun J.S., Chen S.Y., Shen H.H. Augmentation of DMLS Biomimetic Dental Implants with Weight-Bearing Strut to Balance of Biologic and Mechanical Demands: From Bench to Animal. Materials. 2019;12:164. doi: 10.3390/ma12010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brizuela A., Herrero-Climent M., Rios-Carrasco E., Rios-Santos J.V., Perez R.A., Manero J.M., Gil Mur J. Influence of the Elastic Modulus on the Osseointegration of Dental Implants. Materials. 2019;12:980. doi: 10.3390/ma12060980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bae E.B., Yoo J.H., Jeong S.I., Kim M.S., Lim Y.M., Ahn J.J., Lee J.J., Lee S.H., Kim H.J., Huh J.B. Effect of Titanium Implants Coated with Radiation-Crosslinked Collagen on Stability and Osseointegration in Rat Tibia. Materials. 2018;11:2520. doi: 10.3390/ma11122520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alsaeedi R., Ozdemir Z. Evaluation of Chemical Mechanical Polishing-Based Surface Modification on 3D Dental Implants Compared to Alternative Methods. Materials. 2018;11:2286. doi: 10.3390/ma11112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shemtov-Yona K., Rittel D. Fatigue of Dental Implants: Facts and Fallacies. Dent. J. 2016;4:16. doi: 10.3390/dj4020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahmitasari F., Ishida Y., Kurahashi K., Matsuda T., Watanabe M., Ichikawa T. PEEK with Reinforced Materials and Modifications for Dental Implant Applications. Dent. J. 2017;5:35. doi: 10.3390/dj5040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nejem Wakim R., Namour M., Nguyen H.V., Peremans A., Zeinoun T., Vanheusden A., Rompen E., Nammour S. Decontamination of Dental Implant Surfaces by the Er:YAG Laser Beam: A Comparative in Vitro Study of Various Protocols. Dent. J. 2018;6:66. doi: 10.3390/dj6040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao X.-Y., Tian N., Dong X., Cheng C.-K. Implant Coating Manufactured by Micro-Arc Oxidation and Dip Coating in Resorbable Polylactide for Antimicrobial Applications in Orthopedics. Coatings. 2019;9:284. doi: 10.3390/coatings9050284. [DOI] [Google Scholar]
- 45.Variola F., Brunski J.B., Orsini G., Tambasco de Oliveira P., Wazen R., Nanci A. Nanoscale surface modifications of medically relevant metals: State-of-the art and perspectives. Nanoscale. 2011;3:335–353. doi: 10.1039/C0NR00485E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wazen R.M., Currey J.A., Guo H., Brunski J.B., Helms J.A., Nanci A. Micromotion-induced strain fields influence early stages of repair at bone-implant interfaces. Acta Biomater. 2013;9:6663–6674. doi: 10.1016/j.actbio.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guadarrama Bello D., Fouillen A., Badia A., Nanci A. A nanoporous titanium surface promotes the maturation of focal adhesions and formation of filopodia with distinctive nanoscale protrusions by osteogenic cells. Acta Biomater. 2017;60:339–349. doi: 10.1016/j.actbio.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Contreras A., Guadarrama Bello D., Flynn S., Variola F., Wuest J.D., Nanci A. Chemical nanocavitation of surfaces to enhance the utility of stainless steel as a medical material. Colloids Surf. B Biointerfaces. 2018;161:677–687. doi: 10.1016/j.colsurfb.2017.11.051. [DOI] [PubMed] [Google Scholar]
- 49.Bueno Rde B., Adachi P., Castro-Raucci L.M., Rosa A.L., Nanci A., Oliveira P.T. Oxidative nanopatterning of titanium surfaces promotes production and extracellular accumulation of osteopontin. Braz. Dent. J. 2011;22:179–184. doi: 10.1590/S0103-64402011000300001. [DOI] [PubMed] [Google Scholar]
- 50.Maia L.P., Reino D.M., Muglia V.A., Almeida A.L., Nanci A., Wazen R.M., de Oliveira P.T., Palioto D.B., Novaes A.B., Jr. Influence of periodontal tissue thickness on buccal plate remodelling on immediate implants with xenograft. J. Clin. Periodontol. 2015;42:590–598. doi: 10.1111/jcpe.12405. [DOI] [PubMed] [Google Scholar]
- 51.Variola F., Zalzal S.F., Leduc A., Barbeau J., Nanci A. Oxidative nanopatterning of titanium generates mesoporous surfaces with antimicrobial properties. Int. J. Nanomedicine. 2014;9:2319–2325. doi: 10.2147/IJN.S61333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ariganello M.B., Guadarrama Bello D., Rodriguez-Contreras A., Sadeghi S., Isola G., Variola F., Nanci A. Surface nanocavitation of titanium modulates macrophage activity. Int. J. Nanomedicine. 2018;13:8297–8308. doi: 10.2147/IJN.S185436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maturi F.E., Sabio R.M., Silva R.R., Lahoud M.G., Meneguin A.B., Valente G.T., Caface R.A., Leite I.S., Inada N.M., Ribeiro S.J.L. Luminescent Mesoporous Silica Nanohybrid Based on Drug Derivative Terbium Complex. Materials. 2019;12:933. doi: 10.3390/ma12060933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia J.C., Sanz Lobera A., Maresca P., Pareja T.F., Wang C. Some Considerations about the Use of Contact and Confocal Microscopy Methods in Surface Texture Measurement. Materials. 2018;11:1484. doi: 10.3390/ma11081484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmitt C.M., Koepple M., Moest T., Neumann K., Weisel T., Schlegel K.A. In vivo evaluation of biofunctionalized implant surfaces with a synthetic peptide (P-15) and its impact on osseointegration. A preclinical animal study. Clin. Oral Implants Res. 2016;27:1339–1348. doi: 10.1111/clr.12723. [DOI] [PubMed] [Google Scholar]
- 56.Novellino M.M., Sesma N., Zanardi P.R., Lagana D.C. Resonance frequency analysis of dental implants placed at the posterior maxilla varying the surface treatment only: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2017;19:770–775. doi: 10.1111/cid.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mangano C., Shibli J.A., Pires J.T., Luongo G., Piattelli A., Iezzi G. Early Bone Formation around Immediately Loaded Transitional Implants Inserted in the Human Posterior Maxilla: The Effects of Fixture Design and Surface. BioMed Res. Int. 2017;2017:4152506. doi: 10.1155/2017/4152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cannizzaro G., Felice P., Loi I., Viola P., Ferri V., Leone M., Esposito M. Machined versus roughened immediately loaded and finally restored single implants inserted flapless: Preliminary 6-month data from a split- mouth randomised controlled trial. Eur. J. Oral Implantol. 2016;9:155–163. [PubMed] [Google Scholar]
- 59.Schwarz F., Mihatovic I., Becker J., Bormann K.H., Keeve P.L., Friedmann A. Histological evaluation of different abutments in the posterior maxilla and mandible: An experimental study in humans. J. Clin. Periodontol. 2013;40:807–815. doi: 10.1111/jcpe.12115. [DOI] [PubMed] [Google Scholar]
- 60.Corvino V., Iezzi G., Trubiani O., Traini T., Piattelli M. Histological and histomorphometric evaluation of implant with nanometer scale and oxidized surface. In vitro and in vivo study. J. Biol. Regul. Homeost. Agents. 2012;26:19–28. [PubMed] [Google Scholar]
- 61.Karabuda Z.C., Abdel-Haq J Fau - Arisan V., Arisan V. Stability, marginal bone loss and survival of standard and modified sand-blasted, acid-etched implants in bilateral edentulous spaces: A prospective 15-month evaluation. Clin. Oral. Implants Res. 2011;22:840–849. doi: 10.1111/j.1600-0501.2010.02065.x. [DOI] [PubMed] [Google Scholar]
- 62.D’Avila S., Dos Reis L.D., Piattelli A., Aguiar K.C., De Faveri M., Borges F.L., Shibli J.A. Impact of smoking on human bone apposition at different dental implant surfaces: A histologic study in type IV bone. J. Oral Implantol. 2010;36:85–90. doi: 10.1563/AAID-JOI-D-09-00018. [DOI] [PubMed] [Google Scholar]
- 63.Shibli J.A., Grassi S., Piattelli A., Pecora G.E., Ferrari D.S., Onuma T., Iezzi G. Histomorphometric evaluation of bioceramic molecular impregnated and dual acid-etched implant surfaces in the human posterior maxilla. Clin. Implant. Dent. Relat. Res. 2012;12:281–288. doi: 10.1111/j.1708-8208.2009.00174.x. [DOI] [PubMed] [Google Scholar]
- 64.Khang W., Feldman S., Hawley C.E., Gunsolley J. A multi-center study comparing dual acid-etched and machined-surfaced implants in various bone qualities. J. Periodontol. 2001;72:1384–1390. doi: 10.1902/jop.2001.72.10.1384. [DOI] [PubMed] [Google Scholar]
- 65.Menezes H.H.M., Naves M.M., Costa H.L., Barbosa T.P., Ferreira J.A., Magalhães D., Martinez E.F. Effect of Surgical Installation of Dental Implants on Surface Topography and Its Influence on Osteoblast Proliferation. Int. J. Dent. 2018;2018:4089274. doi: 10.1155/2018/4089274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim Y.K., Kim B.S., Yun P.Y., Mun S.U., Yi Y.J., Kim S.G., Jeong K.I. The seven-year cumulative survival rate of Osstem implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014;40:68–75. doi: 10.5125/jkaoms.2014.40.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeong M.A., Kim S.G., Kim Y.K., Oh H.K., Cho Y.S., Kim W.C., Oh J.S. A multicenter prospective study in type IV bone of a single type of implant. Implant. Dent. 2012;21:330–334. doi: 10.1097/ID.0b013e31825cd3d1. [DOI] [PubMed] [Google Scholar]
- 68.Kim Y.K., Lee J.H., Lee J.Y., Yi Y.J. A randomized controlled clinical trial of two types of tapered implants on immediate loading in the posterior maxilla and mandible. Int J. Oral Maxillofac Implants. 2013;28:1602–1611. doi: 10.11607/jomi.3180. [DOI] [PubMed] [Google Scholar]
- 69.Kim Y.K., Ahn K.J., Yun P.Y., Kim M., Yang H.S., Yi Y.J., Bae J.H. Effect of loading time on marginal bone loss around hydroxyapatite-coated implants. J. Korean Assoc. Oral Maxillofac Surg. 2013;39:161–167. doi: 10.5125/jkaoms.2013.39.4.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osman M.S., Ziada H.M., Abubakr N.H., Suliman A.M. Implant impression accuracy of parallel and non-parallel implants: A comparative in-vitro analysis of open and closed tray techniques. Int J. Implant. Dent. 2019;5:4. doi: 10.1186/s40729-019-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tallarico M., Caneva M., Baldini N., Gatti F., Duvina M., Billi M., Iannello G., Piacentini G., Meloni S.M., Cicciu M. Patient-centered rehabilitation of single, partial, and complete edentulism with cemented- or screw-retained fixed dental prosthesis: The First Osstem Advanced Dental Implant Research and Education Center Consensus Conference 2017. Eur J. Dent. 2018;12:617–626. doi: 10.4103/ejd.ejd_243_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tallarico M., Xhanari E., Pisano M., Gatti F., Meloni S.M. Molar replacement with 7 mm-wide diameter implants: To place the implant immediately or to wait 4 months after socket preservation? 1 year after loading results from a randomised controlled trial. Eur J. Oral Implantol. 2017;10:169–178. [PubMed] [Google Scholar]
- 73.Tallarico M., Xhanari E., Pisano M., De Riu G., Tullio A., Meloni S.M. Single post-extractive ultra-wide 7 mm-diameter implants versus implants placed in molar healed sites after socket preservation for molar replacement: 6-month post-loading results from a randomised controlled trial. Eur J. Oral Implantol. 2016;9:263–275. [PubMed] [Google Scholar]
- 74.Sui X., Wei H., Wang D., Han Y., Deng J., Wang Y., Wang J., Yang J. Experimental research on the relationship between fit accuracy and fracture resistance of zirconia abutments. J. Dent. 2014;42:1353–1359. doi: 10.1016/j.jdent.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Jo J.Y., Yang D.S., Huh J.B., Heo J.C., Yun M.J., Jeong C.M. Influence of abutment materials on the implant-abutment joint stability in internal conical connection type implant systems. J. Adv. Prosthodont. 2014;6:491–497. doi: 10.4047/jap.2014.6.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J., Wang K., Liu G., Wang D. Fracture resistance of inter-joined zirconia abutment of dental implant system with injection molding technique. Clin. Oral Implants Res. 2013;24:1247–1250. doi: 10.1111/j.1600-0501.2012.02539.x. [DOI] [PubMed] [Google Scholar]
- 77.Huang J.S., Zhao J.J., Liu Q., Liu T.T. Clinical research of immediate restoration implant with mini-implants in edentulous space. Hua Xi Kou Qiang Yi Xue Za Zhi. 2010;28:412–416. [PubMed] [Google Scholar]
- 78.Jo S.H., Kim K.I., Seo J.M., Song K.Y., Park J.M., Ahn S.G. Effect of impression coping and implant angulation on the accuracy of implant impressions: An in vitro study. J. Adv. Prosthodont. 2010;2:128–133. doi: 10.4047/jap.2010.2.4.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park Y.H., Jung U.W., Kim C.S., Choi S.H., Cho K.S., Lee J.S. Resonance Frequency Analysis of Tapered Implants Placed at Maxillary Posterior Sites After Lateral Sinus Augmentation: A 1.5-year Follow-Up Prospective Study. Implant. Dent. 2019;28:62–67. doi: 10.1097/ID.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 80.Yassin Alsabbagh A., Alsabbagh M.M., Darjazini Nahas B., Rajih S. Comparison of three different methods of internal sinus lifting for elevation heights of 7 mm: An ex vivo study. Int J. Implant. Dent. 2017;3:40. doi: 10.1186/s40729-017-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim Y.K., Yun P.Y., Kim S.G., Kim B.S., Ong J.L. Evaluation of sinus bone resorption and marginal bone loss after sinus bone grafting and implant placement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2009;107:21–28. doi: 10.1016/j.tripleo.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 82.Kim Y.K., Kim S.G., Park J.Y., Yi Y.J., Bae J.H. Comparison of clinical outcomes of sinus bone graft with simultaneous implant placement: 4-month and 6-month final prosthetic loading. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2011;111:164–169. doi: 10.1016/j.tripleo.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 83.Yoo J.M., Ben Amara H., Kim M.K., Song J.D., Koo K.T. Oral tissue response to soft tissue expanders prior to bone augmentation: In vitro analysis and histological study in dogs. J. Periodontal Implant Sci. 2018;48:152–163. doi: 10.5051/jpis.2018.48.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim S.K., Kim S.W., Kim K.W. Effect on bone formation of the autogenous tooth graft in the treatment of peri-implant vertical bone defects in the minipigs. Maxillofac. Plast. Reconstr. Surg. 2015;37:2. doi: 10.1186/s40902-015-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang E.J., Kim S.K., Eom T.G., Choi K.O., Lee T.H. Evaluation of the osteogenic activity of the BMP-2 mimetic peptide, PEP7, in vitro and in vivo. Int. J. Oral Maxillofac. Implants. 2013;28:749–756. doi: 10.11607/jomi.2825. [DOI] [PubMed] [Google Scholar]
- 86.Oliver R. Flapless dental implant surgery may improve hard and soft tissue outcomes. J. Evid. Based Dent. Pract. 2012;12:87–88. doi: 10.1016/j.jebdp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 87.Li Z.R., Liu Z.H., Xu S., Xiao H.J., Zhou W.J. The application of guided bone regeneration technique in the restoration of maxillary lateral incisor with bone defect. Shanghai Kou Qiang Yi Xue. 2012;21:190–193. [PubMed] [Google Scholar]
- 88.Huang H., Xu Z., Shao X., Wismeijer D., Sun P., Wang J., Wu G. Multivariate linear regression analysis to identify general factors for quantitative predictions of implant stability quotient values. PLoS ONE. 2017;12:e0187010. doi: 10.1371/journal.pone.0187010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eom T.G., Kim H.W., Jeon G.R., Yun M.J., Huh J.B., Jeong C.M. Effects of Different Implant Osteotomy Preparation Sizes on Implant Stability and Bone Response in the Minipig Mandible. Int J. Oral Maxillofac. Implants. 2016;31:997–1006. doi: 10.11607/jomi.4165. [DOI] [PubMed] [Google Scholar]
- 90.Park J.C., Ha S.R., Kim S.M., Kim M.J., Lee J.B., Lee J.H. A randomized clinical 1-year trial comparing two types of non-submerged dental implants. Clin. Oral Implants Res. 2010;21:228–236. doi: 10.1111/j.1600-0501.2009.01828.x. [DOI] [PubMed] [Google Scholar]
- 91.Hong J., Lim Y.J., Park S.O. Quantitative biomechanical analysis of the influence of the cortical bone and implant length on primary stability. Clin. Oral Implants Res. 2012;23:1193–1197. doi: 10.1111/j.1600-0501.2011.02285.x. [DOI] [PubMed] [Google Scholar]
- 92.Bansal J., Kedige S., Bansal A., Anand S. A relaxed implant bed: Implants placed after two weeks of osteotomy with immediate loading: A one year clinical trial. J. Oral Implantol. 2012;38:155–164. doi: 10.1563/AAID-JOI-D-10-00036. [DOI] [PubMed] [Google Scholar]
- 93.Cervino G., Romeo U., Lauritano F., Bramanti E., Fiorillo L., D’Amico C., Milone D., Laino L., Campolongo F., Rapisarda S., et al. Fem and Von Mises Analysis of OSSTEM (r) Dental Implant Structural Components: Evaluation of Different Direction Dynamic Loads. Open Dent. J. 2018;12:219–229. doi: 10.2174/1874210601812010219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herekar M., Sethi M., Prithviraj D.R., Bhat K., Fernandes A., Patil V. A Clinical Study Evaluating Changes in the Microbial Flora Around Dental Implants During Various Stages of Implant Restoration. Implant. Dent. 2015;24:527–532. doi: 10.1097/ID.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 95.Kim J.R., Kim S.H., Kim I.R., Park B.S., Kim Y.D. Low-level laser therapy affects osseointegration in titanium implants: Resonance frequency, removal torque, and histomorphometric analysis in rabbits. J. Korean Assoc. Oral Maxillofac. Surg. 2016;42:2–8. doi: 10.5125/jkaoms.2016.42.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Namgoong H., Kim M.D., Ku Y., Rhyu I.C., Lee Y.M., Seol Y.J., Gu H.J., Susin C., Wikesjo U.M., Koo K.T. Bone reconstruction after surgical treatment of experimental peri-implantitis defects at a sandblasted/acid-etched hydroxyapatite-coated implant: An experimental study in the dog. J. Clin. Periodontol. 2015;42:960–966. doi: 10.1111/jcpe.12457. [DOI] [PubMed] [Google Scholar]
- 97.Jeon W.J., Kim S.G., Lim S.C., Ong J.L., Oh D.S. Histomorphometric evaluation of immediately loaded SSII implants of different surface treatments in a dog model. J. Biomed. Mater. Res. A. 2009;90:396–400. doi: 10.1002/jbm.a.32108. [DOI] [PubMed] [Google Scholar]
- 98.Moon S.Y., Kim S.G., Lim S.C., Ong J.L. Histologic and histomorphometric evaluation of early and immediately loaded implants in the dog mandible. J. Biomed. Mater. Res. A. 2008;86:1122–1127. doi: 10.1002/jbm.a.31696. [DOI] [PubMed] [Google Scholar]
- 99.Duncan W.J., Lee M.H., Dovban A.S., Hendra N., Ershadi S., Rumende H. Anodization increases early integration of Osstem implants in sheep femurs. Ann. R. Australas. Coll. Dent. Surg. 2008;19:152–156. [PubMed] [Google Scholar]
- 100.Bramanti E., Cervino G., Lauritano F., Fiorillo L., D’Amico C., Sambataro S., Denaro D., Fama F., Ierardo G., Polimeni A., et al. FEM and Von Mises Analysis on Prosthetic Crowns Structural Elements: Evaluation of Different Applied Materials. Sci. World J. 2017;2017:1029574. doi: 10.1155/2017/1029574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cicciu M., Bramanti E., Matacena G., Guglielmino E., Risitano G. FEM evaluation of cemented-retained versus screw-retained dental implant single-tooth crown prosthesis. Int. J. Clin. Exp. Med. 2014;7:817–825. [PMC free article] [PubMed] [Google Scholar]
- 102.Cicciù M., Cervino G., Bramanti E., Lauritano F., Gudice G.L., Scappaticci L., Rapparini A., Guglielmino E., Risitano G. FEM analysis of mandibular prosthetic overdenture supported by dental implants: Evaluation of different retention methods. Comput. Math. Methods Med. 2015;2015:943839. doi: 10.1155/2015/943839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cicciu M., Cervino G., Herford A.S., Fama F., Bramanti E., Fiorillo L., Lauritano F., Sambataro S., Troiano G., Laino L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs. 2018;16:27. doi: 10.3390/md16010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maridati P., Stoffella E., Speroni S., Cicciu M., Maiorana C. Alveolar antral artery isolation during sinus lift procedure with the double window technique. Open Dent. J. 2014;8:95–103. doi: 10.2174/1874210601408010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beretta M., Cicciù M., Bramanti E., Maiorana C. Schneider membrane elevation in presence of sinus septa: Anatomic features and surgical management. Int. J. Dent. 2012;2012:261905. doi: 10.1155/2012/261905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rancitelli D., Borgonovo A.E., Cicciù M., Re D., Rizza F., Frigo A.C., Maiorana C. Maxillary sinus septa and anatomic correlation with the Schneiderian membrane. J. Craniofac. Surg. 2015;26:1394–1398. doi: 10.1097/SCS.0000000000001725. [DOI] [PubMed] [Google Scholar]
- 107.Poli P.P., Beretta M., Cicciù M., Maiorana C. Alveolar ridge augmentation with titanium mesh. A retrospective clinical study. Open Dent. J. 2014;8:148–158. doi: 10.2174/1874210601408010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lo Giudice G., Iannello G., Terranova A., Lo Giudice R., Pantaleo G., Cicciù M. Transcrestal sinus lift procedure approaching atrophic maxillary ridge: A 60-month clinical and radiological follow-up evaluation. Int. J. Dent. 2015;2015:261652. doi: 10.1155/2015/261652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herford A.S., Cicciù M., Eftimie L.F., Miller M., Signorino F., Famà F., Cervino G., Lo Giudice G., Bramanti E., Lauritano F., et al. rhBMP-2 applied as support of distraction osteogenesis: A split-mouth histological study over nonhuman primates mandibles. Int. J. Clin. Exp. Med. 2016;9:17187–17194. [Google Scholar]
- 110.Crimi S., Fiorillo L., Bianchi A., D’Amico C., Amoroso G., Gorassini F., Mastroieni R., Marino S., Scoglio C., Catalano F., et al. Herpes Virus, Oral Clinical Signs and QoL: Systematic Review of Recent Data. Viruses. 2019;11:463. doi: 10.3390/v11050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lo Giudice G., Cutroneo G., Centofanti A., Artemisia A., Bramanti E., Militi A., Cicciù M. Dentin morphology of root canal surface: A quantitative evaluation based on a scanning electronic microscopy study. Biomed. Res. Int. 2015;2015:164065. doi: 10.1155/2015/164065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Jesus R.N.R., Carrilho E., Antunes P.V., Ramalho A., Moura C.C.G., Stavropoulos A., Zanetta-Barbosa D. Interfacial biomechanical properties of a dual acid-etched versus a chemically modified hydrophilic dual acid-etched implant surface: An experimental study in Beagles. Int J. Implant. Dent. 2018;4:28. doi: 10.1186/s40729-018-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cervino G., Fiorillo L., Herford A.S., Romeo U., Bianchi A., Crimi S., Laino L. Molecular Biomarkers Related to Oral Carcinoma: Clinical Trial Outcome Evaluation in a Literature Review. Dis. Markers. 2019;2019:11. doi: 10.1155/2019/8040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cervino G., Fiorillo L., Laino L., Herford A.S., Lauritano F., Giudice G.L., Fama F., Santoro R., Troiano G., Iannello G., et al. Oral Health Impact Profile in Celiac Patients: Analysis of Recent Findings in a Literature Review. Gastroenterol. Res. Pract. 2018;2018:7848735. doi: 10.1155/2018/7848735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Laino L., Cicciù M., Fiorillo L., Crimi S., Bianchi A., Amoroso G., Monte I.P., Herford A.S., Cervino G. Surgical Risk on Patients with Coagulopathies: Guidelines on Hemophiliac Patients for Oro-Maxillofacial Surgery. Int. J. Environ. Res. Public Health. 2019;16:1386. doi: 10.3390/ijerph16081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fiorillo L., De Stefano R., Cervino G., Crimi S., Bianchi A., Campagna P., Herford A.S., Laino L., Cicciù M. Oral and Psychological Alterations in Haemophiliac Patients. Biomedicines. 2019;7:33. doi: 10.3390/biomedicines7020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cervino G., Terranova A., Briguglio F., De Stefano R., Famà F., D’Amico C., Amoroso G., Marino S., Gorassini F., Mastroieni R., et al. Diabetes: Oral health related quality of life and oral alterations. BioMed Res. Int. 2019;2019:5907195. doi: 10.1155/2019/5907195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghadimi K., Levy J.H., Welsby I.J. Perioperative management of the bleeding patient. Br. J. Anaesth. 2016;117:18–30. doi: 10.1093/bja/aew358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Simurda T., Stanciakova L., Stasko J., Dobrotova M., Kubisz P. Yes or no for secondary prophylaxis in afibrinogenemia? Blood Coagul. Fibrinolysis. 2015;26:978–980. doi: 10.1097/MBC.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 120.Williams B., Indresano A.T., O’Ryan F. Venous thromboembolism in oral and maxillofacial surgery: A review of the literature. J. Oral maxillof. Surg. 2011;69:840–844. doi: 10.1016/j.joms.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 121.Cervino G., Fiorillo L., Monte I.P., De Stefano R., Laino L., Crimi S., Bianchi A., Herford A.S., Biondi A., Cicciù M. Advances in Antiplatelet Therapy for Dentofacial Surgery Patients: Focus on Past and Present Strategies. Materials. 2019;12:1524. doi: 10.3390/ma12091524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rullo R., Scalzone P., Laino L., Russo A., Festa V.M., Fiorillo L., Cicciu M. Solitary Plasmacytoma of the Mandible: Early Diagnosis and Surgical Management. J. Craniofac. Surg. 2019 doi: 10.1097/SCS.0000000000005397. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 123.Matarese G., Ramaglia L., Fiorillo L., Cervino G., Lauritano F., Isola G. Implantology and Periodontal Disease: The Panacea to Problem Solving? Open Dent. J. 2017;11:460–465. doi: 10.2174/1874210601711010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fiorillo L., Cervino G., Herford A., Lauritano F., D’Amico C., Lo Giudice R., Cicciù M. Interferon Crevicular Fluid Profile and Correlation with Periodontal Disease and Wound Healing: A Systemic Review of Recent Data. Int J. Mol. Sci. 2018;19:1908. doi: 10.3390/ijms19071908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Troiano G., Laino L., Cicciu M., Cervino G., Fiorillo L., D’Amico C., Zhurakivska K., Lo Muzio L. Comparison of Two Routes of Administration of Dexamethasone to Reduce the Postoperative Sequelae After Third Molar Surgery: A Systematic Review and Meta-Analysis. Open Dent. J. 2018;12:181–188. doi: 10.2174/1874210601812010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lo Giudice R., Puleio F., Rizzo D., Alibrandi A., Lo Giudice G., Centofanti A., Fiorillo L., Di Mauro D., Nicita F. Comparative investigation of cutting devices on bone blocks: An SEM morphological analysis. Appl. Sci. 2019;9:1908. doi: 10.3390/app9020351. [DOI] [Google Scholar]
- 127.Cervino G., Cicciù M., Biondi A., Bocchieri S., Herford A.S., Laino L., Fiorillo L. Antibiotic Prophylaxis on Third Molar Extraction: Systematic Review of Recent Data. Antibiotics. 2019;8:53. doi: 10.3390/antibiotics8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stacchi C., Berton F., Fiorillo L., Nicolin V., Lombardi T., Cicciù M., Di Lenarda R. Fresh frozen allogeneic bone block in maxillary sinus floor elevation: Histomorphometric analysis of a bone specimen retrieved 15 years after grafting procedure. Appl. Sci. 2019;9:1119. doi: 10.3390/app9061119. [DOI] [Google Scholar]
- 129.Lombardi T., Bernardello F., Berton F., Porrelli D., Rapani A., Camurri Piloni A., Fiorillo L., Di Lenarda R., Stacchi C. Efficacy of Alveolar Ridge Preservation after Maxillary Molar Extraction in Reducing Crestal Bone Resorption and Sinus Pneumatization: A Multicenter Prospective Case-Control Study. Biomed. Res. Int. 2018;2018:9352130. doi: 10.1155/2018/9352130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cervino G., Fiorillo L., Arzukanyan A.V., Spagnuolo G., Cicciu M. Dental Restorative Digital Workflow: Digital Smile Design from Aesthetic to Function. Dent. J. 2019;7:30. doi: 10.3390/dj7020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cervino G., Fiorillo L., Herford A., Laino L., Troiano G., Amoroso G., Cicciù M. Alginate Materials and Dental Impression Technique: A Current State of the Art and Application to Dental Practice. Mar. Drugs. 2018;17:18. doi: 10.3390/md17010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cervino G., Fiorillo L., Spagnuolo G., Bramanti E., Laino L., Lauritano F., Cicciù M. Interface between MTA and Dental Bonding Agents: Scanning Electron Microscope Evaluation. J. Int Soc. Prev. Community Dent. 2017;7:64–68. doi: 10.4103/jispcd.JISPCD_521_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Isola G., Ramaglia L., Cordasco G., Lucchese A., Fiorillo L., Matarese G. The effect of a functional appliance in the management of temporomandibular joint disorders in patients with juvenile idiopathic arthritis. Minerva Stomatol. 2017;66:1–8. doi: 10.23736/S0926-4970.16.03995-3. [DOI] [PubMed] [Google Scholar]
- 134.Isola G., Cicciu M., Fiorillo L., Matarese G. Association Between Odontoma and Impacted Teeth. J. Craniofac. Surg. 2017;28:755–758. doi: 10.1097/SCS.0000000000003433. [DOI] [PubMed] [Google Scholar]
- 135.Bramanti E., Matacena G., Cecchetti F., Arcuri C., Cicciù M. Oral health-related quality of life in partially edentulous patients before and after implant therapy: A 2-year longitudinal study. ORAL Implantol. 2013;6:37–42. doi: 10.11138/orl/2013.6.2.037. [DOI] [PMC free article] [PubMed] [Google Scholar]