Abstract
Purpose:
To correlate aqueous vasoactive protein changes with macular edema after dexamethasone implant in retinal vein occlusion (RVO)
Design:
Prospective, interventional case series
Methods:
Twenty-three central RVO (CRVO) and 17 branch RVO (BRVO) subjects with edema despite prior anti-vascular endothelial growth factor (VEGF) treatment had aqueous taps at baseline, 4 and 16 weeks after dexamethasone implant. Best-corrected visual acuity (BCVA) and center subfield thickness were measured every 4 weeks. Aqueous vasoactive protein levels were measured by protein array or enzyme-linked immunosorbent assay.
Results:
Thirty-two vasoactive proteins were detected in aqueous in untreated eyes with macular edema due to RVO. Reduction in excess foveal thickness after dexamethasone implant correlated with reduction in persephin and pentraxin 3 (Pearson Correlation Coefficients=0.682 and 0.638, p=0.014 and p=0.003). Other protein changes differed among RVO patients as edema decreased, but ≥50% of patients showed reductions in hepatocyte growth factor, endocrine gland-VEGF, insulin-like growth factor binding proteins, or endostatin by ≥30%. Enzyme-linked immunosorbent assay in 18 eyes (12 CRVO, 6 BRVO) showed baseline levels of hepatocyte growth factor and VEGF of 168.2 ± 20.1 pg/ml and 78.7 ± 10.0 pg/ml, and each was reduced in 12 eyes after dexamethasone implant.
Conclusions:
Dexamethasone implants reduce several pro-permeability proteins providing a multitargeted approach in RVO. No single protein in addition to VEGF can be implicated as a contributor in all patients. Candidates for contribution to chronic edema in subgroups of patients that deserve further study include persephin, hepatocyte growth factor, and endocrine gland-VEGF.
Introduction
Central retinal vein occlusion (CRVO) is initiated by thrombotic occlusion of the main outflow vessel of the retina resulting in retinal hemorrhages, variable amounts of retinal nonperfusion, and macular edema. Branch vein occlusion (BRVO) is initiated from thrombotic occlusion of a proximal branch of the central retinal vein that drains ≤50% of the retina. Retinal hemorrhages, variable amounts of retinal nonperfusion, and macular edema also occur after BRVO but on average tend to be less severe, because less of the retina is involved by the occlusion compared to CRVO. While ischemic damage to the macula may contribute, the major cause of reduced visual acuity is macular edema. In patients with relatively recent onset CRVO or BRVO, intraocular injections of a specific antagonist of vascular endothelial growth factor (VEGF) results in dramatic reductions in macular edema and improvements in visual acuity.1 This indicates that VEGF is a major cause of macular edema in patients with RVO. This was confirmed by large multicenter trials and intraocular injections of a VEGF antagonist has become first line therapy for patients with CRVO or BRVO.2-6 Frequent injections of a VEGF antagonist are able to completely eliminate edema in some patients suggesting that VEGF is necessary for edema in those patients; however, it is difficult to maintain a dry retina, because recurrences often occur when the duration between injections is increased. In other patients, it is not possible to achieve complete elimination of the edema despite monthly injections of a VEGF antagonist suggesting an inability to neutralize all VEGF or contributions from other pro-permeability factors.
It was initially thought that the goal of treatment in RVO would be to control edema and maintain vision until recanalization of the occluded vessel allowed for normalization of the underlying disease process and elimination of the need for injections. However, it appears that the occlusion is merely the initiator of a dynamic disease process that is driven by retinal ischemia and high levels of VEGF which promote leukostasis, progression of capillary closure, and increased ischemia.7 This progression of disease makes continued injections necessary to control edema and some patients experience permanent loss of vision from ischemic damage to the macula or damage from chronic/recurrent edema.8 In the RETAIN study, with a mean follow up of 49 months after the initiation of anti-VEGF treatment, only 50% of BRVO patients and 44% of CRVO patients no longer required injections to control edema.9 In many patients, injections of a VEGF antagonist seemed less effective over time, suggesting evolution or change in the disease process such that other pro-permeability factors may play a more important role.
Corticosteroids bind to cytoplasmic receptors that translocate to the nucleus and cause transcriptional repression of a large number of genes whose products participate in inflammation, vascular leakage, and angiogenesis.10-12 The dexamethasone implant reduces edema and improves vision in patients with RVO and has a longer duration of effect than intraocular injection of currently available VEGF antagonists.13 It is an appealing alternative in patients who have residual edema despite anti-VEGF injections or who require frequent injections to control edema. While it is assumed that it reduces many factors that might contribute to edema, there are little data regarding this point. In this study, a vasoactive protein array was used to measure levels of aqueous proteins known to influence vascular cells prior to and after injection of a dexamethasone implant, and changes in protein levels were correlated with changes in edema.
Methods
Study Procedures
The Ozurdex for Retinal Vein Occlusion (ORVO) Study was an investigator-initiated study funded by Allergan, Inc. (Irvine, CA). The protocol was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions and was conducted in compliance with the Declaration of Helsinki, US Code 21 of Federal Regulations, and the Harmonized “Tripartite Guidelines for Good Clinical Practice (1996). The study was registered on February 8, 2013 at www.clinicaltrials.gov ( NCT01790685). All patients provided informed consent. Forty subjects with RVO (17 with BRVO and 23 with CRVO) were enrolled. Disease duration for each patient was calculated from when the patient first developed macular edema until the baseline visit. Because patient reporting is often unreliable, only injections documented in records were used to determine the number of prior anti-VEGF injections. Response to prior anti-VEGF therapy was graded as good, moderate, or poor depending upon whether all intraretinal fluid could be eliminated by monthly injections or how frequently anti-VEGF injections had to be given to maintain a dry macula. Qualitative measurement of nonperfusion at baseline were made using ultra widefield fluorescein angiography (Optos 200Tx) done at or prior to the baseline visit. At baseline and all subsequent visits, subjects had measurement of best-corrected visual acuity (BCVA) using the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol, ophthalmologic examination including measurement of intraocular pressure , spectral domain-optical coherence tomography (SD-OCT) using the Spectralis machine (Heidelberg Engineering, Inc., Carlsbad, CA), and an anterior chamber tap. Aqueous samples were stored at −80°C. Patients were given an intraocular inject ion of a dexamethasone implant in the study eye at baseline. Povidone-iodine was used to clean the conjunctiva and 2% lidocaine was injected under the conjunctiva. The 22-gauge needle of the injector was inserted through the pars plana and the dexamethasone implant was injected into the vitreous cavity.
Functional and anatomic outcomes
The major functional outcome measure was change from baseline BCVA in ETDRS letter score at weeks 4, 8, 12, and 16. One anatomic outcome was the change from baseline center subfield thickness at weeks 4, 8, 12, and 16. Excess foveal thickness (EFT) was calculated for each subject by subtracting the minimum foveal thickness during the course of the disease (edema-free thickness which in each case was < 320μm) from the foveal thickness at baseline and weeks 4, 8, 12 and 16. Percent change in EFT at each time point was calculated using the following formula: % change in EFT at a time point = (EFT at baseline – EFT at time point)/EFT at baseline. In addition, qualitative changes in intraretinal fluid were graded by side-to-side comparisons of baseline, week 4, and week 16 SD-OCT scans.
Vasoactive protein arrays
The levels of vasoactive proteins in aqueous at baseline and week 4 were measured in 11 eyes with BRVO and 11 eyes with CRVO at IMGENEX Corporation (San Diego, CA) using the Human Angiogenesis Antibody Array Kit (catalog number ARY007, R&D Systems, Inc., Minneapolis, MN). Samples obtained at week 16 were also included for 3 eyes with BRVO and one with CRVO. Aqueous samples (115 μl) were blotted on the membrane which was stored for 16 hours at 4°C and further processed according to the manufacturer’s instructions. Each array membrane was exposed to X-ray film for short-term and long-term exposures. The positive signals detected on the developed X-ray film were quantitated using TotalLab Quant software (Gentel Biosciences, Inc., Madison, WI). Results are reported as percent change from baseline.
Enzyme linked immunosorbent assays (ELISAs)
Protein arrays indicated a reduction in aqueous levels of hepatocyte growth factor in several patients between baseline and week 4 and therefore ELISA was used to measure aqueous levels of hepatocyte growth factor and VEGF in another cohort of RVO patients at baseline, week 4, and week 16 (when week 16 samples were available). Levels of hepatocyte growth factor and VEGF were measured in aqueous samples using ELISA kits for each (Abcam, Cambridge, MA) using the manufacturer’s instructions. Briefly, 100 μl of each aqueous sample diluted 1:1 in dilution buffer or 100 μl of hepatocyte growth factor or VEGF protein standard was added to a well of a 96-well plate and incubated at 4°C overnight. After washing wells 4 times, 100 μl of biotinylated anti-hepatocyte growth factor or anti-VEGF antibody was added to each well and incubated for 1 hour at room temperature. After 4 washes, 100 μl of horse radish peroxidase-streptavidin solution was added to each well and incubated for 45 minutes at room temperature. After washing wells 4 times, 100 μl of 3,3'-5,5' tetramethylbenzidine substrate reagent was added to each well and after 30 minutes the reaction was stopped by adding 50 μl of stop solution. Absorbance at 450 nm was measured on a plate reader. The readings from the standards were used to generate standard curves of absorbance versus hepatocyte growth factor or VEGF. The hepatocyte growth factor and VEGF concentration in each sample was calculated by plotting absorbance on the respective standard curve.
Correlation of changes in vasoactive proteins with changes in edema
The diversity of protein levels and center subfield thickness were calculated as percentage change in their measurements at week 4 relative to baseline.
Where, FCProtein and FCCST are the changes in protein level and center subfield thickness, EB, EW4, CSTB and CSTW4 represent the protein level and center subfield thickness at baseline and week 4, respectively. In order to estimate the correlation between percent reduction in protein level and percent reduction in excess foveal thickness, Pearson correlation coefficient was calculated using R.
Results
Demographics and baseline characteristics
The ORVO trial enrolled 40 subjects with macular edema, 17 with BRVO and 23 with CRVO. Most subjects had long-standing macular edema with the median duration 40 months for patients with BRVO and 45 months for patients with CRVO, but there were also a few patients who had a relatively short duration of disease (Table 1). All subjects had previously been treated with anti-VEGF injections with a median of 13.9 (BRVO, n=14) or 18.9 (CRVO, n=21); only documented injections were counted, not those based upon patient history and therefore these means are minimums and actual means may be larger. Detailed information regarding prior response to anti-VEGF injections was available for 12 patients with BRVO and 20 patients with CRVO. During periods of monthly or in some cases less frequent injections, there was minimal residual intraretinal fluid in 4 subjects with BRVO and 11 subjects with CRVO, while in 8 subjects with BRVO and 9 with CRVO there was substantial residual intraretinal fluid even during periods of monthly injections. The median BCVA at baseline in ETDRS letter score was 60 (20/63) in eyes with BRVO and 54 (20/80) in eyes with CRVO. The median central subfield thickness at baseline was 453 μm in eyes with BRVO and 539 μm in eyes with CRVO, but there were differences among patients with thickening and intraretinal fluid ranging from mild to severe.
Table 1.
Patient Demographics and Baseline Characteristics for patients with Retinal Vein Occlusion who received a dexamethasone implant
| Variable | BRVO (N=17) |
CRVO (N=23) |
|---|---|---|
| Age in years median (range) | 72 (54-89) | 74 (54-90) |
| Gender/Females n (%) | 5 (29.4%) | 11 (47.8%) |
| Median disease duration (months) | 40 | 45 |
| Retinal nonperfusion based upon wide angle FA n (%) | ||
| Mild | 9 (52.9) | 12 (52.2) |
| Moderate | 4 (23.5) | 4 (17.4) |
| Severe | 2 (l1.8) | 4 (17.4) |
| No gradable FA | 2 (11.8) | 3 (13.0) |
| Prior anti-VEGF injections* | ||
| Mean (range) | 13.9 (1-36) | 18.9 (2-41) |
| Response to anti-VEGF injections | ||
| Gooda | 4 | 11 |
| Poorb | 8 | 9 |
| Indeterminatec | 5 | 3 |
| Prior Intraocular Steroids n (%) | 5 (29.4%) | 1 (4.3%) |
| Grid laser (%) | 5 (29.4) | 8 (34.8) |
| Scatter Laser photocoagulation (%) | 4 (23.5) | 13 (56.5) |
| Baseline BCVA (letter score) | ||
| Median (range) | 60 (29-71) | 54 (19-76) |
| Baseline CST (μm) | ||
| Median (range) | 453 (225-792) | 539 (251-941) |
| Baseline intraretinal fluid | ||
| Mild | 4 | 1 |
| Moderate | 4 | 9 |
| Severe | 9 | 13 |
Based upon verified observed data and electronic patient records for 14 BRVO and 21 CRVO patients
Good response = elimination of all or most intraretinal fluid during periods of monthly or less frequent injections
Poor response = substantial recurrent/residual intraretinal fluid even during periods of monthly injections
Indeterminate = unable to determine from available data
Abbreviations: BRVO = branch retinal vein occlusion, CRVO = central retinal vein occlusion, BCVA = best-corrected visual acuity, CST = central subfield thickness, VEGF = vascular endothelial growth factor, μm = microns, FA = fluorescein angiogram
Patient disposition
Three patients exited the study early. Subject B7 fell and suffered trauma after the week 4 visit and could not continue, subject B11 withdrew consent after the week 12 visit, and subject C14 was lost to follow up after the week 4 visit. This had little impact on the study because the primary outcome was based upon the correlation between change in central subfield thickness and aqueous vasoactive protein levels between baseline and week 4.
Anatomic and visual outcomes
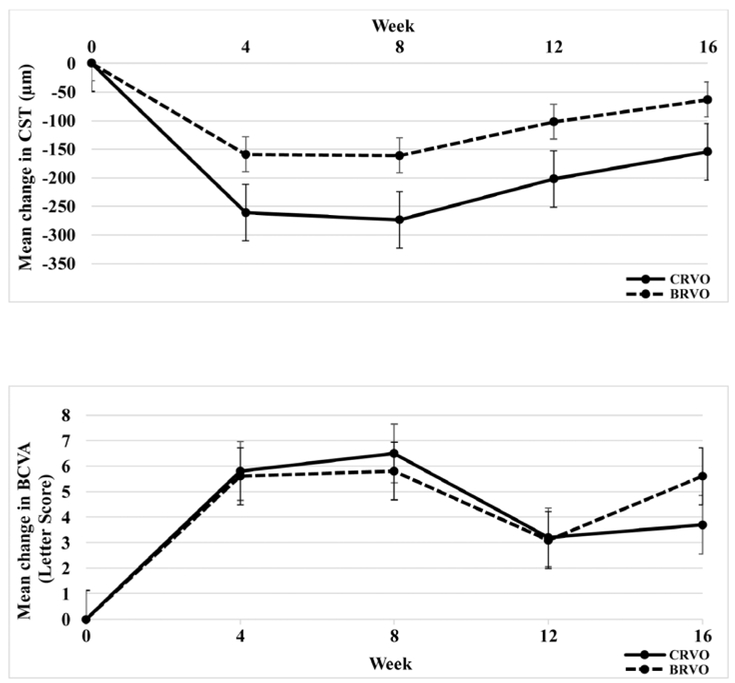
In this population of patients with chronic/recurrent macular edema due to BRVO or CRVO, there were substantial reductions from baseline mean central subfield thickness at weeks 4 and 8, with increases at week 12 (Figure 1, top). This was accompanied by improvements in BCVA at weeks 4 and 8 after injection of a dexamethasone implant with reductions at week 12 and some reversal at week 16 due to rescue injections (Figure 1, bottom).
Figure 1. Improvement in central subfield thickness (top) and best-corrected visual acuity (bottom) after injection of dexamethasone implant in patients with macular edema due to retinal vein occlusion.
Patients with macular edema due to central retinal vein occlusion (CRVO) or branch retinal vein occlusion (BRVO) were given an intravitreous injection of a dexamethasone implant at baseline. Mean (±standard error of the mean) change from baseline central subfield thickness (CST, top) and mean (±standard error of the mean) change from baseline best-corrected visual acuity (BCVA, bottom) are shown at each study visit.
In addition to the mean changes in the subject population, it is important to examine changes in individual subjects considering the differences among patients in duration of disease, severity of edema at baseline, amount of retinal nonperfusion at baseline, and number of prior injections of a VEGF antagonist (Supplemental Tables 1 and 2, available at AJO.com). To assess differences among patients with regard to severity of edema at baseline and response to dexamethasone implant injection, we examined SD-OCT horizontal cross sections through the fovea, BCVA, and center subfield thickness at each study visit (Supplemental Figures 1 and 2, available at AJO.com, supplemental). However, a horizontal cross section may not provide a good indicator of edema in all patients, because in some, intraretinal fluid was slightly above or below the horizontal meridian (Supplemental Table 2, B7). While the anatomic responses were generally very good and in many cases impressive, improvements in BCVA were more variable probably due to macular damage from chronic/recurrent edema or ischemic damage as suggested by macular thinness and/or irregularity after edema reduction in many patients.
Changes in aqueous levels of vasoactive proteins after injection of dexamethasone implant
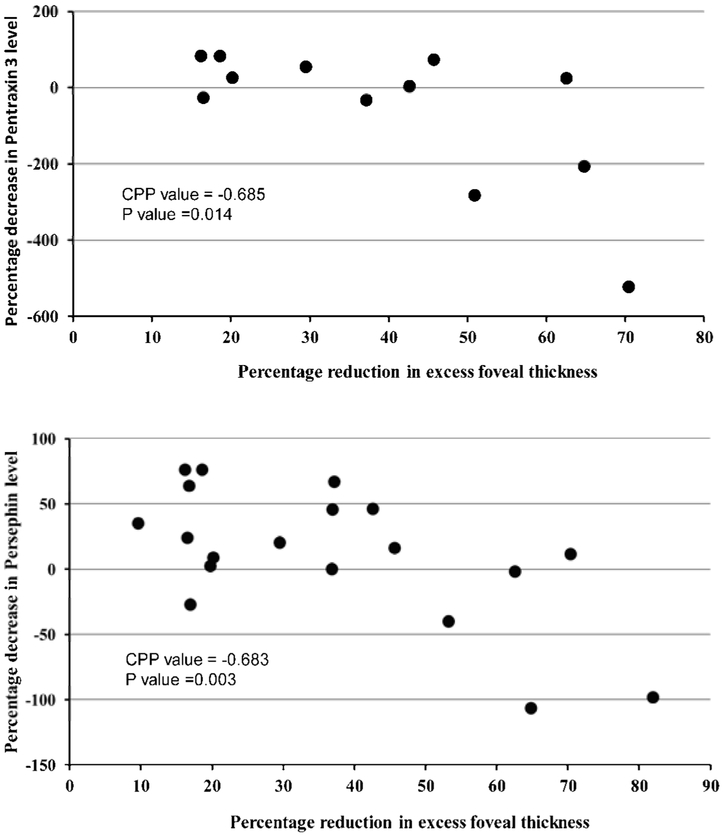
Aqueous samples obtained at baseline and 4 weeks after injection of a dexamethasone implant for 11 subjects with BRVO and 11 subjects with CRVO were run on a vasoactive protein array. Table 2 shows the proteins on the array that were detected in at least some of the aqueous samples of some patients and those that were not detected in any of the samples. The major objective of this study was to determine the relative differences in aqueous vasoactive protein levels between baseline and week 4 and correlate those changes with reduction in edema. To quantitatively assess the correlation between percentage decrease in excess foveal thickness and percentage reduction in each protein between baseline and week 4, percentage decrease in excess foveal thickness was calculated as described in Methods and plotted against percentage change in protein level. The percentage decrease in pentraxin 3 (Pearson Correlation Coefficient= 0.685, p=0.014) and persephin (Pearson Correlation Coefficient = 0.638, p=0.003) correlated with reduction in excess foveal thickness (Figure 2), but for all of the other proteins there was not a significant correlation.
Table 2.
Proteins on array used to test aqueous of patients with macular edema due to retinal vein occlusion who received a dexamethasone implant
| Proteins detected | Proteins consistently undetected |
|---|---|
| Activin A | ADAMTS-1 |
| Angiogenin | Amphiregulin |
| Angiopoietin-1 | Coagulation Factor III |
| Angiopoietin-2 | EGF |
| Angiostatin/Plasminogen | Endoglin |
| Artemin | FGF-1 |
| CXCL4 | FGF-2 |
| CXCL16 | FGF-4 |
| DPP-IV | FGF-7 |
| EG-VEGF | GDNF |
| Endostatin/Collagen XVIII | GM-CSF |
| Endothelin-1 | IL-1β |
| HB-EGF | IL-8 |
| HGF | MCP-1 |
| IGFBP-1 | MIP-1α |
| IGFBP-2 | MMP-8 |
| IGFBP-3 | NRG1-β1 |
| Leptin | PDGF-AB/PDGF-BB |
| MMP-9 | Serpin B5 |
| Pentraxin 3 | TGF-β1 |
| PD-ECGF | uPA |
| PDGF-AA | Vasohibin |
| Persephin | VEGF-C |
| PlGF | |
| Prolactin | |
| Serpin E1 | |
| Serpin F1 | |
| TIMP-1 | |
| TIMP-4 | |
| Thrombospondin-1 | |
| Thrombospondin-2 | |
| VEGF-A |
Abbreviations: CXCL4 = C-X-C motif ligand 4, CXCL16 = C-X-C motif ligand 16, DPP-IV = dipeptidyl peptidase-4, EG-VEGF = endocrine gland-vascular endothelial growth factor, HB-EGF = heparin binding-epidermal growth factor, HGF = hepatocyte growth factor, IGFBP = insulin-like growth factor binding protein, MMP = matrix metalloproteinase, PD-ECGF = platelet-derived endothelial cell growth factor, PDGF = platelet-derived growth factor, PlGF = placental growth factor, TIMP = tissue inhibitor of metalloproteinases, VEGF = vascular endothelial growth factor, ADAMTS-1 = A Disintegrin and Metalloproteinase with Thrombospondin motif protein-1, EGF = epidermal growth factor, FGF = fibroblast growth factor, GDNF = glial cell line-derived neurotrophic factor, GM-CSF = granulocyte macrophage colony-stimulating factor, IL= interleukin, MCP-1 = monocyte chemoattractant protein-1, MIP-1α = macrophage inhibitory protein-1α, NRG1 = neuroregulin 1, TGF-β1 = transforming growth factor-β1, uPA = urokinase
Figure 2. Correlation between reduction from baseline and week 4 in aqueous level of pentraxin 3 (top) or persephin (bottom) and reduction from baseline in excess foveal thickness after dexamethasone implant in patients with retinal vein occlusion.
Patients who had detectable levels of pentraxin 3 (top) or persephin (bottom) at baseline and week 4 had percentage reduction in protein level plotted versus percentage reduction in excess foveal thickness. Pearson Correlation Coefficient was 0.685 for pentraxin 3 (p=0.014) and 0.638 for persephin (p=0.003).
Supplemental Tables 3 and 4 (available at AJO.com) show the proteins for which there were changes between baseline and week 4 for BRVO and CRVO subjects, respectively. The proteins are divided into the following categories: 1) reduced ≥ 70%, 2) reduced ≥ 50%, 3) reduced ≥ 30%, 4) reduced 10-30%, 5) no change (defined as decreased or increased by <10%), 6) increased by ≥10%, or 7) undetected in baseline sample. These data demonstrate that there are a large number of proteins that are reduced in eyes with macular edema after injection of a dexamethasone implant and there is substantial heterogeneity among patients. We selected a 30% reduction as the threshold of a clinically meaningful reduction that potentially could contribute to reduction in edema. The median number of proteins that were decreased ≥ 30% between baseline and week 4 was 7 (range 0-21) in eyes with BRVO and 9 (range 3-17) in eyes with CRVO (Supplemental Tables 3 and 4, available at AJO.com).
Proteins that were reduced by ≥ 30% in the majority of the 22 dexamethasone implant-injected eyes with RVO, were hepatocyte growth factor, endocrine gland-VEGF, insulin-like growth factor binding proteins 1, 2, and 3, and endostatin (Table 3). Considering BRVO (Table 3, top) and CRVO (Table 3, bottom) separately, at least 4 of each showed ≥ 30% reductions in hepatocyte growth factor, endocrine gland-VEGF, insulin-like growth factor binding proteins 1, 2, and 3, activin-A, and endostatin.
Table 3.
Aqueous proteins reduced by ≥30% between baseline and week 4 in at least 30% of patients with retinal vein occlusion treated with dexamethasone implant
| BRVO | |
|---|---|
| Number of patients with ≥30% reduction (N=11) |
Proteins |
| 8 | Persephin |
| 7 | IGFBP-2 IGFBP-3 |
| 6 | Activin-A Endostatin |
| 5 | HGF |
| 4 | EG-VEGF IGFBP-1 |
| CRVO | |
| Number of patients with ≥30% reduction (N=11) |
Proteins |
| 7 | HGF EG-VEGF IGFBP-1 Endostatin |
| 6 | IGFBP-2 IGFBP-3 CXCL16 MMP-9 |
| 5 | HB-EGF Thrombospondin-1 |
| 4 | Activin-A DPP-IV Angiopoietin-1 PDGF-AA |
Abbreviations: IGFBP = insulin-like growth factor binding protein, HGF = hepatocyte growth factor, EG-VEGF = endocrine gland-vascular endothelial growth factor, CXCL16 = C-X-C motif ligand 16, MMP = matrix metalloproteinase, HB-EGF = heparin binding-epidermal growth factor, DPP-IV = dipeptidyl peptidase-4, PDGF = platelet-derived growth factor
The levels of hepatocyte growth factor and VEGF at baseline, week 4, and when available, week 16, were measured in aqueous samples from the 6 remaining eyes with BRVO and the 12 remaining eyes with CRVO by ELISA. Levels of hepatocyte growth factor were greater than those of VEGF in all eyes, but they were in the same range with a mean of 145.9 pg/ml in eyes with CRVO and 212.8 pg/ml in eyes with BRVO for hepatocyte growth factor versus 86.6 pg/ml and 63.0 pg/ml for VEGF. There were 20-64% reductions in hepatocyte growth factor in association with reduced macular edema in 5 of 6 patients with BRVO and in 2 patients for whom samples were available at week 16 there was an increase in hepatocyte growth factor between weeks 4 and 16 associated with recurrent edema (Supplemental Table 5, available at AJO.com). Of the 5 eyes with BRVO that showed a reduction in hepatocyte growth factor, 4 showed 18%-74% concomitant decreases in VEGF. The one BRVO eye that showed a 187% increase in hepatocyte growth factor between baseline and week 4, showed a 37% reduction in VEGF. Of the 12 eyes with CRVO, 9 showed complete or nearly complete resolution of severe or moderate macular edema and 3 showed modest reductions in edema between baseline and week 4. Hepatocyte growth factor was reduced in 7, unchanged in 2, and increased in 3, while VEGF was reduced in 5, unchanged in 2, increased in 4, and not tested in 1 due to insufficient sample (Supplemental Table 6, available at AJO.com). All of the eyes showed a reduction in hepatocyte growth factor or VEGF except one eye in which both were increased and this eye had minimal reduction in central subfield thickness which was 539 μm at baseline and 435μm at week 4.
Effects of dexamethasone implants on intraocular pressure
Three patients (7.5%) had an IOP above 30 during the trial and two of those patients had the increase in IOP after a second rescue injection of a dexamethasone implant. Seven patients (17.5%) required an IOP lowering drop and in 2 it was after a second dexamethasone implant injection.
Discussion
Most patients with BRVO or CRVO receive substantial benefits from injections of a VEGF-neutralizing protein, but prolonged treatment is often needed. In some patients, monthly injections of an anti-VEGF neutralizing protein do not eliminate edema suggesting that in these patients VEGF is not completely neutralized or other pro-permeability factors are contributing to edema. Only 50% of patients with BRVO and 44% of patients with CRVO have resolution of edema with no need for further injections after 4 years of anti-VEGF injections.9 In many of these patients, anti-VEGF injections become less effective at reducing intraretinal fluid over time, suggesting that while VEGF was the predominate cause of leakage initially, other vasoactive factors may contribute to chronic/recurrent edema. Many studies have reported elevated levels of a single or a few vasoactive proteins in eyes with macular edema due to RVO, but the mere presence of a protein does not prove that it contributes to edema, particularly in an eye in which injection of a specific VEGF antagonist is able to eliminate all edema.
In this study, we used a different strategy. First, we focused on patients with chronic/recurrent macular edema secondary to RVO. Some of these patients had elimination of most intraretinal fluid during periods of monthly injections (or in some patients less frequent injections) of a VEGF antagonist; however, some patients chose to reduce visit frequency and tolerate bouts of recurrent edema. Eight patients with BRVO and 9 with CRVO had substantial residual intraretinal or subretinal fluid even during periods of monthly injections. The second aspect of the strategy was to measure levels of 55 vasoactive proteins before and after injection of a dexamethasone implant and correlate improvements in edema with reductions in aqueous proteins.
The first important observation is that injection of a dexamethasone implant resulted in a marked reduction in intraretinal fluid in most patients with chronic/recurrent edema due to RVO, but there were some differences among patients with most having no or minimal residual intraretinal fluid at week 4, while others had substantial residual fluid. In general, benefits were maintained for 8 weeks in almost all patients, 12 weeks in some patients, and 16 weeks in only a few patients. A second important observation is that there is substantial heterogeneity with regard to measureable levels of vasoactive proteins in the aqueous of patients with chronic/recurrent macular edema due to RVO and the manner in which those proteins change in association with reduction in edema. The median number of proteins reduced by ≥ 30% between baseline and week 4 in 11 eyes with BRVO was 7, but there was substantial variability with a range of 0 to 21. In 2 eyes with BRVO in which severe edema was substantially reduced after injection of a dexamethasone implant, none of the 45 proteins on the array were reduced by ≥ 30%, while another eye in which edema was reduced from moderate at baseline to mild at week 4, there was a reduction ≥ 30% in 21 proteins. In 11 eyes with CRVO the number of proteins reduced by ≥ 30% in association with reduced edema ranged from 3 to 17 with a median of 9. These data indicate that there is not one single factor in addition to VEGF that is a major contributor to macular edema in all patients with RVO, but instead there are likely to be different contributors in different patients. Despite the high degree of heterogeneity, there were two factors, persephin and pentraxin-3, for which there was a modest (0.64, 0.68) statistically significant correlation between percentage reduction in protein level and percentage reduction in edema. Persephin belongs to the glial-cell-line-derived neurotrophic factor family of ligands, a subgroup of the transforming growth factor β superfamily.14 Binding of persephin to its receptor results in Ret kinase activation.15 Ret kinase mutations promote papillary thyroid carcinoma and multiple endocrine neoplasia types 2A and 2B.16 Persephin knockout mice are hypersensitive to cerebral ischemia and have a 3-fold increase in infarct volume compared to wild type mice after occlusion of the middle cerebral artery.17 The role of persephin in the retina is uncertain and therefore it is not known if the dexamethasone implant-induced reduction in persephin that correlates with reduction in edema has any physiologic or pathologic effect. Pentraxin-3 is a member of the pentraxin family of fluid phase pattern recognition molecules that is induced in many cell types including endothelial cells and leukocytes by the inflammatory cytokines interleukin-1 β and tumor necrosis factor-α.18, 19 Serum levels of pentraxin 3 provide a biomarker of inflammation particularly that associated with vascular injury.20, 21 Several studies have suggested that an increase in serum pentraxin 3 levels in patients with coronary artery disease suggests vulnerable plaque and is a negative prognostic sign in patients with acute myocardial infarction.22, 23 Previous studies have measured increased levels of pentraxin 3 in the vitreous of patients with BRVO24 or CRVO25. Steroids have been shown to reduce leukocyte-derived pentraxin 3 and increase fibroblast-derived pentraxin 3,26 and thus the reduction of pentraxin 3 after injection of a dexamethasone implant in eyes with RVO may indicate reduced inflammatory cells and/or their activity.
Understanding that RVO patient heterogeneity regarding ocular levels of vasoactive proteins and their correlation with macular edema prevents definitive conclusions as to which other proteins contribute to edema, it is still useful to examine which proteins with known propermeability activity most consistently showed reductions of ≥ 30% to provide candidates for future studies. Those pro-permeability factor that correlate with changes in macular edema in the largest % of patients with RVO are hepatocyte growth factor, endocrine gland-VEGF, and activin-A. The protein array findings for hepatocyte growth factor were confirmed by ELISA, increasing confidence in their validity. Hepatocyte growth factor is is proangiogenic,27 increases permeability through endothelial cell monolayers,28 and causes vascular leakage in the eye.29 Patients with proliferative diabetic retinopathy have increased levels of HGF in the vitreous.30 Under normal conditions, endocrine gland-VEGF is expressed only in endocrine glands,31, 32 so its presence in eyes with macular edema due to RVO is a bit surprising, but perhaps should not be, because aberrant expression is a common feature of diseased tissue. Endocrine gland-VEGF is not structurally related to VEGF and binds to G-protein-coupled receptors rather than tyrosine kinase receptors. Under normal circumstances expression of the receptor may be limited to endothelial cells of endocrine glands contributing to tissue specificity, because injection of adenoviral vectors that increase expression of endocrine gland-VEGF has no effect in skin or skeletal muscle but induces angiogenesis in the ovary;31 therefore, in order for the increased levels of endocrine gland-VEGF in some eyes with RVO to have biologic significance, there must also be aberrant expression of one of the two endocrine gland-VEGF receptors on diseased retinal endothelial cells. If there is, then steroid-induced reduction of endocrine gland-VEGF could contribute to the anti-permeability effects of dexamethasone implants, because stimulation of endocrine gland-VEGF receptors promotes fenestrae and leakage. Activin-A is a glycoprotein that is a member of the transforming growth factor-β superfamily.33 It forms dimers that bind to activin receptors on endothelial cells and stimulates tube formation in vitro.34 Activin A stimulates expression of VEGF and promotes corneal neovascularization.35 Thus it is reasonable to postulate that its reduction after dexamethasone implant injection may contribute to edema reduction.
Our discussion has been limited to proteins that showed changes that correlated with edema in a substantial number of patients, but there were many proteins that showed correlations in a few patients. It is expected that many of these are due to chance, but we cannot rule out the possibility that some of these proteins should also receive consideration and further testing. Protein changes detected in all patients are shown in Supplemental Tables 5 and 6 (available at AJO.com) so that other investigators can give this matter consideration. It should also be noted there are vasoactive proteins that were not included on the protein array and it is possible that some of those deserve consideration.
In summary, our data suggest that patients with chronic/recurrent edema due to RVO differ with respect to the number and identity of vasoactive proteins detectable in the aqueous. This suggests that there may be changes in the disease process over time that could influence therapeutic response. Despite this heterogeneity, intraocular injection of dexamethasone implants are generally quite effective in most patients with chronic/recurrent edema due to RVO causing substantial reduction in edema for about 2-3 months. The reduction in edema is associated with decreases in multiple factors many of which increase as edema recurs. While we cannot say for certain which of these factors contribute to edema, it is likely that multiple proteins contribute and the contributors differ among patients. This indicates that dexamethasone implants provide a useful multitargeted approach. Regardless of the benefit provided by dexamethasone implants in the majority of patients, knowing which of these factors contribute to edema would be useful to design more specific combination treatments, since not all patients can tolerate prolonged use of intraocular steroids due increased intraocular pressure. The most compelling candidates are hepatocyte growth factor, endocrine gland-VEGF, and activin-A. To test the hypothesis that these proteins contribute to edema, it will be necessary to determine the effect of specific antagonists for these proteins on edema in a similar patient population.
Supplementary Material
Acknowledgments/Disclosure
This study was supported by a grant from Allergan. The authors would like to acknowledge the Wilmer Biostatistics Core Grant EY01765.
Financial Disclosures: Peter A. Campochiaro - Consultant- Genentech, South San Francisco, CA; Regeneron, Tarrytown, NY; Aerpio, Cincinnati, OH; Alimera, Atlanta, GA; Allegro, San Diego, CA; Eleven, Cambridge, MA; Kala, Cambridge, MA; Applied Genetic Technologies Corporation, Gainesville, FL; Ocata Therapeutics, Marlborough, MA; and AsclepiX, Baltimore, MD. Institutional Grants- Allergan, Irvine, CA; South San Francisco, CA; Regeneron, Tarrytown, NY; Aerpio, Cincinnati, OH, Abbvie, North Chicago, IL; Genzyme, Cambridge, MA; Oxford Biomedica, Oxford, UK; Roche, Basal, Switzerland; GlaxoSmithKline, Middlesex, UK; AscepiX, Baltimore, MD, Graybug, Baltimore, MD.
Howard S. Ying - Institutional Grant- Regeneron, Tarrytown, NY
Adam Wenick – Consultant –Bristol-Myers Squibb, New York, NY; Institutional Grant – Novo Nordisk, Bagsvaerd, Denmark
Biography

Peter A. Campochiaro is the Eccles Professor of Ophthalmology and Neuroscience at the Wilmer Eye Institute of the Johns Hopkins University School of Medicine. He directs a laboratory focused on elucidating the molecular pathogenesis of ocular neovascularization, macular edema, and retinal degeneration. He conducts early phase and late phase clinical trials and has helped to develop new treatments for neovascular AMD, diabetic macular edema, and macular edema due to retinal vein occlusion.

Gulnar Hafiz MD, MPH is a research faculty member at the Wilmer Eye Institute of Johns Hopkins School of Medicine, who has expertise in Public Health and interventional clinical trials in retinal diseases. She supervises a large clinical trial team and has participated in development of several new treatments for retinal diseases.
Footnotes
Supplemental material available at AJO.com
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions; implication of VEGF as a critical stimulator. Molec Ther 2008;16(4):791–799. [DOI] [PubMed] [Google Scholar]
- 2.Campochiaro PA, Hafiz G, Channa R, et al. Antagonism of vascular endothelial growth factor for macular edema caused by retinal vein occlusion: two-year outcomes. Ophthalmology 2010;117(12):2387–2394. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Campochiaro PA, Singh RP, et al. Efficacy and safety of ranibizumab in the treatment of macular edema secondary to central retinal vein occlusion:6-month results of the phase III CRUISE study. Ophthalmology 2010;117(6):1124–1133. [DOI] [PubMed] [Google Scholar]
- 4.Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011;118(10):2041–2049. [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011. ;118(8):1594–1602. [DOI] [PubMed] [Google Scholar]
- 6.Brown DM, Heier JS, Clark LW, et al. Intravitreal alflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS Study. Am J Ophthalmol 2013;155(3):429–437. [DOI] [PubMed] [Google Scholar]
- 7.Campochiaro PA, Bhistikul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology 2013;120(4):795–802. [DOI] [PubMed] [Google Scholar]
- 8.Sophie R, Hafiz G, Scott A, et al. Long term outcomes in ranibizumab-treated patients with retinal vein occlusion; the role of progression of retinal nonperfusion. Am J Ophthalmol 2013;156(4):693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campochiaro PA, Sophie R, Pearlman J, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: The RETAIN Study. Ophthalmology 2014;121(1):209–219. [DOI] [PubMed] [Google Scholar]
- 10.Yang-Yen HF, Chambard JC, Sun YL, et al. Transciptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 1990;62(6):1205–1215. [DOI] [PubMed] [Google Scholar]
- 11.Schule R, Rangarajan P, Kliewer S, et al. Runctional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell 1990;62(6):1217–1226. [DOI] [PubMed] [Google Scholar]
- 12.Heck S, Kullmann M, Gast A, et al. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP1. EMBO J 1994;13(17):4087–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyer DS, Yoon YH, Belfort RJ, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014;121(10):1904–1914. [DOI] [PubMed] [Google Scholar]
- 14.Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci 1999;13(5):313–325. [DOI] [PubMed] [Google Scholar]
- 15.Lindahl M, Poteryaev D, Yu L, et al. Human glial cell line-derived neurotrophic factor receptor alpha4 is the recepor for persephin and is predominantly expressed in normal and malignant thyroid medullary cells. J Biol Chem 2001. ;276(12):9344–9351. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi M The GdNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev 2001;12(4):361–373. [DOI] [PubMed] [Google Scholar]
- 17.Tomac AC, Agulnick AD, Haughey N, et al. Effects of cerebral ischemia in mice deficient in persephin. Proc Natl Acad Sci USA 2002;99(14):9521–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breviario F, d'Aniello EM, Golay J, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem 1992;267(31):22190–22197. [PubMed] [Google Scholar]
- 19.Han B, Mura M, Andrade CF, et al. TNFalpha-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J Immunol 2005;175(12):8303–8311. [DOI] [PubMed] [Google Scholar]
- 20.Peri G, Introna M, Corradi D, et al. PTX3, a prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans Circulation 2000;102(6):636–641. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S, Takeishi Y, Niizeki T, et al. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am Heart J 2008;155(1):75–81. [DOI] [PubMed] [Google Scholar]
- 22.Rolph MS, Zimmer S, Bottazzi B, et al. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2002;22(5):e10–14. [DOI] [PubMed] [Google Scholar]
- 23.Latini R, Maggioni AP, Peri G, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 2004;110(16):2349–2354. [DOI] [PubMed] [Google Scholar]
- 24.Noma H, Mimura T, Eguchi S. Association of inflammatory factors with macular edema in branch retinal vein occlusion. JAMA Ophthalmol 2013;131(2):160–165. [DOI] [PubMed] [Google Scholar]
- 25.Noma H, Mimura T, Masahara H, Shimada K. Pentraxin 3 and other infalmmatory factors in central retinal vein occlusion and macular edema. Retina 2014;34(2):352–359. [DOI] [PubMed] [Google Scholar]
- 26.Doni A, Mantovani G, Porta C, et al. Cell-specific regulation of PTX3 by glucocorticoid hormones in hematopoietic and nonhematopoietic cells. J Biol Chem 2008;283(44):29983–29992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant DS, Kleinman HK, Goldberg ID, et al. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA 1993;90(5):1937–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang WG, Martin TA, Matsumoto K, Nakamura T, Mansel RE. Hepatocyte growth factor/scatter factor decreases the expression of occludin and transendothelial resistance (TER) and increases paracellular permeability in human vascular endothelial cells. J Cell Physiol 1999;181(2):319–329. [DOI] [PubMed] [Google Scholar]
- 29.Clermont AC, Cahill M, Salti H, et al. Hepatocyte growth factor induces retinal vascular permeability via MAP-kinase and PI-3 kinase without altering retinal hemodynamics. Invest Ophthalmol Vis Sci 2006;47(6):2701–2708. [DOI] [PubMed] [Google Scholar]
- 30.Katsura Y, Okano T, Noritake M, et al. Hepatocyte growth factor in vitreous fluid of patients with proliferative diabetic retinopathy and other retinal disorders. Diabetes Care 1998;21(10):1759–1763. [DOI] [PubMed] [Google Scholar]
- 31.LeCouter J, Kowalski J, Foster J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 2001;412(6850):877–884. [DOI] [PubMed] [Google Scholar]
- 32.LeCouter J, Lin R, Ferrara N. Endocrine gland-derived VEGF and the emerging hypothesis of organ-specific regulation of angiogenesis. Nature Med 2002;8(9):913–917. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy SA, Bicknell R. Activin-A binds to a heterotrimeric receptor complex on the vascular endothelial cell surface. Evidence for a type 3 activin receptor. J Biol Chem 1993;269(6):3909–3912. [PubMed] [Google Scholar]
- 34.Hayashi Y, Maeshima K, Goto F, Kojima I. Activin A as a critical mediator of capillary formation: interaction with the fibroblast growth factor action. Endocrine J 2007;54(2):311–318. [DOI] [PubMed] [Google Scholar]
- 35.Poulaki V, Mitsiades N, Kruse FE, et al. Activin A in the regulation of corneal neovascularization and vascular endothelial growth factor expression. Am J Pathol 2004;164(4): 1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.