Richter transformation (RT) of chronic lymphocytic leukemia (CLL) into high grade lymphoma is a complication in 2–10% of patients. While development of diffuse large B-cell lymphoma (DLBCL) is most common, rare occurrences of Hodgkin lymphoma (HL) have been documented[1, 2]. Targeted therapy has durable antitumor activity in high-risk CLL, but 5–10% of patients treated with ibrutinib and 11–25% of those treated with venetoclax on clinical trials developed RT[3, 4, 5]. Views differ on whether RT occurs prior to, during, or even as a consequence of targeted therapy[1, 4, 6]. Unfortunately, pre-treatment tissue biopsies are often unavailable and the question remains debated. Here we describe two patients with CLL diagnosed with HL after 6 months of Bruton tyrosine kinase inhibitor (BTKi) therapy. In both, pre-treatment biopsies revealed the transformation as pre-existing.
Patient 1: A 55-year-old male with previously untreated CLL with 17p deletion presented with early satiety, weight loss, and frequent infections over the past year. Laboratory studies showed: absolute lymphocyte count (ALC) 68.53 K/μL, lactate dehydrogenase (LDH) of 284 U/L and serum β2-microglobulin (β2M) of 4.6 mg/L. Hemoglobin and platelet count were normal. Bone marrow (BM) biopsy was 65% cellular with a 70% infiltrate of CLL cells. Computed tomography (CT) revealed splenomegaly and diffuse lymphadenopathy. The largest lymph node (LN) measured 6.8 cm x 3.7 cm in the right axilla and had a specific uptake value (SUV) of 9.4 on positron emission tomography (PET) while other LNs had moderate uptake as well (Figure 1A). Core needle biopsy of the hypermetabolic LN showed nodal effacement by small monomorphic CLL cells and no evidence of histologic transformation (Figure 1B).
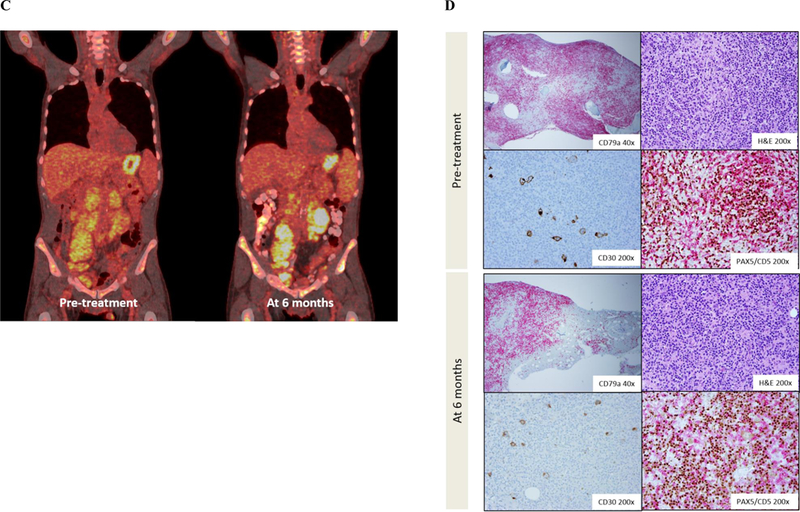
Figure 1: Imaging and histopathologic findings.
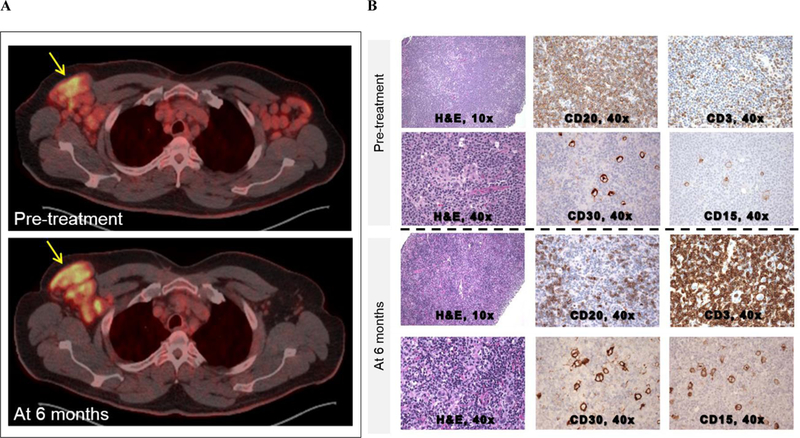
A-B: Patient 1
(A) Pre-treatment PET/CT showed enhancement of bilateral axillary LN with an SUV of 9.4 in the right axillary region. Repeat PET/CT after 6 months showed increased enhancement of the right axillary region (SUV 11.1) and near resolution of disease elsewhere. (B) Pre-treatment LN biopsy using hematoxylin and eosin (H&E) staining at 10x and CD20 and CD3 IHC staining at 40x. After the diagnosis of HL-RT was made, careful inspection of the H&E stain at 40x revealed Reed Sternberg cells amongst a background of diffuse CLL involvement. These Reed-Sternberg cells were positive for CD30 and CD15. After 6 months of treatment, there was a reduction in CLL involvement and more apparent visualization of Reed Sternberg cells.
C-D: Patient 2
(C) Pre-treatment PET/CT showed diffuse enhancement of neck, mediastinal, abdominal and pelvic lymph nodes prior to treatment with acalabrutinib. Maximum SUV of 8.64 was seen in a left iliac node. After 6 months of acalabrutinib, PET/CT showed an interval increase in hypermetabolic activity in retroperitoneal and pelvic LNs. Maximum SUV of 11.16 was seen in retroperitoneal LNs near the left kidney. (B) Bone marrow biopsies were performed before and after 6 months of treatment. CD79a IHC staining of the pre-treatment biopsy at 40x showed extensive bone marrow involvement by CLL. H&E and CD30 stains at 200x revealed Reed Sternberg cells. Double staining for PAX5 (brown) and CD5 (red) highlighted Reed Sternberg cells weakly positive for CD5 amongst a background of CLL cells. Bone marrow biopsy after 6 months of acalabrutinib showed a decrease in CLL involvement and Reed Sternberg cells.
The patient started ibrutinib monotherapy on a phase 2 study (NCT01500733) with rapid resolution of his constitutional symptoms and reduction in splenomegaly and most lymphadenopathy. After 6 months of treatment, however, restaging CT showed persistence of the right axillary LN at 6.7 cm x 3.2 cm. PET demonstrated hypermetabolic activity in this LN (SUV 11.1) concomitant with near resolution of disease in other nodal stations. A LN biopsy showed Reed-Sternberg cells positive for CD30 and CD15, weakly positive for PAX5, and negative for CD20 (Figure 1B), Epstein-Barr virus (EBV)-encoded small RNAs (EBER), and EBV latent membrane protein (LMP). There was also a significant lymphoid infiltrate comprised mostly of T cells and some CLL cells. These findings indicated classical HL amongst a background of residual CLL. To investigate whether RT predated the start of ibrutinib, immunohistochemistry (IHC) for CD15 and CD30 was retrospectively performed on the pre-treatment LN biopsy, revealing a small cluster of Reed-Sternberg cells (Figure 1B). No evidence of HL was observed in the BM at any time. The patient discontinued ibrutinib and started treatment with doxorubicin, bleomycin, vinblastine, and dacarbazine. He achieved a partial response but relapsed within a year and subsequently died of HL.
Patient 2: A 67-year-old male with CLL with 11q deletion presented with urticaria 14 months after achieving a partial response with 2 cycles of fludarabine, cyclophosphamide and ofatumumab complicated by neutropenia. An allergy workup was negative. Over the next year, he developed progressive fatigue, night sweats, unintentional weight loss, abdominal fullness and early satiety. CT showed bulky abdominal lymphadenopathy and splenomegaly. An axillary LN core needle biopsy demonstrated CLL. ALC and β2M were 15.55 K/μL and 6 mg/L, respectively.
He enrolled on a phase 2 study of acalabrutinib for relapsed CLL (NCT02337829). On treatment, his constitutional symptoms and urticaria waxed and waned. After 6 months of acalabrutinib, he was taken off study for grade 3 neutropenia, after which his symptoms rapidly worsened. Although CT showed decreasing lymphadenopathy and splenomegaly, PET revealed hypermetabolic activity in the retroperitoneal and pelvic LNs (maximum SUV 11.66, Figure 1C). BM biopsy showed areas of scattered, large, atypical Reed-Sternberg cells amongst a background of residual CLL cells, histiocytes and mildly increased eosinophils (Figure 1D). The Reed Stenberg cells were positive for CD30, CD15, MUM1, weakly positive for PAX5, and negative for EBER and LMP-1. In a BM biopsy obtained before acalabrutinib therapy, additional IHC identified rare CD30+ CD15+ Reed-Sternberg cells amongst a background of 90% CLL involvement (Figure 1D). No evidence of HL-RT was found in a pre-treatment axillary LN biopsy. The patient was treated with adriamycin, vinblastine, dacarbazine, achieving a complete response with resolution of constitutional symptoms and urticaria. The patient has not required treatment for CLL 12 months after acalabrutinib was discontinued.
Targeted therapy is increasingly used to treat CLL in both salvage and upfront settings. Disease progression on targeted therapy, especially within the first year, often manifests as RT, while progressive CLL tends to emerge after extended treatment[3, 4, 5]. Historically, most patients with RT presented with DLBCL and rarely with HL. On targeted therapy, both DLBCL and HL have been described[1, 2, 3, 4, 7]. Diagnosis of RT amongst a dense background of CLL can be challenging. Recognition of early transformation to HL is aided by the identification of characteristic Reed-Sternberg cells that express IHC markers distinct from CLL cells. Having diagnosed two patients with HL after 6 months on BTKi, we took advantage of this immunophenotype to reexamine pre-treatment tissue biopsies for the presence of Reed-Sternberg cells. Although stains for CD15 and CD30 that helped to identify sites of HL were not part of our routine IHC studies, our experience suggests that they should be included in cases with a suspicion for RT. The delay in clinical diagnosis in our patients fits well with observations from several studies with a median of 4.5–7.5 months from initiation of targeted therapy to the diagnosis of RT[1, 2, 3]. These data suggest that a low burden of transformed disease is already present before starting treatment.
Two types of HL-RT have been described in the literature. Type 1 is characterized by Reed-Sternberg cells scattered in a background of CLL. In type 2 HL-RT, Reed-Sternberg cells are present in a polymorphous, inflammatory background separate from CLL. Cases of HL-RT on BTKi, including the two patients reported here, have been of the type 1 variety and are suggested to represent histologic transformation of the underlying CLL[1, 2]. Known risk factors for RT include prior therapy and EBV reactivation[8]. In a series of 69 HL-RT cases, 76% of patients had been previously treated with 32% receiving fludarabine[9]. Although one of our patients was previously treated with a fludarabine-based regimen, neither were positive for EBV.
The diagnosis of RT requires a high index of suspicion in patients with CLL presenting for treatment. Since diffuse CLL involvement is common in pre-treatment tissue biopsies, a thorough histopathological examination may identify a low burden of transformed disease at an early stage and allow for timely and appropriate management. Interestingly, targeted therapies such as ibrutinib and venetoclax have short-lived activity against DLBCL-RT and de novo DLBCL and HL[10, 11, 12, 13, 14]. Therefore, it is possible that treatment with such agents could only delay the diagnosis of RT due to a transient improvement or delay in progression of disease. Our cases document that RT occurred before the initiation, and not as a result of, BTKi therapy.
Acknowledgements
The authors would like to thank Janet Valdez and Jennifer Lotter for their assistance in the clinic, and Susan Soto, Amanda Bray, and Pia Nierman for protocol support.
References
- 1.Sachanas S, Pangalis GA, Moschogiannis M, et al. Hodgkin Lymphoma Transformation of Chronic Lymphocytic Leukemia Under Ibrutinib Therapy: Chance Association or Therapy-related? Anticancer research. 2017. June;37(6):3277–3280. doi: 10.21873/anticanres.11692. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 2.Glavey S, Quinn J, McCloy M, et al. Emergence of Bruton’s tyrosine kinase-negative Hodgkin lymphoma during ibrutinib treatment of chronic lymphocytic leukaemia. European journal of haematology. 2017. October;99(4):378–380. doi: 10.1111/ejh.12911. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 3.Ahn IE, Underbayev C, Albitar A, et al. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood. 2017. March 16;129(11):1469–1479. doi: 10.1182/blood-2016-06-719294. PubMed PMID: ; PubMed Central PMCID: PMCPMC5356450. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson MA, Tam C, Lew TE, et al. Clinicopathological features and outcomes of progression of CLL on the BCL2 inhibitor venetoclax. Blood. 2017. June 22;129(25):3362–3370. doi: 10.1182/blood-2017-01-763003. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 5.Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 621 ibrutinib-treated chronic lymphocytic leukemia patients in the United States: a real-world analysis. Haematologica. 2018. February 1. doi: 10.3324/haematol.2017.182907. PubMed PMID: ; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CIE. Enigmas in tumor resistance to kinase inhibitors and calculation of the drug resistance index for cancer (DRIC). Semin Cancer Biol. 2017. August;45:36–49. doi: 10.1016/j.semcancer.2016.11.008. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Innocenti I, Rossi D, Trape G, et al. Clinical, pathological, and biological characterization of Richter syndrome developing after ibrutinib treatment for relapsed chronic lymphocytic leukemia. Hematological oncology. 2018. February 27. doi: 10.1002/hon.2502. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 8.Momose H, Jaffe ES, Shin SS, et al. Chronic lymphocytic leukemia/small lymphocytic lymphoma with Reed-Sternberg-like cells and possible transformation to Hodgkin’s disease. Mediation by Epstein-Barr virus. Am J Surg Pathol. 1992. September;16(9):859–67. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 9.Bockorny B, Codreanu I, Dasanu CA. Hodgkin lymphoma as Richter transformation in chronic lymphocytic leukaemia: a retrospective analysis of world literature. British journal of haematology. 2012. January;156(1):50–66. doi: 10.1111/j.1365-2141.2011.08907.x. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 10.Davids MS, Roberts AW, Seymour JF, et al. Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017. March 10;35(8):826–833. doi: 10.1200/jco.2016.70.4320. PubMed PMID: ; PubMed Central PMCID: PMCPMC5455685. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsang M, Shanafelt TD, Call TG, et al. The efficacy of ibrutinib in the treatment of Richter syndrome. Blood. 2015. March 05;125(10):1676–8. doi: 10.1182/blood-2014-12-610782. PubMed PMID: ; PubMed Central PMCID: PMCPMC4351511. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giri S, Hahn A, Yaghmour G, et al. Ibrutinib has some activity in Richter’s syndrome. Blood cancer journal. 2015. January 30;5:e277. doi: 10.1038/bcj.2014.98. PubMed PMID: ; PubMed Central PMCID: PMCPMC5404220. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamadani M, Balasubramanian S, Hari PN. Ibrutinib in Refractory Classic Hodgkin’s Lymphoma. The New England journal of medicine. 2015. October;373(14):1381–2. doi: 10.1056/NEJMc1505857. PubMed PMID: ; eng. [DOI] [PubMed] [Google Scholar]
- 14.Master S, Leary C, Takalkar A, et al. Successful Treatment of Richter Transformation with Ibrutinib in a Patient with Chronic Lymphocytic Leukemia following Allogeneic Hematopoietic Stem Cell Transplant. Case Reports in Oncology. 2017. May-Aug;10(2):534–41. doi: 10.1159/000477338. PubMed PMID: ; PubMed Central PMCID: PMCPMC5498945. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]


