Abstract
Alternative radiolabeled targeted agents are being investigated for children with relapsed neuroblastoma who do not respond to I131-metaiodobenzylguanidine (MIBG) therapy. DOTATATE targets somatostatin receptors (SSTR), particularly SSTR2, which are expressed on neuroblastoma cells. We investigated SSTR2 expression in neuroblastoma tumors (36 high risk; 33 non-high risk patients) and correlated SSTR2 levels with clinical features, norepinephrine transporter (NET) expression and MIBG avidity. SSTR2 and NET immunohistochemistry scores (0-3) were calculated on biopsies using digital image analysis based on staining intensity and distribution. Clinical data were correlated with SSTR2 expression. Median SSTR2 score for 69 patients was 1.31 (0.26-2.55). Non-high risk neuroblastoma was associated with higher SSTR2 score (p=0.032). SSTR2 expression did not correlate with age, INSS stage, MYCN amplification and histology. Higher SSTR2 scores were observed in MIBG-avid versus non-avid NB. SSTR2 score were not significantly associated with NET score (r=−0.062, p=0.62). Twenty-six patients who relapsed or progressed had a median SSTR2 score of 1.33 (0.26-2.55). Patients with neuroblastoma including relapsed or progressive disease showed SSTR2 expression at diagnosis, suggesting they could be candidates for radiolabeled DOTA-conjugated peptide imaging or therapy.
Keywords: Neuroblastoma, somatostatin receptor-2, SSTR2, norepinephrine transporter
Introduction
The outcome for high risk (HR) neuroblastoma (NB) patients who relapse remains poor, with 5-year overall survival (OS) of only 4-8%.1,2 Personalized therapeutic approaches for patients with relapsed NB consist of cytotoxic chemotherapy regimens for example, irinotecan and temozolomide; immunotherapy agents such as dinutuximab; radiolabeled targeted therapy including I131-metaiodobenzylguanidine (I131-MIBG); and molecularly targeted agents for example, crizotinib for ALK-mutated tumors 3–7. Current therapies for relapsed neuroblastoma have response rates of approximately 15-50% with better responses seen in localized non-high-risk patients and those with late relapse.1–3,8–11 However, in the patients that are salvaged from first relapse, 50-90% will have a further relapse within 5 years and time for disease control shortens with each subsequent relapse. 1,11,12 I131-MIBG has shown efficacy and symptomatic clinical response in one-third of patients with relapsed NB, and alternative radioactive targeted agents are being investigated for patients that do not respond to MIBG therapy. 9
Transmembrane G-protein-coupled receptors for somatostatin, a regulatory hypothalamic peptide, are detected in many tissues and are highly expressed in neuroendocrine tumors and other cancers. 13–17 All five somatostatin receptor subtypes have been detected in neuroblastoma tumors, particularly somatostatin receptor-2 (SSTR2), using different in vitro and in vivo techniques including immunohistochemistry, autoradiography (with radioactive somatostatin analogues), Western blot and reverse transcriptase polymerase chain reaction (RT-PCR).15,18–21 There may be intratumoral and intertumoral variations in SSTR expression due to the heterogeneity within neuroblastoma.20 Increased somatostatin and the presence of high affinity SSTRs are associated with more differentiated MYCN-non-amplified neuroblastic tumors and more favorable disease. 14,19,22,23
Somatostatin analogues have been used in diagnosis and treatment of neuroendocrine tumors for many years. 23,24 Radiolabeled DOTA-conjugate peptides bind to specific SSTRs and have demonstrated superior sensitivity and specificity for clinical detection of neuroendocrine tumors when combined with positron emission tomography (PET)/computerized tomography (CT), compared to traditional octreotide scintigraphy.25,26 Early phase studies with radiolabeled-DOTA-conjugate peptides such as 177Lu-DOTATATE and 90Y-DOTATOC have demonstrated efficacy and safety in adults with SSTR-positive tumors. 27
DOTATATE ((DOTA0-Tyr3) octreotate) has a high specificity for SSTR2, and when combined with radionuclide Gallium-68 (68Ga) and Lutetium-177 (177Lu) is a potential candidate for targeted diagnosis and therapy for neuroblastoma.28–30 We recently demonstrated higher uptake of 68Ga-DOTATATE in high-expressing SSTR2 NB xenografts compared to low-expressing SSTR2 NB xenografts, using micro-PET CT and autoradiography. 31 In addition, histological co-localization of SSTR2 and 68Ga-DOTATATE was demonstrated in high-expressing SSTR2 NB xenografts using immunohistochemistry and autoradiography, and tumor growth inhibition was achieved using 177Lu-DOTA-TATE in high-expressing SSTR2 xenograft models. Preliminary studies using DOTA-conjugates for imaging and treatment in children with relapsed and refractory neuroblastoma have demonstrated safety and some clinical benefit; however, the NB patient subgroup most likely to respond to treatment is unknown. 29,30,32 In addition, MIBG non-avid NB patients, who would require alternative therapy to I131-MIBG and who are potential candidates for 177Lu-DOTATATE, have low expression of norepinephrine transporter (NET) 33, but it is not known whether their tumors express SSTR2 or if SSTR2 and NET expression levels are correlated.
This study aimed to describe the prevalence of SSTR2 expression in neuroblastoma tumors and correlate SSTR2 expression with clinical features, clinical outcome, norepinephrine transporter expression and MIBG avidity.
Materials and Methods
Sixty-nine patients with neuroblastoma, 36 high-risk (HR) and 33 non-high risk (NHR), from Children’s Oncology Group (COG) high-risk study A3973 34(N=16) & The Hospital for Sick Children (HSC) (N=53) were included in the study. Tumor samples from diagnosis in the COG study (N=16) were selected from the same cohort of patients (N=27) used in a prior publication from Dubois et al, describing NET protein expression and MIBG avidity.33 For these 16 patients, results of the clinical features and centrally reviewed MIBG scans were obtained from the COG study database. For the remaining patients, clinical features and outcomes were obtained from HSC electronic health records, including local MIBG reports.
15 NB patients from the HSC cohort had post-treatment samples analysed in addition to their diagnostic pre-treatment biopsy. Post-treatment samples were taken at varying time intervals during therapy and not specifically at relapse.
Immunohistochemistry
SSTR2 and NET immunohistochemistry staining was performed for all patient samples (N=69). NET expression was done using the same methodology and antibody as the COG study. 33 Formalin-fixed, paraffin-embedded neuroblastoma specimens were sectioned at 5μm and slides were subjected to heat-induced epitope retrieval with 0.01M Citrate Buffer pH 6 and blocked with 3% methanolic hydrogen peroxide followed by 10% normal horse serum. Slides were then incubated with rabbit anti-SSTR2 antibody (Epitomics #3582-1) (1:100 dilution) or mouse anti-NET antibody (MAb Technologies #NET17-1) (1:1000 dilution) for 60 minutes. Antibody localization was detected using the ImmPRESS Anti-Rabbit or Anti-Mouse Ig Peroxidase Reagent Kit (Vector Laboratories, #MP-7401 or #MP-7452) and visualized with 3,3′-diaminobenzidine. Sections were then counterstained with hematoxylin. Cerebellum and adrenal medulla was used as the positive controls for SSTR2 and NET, respectively. Tonsil was used as the negative for both SSTR2 and NET.
SSTR2 and NET immunostaining were digitally analyzed in a quantitative fashion using Aperio Image Scope v9.1.19.1567 and IHC Membrane Algorithm version 8.001. The staining intensity thresholds were set as follows: strong (3+) 210, moderate (2+) 180, weak (1+) 160. The entire tumour area on each section was scanned by the computer and the total number of cells for each staining intensity calculated. For each antibody, a modified H-score was calculated for each tumor with the following formula: [(1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+)]/100. The scores lay on a continuous scale from 0 to 3.
Statistical analysis
Wilcoxon rank-sum tests were performed to assess the correlation of SSTR2 expression with age at diagnosis (<18 months vs. ≥18 months), INSS stage (non-stage 4 vs. stage 4) 35,36, MYCN status (non-amplified vs. amplified), International Neuroblastoma Pathology Classification (INPC) histology (favorable vs. unfavorable) 37, MIBG avidity (non-avid vs. avid) and risk group (low/intermediate vs. high). (Table 1) The Children’s Oncology Group (COG) neuroblastoma risk stratification definitions were used to describe low-, intermediate- and high-risk as per P9641, A3961 and A3973 studies. 34,38,39
Table 1:
Comparison of clinical features and Somatostatin Receptor-2 (SSTR2) immunohistochemistry scores of mixed-risk neuroblastoma cohort (N=69).
Descriptive statistics for SSTR2 scores included are median, mean and standard deviation. P-value refers to Wilcoxon rank-sum tests for difference between clinical features. COG risk group (low/intermediate vs high) was the only variable with statistically significant SSTR2 scores;
| Clinical Feature | N | % | Median | Mean | SD | P-value | |
|---|---|---|---|---|---|---|---|
| Age | < 18 months | 37 | 53.6% | 1.4174 | 1.4958 | 0.6795 | 0.6346 |
| ≥ 18 months | 32 | 46.4% | 1.2054 | 1.4359 | 0.65 | ||
| INSS Stage | Non-stage 4 | 32 | 46.4% | 1.3339 | 1.4807 | 0.6718 | 0.8804 |
| Stage 4 | 37 | 53.6% | 1.3083 | 1.4571 | 0.662 | ||
| MYCN status | Not amplified | 55 | 79.7% | 1.4174 | 1.5517 | 0.6857 | 0.0992 |
| Amplified | 14 | 20.3% | 1.2325 | 1.1394 | 0.4377 | ||
| INPC Histology | Favorable | 32 | 46.3% | 1.4469 | 1.5752 | 0.6576 | 0.1669 |
| Unfavorable | 36 | 52.2% | 1.2054 | 1.3429 | 0.6388 | ||
| MIBG Avidity | Non-avid | 16 | 23.2% | 0.9544 | 1.2424 | 0.6473 | 0.0767 |
| Avid | 53 | 76.8% | 1.4174 | 1.5362 | 0.6568 | ||
| COG Risk Group | Low/Intermediate | 33 | 47.8% | 1.5409 | 1.6519 | 0.6611 | 0.0320* |
| High | 36 | 52.2% | 1.1563 | 1.2996 | 0.6247 | ||
refers to statistically significant p-value.
INSS: International Neuroblastoma Staging System, INPC: International Neuroblastoma Pathology Classification, MIBG: Meta-iodobenzylguanidine, SD: standard deviation, COG: Children’s Oncology Group.
A Spearman correlation coefficient was calculated between SSTR2 and NET scores. (Figure 2) Wilcoxon ranked sum test was used to compare SSTR2 expression pre and post treatment, as well as NET.
Figure 2:
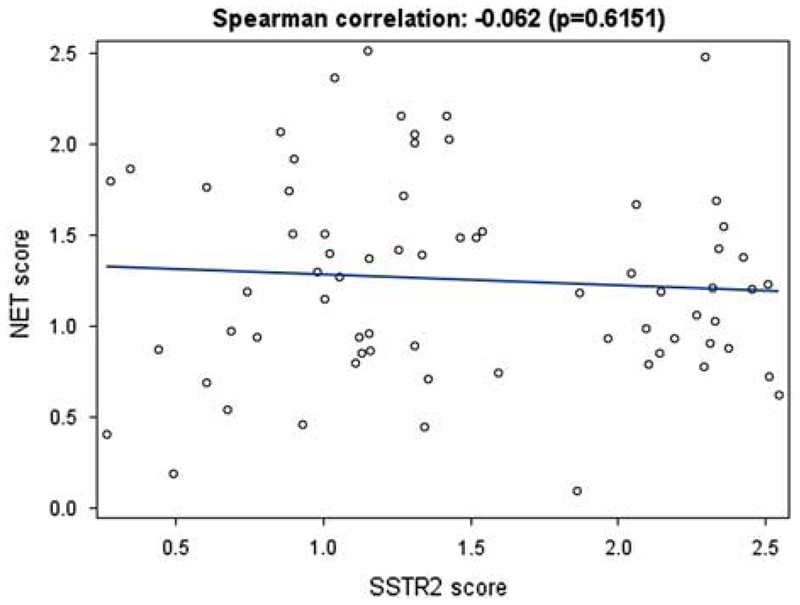
Scatter plot demonstrating the correlation of Somatostatin Receptor-2 (SSTR2) and Norepinephrine Transporter (NET) immunohistochemistry scores using digital analysis from neuroblastoma tumors (N=69). No statistical correlation was demonstrated between SSTR2 scores (X-axis) and NET scores (Y-axis) (r= −0.062, p=0.62).
Results
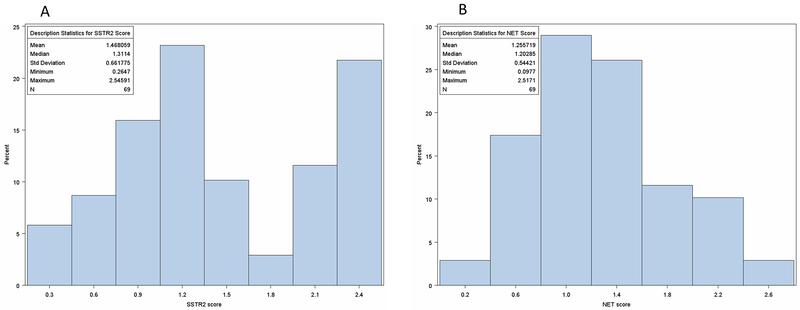
Fifty-two percent of patients had high-risk NB (36/69) with 52% of tumors showing unfavorable histology according to INPC (N=36/68) and 20.3% were MYCN-amplified (N=14). (Table 1) The median score for SSTR2 expression for the whole cohort was 1.31 (range: 0.26-2.55). (Figure 1) Risk group was the only clinical feature significantly associated with SSTR2 score (p=0.032), with lower scores for the 36 high-risk (HR) patients (median=1.16) compared to the 33 non-high risk (NHR) low or intermediate risk patients (median=1.54). (Table 1)
Figure 1:
Distribution of Somatostatin Receptor-2 (SSTR2) (1A) and Norepinephrine Transporter (NET) Receptor (1B) immunohistochemistry scores from 69 high-risk (N=36) and non-high risk tumors (N=33) neuroblastoma tumors from initial diagnostic biopsy. SSTR-2 expression median score of 1.31 (range: 0.26-2.55) and NET expression median score of 1.20 (range: 0.10-2.52).
There were 53 MIBG-avid (76.8%) and 16 MIBG-non-avid (23.1%) patients. There was a trend for higher SSTR2 scores in MIBG-avid (median=1.417) versus non-avid NB patients (median=0.954) but this did not meet statistical significance (p=0.077). (Table 1) SSTR2 scores were not statistically significantly associated with age, INSS stage, MYCN amplification or histology. (Table 1)
The median NET score of the cohort was 1.20 (range: 0.10-2.52). SSTR2 score was not significantly associated with NET score (r=−0.062, p=0.62) (Figure 2). Of the 69 patients in the cohort, there were 20 who relapsed and 6 who progressed. (Table 2) At initial diagnosis, 18/26 of relapsed/refractory patients were high-risk, 5/26 were MYCN-amplified, and 23/26 were MIBG-avid. The median SSTR2 score of the patients who relapsed was 1.32 (range: 0.26-2.55) and median NET score was 1.17 (range: 0.40-1.80). There were 2 patients with events unrelated to NB that were not included: one developed a secondary malignancy and the other died without disease relapse or progression.
Table 2.
Clinical and biological characteristics of the 26 patients who relapsed or progressed with neuroblastoma.
| Clinical Feature | N | % | |||
|---|---|---|---|---|---|
| Event type | Relapse | 20 | 76.9 | ||
| Progression | 6 | 23.1 | |||
| Risk group | Low/intermediate | 8 | 30.8 | ||
| High | 18 | 69.2 | |||
| MYCN status | Non-amplified | 21 | 80.8 | ||
| Amplified | 5 | 19.2 | |||
| MIBG avidity | Non-avid | 3 | 11.5 | ||
| Avid | 23 | 88.5 | |||
| Median | Range | Mean | SD | N | |
| SSTR2 | 1.33 | 0.26-2.55 | 1.47 | 0.73 | 26 |
| NET | 1.17 | 0.40-1.80 | 1.13 | 0.40 | 26 |
MIBG: Meta-iodobenzylguanidine, NET: norepinephrine transporter, SSTR2: Somatostatin Receptor-2.
For the 15 NB patients (9 HR and 6 NHR) from the SickKids cohort who had pre- and post-treatment samples, median SSTR2 score for the pre-treatment group was 1.34 (0.28-2.45) and post-treatment was 1.44 (0.66-2.55). There was no significant difference between pre- and post-treatment SSTR2 scores (p=0.61). Median NET score for pre-treatment group was 1.21 (0.45-1.80) and post-treatment was 2.00 (0.62-2.51). Post-treatment NET scores were significantly higher than pre-treatment (p=0.01).
Discussion
This study used immunohistochemistry (IHC) on tumor samples from a large cohort of mixed-risk neuroblastoma patients at initial diagnosis to determine the prevalence of SSTR2 expression. This is the first study in NB to determine a quantitative SSTR2 IHC score by digital analysis of specimens. We showed a range of SSTR2 expression seen in a variety of neuroblastoma tumors at time of diagnosis. (Figure 1) Although previous smaller studies in NB have demonstrated SSTR2 expression in 77-90% of neuroblastoma tumors, they vary in the definition of SSTR2 positivity, analytical techniques (autoradiography, RT-PCR and IHC) used and stage(s) of disease.15,20,21 In addition, some of the assays only detect total SSTR2 expression of a sample (Western immunoblot and RT-PCR), while IHC can determine both level and percentage of positively stained cells.
The SSTR2 score in this study was calculated on a continuous scale of 0-3 to incorporate the percentage of cells staining at different intensities across the tumor sample. Although there is no standard IHC scoring system for SSTR2, semi-quantitative scoring systems such as Allred score, H-score and immunoreactive score (IRS), have been used to assess protein expression of a range of tumor biomarkers, including epidermal growth factor receptor and estrogen receptors. 40–42 In keeping with the DuBois et al NB study evaluating NET expression and MIBG, we used the methodology for immunohistochemical staining for NET and had a similar approach to NET and SSTR2 scoring by taking into account staining intensity and percentage of positive cells. 33 However, in our study IHC scores were determined by image analysis and quantified the number of tumor cells positive for each intensity (0-3), rather than a product of manual determination of percentage positive cells and average staining intensity (0-300). 33
We found SSTR2 protein expression levels to be significantly higher in low/intermediate risk in comparison to high-risk neuroblastoma tumors. This is consistent with other studies in which SSTRs have been shown to be up-regulated in more differentiated disease, with increased somatostatin concentrations correlating with NB cell differentiation. 14,22,23 SSTRs have also been shown to be down-regulated following disease progression. Although SSTR2 expression has been reported to be associated with favorable histology and prognosis, using our quantitative immunostaining method we did not find SSTR2 to be significantly associated with favorable histology, lack of MYCN amplification, low stage of disease or age < 18 months. 14,15 Moertel et al used autoradiography with radioloabled somatostatin analogues to determine SSTR2 expression in 30 NB specimens and found that expression was associated with lower risk, non-MYCN-amplified disease and favorable outcome.15 In addition, Raggi et al used RT-PCR to detect SSTR2 mRNA in human cell lines and tumors from 54 patients, and determined SSTR2 expression to be positively correlated with OS and EFS, negatively related to tumor stage and MYCN amplification.19 One of the limitations of our study is that as we only had archival specimens available, we did not compare SSTR2 expression using techniques used in other case series other than IHC.
There is no standardized way to clinically interpret the degree of SSTR2 expression of NB tumors in terms of uptake on DOTA-conjugate peptide scans, or to identify which relapsed and progressive patients with NB would benefit from DOTA-conjugate peptide therapy. In our preclinical studies, we found that xenografts expressing high levels of SSTR2 as determined by Western blot and RT-PCR showed a higher uptake of 68Ga-DOTA-TATE PET/CT.31 We also demonstrated co-localization of SSTR2 and DOTATATE uptake using autoradiography-IHC. Early studies in relapsed and refractory NB have used 68Ga-DOTATATE PET/CT to screen for patients for DOTA-conjugate peptide therapy, using a threshold of degree of uptake greater than the liver. 29,30 In addition, Kong et al demonstrated positive staining for SSTR2 by IHC in 5/5 eligible patients with tissue samples.29 They noted some patients had weak SSTR2 expression on biopsy with high 68Ga-DOTATATE uptake, possibly due to tumor heterogeneity and sampling. 29 It will be important to correlate quantitative SSTR2 immunostaining on tissue samples in a prospective study with 68Ga-DOTATATE uptake in larger cohorts of patients to determine optimal cut-offs or thresholds for eligibility for treatments.
The clinical significance of the finding in this study that SSTR2 was more highly expressed in non-high risk NB patients suggests that radiolabeled DOTA-conjugate peptide imaging could be helpful in non-high risk group to detect extent of disease at diagnosis. In clinical studies, sensitivity of 68Ga-DOTA-TOC PET/CT on a per-lesion basis is higher than 131I-MIBG (94.4% vs 76.9%) including detecting marrow disease, and is also a quicker scan that can be done within an hour of injection rather than next day for MIBG. 43,44 However, further studies would be needed to understand if imaging is detecting more differentiated and less clinically significant disease, and the risk-benefit ratio of DOTA-conjugate peptide scanning in non-high-risk patients.
Focusing on the cohort of 18 high-risk and 8 non-high-risk patients with relapsed and progressive NB, who would be the potential candidates for targeted 177Lu-DOTATATE therapy, we found similar SSTR2 expression compared to the total cohort (median 1.33 (0.26-2.55) vs 1.31 (0.26-2.55). (Table 2) For a small group of patients (N=15) who had pre and post-treatment samples analysed, there was no significant difference in SSTR2 expression. Of note, it was beyond the scope of the study to examine tumor biopsy samples at the time of relapse for SSTR2 expression. Studies have demonstrated that SSTR2 can be detected at relapse, and also that the SSTR status may change or SSTR not be expressed at time of progression or after chemotherapy.20,21
Within the cohort we were also interested in whether DOTA-conjugate imaging and treatment could be used as an alternative for MIBG-non-avid patients. However, we did not find a statistically significant difference between SSTR2 scores for MIBG avid versus non-avid NB, and patients who were MIBG-avid had higher median SSTR2 scores than non-avid patients. As expected, most of the patients in the MIBG-non-avid group were non-high-risk patients, so one could have expected the SSTR2 expression to be higher in this group. In addition, all the patients in the relapsed/refractory group who had high SSTR2 tumor expression were MIBG-avid. NET expression is correlated to MIBG avidity but despite our preclinical work finding low NET expression in high SSTR2 NB cell lines, using IHC and western blot detection methods we did not find any correlation between NET expression and SSTR2 expression in our cohort. (Figure 2) 33 Based on these findings of SSTR2 expression in MIBG-avid tumors, it would still be feasible to use DOTA-conjugate imaging and treatment in patients who have not responded to 123I-MIBG.
From this study, we can conclude that SSTR2 expression is present in many NB tumors at time of diagnosis, particularly non-high-risk patients, but expression is variable and does not correlate with prognosis or other clinical and diagnostic features. Further studies will be required to evaluate the prevalence of SSRT2 in relapsed patients with NB and to determine the optimal threshold of immunostaining that correlates with clinical DOTA uptake. The role of DOTA-conjugate peptide imaging in the non-high-risk population still needs to be determined, but this technique can also be helpful in detecting metastatic disease. Moreover, DOTA-conjugate peptide therapy has so far demonstrated minimal toxicity, some efficacy and increased convenience.29,30,45, and has potential as a personalized approach to relapsed or refractory NB with demonstrated SSTR2 expression, particularly in heavily pretreated patients who have not responded to other therapies. A necessary step, however, will involve establishing standardized IHC thresholds for SSTR2 positivity in conjunction with a standard scoring system for DOTA-conjugate peptide imaging, in order to select eligible patients for DOTA-conjugate peptide therapy.
Acknowledgements:
Financial support:
This study was funded by the James Birrell Fund for Neuroblastoma Research and Curtis Chow Memorial Fund
NA was funded by a fellowship from the SickKids Garron Family Cancer Center as the UA Local 46 Childhood Cancer Fellow.
AN and CV were funded by NIH/NCI grant U10 CA180899 for the Children’s Oncology Group Statistics and Data Center.
We thank Dr. Michael Hogarty and the Children’s Oncology Group Neuroblastoma Biology Committee for providing samples per the Material Transfer Agreement terms and acknowledge NCTN Statistics and Data Center Grant U10CA180899.
Abbreviation table
- COG
Children’s Oncology Group
- CT
Computerized tomography
- DOTATATE
(DOTA0-Tyr3)-octreotate
- DOTATOC
(DOTA0-Phe1-Tyr3)octreotide
- EFS
Event-free survival
- 68Ga
Gallium-68
- HR
High-risk
- IHC
Immunohistochemistry
- INPC
International Neuroblastoma Pathology Classification
- IRS
Immunoreactive score
- INSS
International Neuroblastoma Staging System
- 177Lu
Lutetium-177
- MIBG
Metaiodobenzylguanidine
- NB
Neuroblastoma
- NET
Norepinephrine Transporter
- NHR
Non-high risk
- OS
Overall survival
- PET
Positron emission tomography (PET)
- RT-PCR
Reverse Transcriptase – Polymerase Chain Reaction
- SSTR2
Somatostatin receptor 2
Footnotes
Conflicts of interest: SB is disclosing a consulting activity for NEOMED Inc Montreal Quebec.
References
- 1.Basta NO, Halliday GC, Makin G, et al. Factors associated with recurrence and survival length following relapse in patients with neuroblastoma. Br J Cancer. 2016;115(9):1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.London WB, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the International Neuroblastoma Risk Group project. J Clin Oncol 2011;29(24):3286–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagatell R, London WB, Wagner LM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children’s Oncology Group study. J Clin Oncol 2011;29(2):208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgenstern DA, Baruchel S, Irwin MS. Current and future strategies for relapsed neuroblastoma: challenges on the road to precision therapy. J Pediatr Hematol Oncol 2013;35(5):337–347. [DOI] [PubMed] [Google Scholar]
- 5.Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet 2013;45(3):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosse YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol 2013;14(6):472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson JS, Gains JE, Moroz V, et al. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer. 2014;50(4):801–815. [DOI] [PubMed] [Google Scholar]
- 8.Ashraf K, Shaikh F, Gibson P, et al. Treatment with topotecan plus cyclophosphamide in children with first relapse of neuroblastoma. Pediatr Blood Cancer. 2013;60(10):1636–1641. [DOI] [PubMed] [Google Scholar]
- 9.Matthay KK, Yanik G, Messina J, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol 2007;25(9):1054–1060. [DOI] [PubMed] [Google Scholar]
- 10.Garaventa A, Parodi S, De Bernardi B, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian neuroblastoma registry. Eur J Cancer. 2009;45(16):2835–2842. [DOI] [PubMed] [Google Scholar]
- 11.Moreno L, Rubie H, Varo A, et al. Outcome of children with relapsed or refractory neuroblastoma: A meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr Blood Cancer. 2017;64(1):25–31. [DOI] [PubMed] [Google Scholar]
- 12.Santana VM, Furman WL, McGregor LM, et al. Disease control intervals in high-risk neuroblastoma. Cancer. 2008;112(12):2796–2801. [DOI] [PubMed] [Google Scholar]
- 13.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 1999;20(3):157–198. [DOI] [PubMed] [Google Scholar]
- 14.O’Dorisio MS, Chen F, O’Dorisio TM, et al. Characterization of somatostatin receptors on human neuroblastoma tumors. Cell Growth Differ 1994;5(1):1–8. [PubMed] [Google Scholar]
- 15.Moertel CL, Reubi JC, Scheithauer BS, et al. Expression of somatostatin receptors in childhood neuroblastoma. Am J Clin Pathol 1994;102(6):752–756. [DOI] [PubMed] [Google Scholar]
- 16.Reubi JC, Maurer R, von Werder K, et al. Somatostatin receptors in human endocrine tumors. Cancer Res 1987;47(2):551–558. [PubMed] [Google Scholar]
- 17.Reubi JC, Maurer R, Klijn JG, et al. High incidence of somatostatin receptors in human meningiomas: biochemical characterization. J Clin Endocrinol Metab 1986;63(2):433–438. [DOI] [PubMed] [Google Scholar]
- 18.Maggi M, Baldi E, Finetti G, et al. Identification, characterization, and biological activity of somatostatin receptors in human neuroblastoma cell lines. Cancer Res 1994;54(1):124–133. [PubMed] [Google Scholar]
- 19.Raggi CC, Maggi M, Renzi D, et al. Quantitative determination of sst2 gene expression in neuroblastoma tumor predicts patient outcome. J Clin Endocrinol Metab 2000;85(10):3866–3873. [DOI] [PubMed] [Google Scholar]
- 20.Georgantzi K, Tsolakis AV, Stridsberg M, et al. Differentiated expression of somatostatin receptor subtypes in experimental models and clinical neuroblastoma. Pediatr Blood Cancer. 2011;56(4):584–589. [DOI] [PubMed] [Google Scholar]
- 21.Albers AR, O’Dorisio MS, Balster DA, et al. Somatostatin receptor gene expression in neuroblastoma. Regul Pept 2000;88(1-3):61–73. [DOI] [PubMed] [Google Scholar]
- 22.Qualman SJ, O’Dorisio MS, Fleshman DJ, et al. Neuroblastoma. Correlation of neuropeptide expression in tumor tissue with other prognostic factors. Cancer. 1992;70(7):2005–2012. [DOI] [PubMed] [Google Scholar]
- 23.Kogner P, Borgstrom P, Bjellerup P, et al. Somatostatin in neuroblastoma and ganglioneuroma. Eur J Cancer. 1997;33(12):2084–2089. [DOI] [PubMed] [Google Scholar]
- 24.Mojtahedi A, Thamake S, Tworowska I, et al. The value of (68)Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imaging. 2014;4(5):426–434. [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Kan Y, Ge BH, et al. Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol 2014;55(4):389–398. [DOI] [PubMed] [Google Scholar]
- 26.Sadowski SM, Neychev V, Cottle-Delisle C, et al. Detection of insulinoma using (68)Gallium-DOTATATE PET/CT: a case report. Gland Surg 2014;3(4):E1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldherr C, Pless M, Maecke HR, et al. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol 2001;12(7):941–945. [DOI] [PubMed] [Google Scholar]
- 28.Aldridge Matthew D, Walker Caroline, Bomanji Jamshed B, et al. Establishment of a reproducible methodology and results for molecular radiotherapy dosimetric assessment of 177Lu-DOTATATE in neuroblastoma Advances in Neuroblastoma Research Congress 2016, Poster presentation 2016. [Google Scholar]
- 29.Kong G, Hofman MS, Murray WK, et al. Initial Experience With Gallium-68 DOTA-Octreotate PET/CT and Peptide Receptor Radionuclide Therapy for Pediatric Patients With Refractory Metastatic Neuroblastoma. J Pediatr Hematol Oncol 2016;38(2):87–96. [DOI] [PubMed] [Google Scholar]
- 30.Gains JE, Bomanji JB, Fersht NL, et al. 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. J Nucl Med 2011;52(7):1041–1047. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Vines DC, Scollard DA, et al. Correlation of Somatostatin Receptor-2 Expression with Gallium-68-DOTA-TATE Uptake in Neuroblastoma Xenograft Models. Contrast Media Mol Imaging. 2017;2017:9481276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander N, Vali R, Ahmadzadehfar H, et al. Review: The Role of Radiolabeled DOTA-Conjugated Peptides for Imaging and Treatment of Childhood Neuroblastoma. Curr Radiopharm 2018;11(1):14–21. [DOI] [PubMed] [Google Scholar]
- 33.Dubois SG, Geier E, Batra V, et al. Evaluation of Norepinephrine Transporter Expression and Metaiodobenzylguanidine Avidity in Neuroblastoma: A Report from the Children’s Oncology Group. Int J Mol Imaging. 2012;2012:250834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol 2013;14(10):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993;11(8):1466–1477. [DOI] [PubMed] [Google Scholar]
- 36.Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol 1988;6(12):1874–1881. [DOI] [PubMed] [Google Scholar]
- 37.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer. 1999;86(2):364–372. [PubMed] [Google Scholar]
- 38.Strother DR, London WB, Schmidt ML, et al. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: results of Children’s Oncology Group study P9641. J Clin Oncol 2012;30(15):1842–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med 2010;363(14):1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazieres J, Brugger W, Cappuzzo F, et al. Evaluation of EGFR protein expression by immunohistochemistry using H-score and the magnification rule: re-analysis of the SATURN study. Lung Cancer. 2013;82(2):231–237. [DOI] [PubMed] [Google Scholar]
- 41.Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998;11(2):155–168. [PubMed] [Google Scholar]
- 42.Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn Pathol 2014;9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroiss A, Putzer D, Uprimny C, et al. Functional imaging in phaeochromocytoma and neuroblastoma with 68Ga-DOTA-Tyr 3-octreotide positron emission tomography and 123I-metaiodobenzylguanidine. Eur J Nucl Med Mol Imaging. 2011;38(5):865–873. [DOI] [PubMed] [Google Scholar]
- 44.Olivier P, Colarinha P, Fettich J, et al. Guideline for radioiodinated MIBG scintigraphy in children. European Association of Nuclear Medicine;2002. [DOI] [PubMed] [Google Scholar]
- 45.Delpassand ES, Samarghandi A, Zamanian S, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 2014;43(4):518–525. [DOI] [PubMed] [Google Scholar]