Abstract
Rationale: Allosensitization may be a barrier to lung transplant. Currently, consideration is not given to allosensitization when assigning priority on the lung transplant waiting list.
Objectives: We aimed to examine the association between allosensitization and waiting list outcomes.
Methods: We conducted a retrospective single-center cohort study of adults listed for lung transplant at our center between January 1, 2006, and December 31, 2016. We screened candidates for human leukocyte antigen antibodies before listing and examined the association between allosensitization and waiting list outcomes, including likelihood of transplant and death on the waiting list, using a competing risk model. Calculated panel-reactive antibody (CPRA) was used as a continuous measure of allosensitization.
Results: Among 746 candidates who were listed for lung transplant during the study period, 263 (35%) were allosensitized, and 483 (65%) were not. In unadjusted analysis, allosensitized candidates had a decreased likelihood of transplant compared with nonallosensitized candidates (subhazard ratio [sHR], 0.71; 95% confidence interval [CI], 0.60–0.83; P < 0.001) and were more likely to die on the waiting list (sHR, 1.66; 95% CI, 1.08–2.58; P < 0.001). In multivariable modeling, increasing CPRA was associated with an increased risk of death and a decreased likelihood of transplant (sHR for death, 1.15 per 10% increase in CPRA; 95% CI, 1.07–1.22; P < 0.001; sHR for transplant, 0.89 per 10% increase in CPRA; 95% CI, 0.86–0.91; P < 0.001).
Conclusions: Broad allosensitization was associated with longer waiting times, decreased likelihood of transplant, and increased risk of death among candidates on the waiting list for lung transplant. Consideration of allosensitization in organ allocation strategies might help mitigate this increased risk in highly allosensitized candidates.
Keywords: allosensitization, organ allocation, lung transplantation
Lung transplant is a life-prolonging therapy for selected candidates with end-stage lung disease. There were 4,122 lung transplant procedures reported to the International Society of Heart and Lung Transplantation Registry in 2015 (1). Certain lung diseases (e.g., pulmonary fibrosis) follow a more rapidly progressive clinical course than others. Not surprisingly, this resulted in higher death rates on the waiting list (2). To comply with the Organ Procurement and Transplantation Network (OPTN) Final Rule, the Lung Allocation Score (LAS) was implemented in the United States in 2005 with a focus on maximizing transplant benefit by balancing predicted mortality in 1 year on the waiting list and survival in the first year after transplant (3). Although there was an initial decrease in waiting list mortality after implementation of the LAS, waiting list mortality in certain diagnostic categories has increased in recent years (4, 5). A large component of waiting list mortality is likely due to the growing practice of listing older candidates with more advanced lung disease and those requiring mechanical ventilation and extracorporeal life support. However, it is likely that other factors not explicitly considered in the LAS also impact waiting list mortality via an influence on waiting list time. For example, although height is used during LAS calculation to determine body mass index, it is not explicitly considered in the allocation protocol. This is despite multiple studies demonstrating a relationship between height, in particular shorter stature, and length of time spent on the waiting list (6, 7). This has been believed to be a putative cause of decreased transplant rates both for women in comparison with men and for pediatric lung transplant candidates in comparison with adults (5, 8, 9).
Although not accounted for in lung allograft allocation, allosensitization is widely recognized as a barrier to transplant (10–12). In a previous single-center study of lung transplant candidates, those who were allosensitized were less likely to undergo transplant than those who were not, and although they had a nonsignificant trend for a longer waiting time, there was no difference in waiting list mortality between the two groups (13). However, this cohort included only 15 candidates with a calculated panel-reactive antibody (CPRA) greater than 50%, and it is unclear if these results are applicable to highly allosensitized candidates (13). We sought to examine the impact of allosensitization on waiting time to transplant and waiting list mortality using a larger cohort of highly allosensitized candidates. Some of the results of this study were previously reported in abstract form (14).
Methods
Patient Selection
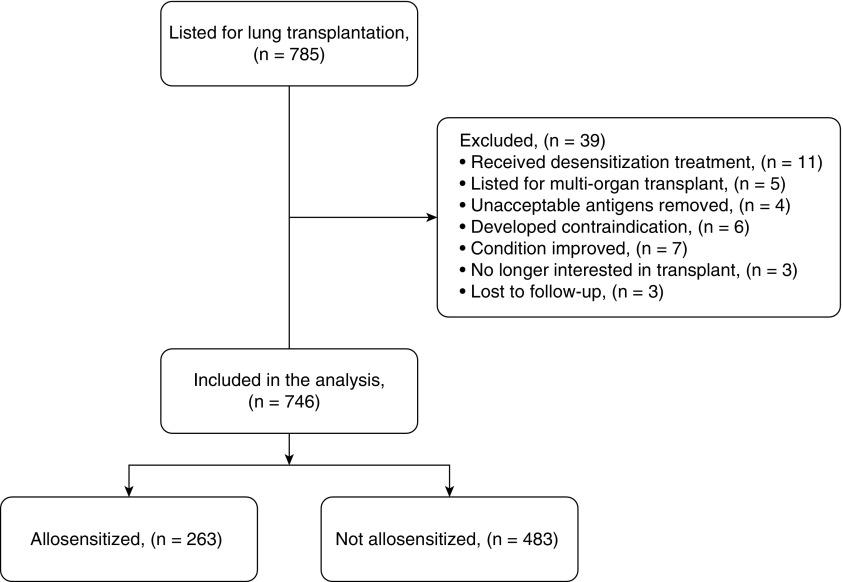
We conducted a single-center retrospective study to assess the relationship between pretransplant allosensitization and outcomes of candidates listed for lung transplant. We identified candidates using the Standard Transplant Analysis and Research database from the United Network for Organ Sharing (UNOS)/OPTN, restricted to those listed for lung transplant at Barnes-Jewish Hospital. We considered all adults listed for lung transplant between January 1, 2006, and December 31, 2016, for inclusion in the analysis. We excluded candidates listed for multiorgan transplants (e.g., lung-liver, heart-lung), those who had unacceptable antigens removed, and those who underwent desensitization therapy before transplant (Figure 1). Follow-up was complete through December 31, 2017. The institutional review board at Washington University in St. Louis approved the study protocol with waiver of informed consent (IRB ID 201801047).
Figure 1.
Flowchart of candidate selection. All candidates listed for lung transplant during the study period were considered for inclusion in the study.
Human Leukocyte Antigen Antibody Screening and Definition of Allosensitization
As part of our routine clinical protocol, we screened all candidates for human leukocyte antigen (HLA) antibodies using the LABScreen Single Antigen assay (One Lambda, Inc.) before listing and every 3 months while they were on the waiting list. Our center’s HLA laboratory defines “HLA antibody positivity” with a mean fluorescence intensity (MFI) threshold greater than or equal to 2,000. This method of screening and positivity threshold was constant over the duration of the study. Based on these results, all reactive HLA are listed in UNet as unacceptable antigens (to be avoided on a virtual crossmatch done before donor organ acceptance). During the study period, four candidates had HLA antibodies identified on historical specimens but not on subsequent tests, and the list of unacceptable antigens in UNet was modified according to the most recent antibody screen; these candidates were excluded from this study, as detailed in Figure 1. We defined “allosensitization” as the presence of any positive HLA antibodies at the time of initial listing and calculated the corresponding CPRA using the OPTN web-based calculator (15). For each patient, allosensitization was determined on the basis of HLA antibodies at the time of listing, and degree of allosensitization was determined by the CPRA. Candidates who developed HLA antibodies subsequent to the initial listing remained defined as nonallosensitized.
Statistical Analyses
We characterized candidates’ demographics using descriptive statistics, and we presented continuous variables as mean ± standard deviation (SD) or median with interquartile range (IQR). We used the Kaplan-Meier method to examine waiting list time for allosensitized versus nonallosensitized candidates and compared groups using the log-rank test. We evaluated waiting list outcomes of transplant versus death using univariable and multivariable competing risk models for allosensitization versus no allosensitization and for degree of allosensitization (CPRA as a continuous variable). Covariates for the multivariable models were chosen on the basis of prior known association with allosensitization or waiting list outcomes. The final multivariable model included CPRA (continuous), age (continuous), LAS (continuous), height (continuous), sex, and diagnostic group. All continuous variables were transformed using restricted cubic splines to allow for nonlinearity. Splines were evaluated with three to five knots and compared with a linear model using the likelihood ratio test statistic to determine the optimal model. In the final multivariable model, CPRA, age, and height were modeled using three knots, and LAS used four knots. Results of competing risk analyses are reported as subhazard ratios (sHRs) with 95% confidence intervals (CIs). We considered a two-sided P < 0.05 statistically significant and conducted all analyses using IBM SPSS Statistics version 24 software (IBM) with the SPSS extension for R work environment (R: A Language and Environment for Statistical Computing; R Core Team 2013, R Foundation for Statistical Computing; www.r-project.org).
Results
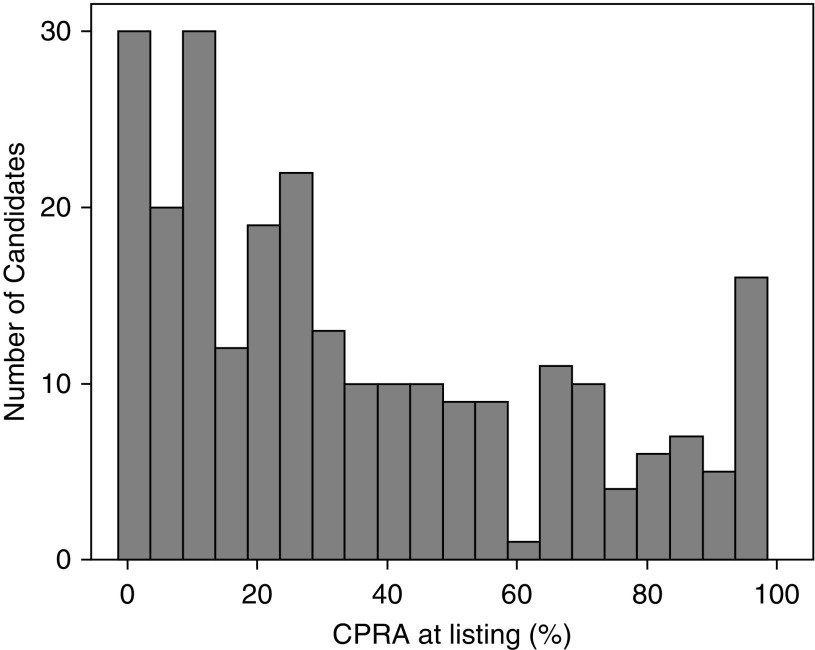
During the study period, 785 candidates were listed for lung transplant at our center. Among these, 39 were excluded from this analysis for the following reasons: 11 received desensitization treatment to deplete HLA antibodies, 5 were listed for multiorgan transplants, 4 had historical unacceptable antigens removed, 6 developed an absolute contraindication to transplant, 7 were removed from the waiting list because their condition improved, 3 were no longer interested in transplant, and 3 were lost to follow-up (Figure 1). The remaining 746 were included in this study. Of the 746 candidates, 263 (35%) were allosensitized, and 483 (65%) were not. Among the 263 allosensitized candidates, the mean (±SD) CPRA was 38 ± 31% (median, 27%; IQR, 53%). The distribution of CPRA among allosensitized candidates is shown in Figure 2. Candidates’ demographics are shown in Table 1.
Figure 2.
Distribution of calculated panel reactive antibody (CPRA) among allosensitized candidates. The total number of candidates represented is 263.
Table 1.
Candidate demographics
| Variable | Not Allosensitized (n = 483) | Allosensitized (n = 263) |
|---|---|---|
| Age at listing, yr, median (IQR) | 58 (45–63) | 55 (41–63) |
| Female sex, n (%) | 192 (40%) | 133 (51%) |
| Race | ||
| White, n (%) | 445 (92%) | 235 (89%) |
| Black, n (%) | 26 (5%) | 23 (9%) |
| Hispanic, n (%) | 8 (2%) | 4 (2%) |
| Asian, n (%) | 4 (1%) | 1 (<1%) |
| ABO blood type | ||
| A, n (%) | 217 (45%) | 107 (41%) |
| AB, n (%) | 18 (4%) | 11 (4%) |
| B, n (%) | 51 (10%) | 24 (9%) |
| O, n (%) | 197 (41%) | 121 (46%) |
| Height, cm, median (IQR) | 170.2 (162.6–177.8) | 167.6 (162.6–175.3) |
| Diagnostic group | ||
| A (obstructive lung disease), n (%) | 145 (30%) | 72 (27%) |
| B (pulmonary vascular disease), n (%) | 13 (3%) | 1 (<1%) |
| C (cystic fibrosis), n (%) | 79 (16%) | 44 (17%) |
| D (restrictive lung disease), n (%) | 246 (51%) | 146 (56%) |
| LAS at listing, median (IQR) | 39.98 (34.27–57.25) | 42.99 (35.73–63.26) |
| Time on the waiting list, d, median (IQR) | 45 (13–143) | 82 (22–207) |
Definition of abbreviations: IQR = interquartile range; LAS = Lung Allocation Score.
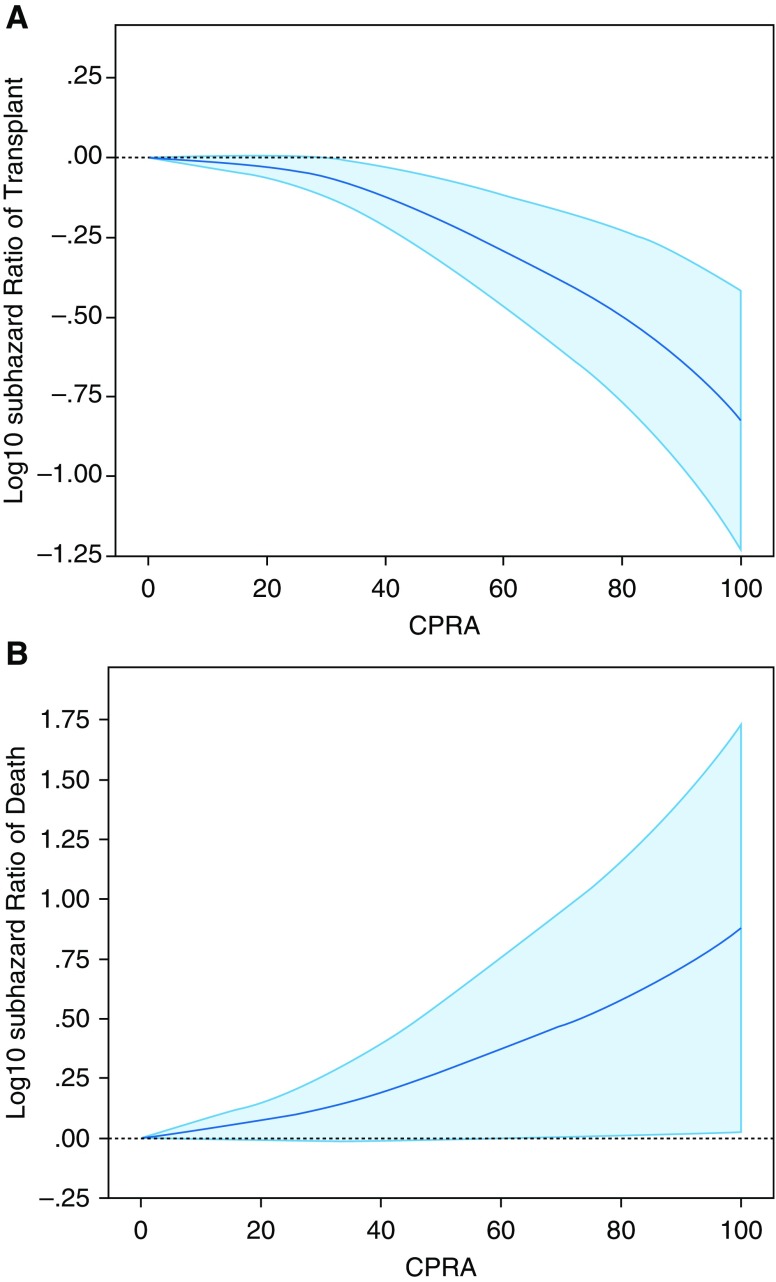
Of the 746 candidates in this cohort, 638 (86%) underwent transplant during the study period, and 108 (14%) died on the waiting list. No candidates were still on the waiting list at the end of follow-up. Of the 483 nonallosensitized candidates, 429 (89%) underwent transplant during the study period compared with 209 of the 263 (79%) allosensitized candidates. Nonallosensitized candidates demonstrated an overall shorter waiting list time than allosensitized candidates (median [95% CI], 45 [38–51] d vs. 82 [64–100] d; P < 0.001). Unadjusted competing risk analysis showed that allosensitization was associated with a 30% lower likelihood of transplant (sHR, 0.71; 95% CI, 0.60–0.83; P < 0.001) and a 66% higher likelihood of death on the waiting list (sHR, 1.66; 95% CI, 1.08–2.58; P < 0.001) (Table 2). Unadjusted competing risk analysis of continuous CPRA at the time of listing showed that it was also associated with a decreased likelihood of transplant (sHR, 0.89 per 10% increase in CPRA; 95% CI, 0.87–0.99; P < 0.001) and an increased likelihood of death on the waiting list (sHR, 1.18 per 10% increase in CPRA; 95% CI, 1.09–1.24; P < 0.001). Multivariable competing risk models demonstrated that cPRA remained associated with both a decreased likelihood of transplant and an increased likelihood of death (Table 2, Figure 3).
Table 2.
Association between allosensitization and waiting list outcomes of transplant and death
| Not Allosensitized (n = 483) | Allosensitized (n = 263) | P Value | Subhazard Ratio per 10% Increase in CPRA | P Value | |
|---|---|---|---|---|---|
| Transplant | |||||
| No. of events | 429 | 209 | |||
| Event rate (95% CI)* | 2.40 (2.18–2.64) | 1.62 (1.41–1.85) | |||
| Unadjusted subhazard ratio (95% CI) | 1 | 0.71 (0.60–0.83) | <0.001 | 0.89 (0.87–0.99) | <0.001 |
| Adjusted subhazard ratio (95% CI)† | 1 | 0.69 (0.59–0.83) | <0.001 | 0.89 (0.86–0.91) | <0.001 |
| Death on the waiting list | |||||
| No. of events | 54 | 54 | |||
| Event rate (95% CI)‡ | 4.18 (3.14–5.46) | 3.02 (2.27–3.94) | |||
| Unadjusted subhazard ratio (95% CI) | 1 | 1.66 (1.08–2.58) | <0.001 | 1.18 (1.09–1.24) | <0.001 |
| Adjusted subhazard ratio (95% CI)† | 1 | 1.63 (1.06–2.52) | 0.026 | 1.15 (1.07–1.22) | <0.001 |
Definition of abbreviations: CI = confidence interval; CPRA = calculated panel-reactive antibody.
Event rate for transplants reported per 10 candidate-years.
Adjusted for age (continuous), Lung Allocation Score (continuous), sex, height (continuous), and Lung Allocation Score diagnostic group.
Event rate for deaths reported per 1 candidate-year.
Figure 3.
Multivariable modeling of subhazard ratio (sHR) for waiting list outcomes, by degree of allosensitization (calculated panel-reactive antibody [CPRA]). Adjusted competing risk analysis of (A) transplant and (B) death. In both analyses, CPRA is represented as a continuous variable with restricted cubic spline transformation along the x-axis. Log10 of sHR values is represented along the y-axis. Solid blue line represents log10 sHR, and light blue–shaded areas represent 95% confidence intervals. Models are adjusted for age (continuous), Lung Allocation Score (continuous), sex, height (continuous), and Lung Allocation Score diagnostic group. For the outcome of transplant, upper limits of 95% confidence intervals remain below log10(sHR) = 0 beginning at CPRA = 30. For the outcome of death, lower limits of 95% confidence intervals remain above log10(sHR) = 0 beginning at CPRA = 76.
After listing, 33 of the 746 (4%) candidates had an increase in CPRA. Eighteen of the 483 (4%) who were not allosensitized when initially listed developed new HLA antibodies, and 15 of the 263 (6%) who were allosensitized developed additional HLA antibodies resulting in an increase in CPRA. Overall, the mean (±SD) increase in CPRA was 25 ± 25% (median, 16%; IQR, 32%). Although an increase in CPRA after listing was associated with a lower likelihood of transplant (hazard ratio, 0.49; 95% CI, 0.33–0.72; P < 0.001), it was not associated with an increased risk of death on the waiting list (hazard ratio, 0.78; 95% CI, 0.36–1.70; P = 0.536). Although some HLA antibodies were no longer detectable in some candidates after listing, we excluded those who had historical unacceptable antigens removed (n = 4) and those treated with desensitization (n = 11) from this cohort, as outlined in Figure 1.
Discussion
In this study, we examined the association between allosensitization and waiting list outcomes. Our data demonstrate that allosensitization prolongs the median waiting time and significantly decreases the likelihood of transplant. In addition, there is a direct relationship between the breadth of allosensitization, as estimated by the CPRA, and waiting time, as well as an inverse relationship with the likelihood of transplant, supporting the hypothesis that allosensitization sufficiently narrows the suitable donor pool so as to prolong waiting list time. This relationship is especially striking at CPRA values greater than 76, when the hazard of death sharply increases. This suggests that low-level or midlevel allosensitization does not impact waiting list mortality, because the donor pool for these candidates remains sufficiently large. In contrast, highly allosensitized candidates have a significantly smaller donor pool, and this prolongs waiting list time, resulting in an increased risk of death and a decreased chance of transplant.
These findings suggest that consideration of allosensitization in organ allocation policies may mitigate the risk of death on the waiting list. To date, this has not been possible in the United States, because transplant centers are not mandated to report details of allosensitization to UNOS, and the large amount of missing data in the UNOS database has been a significant limitation to estimating the risk of death attributable to allosensitization. In addition, as previously stated, the LAS evaluates both risk of death without a transplant and survival benefit with a transplant. We show in the present study that allosensitization conveys an increased risk of death on the waiting list, and our previous data demonstrate that allosensitization is not associated with an increased risk of development of donor-specific antibodies (DSA), acute rejection, chronic lung allograft dysfunction, or death after transplant when donors are selected accordingly (9).
The results of this study are consistent with those of previous studies in kidney and heart transplantation, which showed that allosensitization was associated with increased waiting time, decreased likelihood of transplant, and increased mortality on the waiting list (10, 11, 16, 17). Similarly, our data are consistent with some of the results of a previous study of candidates listed for lung transplant by Kim and colleagues, which illustrated that allosensitized candidates were less likely to undergo transplant than nonallosensitized candidates (13). However, there was no difference in death on the waiting list between the two groups in that study. This may be related to the relatively small number of highly allosensitized candidates in that cohort; indeed, there were only 18 who had a CPRA greater than 50% in that study (13). In contrast, there were 84 candidates who had a CPRA greater than or equal to 50% and 55 who had a CPRA greater than or equal to 70% in our cohort. In addition to the sample size difference, Kim and colleagues used a different donor acceptance algorithm for allosensitized candidates and included candidates who were desensitized in their cohort. Although donor organs were accepted in our study only if the current and historical virtual crossmatches were negative, Kim and colleagues accepted donors if the virtual crossmatch was positive but the complement-dependent cytotoxicity crossmatch was negative.
Although proceeding with transplant with a positive virtual crossmatch may reduce waiting list mortality, there is an increased risk of a positive flow cytometry crossmatch and antibody-mediated rejection after transplant with this approach (13). Moreover, other studies have reported adverse outcomes after transplant in recipients who have pretransplant DSA (i.e., positive virtual crossmatch) (18–22). Brugière and colleagues reported that lung transplant recipients who had retrospectively identified pretransplant class II DSA were significantly more likely to develop bronchiolitis obliterans syndrome and die than those who did not have DSA (19). Similarly, Smith and colleagues noted that lung transplant recipients who had pretransplant DSA had a 1-year survival after transplant of 52%, whereas those who were allosensitized but did not have DSA had a 1-year survival of 78%, and those who were not allosensitized had a 1-year survival of 72% (18). In contrast, we showed that when donor organs were accepted for allosensitized candidates with a virtual crossmatch that was compatible with all previously identified antibodies, allosensitization was not associated with an increased risk of acute cellular rejection, lymphocytic bronchiolitis, DSA development, chronic lung allograft dysfunction, or graft failure (9).
Even with evidence that allosensitization influences both waiting list outcomes and post-transplant outcomes, there remain significant barriers to its implementation in lung allocation strategies. Chief among these is the heterogeneity with which allosensitization data are evaluated and reported across the United States. Different HLA laboratories have different standards and thresholds for positivity. In addition, there is significant data that different HLA antibodies confer different risks to the allograft (23, 24). Although it would be ideal to have a universal standard, this is likely not clinically feasible. An alternative would be to provide an “exception” or additional points to the LAS based on CPRA. Exceptions have been used in heart transplant, with allowance of elective status 1A time for heart transplant candidates with mechanical circulatory devices and, more recently, for lung transplant candidates with an indication of pulmonary hypertension. In addition, allosensitization was recently incorporated into renal allocation protocols with the goal of improving waiting list outcomes for highly sensitized candidates. In this instance, candidates with CPRA greater than prespecified thresholds (99% and 100%) received priority listing (25). This system has been in place for renal allocation for several years, so the first outcome studies are now able to show that although there was an initial decrease in transplants for nonallosensitized candidates, there was no impact on their survival to transplant or on post-transplant outcomes (26). Conversely, as intended, transplant rates for highly allosensitized candidates improved, and waiting list mortality decreased (25, 27).
Another approach to broad allosensitization in solid organ candidates is to subject them to desensitization therapy. Desensitization to deplete HLA antibodies before transplant has been used extensively in kidney transplantation, but experience in lung transplantation has been limited. Furthermore, the results of desensitization among candidates listed for lung transplant have been disappointing. Snyder and colleagues reported their experience with a multimodal regimen consisting of plasmapheresis, intravenous immunoglobulin (IVIG), rituximab, and bortezomib in 18 highly allosensitized candidates (28). Nine of the 18 underwent transplant during the study period: 4 had a negative virtual crossmatch with current and historical antibodies, 3 had a positive virtual crossmatch with current antibodies, and only 2 had a positive virtual crossmatch with historical antibodies. Thus, only 2 of the 18 treated candidates derived a meaningful benefit from desensitization, and the authors concluded that “an aggressive multi-modal desensitization protocol does not significantly reduce pre-transplant HLA antibodies in a broadly sensitized lung candidate cohort” (28). It is also notable that 9 of the 18 patients did not undergo transplant during the study period, and 7 of these were removed from the waiting list (28). Kim and colleagues reported similar findings in a smaller cohort treated with plasmapheresis, IVIG, and rituximab (13). Among four patients treated with this regimen, two had no appreciable change in CPRA, one had a transient decrease in CPRA that did not facilitate transplant, and one had a sustained decrease in CPRA that allowed transplant, but a DSA and a positive flow cytometry crossmatch were noted at the time of transplant (13). Cost and toxicity further confound the limited benefit of pretransplant desensitization.
Tinckam and colleagues reported their experience with a perioperative desensitization regimen consisting of intraoperative and postoperative plasmapheresis, postoperative IVIG, and induction immunosuppression with rabbit antithymocyte globulin (29). In this cohort, 53 of 340 lung transplant recipients had pretransplant DSA, but only 4 (8%) of these had a positive complement-dependent cytotoxicity crossmatch that did not become negative after treatment with dithiothreitol. Importantly, although approximately half of those who had pretransplant DSA had either persistent DSA or developed de novo DSA after transplant, they had no significant difference in acute rejection or allograft survival compared with nonallosensitized recipients (29). It is unclear if the lack of association between DSA and allograft survival reported in this study is related to induction immunosuppression with antithymocyte globulin or to the center’s histocompatibility laboratory’s definition of DSA (30). Nonetheless, additional experience with this approach at other centers is necessary before widespread implementation in clinical practice.
This study has multiple potential limitations. We used a conservative approach to defining the presence of HLA antibodies with an MFI threshold greater than or equal to 2,000. Admittedly, there is considerable variability in the definition of the presence of HLA antibodies across histocompatibility laboratories. However, data from the Clinical Trials in Organ Transplantation antibody core laboratories suggest an optimal MFI cutoff between 1,000 and 1,500 (31). Nevertheless, adopting a higher cutoff to define unacceptable antigens may improve the likelihood of transplant, although this may increase the risk of post-transplant rejection and allograft failure. Clearly, this decision must be based on center histocompatibility laboratory operating procedures, clinical protocols, and patient-specific factors, and our data cannot be used to draw any conclusions about this alternative approach. An additional limitation that is inherent to the single-center design is that we did not validate the statistical models calculating hazards for death and transplant using an independent cohort. This will be necessary to corroborate our findings and to validate the CPRA cutoff associated with an increased risk of death on the waiting list in an external cohort. Finally, the results of this study may not be generalizable to other lung transplant centers, given the heterogeneity in HLA procedures. Unfortunately, this heterogeneity and the limited reporting of allosensitization data to lung transplant databases are strong limitations to conducting large registry studies to validate these findings.
Nevertheless, we conclude that allosensitization can be a barrier to lung transplant and that highly allosensitized candidates have a higher risk of death on the waiting list independent of other potential risk factors. Therefore, we propose that consideration of allosensitization in organ allocation policies may mitigate this increased risk.
Footnotes
Supported by Washington University Division of Pulmonary and Critical Care Medicine grant T32HL007317-39 from the National Institutes of Health (NIH) (L.K.T.). The content is solely the responsibility of the authors and does not necessarily represent the official view of Washington University or the NIH.
Author Contributions: Research design: L.K.T., C.A.W., and R.R.H.; writing of the paper: L.K.T., C.A.W., D.E.B., R.D.Y., P.R.A., H.S.K., K.B.B., K.A.F., V.P., D.K., T.M., E.P.T., and R.R.H.; performance of the research: L.K.T. and C.A.W.; and data analysis: L.K.T. and R.R.H.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chambers DC, Yusen RD, Cherikh WS, Goldfarb SB, Kucheryavaya AY, Khusch K, et al. International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult lung and heart-lung transplantation report—2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017;36:1047–1059. doi: 10.1016/j.healun.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Shearon TH, Qian Y, Kotloff R, Barr ML, Sweet S, et al. Lung transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1047–1068. doi: 10.1111/j.1600-6143.2010.03055.x. [DOI] [PubMed] [Google Scholar]
- 3.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 4.Valapour M, Lehr CJ, Skeans MA, Smith JM, Carrico R, Uccellini K, et al. OPTN/SRTR 2016 annual data report: lung. Am J Transplant. 2018;18:363–433. doi: 10.1111/ajt.14562. [DOI] [PubMed] [Google Scholar]
- 5.Keeshan BC, Rossano JW, Beck N, Hammond R, Kreindler J, Spray TL, et al. Lung transplant waitlist mortality: height as a predictor of poor outcomes. Pediatr Transplant. 2015;19:294–300. doi: 10.1111/petr.12390. [DOI] [PubMed] [Google Scholar]
- 6.Wille KM, Harrington KF, deAndrade JA, Vishin S, Oster RA, Kaslow RA. Disparities in lung transplantation before and after introduction of the lung allocation score. J Heart Lung Transplant. 2013;32:684–692. doi: 10.1016/j.healun.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yusen RD, Lederer DJ. Disparities in lung transplantation. J Heart Lung Transplant. 2013;32:673–674. doi: 10.1016/j.healun.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Sell JL, Bacchetta M, Goldfarb SB, Park H, Heffernan PV, Robbins HA, et al. Short stature and access to lung transplantation in the United States: a cohort study. Am J Respir Crit Care Med. 2016;193:681–688. doi: 10.1164/rccm.201507-1279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosanquet JP, Witt CA, Bemiss BC, Byers DE, Yusen RD, Patterson AG, et al. The impact of pre-transplant allosensitization on outcomes after lung transplantation. J Heart Lung Transplant. 2015;34:1415–1422. doi: 10.1016/j.healun.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kransdorf EP, Kittleson MM, Patel JK, Pando MJ, Steidley DE, Kobashigawa JA. Calculated panel-reactive antibody predicts outcomes on the heart transplant waiting list. J Heart Lung Transplant. 2017;36:787–796. doi: 10.1016/j.healun.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Scornik JC, Bromberg JS, Norman DJ, Bhanderi M, Gitlin M, Petersen J. An update on the impact of pre-transplant transfusions and allosensitization on time to renal transplant and on allograft survival. BMC Nephrol. 2013;14:217. doi: 10.1186/1471-2369-14-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feingold B, Bowman P, Zeevi A, Girnita AL, Quivers ES, Miller SA, et al. Survival in allosensitized children after listing for cardiac transplantation. J Heart Lung Transplant. 2007;26:565–571. doi: 10.1016/j.healun.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Kim M, Townsend KR, Wood IG, Boukedes S, Guleria I, Gabardi S, et al. Impact of pretransplant anti-HLA antibodies on outcomes in lung transplant candidates. Am J Respir Crit Care Med. 2014;189:1234–1239. doi: 10.1164/rccm.201312-2160OC. [DOI] [PubMed] [Google Scholar]
- 14.Witt CA, Byers DE, Yusen RD, Iuppa JA, Bain KB, Patterson GA, et al. Allosensitization increases the risk of death on the lung transplant waiting list [abstract] J Heart Lung Transplant. 2014;33(4 Suppl):S229. [Google Scholar]
- 15.Shaw LM, Korecka M, DeNofrio D, Brayman KL. Pharmacokinetic, pharmacodynamic, and outcome investigations as the basis for mycophenolic acid therapeutic drug monitoring in renal and heart transplant patients. Clin Biochem. 2001;34:17–22. doi: 10.1016/s0009-9120(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 16.Mahle WT, Tresler MA, Edens RE, Rusconi P, George JF, Naftel DC, et al. Pediatric Heart Transplant Study Group. Allosensitization and outcomes in pediatric heart transplantation. J Heart Lung Transplant. 2011;30:1221–1227. doi: 10.1016/j.healun.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Park JE, Kim CY, Park MS, Song JH, Kim YS, Lee JG, et al. Prevalence of pre-transplant anti-HLA antibodies and their impact on outcomes in lung transplant recipients. BMC Pulm Med. 2018;18:45. doi: 10.1186/s12890-018-0606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JD, Ibrahim MW, Newell H, Danskine AJ, Soresi S, Burke MM, et al. Pre-transplant donor HLA-specific antibodies: characteristics causing detrimental effects on survival after lung transplantation. J Heart Lung Transplant. 2014;33:1074–1082. doi: 10.1016/j.healun.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 19.Brugière O, Suberbielle C, Thabut G, Lhuillier E, Dauriat G, Metivier AC, et al. Lung transplantation in patients with pretransplantation donor-specific antibodies detected by Luminex assay. Transplantation. 2013;95:761–765. doi: 10.1097/TP.0b013e31827afb0f. [DOI] [PubMed] [Google Scholar]
- 20.Hadjiliadis D, Chaparro C, Reinsmoen NL, Gutierrez C, Singer LG, Steele MP, et al. Pre-transplant panel reactive antibody in lung transplant recipients is associated with significantly worse post-transplant survival in a multicenter study. J Heart Lung Transplant. 2005;24(7) Suppl:S249–S254. doi: 10.1016/j.healun.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Lau CL, Palmer SM, Posther KE, Howell DN, Reinsmoen NL, Massey HT, et al. Influence of panel-reactive antibodies on posttransplant outcomes in lung transplant recipients. Ann Thorac Surg. 2000;69:1520–1524. doi: 10.1016/s0003-4975(00)01224-8. [DOI] [PubMed] [Google Scholar]
- 22.Gammie JS, Pham SM, Colson YL, Kawai A, Keenan RJ, Weyant RJ, et al. Influence of panel-reactive antibody on survival and rejection after lung transplantation. J Heart Lung Transplant. 1997;16:408–415. [PubMed] [Google Scholar]
- 23.Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, Berry GJ, et al. Antibody-mediated rejection of the lung: a consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2016;35:397–406. doi: 10.1016/j.healun.2016.01.1223. [DOI] [PubMed] [Google Scholar]
- 24.Roux A, Levine DJ, Zeevi A, Hachem R, Halloran K, Halloran PF, et al. Banff Lung Report: current knowledge and future research perspectives for diagnosis and treatment of pulmonary antibody-mediated rejection (AMR) Am J Transplant. 2019;19:21–31. doi: 10.1111/ajt.14990. [DOI] [PubMed] [Google Scholar]
- 25.Jackson KR, Covarrubias K, Holscher CM, Luo X, Chen J, Massie AB, et al. The national landscape of deceased donor kidney transplantation for the highly sensitized: transplant rates, waitlist mortality, and posttransplant survival under KAS. Am J Transplant. 2019;19:1129–1138. doi: 10.1111/ajt.15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parajuli S, Redfield RR, Astor BC, Djamali A, Kaufman DB, Mandelbrot DA. Outcomes in the highest panel reactive antibody recipients of deceased donor kidneys under the new kidney allocation system. Clin Transplant. 2017;31:e12895. doi: 10.1111/ctr.12895. [DOI] [PubMed] [Google Scholar]
- 27.Baxter-Lowe LA, Kucheryavaya A, Tyan D, Reinsmoen N. CPRA for allocation of kidneys in the US: more candidates ≥98% CPRA, lower positive crossmatch rates and improved transplant rates for sensitized patients. Hum Immunol. 2016;77:395–402. doi: 10.1016/j.humimm.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Snyder LD, Gray AL, Reynolds JM, Arepally GM, Bedoya A, Hartwig MG, et al. Antibody desensitization therapy in highly sensitized lung transplant candidates. Am J Transplant. 2014;14:849–856. doi: 10.1111/ajt.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tinckam KJ, Keshavjee S, Chaparro C, Barth D, Azad S, Binnie M, et al. Survival in sensitized lung transplant recipients with perioperative desensitization. Am J Transplant. 2015;15:417–426. doi: 10.1111/ajt.13076. [DOI] [PubMed] [Google Scholar]
- 30.Hachem RR, Reinsmoen NL. What is the definition of a clinically relevant donor HLA-specific antibody (DSA)? Am J Transplant. 2015;15:299–300. doi: 10.1111/ajt.13079. [DOI] [PubMed] [Google Scholar]
- 31.Reed EF, Rao P, Zhang Z, Gebel H, Bray RA, Guleria I, et al. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. Am J Transplant. 2013;13:1859–1870. doi: 10.1111/ajt.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]