Abstract
Rationale: The American Thoracic Society (ATS)/European Respiratory Society defines a positive bronchodilator response (BDR) by a composite of BDR in either forced expiratory volume in 1 second (FEV1) and/or forced vital capacity (FVC) greater than or equal to 12% and 200 ml (ATS-BDR). We hypothesized that ATS-BDR components would be differentially associated with important chronic obstructive pulmonary disease (COPD) outcomes.
Objectives: To examine whether ATS-BDR components are differentially associated with clinical, functional, and radiographic features in COPD.
Methods: We included subjects with COPD enrolled in the COPDGene study. In the main analysis, we excluded subjects with self-reported asthma. We categorized BDR into the following: 1) No-BDR, no BDR in either FEV1 or FVC; 2) FEV1-BDR, BDR in FEV1 but no BDR in FVC; 3) FVC-BDR, BDR in FVC but no BDR in FEV1; and 4) Combined-BDR, BDR in both FEV1 and FVC. We constructed multivariable logistic, linear, zero-inflated negative binomial, and Cox hazards models to examine the association of BDR categories with symptoms, computed tomography findings, change in FEV1 over time, respiratory exacerbations, and mortality. We also created models using the ATS BDR definition (ATS-BDR) as the main independent variable.
Results: Of 3,340 COPD subjects included in the analysis, 1,083 (32.43%) had ATS-BDR, 182 (5.45%) had FEV1-BDR, 522 (15.63%) had FVC-BDR, and 379 (11.34%) had Combined-BDR. All BDR categories were associated with FEV1 decline compared with No-BDR. Compared with No-BDR, both ATS-BDR and Combined-BDR were associated with higher functional residual capacity %predicted, greater internal perimeter of 10 mm, and greater 6-minute-walk distance. In contrast to ATS-BDR, Combined-BDR was independently associated with less emphysema (adjusted beta regression coefficient, −1.67; 95% confidence interval [CI], −2.68 to −0.65; P = 0.001), more frequent respiratory exacerbations (incidence rate ratio, 1.25; 95% CI, 1.03–1.50; P = 0.02) and severe exacerbations (incidence rate ratio, 1.34; 95% CI, 1.05–1.71; P = 0.02), and lower mortality (adjusted hazards ratio, 0.76; 95% CI, 0.58–0.99; P = 0.046). Sensitivity analysis that included subjects with self-reported history of asthma showed similar findings.
Conclusions: BDR in both FEV1 and FVC indicates a COPD phenotype with asthma-like characteristics, and provides clinically more meaningful information than current definitions of BDR.
Keywords: asthma, chronic obstructive pulmonary disease, bronchodilator agents, mortality, spirometry
Chronic obstructive pulmonary disease (COPD) is characterized by airflow obstruction that persists after bronchodilator administration. According to the American Thoracic Society/European Respiratory Society (ATS-ERS), bronchodilator response (BDR) is defined by an increase in forced expiratory volume in 1 second (FEV1) and/or forced vital capacity (FVC) greater than or equal to 12% and 200 ml after bronchodilator administration (1). This definition is simple and easily applicable (2) but its value for phenotyping and for predicting outcomes and treatment response is debatable (1, 3, 4). BDR prevalence rates in COPD range from 4% to 65% (5–9], and the accuracy of BDR in distinguishing between asthma and COPD is low (10–13). The clinical relevance of BDR in COPD is not clear. Some reports have showed that BDR is associated with worse respiratory symptoms (14), reduced exercise capacity (15), greater frequency of respiratory exacerbations (16), lesser amount of emphysema (5, 7, 8), and FEV1 decline (17), whereas others have found no association of BDR with COPD symptoms and outcomes (6, 8, 18).
BDR has also been defined either as an increase in FEV1 alone, or an increase in FEV1 and/or FVC after bronchodilator administration with various cutoffs (3). BDR according to ATS-ERS guidelines (ATS-BDR) is a composite of a positive response in FEV1 and/or FVC. Because ATS-BDR is defined by BDR in either FEV1 and/or FVC, subjects with ATS-BDR may represent a heterogeneous population. BDR in FEV1 may indicate different disease processes associated with COPD than BDR in FVC. BDR in FVC is more common in small airway disease (19), whereas BDR in FEV1 is associated with both large and small airway disease (20). We hypothesized that BDR components would be differentially associated with important COPD outcomes. To test our hypothesis, we analyzed data from the Genetic Epidemiology of COPD study (COPDGene), a large cohort of current and former smokers. We compared the association of BDR components with chronic bronchitis, dyspnea, exercise capacity, and structural lung disease at enrollment. We also examined their predictive value for FEV1 reduction over time, respiratory exacerbations, and mortality.
Methods
Subjects
We retrospectively analyzed data from the COPDGene study, which is an ongoing cohort study that enrolled subjects at 21 clinical centers throughout the United States (http://www.copdgene.org/). The institutional review boards at each participating center approved the study protocol. Details of the study protocol have been published previously (21). Briefly, all subjects provided informed consent before participation in the study. Subjects were self-identified as non-Hispanic whites or African Americans between the ages of 45 and 80 years. They completed a modified ATS Respiratory Epidemiology questionnaire and 6-minute-walk test (6-MWT) at the enrollment visit. Dyspnea was assessed using the modified Medical Research Council scale (21). Subjects performed prebronchodilator and post-bronchodilator spirometry according to ATS-ERS guidelines (22). Subjects were instructed to withhold only short-acting bronchodilators before their visits. After prebronchodilator spirometric maneuvers, post-bronchodilator maneuvers were performed between 15 and 40 minutes after two puffs of albuterol dose inhaler were administered using a spacer (23). We used the National Health and Nutrition Examination Survey III spirometric reference values to calculate % predicted values (24). We included subjects with COPD (post-bronchodilator FEV1/FVC <0.70), and excluded subjects who had undergone lung transplantation or lung volume reduction surgery and subjects with incomplete prebronchodilator and post-bronchodilator spirometry data at enrollment. Subjects performed inspiratory and expiratory chest computed tomography (CT) scans using multidetector CT scanners per protocol (21). Total lung capacity (TLC) was measured at maximal inspiration. Functional residual capacity (FRC) was measured at end expiration. FRC and TLC% predicted were calculated based on the predicted values (25). Emphysema and gas trapping were quantitated using 3D Slicer software (www.airwayinspector.org), and airway dimensions were measured using Pulmonary Workstation 2 (VIDA Diagnostics,) (21). Parametric response mapping was used to calculate functional small airways disease (26, 27).
Approximately 5 years after the enrollment visit, a proportion of subjects had a repeat spirometry at a follow-up visit. Subjects were contacted every 6 months and completed a validated questionnaire regarding respiratory exacerbations. Vital status was also ascertained on follow-up. For the primary analysis, we excluded subjects with self-reported history of asthma at enrollment.
Variables and Outcomes
BDR was defined as an increase in prebronchodilator FEV1 and/or FVC greater than or equal to 12% and greater than or equal to 200 ml after bronchodilator administration (ATS-BDR). We categorized ATS-BDR into the following BDR categories: 1) No-BDR, no BDR by any criteria; 2) FEV1-BDR, BDR in FEV1 but no BDR in FVC; 3) FVC-BDR, BDR in FVC but no BDR in FEV1; and 4) Combined-BDR, BDR in both FEV1 and FVC. All BDR categories had to meet both 12% and 200-ml volume criteria. In separate analyses, we also examined BDR as an increase in FEV1 and/or FVC greater than or equal to 12% (relative percent change), and an increase in FEV1 and/or FVC greater than or equal 200 ml (volume change).
Chronic bronchitis was defined as productive cough for at least 3 consecutive months in the last 2 years (28). Emphysema was defined by the percentage of lung volume at maximal inspiration with attenuation less than −950 HU. Gas trapping was quantified as the percentage of lung volume at end expiration with attenuation less than −856 HU (29). The square root of wall area for a hypothetical airway with an internal perimeter of 10 mm (Pi10) was derived (30). Respiratory exacerbation was defined as an episode of increased cough, phlegm, or shortness of breath that lasted more than 48 hours and required treatment with antibiotics, systemic steroids, or both. Severe exacerbations required an emergency room visit or hospitalization. FEV1 change was calculated as the change in ml/yr between enrollment and follow-up and visits.
Statistical Analysis
We categorized subjects at enrollment into four groups based on BDR category: No-BDR, FEV1-BDR, FVC-BDR, and Combined-BDR. First, we compared the characteristics at enrollment between ATS-BDR and No-BDR subjects. Then we compared characteristics of subjects in FEV1-BDR, FVC-BDR, and Combined-BDR groups with the characteristics of subjects in the No-BDR group. We used Student's t test or Wilcoxon rank sum test for normal and nonnormal continuous variables, respectively, and Fischer exact or chi-square test for categorical variables. We performed multivariable logistic and generalized linear regression models as appropriate for associations between BDR categories and chronic bronchitis, modified Medical Research Council, CT emphysema and gas trapping, 6-MWT distance, and FEV1 change. For exacerbation analysis, we created zero-inflated negative binomial models because exacerbations followed a Poisson distribution and data were overdispersed. Follow-up time was included in the models as an offset. All models included the following covariates: age, sex, race, smoking status, smoking pack-years, body mass index and post-bronchodilator FEV1% predicted. We performed Cox proportional hazards regression analysis to examine the association of BDR categories with mortality, with adjustment for age, sex, race, smoking status, smoking pack-years, body mass index, and post-bronchodilator FEV1% predicted.
We conducted a sensitivity analysis including subjects with self-reported history of asthma (see online supplement). Moreover, because the subjects in the various BDR groups may have a wide range of lung function, we tested additional models by including only subjects with post-bronchodilator FEV1% predicted less than 80% by excluding those with Global Initiative for Chronic Obstructive Lung Disease stage I disease severity. We also tested similar associations for BDR defined as relative percent change and relative volume change in separate models. Finally, we tested additional models adjusted for medication usage. We used R software package (http://www.r-project.org/) for all statistical analysis. Statistical significance was set at a two-sided alpha of 0.05.
Results
The cohort included 4,458 subjects with COPD (see Figure E1 in the online supplement for Consort Diagram). After excluding 1,118 subjects with self-reported history of asthma diagnosis, 3,340 subjects were included in the analysis. Follow-up data for exacerbations and vital status were available in 2,980 and 2,972 subjects, respectively.
Baseline Characteristics at Enrollment (n = 3,340)
Of all 3,340 subjects in the cohort, 1,083 subjects (32.43%) had ATS-BDR. Compared with No-BDR, subjects with ATS-BDR had higher modified Medical Research Council and lower post-bronchodilator FEV1% predicted (see Table E1). In ATS-BDR subjects, there were more Global Initiative for Chronic Obstructive Lung Disease stage III and IV subjects than in No-BDR group. ATS-BDR subjects had more radiographic %gas trapping and functional small airway disease, and greater Pi10 than No-BDR.
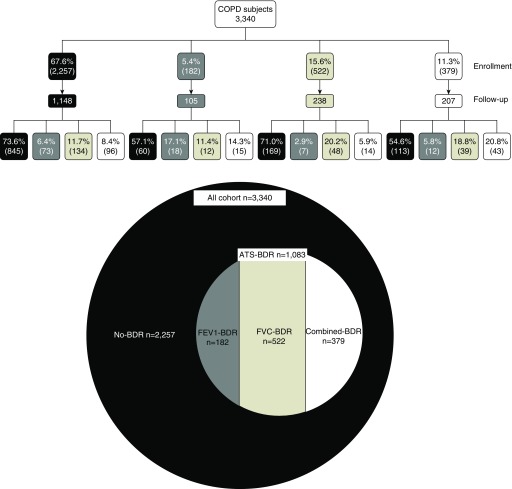
Of the 1,083 ATS-BDR subjects, 182 (5.45%) had FEV1-BDR, 522 (15.63%) had FVC-BDR, and 379 (11.34%) had Combined-BDR (Figure 1). Table 1 shows the characteristics of subjects at enrollment categorized into BDR groups.
Figure 1.
Bronchodilator response rates in subjects with chronic obstructive pulmonary disease at enrollment and follow-up visit. ATS = American Thoracic Society; ATS-BDR = increase in prebronchodilator FEV1 and/or FVC ≥12% and ≥200 ml after bronchodilator administration; BDR = bronchodilator response; Combined-BDR = an increase in both FEV1 and FVC ≥12% and ≥200 ml after bronchodilator administration; COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; FEV1-BDR = increase in FEV1 ≥12% and ≥200 ml but a change in FVC <12% and 200 ml after bronchodilator administration; FVC-BDR = increase in FVC ≥12% and ≥200 ml but a change in FEV1 <12% and 200 ml after bronchodilator administration; No-BDR = a change in both FEV1 and FVC <12% and <200 ml after bronchodilator administration.
Table 1.
Characteristics of subjects with chronic obstructive pulmonary disease at enrollment by BDR groups (n = 3,340)
| FEV1-BDR (n = 182) | FVC-BDR (n = 522) | Combined-BDR (n = 379) | No-BDR (n = 2,257) | |
|---|---|---|---|---|
| Age, yr | 61.55 ± 8.77* | 64.67 ± 8.70* | 63.43 ± 8.90 | 63.50 ± 8.41 |
| Female, n (%) | 62 (34.07) | 256 (49.04) | 134 (35.36) | 920 (40.76) |
| African American, n (%) | 33 (18.13) | 104 (19.92) | 61 (16.09) | 443 (19.63) |
| Body mass index, kg/m2 | 28.84 ± 6.27* | 27.07 ± 5.57 | 27.81 ± 5.54 | 27.62 ± 5.95 |
| Pack-years smoking | 50.23 ± 24.94 | 52.54 ± 27.46 | 54.66 ± 27.26 | 52.66 ± 27.04 |
| Active smokers, n (%) | 87 (47.80) | 208 (39.85) | 180 (47.49) | 998 (44.22) |
| Chronic bronchitis | 50 (27.47) | 122 (23.37) | 112 (29.55)* | 534 (23.66) |
| MMRC | 1.54 ± 1.39 | 2.05 ± 1.50* | 1.67 ± 1.42 | 1.72 ± 1.45 |
| ICS, n (%)† | 55 (30.22)* | 239 (46.31)* | 107 (28.61)* | 854 (38.40) |
| LABA, n (%)† | 49 (26.92)* | 229 (44.64)* | 82 (21.93)* | 852 (38.31) |
| LAMA, n (%)† | 38 (21.47)* | 193 (37.62) | 74 (19.79)* | 760 (34.32) |
| Post-FEV1% predicted | 68.28 ± 16.10* | 51.64 ± 24.39* | 58.38 ± 18.12 | 59.94 ± 23.34 |
| Post-FVC% predicted | 88.68 ± 16.36* | 82.38 ± 22.40 | 84.39 ± 16.86 | 82.55 ± 20.46 |
| GOLD stage | ||||
| I, mild | 34 (18.68) | 80 (15.33) | 53 (13.98) | 497 (22.02) |
| II, moderate | 124 (68.13) | 168 (32.18) | 192 (50.66) | 958 (42.45) |
| III, severe | 23 (12.64) | 153 (29.31) | 119 (31.40) | 532 (23.57) |
| IV, very severe | 1 (0.55) | 121 (23.18) | 15 (3.96) | 270 (11.96) |
| P value‡ | <0.001 | <0.001 | <0.001 | Ref |
| FEV1 change after BD, L | 0.32 ± 0.11* | 0.11 ± 0.10* | 0.34 ± 0.12* | 0.04 ± 0.13 |
| FVC change after BD, L | 0.21 ± 0.16* | 0.50 ± 0.24* | 0.65 ± 0.32* | 0.041 ± 0.21 |
| FEV1/FVC change after BD | 0.057 ± 0.040* | −0.046 ± 0.052* | −0.002 ± 0.067 | 0.005 ± 0.042 |
| Emphysema, % | 8.15 ± 8.71* | 14.94 ± 14.08* | 10.66 ± 10.77 | 12.08 ± 12.45 |
| Gas trapping, % | 27.67 ± 15.49* | 41.77 ± 22.46* | 36.11 ± 18.80 | 34.42 ± 20.48 |
| PRMfSAD, % | 21.6 ± 10.7* | 28.6 ± 13.3* | 28.1 ± 12.0* | 24.5 ± 12.4 |
| Pi10, mm | 3.67 ± 0.15 | 3.71 ± 0.12* | 3.72 ± 0.14* | 3.68 ± 0.13 |
| FRC% predicted | 112.40 ± 23.31* | 130.10 ± 34.72* | 124.20 ± 27.04* | 118.40 ± 30.02 |
| TLC% predicted | 100.10 ± 15.14* | 104.60 ± 17.07* | 103.40 ± 14.48* | 101.60 ± 16.46 |
| 6-min-walk-test distance, ft | 1,363 ± 370.07* | 1,175 ± 407.35 | 1,312 ± 347.35 | 1,249 ± 414.91 |
Definition of abbreviations: BD = bronchodilator; BDR = bronchodilator response; Combined-BDR = an increase in both FEV1 and FVC ≥12% and ≥200 ml after bronchodilator administration; FEV1 = forced expiratory volume in 1 second; FEV1-BDR = increase in FEV1 ≥12% and ≥200 ml but a change in FVC <12% and 200 ml after bronchodilator administration; FRC = functional residual capacity; FVC = forced vital capacity; FVC-BDR = increase in FVC ≥12% and ≥200 ml but a change in FEV1 <12% and 200 ml after bronchodilator administration; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled glucocorticosteroids; LABA = long-acting β agonist; LAMA = long-acting muscarinic antagonist; MMRC = Modified Medical Research Council; No-BDR = a change in both FEV1 and FVC <12% and <200 ml after bronchodilator administration; Pi10 = square root of wall area for a hypothetical airway with an internal perimeter of 10 mm; PRMfSAD = parametric response mapping functional small airway disease; Ref = reference; TLC = total lung capacity.
Continue variables are presented as mean ± SD.
P < 0.05 versus No-BDR using Student’s t test, Wilcoxon, Fischer, or chi-square tests when appropriate.
Data were available for a subset of subjects: For % emphysema and TLC% predicted analysis, data were available for 3,127 subjects. For % gas trapping and FRC% analysis, data were available for 2,788 subjects. For Pi10 data analysis, data were available for 3,102 subjects. For 6-minute-walk-test data analysis, data were available for 3,264 subjects.
Across all GOLD stages shown (vs. No-BDR using chi-square test).
Compared with No-BDR subjects, FEV1-BDR subjects were younger and had higher body mass index and post-bronchodilator FEV1% predicted and FVC% predicted, had less advanced COPD stage, less CT emphysema and CT gas trapping, less functional small airways disease, lower FRC% predicted and TLC% predicted by CT, and covered greater 6-MWT distance. Compared with No-BDR, FVC-BDR subjects were older, and had greater dyspnea, lower post-bronchodilator FEV1% predicted, greater % emphysema and gas trapping, greater functional small airway disease, higher FRC% predicted and TLC% predicted by CT, and shorter 6-MWT distance. Subjects in this category were more likely to have more advanced COPD stage than No-BDR subjects. Compared with No-BDR, Combined-BDR subjects reported a higher frequency of chronic bronchitis, had no difference in CT emphysema and gas trapping, but they had more functional small airway disease and greater Pi10, FRC% predicted, and greater 6-MWT distance.
On multivariable analysis, FEV1-BDR was not associated with any of the outcomes, but FVC-BDR was associated with greater % gas trapping, FRC% predicted, and TLC% predicted (Table 2). Combined-BDR was associated with lower % emphysema, greater functional small airway disease (see Table E2) and Pi10, FRC% and TLC% predicted, and longer 6-MWT distance. ATS-BDR was associated with higher % gas trapping, greater functional small airway disease and Pi10, FRC% and TLC% predicted, and longer 6-MWT distance.
Table 2.
Associations of BDR categories with clinical, functional, and radiographic features at enrollment in subjects with chronic obstructive pulmonary disease (n = 3,340)
| Chronic Bronchitis |
MMRC |
6-MWT (ft) |
Pi10 (mm) |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | Coef (95% CI) | P Value | Coef (95% CI) | P Value | Coef (95% CI) | P Value | |
| No-BDR | Ref | Ref | Ref | Ref | ||||
| FEV1-BDR | 1.30 (0.91 to 1.85) | 0.15 | 0.09 (−0.09 to 0.27) | 0.31 | 30.6 (−19.6 to 80.7) | 0.23 | 0.01 (−0.01 to 0.03) | 0.34 |
| FVC-BDR | 0.94 (0.73 to 1.18) | 0.58 | 0.05 (−0.07 to 0.16) | 0.41 | 6.1 (−25.88 to 38.1) | 0.71 | 0.01 (−0.003 to 0.02) | 0.16 |
| Combined-BDR | 1.24 (0.96 to 1.59) | 0.09 | −0.09 (−0.22 to 0.04) | 0.17 | 70.8 (35.09 to 106.4) | <0.001 | 0.04 (0.02 to 0.05) | <0.001 |
| ATS-BDR* | 1.10 (0.92 to 1.31) | 0.28 | 0.01 (−0.08 to 0.09) | 0.87 | 33.5 (9.5 to 57.5) | 0.01 | 0.02 (0.01 to 0.03) | <0.001 |
| % Emphysema |
% Gas Trapping |
FRC% Predicted |
TLC% Predicted |
|||||
| Coef (95% CI) | P Value | Coef (95% CI) | P Value | Coef (95% CI) | P Value | Coef (95% CI) | P Value | |
| No-BDR | Ref | Ref | Ref | Ref | ||||
| FEV1-BDR | −0.76 (−2.19 to 0.67) | 0.30 | −−0.16 (−2.27 to 1.96) | 0.88 | 1.41 (−2.52 to 5.35) | 0.48 | 0.26 (−2.18 to 2.70) | 0.84 |
| FVC-BDR | 0.13 (−0.76 to 1.02) | 0.77 | 1.47 (0.15 to 2.78) | 0.03 | 4.37 (1.91 to 6.82) | <0.001 | 1.53 (0.003 to 3.05) | 0.0496 |
| Combined-BDR | −1.67 (−2.68 to −0.65) | 0.001 | 1.41 (−0.08 to 2.89) | 0.06 | 5.10 (2.33 to 7.87) | <0.001 | 1.78 (0.05 to 3.51) | 0.043 |
| ATS-BDR* | −0.65 (−1.32 to 0.03) | 0.059 | 1.18 (0.18 to 2.17) | 0.02 | 4.14 (2.28 to 5.99) | <0.001 | 1.40 (0.25 to 2.55) | 0.02 |
Definition of abbreviations: 6-MWT = 6-minute-walk test; ATS = American Thoracic Society; ATS-BDR = increase in prebronchodilator FEV1 and/or FVC ≥12% and ≥200 ml after bronchodilator administration; BDR = bronchodilator response; CI = confidence interval; Coef = coefficient; combined-BDR = an increase in both FEV1 and FVC ≥12% and ≥200 ml after bronchodilator administration; FEV1 = forced expiratory volume in 1 second; FEV1-BDR = increase in FEV1 ≥12% and ≥200 ml but a change in FVC <12% and 200 ml after bronchodilator administration; FRC = functional residual capacity; FVC = forced vital capacity; FVC-BDR = increase in FVC ≥12% and ≥200 ml but a change in FEV1 <12% and 200 ml after bronchodilator administration; MMRC = Modified Medical Research Council; no-BDR = a change in both FEV1 and FVC <12% and <200 ml after bronchodilator administration; OR = odds ratio; Pi10 = square root of wall area for a hypothetical airway with an internal perimeter of 10 mm; Ref = reference; TLC = total lung capacity.
For % emphysema and TLC% predicted analysis, data were available for 3,127 subjects. For % gas trapping and FRC% analysis, data were available for 2,788 subjects. For 6-MWT data analysis, data were available for 3,264 subjects.
All models included the following covariates: age, sex, race, smoking status, smoking pack-years, body mass index, and post-bronchodilator FEV1% predicted.
Multivariable linear and logistic regression models with ATS-BDR binary variable = BDR according to ATS guidelines; Yes or No as the independent variable.
Change in FEV1 between Enrollment and 5-Year Follow-up Visit (n = 1,702)
The mean FEV1 decline for the cohort was −40.39 ± 54.52 ml/yr. In adjusted analysis, FEV1-BDR (adjusted beta regression coefficient [Coef], −18.34; 95% confidence interval [CI], −28.78 to −7.90; P < 0.001), FVC-BDR (Coef, −8.11; 95% CI, −15.49 to −0.73; P = 0.03), and Combined-BDR (Coef, −21.86; 95% CI, −29.60 to −14.11; P < 0.001) were all associated with FEV1 decline over time (Table 3). ATS-BDR was also associated with FEV1 decline (Coef, −15.32; 95% CI, −20.66 to −9.98; P < 0.001). Based on the coefficients, Combined-BDR was associated with greater FEV1 decline.
Table 3.
Association of BDR categories at enrollment with drop in FEV1 between baseline and follow-up visit and respiratory exacerbations in subjects with chronic obstructive pulmonary disease
| Change in FEV1 (n = 1,702) (ml/yr) |
Exacerbations (n = 2,980) |
Severe Exacerbations (n = 2,980) |
||||
|---|---|---|---|---|---|---|
| Coef (95% CI) | P Value | IRR (95% CI) | P Value | IRR (95% CI) | P Value | |
| No-BDR | Ref | Ref | Ref | |||
| FEV1-BDR | −18.34 (−28.78 to −7.90) | <0.001 | 1.18 (0.90 to 1.55) | 0.26 | 0.97 (0.68 to 1.40) | 0.88 |
| FVC-BDR | −8.11 (−15.49 to −0.73) | 0.03 | 1.10 (0.93 to 1.30) | 0.29 | 1.09 (0.88 to 1.35) | 0.42 |
| Combined-BDR | −21.86 (−29.60 to −14.11) | <0.001 | 1.25 (1.03 to 1.50) | 0.02 | 1.34 (1.05 to 1.71) | 0.02 |
| ATS-BDR* | −15.32 (−20.66 to −9.98) | <0.001 | 1.16 (1.02 to 1.32) | 0.02 | 1.16 (0.98 to 1.37) | 0.08 |
Definition of abbreviations: ATS = American Thoracic Society; ATS-BDR = increase in prebronchodilator FEV1 and/or FVC ≥12% and ≥200 ml after bronchodilator administration; BDR = bronchodilator response; CI = confidence interval; Coef = coefficient; Combined-BDR = an increase in both FEV1 and FVC ≥12% and ≥200 ml after bronchodilator administration; FEV1 = forced expiratory volume in 1 second; FEV1-BDR = increase in FEV1 ≥12% and ≥200 ml but a change in FVC <12% and 200 ml after bronchodilator administration; FVC = forced vital capacity; FVC-BDR = increase in FVC ≥12% and ≥200 ml but a change in FEV1 <12% and 200 ml after bronchodilator administration; IRR = incidence rate ratio; No-BDR = a change in both FEV1 and FVC <12% and <200 ml after bronchodilator administration.
All models included the following covariates: age, sex, race, smoking status, smoking pack-years, body mass index, and post-bronchodilator FEV1% predicted.
Multivariable logistic regression models with ATS-BDR binary variable = BDR according to ATS guidelines; Yes or No as the independent variable.
Respiratory exacerbations (n = 2,980)
FEV1-BDR and FVC-BDR were not associated with respiratory exacerbations (Table 3). In contrast, Combined-BDR was associated with respiratory exacerbations (incident rate ratio, [IRR], 1.25; 95% CI, 1.03–1.50; P = 0.02) and severe respiratory exacerbations (IRR, 1.34; 95% CI, 1.05–1.71; P = 0.02) (Table 3). ATS-BDR was associated with respiratory exacerbations (IRR, 1.16; 95% CI, 1.02–1.32; P = 0.02) but it was not associated with severe respiratory exacerbations (IRR, 1.16; 95% CI, 0.98–1.37; P = 0.08).
Mortality (n = 2,972)
Overall, 650 (21.87%) died over a median duration of 2,371 (interquartile range, 2,073–2,652) days follow-up. Mortality was 21.87% (437 of 1,998) in the No-BDR group. Mortality was 21.87% (213 of 974) in the ATS-BDR group, 12.80% (21 of 164) in the FEV1-BDR group, 28.54% (133 of 466) in the FVC-BDR group, and 17.15% (59 of 344) in the Combined-BDR group. After adjusting for demographics, smoking status, and post-bronchodilator FEV1% predicted, FEV1-BDR (adjusted hazards ratio [HR], 0.87; 95% CI, 0.56–1.35; P = 0.53), FVC-BDR (HR, 1.00; 95% CI, 0.83–1.22; P = 0.97), and ATS-BDR (HR, 0.91; 95% CI, 0.77–1.07; P = 0.25) were not associated with mortality, whereas Combined-BDR was associated with lower mortality (HR, 0.76; 95% CI, 0.58–0.99; P = 0.046) (Table 4).
Table 4.
Association of BDR categories at enrollment with mortality in subjects with chronic obstructive pulmonary disease (n = 2,972)
| Mortality |
||
|---|---|---|
| Adjusted HR (95% CI) | P Value | |
| No-BDR | Ref | |
| FEV1-BDR | 0.87 (0.56–1.35) | 0.53 |
| FVC-BDR | 1.00 (0.83–1.22) | 0.97 |
| Combined-BDR | 0.76 (0.58–0.99) | 0.046 |
| ATS-BDR* | 0.91 (0.77–1.07) | 0.25 |
Definition of abbreviations: ATS = American Thoracic Society; ATS-BDR = increase in prebronchodilator FEV1 and/or FVC ≥12% and ≥200 ml after bronchodilator administration; BDR = bronchodilator response; CI = confidence interval; combined-BDR = an increase in both FEV1 and FVC ≥12% and ≥200 ml after bronchodilator administration; FEV1 = forced expiratory volume in 1 second; FEV1-BDR = increase in FEV1 ≥12% and ≥200 ml but a change in FVC <12% and 200 ml after bronchodilator administration; FVC = forced vital capacity; FVC-BDR = increase in FVC ≥12% and ≥200 ml but a change in FEV1 <12% and 200 ml after bronchodilator administration; HR = hazard ratio; no-BDR = a change in both FEV1 and FVC <12% and <200 ml after bronchodilator administration; Ref = reference.
Cox hazard regression models for mortality included the following covariates: age, sex, race, smoking status, smoking pack-years, body mass index, and post-bronchodilator FEV1% predicted.
Cox hazard regression models with ATS-BDR binary variable = BDR according to ATS guidelines; Yes or No as the independent variable.
Bronchodilator Response at Follow-up Visit
1,702 subjects completed a spirometry at follow-up visit. Of all subjects with prebronchodilator and post-bronchodilator spirometry at follow-up visit (n = 1,698), 69.9% had No-BDR and 30.1% had ATS-BDR: 6.5% had FEV1-BDR, 13.7% had FVC-BDR, and 9.9% had Combined-BDR. Of the No-BDR subjects at enrollment, 73.6% had No-BDR at the follow-up visit. Of FEV1-BDR subjects, 17.1% had FEV1-BDR at follow-up visit (Figure 1). Of FVC-BDR subjects at enrollment, 20.2% had FVC-BDR at follow-up. Of Combined-BDR subjects at enrollment, 20.8% had Combined-BDR at follow-up visit.
Sensitivity Analyses
We repeated the analyses in subjects with COPD with and without history of asthma with similar findings (see Tables E3–E5). When subjects with Global Initiative for Chronic Obstructive Lung Disease stage I were excluded from the analyses, Combined-BDR remained associated with less emphysema, higher frequency of exacerbations, and lower mortality (see Tables E6–E8).
When we defined BDR as an increase greater than or equal 12% (without the requirement of 200-ml change) in FEV1 and/or FVC (relative change), we found that combined-percent-BDR was associated with respiratory exacerbations (see Table E10) but not with mortality (see Table E11). When we defined BDR as an increase greater than or equal 200 ml (without the requirement of a 12% change) in FEV1and/or FVC (absolute change), we observed that combined-volume-BDR was associated with mortality (see Table E14) but not with respiratory exacerbations (see Table E13). In additional models adjusted for long-acting inhaled medication use, we found again that Combined-BDR was associated with increased exacerbations, whereas FEV1-BDR and FVC-BDR were not associated with exacerbations (see Table E15).
Discussion
In a cohort of current and former smokers with COPD, we demonstrated that using a more stringent combined BDR in both FEV1 and FVC criterion can identify subjects with lower emphysema who are also at greater risk for exacerbations and lung function decline but are at lower mortality risk than subjects with no BDR.
BDR is often evaluated in patients with respiratory symptoms. Although subjects with asthma have a greater degree of BDR than subjects with COPD (10), and BDR in COPD declines over time as the disease progresses (31), its clinical utility has been debated because BDR does not sufficiently distinguish between asthma and COPD. Current definitions of BDR also do not identify a useful COPD phenotype (3, 32). Multiple prior studies have attempted to identify BDR subtypes with clinical utility. BDR in FVC has been suggested as being a more clinically relevant marker in COPD because it is more common than BDR in FEV1. BDR-FVC is associated with hyperinflation (33–35), which results in dyspnea and lower exercise capacity (36). BDR in FVC has also been shown to be more strongly associated with gas trapping than BDR in FEV1 (37). These findings are in agreement with our results that FVC-BDR is associated with %gas trapping, whereas FEV1-BDR and Combined-BDR are not. The change in FVC after bronchodilator administration is less affected by gas compression during the forced exhalation maneuver, whereas change in FEV1 after bronchodilator administration may be overestimated by gas compression (2). In addition, data from impulse oscillometry and body plethysmography suggest that FEV1-BDR underestimates the change in volume and airway resistance after bronchodilation (38). Newton and colleagues (33) found that in patients with severe COPD and lung hyperinflation, only 11% had a positive FEV1 response, whereas the FVC response was 53%. Furthermore, Ben Saad and coworkers (39) showed that in patients with COPD with reversibility by ATS criteria, FVC response was seen in an additional 45% who did not have FEV1 response. Thus, FVC response seems to be more common in COPD than FEV1 response.
In a COPD cohort, we demonstrated that BDR categories are differentially associated with clinical, functional, and radiographic features of obstructive lung disease. This may reflect different pathophysiologic processes. When emphysema and poor elastic recoil play an important role, FVC-BDR is more common (33, 40). Although the mechanisms underlying isolated FVC response in COPD are not clear, it may be the result of longitudinal traction of airways not being supported by the radial traction of parenchymal tethering, which is impaired at higher lung volumes in emphysema (41). However, in pathophysiologic processes with flow limitation that affect peripheral and central airways (42), FEV1-BDR is more prominent (20). The current ATS-BDR definitions, by stipulating that a positive response in either FEV1 or FVC be met, likely introduce considerable heterogeneity of underlying disease processes, making them less specific. Although, both ATS-BDR and Combined-BDR were associated with thicker airway wall, which is in agreement with prior literature (43), and with higher FRC% predicted, and greater 6-MWT distance compared with No-BDR, Combined-BDR identifies subjects with lower %emphysema, low risk for mortality, but with a heightened exacerbation risk, whereas ATS-BDR does not. This combination of features in the Combined-BDR groups suggests that this is an inherently less impaired group by disease severity and more impaired by disease activity. The inverse association of this “Combined-BDR phenotype” with mortality despite its increased risk for respiratory exacerbations contrasts the prior literature that exacerbations are associated with increased mortality (44). This disagreement could be caused by the fact that the Combined-BDR group had a lower degree of emphysema compared with No-BDR group, and emphysema is a strong predictor of mortality and may be the main driver of survival (45).
We also found that although all the BDR subtypes are associated with FEV1 change over time, Combined-BDR was associated with the greatest decline. Calverley and coworkers (6) reported that BDR in FEV1 is not associated with FEV1 decline, whereas other investigators have shown that BDR in FEV1 is a predictor of FEV1 decline in COPD (17). However, the latter have been criticized because baseline FEV1 was not taken into consideration (3, 18). The mechanisms underlying the stronger association of FEV1 decline with Combined-BDR are not clear, but it should be noted that frequent exacerbations, as noted in this group, are associated with a faster decline in lung function (46).
Furthermore, BDR definitions that include percentage response alone or volume response alone have been proposed (3), but they suffer from the likelihood of meeting BDR criteria easily in mild and severe disease, respectively. For example, a subject with mild disease and a greater than 200-ml response in either FEV1 or FVC is deemed to have BDR. Similarly, a subject with severe disease and low baseline lung function is more likely to meet the percent criteria for FEV1 or FVC. For this reason, ATS-ERS guidelines recommend an increase greater than or equal 12% and greater than or equal 200 ml after bronchodilator administration. We extend the literature by demonstrating that a percentage response coupled with a volume response is superior to either one alone to predict respiratory exacerbations and mortality (see online supplement).
Although, BDR has been used to define asthma-COPD overlap in the past, it does not provide any clinically meaningful information, and currently, there is no consensus definition for the asthma-COPD overlap. Based on our findings, Combined-BDR may prove to be a useful criterion to identify patients with asthma-COPD overlap, although more research is needed to test this criterion. Whether these subjects are more responsive to inhaled corticosteroids with lower risk for pneumonia remains to be tested. We do note that BDR is limited by its variability over time in our study, which is in agreement with previous reports (6, 8). BDR variability may be caused by variability in the spirometric maneuvers, such as differences in coaching and spirometers used, or by factors intrinsic to the subject, such as diurnal variability and changes in mucus production. However, Combined-BDR was more stable than other BDR categories, and its fluctuation over time may be a reflection of the variability in airflow obstruction of this putative COPD phenotype.
Our study has several limitations. First, the cohort included current and former smokers and hence the results may not be generalizable. However, we did perform several sensitivity analyses, including subjects with asthma, and by excluding subjects with mild disease. Second, subjects did not withhold long-acting bronchodilators before the study but did withhold short-acting bronchodilators. Although there were some baseline differences in the use of chronic inhaled medications between BDR categories, models adjusting for their use showed similar associations between BDR categories and outcomes as those in the primary analysis. We did not, however, confirm compliance with use of long-acting medications, and this may introduce some bias. The association of combined-BDR with poorer outcomes is unlikely to be caused by undertreatment because participants with FVC-BDR, despite having a greater proportion of participants on long-acting medications, did not have improved outcomes compared with No-BDR. Third, the repeatability analysis was limited by the fact that we had follow-up spirometry for only half the subjects. Fourth, we did not have data to test asthma-like features including eosinophil counts in blood or sputum, and immunoglobulin levels. Finally, spirometry data were not available at follow-up in some participants because of attrition or mortality. However, as has been previously shown using data from the same cohort by Dransfield and colleagues (46), completers, late, and deceased subjects in the COPDGene study were fairly similar in regards to demographic characteristics and baseline lung function. In addition, the rate of change of FEV1 is very heterogeneous and imputation methods may not reliably capture this change. These limitations do not undermine the strengths of our study, which includes data from a large cohort of participants in whom we had CT and spirometry data that were subject to stringent quality control. The cohort also included a substantial number of women and African Americans.
In conclusion, Combined-BDR is associated with less emphysema and lower mortality but with greater frequency of exacerbations, indicating a putative COPD phenotype with asthma-like characteristics. More research is needed to test whether the Combined-BDR phenotype helps identify patients with the asthma-COPD overlap, and whether targeting patients with this phenotype will result in improved outcomes.
Supplementary Material
Acknowledgments
COPDGene Investigators–Core Units: Administrative Center: James D. Crapo, M.D. (PI); Edwin K. Silverman, M.D., Ph.D. (PI); Barry J. Make, M.D.; and Elizabeth A. Regan, M.D., Ph.D. Genetic Analysis Center: Terri Beaty, Ph.D.; Ferdouse Begum, Ph.D.; Robert Busch, M.D.; Peter J. Castaldi, M.D., M.Sc.; Michael Cho, M.D.; Dawn L. DeMeo, M.D., M.P.H.; Adel R. Boueiz, M.D.; Marilyn G. Foreman, M.D., M.S.; Eitan Halper-Stromberg; Nadia N. Hansel, M.D., M.P.H.; Megan E. Hardin, M.D.; Lystra P. Hayden, M.D., M.Sc.; Craig P. Hersh, M.D., M.P.H.; Jacqueline Hetmanski, M.S., M.P.H.; Brian D. Hobbs, M.D.; John E. Hokanson, M.P.H., Ph.D.; Nan Laird, Ph.D.; Christoph Lange, Ph.D.; Sharon M. Lutz, Ph.D.; Merry-Lynn McDonald, Ph.D.; Margaret M. Parker, Ph.D.; Dandi Qiao, Ph.D.; Elizabeth A. Regan, M.D., Ph.D.; Stephanie Santorico, Ph.D.; Edwin K. Silverman, M.D., Ph.D.; Emily S. Wan, M.D.; and Sungho Won. Imaging Center: Mustafa Al Qaisi, M.D.; Harvey O. Coxson, Ph.D.; Teresa Gray; MeiLan K. Han, M.D., M.S.; Eric A. Hoffman, Ph.D.; Stephen Humphries, Ph.D.; Francine L. Jacobson, M.D., M.P.H.; Philip F. Judy, Ph.D.; Ella A. Kazerooni, M.D.; Alex Kluiber; David A. Lynch, M.B.; John D. Newell, Jr., M.D.; Elizabeth A. Regan, M.D., Ph.D.; James C. Ross, Ph.D.; Raul San Jose Estepar, Ph.D.; Joyce Schroeder, M.D.; Jered Sieren; Douglas Stinson; Berend C. Stoel, Ph.D.; Juerg Tschirren, Ph.D.; Edwin Van Beek, M.D., Ph.D.; Bram van Ginneken, Ph.D.; Eva van Rikxoort, Ph.D.; George Washko, M.D.; and Carla G. Wilson, M.S. PFT QA Center, Salt Lake City, UT: Robert Jensen, Ph.D. Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: Douglas Everett, Ph.D.; Jim Crooks, Ph.D.; Camille Moore, Ph.D.; Matt Strand, Ph.D.; and Carla G. Wilson, M.S. Epidemiology Core, University of Colorado Anschutz Medical Campus, Aurora, CO: John E. Hokanson, M.P.H., Ph.D.; John Hughes, Ph.D.; Gregory Kinney, M.P.H., Ph.D.; Sharon M. Lutz, Ph.D.; Katherine Pratte, M.S.P.H.; and Kendra A. Young, Ph.D.
COPDGene Investigators–Clinical Centers: Ann Arbor VA: Jeffrey L. Curtis, M.D.; Carlos H. Martinez, M.D., M.P.H.; and Perry G. Pernicano, M.D. Baylor College of Medicine, Houston, TX: Nicola Hanania, M.D., M.S.; Philip Alapat, M.D.; Mustafa Atik, M.D.; Venkata Bandi, M.D.; Aladin Boriek, Ph.D.; Kalpatha Guntupalli, M.D.; Elizabeth Guy, M.D.; Arun Nachiappan, M.D.; and Amit Parulekar, M.D. Brigham and Women’s Hospital, Boston, MA: Dawn L. DeMeo, M.D., M.P.H.; Craig Hersh, M.D., M.P.H.; Francine L. Jacobson, M.D., M.P.H.; and George Washko, M.D. Columbia University, New York, NY: R. Graham Barr, M.D., Dr.P.H.; John Austin, M.D.; Belinda D’Souza, M.D.; Gregory D.N. Pearson, M.D.; Anna Rozenshtein, M.D., M.P.H.; and Byron Thomashow, M.D. Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr., M.D.; H. Page McAdams, M.D.; and Lacey Washington, M.D. HealthPartners Research Institute, Minneapolis, MN: Charlene McEvoy, M.D., M.P.H. and Joseph Tashjian, M.D. Johns Hopkins University, Baltimore, MD: Robert Wise, M.D.; Robert Brown, M.D.; Nadia N. Hansel, M.D., M.P.H.; Karen Horton, M.D.; Allison Lambert, M.D., M.H.S.; and Nirupama Putcha, M.D., M.H.S. Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Torrance, CA: Richard Casaburi, Ph.D., M.D.; Alessandra Adami, Ph.D.; Matthew Budoff, M.D.; Hans Fischer, M.D.; Janos Porszasz, M.D., Ph.D.; Harry Rossiter, Ph.D.; and William Stringer, M.D. Michael E. DeBakey VAMC, Houston, TX: Amir Sharafkhaneh, M.D., Ph.D. and Charlie Lan, D.O. Minneapolis VA: Christine Wendt, M.D. and Brian Bell, M.D. Morehouse School of Medicine, Atlanta, GA: Marilyn G. Foreman, M.D., M.S.; Eugene Berkowitz, M.D., Ph.D.; and Gloria Westney, M.D., M.S. National Jewish Health, Denver, CO: Russell Bowler, M.D., Ph.D. and David A. Lynch, M.B. Reliant Medical Group, Worcester, MA: Richard Rosiello, M.D. and David Pace, M.D. Temple University, Philadelphia, PA: Gerard Criner, M.D.; David Ciccolella, M.D.; Francis Cordova, M.D.; Chandra Dass, M.D.; Gilbert D’Alonzo, D.O.; Parag Desai, M.D.; Michael Jacobs, Pharm.D.; Steven Kelsen, M.D., Ph.D.; Victor Kim, M.D.; A. James Mamary, M.D.; Nathaniel Marchetti, D.O.; Aditi Satti, M.D.; Kartik Shenoy, M.D.; Robert M. Steiner, M.D.; Alex Swift, M.D.; Irene Swift, M.D.; and Maria Elena Vega-Sanchez, M.D. University of Alabama, Birmingham, AL: Mark Dransfield, M.D.; William Bailey, M.D.; Surya Bhatt, M.D.; Anand Iyer, M.D.; Hrudaya Nath, M.D.; and J. Michael Wells, M.D. University of California, San Diego, CA: Joe Ramsdell, M.D.; Paul Friedman, M.D.; Xavier Soler, M.D., Ph.D.; and Andrew Yen, M.D. University of Iowa, Iowa City, IA: Alejandro P. Comellas, M.D.; John Newell, Jr., M.D.; and Brad Thompson, M.D. University of Michigan, Ann Arbor, MI: MeiLan K. Han, M.D., M.S.; Ella Kazerooni, M.D.; and Carlos H. Martinez, M.D., M.P.H. University of Minnesota, Minneapolis, MN: Joanne Billings, M.D.; Abbie Begnaud, M.D.; and Tadashi Allen, M.D. University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, M.D.; Jessica Bon, M.D.; Divay Chandra, M.D., M.Sc.; Carl Fuhrman, M.D.; and Joel Weissfeld, M.D., M.P.H. University of Texas Health Science Center at San Antonio, San Antonio, TX: Antonio Anzueto, M.D.; Sandra Adams, M.D.; Diego Maselli-Caceres, M.D.; and Mario E. Ruiz, M.D.
Footnotes
Supported by National Institutes of Health grant K23 HL133438 (S.P.B.) and the COPDGene study (National Heart, Lung, and Blood Institute grant numbers U01 HL089897 and U01 HL089856). The COPDGene study (NCT00608764) is also supported by the COPD Foundation through contributions made to an Industry Advisory Committee comprised of AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, and Sunovion. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Author Contributions: S.F. participated in study conception and design, data analysis and interpretation, drafting the manuscript, and critical revision of the manuscript. S.P.B. participated in study conception and design, data interpretation, drafting the manuscript, and critical revision of the manuscript. All authors participated in data interpretation and critical revision of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: James D. Crapo, Edwin K. Silverman, Barry J. Make, Elizabeth A. Regan, Terri Beaty, Ferdouse Begum, Robert Busch, Peter J. Castaldi, Michael Cho, Dawn L. DeMeo, Adel R. Boueiz, Marilyn G. Foreman, Eitan Halper-Stromberg, Nadia N. Hansel, Megan E. Hardin, Lystra P. Hayden, Craig P. Hersh, Jacqueline Hetmanski, Brian D. Hobbs, John E. Hokanson, Nan Laird, Christoph Lange, Sharon M. Lutz, Merry-Lynn McDonald, Margaret M. Parker, Dandi Qiao, Elizabeth A. Regan, Stephanie Santorico, Edwin K. Silverman, Emily S. Wan, Sungho Won, Mustafa Al Qaisi, Harvey O. Coxson, Teresa Gray, MeiLan K. Han, Eric A. Hoffman, Stephen Humphries, Francine L. Jacobson, Philip F. Judy, Ella A. Kazerooni, Alex Kluiber, David A. Lynch, John D. Newell, Jr., Elizabeth A. Regan, James C. Ross, Raul San Jose Estepar, Joyce Schroeder, Jered Sieren, Douglas Stinson, Berend C. Stoel, Juerg Tschirren, Edwin Van Beek, Bram van Ginneken, Eva van Rikxoort, George Washko, Carla G. Wilson, Robert Jensen, Douglas Everett, Jim Crooks, Camille Moore, Matt Strand, Carla G. Wilson, John E. Hokanson, John Hughes, Gregory Kinney, Sharon M. Lutz, Katherine Pratte, Kendra A. Young, Jeffrey L. Curtis, Carlos H. Martinez, Perry G. Pernicano, Nicola Hanania, Philip Alapat, Mustafa Atik, Venkata Bandi, Aladin Boriek, Kalpatha Guntupalli, Elizabeth Guy, Arun Nachiappan, Amit Parulekar, Dawn L. DeMeo, Craig Hersh, Francine L. Jacobson, George Washko, R. Graham Barr, John Austin, Belinda D’Souza, Gregory D.N. Pearson, Anna Rozenshtein, Byron Thomashow, Neil MacIntyre, Jr., H. Page McAdams, Lacey Washington, Charlene McEvoy, Joseph Tashjian, Robert Wise, Robert Brown, Nadia N. Hansel, Karen Horton, Allison Lambert, Nirupama Putcha, Richard Casaburi, Alessandra Adami, Matthew Budoff, Hans Fischer, Janos Porszasz, Harry Rossiter, William Stringer, Amir Sharafkhaneh, Charlie Lan, Christine Wendt, Brian Bell, Marilyn G. Foreman, Eugene Berkowitz, Gloria Westney, Russell Bowler, David A. Lynch, Richard Rosiello, David Pace, Gerard Criner, David Ciccolella, Francis Cordova, Chandra Dass, Gilbert D’Alonzo, Parag Desai, Michael Jacobs, Steven Kelsen, Victor Kim, A. James Mamary, Nathaniel Marchetti, Aditi Satti, Kartik Shenoy, Robert M. Steiner, Alex Swift, Irene Swift, Maria Elena Vega-Sanchez, Mark Dransfield, William Bailey, Surya Bhatt, Anand Iyer, Hrudaya Nath, J. Michael Wells, Joe Ramsdell, Paul Friedman, Xavier Soler, Andrew Yen, Alejandro P. Comellas, John Newell, Jr., Brad Thompson, MeiLan K. Han, Ella Kazerooni, Carlos H. Martinez, Joanne Billings, Abbie Begnaud, Tadashi Allen, Frank Sciurba, Jessica Bon, Divay Chandra, Carl Fuhrman, Joel Weissfeld, Antonio Anzueto, Sandra Adams, Diego Maselli-Caceres, and Mario E. Ruiz
References
- 1.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 2.Pellegrino R, Brusasco V. Point: Is an increase in FEV1 and/or FVC ≥ 12% of control and ≥ 200 mL the best way to assess positive bronchodilator response? Yes. Chest. 2014;146:536–537. doi: 10.1378/chest.14-0810. [DOI] [PubMed] [Google Scholar]
- 3.Calverley PM, Albert P, Walker PP. Bronchodilator reversibility in chronic obstructive pulmonary disease: use and limitations. Lancet Respir Med. 2013;1:564–573. doi: 10.1016/S2213-2600(13)70086-9. [DOI] [PubMed] [Google Scholar]
- 4.Ward H, Cooper BG, Miller MR. Improved criterion for assessing lung function reversibility. Chest. 2015;148:877–886. doi: 10.1378/chest.14-2413. [DOI] [PubMed] [Google Scholar]
- 5.Han MK, Wise R, Mumford J, Sciurba F, Criner GJ, Curtis JL, et al. NETT Research Group. Prevalence and clinical correlates of bronchoreversibility in severe emphysema. Eur Respir J. 2010;35:1048–1056. doi: 10.1183/09031936.00052509. [DOI] [PubMed] [Google Scholar]
- 6.Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58:659–664. doi: 10.1136/thorax.58.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanania NA, Sharafkhaneh A, Celli B, Decramer M, Lystig T, Kesten S, et al. Acute bronchodilator responsiveness and health outcomes in COPD patients in the UPLIFT trial. Respir Res. 2011;12:6. doi: 10.1186/1465-9921-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albert P, Agusti A, Edwards L, Tal-Singer R, Yates J, Bakke P, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67:701–708. doi: 10.1136/thoraxjnl-2011-201458. [DOI] [PubMed] [Google Scholar]
- 9.Tashkin DP, Celli B, Decramer M, Liu D, Burkhart D, Cassino C, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31:742–750. doi: 10.1183/09031936.00129607. [DOI] [PubMed] [Google Scholar]
- 10.Chhabra SK. Acute bronchodilator response has limited value in differentiating bronchial asthma from COPD. J Asthma. 2005;42:367–372. doi: 10.1081/JAS-62992. [DOI] [PubMed] [Google Scholar]
- 11.Richter DC, Joubert JR, Nell H, Schuurmans MM, Irusen EM. Diagnostic value of post-bronchodilator pulmonary function testing to distinguish between stable, moderate to severe COPD and asthma. Int J Chron Obstruct Pulmon Dis. 2008;3:693–699. doi: 10.2147/copd.s948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesten S, Rebuck AS. Is the short-term response to inhaled beta-adrenergic agonist sensitive or specific for distinguishing between asthma and COPD? Chest. 1994;105:1042–1045. doi: 10.1378/chest.105.4.1042. [DOI] [PubMed] [Google Scholar]
- 13.Meslier N, Racineux JL, Six P, Lockhart A. Diagnostic value of reversibility of chronic airway obstruction to separate asthma from chronic bronchitis: a statistical approach. Eur Respir J. 1989;2:497–505. [PubMed] [Google Scholar]
- 14.Tan WC, Bourbeau J, Hernandez P, Chapman KR, Cowie R, FitzGerald JM, et al. CanCOLD Collaborative Research Group. Bronchodilator responsiveness and reported respiratory symptoms in an adult population. PLoS One. 2013;8:e58932. doi: 10.1371/journal.pone.0058932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortega F, Márquez-Martín E, Valencia B, Cejudo P, Rodriguez A, López-Campos JL, et al. Impact of bronchodilator responsiveness on quality of life and exercise capacity in patients with COPD. Respir Care. 2014;59:81–89. doi: 10.4187/respcare.02399. [DOI] [PubMed] [Google Scholar]
- 16.Cosentino J, Zhao H, Hardin M, Hersh CP, Crapo J, Kim V, et al. COPDGene Investigators. Analysis of asthma-chronic obstructive pulmonary disease overlap syndrome defined on the basis of bronchodilator response and degree of emphysema. Ann Am Thorac Soc. 2016;13:1483–1489. doi: 10.1513/AnnalsATS.201511-761OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 18.Hansen EF, Phanareth K, Laursen LC, Kok-Jensen A, Dirksen A. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1267–1271. doi: 10.1164/ajrccm.159.4.9807121. [DOI] [PubMed] [Google Scholar]
- 19.Gagnon P, Guenette JA, Langer D, Laviolette L, Mainguy V, Maltais F, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:187–201. doi: 10.2147/COPD.S38934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNulty W, Usmani OS. Techniques of assessing small airways dysfunction. Eur Clin Respir J. 2014;1 doi: 10.3402/ecrj.v1.25898. 10.3402/ecrj.v1.25898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 23.Fortis S, Eberlein M, Georgopoulos D, Comellas AP. Predictive value of prebronchodilator and postbronchodilator spirometry for COPD features and outcomes. BMJ Open Respir Res. 2017;4:e000213. doi: 10.1136/bmjresp-2017-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 25.Stocks J, Quanjer PH Official Statement of The European Respiratory Society. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Eur Respir J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 26.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, et al. COPDGene Investigators. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, et al. COPDGene Investigators. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med. 2011;184:57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:519–532. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel BD, Coxson HO, Pillai SG, Agustí AG, Calverley PM, Donner CF, et al. International COPD Genetics Network. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:500–505. doi: 10.1164/rccm.200801-059OC. [DOI] [PubMed] [Google Scholar]
- 31.Tashkin DP, Li N, Kleerup EC, Halpin D, Celli B, Decramer M, et al. Acute bronchodilator responses decline progressively over 4 years in patients with moderate to very severe COPD. Respir Res. 2014;15:102. doi: 10.1186/s12931-014-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, et al. COPDGene Investigators. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121:1042–1050. doi: 10.1378/chest.121.4.1042. [DOI] [PubMed] [Google Scholar]
- 34.Quanjer PH, Ruppel GL, Langhammer A, Krishna A, Mertens F, Johannessen A, et al. Bronchodilator response in FVC is larger and more relevant than in FEV1 in severe airflow obstruction. Chest. 2017;151:1088–1098. doi: 10.1016/j.chest.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Pisi R, Aiello M, Zanini A, Tzani P, Paleari D, Marangio E, et al. Small airway dysfunction and flow and volume bronchodilator responsiveness in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1191–1197. doi: 10.2147/COPD.S82509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:180–184. doi: 10.1513/pats.200508-093DO. [DOI] [PubMed] [Google Scholar]
- 37.Walker PP, Calverley PM. The volumetric response to bronchodilators in stable chronic obstructive pulmonary disease. COPD. 2008;5:147–152. doi: 10.1080/15412550802092928. [DOI] [PubMed] [Google Scholar]
- 38.Jarenbäck L, Eriksson G, Peterson S, Ankerst J, Bjermer L, Tufvesson E. Bronchodilator response of advanced lung function parameters depending on COPD severity. Int J Chron Obstruct Pulmon Dis. 2016;11:2939–2950. doi: 10.2147/COPD.S111573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben Saad H, Préfaut C, Tabka Z, Zbidi A, Hayot M. The forgotten message from gold: FVC is a primary clinical outcome measure of bronchodilator reversibility in COPD. Pulm Pharmacol Ther. 2008;21:767–773. doi: 10.1016/j.pupt.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Chhabra SK, Bhatnagar S. Comparison of bronchodilator responsiveness in asthma and chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2002;44:91–97. [PubMed] [Google Scholar]
- 41.Cerveri I, Pellegrino R, Dore R, Corsico A, Fulgoni P, van de Woestijne KP, et al. Mechanisms for isolated volume response to a bronchodilator in patients with COPD. J Appl Physiol (1985) 2000;88:1989–1995. doi: 10.1152/jappl.2000.88.6.1989. [DOI] [PubMed] [Google Scholar]
- 42.Tulic MK, Christodoulopoulos P, Hamid Q. Small airway inflammation in asthma. Respir Res. 2001;2:333–339. doi: 10.1186/rr83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim V, Desai P, Newell JD, Make BJ, Washko GR, Silverman EK, et al. COPDGene Investigators. Airway wall thickness is increased in COPD patients with bronchodilator responsiveness. Respir Res. 2014;15:84. doi: 10.1186/s12931-014-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19:113–118. doi: 10.1183/09059180.00002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, Coxson H, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187:602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 46.Dransfield MT, Kunisaki KM, Strand MJ, Anzueto A, Bhatt SP, Bowler RP, et al. COPDGene Investigators. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:324–330. doi: 10.1164/rccm.201605-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.