Abstract
Successful fracture healing requires the simultaneous regeneration of both the bone and vasculature; mesenchymal stem cells (MSCs) are directed to replace the bone tissue, while endothelial progenitor cells (EPCs) form the new vasculature that supplies blood to the fracture site. In the elderly, the healing process is slowed, partly due to decreased regenerative function of these stem and progenitor cells. MSCs from older individuals are impaired with regard to cell number, proliferative capacity, ability to migrate, and osteochondrogenic differentiation potential. The proliferation, migration and function of EPCs are also compromised with advanced age. Although the reasons for cellular dysfunction with age are complex and multidimensional, reduced expression of growth factors, accumulation of oxidative damage from reactive oxygen species, and altered signaling of the Sirtuin-1 pathway are contributing factors to aging at the cellular level of both MSCs and EPCs. Because of these geriatric-specific issues, effective treatment for fracture repair may require new therapeutic techniques to restore cellular function. Some suggested directions for potential treatments include cellular therapies, pharmacological agents, treatments targeting age-related molecular mechanisms, and physical therapeutics. Advanced age is the primary risk factor for a fracture, due to the low bone mass and inferior bone quality associated with aging; a better understanding of the dysfunctional behavior of the aging cell will provide a foundation for new treatments to decrease healing time and reduce the development of complications during the extended recovery from fracture healing in the elderly.
Keywords: Fracture healing, Aging, Bone, Angiogenesis, Mesenchymal stem cells, Endothelial progenitor cells
Core tip: Bone fractures in the elderly are a significant issue, due to the prevalence of the problem, the difficulty of treatment, and the severe consequences of the extended healing period. The delay in fracture healing with advanced age has been attributed to both the decreased number and function of mesenchymal stem cells that regenerate the bone and the inferior performance of endothelial progenitor cells that direct angiogenesis. Some suggested avenues for potential treatments include cellular therapies, pharmacological agents, treatments targeting age-related molecular mechanisms, and physical therapeutics.
INTRODUCTION
Aging is the dominant risk factor for fractures, primarily due to low bone mass and poor bone quality in the elderly[1]. While persons 65 years or older currently account for 13% of the United States population[2], they account for more than 50% of hospital admissions with a musculoskeletal injury which are primarily fractures[3]. Fractures in the elderly population are associated with a unique set of geriatric-specific mana-gement challenges. In addition to treatment for a fracture, elderly patients are more likely to be simultaneously treated for additional medical or surgical issues which affect healing and outcomes. In addition, low bone mass and poor bone quality impart technical difficulty in achieving stable internal fixation with plates, screws, nails and wires in surgically treated fractures[4-10]. For example, studies have demonstrated that arthroplasty is typically necessary to avoid predictable healing failure that results from loss of surgical fixation and fracture reduction in elderly fractures of the shoulder, elbow, and hip[4,5,11-13]. In addition, periprosthetic fractures that occur around hip and knee replacement prostheses are increasing exponentially and will continue to increase with the aging population[14-16]. These fractures are particularly challenging for orthopaedic surgeons and healing failure can result in amputation and complete lifelong immobility.
Successful fracture healing requires that both the mineralized tissue and vas-culature regenerate simultaneously to repair the highly vascularized bone (Figure 1). In fact, the processes of bone tissue regeneration and angiogenesis have significant interactions between them during fracture healing. In secondary fracture healing, i.e., in the absence of rigid fixation, the healing process begins when a hematoma forms soon after the injury with subsequent acute inflammation at the fracture site. Inflammatory cytokines as well as growth factors are released to signal the recruitment of mesenchymal stem cells (MSCs) to the injury[17,18]. Resident and infiltrating macrophages also influence the homing and localization of MSCs[18]. The recruited MSCs are multipotent, mesodermally derived cells that are capable of proliferating and differentiating into various cell types including osteoblasts and chondrocytes[19]. Recent evidence supports that the MSCs that home to the fracture site for repair derive primarily from the local periosteum[20,21]. Once the MSCs have reached their target site, circulating growth factors such as bone morphogenetic proteins (BMPs) induce their differentiation into osteoblasts and chondrocytes to initiate the formation of a cartilaginous callus bridge between the bone fragments[21]. Subsequently, the chondrocytes become hypertrophic and undergo endochondral ossification. Both osteoblasts and hypertrophic chondrocytes express high levels of vascular endothelial growth factor (VEGF), a key mediator of angiogenesis and a requisite component of fracture healing[22,23]. VEGF modulates bone repair through the induction of endothelial progenitor cells (EPCs) to increase blood vessel density, providing access for nutrients and cells to the site. With an established vasculature, newly formed osteoblasts begin to replace the soft cartilaginous callus with a stronger osseous one, effectively uniting fragmented bones. Over time, the osseous callus is remodeled into vascularized lamellar bone with a central bone marrow cavity at the diaphysis.
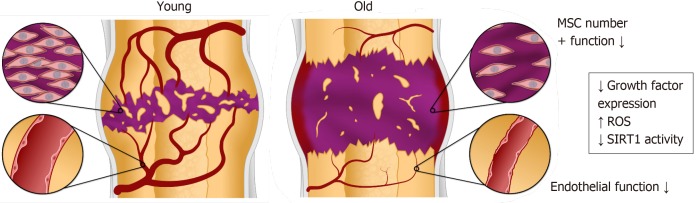
Figure 1.
Fracture healing is impaired with advanced age, including delays in both bone and vascular regeneration due to dysfunction of mesenchymal stem cells and endothelial cells. MSC: Mesenchymal stem cell.
Advanced age is a risk factor for impaired fracture healing[24,25] with increased morbidity and mortality[26-28] as well as increased costs. Increased age has been correlated to healing complications in the tibial shaft[29], clavicle[30], femoral neck[31], and floating knee injuries[32]. Delayed fracture healing, evidenced by a longer time to regain the mechanical strength and mineral content in the bone, has been observed in rodents[33-35]. In general, delayed fracture healing in elderly patients is thought to result from a lower capacity for MSC differentiation and impaired angio-/vasculo-genesis[25]. These phenomena were observed by Lu et al[36], who assessed the molecular, cellular and histological progression of tibia fractures in juvenile, middle-aged and elderly mice and reported delayed chondrocyte differentiation and maturation, vascular invasion, and bone formation in the older animals[36]. The extended healing time may play a role in the development of serious complications that emerge during prolonged immobilization and the consequent high mortality rate with fractures in the geriatric population[37,38].
In this review, we describe the dysfunctional behavior of aging MSCs and EPCs that contribute to impaired fracture healing in the elderly (Figure 1). Although the causes of delayed fracture healing with advanced age are complex and multifactorial, we highlight the reduction in growth factor expression, effects of reactive oxygen species (ROS), and the role of the sirtuin-1 (SIRT1) signaling pathway as significant factors in aging at the cellular level in MSCs and EPCs. Finally, we discuss potential treatments to enhance bone fracture healing that may be beneficial for elderly patients.
MSC IMPAIRMENT WITH AGE
One of the factors for diminished fracture healing in the elderly is the altered behavior of MSCs with respect to number, proliferation, migration ability, and differentiation potential with age[20]. In bone marrow and adipose tissue from different species such as non-human primates[39], humans[40-42], mice[43-45], and rats[46] there was a pronounced age-dependent difference in the number of MSCs based on the colony forming unit (CFU) assay; MSCs from younger individuals were more numerous as they formed up to 50% more CFUs than older individuals[40-44,46]. MSCs have also been cha-racterized by their positive expression of surface markers such CD90, CD44, and CD73. In a study on human marrow-derived MSCs, Stolzing et al[45] found that young cells expressed more CD90, CD105, and Stro-1 and old cells expressed more CD44. The effect of aging on cell surface markers was also observed by Yu et al[39] in MSCs isolated from the bone marrow of rhesus macaques. The MSCs from young and middle-aged individuals had a higher percentage of CD90+ cells than the MSCs derived from older individuals, whereas, the MSCs from older individuals had a higher percentage of CD44+ cells.
The proliferative potential of MSCs also declines with age. The doubling times in MSCs isolated from human bone marrow was 0.9 and 1.7 days in cells from younger and older individuals, respectively[40]. This increase in cell doubling time with age was also observed in MSCs isolated from adipose tissue; cell doubling times increased from approximately 2.6 d in MSCs from younger individuals to 3.8 d in MSCs from older individuals[41,42]. The proliferation rate was also reduced in MSCs isolated from mouse bone marrow by 20% in older animals[44].
The age of the patients not only affects the number and proliferative potential of MSCs but also their ability to migrate to the site of injury, which plays an important role in their regenerative function. It was observed that MSCs from older rats showed lower motility on uncoated filters than those from younger animals[47]. In a different study, twice as many bone marrow-derived MSCs from younger rats migrated towards the chemokine SDF-1 as those from older rats[48]. The decrease in the motility or migration potential of old MSCs may be due to their decreased expression of chemokine receptors[48,49]. In an interesting study on the effect of age on bone marrow microenvironment and migration of MSCs, Yang et al[50] found that co-culture with bone marrow aspirate from old mice reduced the migration of an MSC cell line. The authors also found that the bone marrow aspirate from older mice expressed less SDF-1[50]. Together, these studies suggest that the reduced migratory potential of MSCs from in older individuals may be due reductions in both the MSC expression of chemotactic receptors and in chemotactic cytokines secreted by the older tissue. All of these factors together might contribute toward reduced migration of MSCs to the fracture site in elderly patients leading to poor fracture healing.
An important distinguishing feature of MSCs is their ability to differentiate to the osteogenic and chondrogenic lineages, among others. Various groups studying the age-related changes in differentiation potential of MSCs have concluded differently. Several groups have reported that the osteogenic differentiation potential of the MSCs isolated from either bone marrow or adipose tissue is reduced as age advan-ces[41,42,45,51,52]. Zhang et al[43] reported that osteogenic differentiation capacity of bone marrow-derived MSCs from mice increases in an age-dependent manner to 18 mo of age and decreases rapidly thereafter. In contrast to these studies, other groups found the MSCs maintained their differentiation potential even in aged donors[53,54]. There is also disagreement in the literature on whether age has an effect on the chondrogenic potential of MSCs. Some groups have reported an age-related reduction in their chondrogenic potential[41,42,52]. In other studies, the chondrogenic potential of MSCs was not affected with advanced age[45,51,55]. However, in all cases, the isolated MSCs were cultured and differentiated in vitro where they lack the microenvironment of the native tissue which might be different as the donors age. Conflicting findings in the literature with respect to differentiation potential of MSCs isolated from older individuals require further studies which take tissue microenvironments into consideration to understand any changes in differentiation.
A decline in the expression of growth factors that induce MSC chondrogenic and osteogenic differentiation have been proposed to contribute to impaired fracture healing with age. For example, expression of BMP-2 and Indian hedgehog were at significantly lower levels in the fracture calluses of older rats[56]. Additionally, the response of MSCs to growth factors like BMP-2 may be attenuated with age. As an example, markers of osteogenesis in canine MSCs increased in all animals when treated with BMP-2 in culture, but the increase was less robust in cells from older animals[57]. Similarly, pediatric human iliac crest MSCs were more responsive to exogenous BMP-2 than adult MSCs from the same anatomic location based on the in vitro expression of osteogenic markers[58].
The accumulation of ROS is another factor that may affect MSC function in the aged population, resulting in oxidative damage to DNA, structural lipids and proteins as well as cellular senescence[46]. Oxidative stress has been shown to increase during fracture healing[59-61], however the effect of ROS on MSCs during fracture repair in aging is unclear. In a developmental model of bone formation, chondrogenesis was enhanced by ROS in the developing limb bud, where a cartilage template precedes long bone formation[62]. High levels of ROS have also been associated with hyper-trophic chondrocytes that are undergoing endochondral ossification in vitro[63]. Furthermore, the addition of an antioxidant to cell culture media inhibited chondrocyte hypertrophy, while elevated ROS stimulated chondrocyte hyper-trophy[63]. Osteogenesis through intramembranous ossification, on the other hand, is inhibited by elevated levels of ROS[64-66] and intracellular ROS levels have been observed to dramatically decrease upon osteogenic differentiation due to the upregulation of antioxidant enzymes superoxide dismutase 2 (SOD2) and catalase[66].
Among the molecular regulators of aging, SIRT1, a NAD-dependent histone deacetylase, is of particular importance. SIRT1 expression and activation decrease with age, which modifies a wide range of cellular processes, including MSC pro-liferation and differentiation. For example, SIRT1 knockdown in human marrow- and adipose-derived MSCs resulted in reduced proliferation in vitro[67]. Additionally, MSCs isolated from Sirt1 knock-out mice showed reduced differentiation toward the osteogenic lineage[68]. while Sirt1+/- female mice had reduce bone mass and increased marrow adipogenesis[69]. Differentiation to the chondrogenic lineages were also inhibited in MSCs isolated from Sirt1 knockout mice[68] and with SIRT1 knockdown[70].
IMPAIRED EPCS WITH AGING
Blood supply is critical for fracture healing. Formation of sufficient vasculature at the fracture sites provides oxygen and nutrients for cell survival and proliferation. Aging has negative effects on angiogenesis which can lead to delayed healing or non-union of fractures[36,71]. Vascular changes such as the decline in endothelial function are reliable markers for aging[72-75]. Highly proliferative EPCs, also described as late outgrowth EPCs or endothelial colony forming cells (ECFCs), are believed to play an important role in maintenance of the viable endothelial layer in the vascular sys-tem[76-78].
Aging decreases endothelial cell (EC) proliferation and migration, as well as the expression of EC growth factors and their cognate receptors[79-81]. Aging is also a major cause for endothelial dysfunction and microvascular hypermeability[82,83]. The mechanisms underlying age-related endothelial dysfunction likely involve increased oxidative stress and alterations in molecular pathways affecting common aging processes. Importantly, EPC dysfunction and senescence contribute to oxidative stress[84].
Age related mitochondrial dysfunction is a likely candidate to explain this endothelial progenitor dysfunction. Mitochondria-derived production of ROS results in increased oxidative stress in ECs. Attenuation of mitochondrial oxidative stress in a genetically modified mouse model of overexpression of human catalase in mito-chondria improved endothelial function[85]. Conversely, genetic deletion of the mitochondrial antioxidant proteins, mitochondrial SOD and glutathione peroxidase 1, exacerbated age-related vascular dysfunction[86,87]. Age-related oxidative stress may also be caused by increased activity of NADPH oxidase in ECs[88]. Increased oxidative stress in aged ECs inactivates nitric oxide (NO)[88,89]. Impaired bioavailability of NO negatively affects cell division and survival, mitochondrial function and cellular energy metabolism, and EPCs[90].
SIRT1 is an important molecular regulator in ECs[91] in addition to its role in MSCs. SIRT1 expression and activity decreases with aging in the vasculature. Accordingly, pharmacological activation of SIRT1 significantly improves endothelial function in aged mice[92]. Similarly, cleavage of SIRT1 by cathepsin in EPCs mediates stress-induced premature senescence[93].
Age is also a limiting factor for mobilization of EPCs including ECFCs[94-96]. Thus, it appears that the decrease in number and/or function of ECFCs, a homogenous population of EPCs, may be a major driver for failed fracture repair in elderly pati-ents. Previous studies suggest age-related EPC dysfunction may be reversible by anti-aging intervention[97]. Preclinical studies also showed that the serum factors derived from young rats have beneficial effects on EPCs isolated from aged ones[98,99].
In addition to their role in fracture healing, MSCs share properties with pericytes and are important for vascular network formation[99]. Pericytes have an important role in angiogenesis and could be a novel therapeutic target because of their involvement in regulation of capillary permeability, EC proliferation and extracellular matrix generation[100,101]. In fact, age-related loss of pericyte coverage of microvessels contributes to function and structural impairment of microcirculatory network[100]. Interestingly, when adipose derived mesenchymal and endothelial stem cells are brought in close contact, a Wnt signaling specific mechanism favors osteogenic versus adipogenic differentiation[102,103]. It remains to be elucidated if treatment targeting pericytes could enhance bone healing in aging.
Dysfunction of aged ECs and EPCs lead to endothelial senescence and apoptosis and directly interfered with angiogenesis in aging[82,104-106]. Age-related changes in circulation factors might also contribute to impaired angiogenesis in aging. Pro-angiogenic endocrine factors, growth hormone, insulin like growth factor I, platelet derived growth factor (PDGF) and VEGF, which regulate multiple aspects of angiogenic processes, decline with aging[95,107-109]. This may be explained by reduced expression of, and responsiveness to, HIF-1alpha during aging[110]. Impaired angiogenesis also results in age-related decline in vessel density, impaired adaptation to hypoxia, and ischemia[111].
Impaired angiogenesis during fracture healing creates an ischemic environment at the fracture site and disrupts the interactions between the blood supply and MSCs that are required for bone healing. In a mouse model of fracture accompanied by vascular damage, ischemia significantly decreased the callus size, and the cartilage and bone formation, leading to delayed union[112]. Similar results have been seen in in vitro culture of MSCs in hypoxic environments. Hypoxia was found to be linked to reduced osteogenic potential of MSCs, evidenced by the down regulation of many osteogenic markers[113] and osteogenic pathways such as RUNX2[114]. Hypoxia has also been found to inhibit hypertrophic differentiation of chondrocytes and endochondral ossification[115]. Thus, a disruption to the angiogenesis process due to aging may have profound effects on MSC behavior at the fracture site, leading to delayed fracture healing.
POTENTIAL TREATMENT OPPORTUNITIES FOR IMPROVED FRACTURE HEALING IN AGING
Cell-based therapies
Successful management of bone fractures in the elderly may require special measures not commonly indicated in younger individuals. As native MSCs and EPCs may be compromised with respect to number and/or function with advanced age, delivering these cells to the fracture site is one potential avenue to accelerate fracture repair.
Bone tissue engineering has been investigated intensively for three decades, but efforts to date have not yielded a cell-seeded implant which can be used clinically. Most tissue engineering approaches target intramembranous or direct bone formation, but this approach has had poor outcomes because the cells must initially survive in an avascular hypoxic environment before the invasion of vasculature. Without vasculature, nutrient delivery and waste removal are severely compromised in the center of the implant, causing cell necrosis and failure of cell-seeded implants[113,116]. A relatively new technique to address this issue exploits the tendency of MSCs to undergo a process resembling hypertrophy when cultured under standard chondrogenic differentiation conditions[117,118]. In this regenerative strategy, bone tissue is generated via the endochondral ossification pathway, where a cartilaginous template is first formed and later remodeled into mature bone. One advantage of endochondral bone tissue engineering is that the chondrogenic cells function much better than osteogenic cells in low-oxygen environments such as the avascular region of a bone defect[113,119]. Therefore, the chondrogenic cells are maintained in the implant site until the vasculature invades, at which time the hypertrophic cells induce bone formation, as in secondary native fracture healing. Because the cells undergo a process that resembles hypertrophy, they release an array of growth factors for vascular and bone formation that are spatially and temporally controlled. The feasibility of this technique has been demonstrated using embryonic stem cell[120], marrow- and adipose-derived MSCs[121-129], and the murine, chondrocytic cell line ATDC5[130]. Recently, fracture healing through endochondral ossification using hypertrophically primed MSCs in a collagen construct was demonstrated in a weight bearing femoral defect model in rats[131]. In fact, endochondral ossification has been shown to be a better alternative than intramembranous bone regeneration by Thompson et al[132], where the chondrogenically primed MSCs supported greater repair of a cranial critical-sized defect (CSD)[132].
Another cell-therapy approach to improve bone healing is to enhance angiogenesis with ECFCs. Currently, implantation of ECFCs has been tested in animals and is currently being investigated in human clinical trials for other indications, such as myocardial infarction, ischemic stroke, liver cirrhosis, and diabetic foot[133]. Our group[134] and others[135-140] have recently shown the utility of using ECs for bone repair. ECFCs were selected based on their proliferative potential, expression of CD31 and CD309, as well as their ability to take-up acetylated low-density lipoproteins[141].
ECFCs can induce neovascularization at the bone defect site, and stimulate fracture repair and bone regeneration in young rats[134]. ECFCs (106 cells) were seeded into a type I collagen sponge and transplanted into the bone defect during fracture surgery. The data showed that ECFCs induced more new blood vessels compared to the unseeded type I collagen controls[134]. Furthermore, new bone was formed within the defect area when implanted with ECFCs, but no bone was observed in the controls[134]. Histological examination showed that osteocytes, osteoblasts, and osteoclasts were observed in newly formed bone tissues in ECFC treated animals at 6 weeks[134]. These data suggest that ECFCs can increase neovascularization and stimulate new bone formation in the damaged bone area with a CSD that normally fails to heal.
In another study by our group, hydroxyapatite and tri-calcium phosphate (HA/TCP) scaffolds loaded with ECFCs (106 cells) were placed into the fibula defect. Histological examination showed significantly greater newly formed bone in HA/TCP scaffolds loaded with ECFCs than that observed in the HA/TCP scaffold only animals[134], suggesting that ECFCs may migrate and further enhance bone regeneration inside the scaffold.
Pharmacological agents
Because the endogenous concentrations of bone anabolic agents that facilitate fracture repair can be significantly reduced in the elderly, one obvious remedy hypothesized for enhancement of their healing process has been to supplement the patient’s natural levels of bone anabolic agents. Recognizing that BMP-2 can potently augment the rate of bone fracture repair, Medtronic Inc. has explored the local application of BMP-2 (Infuse) to a fracture surface to accelerate the healing process. While success has been documented for enhancement of recovery from open tibial shaft fractures[142,143], dental and facial reconstruction surgeries[144,145], and spinal fusion procedures[146,147], the same methodology cannot be applied when fracture surfaces cannot be physically exposed. Thus, those fractures that do not require surgical intervention cannot be treated with BMP-2 dosing through local delivery. Moreover, repeated administration of a bone anabolic agent is not possible with this strategy, since the fracture surface is usually only accessible during the initial reconstruction/stabilization surgery.
A second method to augment endogenous levels of osteogenic agents was explored by Eli Lilly and Co. when they examined the use of parathyroid hormone (Forteo) to accelerate the repair rate of tibial[148-150] and hip fractures[151,152]. While measurable improvement in the healing process was documented in many patients, the phase 3 trial failed to reach its clinical endpoint due to concerns over the induced hyper-calcemia that was observed as therapeutically effective concentrations of drug were approached. Although the potentially deleterious consequences of the hypercalcemia forced discontinuation of the clinical trial, the results suggested that a more targeted form of parathyroid hormone might succeed if it could concentrate that drug at the site of the fracture and reduce its concentration in healthy tissues.
Looking to the future, a large number of peptide and protein hormones that are commonly released at the site of a wound have been reported to exhibit bone anabolic activity. These include FGF2[153-156], PTHrP[157-159], PDGF[160-162], Prostaglandins[163-165], IGF[166], VEGF[167,168] and others. Because virtually all of these stimulants are known to have multiple anabolic activities that can cause undesirable changes in healthy tissues, it is unlikely that any will prove useful as bone fracture repair drugs unless they can be applied locally to the fracture surface or targeted to the same fracture surface following systemic administration. Hopefully, with the design of new bone fracture homing ligands, such fracture targeted anabolic agents can be developed for less invasive therapies of fractures in the elderly.
Therapies targeting age-related molecular mechanisms
Aging at the cellular level is associated with increased ROS production and decreased endogenous antioxidant levels, leading to accumulation of oxidative damage and cellular senescence. Therefore, antioxidants have been studied as a therapy to improve a variety of health outcomes, including fracture healing. Antioxidants vitamin E[169,170], melatonin[171,172], and N-acetylcysteine[173] have all been shown to promote fracture healing in animal models. Cellular senescence itself can induce chronic inflammatory disease in mice, and depletion of senescent cells by so called senolytic agents can reduce systemic inflammation and extend life span in small rodent by 37%[174-178]. Importantly, targeting cellular senescence prevents age-related bone loss in mice[179]. Therefore, it appears to be promising to identify ways to reduce the generation and maintenance of senescent progenitor cells. This widely overlooked aspect is of particular interest because a high number of senescent cells with detrimental functions are to be expected during aging and aging-associated infla-mmatory conditions.
Another potential target for improving fracture healing in aging is SIRT1[91]; as described above, it appears to be involved in the age-related decline in both MSC and EC function. Furthermore, crosstalk between SIRT1 activity and ROS production plays a crucial role in the aging process[180]. SIRT1 expression and activity decreases with aging. Accordingly, pharmacological activation of SIRT1 improves the survival of aged MSCs upon transplantation[21] and also significantly improves endothelial function in aged mice[92]. Similarly, cleavage of SIRT1 by cathepsin in EPCs mediates stress-induced premature senescence[93]. Most notably, pharmacological activation of SIRT1 increased bone mass in mouse models of osteoporosis[181].
Physical therapeutics
Physical therapeutics are non-invasive and non-pharmacological treatments that cause physiologic cascades in the body to affect measurable change in molecular and tissue function, leading to improved functional outcomes[182-184]. Movement therapies and therapeutic modalities are two approaches frequently used in the clinic that should be considered within the context of fracture healing and aging.
Movement approaches may include ambulation and therapeutic exercise, both of which mobilize physiological responses secondary to mechanical loading. While the effects of these treatments have not been fully explored in humans, it has been shown that mechanical loading of cells in vitro can impact gene expression and bone-derived mesenchymal cell (MSC) differentiation into three types of tissue: Fat, bone, and cartilage[185]. Even short durations of compression can cause an increase in diffe-rentiation and calcium mineralization in certain cultures[186]. Another benefit of mechanical loading during cyclical compression, which mimics gait, of human MSCs is an improvement in oxygenation of the fracture’s hematoma that benefits cellular metabolism and the ability to heal[187]. One study investigated the effects of functional mechanical loading on large bone defect regeneration in vivo. Bone CSDs in rat femora were stabilized using either stiff or compliant fixation plates that allowed compressive loading during ambulation. Findings demonstrated that functional transfer of axial loads during segmental bone repair enhanced bone formation and regeneration[188]. Meanwhile, a retrospective cohort study observed that early ambulation/mobilization of elderly patients with fractures improve outcomes faster than those who delay mobilization[189]. In contrast, immobility was associated with higher mortality and lower function[190].
Several therapeutic modalities are used clinically to facilitate fracture healing, including whole-body vibration (WBV) and pulsed ultrasound. Recent systematic reviews suggest WBV is a safe and effective treatment. Pre-clinical trials with ovariectomized rats have shown those with diminished estrogen respond better to WBV than those with normal levels[191-193]. Interestingly, a study observed an increase in osteogenic potential of bone marrow with WBV during a period of hindlimb unloading compared to those with no treatment; this increase was expounded upon later during re-ambulation and concurrent WBV[194]. Pulsed ultrasound may offer another option for fracture healing in the elderly. Research has shown low-intensity pulsed ultrasound to decrease osteoclastic gene expression[195] decrease MSC adipocyte differentiation[195], and foster MSC’s commitment to osteogenesis[196,197]. However, according to recent systematic reviews, there is a low level of evidence to support its use in the early phases of fracture healing in elderly humans[198] and in those undergoing distraction osteogenesis[199].
CONCLUSION
Bone fractures in the elderly are a significant issue, due to the prevalence of the problem, the difficulty of treatment, and the severe consequences of the extended healing period. The delay in fracture healing with advanced age has been attributed to the decreased number and function of MSCs that regenerate the bone and the inferior performance of EPCs that participate in angiogenesis. The causes of cellular aging and the concomitant decline in functionality are wide-ranging, but provide some intriguing indications of potential targets for speeding fracture healing in older individuals. In the future, cell therapies that supplement the inadequate native cellular response with MSCs or ECFCs; bone anabolic pharmacological agents, particularly in combination with strategies to localize their delivery to the bone fracture; drugs that reduce oxidative stress, cellular senescence, or activate SIRT1; and/or physical therapeutics may prove effective in promoting fracture healing in the elderly.
ACKNOWLEDGEMENTS
We would like to thank Sue Samson for her administrative support.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: March 22, 2019
First decision: April 11, 2019
Article in press: June 12, 2019
Specialty type: Cell and tissue engineering
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good):
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kahveci R, Yukata K, Wang YH S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
Contributor Information
Diane R Wagner, Department of Mechanical and Energy Engineering, Indiana University-Purdue University Indianapolis, Indianapolis, IN 46202, United States. wagnerdi@iupui.edu.
Sonali Karnik, Department of Mechanical and Energy Engineering, Indiana University-Purdue University Indianapolis, Indianapolis, IN 46202, United States.
Zachary J Gunderson, Department of Orthopaedic Surgery, Indiana University School of Medicine, Indianapolis, IN 46202, United States.
Jeffery J Nielsen, Department of Medicinal Chemistry and Molecular Pharmacology, Purdue University, West Lafayette, IN 47907, United States.
Alanna Fennimore, Department of Physical Therapy, Indiana University-Purdue University Indianapolis, Indianapolis, IN 46202, United States.
Hunter J Promer, Division of Biomedical Science, Marian University College of Osteopathic Medicine, Indianapolis, IN 46222, United States.
Jonathan W Lowery, Division of Biomedical Science, Marian University College of Osteopathic Medicine, Indianapolis, IN 46222, United States.
M Terry Loghmani, Department of Physical Therapy, Indiana University-Purdue University Indianapolis, Indianapolis, IN 46202, United States.
Philip S Low, Department of Chemistry, Purdue University, West Lafayette, IN 47907 United States.
Todd O McKinley, Department of Orthopaedic Surgery, Indiana University School of Medicine, Indianapolis, IN 46202, United States.
Melissa A Kacena, Department of Orthopaedic Surgery, Indiana University School of Medicine, Indianapolis, IN 46202, United States; Richard L. Roudebush VA Medical Center, Indianapolis, IN 46202, United States.
Matthias Clauss, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202, United States.
Jiliang Li, Department of Biology, Indiana University-Purdue University Indianapolis, Indianapolis, IN 46202, United States.
References
- 1.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US census. The Older Population: 2010. Available from: https://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf. [Google Scholar]
- 3.USA BaJI. 2015. The Burden of Musculoskeletal Diseases in the United States. Available from: http://www.boneandjointburden.org. [Google Scholar]
- 4.Chun YM, Kim DS, Lee DH, Shin SJ. Reverse shoulder arthroplasty for four-part proximal humerus fracture in elderly patients: can a healed tuberosity improve the functional outcomes? J Shoulder Elbow Surg. 2017;26:1216–1221. doi: 10.1016/j.jse.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Kurar L. Clinical audit of ankle fracture management in the elderly. Ann Med Surg (Lond) 2016;6:96–101. doi: 10.1016/j.amsu.2015.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pidhorz L, Alligand-Perrin P, De Keating E, Fabre T, Mansat P Société française de chirurgie orthopédique et traumatologie (SoFCOT) Distal humerus fracture in the elderly: does conservative treatment still have a role? Orthop Traumatol Surg Res. 2013;99:903–907. doi: 10.1016/j.otsr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Crespo AM, Rautenberg AF, Siev N, Saadeh P, Egol KA. Fibulectomy, tibial shortening, and ankle arthrodesis as an alternative treatment of nonhealing wounds following open ankle fracture in compromised elderly adults. Foot Ankle Int. 2015;36:103–107. doi: 10.1177/1071100714550653. [DOI] [PubMed] [Google Scholar]
- 8.Hinds RM, Capo JT, Kakar S, Roberson J, Gottschalk MB. Early Complications Following Osteosynthesis of Distal Radius Fractures: A Comparison of Geriatric and Nongeriatric Cohorts. Geriatr Orthop Surg Rehabil. 2017;8:30–33. doi: 10.1177/2151458516681636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim GD, Chae SU, Cha MS. Medial malleolar insufficiency fracture of the ankle in an elderly patient with osteoporosis. J Bone Metab. 2013;20:119–122. doi: 10.11005/jbm.2013.20.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian W, He C, Jia J. Total elbow joint replacement for the treatment of distal humerus fracture of type c in eight elderly patients. Int J Clin Exp Med. 2015;8:10066–10073. [PMC free article] [PubMed] [Google Scholar]
- 11.Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop Relat Res. 2001:229–242. doi: 10.1097/00003086-200102000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Kos N, Burger H, Vidmar G. Mobility and functional outcomes after femoral neck fracture surgery in elderly patients: a comparison between hemiarthroplasty and internal fixation. Disabil Rehabil. 2011;33:2264–2271. doi: 10.3109/09638288.2011.568665. [DOI] [PubMed] [Google Scholar]
- 13.Puvanesarajah V, Amin R, Qureshi R, Shafiq B, Stein B, Hassanzadeh H, Yarboro S. Outcomes following surgical management of femoral neck fractures in elderly dialysis-dependent patients. Arch Orthop Trauma Surg. 2018;138:757–764. doi: 10.1007/s00402-018-2898-9. [DOI] [PubMed] [Google Scholar]
- 14.Chitre A, Wynn Jones H, Shah N, Clayson A. Complications of total hip arthroplasty: periprosthetic fractures of the acetabulum. Curr Rev Musculoskelet Med. 2013;6:357–363. doi: 10.1007/s12178-013-9188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gondalia V, Choi DH, Lee SC, Nam CH, Hwang BH, Ahn HS, Ong AC, Park HY, Jung KA. Periprosthetic supracondylar femoral fractures following total knee arthroplasty: clinical comparison and related complications of the femur plate system and retrograde-inserted supracondylar nail. J Orthop Traumatol. 2014;15:201–207. doi: 10.1007/s10195-014-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuurmond RG, van Wijhe W, van Raay JJ, Bulstra SK. High incidence of complications and poor clinical outcome in the operative treatment of periprosthetic femoral fractures: An analysis of 71 cases. Injury. 2010;41:629–633. doi: 10.1016/j.injury.2010.01.102. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013;37:2491–2498. doi: 10.1007/s00264-013-2059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibon E, Lu L, Goodman SB. Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther. 2016;7:44. doi: 10.1186/s13287-016-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Xia X, Li B. Mesenchymal stem cell aging: Mechanisms and influences on skeletal and non-skeletal tissues. Exp Biol Med (Maywood) 2015;240:1099–1106. doi: 10.1177/1535370215591828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadjiargyrou M, O'Keefe RJ. The convergence of fracture repair and stem cells: interplay of genes, aging, environmental factors and disease. J Bone Miner Res. 2014;29:2307–2322. doi: 10.1002/jbmr.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, Zhang X, Bikle DD. Osteogenic Differentiation of Periosteal Cells During Fracture Healing. J Cell Physiol. 2017;232:913–921. doi: 10.1002/jcp.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29:560–564. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 24.Foulke BA, Kendal AR, Murray DW, Pandit H. Fracture healing in the elderly: A review. Maturitas. 2016;92:49–55. doi: 10.1016/j.maturitas.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Exp Gerontol. 2006;41:1080–1093. doi: 10.1016/j.exger.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Huntjens KM, Kosar S, van Geel TA, Geusens PP, Willems P, Kessels A, Winkens B, Brink P, van Helden S. Risk of subsequent fracture and mortality within 5 years after a non-vertebral fracture. Osteoporos Int. 2010;21:2075–2082. doi: 10.1007/s00198-010-1178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J. 2006;6:479–487. doi: 10.1016/j.spinee.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Lau E, Ong K, Kurtz S, Schmier J, Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am. 2008;90:1479–1486. doi: 10.2106/JBJS.G.00675. [DOI] [PubMed] [Google Scholar]
- 29.Claes L, Grass R, Schmickal T, Kisse B, Eggers C, Gerngross H, Mutschler W, Arand M, Wintermeyer T, Wentzensen A. Monitoring and healing analysis of 100 tibial shaft fractures. Langenbecks Arch Surg. 2002;387:146–152. doi: 10.1007/s00423-002-0306-x. [DOI] [PubMed] [Google Scholar]
- 30.Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004;86:1359–1365. doi: 10.2106/00004623-200407000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Parker MJ, Raghavan R, Gurusamy K. Incidence of fracture-healing complications after femoral neck fractures. Clin Orthop Relat Res. 2007;458:175–179. doi: 10.1097/BLO.0b013e3180325a42. [DOI] [PubMed] [Google Scholar]
- 32.Hee HT, Wong HP, Low YP, Myers L. Predictors of outcome of floating knee injuries in adults: 89 patients followed for 2-12 years. Acta Orthop Scand. 2001;72:385–394. doi: 10.1080/000164701753542050. [DOI] [PubMed] [Google Scholar]
- 33.Meyer RA, Jr, Tsahakis PJ, Martin DF, Banks DM, Harrow ME, Kiebzak GM. Age and ovariectomy impair both the normalization of mechanical properties and the accretion of mineral by the fracture callus in rats. J Orthop Res. 2001;19:428–435. doi: 10.1016/S0736-0266(00)90034-2. [DOI] [PubMed] [Google Scholar]
- 34.Lopas LA, Belkin NS, Mutyaba PL, Gray CF, Hankenson KD, Ahn J. Fractures in geriatric mice show decreased callus expansion and bone volume. Clin Orthop Relat Res. 2014;472:3523–3532. doi: 10.1007/s11999-014-3829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bak B, Andreassen TT. The effect of aging on fracture healing in the rat. Calcif Tissue Int. 1989;45:292–297. doi: 10.1007/BF02556022. [DOI] [PubMed] [Google Scholar]
- 36.Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C, Marcucio RS. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–1307. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roche JJ, Wenn RT, Sahota O, Moran CG. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331:1374. doi: 10.1136/bmj.38643.663843.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Streubel PN, Ricci WM, Wong A, Gardner MJ. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469:1188–1196. doi: 10.1007/s11999-010-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu JM, Wu X, Gimble JM, Guan X, Freitas MA, Bunnell BA. Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell. 2011;10:66–79. doi: 10.1111/j.1474-9726.2010.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 41.Ye X, Liao C, Liu G, Xu Y, Tan J, Song Z. Age-Related Changes in the Regenerative Potential of Adipose-Derived Stem Cells Isolated from the Prominent Fat Pads in Human Lower Eyelids. PLoS One. 2016;11:e0166590. doi: 10.1371/journal.pone.0166590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. doi: 10.1186/1479-5876-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Ou G, Hamrick M, Hill W, Borke J, Wenger K, Chutkan N, Yu J, Mi QS, Isales CM, Shi XM. Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J Bone Miner Res. 2008;23:1118–1128. doi: 10.1359/JBMR.080304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mutyaba PL, Belkin NS, Lopas L, Gray CF, Dopkin D, Hankenson KD, Ahn J. Notch signaling in mesenchymal stem cells harvested from geriatric mice. J Orthop Trauma. 2014;28 Suppl 1:S20–S23. doi: 10.1097/BOT.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 45.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 46.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5:213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 47.Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kühnisch J, Tschirschmann M, Kaspar K, Perka C, Duda GN, Klose J. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells. 2009;27:1288–1297. doi: 10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- 48.Sanghani-Kerai A, Osagie-Clouard L, Blunn G, Coathup M. The influence of age and osteoporosis on bone marrow stem cells from rats. Bone Joint Res. 2018;7:289–297. doi: 10.1302/2046-3758.74.BJR-2017-0302.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bustos ML, Huleihel L, Kapetanaki MG, Lino-Cardenas CL, Mroz L, Ellis BM, McVerry BJ, Richards TJ, Kaminski N, Cerdenes N, Mora AL, Rojas M. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med. 2014;189:787–798. doi: 10.1164/rccm.201306-1043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang YM, Li P, Cui DC, Dang RJ, Zhang L, Wen N, Jiang XX. Effect of aged bone marrow microenvironment on mesenchymal stem cell migration. Age (Dordr) 2015;37:16. doi: 10.1007/s11357-014-9743-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaim M, Karaman S, Cetin G, Isik S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol. 2012;91:1175–1186. doi: 10.1007/s00277-012-1438-x. [DOI] [PubMed] [Google Scholar]
- 52.Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Justesen J, Stenderup K, Eriksen EF, Kassem M. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int. 2002;71:36–44. doi: 10.1007/s00223-001-2059-x. [DOI] [PubMed] [Google Scholar]
- 54.Fickert S, Schröter-Bobsin U, Gross AF, Hempel U, Wojciechowski C, Rentsch C, Corbeil D, Günther KP. Human mesenchymal stem cell proliferation and osteogenic differentiation during long-term ex vivo cultivation is not age dependent. J Bone Miner Metab. 2011;29:224–235. doi: 10.1007/s00774-010-0215-y. [DOI] [PubMed] [Google Scholar]
- 55.Scharstuhl A, Schewe B, Benz K, Gaissmaier C, Bühring HJ, Stoop R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 2007;25:3244–3251. doi: 10.1634/stemcells.2007-0300. [DOI] [PubMed] [Google Scholar]
- 56.Meyer RA, Jr, Meyer MH, Tenholder M, Wondracek S, Wasserman R, Garges P. Gene expression in older rats with delayed union of femoral fractures. J Bone Joint Surg Am. 2003;85:1243–1254. doi: 10.2106/00004623-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Volk SW, Diefenderfer DL, Christopher SA, Haskins ME, Leboy PS. Effects of osteogenic inducers on cultures of canine mesenchymal stem cells. Am J Vet Res. 2005;66:1729–1737. doi: 10.2460/ajvr.2005.66.1729. [DOI] [PubMed] [Google Scholar]
- 58.Osyczka AM, Damek-Poprawa M, Wojtowicz A, Akintoye SO. Age and skeletal sites affect BMP-2 responsiveness of human bone marrow stromal cells. Connect Tissue Res. 2009;50:270–277. doi: 10.1080/03008200902846262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeler H, Tahtabas F, Candan F. Investigation of oxidative stress during fracture healing in the rats. Cell Biochem Funct. 2005;23:137–139. doi: 10.1002/cbf.1199. [DOI] [PubMed] [Google Scholar]
- 60.Turgut A, Göktürk E, Köse N, Kaçmaz M, Oztürk HS, Seber S, Acar S. Oxidant status increased during fracture healing in rats. Acta Orthop Scand. 1999;70:487–490. doi: 10.3109/17453679909000986. [DOI] [PubMed] [Google Scholar]
- 61.Prasad G, Dhillon MS, Khullar M, Nagi ON. Evaluation of oxidative stress after fractures. A preliminary study. Acta Orthop Belg. 2003;69:546–551. [PubMed] [Google Scholar]
- 62.Johnson CS, Blanton MR, Hunter ES., 3rd Effects of ethanol and hydrogen peroxide on mouse limb bud mesenchyme differentiation and cell death. In Vitro Cell Dev Biol Anim. 2004;40:108–112. doi: 10.1290/1543-706x(2004)040<0108:eoeahp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 63.Morita K, Miyamoto T, Fujita N, Kubota Y, Ito K, Takubo K, Miyamoto K, Ninomiya K, Suzuki T, Iwasaki R, Yagi M, Takaishi H, Toyama Y, Suda T. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med. 2007;204:1613–1623. doi: 10.1084/jem.20062525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tahara EB, Navarete FD, Kowaltowski AJ. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med. 2009;46:1283–1297. doi: 10.1016/j.freeradbiomed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Lee DH, Lim BS, Lee YK, Yang HC. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 2006;22:39–46. doi: 10.1007/s10565-006-0018-z. [DOI] [PubMed] [Google Scholar]
- 66.Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 67.Yuan HF, Zhai C, Yan XL, Zhao DD, Wang JX, Zeng Q, Chen L, Nan X, He LJ, Li ST, Yue W, Pei XT. SIRT1 is required for long-term growth of human mesenchymal stem cells. J Mol Med (Berl) 2012;90:389–400. doi: 10.1007/s00109-011-0825-4. [DOI] [PubMed] [Google Scholar]
- 68.Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, Baron R, Scadden D, Schipani E, Guarente L. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating β-catenin. EMBO Mol Med. 2013;5:430–440. doi: 10.1002/emmm.201201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen-Kfir E, Artsi H, Levin A, Abramowitz E, Bajayo A, Gurt I, Zhong L, D'Urso A, Toiber D, Mostoslavsky R, Dresner-Pollak R. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology. 2011;152:4514–4524. doi: 10.1210/en.2011-1128. [DOI] [PubMed] [Google Scholar]
- 70.Buhrmann C, Busch F, Shayan P, Shakibaei M. Sirtuin-1 (SIRT1) is required for promoting chondrogenic differentiation of mesenchymal stem cells. J Biol Chem. 2014;289:22048–22062. doi: 10.1074/jbc.M114.568790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu C, Hansen E, Sapozhnikova A, Hu D, Miclau T, Marcucio RS. Effect of age on vascularization during fracture repair. J Orthop Res. 2008;26:1384–1389. doi: 10.1002/jor.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Treitinger A, Spada C, da Silva LM, Hermes EM, Amaral JA, Abdalla DS. Lipid and acute-phase protein alterations in HIV-1 infected patients in the early stages of infection: correlation with CD4+ lymphocytes. Braz J Infect Dis. 2001;5:192–199. doi: 10.1590/s1413-86702001000400005. [DOI] [PubMed] [Google Scholar]
- 73.Savarino A, Bottarel F, Calosso L, Feito MJ, Bensi T, Bragardo M, Rojo JM, Pugliese A, Abbate I, Capobianchi MR, Dianzani F, Malavasi F, Dianzani U. Effects of the human CD38 glycoprotein on the early stages of the HIV-1 replication cycle. FASEB J. 1999;13:2265–2276. doi: 10.1096/fasebj.13.15.2265. [DOI] [PubMed] [Google Scholar]
- 74.Simm M, Pekarskaya O, Volsky DJ. Synthesis of full-length viral DNA in CD4-positive membrane vesicles exposed to HIV-1. A model for studies of early stages of the hiv-1 life cycle. J Biol Chem. 1996;271:28266–28270. doi: 10.1074/jbc.271.45.28266. [DOI] [PubMed] [Google Scholar]
- 75.Gisslén M, Hagberg L, Norkrans G, Lekman A, Fredman P. Increased cerebrospinal fluid ganglioside GM1 concentrations indicating neuronal involvement in all stages of HIV-1 infection. J Neurovirol. 1997;3:148–152. doi: 10.3109/13550289709015804. [DOI] [PubMed] [Google Scholar]
- 76.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 77.Op den Buijs J, Musters M, Verrips T, Post JA, Braam B, van Riel N. Mathematical modeling of vascular endothelial layer maintenance: the role of endothelial cell division, progenitor cell homing, and telomere shortening. Am J Physiol Heart Circ Physiol. 2004;287:H2651–H2658. doi: 10.1152/ajpheart.00332.2004. [DOI] [PubMed] [Google Scholar]
- 78.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 80.Edelberg JM, Reed MJ. Aging and angiogenesis. Front Biosci. 2003;8:s1199–s1209. doi: 10.2741/1166. [DOI] [PubMed] [Google Scholar]
- 81.Moriya J, Minamino T. Angiogenesis, Cancer, and Vascular Aging. Front Cardiovasc Med. 2017;4:65. doi: 10.3389/fcvm.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, Geng YJ, Sato N, Nazareno JB, Vatner DE, Natividad F, Bishop SP, Vatner SF. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 83.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imanishi T, Tsujioka H, Akasaka T. Endothelial progenitor cells dysfunction and senescence: contribution to oxidative stress. Curr Cardiol Rev. 2008;4:275–286. doi: 10.2174/157340308786349435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 86.Wenzel P, Schuhmacher S, Kienhöfer J, Müller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K, Münzel T, Bürkle A, Bachschmid MM, Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res. 2008;80:280–289. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oelze M, Kröller-Schön S, Steven S, Lubos E, Doppler C, Hausding M, Tobias S, Brochhausen C, Li H, Torzewski M, Wenzel P, Bachschmid M, Lackner KJ, Schulz E, Münzel T, Daiber A. Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension. 2014;63:390–396. doi: 10.1161/HYPERTENSIONAHA.113.01602. [DOI] [PubMed] [Google Scholar]
- 88.Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol. 2003;285:H1015–H1022. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- 89.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004;286:H2249–H2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15:555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gano LB, Donato AJ, Pasha HM, Hearon CM, Jr, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2014;307:H1754–H1763. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen J, Xavier S, Moskowitz-Kassai E, Chen R, Lu CY, Sanduski K, Špes A, Turk B, Goligorsky MS. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am J Pathol. 2012;180:973–983. doi: 10.1016/j.ajpath.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoetzer GL, Van Guilder GP, Irmiger HM, Keith RS, Stauffer BL, DeSouza CA. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol (1985) 2007;102:847–852. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- 95.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 96.Williamson KA, Hamilton A, Reynolds JA, Sipos P, Crocker I, Stringer SE, Alexander YM. Age-related impairment of endothelial progenitor cell migration correlates with structural alterations of heparan sulfate proteoglycans. Aging Cell. 2013;12:139–147. doi: 10.1111/acel.12031. [DOI] [PubMed] [Google Scholar]
- 97.Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD, Poole-Wilson PA, Borlak J, Ertl G, Bauersachs J. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100:434–443. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 98.Zhu G, Song M, Wang H, Zhao G, Yu Z, Yin Y, Zhao X, Huang L. Young environment reverses the declined activity of aged rat-derived endothelial progenitor cells: involvement of the phosphatidylinositol 3-kinase/Akt signaling pathway. Ann Vasc Surg. 2009;23:519–534. doi: 10.1016/j.avsg.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 99.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 100.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kelly-Goss MR, Sweat RS, Stapor PC, Peirce SM, Murfee WL. Targeting pericytes for angiogenic therapies. Microcirculation. 2014;21:345–357. doi: 10.1111/micc.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taipaleenmäki H, Abdallah BM, AlDahmash A, Säämänen AM, Kassem M. Wnt signalling mediates the cross-talk between bone marrow derived pre-adipocytic and pre-osteoblastic cell populations. Exp Cell Res. 2011;317:745–756. doi: 10.1016/j.yexcr.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 103.Rajashekhar G, Traktuev DO, Roell WC, Johnstone BH, Merfeld-Clauss S, Van Natta B, Rosen ED, March KL, Clauss M. IFATS collection: Adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: role of canonical Wnt signaling. Stem Cells. 2008;26:2674–2681. doi: 10.1634/stemcells.2008-0277. [DOI] [PubMed] [Google Scholar]
- 104.Ungvari Z, Podlutsky A, Sosnowska D, Tucsek Z, Toth P, Deak F, Gautam T, Csiszar A, Sonntag WE. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci. 2013;68:1443–1457. doi: 10.1093/gerona/glt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Green LA, Njoku V, Mund J, Case J, Yoder M, Murphy MP, Clauss M. Endogenous Transmembrane TNF-Alpha Protects Against Premature Senescence in Endothelial Colony Forming Cells. Circ Res. 2016;118:1512–1524. doi: 10.1161/CIRCRESAHA.116.308332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y, Herbert BS, Rajashekhar G, Ingram DA, Yoder MC, Clauss M, Rehman J. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-alpha via the p38 mitogen-activated protein kinase pathway. FASEB J. 2009;23:1358–1365. doi: 10.1096/fj.08-110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khan AS, Lynch CD, Sane DC, Willingham MC, Sonntag WE. Growth hormone increases regional coronary blood flow and capillary density in aged rats. J Gerontol A Biol Sci Med Sci. 2001;56:B364–B371. doi: 10.1093/gerona/56.8.b364. [DOI] [PubMed] [Google Scholar]
- 108.Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 2016;38:273–289. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 110.Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. Decreased vascular repair and neovascularization with ageing: mechanisms and clinical relevance with an emphasis on hypoxia-inducible factor-1. Curr Mol Med. 2008;8:754–767. doi: 10.2174/156652408786733685. [DOI] [PubMed] [Google Scholar]
- 111.Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51:1119–1130. doi: 10.1177/002215540305100902. [DOI] [PubMed] [Google Scholar]
- 112.Lu C, Miclau T, Hu D, Marcucio RS. Ischemia leads to delayed union during fracture healing: a mouse model. J Orthop Res. 2007;25:51–61. doi: 10.1002/jor.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Potier E, Ferreira E, Andriamanalijaona R, Pujol JP, Oudina K, Logeart-Avramoglou D, Petite H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078–1087. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 114.Yang DC, Yang MH, Tsai CC, Huang TF, Chen YH, Hung SC. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS One. 2011;6:e23965. doi: 10.1371/journal.pone.0023965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leijten JC, Moreira Teixeira LS, Landman EB, van Blitterswijk CA, Karperien M. Hypoxia inhibits hypertrophic differentiation and endochondral ossification in explanted tibiae. PLoS One. 2012;7:e49896. doi: 10.1371/journal.pone.0049896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007;13:1325–1331. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- 117.Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhong L, Huang X, Karperien M, Post JN. The Regulatory Role of Signaling Crosstalk in Hypertrophy of MSCs and Human Articular Chondrocytes. Int J Mol Sci. 2015;16:19225–19247. doi: 10.3390/ijms160819225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khan WS, Adesida AB, Hardingham TE. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther. 2007;9:R55. doi: 10.1186/ar2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jukes JM, Both SK, Leusink A, Sterk LM, van Blitterswijk CA, de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:6840–6845. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 122.Scotti C, Piccinini E, Takizawa H, Todorov A, Bourgine P, Papadimitropoulos A, Barbero A, Manz MG, Martin I. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci U S A. 2013;110:3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, Lopez-Rios J, Zeller R, Barbero A, Martin I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A. 2010;107:7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Farrell E, Both SK, Odörfer KI, Koevoet W, Kops N, O'Brien FJ, Baatenburg de Jong RJ, Verhaar JA, Cuijpers V, Jansen J, Erben RG, van Osch GJ. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord. 2011;12:31. doi: 10.1186/1471-2474-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sheehy EJ, Vinardell T, Buckley CT, Kelly DJ. Engineering osteochondral constructs through spatial regulation of endochondral ossification. Acta Biomater. 2013;9:5484–5492. doi: 10.1016/j.actbio.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 126.Harada N, Watanabe Y, Sato K, Abe S, Yamanaka K, Sakai Y, Kaneko T, Matsushita T. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials. 2014;35:7800–7810. doi: 10.1016/j.biomaterials.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 127.Weiss-Bilka HE, McGann ME, Meagher MJ, Roeder RK, Wagner DR. Ectopic models for endochondral ossification: comparing pellet and alginate bead culture methods. J Tissue Eng Regen Med. 2018;12:e541–e549. doi: 10.1002/term.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dang PN, Herberg S, Varghai D, Riazi H, Varghai D, McMillan A, Awadallah A, Phillips LM, Jeon O, Nguyen MK, Dwivedi N, Yu X, Murphy WL, Alsberg E. Endochondral Ossification in Critical-Sized Bone Defects via Readily Implantable Scaffold-Free Stem Cell Constructs. Stem Cells Transl Med. 2017;6:1644–1659. doi: 10.1002/sctm.16-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Osinga R, Di Maggio N, Todorov A, Allafi N, Barbero A, Laurent F, Schaefer DJ, Martin I, Scherberich A. Generation of a Bone Organ by Human Adipose-Derived Stromal Cells Through Endochondral Ossification. Stem Cells Transl Med. 2016;5:1090–1097. doi: 10.5966/sctm.2015-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weiss HE, Roberts SJ, Schrooten J, Luyten FP. A semi-autonomous model of endochondral ossification for developmental tissue engineering. Tissue Eng Part A. 2012;18:1334–1343. doi: 10.1089/ten.TEA.2011.0602. [DOI] [PubMed] [Google Scholar]
- 131.Matsiko A, Thompson EM, Lloyd-Griffith C, Cunniffe GM, Vinardell T, Gleeson JP, Kelly DJ, O'Brien FJ. An endochondral ossification approach to early stage bone repair: Use of tissue-engineered hypertrophic cartilage constructs as primordial templates for weight-bearing bone repair. J Tissue Eng Regen Med. 2018;12:e2147–e2150. doi: 10.1002/term.2638. [DOI] [PubMed] [Google Scholar]
- 132.Thompson EM, Matsiko A, Kelly DJ, Gleeson JP, O'Brien FJ. An Endochondral Ossification-Based Approach to Bone Repair: Chondrogenically Primed Mesenchymal Stem Cell-Laden Scaffolds Support Greater Repair of Critical-Sized Cranial Defects Than Osteogenically Stimulated Constructs In Vivo. Tissue Eng Part A. 2016;22:556–567. doi: 10.1089/ten.TEA.2015.0457. [DOI] [PubMed] [Google Scholar]
- 133.All Clinical Trials Related to Endothelial Progenitor Cells [Internet] Available from: www.clinicaltrials.gov. [Google Scholar]
- 134.Chandrasekhar KS, Zhou H, Zeng P, Alge D, Li W, Finney BA, Yoder MC, Li J. Blood vessel wall-derived endothelial colony-forming cells enhance fracture repair and bone regeneration. Calcif Tissue Int. 2011;89:347–357. doi: 10.1007/s00223-011-9524-y. [DOI] [PubMed] [Google Scholar]
- 135.Atesok K, Li R, Stewart DJ, Schemitsch EH. Endothelial progenitor cells promote fracture healing in a segmental bone defect model. J Orthop Res. 2010;28:1007–1014. doi: 10.1002/jor.21083. [DOI] [PubMed] [Google Scholar]
- 136.Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, Iwasaki H, Miwa M, Horii M, Hayashi S, Oyamada A, Nishimura H, Murasawa S, Doita M, Kurosaka M, Asahara T. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. 2006;169:1440–1457. doi: 10.2353/ajpath.2006.060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kawakami Y, Ii M, Matsumoto T, Kuroda R, Kuroda T, Kwon SM, Kawamoto A, Akimaru H, Mifune Y, Shoji T, Fukui T, Kurosaka M, Asahara T. SDF-1/CXCR4 axis in Tie2-lineage cells including endothelial progenitor cells contributes to bone fracture healing. J Bone Miner Res. 2015;30:95–105. doi: 10.1002/jbmr.2318. [DOI] [PubMed] [Google Scholar]
- 138.Lee DY, Cho TJ, Kim JA, Lee HR, Yoo WJ, Chung CY, Choi IH. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone. 2008;42:932–941. doi: 10.1016/j.bone.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 139.Li R, Nauth A, Gandhi R, Syed K, Schemitsch EH. BMP-2 mRNA expression after endothelial progenitor cell therapy for fracture healing. J Orthop Trauma. 2014;28 Suppl 1:S24–S27. doi: 10.1097/BOT.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 140.Matsumoto T, Mifune Y, Kawamoto A, Kuroda R, Shoji T, Iwasaki H, Suzuki T, Oyamada A, Horii M, Yokoyama A, Nishimura H, Lee SY, Miwa M, Doita M, Kurosaka M, Asahara T. Fracture induced mobilization and incorporation of bone marrow-derived endothelial progenitor cells for bone healing. J Cell Physiol. 2008;215:234–242. doi: 10.1002/jcp.21309. [DOI] [PubMed] [Google Scholar]
- 141.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res. 2004;67:139–151. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 142.Swiontkowski MF, Aro HT, Donell S, Esterhai JL, Goulet J, Jones A, Kregor PJ, Nordsletten L, Paiement G, Patel A. Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from two prospective randomized studies. J Bone Joint Surg Am. 2006;88:1258–1265. doi: 10.2106/JBJS.E.00499. [DOI] [PubMed] [Google Scholar]
- 143.Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX, Pollak AN, Golden JD, Valentin-Opran A BMP-2 Evaluation in Surgery for Tibial Trauma-Allgraft (BESTT-ALL) Study Group. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1431–1441. doi: 10.2106/JBJS.E.00381. [DOI] [PubMed] [Google Scholar]
- 144.Fiorellini JP, Howell TH, Cochran D, Malmquist J, Lilly LC, Spagnoli D, Toljanic J, Jones A, Nevins M. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005;76:605–613. doi: 10.1902/jop.2005.76.4.605. [DOI] [PubMed] [Google Scholar]
- 145.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31:729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burkus JK, Sandhu HS, Gornet MF, Longley MC. Use of rhBMP-2 in combination with structural cortical allografts: clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am. 2005;87:1205–1212. doi: 10.2106/JBJS.D.02532. [DOI] [PubMed] [Google Scholar]
- 147.Burkus JK, Heim SE, Gornet MF, Zdeblick TA. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16:113–122. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 148.Aspenberg P, Johansson T. Teriparatide improves early callus formation in distal radial fractures. Acta Orthop. 2010;81:234–236. doi: 10.3109/17453671003761946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim SM, Kang KC, Kim JW, Lim SJ, Hahn MH. Current Role and Application of Teriparatide in Fracture Healing of Osteoporotic Patients: A Systematic Review. J Bone Metab. 2017;24:65–73. doi: 10.11005/jbm.2017.24.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aspenberg P, Genant HK, Johansson T, Nino AJ, See K, Krohn K, García-Hernández PA, Recknor CP, Einhorn TA, Dalsky GP, Mitlak BH, Fierlinger A, Lakshmanan MC. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25:404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- 151.Bhandari M, Jin L, See K, Burge R, Gilchrist N, Witvrouw R, Krohn KD, Warner MR, Ahmad QI, Mitlak B. Does Teriparatide Improve Femoral Neck Fracture Healing: Results From A Randomized Placebo-controlled Trial. Clin Orthop Relat Res. 2016;474:1234–1244. doi: 10.1007/s11999-015-4669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peichl P, Holzer LA, Maier R, Holzer G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am. 2011;93:1583–1587. doi: 10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- 153.Kawaguchi H, Oka H, Jingushi S, Izumi T, Fukunaga M, Sato K, Matsushita T, Nakamura K TESK Group. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: A randomized, placebo-controlled trial. J Bone Miner Res. 2010;25:2735–2743. doi: 10.1002/jbmr.146. [DOI] [PubMed] [Google Scholar]
- 154.Kawaguchi H, Nakamura K, Tabata Y, Ikada Y, Aoyama I, Anzai J, Nakamura T, Hiyama Y, Tamura M. Acceleration of fracture healing in nonhuman primates by fibroblast growth factor-2. J Clin Endocrinol Metab. 2001;86:875–880. doi: 10.1210/jcem.86.2.7199. [DOI] [PubMed] [Google Scholar]
- 155.Kitamura M, Akamatsu M, Machigashira M, Hara Y, Sakagami R, Hirofuji T, Hamachi T, Maeda K, Yokota M, Kido J, Nagata T, Kurihara H, Takashiba S, Sibutani T, Fukuda M, Noguchi T, Yamazaki K, Yoshie H, Ioroi K, Arai T, Nakagawa T, Ito K, Oda S, Izumi Y, Ogata Y, Yamada S, Shimauchi H, Kunimatsu K, Kawanami M, Fujii T, Furuichi Y, Furuuchi T, Sasano T, Imai E, Omae M, Yamada S, Watanuki M, Murakami S. FGF-2 stimulates periodontal regeneration: results of a multi-center randomized clinical trial. J Dent Res. 2011;90:35–40. doi: 10.1177/0022034510384616. [DOI] [PubMed] [Google Scholar]
- 156.Chen WJ, Jingushi S, Aoyama I, Anzai J, Hirata G, Tamura M, Iwamoto Y. Effects of FGF-2 on metaphyseal fracture repair in rabbit tibiae. J Bone Miner Metab. 2004;22:303–309. doi: 10.1007/s00774-003-0487-6. [DOI] [PubMed] [Google Scholar]
- 157.Campbell EJ, Campbell GM, Hanley DA. The effect of parathyroid hormone and teriparatide on fracture healing. Expert Opin Biol Ther. 2015;15:119–129. doi: 10.1517/14712598.2015.977249. [DOI] [PubMed] [Google Scholar]
- 158.Babu S, Sandiford NA, Vrahas M. Use of Teriparatide to improve fracture healing: What is the evidence? World J Orthop. 2015;6:457–461. doi: 10.5312/wjo.v6.i6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Miao D, He B, Jiang Y, Kobayashi T, Sorocéanu MA, Zhao J, Su H, Tong X, Amizuka N, Gupta A, Genant HK, Kronenberg HM, Goltzman D, Karaplis AC. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1-34. J Clin Invest. 2005;115:2402–2411. doi: 10.1172/JCI24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Solheim E. Growth factors in bone. Int Orthop. 1998;22:410–416. doi: 10.1007/s002640050290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tyndall WA, Beam HA, Zarro C, O'Connor JP, Lin SS. Decreased platelet derived growth factor expression during fracture healing in diabetic animals. Clin Orthop Relat Res. 2003:319–330. doi: 10.1097/00003086-200303000-00043. [DOI] [PubMed] [Google Scholar]
- 162.Hollinger JO, Onikepe AO, MacKrell J, Einhorn T, Bradica G, Lynch S, Hart CE. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-BB and an injectable beta-tricalcium phosphate/collagen matrix. J Orthop Res. 2008;26:83–90. doi: 10.1002/jor.20453. [DOI] [PubMed] [Google Scholar]
- 163.Weinreb M, Suponitzky I, Keila S. Systemic administration of an anabolic dose of PGE2 in young rats increases the osteogenic capacity of bone marrow. Bone. 1997;20:521–526. doi: 10.1016/s8756-3282(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 164.Jee WS, Mori S, Li XJ, Chan S. Prostaglandin E2 enhances cortical bone mass and activates intracortical bone remodeling in intact and ovariectomized female rats. Bone. 1990;11:253–266. doi: 10.1016/8756-3282(90)90078-d. [DOI] [PubMed] [Google Scholar]
- 165.Xie C, Liang B, Xue M, Lin AS, Loiselle A, Schwarz EM, Guldberg RE, O'Keefe RJ, Zhang X. Rescue of impaired fracture healing in COX-2-/- mice via activation of prostaglandin E2 receptor subtype 4. Am J Pathol. 2009;175:772–785. doi: 10.2353/ajpath.2009.081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tahimic CG, Wang Y, Bikle DD. Anabolic effects of IGF-1 signaling on the skeleton. Front Endocrinol (Lausanne) 2013;4:6. doi: 10.3389/fendo.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tarkka T, Sipola A, Jämsä T, Soini Y, Ylä-Herttuala S, Tuukkanen J, Hautala T. Adenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J Gene Med. 2003;5:560–566. doi: 10.1002/jgm.392. [DOI] [PubMed] [Google Scholar]
- 168.Li R, Stewart DJ, von Schroeder HP, Mackinnon ES, Schemitsch EH. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. J Orthop Res. 2009;27:8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 169.Turk C, Halici M, Guney A, Akgun H, Sahin V, Muhtaroglu S. Promotion of fracture healing by vitamin E in rats. J Int Med Res. 2004;32:507–512. doi: 10.1177/147323000403200508. [DOI] [PubMed] [Google Scholar]
- 170.Durak K, Sonmez G, Sarisozen B, Ozkan S, Kaya M, Ozturk C. Histological assessment of the effect of alpha-tocopherol on fracture healing in rabbits. J Int Med Res. 2003;31:26–30. doi: 10.1177/147323000303100104. [DOI] [PubMed] [Google Scholar]
- 171.Halıcı M, Öner M, Güney A, Canöz Ö, Narin F, Halıcı C. Melatonin promotes fracture healing in the rat model. Eklem Hastalik Cerrahisi. 2010;21:172–177. [PubMed] [Google Scholar]
- 172.Dong P, Gu X, Zhu G, Li M, Ma B, Zi Y. Melatonin Induces Osteoblastic Differentiation of Mesenchymal Stem Cells and Promotes Fracture Healing in a Rat Model of Femoral Fracture via Neuropeptide Y/Neuropeptide Y Receptor Y1 Signaling. Pharmacology. 2018;102:272–280. doi: 10.1159/000492576. [DOI] [PubMed] [Google Scholar]
- 173.Volkmer DL, Sears B, Lauing KL, Nauer RK, Roper PM, Yong S, Stover M, Callaci JJ. Antioxidant therapy attenuates deficient bone fracture repair associated with binge alcohol exposure. J Orthop Trauma. 2011;25:516–521. doi: 10.1097/BOT.0b013e31821f65cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schmitt R. Senotherapy: growing old and staying young? Pflugers Arch. 2017;469:1051–1059. doi: 10.1007/s00424-017-1972-4. [DOI] [PubMed] [Google Scholar]
- 175.Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Demaria M. Senescent cells: New target for an old treatment? Mol Cell Oncol. 2017;4:e1299666. doi: 10.1080/23723556.2017.1299666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Del Pinto R, Ferri C. Inflammation-Accelerated Senescence and the Cardiovascular System: Mechanisms and Perspectives. Int J Mol Sci. 2018:19. doi: 10.3390/ijms19123701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23:1072–1079. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Salminen A, Kaarniranta K, Kauppinen A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int J Mol Sci. 2013;14:3834–3859. doi: 10.3390/ijms14023834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zainabadi K, Liu CJ, Caldwell ALM, Guarente L. SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis. PLoS One. 2017;12:e0185236. doi: 10.1371/journal.pone.0185236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rando TA, Ambrosio F. Regenerative Rehabilitation: Applied Biophysics Meets Stem Cell Therapeutics. Cell Stem Cell. 2018;22:306–309. doi: 10.1016/j.stem.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Thompson WR, Scott A, Loghmani MT, Ward SR, Warden SJ. Understanding Mechanobiology: Physical Therapists as a Force in Mechanotherapy and Musculoskeletal Regenerative Rehabilitation. Phys Ther. 2016;96:560–569. doi: 10.2522/ptj.20150224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rose LF, Wolf EJ, Brindle T, Cernich A, Dean WK, Dearth CL, Grimm M, Kusiak A, Nitkin R, Potter K, Randolph BJ, Wang F, Yamaguchi D. The convergence of regenerative medicine and rehabilitation: federal perspectives. NPJ Regen Med. 2018;3:19. doi: 10.1038/s41536-018-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sarraf CE, Otto WR, Eastwood M. In vitro mesenchymal stem cell differentiation after mechanical stimulation. Cell Prolif. 2011;44:99–108. doi: 10.1111/j.1365-2184.2010.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sittichokechaiwut A, Edwards JH, Scutt AM, Reilly GC. Short bouts of mechanical loading are as effective as dexamethasone at inducing matrix production by human bone marrow mesenchymal stem cell. Eur Cell Mater. 2010;20:45–57. doi: 10.22203/ecm.v020a05. [DOI] [PubMed] [Google Scholar]
- 187.Witt F, Duda GN, Bergmann C, Petersen A. Cyclic mechanical loading enables solute transport and oxygen supply in bone healing: an in vitro investigation. Tissue Eng Part A. 2014;20:486–493. doi: 10.1089/ten.TEA.2012.0678. [DOI] [PubMed] [Google Scholar]
- 188.Boerckel JD, Kolambkar YM, Stevens HY, Lin AS, Dupont KM, Guldberg RE. Effects of in vivo mechanical loading on large bone defect regeneration. J Orthop Res. 2012;30:1067–1075. doi: 10.1002/jor.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang Y, Wang M, Chen H, Li G, Li W, Luo J, Li H. Early Out-of-Bed Functional Exercise Benefits Elderly Patients Following Hip Fracture: A Retrospective Cohort Study. Tohoku J Exp Med. 2018;246:205–212. doi: 10.1620/tjem.246.205. [DOI] [PubMed] [Google Scholar]
- 190.Siu AL, Penrod JD, Boockvar KS, Koval K, Strauss E, Morrison RS. Early ambulation after hip fracture: effects on function and mortality. Arch Intern Med. 2006;166:766–771. doi: 10.1001/archinte.166.7.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen J, Ruan H, Liu Y, Bao J, Xu H, Yao M, Cui X, Liang Q, Wang Y. Therapeutic effects of whole-body vibration on fracture healing in ovariectomized rats: a systematic review and meta-analysis. Menopause. 2018 doi: 10.1097/GME.0000000000001285. [DOI] [PubMed] [Google Scholar]
- 192.Wang J, Leung KS, Chow SK, Cheung WH. The effect of whole body vibration on fracture healing - a systematic review. Eur Cell Mater. 2017;34:108–127. doi: 10.22203/eCM.v034a08. [DOI] [PubMed] [Google Scholar]
- 193.Haffner-Luntzer M, Kovtun A, Lackner I, Mödinger Y, Hacker S, Liedert A, Tuckermann J, Ignatius A. Estrogen receptor α- (ERα), but not ERβ-signaling, is crucially involved in mechanostimulation of bone fracture healing by whole-body vibration. Bone. 2018;110:11–20. doi: 10.1016/j.bone.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 194.Ozcivici E, Luu YK, Rubin CT, Judex S. Low-level vibrations retain bone marrow's osteogenic potential and augment recovery of trabecular bone during reambulation. PLoS One. 2010;5:e11178. doi: 10.1371/journal.pone.0011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Meng J, Hong J, Zhao C, Zhou C, Hu B, Yang Y, Jiang G, Li S, Shi Z, Cai X, Yan S. Low-intensity pulsed ultrasound inhibits RANKL-induced osteoclast formation via modulating ERK-c-Fos-NFATc1 signaling cascades. Am J Transl Res. 2018;10:2901–2910. [PMC free article] [PubMed] [Google Scholar]
- 196.Kusuyama J, Bandow K, Shamoto M, Kakimoto K, Ohnishi T, Matsuguchi T. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem. 2014;289:10330–10344. doi: 10.1074/jbc.M113.546382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Costa V, Carina V, Fontana S, De Luca A, Monteleone F, Pagani S, Sartori M, Setti S, Faldini C, Alessandro R, Fini M, Giavaresi G. Osteogenic commitment and differentiation of human mesenchymal stem cells by low-intensity pulsed ultrasound stimulation. J Cell Physiol. 2018;233:1558–1573. doi: 10.1002/jcp.26058. [DOI] [PubMed] [Google Scholar]
- 198.Rutten S, van den Bekerom MP, Sierevelt IN, Nolte PA. Enhancement of Bone-Healing by Low-Intensity Pulsed Ultrasound: A Systematic Review. JBJS Rev. 2016:4. doi: 10.2106/JBJS.RVW.O.00027. [DOI] [PubMed] [Google Scholar]
- 199.Raza H, Saltaji H, Kaur H, Flores-Mir C, El-Bialy T. Effect of Low-Intensity Pulsed Ultrasound on Distraction Osteogenesis Treatment Time: A Meta-analysis of Randomized Clinical Trials. J Ultrasound Med. 2016;35:349–358. doi: 10.7863/ultra.15.02043. [DOI] [PubMed] [Google Scholar]