Version Changes
Revised. Amendments from Version 1
Some important data from Terasawa’s laboratory on the relationship between neuropeptide Y and GnRH pulse generation were overlooked in the original version of the Review. This omission is rectified in version 2.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
Abstract
This review recounts the origins and development of the concept of the hypothalamic gonadotropin-releasing hormone (GnRH) pulse generator. It starts in the late 1960s when striking rhythmic episodes of luteinizing hormone secretion, as reflected by circulating concentrations of this gonadotropin, were first observed in monkeys and ends in the present day. It is currently an exciting time witnessing the application, primarily to the mouse, of contemporary neurobiological approaches to delineate the mechanisms whereby Kiss1/NKB/Dyn (KNDy) neurons in the arcuate nucleus of the hypothalamus generate and time the pulsatile output of kisspeptin from their terminals in the median eminence that in turn dictates intermittent GnRH release and entry of this decapeptide into the primary plexus of the hypophysial portal circulation. The review concludes with an examination of questions that remain to be addressed.
Keywords: Kisspeptin, neurokinin B, dynorphin, GnRH, pulse generation
Introduction
The intermittent or pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus into the hypophysial portal circulation is obligatory for driving gonadotropin secretion from the anterior pituitary and therefore for gonadal development and maturation, puberty, and fertility in adulthood 1. The intermittent pattern of GnRH release is imposed upon the diffusely distributed hypothalamic GnRH neurons by a neural network termed the GnRH pulse generator. Realization of the existence of such a pulse-generating mechanism in the hypothalamus may be traced to 1970. However, it was not until 2003, following the discovery that loss-of-function mutations of the gene encoding the kisspeptin receptor (KISS1R), a G protein–coupled receptor (GPR, specifically GPR54), were associated with delayed or absent puberty that major advances in our understanding of the neurobiological components of the GnRH pulse generator began to emerge. Moreover, another decade passed before the contemporary view that kisspeptin represents the output of the GnRH pulse generator was proposed. The purpose of this article is to review the signal developments at both the empirical and conceptual levels that have led, over half a century of scientific endeavor, to our present understanding of how hypothalamic GnRH pulses are generated by kisspeptin neurons in the arcuate nucleus.
The early years
In attempting to resolve whether the marked differences in daily concentrations of circulating luteinizing hormone (LH) observed in ovariectomized monkeys were the consequence of physiology or assay error, Knobil’s group in the late 1960s measured LH levels in sequential plasma samples collected every 10 or 20 minutes from such animals 2 I. The group reported a striking sawtooth pattern in plasma LH concentrations. Although unstable levels of circulating LH levels had been revealed by hourly sampling of the castrate male rat 3, it was Knobil’s laboratory that correctly posited that the discrete, roughly hourly episodes of LH secretion that produced the pulses in the circulating gonadotropin were due to intermittent signals from the brain that were relayed to the anterior pituitary by an LH-releasing factor. This was in the year before the structure of the LH-releasing factor (the decapeptide now termed GnRH) was finally reported 4, 5, and it was another 10 years before Clarke and Cummins 6 described the temporal correlation between discharges of GnRH in portal blood and LH pulses in the peripheral circulation, thereby providing empirical confirmation for the hypothesis proposed by Dierschke et al. 2 II.
That the intermittent signal generating GnRH pulses originated within the mediobasal hypothalamus (MBH) was suggested by the finding that, in ovariectomized female rats and monkeys, pulsatile LH secretion was not interrupted by surgical isolation of the MBH 9, 10. Knobil’s group later reported, again in the ovariectomized rhesus monkey, that discrete bilateral radiofrequency lesions of the arcuate nucleus at the base of the third ventricle (also known as infundibular nucleus in humans) abolished LH secretion but that larger lesions of the MBH that spared this nucleus were noticeably less disruptive 11. These findings suggested that the arcuate nucleus, which is located immediately dorsal to the primary plexus of the hypophysial portal circulation, was essential for generating the intermittent GnRH signals driving pulsatile LH release.
Evolution of the concept of the hypothalamic GnRH pulse generator
Although the “black box” concept of a hypothalamic pulse generator or arcuate oscillator driving pulsatile GnRH and LH secretion appeared in the literature in the early 1980s 12, 13, it was not until the recognition that pulsatile LH secretion was tightly correlated with electrical activity in the MBH recorded with multiunit electrodes (multiunit activity, MUA) ( Figure 1) 14– 16 III that the concept of the hypothalamic GnRH pulse generator became a cornerstone of the control system governing the reproductive axis. One school of thought argued that pulsatility was intrinsic to the GnRH neurons themselves whereas another school proposed that this mode of secretion was imposed on the network of GnRH neurons by non-GnRH cells in the MBH 17. Evidence for the former idea was provided by the finding that immortalized GnRH neurons in culture produced a discontinuous pattern of release of GnRH into the media 18 reminiscent of the pulsatile mode of GnRH secretion in vivo. However, the neurobiological mechanisms that underpin GnRH release in vitro are not operative in vivo 17. Compelling support for extrinsic pulse generation was provided by two studies conducted in the 1990s. Ohkura et al. 19 reported that the acute suppression of LH pulsatility, which was produced in ovariectomized rats following a surgical 180° hypothalamic deafferentation and which interrupted anterior inputs (including those from GnRH cell bodies) to the arcuate nucleus at its rostral aspect, was prevented by prior transplantation at the base of the third ventricle of fetal MBH devoid of GnRH neurons. Purnelle et al. 20 subsequently demonstrated that retrochiasmatic hypothalamic explants from the rat that contained the arcuate nucleus and GnRH fiber projections to the median eminence, but not GnRH cell bodies, exhibited pulsatile GnRH release in culture.
Figure 1. Pulsatile luteinizing hormone secretion is tightly correlated with electrophysiological activity in the monkey mediobasal hypothalamus.
The relationship between luteinizing hormone (LH) “pulses”, observed in the peripheral circulation (open data points), and multiunit activity (MUA) (continuous trace) recorded in the mediobasal hypothalamus of two ovariectomized rhesus monkeys before (Panel A) and after (Panel B) anesthesia was induced with thiopental. Note the high-fidelity relationship between the two parameters regardless of frequency of pulsatile LH secretion. Reproduced from Wilson et al. 16 with permission of Karger Publishers. Copyright © (1984) Karger Publishers, Basel, Switzerland.
The first real insight into this black box came as a result of the 2003 discovery that humans and mice with loss-of-function mutations of KISS1R were hypogonadotropic and had delayed or absent puberty 24, 25. Moreover, in addition to the very low circulating LH levels associated with the mutated gene, secretion of LH in human subjects bearing the mutation could be induced by intermittent exogenous GnRH administration 25, suggesting that the impact of the mutation was manifest at a supra-pituitary level. Shortly thereafter, expression of KISS1R by GnRH neurons was identified in mouse by in situ hybridization 26, 27, and kisspeptin, the endogenous ligand of KISS1R, was demonstrated to elicit (1) an increase of GnRH levels in the cerebrospinal fluid of sheep 28; (2) tetrodotoxin-independent depolarization of green fluorescent protein (GFP)-expressing GnRH neurons in perfused slices of hypothalamus from transgenic mice 27 and release of GnRH from mouse MBH explants in culture 29, suggesting a major site of kisspeptin action at the level of both the GnRH perikaryon and nerve terminal in the median eminence; and (3) robust and GnRH-dependent increases of circulating LH concentrations in both rodent and primate species 26, 30, 31. Moreover, repetitive hourly intravenous (IV) administration of kisspeptin for 48 hours in the juvenile male monkey, in which kisspeptin content of the arcuate nucleus/median eminence is low and hypothalamic GnRH release is profoundly restrained, evokes—when the pituitary is first primed with pulsatile GnRH—a sustained train of GnRH-dependent LH pulses that mimics that observed spontaneously in adult animals with unrestrained GnRH release ( Figure 2) 32. Intermittent release of kisspeptin in the region of the arcuate nucleus and median eminence of the ovariectomized monkey was observed and about 75% of these events were associated with, or immediately preceded by, a GnRH discharge 33, whereas administration of a KISS1R antagonist directly to this hypothalamic area of the monkey resulted in suppression of GnRH release 34. Not surprisingly, mice null for Kiss1 exhibited a phenotype similar to that of the receptor knockout, and the LH-releasing action of kisspeptin was preserved in these transgenic animals 35.
Figure 2. Intermittent kisspeptin administration drives a corresponding pattern of GnRH dependent luteinizing hormone discharges in monkey.
Intermittent intravenous administration of kisspeptin-10 (2 μg/min for 1 min/hour starting at 11 a.m. on day 1 and continuing for 48 hours, closed data points) induces a sustained train of luteinizing hormone (LH) “pulses” in a naturally GnRH-deficient primate model (juvenile male rhesus monkey) that matches that generated by an antecedent intermittent GnRH infusion, also administered at 1 pulse/hour before kisspeptin administration (9 to 11 a.m., day 1). The LH response to kisspeptin was abolished by prior treatment with a GnRH receptor antagonist (not shown). Results for vehicle are shown in the open data points. Although kisspeptin-10 or vehicle was administered every hour for 48 hours, LH responses were tracked for only two or three pulses per day. Black arrows indicate times of pulse infusions of kisspeptin-10 or vehicle that were selected for monitoring the LH response. White arrows indicate time of GnRH pulse infusions. Values are presented as mean ± standard error. GnRH, gonadotropin-releasing hormone. Reproduced from Plant et al. 32 with permission of the Endocrine Society.
Taken together, the above findings led to the notions that the GnRH pulse generator was extrinsic to the GnRH neuron and that kisspeptin was likely a key component of the black box. These ideas were reinforced by several later findings. Kisspeptin was shown to induce GnRH release into the hypophysial portal circulation in sheep 36, 37, and transgenic approaches demonstrated that LH pulsatility was absent in Kiss1R-null mice 38 and Kiss1-null rats 39. Most recently, LH pulsatility was restored in the former animal model by selective rescue of expression of the kisspeptin receptor in GnRH neurons 40.
Kisspeptin neurons in the arcuate nucleus provide the output of the hypothalamic GnRH pulse generator
Attention to the arcuate nucleus as the likely site of the hypothalamic GnRH pulse generator was reignited when it was demonstrated that in several species the major site of Kiss1/ KISS1-expressing and kisspeptin-containing neurons within the MBH resided in this nucleus 29, 30, 41– 44. Indeed, the lesions earlier placed in the arcuate nucleus of the monkey that produced a profound decline in gonadotropin secretion 11 would have destroyed most of the kisspeptin perikarya.
Kisspeptin neurons in the arcuate nucleus may be distinguished from other kisspeptin neurons in the hypothalamus by the co-expression of neurokinin B and the neurokinin B receptor (TAC3R) 45, 46 and this feature of the arcuate cells has been exploited in studies of their function. Specifically, selective destruction of arcuate kisspeptin neurons using TAC3R-targeted uptake of saporin, a ribosome-inactivating toxin, resulted in a profound reduction in LH secretion in the ovariectomized rat 47, thus mimicking the effects of earlier indiscriminate neuronal destruction in the arcuate nucleus of the monkey. In addition, many kisspeptin axons and terminals in the median eminence contain neurokinin B 48– 50, indicating the arcuate nucleus as the origin of these projections, a finding confirmed by conditional viral tract-tracing studies in the mouse 51. Kisspeptin fibers and terminals are found throughout the internal zone of the median eminence 52, where they intermingle in an intimate fashion with GnRH projections ( Figure 3) 44, and kisspeptin-induced calcium currents in GnRH terminals and projections in and around the median eminence were recently described ( Figure 4) 53. Interestingly, studies of GnRH projections to the median eminence in the mouse indicate that they exhibit properties characteristic of both axons and dendrites, and the term dendron was coined by Herbison’s group to describe these processes 54 IV. Such functional morphology would facilitate control of GnRH release by kisspeptin (and other neuropeptides) at the level of the median eminence. It should be noted that GnRH perikarya in the MBH in the ewe also appear to receive synaptic input from arcuate kisspeptin neurons 55, and the fos proto-oncogene protein (FOS, a marker of cell activation) was induced in these GnRH neurons in association with stimulation of LH pulses that followed naloxone administration 56.
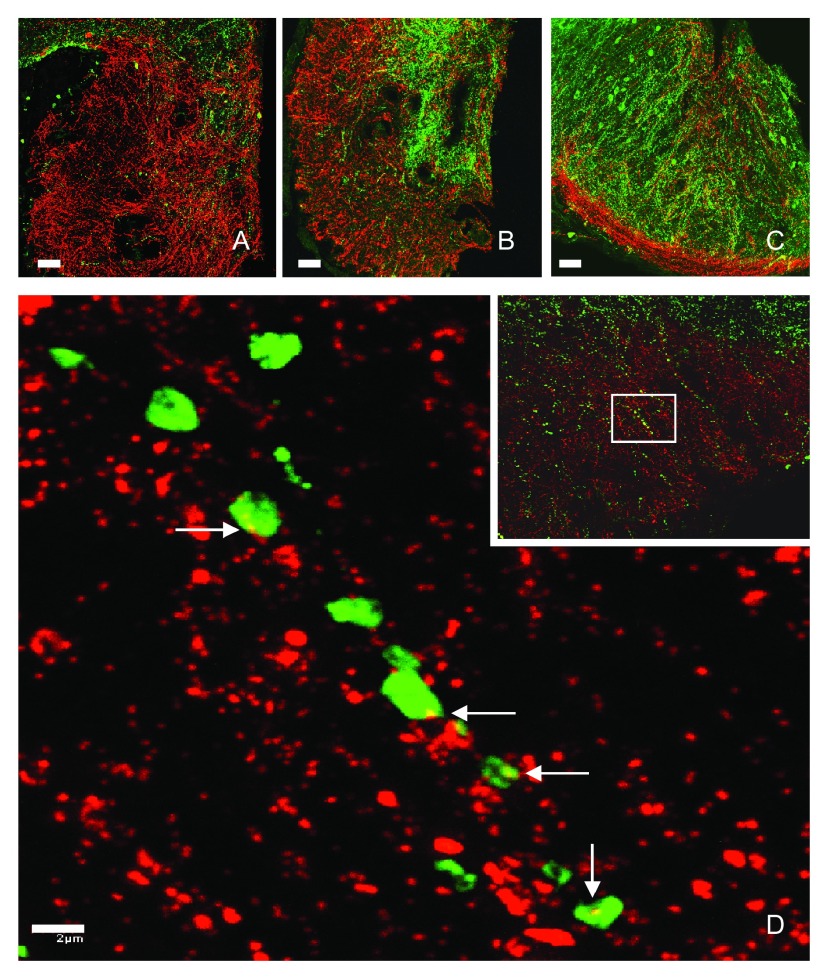
Figure 3. Kisspeptin projections to the median eminence intermingle intimately with GnRH fibers in the monkey.
Interactions between kisspeptin (green fluorescence) and GnRH (red fluorescence) fibers in hemi-coronal sections at the anterior ( A), mid-tuberal ( B), and posterior ( C) aspects of the median eminence of a castrated adult male rhesus monkey. Note, in panel B, the heavy kisspeptin innervation of the internal zone of the median eminence and GnRH innervation of both the internal and external zones. Panel D shows high-power magnification of kisspeptin axonal beads in contact with GnRH fibers (white arrows) in the external zone of the median eminence shown in the inset. GnRH, gonadotropin-releasing hormone. Reproduced from Ramaswamy et al. 44 with permission of the Endocrine Society.
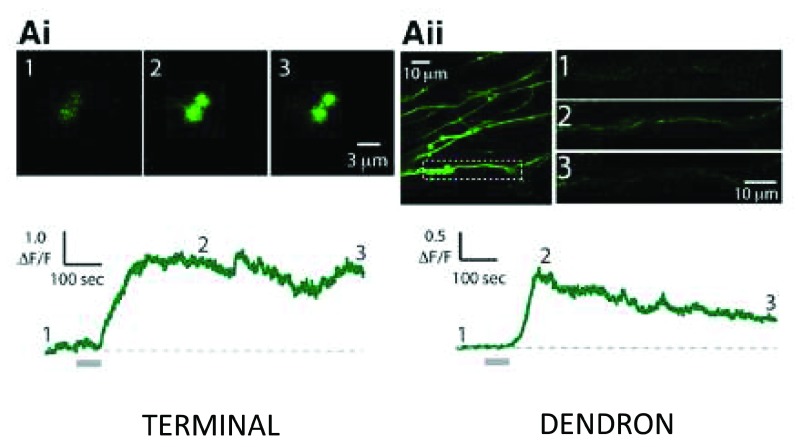
Figure 4. Kisspeptin elicits Ca ++ increases in GnRH dendrons and terminals in the mouse median eminence.
One-minute discharges (“puffs”) of kisspeptin from a pipette locally applied to GnRH nerve terminals and dendrons in hypothalamic slices elicit increases in intracellular Ca ++ (the trigger for GnRH release) monitored by the Ca ++ indicator, GCaMP6 (green fluorescence), expressed in GnRH neurons of transgenic mice. Lower traces, Ca ++ responses to a kisspeptin puff (horizontal gray bar) on a GnRH terminal ( Ai) or dendron ( Aii). The fluorescent signals observed at times 1, 2, and 3 on the Ca ++ traces are shown above. ΔF/F, relative increase in fluorescence over baseline; GnRH, gonadotropin-releasing hormone. Reproduced from Iremonger et al. 53 with permission of the Society for Neuroscience.
Definitive evidence that kisspeptin neurons provide the output of the hypothalamic GnRH pulse generator was recently provided by Herbison’s group 57. Using transgenic mice in which a Ca ++ reporter was targeted to arcuate kisspeptin neurons, these investigators demonstrated in vivo that abrupt Ca ++ signals in these neurons, like the volleys of MUA recorded earlier in the MBH, were temporally correlated with high fidelity to pulses of LH secretion 57 ( Figure 5). Moreover, optogenetic activation of arcuate kisspeptin neurons in vivo for periods equivalent to the duration of the endogenous Ca ++ events elicited LH pulses comparable to those observed spontaneously, whereas optogenetic silencing resulted in a decrease in the frequency and amplitude of LH pulsatility 57. Termination of a brief period of optogenetic silencing resulted in an immediate discharge of LH, followed in all mice examined by a spontaneous LH pulse after a “time-locked” interval, indicating that the Kiss1/NKB/Dyn (KNDy) neurons are the pacemaker of the GnRH pulse generator rather than the relay of an upstream timing signal.
Figure 5. Electrophysiological activity in KNDy neurons is tightly correlated with discharges of luteinizing hormone in mouse.
High-fidelity correlation between electrophysiological activity (reflected by intracellular Ca ++) of kisspeptin (KNDy) neurons in the arcuate nucleus of gonadectomized male mice monitored in vivo using an optogenetic approach (black trace) and the pulsatile secretion of luteinizing hormone (LH) (red) as tracked by measuring plasma concentrations of the gonadotropin. Reproduced from Clarkson et al. 57 with permission from the National Academy of Sciences.
Mechanism timing pulsatile arcuate kisspeptin release in the median eminence
In 2006, two groups found that neurokinin B axons and nerve terminals in the arcuate nucleus of rat co-localized dynorphin and abutted cell bodies that also co-expressed these two neuropeptides 58, 59. At the time, dynorphin and neurokinin B were viewed as inhibitory and stimulatory, respectively, to LH secretion. The group of Rance 58 also reported that arcuate dynorphin neurons expressed TAC3R and proposed that autofeedback within this population of neurons likely provides a mechanism to synchronize their activity 58. Three years later, Topaloglu et al. 60 reported that loss-of-function mutations of TAC3R or TAC3, the gene encoding neurokinin B, were associated with hypogonadotropic hypogonadism and delayed or absent puberty in humans; this phenotype is very similar to that reported for inactivating mutations of KISS1R six years earlier 24, 25. Excitement surrounding the finding by Topaloglu et al. was amplified by an earlier report that neurokinin B in sheep was expressed in the same arcuate neurons as those that synthesize kisspeptin 46 because this immediately led to the realization that two neuropeptides expressed by the same hypothalamic cells were both required for puberty and presumably therefore for pulsatile GnRH release 61. Because double-label immunohistochemistry indicated that most kisspeptin neurons in the arcuate nucleus of the ewe expressed both neurokinin B and dynorphin 46, it became apparent that the arcuate kisspeptin neurons and the neurokinin B/dynorphin neurons described earlier were one of a kind and these neurons were later assigned the acronym, KNDy 62.
The idea of autofeedback within this population of arcuate neurons was further pursued by Steiner’s group 45. Employing in situ hybridization, they showed that, in mouse, 20% of arcuate Kiss1 neurons also express Kor, the gene encoding the kappa opioid receptor (KOR, a dynorphin receptor) V, and proposed an early KNDy model of GnRH pulse generation 45. In essence, this model posited that KNDy in the arcuate nucleus possess an intrinsic tendency to activate. Activation was proposed to trigger synaptic release of neurokinin B within the nucleus, which in turn would amplify and synchronize firing by the network, resulting in the output of kisspeptin in the median eminence; this view is consistent with later findings that the action of neurokinin B to elicit GnRH-dependent LH discharges in the monkey lies upstream of KISS1R 63 and that intracerebro-ventricular (ICV) administration of TAC3R agonists in the rat and ewe induced the expression of FOS in KNDy neurons 64, 65. Dynorphin was also posited to be concomitantly released, and with a small phase lag this neuropeptide was proposed to terminate the activity of the network. Another episode of synchronized activity in the KNDy network would be triggered following the waning of the inhibitory activity of dynorphin.
Evidence that kisspeptin itself is involved in generating synchronized activity within the KNDy network is largely negative. It is now generally recognized that KNDy neurons do not express Kiss1R/KISS1R 67, and in studies of hypothalamic slices from mouse, the electrical activity of arcuate kisspeptin (KNDy) neurons expressing GFP was unaffected by kisspeptin 68. Similarly, kisspeptin-induced LH release in the rat and goat occurred without eliciting volleys of MUA in the arcuate nucleus 69, 70, which are now recognized to be generated by KNDy neurons (see below).
On the other hand, intra-arcuate injection of a KISS1R antagonist resulted in a modest slowing of LH pulse frequency in sheep and rat 71, 72 whereas that of kisspeptin induced an LH discharge in the rat. However, the possibility that the site of action of the KISS1R ligands administered directly to the arcuate nucleus is exerted at the level of the GnRH dendron has been raised 40. It will be important to clarify this uncertainty as the LH response to intra-arcuate–administered KISS1R antagonist in the absence of KISS1R expression by KNDy neurons led Goodman et al. 73 to incorporate a non-KNDy neuronal phenotype into the KNDy model of GnRH pulse generation.
An argument for a role of kisspeptin in determining the frequency of the GnRH pulse generator has also been proposed on the basis of findings that continuous infusions or a subcutaneous bolus injection of kisspeptin to healthy men and women and to women with hypothalamic amenorrhea resulted in an increase (not always statistically significant) in the number of LH discharges 74– 79. When evaluating these data, it should be noted that when LH levels are low, as in young gonad-intact adults, identification of LH pulses (and therefore their precision in reflecting GnRH pulse frequency) may be less reliable than in the castrate or agonadal situation where LH secretion is elevated and discharges of the gonadotropin are generally robust. Therefore, the possibility that exogenous kisspeptin provided in these clinical studies has in some cases increased the responsivity of the GnRH neuronal network to endogenous kisspeptin pulses should be considered.
The possibility that glia also comprise a component of the KNDy model of pulse generation has been proposed as a result of the in vitro finding that cultured GFP-expressing kisspeptin cells and neighboring glia isolated from fetal mouse hypothalamus exhibit synchronized Ca ++ oscillations in response to the neurokinin B agonist, Senktide 80.
The recent acceptance that the MUA recorded in the vicinity of the arcuate nucleus is generated by KNDy neurons 40, thereby confirming the view long held by some that MUA is indeed an electrophysiological property of the GnRH pulse generator, adds considerable importance to earlier goat studies that had monitored this activity while pharmacologically probing signaling by KNDy peptides within the arcuate network of KNDy neurons. Specifically, ICV injection of dynorphin to ovariectomized animals interrupted spontaneous volleys of MUA whereas administration of a KOR antagonist (nor-binaltorphimine, or nor-BNI) resulted in a marked acceleration in this electrophysiological parameter and an increase in the duration of the volley 81, 82 VI. On the other hand, neurokinin B evoked an immediate MUA volley or train of such volleys. It is to be noted that, in the ewe, local pharmacological manipulation of KNDy signaling within the arcuate nucleus modulated pulsatile LH release in a manner generally consistent with that observed when MUA was used to monitor the GnRH pulse generator in the goat 72, 82. Additionally, neurokinin B and dynorphin increased and decreased, respectively, the firing of arcuate kisspeptin (KNDy) neurons in studies of hypothalamic slices in mice 68. Finally, ICV or IV administration of TAC3R antagonists (MRK-08 or ESN 364, respectively) in the ovariectomized ewe resulted in the interruption of pulsatile LH secretion 82, 83, and various parameters of LH secretion were suppressed by chronic oral administration of ESN 364 to castrate male monkeys, or MLE4901 (another TAC3R antagonist), to men and women 84– 86.
Data on the action of endogenously released arcuate neurokinin B and dynorphin have recently become available and are consistent with those derived from earlier classic pharmacological approaches. An elegant electrophysiological study by Kelly and Rønnekleiv et al. 87, using brain slices from transgenic mice, demonstrated that photostimulation of arcuate KNDy neurons for 10 seconds resulted, in these neurons, in the development of a slow excitatory post-synaptic potential (EPSP) with a time course of several minutes. The slow EPSP was blocked by a TACR3 antagonist, indicating that the potential is driven by neurokinin B released from KNDy cells, and presumably underlies the Ca ++ currents and volleys of MUA that result in the discharge of kisspeptin from KNDy terminals in the median eminence. Optogenetic activation of KNDy neurons also elicited dynorphin release as indicated by the finding that concomitant administration of a KOR antagonist potentiated the slow EPSP; the action of dynorphin was argued to be pre-synaptic because the response to an NKB agonist was not suppressed 87. Weems et al. 88 have also argued that dynorphin is released in the arcuate nucleus of the sheep during the generation of a GnRH pulse. Their conclusion is based on an increase in immunofluorescent-identified KOR internalization in the cell body of KNDy neurons in association with a GnRH pulse induced by ICV administration of neurokinin B. This finding would suggest a post-synaptic action of dynorphin in contrast to the pre-synaptic action earlier proposed in mice 87.
As the foregoing data strongly implicate a role for dynorphin in terminating the pulse of activity in KNDy neurons, the findings that, in the rat, intra-arcuate or ICV administration of nor-BNI influenced neither MUA recorded in this nucleus nor pulsatile LH secretion 90, 91 require an explanation. In this regard, Goodman et al. 82 suggested that there may be either a redundancy in opioid signaling underlying pulse termination in the rat or an alternative mechanism in this species.
The arcuate nucleus is a bilateral structure at the base of the third ventricle, and evidence for communication between KNDy neurons on either side of the MBH, and by inference synchronization of activity, was initially provided in the rat by the finding that anterograde-labelled neurokinin B neurons on one side of the hypothalamus projected to the contralateral arcuate nucleus. In one animal close apposition between labeled neurokinin B fibers and contralateral neurokinin B perikarya was observed 92. The latter observation was confirmed and extended in a goat study, which also directly demonstrated synchronization of MUA monitored simultaneously in the arcuate nucleus on either side of the third ventricle 93. More recently, electrophysiological studies have demonstrated that photostimulation of kisspeptin fibers from cell bodies on one side of the arcuate nucleus evokes in kisspeptin neurons in the contralateral nucleus the same neurokinin B–dependent small EPSPs described above 87. A simple schematic of the KNDy model for GnRH pulse generation is shown in Figure 6.
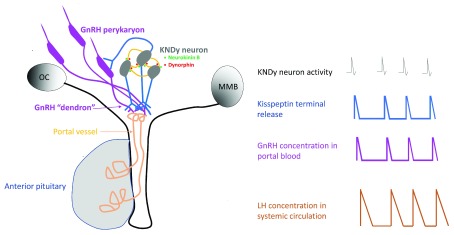
Figure 6. Simple schematic of the KNDy neuron model of the GnRH pulse generator.
Small green and red filled circles indicate neurokinin B and dynorphin auto-synaptic release, respectively, within the arcuate nucleus. The temporal dynamics of the release of these two neuropeptides at this location are unknown. GnRH, gonadotropin-releasing hormone; MMB, mammillary body; OC, optic chiasm.
Questions that remain to be addressed
Are the three KNDy peptides the sole interneuron signals used by the arcuate GnRH pulse generator?
Arcuate kisspeptin neurons likely release neurally active substances in addition to the KNDy peptides 40 and therefore it is reasonable to ask the question: do these contribute to GnRH pulse generation? One neurotransmitter of particular interest is glutamate. This is because (1) cell bodies and terminals of KNDy neurons are positive for the vesicular glutamate transporter type 2 (VGLUT2) or the mRNA encoding the protein 55, 94, 95, and glutamate release by arcuate KNDy neurons has been demonstrated in the mouse 87; (2) VGLUT2-positive terminals are in close contact with KNDy perikarya in the sheep 55, 96; (3) glutamate and the glutamate analog, N-methyl D-aspartate (NMDA), induced burst firing in arcuate kisspeptin neurons in vitro 97; (4) DL-2-amino-5-phosphonopentanoic acid, an antagonist of the ionotropic glutamate receptor known as the NMDA receptor, interrupted LH pulsatility in the castrate male rat 98; and (5) repetitive IV administration of NMDA, an agonist of the cognate receptor, closely mimics the GnRH-dependent LH-releasing action of intermittent kisspeptin stimulation in the juvenile male monkey model described above 99. The findings that peripheral administration of NMDA failed to induce LH release in mice null for Kiss1 and Kiss1R 100 suggest that the GnRH-releasing action of NMDA may be mediated by arcuate KNDy neurons. In this regard, it is interesting to note that expression of c-FOS was reported in glutamate neurons in the arcuate nucleus of the ewe during a spontaneous GnRH pulse 96. However, the notion that glutamate is involved in the GnRH pulse generator is contradicted by the finding that in the ewe an intra-arcuate injection of MK801, also an NMDA receptor antagonist, failed to influence LH pulse frequency or amplitude 72. Clearly, the role of glutamate in GnRH pulse generation demands further exploration.
Before the advent of the kisspeptinocentric era of reproductive neuroendocrinology, the catecholamine, norepinephrine, was recognized as an important factor in the regulation of LH secretion 101. Nearly half a century ago, Knobil’s laboratory reported that IV administration of the alpha-adrenergic blockers, phentolamine or phenoxybenzamine, resulted in a profound interruption of pulsatile LH secretion in the ovariectomized monkey 102. This effect could not be attributed to a systemic hypotension or to an action of the blockers to inhibit GnRH responsiveness at the pituitary level 103. More recently, an action on the KNDy GnRH pulse generator was confirmed by the finding that volleys of MUA in the vicinity of the arcuate nucleus were arrested or retarded following administration of phentolamine in the ovariectomized monkey and rat 89, 104. These striking effects of alpha-adrenergic receptor blockers on GnRH pulse generator activity remain difficult to reconcile with the finding that surgical isolation of the MBH does not interrupt GnRH pulse generation over the long term (see above). This is because the major source of hypothalamic adrenergic tone is provided by projections from the brainstem 101 and therefore isolation of the MBH would be expected to greatly reduce any adrenergic input to this region of the hypothalamus and therefore mimic the effect of alpha blockers on LH secretion. As described above, this is not the case. One explanation for this paradox is that the impact of a loss in adrenergic input to the KNDy network, effected (for example) by surgical isolation of the MBH, is acute and that after an ill-defined time the KNDy network, because of plasticity posited to characterize the neural regulation of GnRH release 101, re-acquires the ability to generate kisspeptin pulses in the absence of a normal adrenergic input. Such a scenario would be easy to test empirically. With the extant data, however, it seems reasonable to propose that although adrenergic input may be viewed as permissive for pulse generation, it appears over the short term to be obligatory rather than modulatory and therefore examination of the neurobiology underlying the adrenergic influence on the KNDy network is of considerable importance.
Somewhat later in the pre-kisspeptin era, the laboratory of Terasawa studying gonadectomized adult rhesus monkeys described striking intermittent increments in neuropeptide Y (NPY) content in sequential perfusates of stalk median eminence (S-ME) that were concomitantly tightly correlated with discharges of GnRH 105. Moreover, local delivery of an NPY antibody into the S-ME resulted in a reduction in the overall content of NPY suggesting that signaling by this neuropeptide was in part responsible for the pulsatile release of GnRH. Although these findings in the monkey are unambiguous, they have been largely overlooked; perhaps because 1) mice null for NPY are fertile 106, and 2) the ever increasing importance that has been assigned to kisspeptin in GnRH pulse generation since 2003. Recently, however, the relationship between NPY and GnRH release described in the 1990s in the S-ME of the monkey, has been resurrected and correctly identified by Terasawa 107 as presenting an “unresolved question” that should be addressed when further evaluating the KNDy model for GnRH pulse generation. The presumption that NPY neurons in the arcuate neurons 108, 109 are the principal source of NPY in the S-ME is reasonable, but multiple mechanisms may be posited to underlie the synchrony reported for NPY and GnRH pulses in the monkey. Clearly it would be of considerable interest to determine whether this phenomenon is found in the mouse and, if so, to explore with contemporary neurobiological approaches its relationship to KNDy neuron activity.
What is the peptide makeup of KNDy neuron terminals and what is the temporal pattern of their release from these sites?
Because KNDy neurons synthesize at least three peptides, one question that needs to be addressed is the terminal composition of their peptides and the dynamics of release both within the arcuate nucleus and at the level of GnRH processes in the median eminence. As discussed by Herbison 40, the dogma is that a particular neuronal phenotype releases the same set of transmitters from all of their synapses (Dale’s principal). Whether this applies to the KNDy neurons is not known. It appears unlikely that kisspeptin, if released within the arcuate nucleus along with neurokinin B and dynorphin, has a critical action on KNDy neurons (see above). On the other hand, at the level of the median eminence, dynorphin (if released) may well modulate the action of kisspeptin at the GnRH terminal, as GnRH neurons have been reported to express KOR in some species 67. In this regard, dynorphin release from KNDy nerve terminals onto GnRH cell bodies in the MBH of sheep was recently proposed to play a role in GnRH pulse termination in this species 88. The situation with TAC3R is less clear. Although this receptor has not been consistently detected in GnRH soma 67, GnRH processes in the median eminence of the rat co-express TAC3R 110, and in a study employing hypothalamic slices from mice, application to the bath of Senktide, a TAC3R agonist 111, elicited GnRH release from terminals in the median eminence. Therefore, neurokinin B, if released from KNDy terminals in the median eminence, is likely to modulate GnRH release. Neurokinin B is a member of the tachykinin family of peptides that include substance P and neurokinin A, and the issue of redundancy in neurokinin signaling in GnRH pulse generation, particularly in rodents, is of emerging interest. This subject was recently reviewed by Lehman and Goodman et al. 72 and will not be discussed here.
Although there is evidence from ultrastructure studies of distinct neurosecretory granules for each of the KNDy peptides 112, essentially nothing is known regarding the trafficking of the KNDy peptides from their site of synthesis in cell bodies in the arcuate nucleus to terminals within this nucleus and those in the median eminence.
Is the KNDy model of GnRH pulse generation applicable to both sexes and all mammalian species?
Another question is whether the KNDy model of GnRH pulse generation may be applied universally across all mammalian species, and most importantly to humans. The cornerstone of the view that this may not be the case stems from the finding that only a small proportion of kisspeptin neurons in the infundibular (arcuate) nucleus of young men and post-menopausal women are immunopositive for dynorphin 113, 114. However, alternative splicing and processing of prodynorphin transcripts in the human genome are relatively complex 115, and detection of the encoded peptides by available antibodies may be limited. Consistent with this possibility is the finding that expression patterns of the gene that encodes dynorphin (also known as PDYN) in humans do not parallel those of the immunoactive protein. With in situ hybridization, the prodynorphin (PDYN) mRNA was detected in a large number of neurons in the infundibular nucleus in both pre- and post-menopausal human brains 116, 117. Moreover, the number of infundibular neurons expressing this message was smaller in the post-menopausal brain where the transcript was found in hypertrophied cell bodies: a pattern and location of expression suggesting that the PDYN mRNA-expressing neurons are indeed KNDy neurons. Thus, at present, it would appear inappropriate to reject the KNDy model of GnRH pulse generation as fundamentally applicable to all mammalian species.
Although most laboratories have employed female animals to study the KNDy model of GnRH pulse generation, available data suggest that the organizational or programming action of perinatal gonadal steroids on the arcuate population of kisspeptin neurons is less marked than that on the more anterior population of these cells in the preoptic area 62, 118. Therefore, it seems reasonable to propose that the neurobiological mechanisms underlying the operation of the GnRH pulse generator in the male, the electrophysiological activity (Ca ++ currents) of which was recently described in the male mouse 119, are not fundamentally different from those in the female. The somewhat sensational finding that GnRH pulse generation by a male monkey was capable of driving folliculogenesis, ovulation, and corpus luteum function in ovarian tissue transplanted subcutaneously 120 may reflect an absence of major sex differences in primate GnRH pulse generation.
Can GnRH pulsatility be maintained in the absence of an intermittent kisspeptin input to the GnRH network?
An initial report that stimulated interest in this possibility was provided by Mayer and Boehm 121, who ablated kisspeptin neurons during early development by generating mice which expressed diphtheria A toxin in cells expressing the Kiss1 gene. When the hypothalamus of these animals was examined on postnatal day 15, immunoactive kisspeptin neurons in the arcuate nucleus were absent. Remarkably, such female mice progressed through puberty and became fertile adults; this finding led to what might be viewed as a heretical conclusion (in light of the evidence presented above) that KNDy neurons are not essential to GnRH pulse generation. On the other hand, when kisspeptin neurons were ablated post-pubertally, ovarian cyclicity was profoundly interrupted, as would have been predicted from the KNDy model for GnRH pulse generation. The authors hypothesized that, during early development of the hypothalamus, a neuronal circuit devoid of kisspeptin neurons is formed that compensates for the ablation of KNDy neurons and drives GnRH pulsatility at and after puberty. However, there is an alternative explanation, namely that the plasticity induced by kisspeptin neuron ablation lies distal to the KNDy neuron (that is, in the GnRH neuron–pituitary–ovarian axis). This may take one of two forms. First, GnRH neurons in the absence of kisspeptin neurons might develop the intrinsic ability to generate GnRH pulses; this is not unreasonable in view of studies of GnRH neurons in culture 17. Second, the GnRH neuron–pituitary–ovarian axis is able to support cyclicity in the absence of pulsatility. Both of these possibilities are compatible with the concomitant finding that ablation of KISS1R cells during early development did not prevent puberty and subsequent fertility. This is because a small number of GnRH neurons were unaffected by the latter genetic strategy, and it has been recognized for many years that only a small number of GnRH neurons are required to drive the pituitary–ovarian axis 17. An insight into these two lines of speculation might be gained by examining moment-to-moment changes in LH concentrations in the transgenic animals of Mayer and Boehm.
Two other approaches aimed at generating an experimental model in which kisspeptin pulsatility is absent or interrupted have been employed. In the first, human subjects bearing loss-of-function mutations in either TAC3 or TAC3R and therefore lacking the neurokinin B signal posited by the KNDy model to trigger pulses of kisspeptin were studied 122. In such individuals, continuous IV administration of kisspeptin for 12 hours resulted in a marked increase in the number of LH pulses detected over the duration of the infusion. In the second, Clarke et al. 123, employing the ovariectomized ewe, administered a neurokinin B antagonist ICV with the aim of abolishing the intermittent kisspeptin output of the pulse generator. As was to be expected, LH pulsatility was interrupted but a continuous IV infusion of kisspeptin during the last 3 to 4 hours of neurokinin B antagonist administration precisely restored the pattern of LH pulsatility to that observed in the control period. If the pulsatile kisspeptin output to the GnRH system was indeed silenced in the foregoing models, it must be argued that a background of continuous kisspeptin signaling allows the GnRH network to pulse at a frequency identical to that which is normally dictated by pulses of kisspeptin. Although this possibility does not detract from the fundamental significance of the KNDy neurons in driving pulsatile GnRH secretion under physiological conditions, it is an unexpected result that merits confirmation and then further study because of its potential relevance to therapeutic approaches used in the infertility clinic 123. Additional studies that examine the action of continuous kisspeptin administration in models that employ a different approach to abrogating the intermittent kisspeptin output of the pulse generator (for example, KNDy neuron ablation or silencing) are likely to be particularly helpful. In this regard, in the anestrous ewe, pulsatile GnRH release is greatly repressed by seasonal cues, but Caraty et al. 37 were unable to induce pulsatile LH secretion in such a model by a 4-hour infusion of kisspeptin. Although there are differences between the ovine models used by the groups of Clarke 123 and Caraty 37, a potential explanation to reconcile the contradictory results is not forthcoming at present.
Acknowledgments
The author is most grateful to Allan Herbison (University of Otago) for sharing his recent review of the GnRH pulse generator 40 before publication and for comments on an earlier draft of this manuscript. The author would like to recognize Kevin O’Byrne (King’s College London) for his insightful comments on an earlier draft of the manuscript, Robert Goodman (West Virginia University) for his help in resolving a question or two before submission, and the formal reviewers for their insightful comments which led to an improvement in the scholarship of the final manuscript.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 4 approved]
Footnotes
I. As was noted by Knobil in 1992 7, the pulsatile nature of gonadotropin secretion was also reported in 1970 by a group of French investigators studying men and women 8 but this clinical observation commanded little attention from the neuroendocrine community.
II. A pulsatile pattern in GnRH concentrations in hypophysial portal blood had been reported earlier for the hypophysectomized monkey and rat by Carmel et al. 21 and Sarkar and Fink 22, respectively. However, the experimental models employed by these investigators prevented correlation of GnRH levels with episodic LH release.
III. Pioneering studies of MUA in the MBH were initiated by Knobil’s laboratory in the latter half of the 1970s 23.
IV. To date, the anatomical structure termed “dendron” has not been described for species other than mouse.
V. A more recent study of ovine and rat hypothalamus employing dual-label immunofluorescence revealed a much higher co-localization of KOR and kisspeptin in neurons in the arcuate nucleus 66.
VI. Earlier, Nishihara et al. 89 had found that the frequency of MUA volleys in rat was accelerated following administration of naloxone, a non-selective opioid antagonist.
References
- 1. Belchetz PE, Plant TM, Nakai Y, et al. : Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631–3. 10.1126/science.100883 [DOI] [PubMed] [Google Scholar]
- 2. Dierschke DJ, Bhattacharya AN, Atkinson LE, et al. : Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87(5):850–3. 10.1210/endo-87-5-850 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Gay VL, Midgley AR, Jr: Response of the adult rat to orchidectomy and ovariectomy as determined by LH radioimmunoassay. Endocrinology. 1969;84(6):1359–64. 10.1210/endo-84-6-1359 [DOI] [PubMed] [Google Scholar]
- 4. Matsuo H, Baba Y, Nair RM, et al. : Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43(6):1334–9. 10.1016/s0006-291x(71)80019-0 [DOI] [PubMed] [Google Scholar]
- 5. Amoss M, Burgus R, Blackwell R, et al. : Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun. 1971;44(1):205–10. 10.1016/s0006-291x(71)80179-1 [DOI] [PubMed] [Google Scholar]
- 6. Clarke IJ, Cummins JT: The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–9. 10.1210/endo-111-5-1737 [DOI] [PubMed] [Google Scholar]
- 7. Knobil E: Remembrance: the discovery of the hypothalamic gonadotropin-releasing hormone pulse generator and of its physiological significance. Endocrinology. 1992;131(3):1005–6. 10.1210/endo.131.3.1505445 [DOI] [PubMed] [Google Scholar]
- 8. Dolais J, Valleron AJ, Grapin AM, et al. : [Study of human luteinizing hormone (HLH) during the nycthemeron]. C R Acad Sci Hebd Seances Acad Sci D. 1970;270(25):3123–6. [PubMed] [Google Scholar]
- 9. Blake CA, Sawyer CH: Effects of hypothalamic deafferentation on the pulsatile rhythm in plasma concentrations of luteinizing hormone in ovariectomized rats. Endocrinology. 1974;94(3):730–6. 10.1210/endo-94-3-730 [DOI] [PubMed] [Google Scholar]
- 10. Krey LC, Butler WR, Knobil E: Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology. 1975;96(5):1073–87. 10.1210/endo-96-5-1073 [DOI] [PubMed] [Google Scholar]
- 11. Plant TM, Krey LC, Moossy J, et al. : The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey ( Macaca mulatta). Endocrinology. 1978;102(1):52–62. 10.1210/endo-102-1-52 [DOI] [PubMed] [Google Scholar]
- 12. Knobil E: The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. 10.1016/B978-0-12-571136-4.50008-5 [DOI] [PubMed] [Google Scholar]
- 13. Goodman RL, Karsch FL: The hypothalamic pulse generator: a key determinant of reproductive cycles in sheep. In: Follett, B.K. and Follett, D.K. (eds.) Biological Clocks in Seasonal Reproductive Cycles John Wright & Sons Ltd., Bristol,1981;223–236. [Google Scholar]
- 14. Thiéry JC, Pelletier J: Multiunit activity in the anterior median eminence and adjacent areas of the hypothalamus of the ewe in relation to LH secretion. Neuroendocrinology. 1981;32(4):217–24. 10.1159/000123162 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Kawakami M, Uemura T, Hayashi R: Electrophysiological correlates of pulsatile gonadotropin release in rats. Neuroendocrinology. 1982;35(1):63–7. 10.1159/000123356 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Wilson RC, Kesner JS, Kaufman JM, et al. : Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39(3):256–60. 10.1159/000123988 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Herbison AE: Physiology of the adult gonadotropin-releasing hormone neuronal network. In: Plant TM and Zeleznik AJ (eds), Knobil and Neill’s Physiology of Reproduction 4th edn. Elsevier, San Diego, CA,2015;399–467. [Google Scholar]
- 18. Weiner RI, Wetsel W, Goldsmith P, et al. : Gonadotropin-releasing hormone neuronal cell lines. Front Neuroendocrinol. 1992;13(2):95–119. [PubMed] [Google Scholar]
- 19. Ohkura S, Tsukamura H, Maeda K: Effects of transplants of fetal mediobasal hypothalamus on luteinizing hormone pulses impaired by hypothalamic deafferentation in adult ovariectomized rats. Neuroendocrinology. 1992;55(4):422–6. 10.1159/000126153 [DOI] [PubMed] [Google Scholar]
- 20. Purnelle G, Gérard A, Czajkowski V, et al. : Pulsatile secretion of gonadotropin-releasing hormone by rat hypothalamic explants without cell bodies of GnRH neurons [corrected]. Neuroendocrinology. 1997;66(5):305–12. 10.1159/000127253 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Carmel PW, Araki S, Ferin M: Pituitary stalk portal blood collection in rhesus monkeys: evidence for pulsatile release of gonadotropin-releasing hormone (GnRH). Endocrinology. 1976;99(1):243–8. 10.1210/endo-99-1-243 [DOI] [PubMed] [Google Scholar]
- 22. Sarkar DK, Fink G: Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: effects of steroids. J Endocrinol. 1980;86(3):511–24. 10.1677/joe.0.0860511 [DOI] [PubMed] [Google Scholar]
- 23. Dufy B, Dufy-Barbe L, Vincent JD, et al. : [Electrophysiological study of hypothalamic neurons and gonadotropin regulation in rhesus monkey (author's transl)]. J Physiol (Paris). 1979;75(1):105–8. [PubMed] [Google Scholar]; F1000 Recommendation
- 24. de Roux N, Genin E, Carel JC, et al. : Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–6. 10.1073/pnas.1834399100 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Seminara SB, Messager S, Chatzidaki EE, et al. : The GPR54 Gene as a Regulator of Puberty. N Engl J Med. 2003;349(17):1614–27. 10.1056/NEJMoa035322 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Irwig MS, Fraley GS, Smith JT, et al. : Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–72. 10.1159/000083140 [DOI] [PubMed] [Google Scholar]
- 27. Han SK, Gottsch ML, Lee KJ, et al. : Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–56. 10.1523/JNEUROSCI.3328-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Messager S, Chatzidaki EE, Ma D, et al. : Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761–6. 10.1073/pnas.0409330102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, et al. : Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149(8):3926–32. 10.1210/en.2007-1487 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Gottsch ML, Cunningham MJ, Smith JT, et al. : A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–7. 10.1210/en.2004-0431 [DOI] [PubMed] [Google Scholar]
- 31. Shahab M, Mastronardi C, Seminara SB, et al. : Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102(6):2129–34. 10.1073/pnas.0409822102 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Plant TM, Ramaswamy S, DiPietro MJ: Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey ( Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147(2):1007–13. 10.1210/en.2005-1261 [DOI] [PubMed] [Google Scholar]
- 33. Keen KL, Wegner FH, Bloom SR, et al. : An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–7. 10.1210/en.2008-0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roseweir AK, Kauffman AS, Smith JT, et al. : Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–9. 10.1523/JNEUROSCI.5740-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. d'Anglemont de Tassigny X, Fagg LA, Dixon JP, et al. : Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104(25):10714–9. 10.1073/pnas.0704114104 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Li Q, Roa A, Clarke IJ, et al. : Seasonal variation in the gonadotropin-releasing hormone response to kisspeptin in sheep: possible kisspeptin regulation of the kisspeptin receptor. Neuroendocrinology. 2012;96(3):212–21. 10.1159/000335998 [DOI] [PubMed] [Google Scholar]
- 37. Caraty A, Lomet D, Sébert ME, et al. : Gonadotrophin-releasing hormone release into the hypophyseal portal blood of the ewe mirrors both pulsatile and continuous intravenous infusion of kisspeptin: an insight into kisspeptin's mechanism of action. J Neuroendocrinol. 2013;25(6):537–46. 10.1111/jne.12030 [DOI] [PubMed] [Google Scholar]
- 38. Steyn FJ, Wan Y, Clarkson J, et al. : Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–45. 10.1210/en.2013-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uenoyama Y, Nakamura S, Hayakawa Y, et al. : Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol. 2015;27(3):187–97. 10.1111/jne.12257 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Herbison AE: The Gonadotropin-Releasing Hormone Pulse Generator. Endocrinology. 2018;159(1):3723–36. 10.1210/en.2018-00653 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Smith JT, Cunningham MJ, Rissman EF, et al. : Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–92. 10.1210/en.2005-0488 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Clarkson J, Herbison AE: Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–25. 10.1210/en.2006-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith JT, Clay CM, Caraty A, et al. : KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148(3):1150–7. 10.1210/en.2006-1435 [DOI] [PubMed] [Google Scholar]
- 44. Ramaswamy S, Guerriero KA, Gibbs RB, et al. : Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey ( Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149(9):4387–95. 10.1210/en.2008-0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Navarro VM, Gottsch ML, Chavkin C, et al. : Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–66. 10.1523/JNEUROSCI.1569-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Goodman RL, Lehman MN, Smith JT, et al. : Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–60. 10.1210/en.2007-0961 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. : Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–12. 10.1210/en.2012-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Ramaswamy S, Seminara SB, Ali B, et al. : Neurokinin B stimulates GnRH release in the male monkey ( Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494–503. 10.1210/en.2010-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. True C, Kirigiti M, Ciofi P, et al. : Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23(1):52–64. 10.1111/j.1365-2826.2010.02076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smith JT, Li Q, Yap KS, et al. : Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–12. 10.1210/en.2010-1225 [DOI] [PubMed] [Google Scholar]
- 51. Yip SH, Boehm U, Herbison AE, et al. : Conditional Viral Tract Tracing Delineates the Projections of the Distinct Kisspeptin Neuron Populations to Gonadotropin-Releasing Hormone (GnRH) Neurons in the Mouse. Endocrinology. 2015;156(7):2582–94. 10.1210/en.2015-1131 [DOI] [PubMed] [Google Scholar]
- 52. Lehman MN, Coolen LM, Goodman RL: Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–89. 10.1210/en.2010-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iremonger KJ, Porteous R, Herbison AE: Spike and Neuropeptide-Dependent Mechanisms Control GnRH Neuron Nerve Terminal Ca 2+ over Diverse Time Scales. J Neurosci. 2017;37(12):3342–51. 10.1523/JNEUROSCI.2925-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Herde MK, Iremonger KJ, Constantin S, et al. : GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J Neurosci. 2013;33(31):12689–97. 10.1523/JNEUROSCI.0579-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Merkley CM, Coolen LM, Goodman RL, et al. : Evidence for Changes in Numbers of Synaptic Inputs onto KNDy and GnRH Neurones during the Preovulatory LH Surge in the Ewe. J Neuroendocrinol. 2015;27(7):624–35. 10.1111/jne.12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boukhliq R, Goodman RL, Berriman SJ, et al. : A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology. 1999;140(12):5929–36. 10.1210/endo.140.12.7216 [DOI] [PubMed] [Google Scholar]
- 57. Clarkson J, Han SY, Piet R, et al. : Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A. 2017;114(47):E10216–E10223. 10.1073/pnas.1713897114 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Burke MC, Letts PA, Krajewski SJ, et al. : Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–26. 10.1002/cne.21086 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Foradori CD, Amstalden M, Goodman RL, et al. : Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18(7):534–41. 10.1111/j.1365-2826.2006.01445.x [DOI] [PubMed] [Google Scholar]
- 60. Topaloglu AK, Reimann F, Guclu M, et al. : TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–8. 10.1038/ng.306 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Plant TM: 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-pituitary-gonadal axis. J Endocrinol. 2015;226(2):T41–T54. 10.1530/JOE-15-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cheng G, Coolen LM, Padmanabhan V, et al. : The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301–11. 10.1210/en.2009-0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ramaswamy S, Seminara SB, Plant TM: Evidence from the agonadal juvenile male rhesus monkey ( Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94(3):237–45. 10.1159/000329045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sakamoto K, Murata K, Wakabayashi Y, et al. : Central administration of neurokinin B activates kisspeptin/NKB neurons in the arcuate nucleus and stimulates luteinizing hormone secretion in ewes during the non-breeding season. J Reprod Dev. 2012;58(6):700–6. 10.1262/jrd.2011-038 [DOI] [PubMed] [Google Scholar]
- 65. Navarro VM, Castellano JM, McConkey SM, et al. : Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300(1):E202–10. 10.1152/ajpendo.00517.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weems PW, Witty CF, Amstalden M, et al. : κ-Opioid Receptor Is Colocalized in GnRH and KNDy Cells in the Female Ovine and Rat Brain. Endocrinology. 2016;157(6):2367–79. 10.1210/en.2015-1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moore AM, Coolen LM, Porter DT, et al. : KNDy Cells Revisited. Endocrinology. 2018;159(9):3219–34. 10.1210/en.2018-00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. de Croft S, Boehm U, Herbison AE: Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–60. 10.1210/en.2013-1231 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Kinsey-Jones JS, Li XF, Luckman SM, et al. : Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology. 2008;149(3):1004–8. 10.1210/en.2007-1505 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Ohkura S, Takase K, Matsuyama S, et al. : Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21(10):813–21. 10.1111/j.1365-2826.2009.01909.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Li XF, Kinsey-Jones JS, Cheng Y, et al. : Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;4(12):e8334. 10.1371/journal.pone.0008334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Goodman RL, Hileman SM, Nestor CC, et al. : Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–69. 10.1210/en.2013-1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Goodman RL, Coolen LM, Lehman MN: A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology. 2014;99(1):18–32. 10.1159/000355285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. George JT, Veldhuis JD, Roseweir AK, et al. : Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab. 2011;96(8):E1228–E1236. 10.1210/jc.2011-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Jayasena CN, Comninos AN, Veldhuis JD, et al. : A single injection of kisspeptin-54 temporarily increases luteinizing hormone pulsatility in healthy women. Clin Endocrinol (Oxf). 2013;79(4):558–63. 10.1111/cen.12179 [DOI] [PubMed] [Google Scholar]
- 76. Jayasena CN, Abbara A, Veldhuis JD, et al. : Increasing LH pulsatility in women with hypothalamic amenorrhoea using intravenous infusion of Kisspeptin-54. J Clin Endocrinol Metab. 2014;99(6):E953–61. 10.1210/jc.2013-1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Narayanaswamy S, Jayasena CN, Ng N, et al. : Subcutaneous infusion of kisspeptin-54 stimulates gonadotrophin release in women and the response correlates with basal oestradiol levels. Clin Endocrinol (Oxf). 2016;84(6):939–45. 10.1111/cen.12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Narayanaswamy S, Prague JK, Jayasena CN, et al. : Investigating the KNDy Hypothesis in Humans by Coadministration of Kisspeptin, Neurokinin B, and Naltrexone in Men. J Clin Endocrinol Metab. 2016;101(9):3429–36. 10.1210/jc.2016-1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Millar RP, Sonigo C, Anderson RA, et al. : Hypothalamic-Pituitary-Ovarian Axis Reactivation by Kisspeptin-10 in Hyperprolactinemic Women With Chronic Amenorrhea. J Endocr Soc. 2017;1(11):1362–71. 10.1210/js.2017-00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ikegami K, Minabe S, Ieda N, et al. : Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J Neuroendocrinol. 2017;29(6). 10.1111/jne.12480 [DOI] [PubMed] [Google Scholar]
- 81. Wakabayashi Y, Nakada T, Murata K, et al. : Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–32. 10.1523/JNEUROSCI.5848-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Goodman RL, Okhura S, Okamura H, et al. : KNDy Hypothesis for Generation of GnRH Pulses: Evidence from Sheep and Goats. In Herbison AE Plant TM eds. The GnRH Neuron and its Control Masterclass in Neuroendocrinology Series. Wiley Blackwell;2017;289–324. 10.1002/9781119233275.ch12 [DOI] [Google Scholar]
- 83. Li Q, Millar RP, Clarke IJ, et al. : Evidence that Neurokinin B Controls Basal Gonadotropin-Releasing Hormone Secretion but Is Not Critical for Estrogen-Positive Feedback in Sheep. Neuroendocrinology. 2015;101(2):161–74. 10.1159/000377702 [DOI] [PubMed] [Google Scholar]
- 84. Fraser GL, Hoveyda HR, Clarke IJ, et al. : The NK3 Receptor Antagonist ESN364 Interrupts Pulsatile LH Secretion and Moderates Levels of Ovarian Hormones Throughout the Menstrual Cycle. Endocrinology. 2015;156(11):4214–25. 10.1210/en.2015-1409 [DOI] [PubMed] [Google Scholar]
- 85. Skorupskaite K, George JT, Veldhuis JD, et al. : Neurokinin B Regulates Gonadotropin Secretion, Ovarian Follicle Growth, and the Timing of Ovulation in Healthy Women. J Clin Endocrinol Metab. 2018;103(1):95–104. 10.1210/jc.2017-01306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Skorupskaite K, George JT, Veldhuis JD, et al. : Neurokinin 3 receptor antagonism decreases gonadotropin and testosterone secretion in healthy men. Clin Endocrinol (Oxf). 2017;87(6):748–56. 10.1111/cen.13445 [DOI] [PubMed] [Google Scholar]
- 87. Qiu J, Nestor CC, Zhang C, et al. : High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5: pii: e16246. 10.7554/eLife.16246 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Weems PW, Coolen LM, Hileman SM, et al. : Evidence That Dynorphin Acts Upon KNDy and GnRH Neurons During GnRH Pulse Termination in the Ewe. Endocrinology. 2018;159(9):3187–99. 10.1210/en.2018-00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nishihara M, Hiruma H, Kimura F: Interactions between the noradrenergic and opioid peptidergic systems in controlling the electrical activity of luteinizing hormone-releasing hormone pulse generator in ovariectomized rats. Neuroendocrinology. 1991;54(4):321–6. 10.1159/000125909 [DOI] [PubMed] [Google Scholar]
- 90. Kinsey-Jones JS, Grachev P, Li XF, et al. : The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153(1):307–15. 10.1210/en.2011-1641 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Grachev P, Li XF, Kinsey-Jones JS, et al. : Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153(10):4894–904. 10.1210/en.2012-1574 [DOI] [PubMed] [Google Scholar]
- 92. Krajewski SJ, Burke MC, Anderson MJ, et al. : Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680–97. 10.1016/j.neuroscience.2009.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wakabayashi Y, Yamamura T, Sakamoto K, et al. : Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among Kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in goats. J Reprod Dev. 2013;59(1):40–8. 10.1262/jrd.2012-136 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Ciofi P, Leroy D, Tramu G: Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience. 2006;141(4):1731–45. 10.1016/j.neuroscience.2006.05.041 [DOI] [PubMed] [Google Scholar]
- 95. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. : Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. 10.1016/j.neuroscience.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ezzat A, Pereira A, Clarke IJ: Kisspeptin is a component of the pulse generator for GnRH secretion in female sheep but not the pulse generator. Endocrinology. 2015;156(5):1828–37. 10.1210/en.2014-1756 [DOI] [PubMed] [Google Scholar]
- 97. Gottsch ML, Popa SM, Lawhorn JK, et al. : Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152(11):4298–309. 10.1210/en.2011-1521 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Arslan M, Pohl CR, Plant TM: DL-2-amino-5-phosphonopentanoic acid, a specific N-methyl-D-aspartic acid receptor antagonist, suppresses pulsatile LH release in the rat. Neuroendocrinology. 1988;47(5):465–8. 10.1159/000124951 [DOI] [PubMed] [Google Scholar]
- 99. Gay VL, Plant TM: Sustained intermittent release of gonadotropin-releasing hormone in the prepubertal male rhesus monkey induced by N-methyl-DL-aspartic acid. Neuroendocrinology. 2004;48(2):147–52. 10.1159/000125002 [DOI] [PubMed] [Google Scholar]
- 100. d'Anglemont de Tassigny X, Ackroyd KJ, Chatzidaki EE, et al. : Kisspeptin signaling is required for peripheral but not central stimulation of gonadotropin-releasing hormone neurons by NMDA. J Neurosci. 2010;30(25):8581–90. 10.1523/JNEUROSCI.5486-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Herbison AE: Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19(3):302–30. 10.1210/edrv.19.3.0332 [DOI] [PubMed] [Google Scholar]
- 102. Bhattacharya AN, Dierschke DJ, Yamaji T, et al. : The pharmacologic blockade of the circhoral mode of LH secretion in the ovariectomized rhesus monkey. Endocrinology. 1972;90(3):778–86. 10.1210/endo-90-3-778 [DOI] [PubMed] [Google Scholar]
- 103. Plant TM, Nakai Y, Belchetz P, et al. : The sites of action of estradiol and phentolamine in the inhibition of the pulsatile, circhoral discharges of LH in the rhesus monkey (Macaca mulatta). Endocrinology. 1978;102(4):1015–8. 10.1210/endo-102-4-1015 [DOI] [PubMed] [Google Scholar]
- 104. Kaufman JM, Kesner JS, Wilson RC, et al. : Electrophysiological manifestation of luteinizing hormone-releasing hormone pulse generator activity in the rhesus monkey: influence of alpha-adrenergic and dopaminergic blocking agents. Endocrinology. 1985;116(4):1327–33. 10.1210/endo-116-4-1327 [DOI] [PubMed] [Google Scholar]
- 105. Woller MJ, McDonald JK, Reboussin DM, et al. : Neuropeptide Y is a neuromodulator of pulsatile luteinizing hormone-releasing hormone release in the gonadectomized rhesus monkey. Endocrinology. 1992;130(4):2333–2342. 10.1210/endo.130.4.1547745 [DOI] [PubMed] [Google Scholar]
- 106. Palmiter RD, Erickson JC, Hollopeter G, et al. : Life without neuropeptide Y. Rec Prog Horm Res. 1998;53:163–199. [PubMed] [Google Scholar]
- 107. Terasawa E: Mechanism of pulsatile GnRH release in primates: Unresolved questions. Mol Cell Endocrinol. 2019;498:110578. 10.1016/j.mce.2019.110578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. El Majdoubi M, Sahu S, Ramaswamy S, et al. : Neuropeptide Y: A hypothalamic brake restraining the onset of puberty in primates. Proc Nat Acad Sci. 2000;97(11):6179–6184. 10.1073/pnas.090099697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. True C, Takahashi D, Kirigiti M, et al. : Arcuate nucleus neuropeptide coexpression and connections to gonadotrophin-releasing hormone neurones in the female rhesus macaque. J Neuroendocrinol. 2017;29(6):1–15. 10.1111/jne.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Krajewski SJ, Anderson MJ, Iles-Shih L, et al. : Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–86. 10.1002/cne.20626 [DOI] [PubMed] [Google Scholar]
- 111. Gaskins GT, Glanowska KM, Moenter SM: Activation of neurokinin 3 receptors stimulates GnRH release in a location-dependent but kisspeptin-independent manner in adult mice. Endocrinology. 2013;154(11):3984–9. 10.1210/en.2013-1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Murakawa H, Iwata K, Takeshita T, et al. : Immunoelectron microscopic observation of the subcellular localization of kisspeptin, neurokinin B and dynorphin A in KNDy neurons in the arcuate nucleus of the female rat. Neurosci Lett. 2016;612:161–6. 10.1016/j.neulet.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 113. Hrabovszky E, Sipos MT, Molnár CS, et al. : Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology. 2012;153(10):4978–89. 10.1210/en.2012-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Skrapits K, Borsay BÁ, Herczeg L, et al. : Neuropeptide co-expression in hypothalamic kisspeptin neurons of laboratory animals and the human. Front Neurosci. 2015;9:29. 10.3389/fnins.2015.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nikoshkov A, Hurd YL, Yakovleva T, et al. : Prodynorphin transcripts and proteins differentially expressed and regulated in the adult human brain. FASEB J. 2005;19(11):1543–5. 10.1096/fj.05-3743fje [DOI] [PubMed] [Google Scholar]
- 116. Rometo AM, Rance NE: Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20(12):1376–81. 10.1111/j.1365-2826.2008.01796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rance NE, Krajewski SJ, Smith MA, et al. : Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–28. 10.1016/j.brainres.2010.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kauffman AS, Gottsch ML, Roa J, et al. : Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774–83. 10.1210/en.2006-1540 [DOI] [PubMed] [Google Scholar]
- 119. Han SY, Kane G, Cheong I, et al. : Characterization of GnRH Pulse Generator Activity in Male Mice Using GCaMP Fiber Photometry. Endocrinology. 2019;160(3):557–67. 10.1210/en.2018-01047 [DOI] [PubMed] [Google Scholar]
- 120. Norman RL, Spies HG: Cyclic ovarian function in a male macaque: additional evidence for a lack of sexual differentiation in the physiological mechanisms that regulate the cyclic release of gonadotropins in primates. Endocrinology. 1986;118(6):2608–10. 10.1210/endo-118-6-2608 [DOI] [PubMed] [Google Scholar]
- 121. Mayer C, Boehm U: Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14(6):704–10. 10.1038/nn.2818 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 122. Young J, George JT, Tello JA, et al. : Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology. 2013;97(2):193–202. 10.1159/000336376 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 123. Clarke IJ, Li Q, Henry BA, et al. : Continuous Kisspeptin Restores Luteinizing Hormone Pulsatility Following Cessation by a Neurokinin B Antagonist in Female Sheep. Endocrinology. 2018;159(2):639–46. 10.1210/en.2017-00737 [DOI] [PubMed] [Google Scholar]