Mutations in STXBP1, a gene involved in the exocytosis of synaptic vesicles, cause early infantile epileptic encephalopathy with burst-suppression (Otahara syndrome)1, or infantile spasms with non-syndromic encephalopathy2. Recent descriptions of STXBP1 mutations in Dravet syndrome3 and documentation of spasticity and childhood onset ataxia4 indicate a broad and progressive disease phenotype. Here we report a patient with a novel de novo mutation in STXBP1 who presented with infantile onset epilepsy and subsequent ataxia and adolescent onset parkinsonism. Muscle biopsy showed a profound defect in complex I of the respiratory chain, implicating a mitochondrial mechanism in the pathogenesis.
Case report
A full term neonate born by normal delivery to non-consanguineous parents, presented on day 5 with intermittent generalized seizures. Routine bloods, CT head and lumbar puncture were normal. Her EEG at age 3 weeks showed bilateral multifocal epileptiform activity imposed on normal background activity, and by age 6 weeks she had a mixture of tonic seizures and intermittent focal seizures of her left arm and right leg. By 4 months of age her seizures were controlled with phenobarbitone.
Assessment aged one year revealed global developmental delay. Aged 6 she was markedly ataxic, and at age 7 she developed head nodding stereotypies. Seizures remained infrequent and an EEG was diffusely abnormal with bifrontal slow waves and a mixture of moderately high amplitude semi-rhythmical theta and delta activity. Aged 9 she developed dystonic posturing of her legs and a prominent asymmetrical dystonic tremor of her upper limbs. By aged 12, this had progressed to an overt extra-pyramidal syndrome with a fine resting tremor, cogwheel rigidity and hypomimia. There were additional pyramidal features with global hyperreflexia and upgoing plantars bilaterally. Language and cognition remained severely impaired. Brain MR images aged 4 months and 6 years were normal, as were serum lactate, pyruvate, very long chain fatty acids, phenylalanine, white cell enzymes, alpha-fetoprotein, vitamin E, acanthocytes, and karyotype. Molecular analysis for SCA 1,2,3,6,7 and 17, Friedreich’s ataxia, MECP2, DRPLA, POLG, CDKL5 and screening for ataxia telangiectasia, oculomotor apraxia type 1 and 2 were normal.
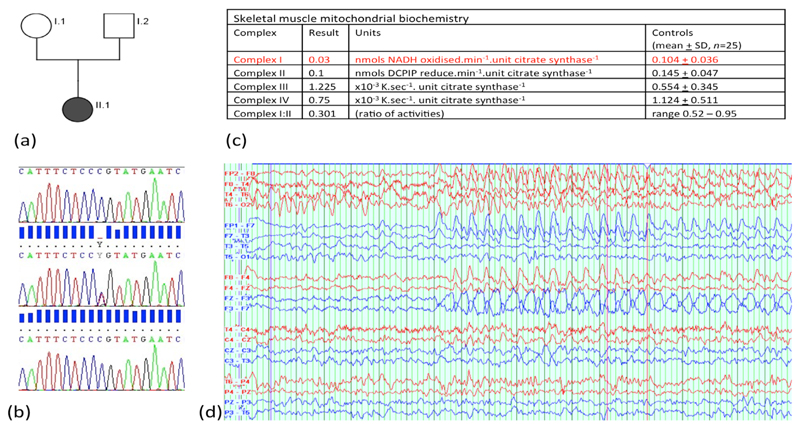
Muscle histology and histochemistry were normal, but mitochondrial biochemical studies showed a profound defect of respiratory chain complex I activity (figure 1C). Muscle mitochondrial DNA levels were normal (20.5, reference range 9-40), there were no mtDNA rearrangements, and no pathogenic mutations were found on whole mitochondrial DNA genome sequencing.
(a) Family pedigree indicating STXBP1 mutation status and phenotype (shaded grey =c.416C>T, p.P139L, clinically affected. Clear – wild type allele, unaffected). (b) Sanger sequencing of c.410-423 of STXBP1. Top trace case I.1, middle trace case I.2, lower trace II.1 (index case) indicating the de novo nature of the mutation. (c) Muscle mitochondrial biochemical studies showing a profound defect in complex I. (d) An ictal EEG during an absence episode with motor arrest showing predominantly frontal spike and wave activity.
With further informed parental consent, whole exome sequencing was performed, revealing a heterozygous missense mutation in exon 6 of the STXBP1 gene (c.416C>T, p.P139L) not seen in either the 1000genome or ESP6500 public databases, or 300 in-house controls. c.416C>T was predicted to be pathogenic by PolyPhen2, SIFT, LRT and MutationTaster software, and sits in exon 6 where pathogenic mis-sense mutations have previously been described. The mutation was not present in parental blood samples (Figure 1(b)).
Discussion
We report the first case of a pathogenic mutation in STXBP1 causing both juvenile onset parkinsonism and significant impairment of complex I of the mitochondrial respiratory chain in addition to the recognized features of seizures, encephalopathy and ataxia.
It is recognized that a primary defect in mitochondrial function may impair synaptic function5, however, our patient shows the inverse situation may also occur, namely that primary defects in exocytosis may impair mitochondrial function. The mechanism underlying this is unclear, but may be mediated through phospholipase D related pathways, as both mitochondrial fusion and synaptic exocytosis, despite being relatively discrete cellular processes, are closely linked through these pathways 6. Our case also suggests that secondary mitochondrial impairment may contribute to disease progression and may underlie the development of the broad neurological phenotype seen in our patient. In this respect, the development of Parkinsonism in our patient is particularly intriguing given the strong association between complex I deficiency and sporadic Parkinson’s disease 7.
In conclusion, STXBP1 should be considered a nuclear gene causing impaired mitochondrial function and secondary mitochondrial impairment may contribute to disease progression in patients with STXBP1 mutations. Treatments aimed at supporting mitochondrial function in patients may be efficacious.
Study funding
Professor Chinnery is a Wellcome Trust Senior Fellow in Clinical Science and National Institute for Health Research Senior Investigator. He receives funding from the Medical Research Council and the National Institute for Health Research Biomedical Research Centre for Ageing and Age-Related Disease award to the Newcastle upon Tyne Foundation Hospitals National Health Service Trust.
Footnotes
Disclosure: The authors report no disclosures relevant to the manuscript.
References
- 1.Saitsu H, Kato M, Mizuguchi T, et al. De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nature genetics. 2008;40:782–788. doi: 10.1038/ng.150. [DOI] [PubMed] [Google Scholar]
- 2.Mignot C, Moutard ML, Trouillard O, et al. STXBP1-related encephalopathy presenting as infantile spasms and generalized tremor in three patients. Epilepsia. 2011;52:1820–1827. doi: 10.1111/j.1528-1167.2011.03163.x. [DOI] [PubMed] [Google Scholar]
- 3.Carvill GL, Weckhuysen S, McMahon JM, et al. GABRA1 and STXBP1: Novel genetic causes of Dravet syndrome. Neurology. 2014 doi: 10.1212/WNL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deprez L, Weckhuysen S, Holmgren P, et al. Clinical spectrum of early-onset epileptic encephalopathies associated with STXBP1 mutations. Neurology. 2010;75:1159–1165. doi: 10.1212/WNL.0b013e3181f4d7bf. [DOI] [PubMed] [Google Scholar]
- 5.Ivannikov MV, Sugimori M, Llinas RR. Synaptic vesicle exocytosis in hippocampal synaptosomes correlates directly with total mitochondrial volume. Journal of molecular neuroscience : MN. 2013;49:223–230. doi: 10.1007/s12031-012-9848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nature cell biology. 2006;8:1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- 7.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Annals of neurology. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]