Abstract
Sleep is a universal phenomenon necessary for maintaining homeostasis and function across a range of organs. Lack of sleep has severe health-related consequences affecting whole-body functioning, yet no other organ is as severely affected as the brain. The neurophysiological mechanisms underlying these deficits are poorly understood. Here, we characterize the dynamic changes in brain connectivity profiles inflicted by sleep deprivation and how they deviate from regular daily variability. To this end, we obtained functional magnetic resonance imaging data from 60 young, adult male participants, scanned in the morning and evening of the same day and again the following morning. 41 participants underwent total sleep deprivation before the third scan, whereas the remainder had another night of regular sleep. Sleep deprivation strongly altered the connectivity of several resting-state networks, including dorsal attention, default mode, and hippocampal networks. Multivariate classification based on connectivity profiles predicted deprivation state with high accuracy, corroborating the robustness of the findings on an individual level. Finally, correlation analysis suggested that morning-to-evening connectivity changes were reverted by sleep (control group) – a pattern which did not occur after deprivation. We conclude that both, a day of waking and a night of sleep deprivation dynamically alter the brain functional connectome.
Keywords: Sleep deprivation, fMRI-based connectivity, circadian variability, machine learning
1. Introduction
Although we spend roughly one third of our lives sleeping, the neurobiological mechanisms of sleep and sleep deprivation remain poorly understood. Whereas the quality, intensity and functions of sleep vary across species (Siegel, 2008), the almost universal presence of some form of sleep behaviour strongly promotes sleep as an evolutionary conserved phenomenon of immense implications (Cirelli and Tononi, 2008). Lack of sleep has been associated with a long list of health-related and cognitive consequences which can only be compensated with sleep itself (Rogers et al., 2003). Although sleep deprivation affects whole-body functioning, it is evident that no other organ is as severely affected as the brain (Cirelli and Tononi, 2008).
Not only is sleep essential to consolidate memory acquired prior to sleep (Walker and Stickgold, 2004), but it is also needed to prepare the brain for acquiring next-day’s memories (Yoo et al., 2007). In line with these important roles of sleep in memory functions, a single night of sleep deprivation has been shown to perturb functional connectivity in hippocampal circuits (Yoo et al., 2007). Furthermore, sleep deprivation is associated with mood alterations and biased emotional appraisal (Anderson and Platten, 2011). Recent imaging data illustrated disturbed functional connectivity in amygdala circuits, particularly connections between amygdala and executive control regions (dorsolateral prefrontal, anterior cingulate, inferior frontal; Shao et al., 2014), and increased connectivity between the dorsal nexus and dorsolateral prefrontal cortex, thereby suggesting a mechanism for the putative therapeutic utility in depression (Bosch et al., 2013). Importantly, emotional effects of sleep deprivation are not only associated with altered response patterns to negative stimuli but also enhanced reactivity toward pleasure-evoking stimuli (Gujar et al., 2011). Apart from its impact on memory and emotional processing, sleep deprivation strongly affects vigilance and attentional capacities (Lim and Dinges, 2008), likely in parts originating from decreased activation and altered patterns of connectivity of the attention and salience networks of the brain (Ma et al., 2014). In line with the notion that lack of sleep affects patterns of large-scale brain connectivity, sleep deprivation was found to reduce the frequently reported anti-correlation between the task-positive dorsal attention network (DAN) and the default-mode network (DMN) in addition to diminished within-DMN connectivity (De Havas et al., 2012; Yeo et al., 2015).
With this body of literature associating sleep deprivation with pronounced connectivity changes throughout the brain, it is important to distinguish connectivity changes that appear solely due to deprivation from those that appear due to morning-to-evening or morning-to-morning variability. Previous results suggest that, in the context of memory retrieval, functional connectivity of medio-temporal regions changes during a regular day from local within-regional connections to global across-regional connections (Shannon et al., 2013). These findings clearly emphasize the need to account for diurnal variability when studying sleep deprivation. Furthermore, imaging studies on sleep deprivation often lack a control group.
Thus, to help us understand the alterations inflicted on the functional organization of the brain by lack of sleep, we estimated patterns of large-scale between-network resting-state brain connectivity using fMRI data obtained at three time points: In the morning after a regular night’s sleep, in the evening of the same day and the next morning after a night of total sleep deprivation. We included a control group that had another night of regular sleep between the evening and the second morning. Furthermore, we included behavioural assessments of vigilance and visual attention in a subgroup. This 2 group x 3 time points design allowed us to directly assess and differentiate diurnal variability from the effects of sleep deprivation. In order to assess the robustness and predictability of network alterations, we combined data-driven definitions of brain network nodes, large-scale network modelling by means of regularized partial correlations, and multivariate machine-learning techniques with cross-validation and permutation testing. Finally, we employed an automated wake and sleep staging using a previously validated connectivity-based classifier (Tagliazucchi and Laufs, 2014) to assess wakefulness probabilities based on connectivity profiles.
2. Materials and Methods
2.1. Sample and ethical approval
We included 60 male, healthy participants and assigned them either to the deprived group (N=41, mean age: 21.8 years, SD= 2.4, range 18-26) or control group (N=19, mean age: 22.7 year, SD= 2.2, range 19-26). The groups did not differ in age (t=1.4, p=.16). Before study inclusion, all subjects were screened for current and previous psychiatric and somatic illnesses, either by a medical doctor (TE or NZ) or a student in the final year of medical school (PØP). Exclusion criteria were any history of sleep disorder, neurological or other chronic and acute somatic disorder, including infections, psychiatric illness, alcohol or drug use disorder, previous head injury with loss of consciousness for more than one minute, metallic implants, and previous or current use of psychotropic drugs. Participants reported an average sleep duration of 7.4 ± 1.0 hours per night the week prior to the study, 7.6 ± 0.9 hours per night the month prior to the study, and 6.6 ± 1.2 hours the night before the first scanning using a self-reporting questionnaire. The self-reported sleep durations of the participants the last week and month are consistent with average sleep duration in a recently published self-reporting-based sleep duration study of young Norwegian adults (Hayley et al., 2015). The sleep duration estimates did not differ between the two groups (all p>.71). Self-reported sleep duration the night before the study was significantly shorter than the self-reported average sleep duration per night the last month in both groups (p<.001). Furthermore, the control group reported an average sleep duration of 6.1 ± 0.9 hours in the night before the third scan, which is significantly shorter than their reported sleep duration the last month (p<.05).
Appropriate ethical approval was obtained and all procedures were in line with the declaration of Helsinki. All participants signed informed consent prior to enrolment.
2.2. Study protocol
From each participant, we collected resting state fMRI scans at three time points (TP): TP1 (first morning after normal sleep), TP2 (evening after a regular day of waking) and TP3 (next morning). While TP1 and TP2 were similar for all participants in terms of waking length, TP3 differed between groups. Participants of the control group went home after TP2 and came back at TP3 after another night of regular sleep. In contrast, participants of the deprivation group were kept awake the entire night, continuously monitored by a research assistant. To control for light exposure, participants of the deprivation group stayed in the same room with constant, normal light intensity between TP2 and TP3. The room had no windows, measured approximately 5 x 4 meters, had chairs and a table, and a TV set. During the night, participants of this group were playing video games, watching movies, reading books, and talking with the research assistant. Independent of group assignment, all participants had to refrain from any consumption of caffeine or energy drinks and had no exercise between TP1 and TP3.
Scan times were adjusted to the participants’ usual sleep-wake cycles. Average scan times per time point were 8:15AM (SD: 37min) for TP1 (across groups), 10:05PM (SD: 44min) for TP2 (across groups) and for TP3 average scan times were 6:47AM (SD: 27min) for the sleep deprivation group and 8:03AM (SD: 56min) for the control group. For the morning scans, participants came to the scanning facilities immediately after waking up.
In order to explore the effects of sleep deprivation on attentional functions and vigilance, and its associations with large-scale brain network connectivity changes, subjects performed the attention network task (ANT; (Fan et al., 2002)) at each session, directly after MRI acquisition. ANT yields reaction time (RT) measures in three different conditions by flankering target stimuli with congruent, incongruent or neutral stimuli, thereby altering the difficulty of the task (neutral < congruent < incongruent). Since we were mainly interested in vigilance and attentional lapses as markers of sleep deprivation (Lim and Dinges, 2008), we primarily targeted the inter-individual variability in reaction time (IIV-RT), known to increase substantially with tiredness (Corfitsen, 1994), defined as the coefficient of variation in response time across all correct trials across conditions. In addition, we investigated changes in mean reaction time in addition to the conflict, orienting and alerting scores based on differences in mean reaction times between conditions (Fan et al., 2002; Westlye et al., 2011). Both IIV-RT and reaction times were assessed within and across the 3 ANT flanker conditions (congruent, incongruent and neutral).
Furthermore, we assessed subjective sleepiness using the self-reported Karolinska Sleepiness Scale (KSS; (Akerstedt and Gillberg, 1990)), a nine-point Likert-type scale ranging from “Extremely alert” (score = 1) to “extremely sleepy, fighting sleep” (score = 9). The KSS was administered immediately after the ANT. IIV-RT and KSS data was available for N=39 subjects (all 19 controls, 20 of the deprived group).
2.3. MRI data collection
MRI scans were obtained from a Philips Achieva 3.0T scanner (Philips Healthcare, The Netherlands) with an 8-channel head coil at Oslo University Hospital. We acquired structural MRI with a T1-weighted 3D turbo field echo sequence (TFE; TR: 6.7 ms; TE: 3.1 ms; FA: 8°; voxel size: 1x1x1.2 mm; slices: 170; FOV: 256 mm2) and functional MRI data with a T2*-weighted 2D gradient echo planar imaging sequence (EPI) with 200 volumes (TR: 2.5 s; TE: 30 ms; FA: 80°; voxel size: 2.625x2.625x3 mm; slices: 45). Participants were instructed to keep their eyes open and focus on a fixation cross during the scan.
2.4. MRI processing and network estimation
We used the FMRI Expert Analysis Tool (FEAT) from the FMRIB Software Library (FSL, (Smith et al., 2004)) to analyse fMRI data. The processing pipeline included spatial smoothing (FWHM=6 mm), high-pass filtering (100 s), motion correction (MCFLIRT) and single-session independent component analysis (ICA) using MELODIC (Beckmann and Smith, 2004). We used FIX (ICA-based Xnoisefier (Salimi-Khorshidi et al., 2014), recently verified for its reliability (Pruim et al., 2015)) to identify and remove noise components on an individual level using a machine learning approach (custom training data set for Philips scanner, threshold: 20). Influence of motion on the reported results was carefully investigated and is addressed in the results section. Next, we extracted brain masks from the T1-weighted images using automated brain segmentation in Freesurfer (Fischl et al., 2002). These were used for registration to standard coordinate space using FLIRT (Jenkinson and Smith, 2001) with boundary-based registration (BBR, (Greve and Fischl, 2009)) and FNIRT (Andersson et al., 2010). After registration, we ran an independent component analysis using a novel group-PCA approach for large data sets (Smith et al., 2014) in MELODIC including all scans with 40 components requested. We chose a model order of 40 components as it provides a good balance between adequate spatial segmentation and sufficiently low number of components, the latter known to decrease the risk of false positives (Abou Elseoud et al., 2011). SI-Figure 1 provides an overview of all components. Next, for each subject we computed individual time series and component spatial maps using dual regression (Filippini et al., 2009). Based on the spatial distribution of the component maps and/or the frequency spectrum of the components’ time series, we identified five classical noise components and regressed the time-series from these five out of the remaining components. Next, we identified another eight components of which spatial maps were not corresponding to any interpretable neuronal origin or were outside the mask, and these were therefore not included in further analyses. The remaining 27 clean components constituted the nodes in subsequent network analyses, whereas the corresponding 351 time series partial correlations between each component pairs formed the edges (connections) of the full network. For each subject, we estimated these networks using L1-regularized partial correlations with a lambda of 0.025 in FSLNets (Smith et al., 2011). In addition, we compared the results to a novel method that automatically estimates regularization strength on the individual subject level following the Ledoit & Wolf theorem (Ledoit and Wolf, 2003). Recent studies suggested influence of vigilance and sleep deprivation on the global fMRI signal (Wong et al., 2013; Yeo et al., 2015) indicating that under certain conditions a global signal regression (GSR) may be beneficial (Yeo et al., 2015). Here, we did not regress out the global signal, as global signal regression likely decreases the signal to noise ratio (Pruim et al., 2015). Rather, we used regularized partial correlation matrices to infer connectivity, known to be relatively unaffected by global signal (Smith et al., 2011).
2.5. Machine learning
We performed several classification tasks using the 351 edges from the above described networks (27 components) as features in a regularized linear discriminant analysis classifier (rLDA; Friedman, 1989; Schäfer and Strimmer, 2005)), similar to the methods applied in Alnaes et al. (2015) and Kaufmann et al. (2015). Among the advantages of rLDA compared to other classification techniques is that it does not rely on an external feature selection procedures (this is taken care of by the regularisation) and that it is fast and efficient. The regularization parameter was optimized from the training data following the analytical procedure by Ledoit and Wolf (2003) and described in Schäfer and Strimmer (2005). First, we classified time points within groups based on edgewise connectivity strength utilizing leave-one-out (LOO) cross validation procedures. Next, we further tested the specificity of features by training a classifier on the deprived group and testing it on the control group, and vice versa. In addition, we used binary classification within groups to assess if the third session is more similar to the first or second session, by training a classifier on TP1 and TP2 and testing it on TP3. Finally, we assessed morning-to-evening effects on functional connectivity by merging participants of both groups into one sample since conditions were identical between groups for TP1 and TP2 and utilized LOO in a binary classification task (TP1 vs. TP2). In all classification tasks, significance was assessed based on permutation tests across 10000 iterations, each randomly permuting assignment to the three time points within subjects.
2.6. Automated sleep staging
We performed prediction of wake/sleep stages based on fMRI data using the procedures described in (Tagliazucchi and Laufs, 2014). Briefly, we extracted the time series from the mean BOLD signals of regions of interest defined by the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). Whereas we used ICA based networks for all other analyses, using AAL-based parcellation allowed us to directly apply the support vector machine classifier trained on carefully sleep-staged samples (Tagliazucchi and Laufs, 2014). Using a sliding window of 2 minutes on the continuous fMRI time series, the classifier classified each data point into either wakefulness, N1, N2 or N3 sleep without the need for EEG data. To minimize the probability that our main classification results (section 2.5) were driven by sleep, we excluded TRs based on sleep probability and reran the classification of TPs. Sleep stage classification output at each TR indicated if the last 2 minutes were most likely awake, N1, N2 or N3. First, we identified all TRs per scan that were classified as N1, N2 or N3 sleep. Next, we applied three different exclusion criteria. The full 2 minutes prior to the identified TRs were excluded, if the decision indicated sleep for at least 30s, 60s or 90s in a row respectively (12, 24 and 36 TRs, with sleep staging based on 48 TRs). Next, if the remaining TRs in either of the three time points comprised less than 3 minutes of scan duration, the whole subject was excluded to avoid unreliable network estimations. Finally, for the remaining subjects with long enough scan duration of TRs classified as awake we computed the networks.
2.7. Univariate network analysis and alpha adjustment
We assessed the effects of sleep deprivation on between-node connectivity using repeated measures analysis of variance (ANOVA) on each network edge, as well as post-hoc independent-sample t-tests. We adjusted the 5% alpha level using false discovery rate (FDR) for each test separately with a false discovery rate level q=0.05 and a threshold based on the assumption of independence or positive dependence (Nichols, 2009; Nichols and Hayasaka, 2003). For transparency, we report raw p-values. In addition, we associated subjective sleepiness ratings and IIV-RT with edgewise connectivity strength across time points using repeated measures correlations (Bland and Altman, 1995).
2.8. Motion as a potential confounder of results
Whereas we cleaned the raw individual fMRI data using the machine-learning based cleaning tool FIX (Salimi-Khorshidi et al., 2014), to ensure that potentially retaining motion confounds could not explain the main effects on connectivity, we also tested if the effects remained when including estimated relative motion as a covariate in the repeated measures analysis. We used linear mixed effects modelling in SPSS (IBM SPSS Inc.) and included motion as a covariate with first-order, autoregressive covariance matrix (AR1).
3. Results
3.1. Effects of sleep deprivation on reaction time and subjective sleepiness
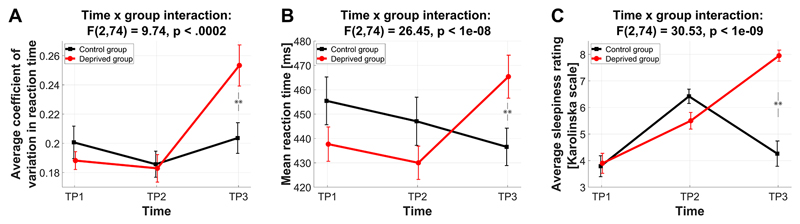
Sleep deprivation strongly affected reaction time measures and subjective sleepiness. IIV-RT displayed a significant group x time interaction (F(2,74)=9.74, p<.0002) due to increased IIV-RT at TP3 in the deprived group (t=2.8, p<.008, Figure 1A). IIV-RT at TP1 and TP2 did not differ between groups (both p>.3). Similarly, mean RT decreased from TP1 to TP2 and in the control group further at TP3, yet despite such potential training effects sleep deprivation strongly affected mean RT, yielding higher mean RT at TP3 for the deprived group than at TP1 (t=2.45, p=.02; Figure 1B). These patterns of deprivation effects were also present within each of the three ANT flanker conditions alone (SI-Figure 2), with group differences at TP3 being largest for congruent and incongruent conditions (IIV-RT: t=2.91, p=.006, t=2.84, p=.007, t=1.86, p=.07 for congruent, incongruent and neutral respectively; mean RT: t=2.58, p=.01, t=2.34, p=.025, t=2.10, p=.04). In line with the global effects on RT, no effect of sleep deprivation on any of the three ANT component scores (alerting, orienting and conflict) was found (all p>.35; SI-Figure 3).
Figure 1. Sleep deprivation results in higher reaction time variability and increased reaction times.
TP1: first morning, TP2: evening, TP3: second morning. (A) Coefficient of variation in reaction time. (B) Mean reaction times (C) Self-reported subjective sleepiness obtained from the Karolinska Sleepiness Scale (Akerstedt and Gillberg, 1990).
Furthermore, a repeated measures ANOVA on subjective sleepiness scales yielded a significant group x time interaction (F(2,74)=30.53, p<1e-09), indicating higher scores for the deprived group at TP3 (t=7.2, p<.1e-07, Figure 1C).
3.2. Effects of sleep deprivation on functional brain connectivity
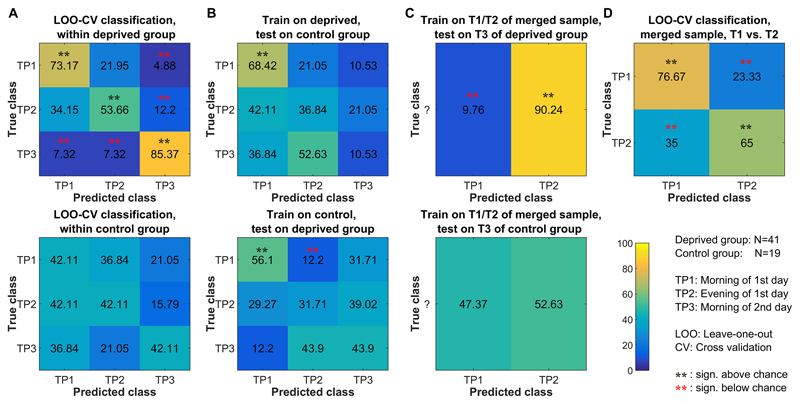
Sleep deprivation robustly affected functional brain network connectivity. Within the deprived group, multivariate classification of time points yielded accuracy significantly above chance across sessions (morning: 73.2%, p<.0001; evening 53.7%, p<.0015; next morning (deprived): 85.4%, p<.0001). Figure 2A (upper row) depicts the resulting confusion matrix illustrating that the deprived session is almost never mistaken for a morning session and a morning session almost never for a deprived session (all p<.002), i.e. brain connectivity following sleep deprivation is distinct from the non-deprived state. The uncertainty clearly lies in the evening condition, with biases toward the morning session. A similar classification task within the control group was expected to result in low classification performance, since conditions at TP1 and TP3 were similar (both scans performed in the morning after regular sleep). Indeed, the resulting accuracies were not above chance (Figure 2A, lower row).
Figure 2. Functional connectivity based classification of morning, evening and sleep deprivation scanning sessions.
The figure illustrates confusion matrices from various classification tasks. Significance was assessed based on permutation testing across 10000 iterations, each randomly permuting assignment to the three time points within subjects. (A) Within-group classifications of the three time points. (B) Classification of the three time points with classifiers trained on data from one group and tested on data from the other group. (C) Classification of TP3 with classifiers trained on TP1 and TP2. This allows to assess if TP3 is more similar to TP1 or TP2. (D) Classification of TP1 and TP2 in the merged sample (N=60, morning-to-evening variability, independent of sleep deprivation). SI-Figure 4 depicts similar classification patterns for networks based on partial correlations with automatic optimization of regularization strength on the single subject level.
We further tested the specificity of the features across groups. With a classifier trained on the deprived group, sessions of the control group were least often classified as being deprived (Figure 2B, upper row). As expected, training the classifier on the control group did not result in accuracies above chance for the deprived group, except for classification of TP1 (lower row).
Finally, in a binary classification task we assessed if the third session is more similar to the first or second session. Within the deprived group, TP3 was clearly classified as being evening-like (90.2%, p<.0001) rather than morning-like (9.8%, p<.0001, Figure 2C, upper row). In contrast, within the control group, the third session was equally classified as either morning (47%) or evening (53%, Figure 2C, lower row).
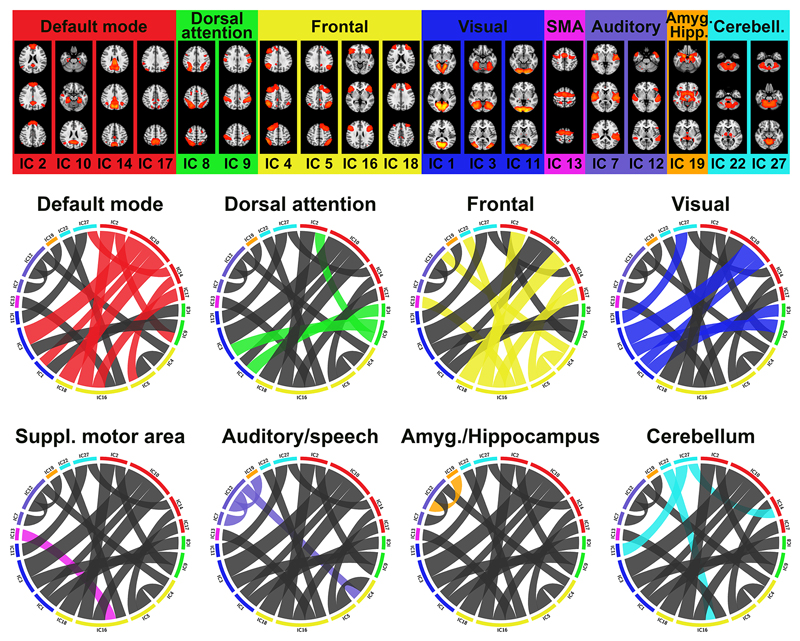
The above reported classification results indicated robust differences in connectivity profiles between the deprived and non-deprived state. We further assessed which edges were mostly affected using edge-wise repeated measures ANOVA and post-hoc independent-sample t-tests on each edge of the functional connectivity matrices. 17 out of 351 edges showed a significant group x time interaction at FDR level (p=.0023; SI-Table 1 for F statistics), involving default mode, dorsal attention, frontal, visual, auditory and motor networks as well as amygdala, hippocampus and the cerebellum. Figure 3 depicts the locality of significantly altered connection grouped by their functional units. Post-hoc t-tests revealed no significant difference between groups at the first morning scan, and no significant difference between groups in the evening for any of the 17 edges showing group by time interactions, which were all due to group differences at the second morning (SI-Table 1). A few edges indicated nominal (non-significant after corrections for multiple comparisons) differences at TP1 and TP2, yet the strongest effect was always seen at TP3.
Figure 3. Sleep deprivation altered brain connectivity in a range of functional network units.
The figure illustrates the 17 edges showing a significant group x time interaction effect in connectivity (due to deprivation at TP3), grouped by their functional sub-networks. The width of a link in each circular plot reflects the strength of the group x time interaction effect (ηpartial2 effect size). Each circular plot highlights the significant connections of one sub-network only, comprising default mode (red), dorsal attention (green), frontal (yellow), visual (blue), SMA (magenta), auditory/speech (purple), amygdala/hippocampus (orange) and cerebellum (cyan). The spatial maps of the 19 involved nodes (independent components) are shown on top. For surface-maps of all components, see SI-Figure 1. TP1: first morning, TP2: evening, TP3: second morning.
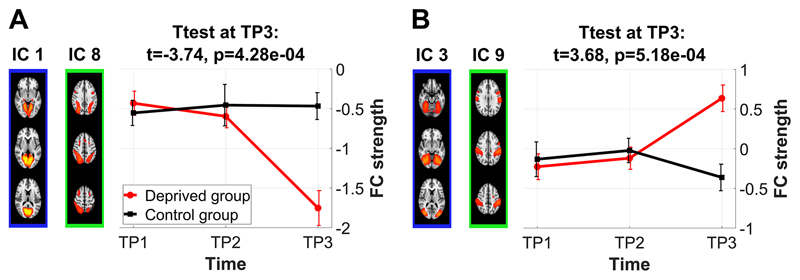
As depicted in Figure 4, edge connectivity in 13 of 17 edges shifted toward more positive correlations following deprivation (above diagonal in Figure 4) and only four of 17 edges shifted toward more negative correlations (IC 1-8, 11-27, 16-17, 16-22; below the diagonal in Figure 4). These four edges increased magnitude of negative correlations whereas five other edges showed reduced magnitude of negative correlations (IC 2-9, 2-16, 4-12, 10-18, 14-27; four of them involving the DMN, including a DMN-DAN edge IC 2-9). The remainder showed positive correlations at TP3, with five of them flipping the sign from negative to positive correlations (IC 3-9, 3-10, 4-5, 7-12, 13-16) and three edges increasing magnitude of positive correlations (IC 1-10, 5-14, 12-19).
Figure 4. Scatter plots of connectivity strength between TP1 and TP3 show a drift in connectivity strength following sleep deprivation.
The figure presents the 17 edges showing a group x time interaction (corresponding to figure 3). Colour indicates connectivity at TP2 and the white line indicates the diagonal. TP1: first morning, TP2: evening, TP3: second morning.
Figure 5 illustrates the temporal connectivity patterns of the two visual-DAN edges, recently shown to be particularly sensitive to attentional load level (Alnaes et al., 2015), showing remarkable overlap with the behavioural profiles of attentional vigilance and subjective sleepiness (Figure 1). This overlap was corroborated by significant edgewise repeated measures correlations (Bland and Altman, 1995). Functional connectivity in five of the 17 edges, including the visual-DAN edge IC 1-8, was significantly (FDR) correlated with IIV-RT (IC 1-8, 2-16, 3-10, 4-12, 11-27; SI-Table 2) and connectivity in six of the 17 edges, including both visual-DAN edges, was significantly correlated with mean-RT (IC 1-8, 2-16, 3-9, 3-10, 4-12, 11-27; SI-Table 3). Furthermore, connectivity in ten of the 17 edges was significantly associated with sleepiness ratings (IC 1-8, 1-10, 2-16, 3-10, 4-5, 4-12, 7-12, 13-16, 16-17, 16-22; SI-Table 4). Note that these repeated measures correlations corroborate the strength of effects at TP3, but do not necessarily imply a simple link between brain connectivity and vigilance and sleepiness, as correlations vanished when testing within time points or within the control group.
Figure 5. Temporal connectivity profiles of the two visual-DAN edges show a remarkable overlap with the profiles of attentional vigilance and subjective sleepiness (Figure 1).
Connectivity strength of IC 1-8 was significantly associated with IIV-RT and KSS scores. Connectivity strength of IC 3-9 was significantly correlated with mean RT in the ANT task (performed outside of scanner). TP1: first morning, TP2: evening, TP3: second morning.
3.3. Automated sleep staging from fMRI data to assess wakefulness probabilities
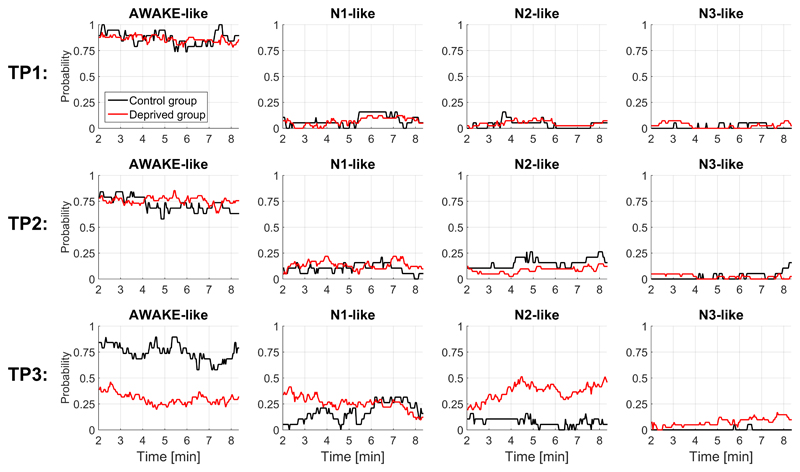
Next, we detected patterns of wakefulness and non-REM sleep stages 1-3 in the continuous fMRI data, using a recently published and validated pre-trained classifier (Tagliazucchi and Laufs, 2014). Figure 6 depicts the wakefulness/sleep probabilities as a function of recording time for each group and session. While there was no difference between groups at TP1 and TP2, wakefulness probabilities decreased and sleep probabilities increased at TP3 in the deprived group. We investigated if our main classification results (Figure 2) could potentially be driven by sleep, as indicated by the automated sleep classification. Independent of the exclusion criteria applied (Methods section 2.6), we retained similar classification results (SI-Figures 5-7).
Figure 6. Prediction of wakefulness and sleep stages (N1, N2 and N3) based on the connectivity profiles of the continuous fMRI data showed increased sleep probability at TP3 in the deprived group.
We used the classifier from Tagliazucchi and Laufs (2014).
3.4. Connectivity differences between morning and evening
Finally, we assessed morning-to-evening effects on functional connectivity in the merged sample in a binary classification task (TP1 vs. TP2). We classified time points at high accuracies with 76.7% of morning sessions being correctly identified as morning (p<.0001), and 65.0% of evenings correctly identified as evening (p<.004, Figure 2D).
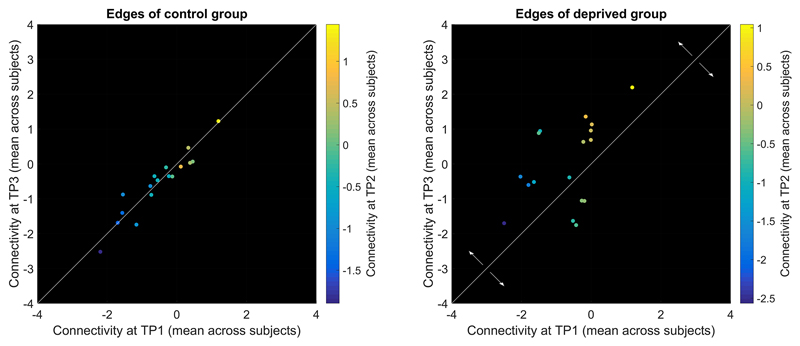
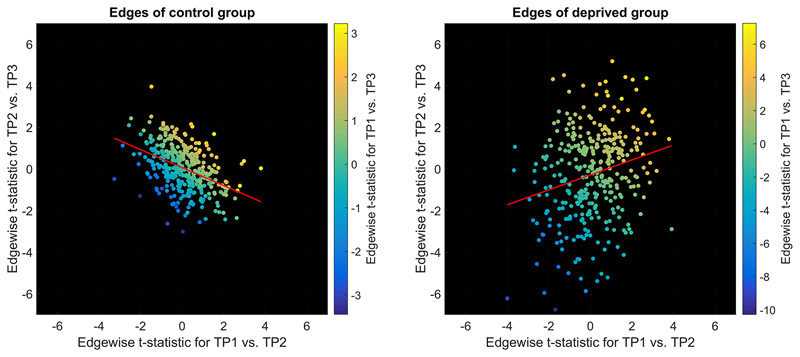
We further assessed diurnal connectivity alterations using edge-wise paired-sample t-tests. Figure 7 compares t-statistics between the two groups. For the deprived group, the t-statistic representing the difference between TP1 and TP2 was positively correlated to the t-statistic representing the difference between TP2 and TP3 (r=0.23, p<1e-05). Similar correlation in the control group flipped the sign (r=-0.43, p<1e-16). The regression slopes were significantly different (t=8.49, p<.05) between the two groups.
Figure 7. Correlation of the t-statistics reflecting morning-to-evening variability (TP1 vs. TP2) to t-statistics reflecting evening-to-next-morning variability (TP2 vs. TP3) indicated the impact of sleep on diurnal connectivity alterations (sign flip of the regression slope).
Least square regression lines are depicted in red. Colour indicates t-statistics for TP1 vs. TP3. TP1: first morning, TP2: evening, TP3: second morning
As can be seen from the magnitude of t-values in Figure 7, diurnal connectivity alterations were weak compared to deprivation induced alterations. When tested within groups separately, no diurnal alterations survived FDR correction. As t-statistics for TP1 vs. TP2 across all edges were significantly correlated between groups (r=0.24, p<1e-05) and as conditions were identical for both groups at TP1 and TP2, we also merged groups. In the merged sample, diurnal connectivity alterations in six edges survived FDR correction (visual-insula/cingulum: IC 3-15, t=3.8, p=4e-04; DMN-hippocampus/amygdala: IC 10-19, t=3.9, p=3e-04; auditory/speech-frontal: IC 12-16, t=4.6, p=3e-05; frontal-sensorimotor: IC 18-20, t=3.8, p=4e-04; within-auditory/speech: IC 7-12, t=-3.8, p=4e-04; DAN-auditory/speech: IC 9-26, t=-4.2, p=1e-04).
3.5. Motion as a potential confounder of results
The deprived group showed significantly more motion (defined as the average root mean square of the displacement from one frame to its previous frame) at TP3 compared to TP1 (t=-6.2, p<.0001) and TP2 (t=-4.8, p<.0001) whereas time points of the control group did not differ in motion (all p>.6). However, group x time interaction effects in all of the 17 edges remained significant when accounting for mean estimated motion in linear mixed effects models.
4. Discussion
4.1. Brain connectivity robustly altered by lack of sleep
Utilizing a combination of univariate statistics and machine learning, we have demonstrated widespread and robust functional connectivity alterations following sleep deprivation and assessed how these alterations integrate with regular diurnal variability. Sleep deprivation reduced attentional vigilance, as expected, and we identified a set of 17 brain network connections showing significant group x time interactions of brain connectivity alterations. Furthermore, a comparison of morning-to-evening connectivity alterations to alterations between evening and next morning suggests that sleep reverted diurnal connectivity changes whereas sleep deprivation did not show this effect, thereby potentially indicating the impact of sleep on diurnal connectivity alterations.
A few previous studies have investigated connectivity alterations following sleep deprivation (e.g., Bosch et al., 2013; De Havas et al., 2012; Shao et al., 2014; Yeo et al., 2015; Yoo et al., 2007), yet the lack of a control group or evening scans has made it difficult to distinguish deprivation induced effects from morning-to-evening and morning-to-morning variability. With classification of time points, we first showed that connectivity profiles following sleep deprivation yield significant classification accuracies, largely outperforming those based on regular morning/evening profiles. Next, studying edge-wise group x time interactions, we identified 17 edges showing significant effects of sleep deprivation on connectivity, involving DMN, DAN, frontal, visual, SMA, auditory/speech, amygdala/hippocampus and cerebellar networks.
More specifically, connectivity of the dorsal attention network with visual areas was strengthened following sleep deprivation (IC 1-8 and 3-9). Connectivity profiles in one of these edges (IC 1-8, Figure 5) were significantly correlated with profiles of attentional vigilance, as measured using ANT (Figure 1A). These repeated measures correlations do not necessarily imply a deprivation-independent association between brain connectivity and attentional vigilance, as they were not observed when testing within time points or within the control group. However, they fit well the recent report on increased DAN-visual connectivity related to increased attentional effort (Alnaes et al., 2015). Furthermore, replicating previous findings (De Havas et al., 2012; Yeo et al., 2015), the anti-correlation between DAN and DMN decreased after sleep deprivation. In addition, connectivity alterations involving the DMN included edges to visual and frontal networks, whereas, irrespective of earlier findings, within-DMN alterations were not present among the group x time interactions. A recent study suggested that the strength in alteration of visual-DMN connections may depend on subjects’ vulnerability to sleep deprivation (Yeo et al., 2015). This may well be supported by our data, as the edge yielding the strongest interaction effect was visual-DMN (IC 3-10), thereby indicating particularly strong impact of sleep deprivation. Among the frontal networks, one within-network connection between the left and right fronto-parietal network was significantly strengthened following deprivation, whereas other altered frontal connections linked to SMA, cerebellar and auditory/speech networks. Finally, the auditory/speech network showed several altered connections, one within-auditory, one to the fronto-parietal network and one to hippocampus/amygdala. These novel results match well with recent reports of impaired auditory processing following 24h of sleep deprivation (Liberalesso et al., 2012).
Although diurnal connectivity alterations were weaker than deprivation-induced alterations, we significantly discriminated between morning and evening connectivity profiles. These findings add to a growing body of evidence indicating that changes in functional brain connectivity can occur within hours of prolonged wakefulness (Park et al., 2012; Shannon et al., 2013). Our results indicate that diurnal connectivity alterations are reverted after sleep, a pattern that only appeared in the control group. These results emphasise the importance of accounting for regular diurnal variability when studying sleep deprivation (e.g. by employing studies with deprivation durations of 36 hours). Furthermore, by suggesting an important role of time-of-day on brain connectivity, they have potentially important implications for a range of applications, including a potential bias in scientific studies when group assignments are not balanced for time-of-day, and provide important information for researchers designing longitudinal studies. Diurnal changes after a day of waking could either be related to circadian rhythms or to waking-related homeostatic processes. To further disentangle these mechanisms, future research would benefit from increasing the number of scans during the day, both before and after sleep deprivation, and to sleep deprive until the following evening.
4.2. Limitations
As for any study on sleep deprivation, it is difficult to assess the degree to which effects reflect compensatory mechanisms. Clearly, an edge could be altered as a direct consequence of deprivation or due to compensation of deprivation, e.g. related to increased effort to stay awake. We chose to incorporate a uniform sample of young males, as there is recent evidence that compensatory effects in the brain following sleep deprivation are more pronounced among the elderly (Almklov et al., 2014). Consequently, age and gender should not confound the results in this study and compensatory effects are likely kept low. Yet future research could make use of the age-related increase in compensatory effects to distinguish between compensatory and non-compensatory deprivation-induced connectivity alterations. Furthermore, assessment of sleep behaviour in our study relies on self-reports, which may yield less accurate measures than objective measures of sleep behaviour based on polysomnography or actigraphy. Future studies may employ such methods to verify sleep duration both before and during similar experiments. Due to the scanning schedule, participants of the control group reported significantly less sleep than usual between TP2 and TP3 (average: 6.1 ± 0.9 hours). Should the decreased sleep duration have induced any bias, it is however likely that this study underestimates (rather than overestimates) the true size of sleep deprivation effects.
In addition, due to the absence of eye tracking or EEG during scanning we cannot fully rule out confounds by brief periods of sleep, especially considering recent research indicating drifts between wakefulness and sleep during resting state MRI recordings, even in the non-deprived state (Tagliazucchi and Laufs, 2014). However, we have made great efforts to account for this lack by applying a multivariate connectivity-based wake and non-REM sleep classifier for fMRI data (Tagliazucchi and Laufs, 2014). We found increased sleep probabilities in the deprived group at TP3. Note that the sleep staging classifier has not been trained on data collected during sleep deprivation, and it can only select between “awake-like” and “sleep-like”. Therefore, classification of sleep does not necessarily imply true sleep, and an alternative explanation is that sleep deprived wakefulness shares connectivity features with sleep to such an extent that true wake periods are classified as sleep. Also, the fact that all subjects specifically reported that they had not fallen asleep during the fMRI and that N2/N3 sleep – both classified repeatedly in our data – are unlikely to occur in an eight-minute scan, increases the uncertainty in the automated sleep classification. Lastly, the classification algorithm pertains to probabilities of one class versus another, and the results should not be interpreted as deterministic. However, even when excluding time series snips or whole subjects with high sleep probabilities as indicated by the automated algorithm based on connectivity features, we retained the reported patterns of classification of time points, strongly supporting that the high accuracies in classifying the sleep deprived state are not purely driven by sleep.
Finally, our results are based on networks derived from an independent component analysis across all scans, which could bias the results due to unbalanced group sizes. We preferred to have all scans represented in the decomposition to the alternative of basing the analysis on fewer subjects or time points only. Future studies may be able to benefit from ongoing developments to increase the reliability and therefore also the sensitivity of brain connectivity estimates, both related to data acquisition and analysis (Smith, 2012).
4.3. Conclusion
The present results underline the major impact of sleep deprivation on the functional human brain connectome, particularly alterations in the dorsal attention, default mode, visual, frontal, auditory, cerebellar, motor and hippocampal networks. The effects proved robust on the single-subject level, as verified using cross-validated classification procedures and permutation testing. Finally, our analyses indicated that morning-to-evening connectivity alterations are reverted after sleep – a pattern that did not occur after deprivation.
Supplementary Material
Acknowledgements
The authors would like to thank the participants of the study for their contribution. The study was financially supported by the Research Council of Norway (#213837, #223273, #204966/F20), the South-Eastern Norwegian Regional Health Authorities (#2014-097, #2013-123), Oslo University Hospital – Rikshospitalet, a research grant from Mrs. Aslaug Throne-Holst, a research grant from the Ebbe Frøland Foundation and by the KG Jebsen Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript and the authors declare no conflict of interest.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Abou Elseoud A, Littow H, Remes J, Starck T, Nikkinen J, Nissila J, Timonen M, Tervonen O, Kiviniemi V. Group-ICA Model Order Highlights Patterns of Functional Brain Connectivity. Front Syst Neurosci. 2011;5:37. doi: 10.3389/fnsys.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- Almklov EL, Drummond SP, Orff H, Alhassoon OM. The Effects of Sleep Deprivation on Brain Functioning in Older Adults. Behav Sleep Med. 2014:1–22. doi: 10.1080/15402002.2014.905474. [DOI] [PubMed] [Google Scholar]
- Alnaes D, Kaufmann T, Richard G, Duff EP, Sneve MH, Endestad T, Nordvik JE, Andreassen OA, Smith SM, Westlye LT. Attentional load modulates large-scale functional brain connectivity beyond the core attention networks. Neuroimage. 2015;109:260–272. doi: 10.1016/j.neuroimage.2015.01.026. [DOI] [PubMed] [Google Scholar]
- Anderson C, Platten CR. Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behav Brain Res. 2011;217:463–466. doi: 10.1016/j.bbr.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation. FMRIB technical report TR07JA2. 2010 [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1--Correlation within subjects. BMJ. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OG, Rihm JS, Scheidegger M, Landolt HP, Stampfli P, Brakowski J, Esposito F, Rasch B, Seifritz E. Sleep deprivation increases dorsal nexus connectivity to the dorsolateral prefrontal cortex in humans. Proc Natl Acad Sci U S A. 2013;110:19597–19602. doi: 10.1073/pnas.1317010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfitsen MT. Tiredness and Visual Reaction-Time among Young Male Nighttime Drivers - a Roadside Survey. Accident Analysis and Prevention. 1994;26:617–624. doi: 10.1016/0001-4575(94)90023-x. [DOI] [PubMed] [Google Scholar]
- De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59:1745–1751. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon 4 allele. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Friedman JH. Regularized Discriminant-Analysis. Journal of the American Statistical Association. 1989;84:165–175. [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. Journal of Neuroscience. 2011;31:4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley AC, Skogen JC, Overland S, Wold B, Williams LJ, Kennedy GA, Sivertsen B. Trajectories and stability of self-reported short sleep duration from adolescence to adulthood. J Sleep Res. 2015;24:621–628. doi: 10.1111/jsr.12316. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Skatun KC, Alnaes D, Doan NT, Duff EP, Tonnesen S, Roussos E, Ueland T, Aminoff SR, Lagerberg TV, Agartz I, et al. Disintegration of Sensorimotor Brain Networks in Schizophrenia. Schizophr Bull. 2015 doi: 10.1093/schbul/sbv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoit O, Wolf M. Improved estimation of the covariance matrix of stock returns with an application to portfolio selection. J Empir Finance. 2003;10:603–621. [Google Scholar]
- Liberalesso PB, D'Andrea KF, Cordeiro ML, Zeigelboim BS, Marques JM, Jurkiewicz AL. Effects of sleep deprivation on central auditory processing. BMC Neurosci. 2012;13:83. doi: 10.1186/1471-2202-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Ma N, Dinges DF, Basner M, Rao H. How Acute Total Sleep Loss Affects the Attending Brain: A Meta-analysis of Neuroimaging Studies. Sleep. 2014 doi: 10.5665/sleep.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, T., 2009. FDR implementation provided online by Tom Nichols at http://www-personal.umich.edu/~nichols/FDR/.
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Park B, Kim JI, Lee D, Jeong SO, Lee JD, Park HJ. Are brain networks stable during a 24-hour period? Neuroimage. 2012;59:456–466. doi: 10.1016/j.neuroimage.2011.07.049. [DOI] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, Buitelaar JK, Beckmann CF. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.02.063. [DOI] [PubMed] [Google Scholar]
- Rogers NL, Dorrian J, Dinges DF. Sleep, waking and neurobehavioural performance. Front Biosci. 2003;8:s1056–1067. doi: 10.2741/1174. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer J, Strimmer K. A shrinkage approach to large-scale covariance matrix estimation and implications for functional genomics. Stat Appl Genet Mol Biol. 2005;4 doi: 10.2202/1544-6115.1175. Article32. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Dosenbach RA, Su Y, Vlassenko AG, Larson-Prior LJ, Nolan TS, Snyder AZ, Raichle ME. Morning-evening variation in human brain metabolism and memory circuits. J Neurophysiol. 2013;109:1444–1456. doi: 10.1152/jn.00651.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Lei Y, Wang L, Zhai T, Jin X, Ni W, Yang Y, Tan S, Wen B, Ye E, Yang Z. Altered resting-state amygdala functional connectivity after 36 hours of total sleep deprivation. PLoS One. 2014;9:e112222. doi: 10.1371/journal.pone.0112222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Do all animals sleep? Trends Neurosci. 2008;31:208–213. doi: 10.1016/j.tins.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. The future of FMRI connectivity. Neuroimage. 2012;62:1257–1266. doi: 10.1016/j.neuroimage.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Smith SM, Hyvarinen A, Varoquaux G, Miller KL, Beckmann CF. Group-PCA for very large fMRI datasets. Neuroimage. 2014;101:738–749. doi: 10.1016/j.neuroimage.2014.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, Ramsey JD, Woolrich MW. Network modelling methods for FMRI. Neuroimage. 2011;54:875–891. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E, Laufs H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron. 2014;82:695–708. doi: 10.1016/j.neuron.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cereb Cortex. 2011;21:345–356. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O, Liu TT. The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage. 2013;83:983–990. doi: 10.1016/j.neuroimage.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Tandi J, Chee MW. Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage. 2015;111:147–158. doi: 10.1016/j.neuroimage.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.