Abstract
The ability of vertebrates to occupy diverse niches has been linked to the spectral properties of rhodopsin, conferring rod-based vision in low-light conditions. More recent insights have come from nonspectral kinetics, including the retinal release rate of the active state of rhodopsin, a key aspect of scotopic vision that shows strong associations with light environments in diverse taxa. We examined the retinal release rates in resurrected proteins across early vertebrates and show that the earliest forms were characterized by much faster rates of retinal release than more recent ancestors. We also show that scotopic vision at the origin of tetrapods is a derived state that arose via at least 4 major shifts in retinal release rate. Our results suggest that early rhodopsin had a function intermediate to that of modern rod and cone pigments and that its well-developed adaptation to low light is a relatively recent innovation since the origin of tetrapods.
Keywords: rhodopsin, metarhodopsin II, vertebrates, evolution
Vision in vertebrates at dim-light (mesopic) and very low-light (scotopic) conditions is mediated by the visual pigment rhodopsin (RH1), expressed in rod cells. RH1 evolved from cone pigments by gene duplication before the split between jawed vertebrates and lampreys (1). This origin of RH1 accords with suggestions that the earliest vertebrates were likely diurnal and lived in shallow water and that the transition to lower light niches occurred later in their evolution (1).
Most insights into rod vision evolution come from data on spectral tuning (2). More recently, attention has focused on nonspectral adaptations (3–5). After photobleaching, the slow dissociation rate of the retinal (half-life of retinal release rate, or t1/2) from the light-activated RH1 (metarhodopsin II) leads to an extended signaling state, and comparative analyses suggest that retinal release rates correlate with photic niches, with long half-lives adaptive for low light (4, 5). However, few studies have examined nonspectral properties in ancestral RH1 pigments (3, 4). To obtain insight into the vision of early vertebrates, we performed reconstructions of ancestral RH1 for the major vertebrate lineages and examined their retinal release rates.
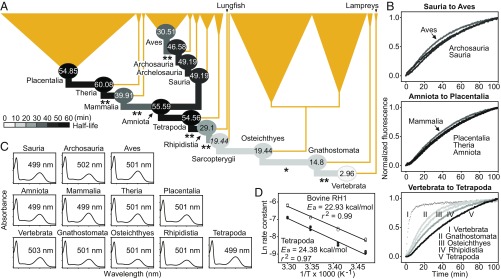
We recorded dramatic changes in retinal release rates of our ancestral RH1 pigments, compared with only minor differences (1 to 4 nm) in the wavelengths of maximum absorption (λmax) (Fig. 1). The common ancestor of vertebrates had a half-life around an order of magnitude shorter than that of the cow (t1/2 = 2.96 versus 21.82 min, respectively; P < 0.01), implying that it was likely less adapted to low light and its retinal release rate was functionally equivalent to that of a rod–cone intermediate (6). Our results reveal 2 major shifts in half-life during the subsequent diversification of nonterrestrial vertebrates, each toward a lower-light niche (Fig. 1). The first increase in half-life (from 2.96 to 14.8 min) occurred near the origin of jawed vertebrates (Gnathostomata) and involved a key residue (189) (4). Repeating our assay with RH1 proteins reconstructed using amino acids based on a codon model gave a greater shift (from 2.29 to 15.27 min; P < 0.01), although effects of inferred rarer variants were not tested. We found further, less dramatic half-life increases in the ancestors of bony fishes (Osteichthyes) and lobe-finned fishes (Sarcopterygii) (from 14.8 to 19.44 min). None of these values were greater than the cow’s t1/2, again suggesting that these ancestors were less low-light adapted.
Fig. 1.
Retinal release rates and spectral sensitivities of ancestral RH1 pigments inferred from an amino acid model. (A) Species tree with focal ancestors (circles) and their RH1 half-life values. Sequences for the ancestral Sauria and Archelosauria were identical, and the Sarcopterygii value (shown in italics) was predicted from a near-identical sequence. Significant differences between t1/2 values for adjacent nodes are shown (*P < 0.05; **P < 0.01). (B) Evolutionary changes in RH1 retinal release rate, with plots based on mean values from 3 to 4 assays. (C) Spectral sensitivities and λmax values. (D) Arrhenius activation energies for Tetrapoda (filled circles) and bovine (open circles) RH1.
Two more dramatic shifts toward increasingly scotopic vision occurred during the transition of early vertebrates to a terrestrial niche. We detected jumps in t1/2 from 19.44 min in the ancestor of Sarcopterygii to 29.1 min in that of Rhipidistia (tetrapods plus lungfish), and then to 54.56 min in the Tetrapoda (a value 2 times longer than the cow’s t1/2) (Fig. 1), and these findings held for RH1 based on the codon model (31.48 to 49.39 min; P < 0.01). Overall, the apparently slower retinal release rates after the origin of tetrapods implies selection for more scotopic activity, supporting suggestions that the tetrapod ancestor was nocturnal (7).
The absence of clear shifts in retinal release rate between the ancestors of tetrapods (54.56 min) and amniotes (55.59 min) suggests that the latter were, like the former, mainly active in light-limited environments (7). In contrast, shifts in t1/2 occurred following the split between early mammals and saurians (Fig. 1). The t1/2 value of 39.91 min at the origin of Mammalia augments ideas that the earliest mammals were not strictly nocturnal but instead occupied both nocturnal and crepuscular niches (8). While our results support claims that early synapsids likely exhibited diverse diel activity patterns at day/night (9), they contradict findings based on fewer taxa that the retinal release rate slowed at the origin of mammals (4). We find that the half-life reaches a maximum at the origin of Theria (60.08 min; Fig. 1), implying that this form was perhaps most adapted to scotopic conditions.
RH1 retinal release rates in the Sauria showed a more consistent evolutionary trajectory, except for a faster rate at the origin of Aves (Fig. 1). This trend (from 46.58 to 30.51 min) supports suggestions that early saurians and archosaurs were nocturnal, based on reconstructed diel activity patterns (7). Nocturnality in archosaurs has also been inferred from G protein activation kinetics of ancestral pigments (3), while morphological analyses of Mesozoic fossils point to both day and night niches (10). By adding to the evidence that the ancestral archosaur had scotopic vision, consistent with nocturnality (7), our data on retinal release rates help to overturn earlier ideas that mammals evolved from diurnal dinosaurs (11). On the other hand, our data show that the earliest birds were adapted to bright light conditions (7).
Our results on retinal release rates extend current understanding of adaptive change in RH1 pigments to the evolution of the earliest vertebrates. By widening taxonomic breadth, we are able to show that these early taxa were poorly adapted to scotopic vision. Thus, well-developed scotopic vision at the origin of tetrapods may represent a relatively recent innovation.
Materials and Methods
Vertebrate RH1 genes were obtained from GenBank. We reconstructed ancestral RH1 sequences under amino acid (LG+Γ) model using a published tree topology in CODEML (12) and used these to express proteins for functional analyses (Datasets S1–S4).
Briefly, RH1 was expressed, regenerated (adding 11-cis-retinal), and purified in an elution buffer (40 μM RH1 epitope, 50 mM Hepes at pH = 6.6, 0.1% n-dodecyl β-d-maltoside, 20% glycerol, 140 mM NaCl, 3 mM MgCl2). The λmax and retinal release rate of the pigments were then measured. Retinal release was measured every 30 s at 20 °C (13). Curves were fitted using y = y0 + a(1 − e−bx), and the half-lives of retinal release were calculated (t1/2 = ln2/b) (4). The r2 for all fitted curves was >0.99, except for Vertebrata (0.87 to 0.98), as also reported for the cone pigment that shows much faster retinal release (6). We compared t1/2 values between pairs of ancestral pigments (adjacent nodes) in the tree with 2-tailed t tests. As a control, we synthesized the bovine RH1 (4, 5). We consider the cow to be better adapted to low light than other day-active species; they are diurnal but also show crepuscular activity, and they possess duplex retinas with rods that show intermediate patterns between those of nocturnal and diurnal species (14).
To ensure that our results were robust to model choice, we resynthesized and measured the t1/2 of RH1 of 4 focal ancestral taxa using sequences from the codon (“free-ratio”) model, which differed by 4 to 17 residues from the amino acid model. For Tetrapoda and bovine RH1, we also estimated the Arrhenius activation energies (Ea) of Schiff base hydrolysis (6) as 24.38 and 22.93 kcal/mol, respectively, based on retinal release rates at 15, 20, 25, and 30 °C.
Supplementary Material
Acknowledgments
We thank R. Crouch (Medical University of South Carolina) and L. Neuhold (NIH) for the 11-cis-retinal. This work was funded by National Natural Science Foundation of China grants to Y.L. (31601855) and S.Z. (31570382) and by a European Research Council Starting grant (310482) to S.J.R.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1900481116/-/DCSupplemental.
References
- 1.Pisani D., Mohun S. M., Harris S. R., McInerney J. O., Wilkinson M., Molecular evidence for dim-light vision in the last common ancestor of the vertebrates. Curr. Biol. 16, PR318–R319 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Yokoyama S., Tada T., Zhang H., Britt L., Elucidation of phenotypic adaptations: Molecular analyses of dim-light vision proteins in vertebrates. Proc. Natl. Acad. Sci. U.S.A. 105, 13480–13485 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang B. S., Jönsson K., Kazmi M. A., Donoghue M. J., Sakmar T. P., Recreating a functional ancestral archosaur visual pigment. Mol. Biol. Evol. 19, 1483–1489 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Bickelmann C., et al. , The molecular origin and evolution of dim-light vision in mammals. Evolution 69, 2995–3003 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez E. A., et al. , Functional shifts in bat dim-light visual pigment are associated with differing echolocation abilities and reveal molecular adaptation to photic-limited environments. Mol. Biol. Evol. 35, 2422–2434 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Chen M. H., Kuemmel C., Birge R. R., Knox B. E., Rapid release of retinal from a cone visual pigment following photoactivation. Biochemistry 51, 4117–4125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson S. R., Wiens J. J., Out of the dark: 350 million years of conservatism and evolution in diel activity patterns in vertebrates. Evolution 71, 1944–1959 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Liu Y., Chi H., Li L., Rossiter S. J., Zhang S., Molecular data support an early shift to an intermediate-light niche in the evolution of Mammals. Mol. Biol. Evol. 35, 1130–1134 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Angielczyk K. D., Schmitz L., Nocturnality in synapsids predates the origin of mammals by over 100 million years. Proc. Biol. Sci. 281, 20141642 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz L., Motani R., Nocturnality in dinosaurs inferred from scleral ring and orbit morphology. Science 332, 705–708 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Crompton A. W., Taylor C. R., Jagger J. A., Evolution of homeothermy in mammals. Nature 272, 333–336 (1978). [DOI] [PubMed] [Google Scholar]
- 12.Yang Z., PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Farrens D. L., Khorana H. G., Structure and function in rhodopsin. Measurement of the rate of metarhodopsin II decay by fluorescence spectroscopy. J. Biol. Chem. 270, 5073–5076 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Solovei I., et al. , Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137, 356–368 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.