Significance
Suppression of a learned response is critical to survival and adaptation and is impaired in various diseases. Conditioned inhibition is proposed to require learning and inhibitory processes, but its exact nature is poorly understood. Here we studied safety cue-triggered fear suppression and found that its learning process requires plasticity in the ventral tegmental area (VTA), leading to an enhanced dopaminergic (DA) neuron activity by safety cue, and its inhibitory process requires VTA DA neuron inputs to parvalbumin neurons in the dorsomedial prefrontal cortex (dmPFC) to reduce dmPFC activity and fear responses. This DA-dependent learning process is impaired in a posttraumatic stress disorder model. Thus, conditioned inhibition requires complex interactions between DA and GABAergic signaling to suppress learned responses.
Keywords: dopamine, prefrontal cortex, ventral tegmental area, parvalbumin neurons, safety learning
Abstract
Conditioned inhibition is an important process to suppress learned responses for optimal adaptation, but its underlying biological mechanism is poorly understood. Here we used safety learning (SL)/fear discrimination after fear conditioning as a conditioned inhibition model because it demonstrates the essential properties of summation and retardation. Activity of the dorsomedial prefrontal cortex (dmPFC) parvalbumin (PV) neurons bidirectionally regulates spiking levels of dmPFC excitatory neurons and fear states. Responses to safety cues are increased in dopaminergic (DA) neurons in the ventral tegmental area (VTA) and in PV neurons in dmPFC after SL. Plasticity in the VTA is implicated, since SL requires activation of N-methyl-d-aspartate receptors. Furthermore, in a posttraumatic stress disorder model, impaired SL is associated with impaired potentiation of VTA DA neuron activity. Our results demonstrate a DA-dependent learning process that targets prefrontal inhibitory neurons for suppression of learned responses, and have implications for the pathogenesis and treatment of various psychiatric diseases.
Learning, especially emotional learning, is not and should not be precise in nature to allow optimal adaptation, since very rarely the exact same circumstance occurs repeatedly in the real world. Appropriate generalization of learned responses has adaptive value (1, 2); for example, adequate generalization of fear memory may enable the expression of these responses under similar situations when a danger is eminent or predicted (3, 4). On the other hand, discrimination between safe and dangerous circumstances/cues is critical to survival. An animal can learn to stop responding or to suppress learned responses in the presence of a safety cue, a form of learning termed conditioned inhibition (1, 2, 5, 6).
Conditioned inhibition has been recognized as an important biological process for survival and adaptation, as an animal learns to take advantage of safety in its environment (7, 8), and safety learning (SL) has antidepressant effects (9, 10) and adaptive value for behavioral flexibility (2). Inability to suppress fear by safety cues results in excessive generalization of fear responses to harmless stimuli, which has been proposed as a core symptom of anxiety disorders (4, 11, 12). Although suppression of learned responses has been modeled as a conditioned inhibitor (6), the underlying biological process remains poorly understood.
The core feature of conditioned inhibition is suppression of learned behavior through learning. SL can be a good model system for studying conditioned inhibition due to its robust nature and clear relevance to various psychiatric disorders. Opposite changes in neuronal spiking and dendritic spine size have been reported after fear conditioning (FC) and SL (13, 14). Evidence both for and against the contribution of the medial prefrontal cortex (mPFC) to SL/fear discrimination have been reported (15–18). Spiking of a dorsomedial prefrontal cortex (dmPFC) subpopulation of neurons correlates with fear responses after auditory conditioning, implicating their regulation of freezing level in a bidirectional manner (19, 20). These results identify the PFC as a key brain region mediating inhibitory control.
Either reward or aversive stimuli causes the release of dopamine (21). Dopamine signaling is important in fear conditioning (FC), generalization, and discrimination (22). Importantly, ventral tegmental area (VTA) dopaminergic (DA) neurons contribute to the learning process in a projection-dependent manner (23). Efferents of DA neurons to the PFC target both excitatory and inhibitory neurons and likely activate both D1 and D2 subtype DA receptors (24, 25). Both in vitro and in vivo studies have shown that DA inputs directly activate inhibitory neurons, particularly fast-spiking interneurons, in the PFC, resulting in feed-forward inhibition of principal excitatory neurons (24, 25). Thus, a DA-mediated increase in inhibition may contribute or even mediate conditioned inhibition/fear discrimination. Given that altering DA receptor activity in the amygdala affects conditioned inhibition or fear discrimination (26, 27), it will be of great interest to examine whether DA signaling in the PFC contributes to SL/conditioned inhibition by recruiting inhibitory neurons.
In this study, we found that SL exhibits essential features of conditioned inhibition, namely summation and retardation. Fear suppression requires activation of dmPFC parvalbumin (PV) neurons in an conditioned stimulus (CS)-dependent manner. This association between safety cue and fear suppression is mediated by increased activity of VTA DA neurons, requires N-methyl-d-aspartate receptor (NMDAR) activity during learning, and is impaired in a mouse model of posttraumatic stress disorder (PTSD). The finding that conditioned inhibition requires interactions between DA and GABAergic signaling sheds light on the mechanisms underlying emotional regulation and its dysregulation in diseases.
Results
Spiking Activity in dmPFC Neurons Discriminates Between Reinforced CS (CS+) and Nonreinforced CS (CS−) After SL.
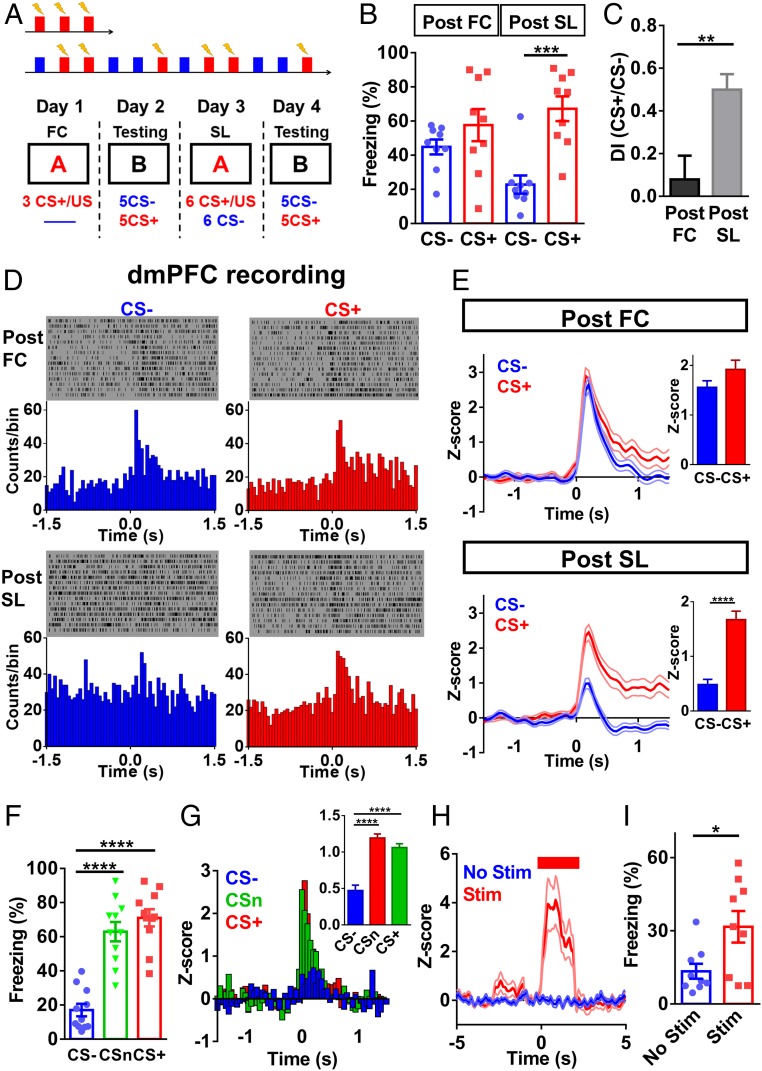
Spiking in a subpopulation of dmPFC neurons was increased during reinforced CS (CS+; danger cue) and low during nonreinforced CS (CS−; safety cue) after FC (20). We simultaneously measured spike rates of dmPFC neurons and fear responses (freezing percent) in the same behaving mouse. Our recording region included the prelimbic cortex and cingulate cortex area 1. To best understand changes in neuronal activity, we monitored spiking activity from the same neurons under different experimental conditions over several days. The recorded spikes were distinct and stable, as shown by spike waveforms (SI Appendix, Figs. S1 A–C) and principal component space cylinder (SI Appendix, Fig. S1D). After FC (Fig. 1A), freezing levels were high during both CS+ and CS− [unconditioned CS at this time], with no significant difference between them (Fig. 1B). This state of fear generalization can also be quantified as a low discrimination index (Fig. 1C). In contrast, SL (i.e., discriminative learning) led to significantly lower freezing during CS− (Fig. 1 A and B) and a higher discrimination index (Fig. 1C). In the same mice, dmPFC neurons (∼25% of all recorded neurons) showed similar spike rate increases during CS+ and CS− after FC (Fig. 1 D and E) but much lower spike increases during CS− than during CS+ after SL (Fig. 1 D and E). Thus, there is a good correlation between changes in dmPFC spike rate and freezing level. After SL, responses to a third CS (CSn, to which mice had not been exposed) were similar to the responses to CS+ but significantly higher than those to CS− in terms of both freezing level (Fig. 1F) and dmPFC spike rate (Fig. 1G), demonstrating the selectivity of SL to CS−.
Fig. 1.
Differential fear responses and dmPFC neuronal activity associated with fear generalization and SL. (A) Experimental procedure for FC and SL. (B) Freezing levels during CS+ and CS− after FC and after SL [two-way repeated-measures (RM) ANOVA; interaction, F(1,8) = 12.62, P < 0.01; stimulus, F(1,8) = 16.37, P < 0.01; training, F(1,8) = 2.746, P = 0.136; n = 9 mice; post-FC, CS− vs. CS+, P = 0.15, post-SL, CS− vs. CS+, P < 0.001, Bonferroni’s posttest]. (C) Discrimination index post-FC and post-SL (two-tailed paired t test, t = 3.608, df = 8; n = 9 mice, post-FC vs. post-SL, P < 0.01). (D) Sample recording of dmPFC neuronal spikes post-FC (Upper) and post-SL (Lower). (E) Population data on dmPFC neuronal spike change post-FC (Upper) and post-SL (Lower), with corresponding average z-scores (two-tailed paired t test; post-FC, t = 1.856, df = 49; post-SL, t = 10.95, df = 49; n = 50 units/11 mice; post-FC, CS− vs. CS+, P = 0.07; post-SL, CS− vs. CS+, P < 0.0001). In this and subsequent figures, thick lines represent mean and thin lines represent SEM. (F) Fear suppression was selective to CS− after SL [one-way RM ANOVA, F(2, 20) = 40.18, P < 0.0001; n = 11 mice, CS− vs. CSn, P < 0.0001; CS− vs. CS+, P < 0.0001; CSn vs. CS+, P = 0.82, Bonferroni’s posttest]. (G) Smaller dmPFC neuronal spike change occurred only during CS− after SL [one-way RM ANOVA, F(2, 72) = 46.52, P < 0.0001; n = 37 units/7 mice; CS− vs. CSn, P < 0.0001; CS− vs. CS+, P < 0.0001; CSn vs. CS+, P = 0.28, Bonferroni’s posttest]. (H) Electrical stimulation in the dmPFC elevated dmPFC spiking (n = 30 units/7 mice). (I) Elevated freezing level during electrical stimulation in dmPFC (two-tailed paired t test, t = 2.57, df = 8; n = 9 mice, no stim vs. stim, P < 0.05). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Data represent mean ± SEM.
Although the foregoing results are consistent with dmPFC neurons driving fear behavior (28, 29), the observed dmPFC spike changes could be responding to elevated freezing. Thus, we stimulated dmPFC neurons during CS− after SL via the implanted stimulating electrodes and found elevated dmPFC spike rates (Fig. 1H) and freezing levels (Fig. 1I), while stimulation in the absence of CS did not change freezing levels (SI Appendix, Fig. S2).
SL Exhibits Essential Properties of Conditioned Inhibition.
Conditioned inhibition has two fundamental properties: (i) summation, reduced fear response when CS+ and CS− are presented simultaneously (5, 6), and (ii) retardation, lower fear responses when safety cue is paired to the same US (6, 30). We tested whether these two properties are met at both behavioral and neuronal activity levels after SL. First, when both CS+ and CS− were presented simultaneously, freezing level was between the levels seen during either CS+ or CS− alone (Fig. 2A), indicating suppression of CS+-induced fear response by CS−. In the same mice, changes in the spike rate of dmPFC neurons were of a similar pattern, that is, the responses to CS+/CS− were between the responses to CS+ or CS− alone (Fig. 2B).
Fig. 2.
SL meets the criteria for conditioned inhibition. (A) Summation effect at the behavioral level after SL [one-way RM ANOVA, F(2, 38) = 131.6, P < 0.0001; n = 20 mice, CS− vs. CS+, P < 0.0001; CS− vs. CS+/−, P < 0.0001; CS+ vs. CS+/−, P < 0.0001, Bonferroni’s posttest]. (B) Summation effect at neuronal activity level after SL [one-way RM ANOVA, F(2, 106) = 36.53, P < 0.0001; n = 54 units/12 mice; CS− vs. CS+, P < 0.0001; CS− vs. CS+/−, P < 0.001; CS+ vs. CS+/−, P < 0.001, Bonferroni’s posttest]. (C) Retardation effect at behavioral level after pairing of CS− with US [one-way RM ANOVA, F(2, 18) = 41.94, P < 0.0001; n = 10 mice, SL (CS+) vs. CS−/US, P < 0.01, Bonferroni’s posttest]. (D) Sample recording in the dmPFC after pairing CS− with US. (E) Retardation effect at the neuronal activity level after pairing of CS− with US [one-way RM ANOVA, F(2, 72) = 18.95, P < 0.0001; n = 37 units/10 mice; SL (CS+) vs. CS−/US, P < 0.001, Bonferroni’s posttest].
Retardation was tested by pairing CS− with foot shock using the same protocol as for the CS+/US pairing. The freezing level after the CS−/US pairing was significantly lower than that achieved with CS as a neutral cue during pairing [i.e., SL (CS+) in Fig. 2C]. In the same mice, the increase in dmPFC neuronal spiking after US pairing was lower than that seen with CS as a neutral cue (Fig. 2 D and E). We failed to reverse the discriminative fear responses to generalized responses by using a greater foot shock current (SI Appendix, Fig. S3; but see ref. 13). Taken together, our SL protocol establishes an adequate conditioned inhibition model and allows us to explore the cellular basis of conditioned inhibition.
Dopamine Signaling from the VTA to dmPFC Is Required for SL.
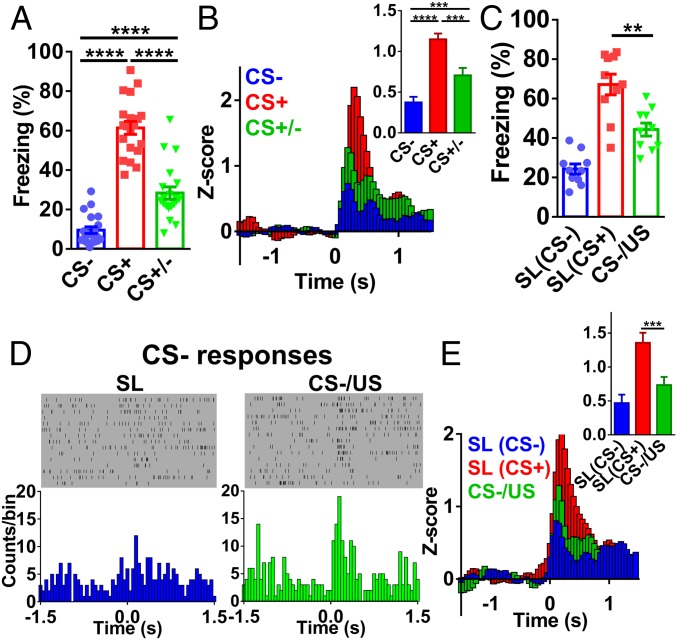
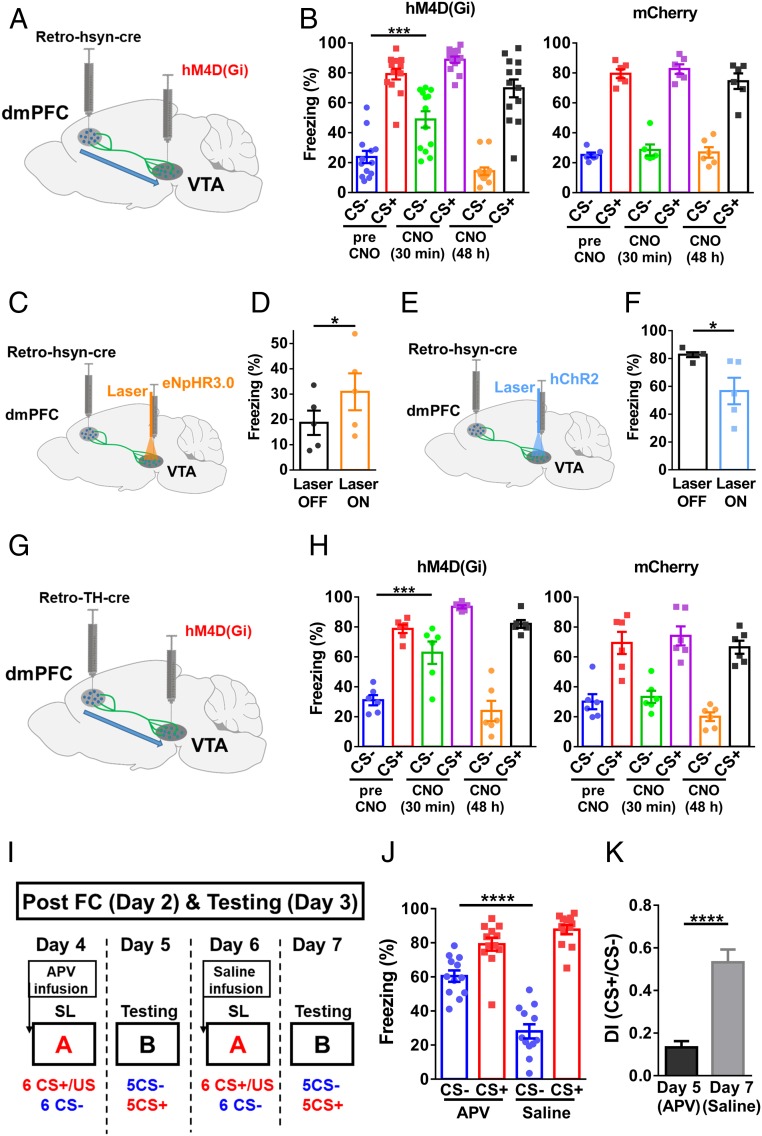
Dopamine signaling has been implicated in SL/fear generalization (27, 31, 32). Consistent with the importance of DA for SL, we found that i.p. injection of D1 receptor antagonist SCH 23390 (0.5 mg/kg) resulted in similar dmPFC spike changes during CS+ and CS− after SL (Fig. 3A). Freezing levels were not different between CS+ and CS− (SI Appendix, Fig. S4A), likely due to the increased freezing response after SCH 23390 injection (SI Appendix, Fig. S4B). To circumvent this problem, we infused SCH 23390 locally into dmPFC (0.5 μg/per side, bilateral). In these experiments, we used SL protocol consisted of six pairings of CS+/CS−. We found that differential fear responses disappeared after SCH 23390 infusion and reappeared after saline infusion (Fig. 3B). In similar experiments, local infusion of D2 receptor antagonist raclopride had no effect (SI Appendix, Fig. S4C). Thus, DA D1 receptor-coupled signaling in the dmPFC is required for SL.
Fig. 3.
DA in the dmPFC is required for differential fear response and dmPFC spiking. (A) Spike change during CS+ and CS− before and after SCH23390 i.p. injection (two-tailed paired t test, before SCH, t = 8.359, df = 30 SCH, t = 0.547, df = 30; n = 31 units/9 mice; CS− vs. CS+, P < 0.0001 before SCH; CS−/SCH vs. CS+/SCH, P = 0.59). (B) Freezing levels after local SCH23390 or saline infusion [two-way RM ANOVA; interaction, F(2,18) = 10.40, P = 0.001; treatment, F(2,18) = 58.91, P < 0.0001; stimulus, F(1,9) = 36.26, P < 0.001; n = 10 mice, CS− vs. CS+, P < 0.0001 (before SCH); CS− vs. CS+, P = 0.07 (SCH); CS− vs. CS+, P < 0.0001 (Sal), Bonferroni’s posttest]. (C) Schematic illustration of optogenetic inhibition of VTA DA projections in the dmPFC. (D) Optogenetic inhibition after SL elevated freezing levels during CS− (two-tailed paired t test; eNpHR3.0, t = 4.913, df = 4; eYFP, t = 1.997, df = 5; Left, eNpHR3.0, n = 5 mice, laser off vs. laser on, P < 0.01; Right, eYFP, n = 6 mice; laser off vs. laser on, P = 0.10). (E) Schematic illustration of optogenetic excitation of VTA DA projections in the dmPFC. (F) Optogenetic excitation after SL reduced freezing levels during CS+ (two-tailed paired t test; hChR2, t = 6.770, df = 5; eYFP, t = 0.5894, df = 5; Left, hChR2, n = 6 mice, laser off vs. laser on, P < 0.01; Right, eYFP, n = 6 mice, laser off vs. laser on, P = 0.58).
To understand whether dynamic modulation of DA release in the dmPFC can affect SL, we injected AAV2/9-TH-NLS-cre and AAV2/9-Ef1α-DIO-eNpHR3.0 viruses in the VTA and used yellow laser to suppress dmPFC DA axonal terminals from the VTA (Fig. 3C and SI Appendix, Fig. S5A). The reason for selecting TH-cre over DA transporter (DAT)-cre is the observation that PFC-projecting VTA DA neurons express significantly lower levels of DAT (33). After SL, freezing during CS− was significantly elevated with laser on compared with laser off (Fig. 3D). Freezing levels did not differ between laser on and laser off in mice injected with eYFP virus (Fig. 3D), and yellow laser stimulation without CS did not alter freezing levels (SI Appendix, Fig. S5B). Thus, suppression of fear responses by CS− requires VTA DA release in the dmPFC. In the converse experiment, AAV2/9-TH-NLS-cre and AAV2/9-Ef1α-DIO-hChR2 viruses were injected in the VTA (Fig. 3E), and the efficacy of optogenetic manipulation was confirmed by slice electrophysiology (SI Appendix, Fig. S5C). Blue laser stimulation in the dmPFC significantly reduced freezing level during CS+ after SL (Fig. 3F), while no difference in freezing level was seen between laser on and off in eYFP-injected mice (Fig. 3F), and laser stimulation without CS did not alter freezing level (SI Appendix, Fig. S5D).
VTA DA Neurons Show Enhanced Activation by CS− After SL.
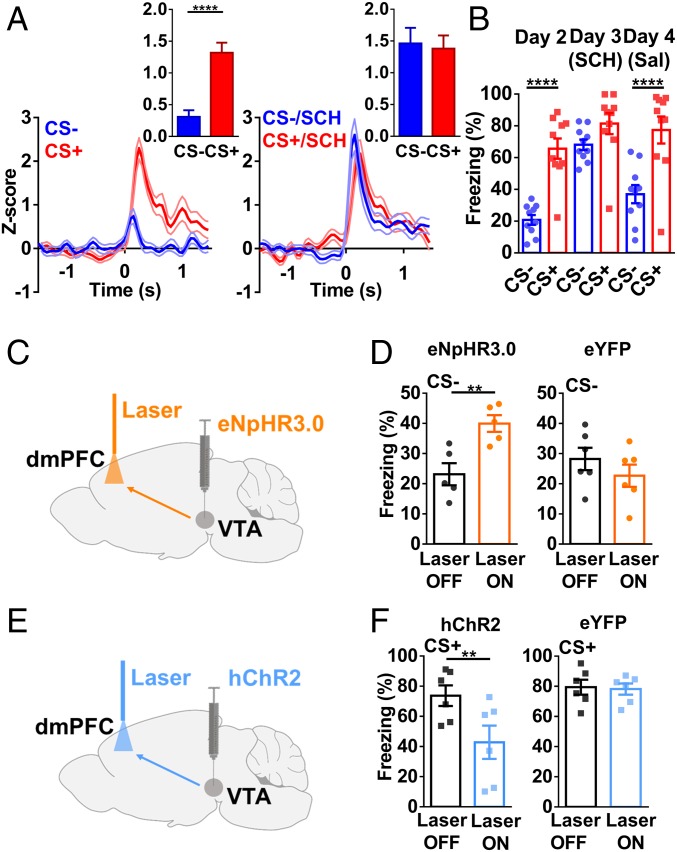
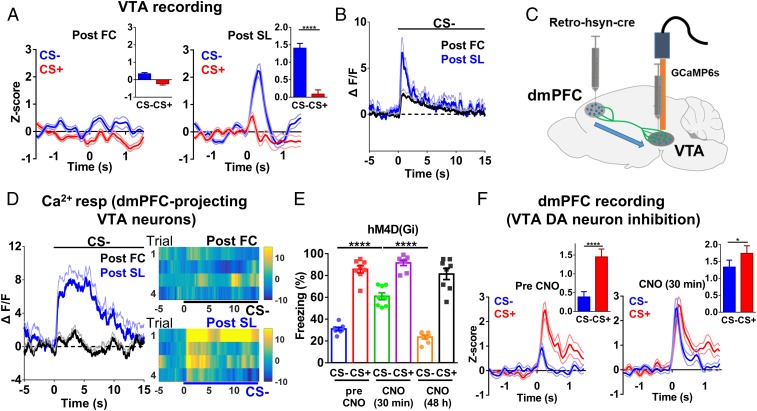
The results presented so far suggest that DA release from VTA DA neurons is required for SL, but do not distinguish whether this is due to constitutive, activity-independent release or to DA neuronal activity-dependent release. A subgroup of VTA neurons (∼28% of all recorded neurons) showed minimal responses during CS+ or CS− after FC (Fig. 4A), with responses increasing significantly only during CS− after SL (Fig. 4A). These changes in spiking were correlated with a larger discrimination index (SI Appendix, Fig. S6A). This subtype of VTA neurons had a relatively high firing frequency (28.74 ± 5.75 Hz), which is uncharacteristic of typical dopamine neuron spiking frequency (34) but is consistent with the characteristics of PFC-projecting VTA DA neurons (33, 35).
Fig. 4.
VTA DA neurons are selectively activated by CS− after SL. (A) Population spike change in VTA neurons after FC (Left) and after SL (Right) (two-tailed paired t test; post-FC, t = 1.004, df = 32; post-SL, t = 7.857, df = 32; n = 33 units/5 mice; post-FC, CS− vs. CS+, P = 0.32; post-SL, CS− vs. CS+, P < 0.0001). (B) Peri-event plot of averaged Ca2+ responses during CS− post-FC and post-SL (two-tailed paired t test, t = 3.554, df = 11; n = 12 mice; post-FC vs. post-SL, P < 0.01). (C) Schematic illustration of recording Ca2+ responses in dmPFC-projecting VTA neurons. (D) Averaged Ca2+ responses during CS− post-FC and post-SL (two-tailed paired t test, t = 5.211, df = 6; n = 7 mice; post-FC vs. post -SL, P < 0.01). (E) Freezing levels during CS− after CNO injection [two-way RM ANOVA; interaction, F(2,14) = 13.53, P < 0.001; stimulus, F(1,7) = 387.1, P < 0.0001; treatment, F(2,14) = 39.11, P < 0.0001; n = 8 mice, CS− (pre CNO) vs. CS− (CNO 30 min), P < 0.0001; CS− (CNO 30 min) vs. CS− (CNO 48 h), P < 0.0001, Bonferroni’s posttest]. (F) Spike change of dmPFC neurons before (Left) and 30 min after (Right) CNO injection [two-tailed paired t test; pre-CNO, t = 7.373, df = 20; CNO (30 min), t = 2.534, df = 20; n = 21 units/5 mice; pre-CNO, CS− vs. CS+, P < 0.0001; CNO (30 min), CS− vs. CS+, P < 0.05].
To further verify the activation of DA neurons, we recorded population activity in VTA neurons infected with AAV2/9-TH-NLS-Cre and AAV2/9-Ef1α-DIO-GCaMP6s viruses, using Ca2+ imaging. Small Ca2+ increases were seen during CS− before SL, with larger increases seen after SL (Fig. 4B; freezing levels in SI Appendix, Fig. S6B). In contrast, no significant changes in Ca2+ response (SI Appendix, Fig. S6C) or fear response during CS+ (SI Appendix, Fig. S6D) were observed after SL. In addition, we injected AAV2-Retro-hsyn-Cre virus in the dmPFC and AAV2/9-Ef1α-DIO-GCaMP6s virus in the VTA to measure Ca2+ responses of dmPFC-projecting VTA neurons (Fig. 4C). Increased Ca2+ responses during CS− were seen after SL, but not before SL (Fig. 4D), corresponding to the reduced freezing level after SL (SI Appendix, Fig. S6E). Calcium responses and freezing levels remained unchanged during CS+ (SI Appendix, Fig. S6 F and G).
If elevated VTA DA neuronal activity is required for discriminative fear responses to emerge, inhibiting VTA DA neuron activity should block SL. To test this, we injected AAV2/9-TH-NLS-Cre and AAV2/9-Ef1α-DIO-hM4D (Gi) viruses in the VTA. Injection of clozapine-N-oxide (CNO; 3 mg/kg, i.p.) at 30 min before fear recall led to significantly higher freezing during CS− compared with levels seen before or 48 h after CNO injection (Fig. 4E and SI Appendix, Fig. S6H). No significant change was seen during CS− in mice injected with mCherry virus (SI Appendix, Fig. S6I). Furthermore, similar spike changes in the dmPFC were seen during CS+ and CS− at 30 min after CNO injection (Fig. 4F), in contrast to the low spike changes seen during CS− before or 48 h after CNO injection (Fig. 4F and SI Appendix, Fig. S6J). Thus, VTA DA neuronal activity is required for the expression of differential freezing responses and dmPFC activity after SL. Viral expression was largely restricted to the VTA DA neurons (SI Appendix, Fig. S6K). The efficacy of CNO on spiking in VTA DA neurons was also verified in slices (SI Appendix, Fig. S7 A and B).
In further experiments, we found that inhibiting dmPFC-projecting VTA neurons using pharmacogenetic approaches significantly increased freezing levels during CS− (Fig. 5 A and B). In optogenetic experiments in which dmPFC-projecting VTA neurons were inhibited, increased freezing levels during CS− were also observed (Fig. 5 C and D). In addition, optogenetic excitation of dmPFC-projecting VTA neurons decreased freezing levels during CS+ (Fig. 5 E and F), suggesting that high activity in dmPFC-projecting VTA neurons is sufficient to reduce fear.
Fig. 5.
Activation of dmPFC-projecting VTA neurons is required for fear suppression after SL, and NMDAR in VTA is required for SL. (A) Schematic illustration of pharmacogenetic inhibition of dmPFC-projecting VTA neurons. (B) Freezing levels during CS+ and CS− before, 30 min after, and 48 h after CNO injection in hM4D (Gi)- and mCherry-injected mice [two-way RM ANOVA; Left: interaction, F(2,24) = 2.524, P = 0.101; stimulus, F(1,12) = 130.1, P < 0.0001; treatment, F(2,24) = 57.33, P < 0.0001; Right: interaction, F(2,10) = 3.52, P = 0.0696; stimulus, F(1,5) = 309.9, P < 0.0001; treatment, F(2,10) = 1.992, P = 0.187; Left: hM4D (Gi), n = 13 mice; CS−, pre-CNO vs. CNO (30 min), P < 0.001; Right: mCherry, n = 6 mice; CS−, pre-CNO vs. CNO (30 min), P = 0.40, Bonferroni’s posttest]. (C) Schematic drawing of optogenetic inhibition of dmPFC-projecting VTA neurons. (D) Freezing levels during yellow laser stimulation and CS− in eNpHR3.0-injected mice (two-tailed paired t test, t = 4.555, df = 4; n = 5 mice; laser off vs. laser on, P < 0.05). (E) Schematic drawing of optogenetic excitation of dmPFC-projecting VTA neurons. (F) Freezing levels during blue laser stimulation and CS+ in hChR2-injected mice (two-tailed paired t test, t = 2.896, df = 4; n = 5 mice; laser off vs. laser on, P < 0.05). (G) Schematic illustration of pharmacogenetic inhibition of dmPFC-projecting VTA DA neurons. (H) Freezing levels during CS+ and CS− before, 30 min after, and 48 h after CNO injection in hM4D (Gi)- and mCherry-injected mice [two-way RM ANOVA; Left: interaction, F(2,10) = 7.165, P = 0.0117; stimulus, F(1,5) = 85.14, P = 0.0003; treatment, F(2,10) = 19.32, P = 0.0004; Right: interaction, F(2,10) = 1.441, P = 0.2818; stimulus, F(1,5) = 129.5, P < 0.0001; treatment, F(2,10) = 3.085, P = 0.0905; Left: hM4D (Gi), n = 6 mice; CS−, pre-CNO vs. CNO (30 min), P < 0.001; Right: mCherry, n = 6 mice; CS−, pre-CNO vs. CNO (30 min), P = 0.98, Bonferroni’s posttest]. (I) Experimental procedure for APV and saline infusion in the VTA. (J) Infusion of APV blocked SL [two-way RM ANOVA; interaction, F(1,11) = 60.46, P < 0.0001; stimulus, F(1,11) = 100.4, P < 0.0001; treatment, F(1,11) = 10.09, P = 0.0088; n = 12 mice; CS−, APV vs. saline, P < 0.0001; CS+, APV vs. saline, P = 0.08, Bonferroni’s posttest]. (K) Discrimination index after SL with APV and saline infusion (two-tailed paired t test, t = 6.481, df = 11; n = 12 mice; APV vs. saline, P < 0.0001).
We next tested whether activation of dmPFC-projecting VTA DA neurons is required for SL. We injected AAV2-Retro-TH-NLS-Cre virus into the dmPFC and AAV2/9-Ef1α-DIO-hM4D (Gi)-mCherry virus into the VTA for pharmacogenetic inhibition of dmPFC-projecting VTA DA neurons (Fig. 5G), and this manipulation significantly increased freezing level during CS− (Fig. 5H).
The selectively enhanced responses to CS− after SL in dmPFC-projecting VTA DA neurons suggest that CS− leads to elevated VTA DA neuronal spiking after SL. One possible mechanism for such enhancement is the NMDAR-dependent plasticity in the VTA (21, 36). To test this possibility, the competitive NMDAR antagonist d-APV (1.0 μg/per side, bilateral) was infused into the VTA at 15 min before SL (Fig. 5I). No significant difference in freezing levels between CS− and CS+ was seen in the d-APV–infused mice (Fig. 5J), corresponding to a low discrimination index (Fig. 5K). In contrast, subsequent infusion of saline in the same mice at 48 h after APV infusion resulted in a significant decrease in freezing level (Fig. 5J) and a large discrimination index (Fig. 5K). Thus, an NMDAR-dependent plasticity process in the VTA likely occurs during SL, which may underlie the selective elevation of VTA DA neuron activity by CS− (37).
PV-Positive Neurons in the dmPFC Mediate SL.
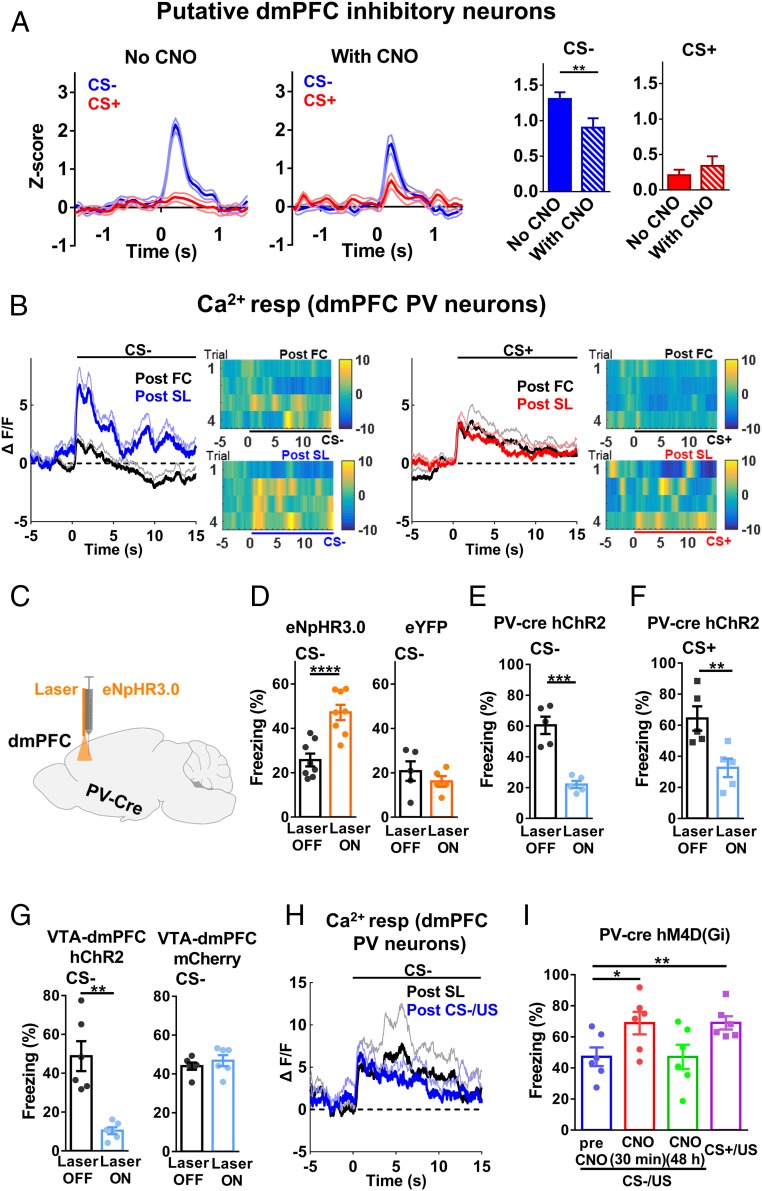
What are the targets of VTA DA neurons in the dmPFC? The dmPFC neurons showing spiking reduction appear to be excitatory neurons based on their spike properties; thus, a likely candidate for causing this reduction is inhibitory GABAergic neurons in the PFC, especially those containing PV, which has a powerful perisomatic action that suppresses the spiking activity of excitatory neurons (38). We encountered a few dmPFC neurons with waveforms and basal spiking frequency resembling those of inhibitory neurons (SI Appendix, Fig. S8A). They showed lower spike changes to CS− with CNO than without CNO (Fig. 6A, neurons from experiments in Fig. 4). These results prompted us to examine PV neurons in the dmPFC.
Fig. 6.
Contribution of dmPFC PV neurons to the expression of SL and the retardation effect. (A) Putative dmPFC inhibitory neurons showed reduced changes in spike change during CS− after CNO injection to inhibit DA neuron activity (two-tailed paired t test; CS−, t = 2.870, df = 24; CS+, t = 0.7844, df = 24; n = 25 units/10 mice; CS−, no CNO vs. with CNO, P < 0.01; CS+, no CNO vs. with CNO, P = 0.44). (B) Averaged Ca2+ responses during CS− or CS+ post-FC and post-SL (two-tailed paired t test; CS−, t = 3.734, df = 14; CS+, t = 0.5759, df = 14; CS−, n = 15 mice, post-FC vs. post-SL, P < 0.01; CS+, n = 15 mice, post-FC vs. post-SL, P = 0.57). (C) Schematic illustration of optogenetic inhibition of PV neurons in the dmPFC. (D) In PV-cre mice injected with eNpHR3.0, freezing levels during CS− was elevated by yellow laser illumination (two-tailed paired t test; eNpHR3.0, t = 8.919, df = 7; eYFP, t = 1.401, df = 4; Left: eNpHR3.0, n = 8 mice, laser off vs. laser on, P < 0.0001; Right: eYFP, n = 5 mice, laser off vs. laser on, P = 0.23). (E) In PV-cre mice injected with hChR2, freezing levels during CS− post-FC (two-tailed paired t test, t = 9.941, df = 4; n = 5 mice; laser off vs. laser on, P < 0.001). (F) In PV-cre mice injected with hChR2, freezing levels during CS+ post-FC (two-tailed paired t test, t = 5.199, df = 4; n = 5 mice; laser off vs. laser on, P < 0.01). (G) Optogenetic excitation of VTA DA terminals in the dmPFC during CS− in hChR2- and mCherry-injected mice post-FC (two-tailed paired t test; hChR2, t = 5.276, df = 5; mCherry, t = 1.861, df = 5; Left: hChR2, n = 6 mice, laser off vs. laser on, P < 0.01; Right: mCherry, n = 6 mice, laser off vs. laser on, P = 0.12). (H) Averaged Ca2+ responses in dmPFC PV neurons post-SL and post-CS−/US (foot shock) pairing (retardation) (two-tailed paired t test, t = 0.849, df = 4; n = 5 mice; post-SL vs. CS−/US, P = 0.44). (I) A retardation effect was absent with pharmacogenetic inhibition of dmPFC PV neurons (two-tailed paired t test, t = 3.45, df = 5; n = 6 mice, CS−/US, pre-CNO vs. CNO 30 min, P < 0.05; two-tailed paired t test, t = 4.635, df = 5, CS−/US, pre-CNO vs. CS+/US, P < 0.01).
To further verify the dmPFC PV neurons, we monitored Ca2+ responses in PV neurons in the dmPFC (GCaMP6s virus injected in the dmPFC of PV-cre mice; SI Appendix, Fig. S8 B and C). The selectivity of GCaMP6s expression in PV neurons was confirmed using immunohistochemistry (SI Appendix, Fig. S8D). Responses were small during CS− before SL and became significantly larger after SL (Fig. 6B), corresponding to decreased freezing level (SI Appendix, Fig. S9A). In contrast, Ca2+ responses during CS+ were not significantly altered by SL in the same set of mice (Fig. 6B), nor was freezing level (SI Appendix, Fig. S9B). These results suggest that SL selectively elevates PV neuron activity in the dmPFC during CS−.
We next tested whether ongoing PV neuron activity is required for the expression of SL. By injecting DIO-eNpHR3.0-eYFP virus into PV-cre mice, we inhibited PV neurons with yellow laser during CS− (Fig. 6C). Freezing level during CS− was significantly higher with the laser on than with it off (Fig. 6D) after SL, in contrast to no difference between laser on and off in mice injected with eYFP virus (Fig. 6D). Yellow laser stimulation without CS did not alter freezing level (SI Appendix, Fig. S9C). Thus, fear level is likely modulated bidirectionally by the activity of dmPFC PV neurons.
Elevated dmPFC PV Neuron Activity Mimics the Effects of SL.
Although the foregoing results indicate that PV neuron activity is required for SL expression, they do not show whether elevated PV neuron activity alone is sufficient to mimic the effect of SL. To test this, we injected DIO-hChR2-eYFP/mCherry virus into dmPFC of PV-cre mice after FC. The locations of virus expression and implanted optic fiber were verified after experiments (SI Appendix, Fig. S10A).When PV neurons were excited during CS−, freezing level was significantly lower with laser on, in contrast to no difference between laser on and laser off in mCherry-injected mice (Fig. 6E and SI Appendix, Fig. S10B), indicating that increasing PV neuron activity is sufficient to reduce fear expression. We obtained a similar result when the same experiment was repeated with CS+ (Fig. 6F and SI Appendix, Fig. S10B). Furthermore, laser stimulation of VTA-dmPFC DA terminals in mice that only received FC also led to reduced freezing (Fig. 6G). Taken together, these results indicate that fear responses associated with a given CS are determined by DA release in the dmPFC and the resulted activity of PV neurons. Since increasing the activity of dmPFC DA terminals or dmPFC PV neurons reduces fear in a similar way as SL, these results suggest that this portion of the circuitry is constitutively active and unlikely affected by SL.
Since retardation effect is a key feature of SL, we next examined the role of PV neuronal activity in retardation. Two possible mechanisms could underlie the retardation effect: reduced association between CS− and US during CS−/US pairing, or reduced fear expression due to suppression by CS− (i.e., it functions as a safety cue). To distinguish between these two possibilities, we first compared the Ca2+ response of PV neurons during CS− after SL and after pairing of CS− with US in the same mice. We found no significant difference (Fig. 6H), which suggests that PV neuronal responses are likely not altered by pairing. Thus, we asked whether inhibiting PV neurons could abolish the retardation effect. PV neurons in the dmPFC were inhibited in mice injected with DIO-hM4D (Gi)-mCherry virus with CNO injection before CS− recall. The efficacy of CNO in inhibiting PV neuron spiking was confirmed in slices (SI Appendix, Fig. S10C). This manipulation resulted in a significant increase in freezing level, which was similar to the freezing level achieved by CS+/US pairing (Fig. 6I). In contrast, there was no significant change in freezing level in mice injected with mCherry (SI Appendix, Fig. S10D). Thus, retardation is mediated by a reduced fear expression via activation of dmPFC PV neurons. This result thus indicates that CS− still functions as a safety cue after pairing with US.
Deficits in Elevated VTA DA Signaling Underlies the Impaired SL in a PTSD Model.
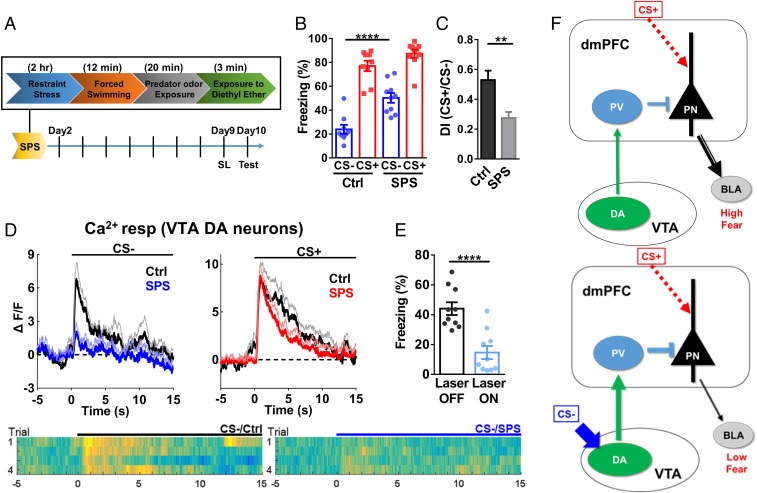
Clinical evidence suggests that fear discrimination or SL is impaired in PTSD patients (11, 39), but the underlying mechanisms are poorly understood. In a single prolonged stress (SPS) model of PTSD (Fig. 7A) (40), we found a significant increase in freezing level during CS− (Fig. 7B) and a low discrimination index (Fig. 7C), compared with control mice after SL. Thus, similar to human PTSD patients, SPS mice also exhibited deficits in SL. Since elevated VTA DA and dmPFC PV neuronal activities are required for SL, we examined which signaling may be altered in SPS mice. In SPS mice after SL, significantly lower Ca2+ responses in VTA DA neurons were seen during CS−, while responses during CS+ were not altered (Fig. 7D; freezing levels in SI Appendix, Fig. S11). Activating VTA DA projection terminals in the dmPFC with blue laser excitation during CS− reduced the freezing level in SPS mice to a level similar to that in control mice (Fig. 7E), suggesting that these outputs are largely unaltered and thus PV neuronal functions remain largely intact in SPS mice. These findings indicate that the SL-associated increase in VTA DA neuronal activity is selectively impaired in SPS mice, which may underlie the impaired SL in these mice.
Fig. 7.
Impaired SL and reduced increase in VTA DA signaling in a single prolonged stress model of PTSD. (A) Procedure for establishing the SPS/PTSD model. (B) Freezing levels during CS− [two-way RM ANOVA; interaction, F(1,17) = 4.148, P = 0.0576; stimulus, F(1,17) = 130.1, P < 0.0001; group, F(1,17) = 21.83, P < 0.001; n = 9 mice for control (Ctrl), 10 mice for SPS; CS−, Ctrl vs. SPS, P < 0.0001; CS+, Ctrl vs. SPS, P = 0.15. Bonferroni’s posttest]. (C) Discrimination index in Ctrl and SPS mice (two-tailed unpaired t test, t = 3.470, df = 17; Ctrl vs. SPS, P < 0.01; same set of mice as in B). (D) VTA DA Ca2+ responses during CS− (Left) or CS+ (Right) post-SL in SPS and Ctrl mice [two-tailed unpaired t test; CS−, t = 2.319, df = 10; CS+, t = 0.0158, df = 10; n = 7 mice (Ctrl), 5 mice (SPS); CS−, Ctrl vs. SPS, P < 0.05; CS+, Ctrl vs. SPS, P = 0.99]. Heat maps showed corresponding Ca2+ responses during CS−. (E) Reduced freezing levels during CS− by excitation of VTA DA terminals in the dmPFC after SL in SPS mice (two-tailed paired t test, t = 10.90, df = 9; n = 10 mice; laser off vs. laser on, P < 0.0001). (F) Model of conditioned inhibition. (Left) CS+ (danger signal) leads to high activity in principal neurons (PN) in the dmPFC and high fear via outputs to subcortical regions, such as the basolateral amygdala, (BLA). (Right) After SL, CS− (safety cue) leads to stronger activation of VTA DA neurons, which results in stronger PV neuron activity and reduced PN activity, with low fear expression, in the presence of CS+. The main consequence of SL is enhanced VTA DA neurons from the CS− input, which leads to enhanced transmission from VTA DA neurons to PV neurons and reduced PN neuronal activity (illustrated by thicker lines).
Discussion
In this study, we found that conditioned inhibition in the form of suppression of fear responses by safety cues is acquired through a learning process that results in elevated DA neuron activity in the VTA and enhanced parvalbumin neuron activity in the dmPFC. This enhanced inhibition reduces spiking of excitatory (principal) neurons in the dmPFC to suppress fear expression (Fig. 7F). This mechanism confirms the long-held belief that conditioned inhibition is inhibitory in nature, and further emphasizes the critical contribution of DA signaling during learning. Interestingly, this DA contribution is impaired in a PTSD model, which may underlie the impaired SL in this model. These insights reveal the complex nature of conditioned inhibition, which requires interactions between DA and GABAergic systems. These findings may also provide new thinking and therapeutic targets for treating various psychiatric diseases, especially those with altered anxiety and/or fear.
Based on our findings, we propose the following model for how conditioned inhibition mediates fear discrimination (Fig. 7F): (i) the activity of dmPFC excitatory neurons bidirectionally determines fear responses, and this activity is modulated by the local GABAerigc (especially PV-containing) neurons; (ii) when fear is generalized, CS+ or CS− leads to high-level dmPFC spiking and high fear; (iii) SL selectively increases CS− -induced VTA DA neuron activity and spiking of PV neurons; (iv) activation of PV neurons reduces spiking of dmPFC excitatory neurons and results in the suppression of fear responses during CS− (discrimination) or simultaneous presentation of CS+ and CS− (summation); (v) plasticity in the VTA, via an NMDAR-dependent mechanism, is required for SL; (ix) a retardation effect is mediated by fear suppression as CS− functions as both danger and safety cues, and summation of these two cues determines fear responses. This model emphasizes the associative nature of conditioned inhibition and interactions among multiple important neurotransmitter/neuromodulator systems.
Conditioned Inhibition and Fear Discrimination.
It is critical to consider the similarities and differences between these two processes, as these differences could explain the differences between our findings and those reported by others. Fear discrimination occurs with a sufficiently great difference between responses to CS+ and CS−, and was tested by giving separate CS+ and CS− in most studies. Conditioned inhibition or SL uses CS− as a safety cue to reduce responses to CS+ and was tested with simultaneous presentation of CS+ and CS− (summation). Thus, interaction between CS+ and CS− is an essential property of conditioned inhibition but might not be necessary for discrimination. For example, Jo et al. (31) suggested that discriminative training is effective in preventing the occurrence of fear generalization, but whether CS− inhibits CS+ response is unclear. Thus, it is possible that different conditioning/training protocols may result in either discrimination or conditioned inhibition with distinct underlying mechanisms.
Contributions of PV-Containing GABAergic Neurons.
Parvalbumin-neurons are known to provide powerful, perisomatic inhibition to effectively suppress the spiking activity of their targets (38). Both D1 and D2 DA receptors are expressed on both excitatory and inhibitory neurons, with D1 receptors the most abundantly expressed (41). Inputs from VTA DA neurons directly activate/excite PFC PV neurons in vivo, activate D1 receptors, and result in enhanced inhibition in the PFC excitatory neurons in vitro (24, 25), consistent with our present findings. Since enhancement of VTA DA-dmPFC connections switched high fear to low fear regardless of whether SL occurred (Fig. 6G), it is likely that the section of inhibitory circuitry functions in a default mode to mediate conditioned inhibition. Marek et al. (42) recently reported that ventral hippocampal projections to infralimbic PFC contribute to fear extinction by driving local PV neurons, suggesting that a similar inhibitory process may mediate fear suppression after extinction training. Different from the reduced responses in PV neurons during CS reported by Courtin et al. (20), we found enhanced PV neuron activity after SL. Nonetheless, both studies found higher PV neuron activity during CS− than during CS+.
Fear responses can be suppressed at multiple levels. Increased inhibition of fear output neurons of the central amygdala was reported after fear extinction training via potentiation of synaptic inputs to the inhibitory GABAergic intercalated neurons in the amygdala (43). Some studies implicated mPFC in SL/fear discrimination (16–18), while others did not (15, 44). Our findings indicate that computation and decision to allow or suppress fear expression may occur in the PFC, with this decision then relayed to the subcortical regions for execution. Outputs of dmPFC excitatory neurons can mediate fear responses in a bidirectional manner, and dmPFC-driven oscillatory events in amygdala neurons may mediate fear expression (20, 45). Whether and how circuits at cortical and subcortical levels may coordinate or interact to generate coherent and appropriate responses is an interesting and important topic for further exploration.
It is also interesting and important to note the similarities between conditioned inhibition and fear extinction. First, both are suppressive in nature, mediated by enhanced inhibition, although the exact location and/or cell types mediating this inhibition might differ (i.e., PV neurons in dmPFC for conditioned inhibition, GABAergic neurons in the hippocampus and/or intercalated amygdala for fear extinction) (42, 46–48). In the absence of this inhibition, fear reappears/returns. Second, the suppression of fear is selective to the specific condition/cue/context, with a safety cue for conditioned inhibition and extinction context for fear extinction (47, 49, 50).
Contributions of DA Signaling.
Previous studies have demonstrated a critical role of DA in FC (37, 51). Ng et al. (27) provided evidence that DA signaling in the amygdala influences the effectiveness of safety cues. As for reward learning, responses of DA neurons were reduced by reward omission, which functions as a conditioned inhibitor (52). The omission of an aversive stimulus (foot shock) indicated by safety cue can be considered a reward signal, and thus the contribution of DA signaling may be of a similar nature for both reward and aversive stimuli. In addition, our findings indicate that DA signaling is required for the expression of SL, and future work is needed to examine whether a plasticity process underlies the potentiation of CS− inputs to VTA DA neurons. Previous studies have shown that knockout of NMDARs on DAT-expressing DA neurons led to poor fear discrimination (32), while activation of DAT-DA neurons prevented fear generalization (31). The foregoing results appear to be different from ours, but the differences may be explained by the following: (i) different groups of DA neurons being targeted: we targeted TH-positive, PFC-projecting DA neurons while Jones et al. (32) and Jo et al. (31) targeted DAT-positive neurons which likely innervate subcortical regions. (ii) Differences in the conditioning/learning protocols: we used high US (shock) intensity to induce fear generalization and SL started from a generalization state, while Jones et al. (32) and Jo et al. (31) used low US intensity to avoid generalization. Different DA populations might contribute to different aspects of fear generalization/discrimination, with activation of DAT-DA neurons preventing fear generalization at a state of fear discrimination and activation of TH-positive neurons leading to fear discrimination at a state of fear generalization. This distinction of DA subgroups could also be subcortical vs. cortical, which should be examined in future studies.
Implications for Psychiatric Disorders.
Deficits in SL/fear discriminating have been found in certain psychiatric disorders, especially anxiety disorders, and has been proposed as a biomarker (11, 53, 54). Defective DA signaling has been implicated in PTSD, including deficits in the reward and reinforcement circuits, decreased reward anticipation and reduced hedonic responses, reduced DA metabolites in cerebral spinal fluid following traumatic reminders, altered expression of striatal DA transporter, and polymorphisms in genes encoding DAT and DA receptors in PTSD patients (40). A reduced basal spiking rate of VTA DA neurons (55) and altered DA level (56) were reported in PTSD models. We have provided clear evidence of impaired fear suppression and the absence of potentiated activation of VTA DA neurons after SL, while DA-dmPFC connections appear to be largely intact.
One important implication from our study is that multiple distinct causes can result in the observed deficits in conditioned inhibition. Impaired DA signaling, PV signaling, or both, can occur, each with distinct pathological implications and treatment strategies. The required participation of both DA and GABAergic systems also provides an explanation for the diversity of impaired functions in PTSD patients, including motivation, reward, and fear discrimination, and raises the possibility that deficits in seemingly distinct domains of neural functions are actually driven by deficits in different sections of the same circuitry. Thus, identifying the exact cause/deficit may enable more precise and effective therapy. For example, treating impaired DA functions may require targeting the VTA, while ameliorating reduced inhibitory functions may require enhancing GABAergic function.
Major Unresolved Questions.
Although we have provided strong evidence for the involvement of DA and GABA signaling in conditioned inhibition, several key questions need to be addressed in future studies. First, how are DA neurons activated during SL and become associated with the safety cue? What is the role of CS+/US in SL? Second, are the DA projections to PV neurons a separate pathway distinct from DA projections to the excitatory neurons? Third, is there any contribution from the subcortical (such as amygdala) circuitry to SL and conditioned inhibition? If so, how is this circuitry coordinated with the PFC circuitry examined in this study?
Conclusions
In summary, our findings reveal the biological basis of conditioned inhibition and how this important biological process is malfunctioning in relevant diseases. The fact that conditioned inhibition involves two of the most important players in neural function, plasticity, and disease—namely dopamine and GABA—is clearly consistent with their critical contributions to both adaptive physiological functions and pathogenesis of various psychiatric disorders. The insights gained from this study may also help us design better treatments based on a more precise understanding of the underlying pathology. All animal experiments were performed in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines on the Care and Use of Experimental Animals, approved by the Peking University Shenzhen Graduate School Animal Care and Use Committee.
Materials and Methods
Complete descriptions of the study methodology are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Meifang Ma for excellent technical support and help with in vivo recording and data analysis, Xiaoyan Ma for help with slice recording, Dr. Weidong Yao for comments and suggestions, Yangmei Huang for help with immunofluorescence staining, and Dr. Miao He (Fudan University) for the PV-cre mice. This work was supported by the Shenzhen Science and Technology Innovation Fund (Grants KQTD2015032709315529 and JCYJ20170412150845848).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1901902116/-/DCSupplemental.
References
- 1.Christianson J. P., et al. , Inhibition of fear by learned safety signals: A mini-symposium review. J. Neurosci. 32, 14118–14124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kong E., Monje F. J., Hirsch J., Pollak D. D., Learning not to fear: Neural correlates of learned safety. Neuropsychopharmacology 39, 515–527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunsmoor J. E., Paz R., Fear generalization and anxiety: Behavioral and neural mechanisms. Biol. Psychiatry 78, 336–343 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Kheirbek M. A., Klemenhagen K. C., Sahay A., Hen R., Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nat. Neurosci. 15, 1613–1620 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond L. J., A traditional demonstration of the active properties of Pavlovian inhibition using differential CER. Psychon. Sci. 9, 65–66 (1967). [Google Scholar]
- 6.Rescorla R. A., Pavlovian conditioned inhibition. Psychol. Bull. 72, 77–94 (1969). [Google Scholar]
- 7.Cándido A., González F., de Brugada I., Safety signals from avoidance learning but not from yoked classical conditioning training pass both summation and retardation tests for inhibition. Behav. Processes 66, 153–160 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Dinsmoor J. A., Stimuli inevitably generated by behavior that avoids electric shock are inherently reinforcing. J. Exp. Anal. Behav. 75, 311–333 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollak D. D., et al. , An animal model of a behavioral intervention for depression. Neuron 60, 149–161 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogan M. T., Leon K. S., Perez D. L., Kandel E. R., Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron 46, 309–320 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Jovanovic T., Kazama A., Bachevalier J., Davis M., Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62, 695–704 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahan A. L., Ressler K. J., Fear conditioning, synaptic plasticity and the amygdala: Implications for posttraumatic stress disorder. Trends Neurosci. 35, 24–35 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh S., Chattarji S., Neuronal encoding of the switch from specific to generalized fear. Nat. Neurosci. 18, 112–120 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Ostroff L. E., Cain C. K., Bedont J., Monfils M. H., Ledoux J. E., Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 107, 9418–9423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christianson J. P., et al. , The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. J. Neurosci. 28, 13703–13711 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sangha S., Robinson P. D., Greba Q., Davies D. A., Howland J. G., Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices. Neuropsychopharmacology 39, 2405–2413 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vieira P. A., et al. , Prefrontal NMDA receptors expressed in excitatory neurons control fear discrimination and fear extinction. Neurobiol. Learn. Mem. 119, 52–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira P. A., et al. , Prefrontal consolidation supports the attainment of fear memory accuracy. Learn. Mem. 21, 394–405 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgos-Robles A., Vidal-Gonzalez I., Quirk G. J., Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J. Neurosci. 29, 8474–8482 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courtin J., et al. , Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505, 92–96 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Pignatelli M., Bonci A., Role of dopamine neurons in reward and aversion: A synaptic plasticity perspective. Neuron 86, 1145–1157 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Pezze M. A., Feldon J., Mesolimbic dopaminergic pathways in fear conditioning. Prog. Neurobiol. 74, 301–320 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Lammel S., Lim B. K., Malenka R. C., Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76, 351–359 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seamans J. K., Yang C. R., The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 74, 1–58 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Tritsch N. X., Sabatini B. L., Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76, 33–50 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Bundel D., et al. , Dopamine D2 receptors gate generalization of conditioned threat responses through mTORC1 signaling in the extended amygdala. Mol. Psychiatry 21, 1545–1553 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng K. H., Pollock M. W., Urbanczyk P. J., Sangha S., Altering D1 receptor activity in the basolateral amygdala impairs fear suppression during a safety cue. Neurobiol. Learn. Mem. 147, 26–34 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Sotres-Bayon F., Quirk G. J., Prefrontal control of fear: More than just extinction. Curr. Opin. Neurobiol. 20, 231–235 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giustino T. F., Maren S., The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front. Behav. Neurosci. 9, 298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rescorla R. A., Summation and retardation tests of latent inhibition. J. Comp. Physiol. Psychol. 75, 77–81 (1971). [DOI] [PubMed] [Google Scholar]
- 31.Jo Y. S., Heymann G., Zweifel L. S., Dopamine neurons reflect the uncertainty in fear generalization. Neuron 100, 916–925.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones G. L., et al. , A genetic link between discriminative fear coding by the lateral amygdala, dopamine, and fear generalization. eLife 4, e8969 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh J. J., Han M. H., The heterogeneity of ventral tegmental area neurons: Projection functions in a mood-related context. Neuroscience 282, 101–108 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungless M. A., Grace A. A., Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 35, 422–430 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lammel S., et al. , Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57, 760–773 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Lüscher C., Malenka R. C., Drug-evoked synaptic plasticity in addiction: From molecular changes to circuit remodeling. Neuron 69, 650–663 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pignatelli M., et al. , Synaptic plasticity onto dopamine neurons shapes fear learning. Neuron 93, 425–440 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Freund T. F., Katona I., Perisomatic inhibition. Neuron 56, 33–42 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Kaczkurkin A. N., et al. , Neural substrates of overgeneralized conditioned fear in PTSD. Am. J. Psychiatry 174, 125–134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lisieski M. J., Eagle A. L., Conti A. C., Liberzon I., Perrine S. A., Single-prolonged stress: A review of two decades of progress in a rodent model of post-traumatic stress disorder. Front. Psychiatry 9, 196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santana N., Mengod G., Artigas F., Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex 19, 849–860 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Marek R., et al. , Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat. Neurosci. 21, 384–392 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amano T., Unal C. T., Paré D., Synaptic correlates of fear extinction in the amygdala. Nat. Neurosci. 13, 489–494 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gewirtz J. C., Falls W. A., Davis M., Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medical prefrontal cortex in rats. Behav. Neurosci. 111, 712–726 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Karalis N., et al. , 4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior. Nat. Neurosci. 19, 605–612 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duvarci S., Pare D., Amygdala microcircuits controlling learned fear. Neuron 82, 966–980 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quirk G. J., Mueller D., Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herry C., et al. , Neuronal circuits of fear extinction. Eur. J. Neurosci. 31, 599–612 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Maren S., Phan K. L., Liberzon I., The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14, 417–428 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouton M. E., Context and behavioral processes in extinction. Learn. Mem. 11, 485–494 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Fadok J. P., Dickerson T. M., Palmiter R. D., Dopamine is necessary for cue-dependent fear conditioning. J. Neurosci. 29, 11089–11097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tobler P. N., Dickinson A., Schultz W., Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. J. Neurosci. 23, 10402–10410 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jovanovic T., Ressler K. J., How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry 167, 648–662 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lissek S., et al. , Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol. Psychiatry 75, 909–915 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corral-Frias N. S., Lahood R. P., Edelman-Vogelsang K. E., French E. D., Fellous J. M., Involvement of the ventral tegmental area in a rodent model of post-traumatic stress disorder. Neuropsychopharmacology 38, 350–363 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin C. C., Tung C. S., Liu Y. P., Escitalopram reversed the traumatic stress-induced depressed and anxiety-like symptoms but not the deficits of fear memory. Psychopharmacology (Berl.) 233, 1135–1146 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.