Significance
Viroids are the only known autonomously replicating pathogenic agents that do not encode proteins. As viroids are known only to naturally infect plants, their infectivity and pathogenicity in other eukaryotes are largely unexplored. Herein, we demonstrate the stable infection of three viroid species in different plant pathogenic filamentous fungi and show that viroid infection can reduce the growth and virulence of fungi. In addition to successful viroid RNA inoculation of fungal spheroplasts, viroid infection of fungus could occur through viroid transmission from the plant and when viroid RNAs are directly applied to fungal mycelia. These findings are relevant to our understanding of viroid replication, transmission, and pathogenicity.
Keywords: plant viroid, fungus, transmission, pathogenicity
Abstract
Viroids are pathogenic agents that have a small, circular noncoding RNA genome. They have been found only in plant species; therefore, their infectivity and pathogenicity in other organisms remain largely unexplored. In this study, we investigate whether plant viroids can replicate and induce symptoms in filamentous fungi. Seven plant viroids representing viroid groups that replicate in either the nucleus or chloroplast of plant cells were inoculated to three plant pathogenic fungi, Cryphonectria parasitica, Valsa mali, and Fusarium graminearum. By transfection of fungal spheroplasts with viroid RNA transcripts, each of the three, hop stunt viroid (HSVd), iresine 1 viroid, and avocado sunblotch viroid, can stably replicate in at least one of those fungi. The viroids are horizontally transmitted through hyphal anastomosis and vertically through conidia. HSVd infection severely debilitates the growth of V. mali but not that of the other two fungi, while in F. graminearum and C. parasitica, with deletion of dicer-like genes, the primary components of the RNA-silencing pathway, HSVd accumulation increases. We further demonstrate that HSVd can be bidirectionally transferred between F. graminearum and plants during infection. The viroids also efficiently infect fungi and induce disease symptoms when the viroid RNAs are exogenously applied to the fungal mycelia. These findings enhance our understanding of viroid replication, host range, and pathogenicity, and of their potential spread to other organisms in nature.
Viroids are infectious pathogenic agents possessing small, nonencapsidated, circular single-stranded RNAs that, to date, have been found to naturally infect only plants (1, 2). Viroids infect a wide variety of higher plant species, causing devastating diseases in many crops, particularly vegetables, fruits, and ornamental plants (3). In crop plants, viroids are known to spread by vegetative propagation; by mechanical agricultural practices; and, in certain cases, through seeds, pollen, and insect transmission (3, 4). As viroids do not encode any proteins and do not require a helper agent for their multiplication and survival, the biological activities of viroids are entirely dependent on direct interactions of their RNA genome or its derivatives with cellular host components (5–9). Viroid replication or processing of its RNAs in the yeast, Saccharomyces cerevisiae, and cyanobacterium, Nostoc (Nostocales), have been demonstrated (10–12).
Currently, at least 34 viroid species have been identified and are classified into two families, Avsunviroidae and Pospiviroidae (13–15). The members of Avsunviroidae (type species: Avocado sunblotch viroid) replicate in the chloroplasts or plastids through symmetric rolling-circle replication using the host nuclear-encoded polymerase. Their RNAs form highly branched secondary structures and have ribozyme activities. Members of Pospiviroidae (type species: Potato spindle tuber viroid) replicate and accumulate in the nucleus through asymmetric rolling-circle replication using host RNA polymerase II (Pol II) as the replication enzyme. Their RNAs form rod-shaped secondary structures but likely lack ribozyme activities (2, 16). Potato spindle tuber viroid (PSTVd) requires a unique splicing variant of transcription factor IIIA (TFIIIA-7ZF) to replicate by Pol II (17) and optimizes expression of TFIIIA-7ZF through a direct interaction with a TFIIIA splicing regulator (ribosomal protein L5, a negative regulator of viroid replication) (18). The molecular basis of viroid pathogenicity is not fully understood, although some mechanisms have been suggested, including the down-regulation of host gene expression via RNA silencing-mediated degradation guided by viroid-derived small RNAs (19–21).
The majority of plant diseases are caused by fungi and fungus-like organisms (oomycetes). Plant pathogenic fungi mainly belong to Ascomycetes and Basidiomycetes, and are generally categorized according to whether they absorb sugar or nutrients from the dead host cells (necrotrophic) or living host cells (biotrophic) (22, 23). It has been long known that pathogenic fungi, particularly the biotrophs, secrete effector proteins into host cells to promote fungal infection (24, 25). More recently, it was determined that pathogenic fungi transfer small RNA effectors to suppress host defense-related genes (26, 27) as well as acquire small RNAs transferred from plants during infection (28, 29), revealing bidirectional horizontal transfers of genetic information between fungi and plant hosts (30).
Like other higher eukaryotes, fungi could host other parasites, including fungi (31), bacteria (32, 33), and viruses (34). Mycoviruses infecting phytopathogenic fungi have been extensively characterized, in part, owing to their prospect for the use as biological control agents of plant fungal diseases (34–36). Indeed, there are a number of examples of the successful or promising use of mycoviruses to control fungal diseases (37–39). Interestingly, a number of studies have recently demonstrated that phytopathogenic fungi could be a suitable host of some plant viruses (40–42), which extends the previously known compatibility of plant viruses and yeast (S. cerevisiae) as hosts (43, 44). Conversely, mycoviruses originating from marine fungi (Penicillium aurantiogriseum var. viridicatum) associated with sea plants were shown to replicate in plant cells (45). Together, these observations give rise to an interesting notion that certain parasitic agents could cross kingdom barriers and invade both plants and fungi.
In this study, we demonstrate the stable replication of three plant viroids in phytopathogenic fungi. Importantly, viroid infection could reduce the growth and virulence of a defined pathogenic fungus. Moreover, we found that viroid infections of fungi could be established through in planta transmission during fungal infection or when the viroid RNAs were directly applied to fungal mycelia. We discuss the significance of these findings for extending our knowledge of the host ranges and pathogenesis of viroids.
Results
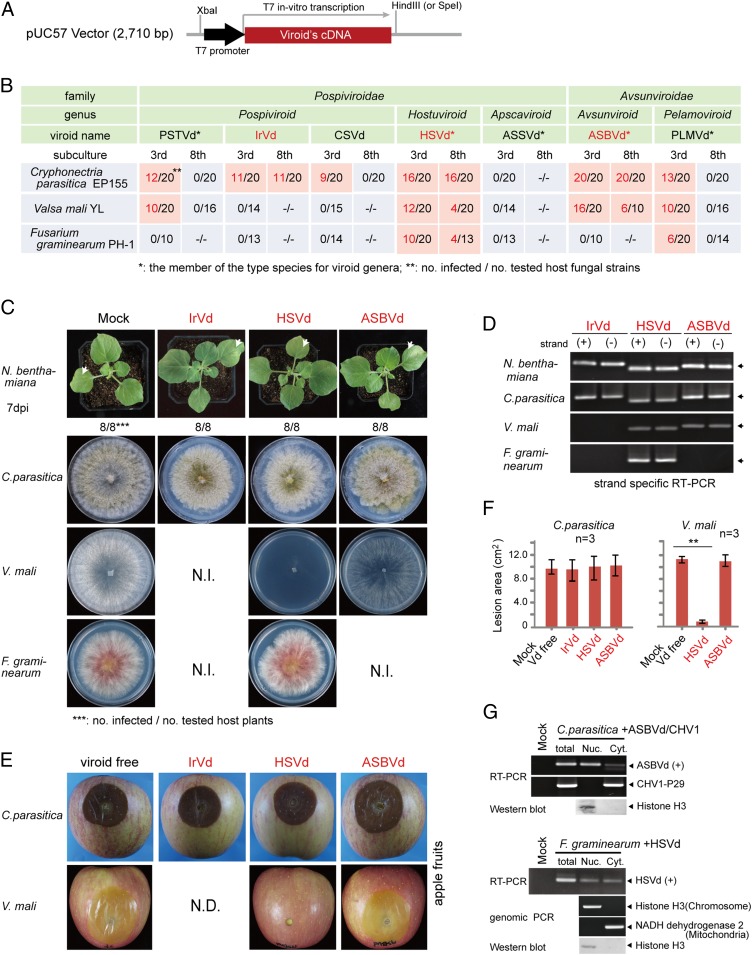
Production of Infectious Viroid cDNA Clones.
Using oligonucleotide synthesis and cloning technique, we produced full-length monomeric cDNA clones of seven plant viroids belonging to the Pospiviroidae [PSTVd; iresine 1 viroid (IrVd-1), chrysanthemum stunt viroid (CSVd), hop stunt viroid (HSVd), and apple scar skin viroid (ASSVd)] and Avsunviroidae [avocado sunblotch viroid (ASBVd) and peach latent mosaic viroid (PLMVd)]. To facilitate in vitro transcription, T7 RNA polymerase promoter sequences and a restriction site (HindIII or SpeI) were incorporated into the 5′ and 3′ termini, respectively, of each cDNA clone (Fig. 1A and SI Appendix, Fig. S1). First, the infectivity of uncapped plus (+) strand in vitro transcripts of viroid cDNA clones was tested by mechanical inoculation to the leaves of Nicotiana benthamiana (Solanaceae) plants. At 7 d postinoculation, viroid RNAs were detected by RT-PCR in the upper systemic leaves of all plants inoculated with the seven viroid transcripts, although no visible symptoms were observed on these plants (Fig. 1 C and D and SI Appendix, Fig. S2 A and B). When the viroid transcripts were introduced to yeast cells (S. cerevisiae strain AH109) by transfection, viroid RNA accumulations were detected by RT-PCR after successive subcultures of the cells (SI Appendix, Fig. S2 C and D), indicating viroid replication in this host similar to what was previously demonstrated for ASBVd (10). These results confirm the infectivity of in vitro RNA transcripts derived from all seven viroid cDNA clones.
Fig. 1.
Viroid infection and pathogenicity in filamentous fungi. (A) Sequence construct for generating plus (+) strand in vitro transcripts of viroid cDNA clones. (B) Detection of viroid in fungal isolates regenerated from fungal spheroplasts that have been transfected using in vitro transcripts of viroid cDNA clones. Viroid RNA accumulations were detected using RT-PCR at third and eighth fungal subcultures. (C) Phenotypic growth of plants and fungi infected with viroids. Fungi were grown on PDA medium (10-cm plate) for 3 d and photographed. N.I., not infected. (D) RT-PCR analysis of viroid RNA accumulation in the plants and fungi described in C. (E) Fungal virulence assay on apple. Apple fruits were inoculated with mycelial plugs, and fungal lesions were photographed 5 d after inoculation. N.D., no data. (F) Fungal lesion area measured on inoculated apple fruits described in E. **P < 0.01 (Student’s t test). Vd, viroid. (G) Accumulation of viroids in nuclear (Nuc.)- and cytoplasmic (Cyt.)-enriched fractions. Spheroplasts of infected fungi were subjected to cell fractionation using differential centrifugation. Viroid (ASBVd or HSVd) and virus (CHV1) RNAs were detected by RT-PCR, while chromosomal and mitochondrial DNAs (cytosolic DNAs) were detected by genomic PCR. The presence of histone H3 protein in the nuclear fraction was confirmed by Western blotting analysis.
Infection of Plant Pathogenic Fungi with Viroids.
We investigated the possibility of viroid infection in three phytopathogenic ascomycete fungi, Cryphonectria parasitica, Valsa mali, and Fusarium graminearum, which are the causative agents of chestnut blight, apple tree canker, and wheat/barley head blight and maize ear rot diseases, respectively (46–48). Spheroplasts prepared from these three fungi were transfected with in vitro RNA transcripts derived from each of the seven viroid cDNA clones and then regenerated and followed by subcultures (SI Appendix, Fig. S3A). Several fungal transfectants (10–20 isolates) derived from each transfection (total of 21 viroid–fungal host combinations) were analyzed for the presence of viroid RNAs using RT-PCR and sequencing of the amplification products. During the first round of screening (third fungal subcultures), the majority of viroid–fungus combinations (12 of 21 transfections with six viroids) yielded fungal transfectants that were carrying viroids (Fig. 1B). After the third fungal subculture, C. parasitica was infected by six of seven viroids tested, V. mali by four of seven, and F. graminearum by two of seven (Fig. 1B). However, after the eighth subculture, only three viroids were stably maintained in these filamentous fungi: HSVd accumulated in all three fungi, while ASBVd accumulated in C. parasitica and V. mali, and IrVd-1 could be detected only in C. parasitica (Fig. 1 B and D and SI Appendix, Fig. S3C). RNA blotting analysis was able to detect HSVd and ASBVd RNA accumulation in N. benthamiana plants, but not in fungi (SI Appendix, Fig. S4A). The negative (−) strand viroid RNAs were also detected by RT-PCR (Fig. 1D and SI Appendix, Fig. S3C; confirmation of strand specificity of RT-PCR is shown in SI Appendix, Fig. S3B). Likewise, circular (and/or linear oligomeric) (+) genomic RNAs were detected by inverse RT-PCR (SI Appendix, Fig. S5). Moreover, viroids were efficiently transmitted through hyphal anastomosis and conidia (SI Appendix, Fig. S6), which are similar to horizontal and vertical transmissions of mycoviruses (49). Thus, taken together with the stable accumulation of these viroid RNAs, these observations strongly indicate the replication of these viroids in these fungal hosts.
C. parasitica infected with IrVd-1, HSVd, or ASBVd exhibited slightly reduced growth on potato dextrose agar (PDA) medium compared with viroid-free fungus (Fig. 1C and SI Appendix, Fig. S3D), but viroid infections had no effect on C. parasitica virulence in an apple-fruit inoculation assay (Fig. 1 E and F). Likewise, viroid infections had no apparent effect on growth and virulence of V. mali (ASBVd) and F. graminearum (HSVd), respectively (Figs. 1 C, E, and F and 2B and SI Appendix, Fig. S3D). In contrast, HSVd infection severely debilitated V. mali growth and virulence (Fig. 1 C, E, and F and SI Appendix, Fig. S3D), indicating that HSVd confers pathogenicity to V. mali.
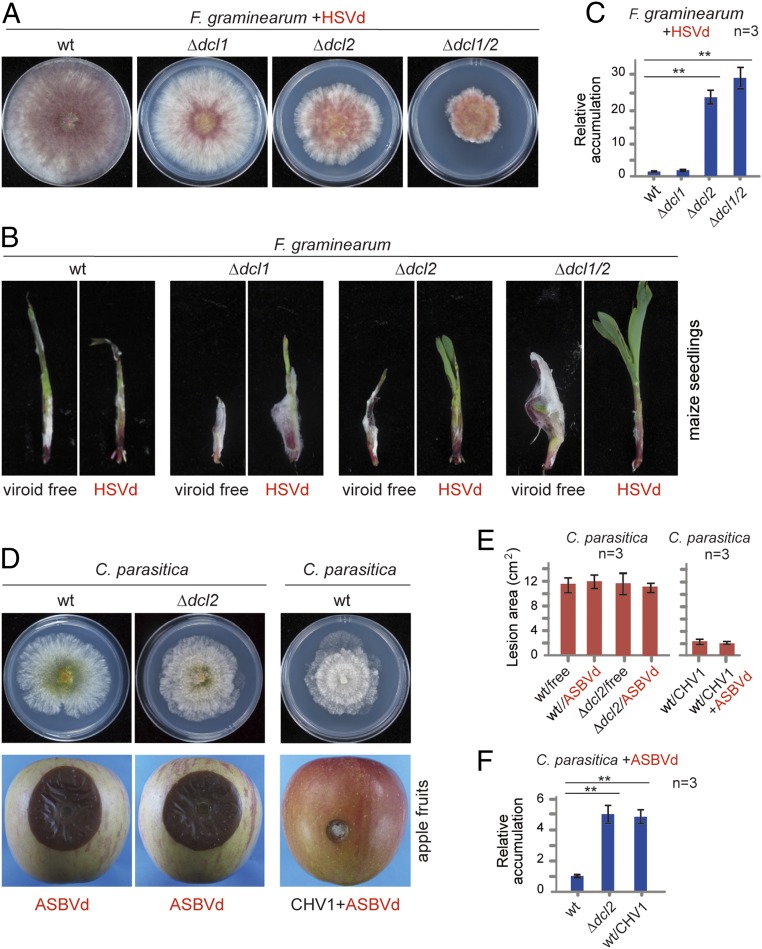
Fig. 2.
Viroid accumulation and pathogenicity in dcl mutant fungal strains. (A) Phenotypic growth of wild-type (wt) and dcl mutants of F. graminearum infected with HSVd. Fungi were grown on PDA medium (6-cm plate) for 3 d and photographed. (B) Disease severities on maize seedlings induced by infection of the fungal strain described in A. Seedlings were photographed 7 d after inoculation. (C) Quantitative RT-PCR analysis of HSVd accumulation in F. graminearum dcl mutants. **P < 0.01 (Student’s t test). (D) Phenotypic growth and virulence on apple of wt and dcl2 mutants of C. parasitica infected with ASBVd and wt C. parasitica coinfected with ASBVd and CHV1. Fungi were grown on PDA medium for 3 d and photographed. Fungal lesions on apples were photographed 5 d after inoculation. (E) Fungal lesion area measured on inoculated apple described in D. (F) Quantitative RT-PCR analysis of ASBVd accumulation in C. parasitica strains described in D. **P < 0.01 (Student’s t test).
As a member of the Avsunviroidae, ASBVd replicates in the chloroplasts of plant cells (5). As fungal cells do not have chloroplasts or plastids (nonphotosynthetic organisms), it is thus not predictable where ASBVd replication takes place within the fungal cell. To investigate this aspect, spheroplasts prepared from C. parasitica coinfected with ASBVd and Cryphonectria parasitica hypovirus 1 [CHV1, a well-studied single-stranded RNA (ssRNA) mycovirus belonging to the genus Hypovirus] were subjected to a cell fractionation procedure to separate nuclear and cytoplasmic contents. RT-PCR detection showed that ASBVd RNAs were mainly present in the nuclear-enriched fraction, whereas the coinfecting CHV1 RNAs were exclusively detected in the cytoplasmic fraction (Fig. 1G), which is expected from cytoplasmically replicating ssRNA viruses (50). In a fractionation experiment using F. graminearum, HSVd, a nuclear-replicating viroid, was shown to be present in both nuclear and cytoplasmic fractions (Fig. 1G). These findings suggest that both types of plant viroids replicate in the nuclear compartment of fungal cells and might utilize fungal nuclear polymerase for their replication (10).
RNA Silencing Is Implicated in Defense Against Viroids in Fungi.
RNA silencing is the primary defense mechanism against viruses in plants, fungi, and insects (51–53). This mechanism has been shown to operate against viroids in plants (19), and it could conceivably also operate in fungi. To elucidate this aspect, HSVd was introduced to F. graminearum knockout mutants defective in the gene encoding for Dicer-like (DCL) protein, essential for RNA silencing through generation of small-interfering RNAs (54). Many filamentous fungi encode two dcl genes, dcl1 and dcl2 (55). HSVd was inoculated to F. graminearum Δdcl1, Δdcl2, and Δdcl1/2 double mutants via transfection of spheroplasts with viroid RNAs. Relative to HSVd-infected wild-type strain, infected Δdcl1 strain exhibited only slightly reduced growth on PDA medium, while infected Δdcl2 and, Δdcl1/2 strains showed a strong reduction in growth (Fig. 2A); furthermore, dcl knockout did not noticeably alter the growth of viroid-free F. graminearum (SI Appendix, Fig. S7A). A fungal inoculation assay on maize seedlings demonstrated that HSVd-infected Δdcl2 and Δdcl1/2 strains, but not Δdcl1 strain, showed reduced virulence (Fig. 2B). Accordingly, HSVd RNA accumulation was more than 20-fold higher in Δdcl2 and Δdcl1/2 than in Δdcl1 and wild-type strains (Fig. 2C), and was readily detectable in the Δdcl1/2 mutant by RNA blotting (SI Appendix, Fig. S4B), indicating that DCL2 is the primary DCL component for defense against viroids in F. graminearum. In addition, inactivation of dcl1 or dcl2 enhanced the efficiency of HSVd transmission through conidia (SI Appendix, Fig. S6B).
To further investigate whether DCL2 protein also plays a defense role against viroids in other fungi, ASBVd was inoculated to the C. parasitica Δdcl2 mutant (56). ASBVd-infected wild-type and Δdcl2 mutant strains of C. parasitica showed similar levels of growth and virulence (Fig. 2 D and E and SI Appendix, Fig. S7B); nevertheless, ASBVd RNAs accumulated around fivefold higher in Δdcl2 mutant versus wild-type strain (Fig. 2F), and ASBVd RNAs in Δdcl2 mutant were detectable by RNA blotting (SI Appendix, Fig. S4B), showing that C. parasitica DCL2 also contributes to defense against viroid infection, similar to mycovirus infections (56, 57). CHV1 infection was previously shown to have enhancing effects on a coinfecting double-stranded RNA (dsRNA) mycovirus through the activity of CHV1 p29, an RNA-silencing suppressor (58). Interestingly, coinfection with CHV1 also elevated ASBVd RNA accumulation (Fig. 2F). This result further underlines the role of RNA silencing in fungal host defense against viroid infection.
Viroids Could Be Bidirectionally Transferred Between Plants and Fungi.
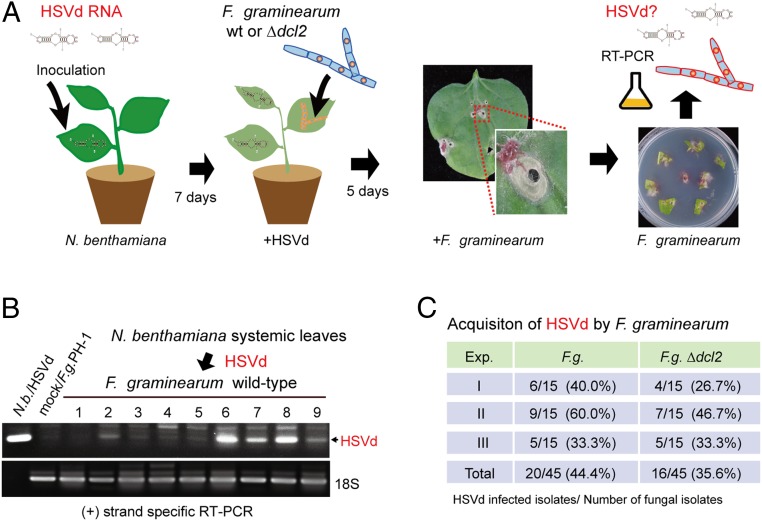
The finding that plant viroids can infect phytopathogenic fungi raises the question of whether viroids could be horizontally transferred from the plant to fungus during colonization of plant tissue by the fungal pathogens. In fact, under laboratory conditions, we have previously demonstrated the transfer of a plant RNA virus from the plant to fungus, an event that possibly occurs in natural settings (42). To explore this possibility, we inoculated viroid-free wild-type and Δdcl2 mutant strains of F. graminearum to HSVd-infected N. benthamiana plants. After allowing F. graminearum to infect and colonize the leaf tissue, the fungus was then reisolated from plants and subcultured before viroid detection using RT-PCR (Fig. 3A). Analysis showed that, respectively, ∼44% and 36% of wild-type and Δdcl2 fungal isolates derived from HSVd-infected plants contained HSVd (Fig. 3 B and C), indicating that F. graminearum could acquire HSVd from plants during infection.
Fig. 3.
Acquisition of HSVd by F. graminearum from plants. (A) Experimental procedure for investigating HSVd acquisition by wild-type (wt) and dcl2 mutants of F. graminearum. (B) RT-PCR detection of HSVd accumulation in F. graminearum (F.g.) strains isolated from inoculated leaves of N. benthamiana (N.b.) plants. (C) Efficiency of HSVd acquisition by wt and dcl2 mutants of F.g. from N. benthamiana plants. Exp., experiment.
Next, we investigated whether F. graminearum could introduce HSVd to the plant by inoculating HSVd-infected fungus to viroid-free N. benthamiana plants. Seven days later, viroid RNAs were detected in the uninoculated upper leaves by RT-PCR (SI Appendix, Fig. S8A). RT-PCR detected HSVd RNAs in the upper systemic leaves of all fungal inoculated N. benthamiana plants (SI Appendix, Fig. S8B). Lack of spread of the fungus to the upper leaves was confirmed by RT-PCR for detection of F. graminearum 18S rRNA (SI Appendix, Fig. S8B), as well as no growth of fungal mycelia from the portions of uninoculated upper leaves placed on medium (SI Appendix, Fig. S8C). This result demonstrates that viroid-infected F. graminearum can efficiently transmit HSVd to a plant.
Viroid Infection Through Direct Inoculation of Fungal Mycelia.
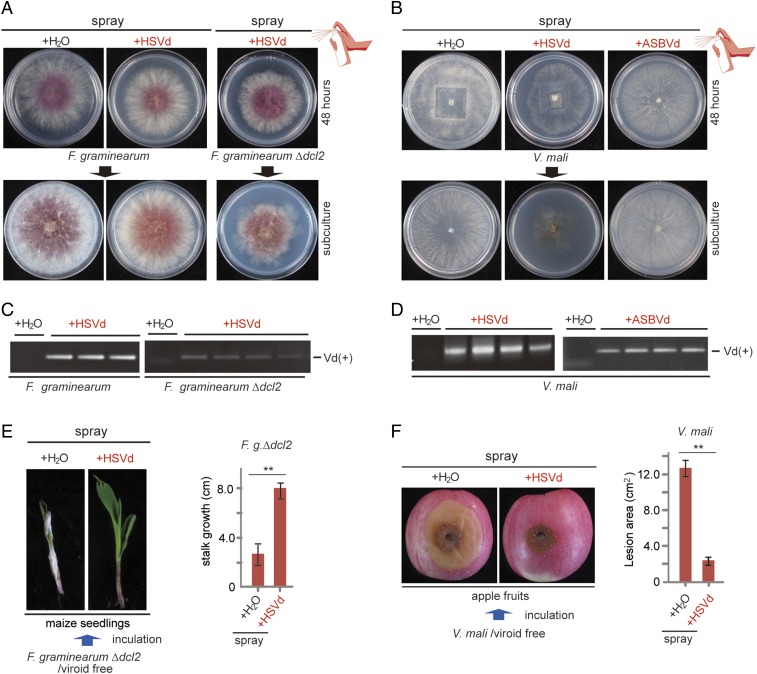
Previous studies showed that fungal and plant viruses can infect fungi when virions are externally applied to mycelia (38, 41), but it is not known whether naked viral RNAs are infectious when similarly applied to mycelia. However, there have been reports showing that various fungi can uptake exogenous dsRNAs (59, 60). We therefore investigated whether viroid RNA could be introduced to the fungus through exogenous application. As a first attempt, solutions containing HSVd or ASBVd RNAs (100 μg/μL) were directly applied to the mycelia of F. graminearum and V. mali grown on PDA medium by spraying with the viroid solutions or water. After allowing the fungus to grow more for 2 d, several mycelial plugs were taken and subcultured for phenotyping and RT-PCR analysis (SI Appendix, Fig. S9A). Fungal isolates derived from F. graminearum and V. mali that were sprayed with HSVd and ASBVd RNAs, respectively, were confirmed to be infected with the corresponding viroids, although no clear symptoms were observed (Fig. 4 A–D). In a parallel experiment, the presence of viroid RNAs was not detected when a similar spray inoculation procedure was carried out using ASSVd RNAs and V. mali (SI Appendix, Fig. S9 B and C), which are incompatible viroid and fungal host combinations (Fig. 1B), ruling out the possibility that RT-PCR amplified RNA in the inoculum that was applied to the cultures.
Fig. 4.
Viroid infection through exogenous inoculation. (A and B) Phenotype of fungal strains after direct application of viroid RNAs on mycelia (as illustrated in SI Appendix, Fig. S9). (C and D) RT-PCR detection of viroid (Vd) accumulations in the fungal strains described in A and B. (E and F) Disease severities of fungal strains grown on maize seedlings (E) and apple fruits (F) following direct application of viroid RNAs are illustrated. **P < 0.01 (Student’s t test).
Next, we extended the experiment by inoculating HSVd RNAs to F. graminearum Δdcl2 mutant and V. mali, which are the susceptible hosts of HSVd (Figs. 1C and 2A), so that the effects of viroid infection on the host fungi would be visible following the viroid RNA spray. As expected, F. graminearum Δdcl2 and V. mali exhibited an obvious reduction in growth following HSVd RNA treatments (Fig. 4 A and B), and HSVd infection was confirmed by RT-PCR (Fig. 4 C and D). Prompted by these results, we then sprayed HSVd RNAs on F. graminearum Δdcl2 and V. mali colonies that grew on maize seedlings and apples, respectively. Strikingly, compared with water treatment (+H2O), HSVd RNA treatment (+HSVd) reduced fungal growth and virulence, as indicated by the larger growth of F. graminearum-infected maize seedlings (Fig. 4E) and the reduced size of V. mali lesions on apples (Fig. 4F). Taken together, these results demonstrate the direct application of viroid RNAs on fungal mycelia as an effective viroid inoculation method.
Discussion
Viroids are considered as the smallest and simplest infectious pathogen. Despite the small size (∼250–400 nucleotides) and noncoding nature of their RNA genome, viroids are capable of self-replication, cell-to-cell and long-distance spread, and inducing disease symptoms in host plants. PSTVd (a pospiviroid) is the most intensively studied viroid, and its RNA secondary structure is well understood. Mutational analyses have identified the loops in PSTVd’s structure that are critical for its replication, along with its cell-to-cell and systemic spread (61, 62), demonstrating the direct role of the RNA genome in regulating biological activities of viroids. Although viroids generally infect a limited range of natural plant hosts, experimentally, they have been shown to replicate in a wide range of plant species (63), yeast (10), and cyanobacteria (12). In this study, we have shown they can also infect filamentous fungi. These results indicate the ability of some viroids to infect a wide variety of organisms across biological kingdoms. Thus, viroids probably coopt highly conserved polymerases and other factors to replicate.
In this study, seven viroids were inoculated to three filamentous phytopathogenic fungi belonging to different families. Unlike the full compatibility of all viroids with a unicellular fungus (yeast) (SI Appendix, Fig. S2), viroid infection in filamentous fungi was apparently more host-specific and also seems to be affected by host immune responses (Fig. 1). We found that not all viroids tested were infectious in the same fungus, suggesting incompatibility with the host factors required for replication in some viroid–fungus combinations (5–9). In addition, some viroids initially accumulated in the specific fungal hosts, but they were then eliminated after successive fungal cultures, likely by the activities of a fungal defense mechanism(s) against invading pathogens. For example, PLMVd (a pelamoviroid), was detected in all three fungi in the third subculture but not in the eighth subculture, suggesting that PLMVd is sensitive to the fungal immune responses. Only one viroid, HSVd (a hostuviroid), was able to stably replicate in all three fungi, while ASBVd (an avsunviroid) replicated in C. parasitica and V. mali, which both belong to the order Diaporthales, but not in F. graminearum (order Hypocreales), suggesting that HSVd has a rather wide fungal host range, while the host range of ASBVd is more limited. We showed that both nuclear (HSVd)- and chloroplast (ASBVd)-replicating plant viroids replicate in filamentous fungi, and were both detected in fungal nuclear fractions (Fig. 1), suggesting the adaptability of both types of plant viroids to the nuclear environment of fungal cells.
Among the five fully compatible viroid–fungus combinations, only HSVd infection in V. mali caused severe disease symptoms, as seen by the drastic reduction of fungal growth (Fig. 1). This viroid-induced phenotypic symptom is quite similar to the commonly observed reduced growth and “hypovirulence” associated with mycovirus infection in filamentous fungi (64). In fact, the symptom diseases induced by viroid infection in plants quite faithfully resemble those caused by plant viruses, such as plant stunting, leaf variegation, yellowing, necrosis, and sometimes plant death (3). Viral pathogenicity proteins are commonly responsible for development of viral symptoms through disturbances of various biological processes, such as gene expression, metabolism, and immune responses (65), while the mechanism underlying viroid pathogenesis is not well understood. However, it is generally known that viroid infection is associated with the alteration of expression of host plant genes (66–68) and accumulation of viroid-derived small RNAs (69, 70). Some studies suggest the role of viroid-derived small RNAs in modulating viroid symptoms (20, 21, 71, 72). Further work is needed to investigate whether the RNA-silencing pathway or other mechanisms are involved in HSVd pathogenicity in V. mali. Given the practicality of filamentous fungi as an experimental model that has a short life cycle and allowance of productive genetic manipulations, a viroid–fungus pathosystem would provide an advantageous platform to elucidate the molecular mechanism underlying pathogenic effects of viroid infection on the nonplant host.
Inactivation of genetic components of the RNA-silencing pathway usually lead to enhanced host susceptibility manifested as an increase of virus accumulation and symptoms (56, 73, 74). Plants encode multiple DCL proteins that are implicated in distinct RNA-silencing pathways (75–77). For example, experimental model plants, such as Arabidopsis thaliana and the Nicotiana species, encode four DCL proteins (75, 78). Studies of PSTVd using transgenic N. benthamiana plants with down-regulated dcl genes revealed a complex relationship between DCL proteins and viroid infections. DCL1 and DCL4, which play essential roles in the microRNA pathway and antiviral defense against RNA viruses, respectively, are required for optimum PSTVd accumulation, whereas the combined activity of DCL2 and DCL3 inhibits PSTVd accumulation (79–81). Hence, different from plant RNA viruses, viroids may utilize particular DCL proteins to support their infection; meanwhile, they may be suppressed by other DCL components. Our inoculation experiment using dcl mutant strains of F. graminearum and V. mali indicated that DCL2, but not DCL1, is likely the primary DCL component in fungal defense against viroids (Fig. 2). Similarly, DCL2 is also critical for antiviral defense in C. parasitica and Colletotrichum higginsianum (a hemibiotrophic ascomycete fungus) (56, 82); in other fungi (F. graminearum), DCL1 and DCL2 can functionally complement each other (83). Therefore, it seems that the filamentous fungi exert similar RNA-silencing mechanisms to suppress viral and viroid infections, although it is necessary to further investigate the roles of other core components of the RNA-silencing pathway, such as Argonaute and RNA-dependent RNA polymerase, which have been shown to contribute to defense against viroids in plants (84, 85).
We previously discovered a natural infection of a plant virus, cucumber mosaic virus [CMV; a plant alpha-like (+) ssRNA virus superfamily] in a strain of the phytopathogenic basidiomycete fungus, Rhizoctonia solani, obtained from the field (42). Moreover, under laboratory conditions, we demonstrated that R. solani can acquire CMV from an infected plant and, vice versa, can transmit CMV to the plant. Thus, based on our current findings on the potential viroid transfer between plants and fungi (Fig. 3 and SI Appendix, Fig. S8), it is tempting to speculate the occurrence of viroid infections in plant-associated fungi in the natural environment. Thus, varieties of fungi, including phytopathogenic, mycorrhizal, and endophytic fungi, could possibly serve as the reservoir or biological vector of viroids. Except for certain mycoviruses with viroid-like RNA satellites (86, 87), viroid or self-replicating viroid-like agents have not been found in fungi. Presumably, the common use of dsRNA fractions that exclude low-molecular-weight RNAs for mycovirus identifications or viral metagenomics may have prevented the discovery of viroids from fungi.
The application of mycoviruses for biological control of plant fungal diseases is hampered by the lack of practical methods to introduce the hypovirulence-causing virus to pathogenic fungi in the field (38). Although mycoviruses could be transmitted intracellularly via hyphal anastomosis, different fungal isolates often show vegetative incompatibility that prevents the spread of the virus (88, 89). Therefore, exogenous inoculation through direct application of the virus, such as successfully demonstrated for inoculation of a circular ssDNA mycovirus, Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 (family Genomoviridae, nonenveloped spherical virion), to the phytopathogenic ascomycete fungus Sclerotinia sclerotiorum (38), is a promising method for biocontrol application. Moreover, fungal cells could uptake external dsRNAs that activate the down-regulation of fungal mRNA targets through an RNA-silencing mechanism, and this has been exploited as an alternative fungal control method (59, 90, 91). Our inoculation experiments showed efficient viroid infection of the fungi when the viroid RNAs were directly applied to the mycelia (Fig. 4). The high stability of dsRNAs and highly structured viroid RNAs in unprotective extracellular conditions may partly facilitate efficient entry of those RNA molecules to fungal cells. Importantly, reduced growth and virulence were apparent on fungal colonies grown on plants or fruit following exogenous inoculation of viroid RNAs (Fig. 4). Considering that certain viroids are not infectious or pathogenic to particular crop plants, this implies the potential use of viroids for biocontrol of fungal diseases, but biosafety considerations need to be resolved prior to using viroids for such applications.
Materials and Methods
Fungal Strains, Virus Isolate, and Plant Materials.
C. parasitica strain EP155 virus-free, infected with CHV1, and Δdcl2 mutant strains (56, 92) were generous gifts from Nobuhiro Suzuki, Okayama University. V. mali YL strain was described previously (42). F. graminearum PH1, Δdcl1, Δdcl2, and Δdcl1/Δdcl2 mutants (93) were kindly provided by Jinrong Xu, Northwest A&F University. All fungal strains were grown on PDA medium for 3–6 d at 24–26 °C for morphological observation or on cellophane-covered PDA medium for RNA, DNA, and protein extractions. Zea mays (maize) var. Luoyu 818 and N. benthaniana plants were grown in a growth room at 22 ± 2 °C with a photoperiod of 16 h/8 h (day/night).
Generation of Viroid cDNA Clones.
The full-length monomeric cDNAs of seven plant viroids were generated by oligonucleotide synthesis and performed by Jin Weizhi Co. To construct the infectious cDNA clone, the full-length monomeric cDNA fragments of viroids were PCR-amplified with primers incorporating the restriction site, XbaI, and T7 promoter sequences at 5′ and HindIII/SpeI (Fig. 1A and SI Appendix, Fig. S1). The PCR products were inserted into the pUC57 Vector (GenScript, Inc.). After linearization of plasmids with restriction enzymes, the monomeric viroid RNAs were synthesized using an in vitro transcription kit (RiboMAXLarge Scale RNA Production Systems-SP6 and T7; Promega) according to the manufacturer’s instructions.
Yeast and Fungal Protoplast Isolation and Viroid Transfection.
Preparation of competent cells of S. cerevisiae AH109 (Clontech) and transfection of in vitro transcripts of viroid RNAs (0.1 μg) using plasmid transformation methods were carried out according to the CLONTECH yeast protocols handbook. The transfected yeast cells were grown on yeast peptone dextrose agar (YPDA; Sigma) and incubated at 28 °C for roughly 72 h. Four independent colonies were randomly selected and cultured in the YPD liquid medium for 72 h. Protoplasts of V. mali, C. parasitica, and F. graminearum strains were prepared following the method described previously (94). Transfection of fungal protoplasts with in vitro-transcribed RNAs (0.1 μg) was performed as described previously (95).
Viroid and Fungal Inoculations to Plants.
For mechanical inoculation of viroids to plants, in vitro transcripts of viroid cDNA clones (0.1 μg/μL) were rubbed onto carborundum-dusted leaves of N. benthamiana plants. For inoculation of F. graminearum strains to N. benthamiana plants, lower leaves were wounded with sterilized toothpicks, and mycelia-containing gel plugs (∼0.5 × 1 cm), which were picked up from the edge of a 3-d-old culture colony, were placed on the wounded area. For inoculation of F. graminearum to maize plants, maize seeds were germinated on sterilized Petri plates layered with wet filter papers. After the shoots reached a height of 4 cm, the stems were wounded and mycelia-containing gel plugs were placed on the wounds. The inoculated part of the stem was wrapped with parafilm for 24 h. Inoculated plants were grown in pots with soil at 23 ± 2 °C at 70–80% humidity and a photoperiod of 16 h/8 h (day/night).
Exogenous Inoculation of Fungal Mycelia with Viroid RNAs.
In vitro transcripts of viroid RNAs were diluted in RNase-free water (100 μg/μL), and solution was then transferred to a small spray bottle (∼1 mL). The viroid RNA solution (∼200 μL) was directly sprayed onto fungal colonies grown on PDA plates (1-d-old), apples (2 d), or maize seedlings (2 d) until the solution visibly covered the mycelial area. For subculturing the fungal colonies, mycelial plugs outside the sprayed region were transferred to new PDA plates.
Viroid Acquisition by F. graminearum.
To investigate HSVd acquisition by F. graminearum, lower leaves of N. benthamiana plants were first mechanically rub-inoculated with HSVd (12–16 plants were inoculated in each experiment), and viroid infection was then confirmed by RT-PCR at 7 d after inoculation. Virus-free F. graminearum was then inoculated onto the noninoculated upper leaves. Seven days after fungal inoculation, F. graminearum was retrieved from the inoculated plants with a similar procedure as described previously (42).
RNA Extraction, RT-PCR, and RNA Blot Analysis.
Extraction of ssRNA and total RNA from fungal mycelia followed the procedure described previously (96). Total RNAs were extracted from leaves of N. benthamiana plants using TRIzol (Invitrogen). For RT-PCR detection, first-strand cDNAs were synthesized using ReverTra Ace reverse transcriptase (Toyobo) and amplified by using 2× mixture DNA polymerase (Kangwei). For quantitative RT-PCR, the 18S RNA of C. parasitica and F. graminearum was employed as an internal control. Quantitative RT-PCR was performed with the GoTaq Green Master Mix kit (Promega) on a CFX96TM Real-Time PCR Detection System apparatus (Bio-Rad). Three biological replicate samples were analyzed. For Northern blot analysis, total RNAs were separated on 7.5% polyacrylamide gel electrophoresis (PAGE) or denaturing agarose gel (2%). Digoxigenin (DIG)-labeled DNA probes specific for HSVd (nucleotides 2–301) or ASBVd (nucleotides 6–233) were used for detection. The probes were prepared using the PCR DIG Probe Synthesis Kit (Roche). Gel electrophoresis and blotting were carried out as described previously (97). Hybridization conditions and detection of mRNAs were as described in the DIG application manual supplied by Roche. All of the primers used in this study are listed in SI Appendix, Table S1.
Fractionation of Cytosolic and Nuclear Components and Western Blot Analysis.
Fractionations of cytosolic and nuclear components were conducted according to the subcellular fractionation protocol provided by Abcam (https://www.abcam.com/ps/pdf/protocols/subcellular_fractionation.pdf). Briefly, fungal spheroplasts were suspended in a fractionation buffer [250 mM sucrose, 20 mM Hepes (pH 7.4), 10 mM KCl, 1.5 mM MgCl2, 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 1 mM dithiothreitol, and protease inhibitor mixture (Roche)] and passed through a 25-gauge needle 10 times. Nuclear pellet (nuclear-enriched fraction, P1) was obtained by centrifugation at 720 × g for 5 min. The supernatant was centrifuged at 10,000 × g for 15 min, and the resulting pellet was used as the cytoplasmic fraction.
Preparation of protein samples, sodium dodecyl sulfate/PAGE, electroblotting, and immunodetection for Western blot analysis were carried out as described previously (96). Histone H3 was detected using primary polyclonal anti-Histone H3 antibody (1:2,000; Abcam) and secondary polyclonal horseradish peroxidase-conjugated mouse anti-rabbit IgG (1:10,000; Abcam). Protein bands were visualized with a western ECL substrate kit (Bio-Rad).
Fungal Pathogenicity Assays.
The virulence assay for C. parasitica and V. mali on apples was described previously (95). The pathogenicity assay for F. graminearum on maize shoots followed the method described previously (98).
Supplementary Material
Acknowledgments
We thank Drs. N. Suzuki and J. Xu for providing research materials; Dr. C. Han for helpful discussions; and Drs. L. Torrance and M. Keller for valuable comments and English editing of the manuscript. This work was supported, in part, by the National Key Research and Development Program of China (Grant 2017YFD0201100), National Natural Science Foundation of China (Grant U1703113), 111 Project of the Ministry of Education of China (Grant B07049), and Science Foundation of Shaanxi (Grant 2016KW-069) (all to L.S.), and by Grants-in-Aid for Scientific Research on Innovative Areas from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Grants 16H06436, 16H06429, and 16K21723 to H.K.).
Footnotes
Conflict of interest statement: B.I.H. has coauthored papers with H.K. and I.B.A. within the past 48 months. They did not collaborate directly on the papers.
This article is a PNAS Direct Submission. B.I.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1900762116/-/DCSupplemental.
References
- 1.Tsagris E. M., Martínez de Alba A. E., Gozmanova M., Kalantidis K., Viroids. Cell. Microbiol. 10, 2168–2179 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Flores R., Gago-Zachert S., Serra P., Sanjuán R., Elena S. F., Viroids: Survivors from the RNA world? Annu. Rev. Microbiol. 68, 395–414 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Flores R., Hernández C., Martínez de Alba A. E., Daròs J. A., Di Serio F., Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 43, 117–139 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Palukaitis P., What has been happening with viroids? Virus Genes 49, 175–184 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Navarro J. A., Vera A., Flores R., A chloroplastic RNA polymerase resistant to tagetitoxin is involved in replication of avocado sunblotch viroid. Virology 268, 218–225 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Schindler I. M., Muhlbach H. P., Involvement of nuclear DNA-dependent RNA-polymerases in potato spindle tuber viroid replication–A reevaluation. Plant Sci. 84, 221–229 (1992). [Google Scholar]
- 7.Gas M. E., Hernández C., Flores R., Daròs J. A., Processing of nuclear viroids in vivo: An interplay between RNA conformations. PLoS Pathog. 3, e182 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nohales M. A., Flores R., Daròs J. A., Viroid RNA redirects host DNA ligase 1 to act as an RNA ligase. Proc. Natl. Acad. Sci. U.S.A. 109, 13805–13810 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nohales M. A., Molina-Serrano D., Flores R., Daròs J. A., Involvement of the chloroplastic isoform of tRNA ligase in the replication of viroids belonging to the family Avsunviroidae. J. Virol. 86, 8269–8276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delan-Forino C., Maurel M. C., Torchet C., Replication of avocado sunblotch viroid in the yeast Saccharomyces cerevisiae. J. Virol. 85, 3229–3238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friday D., Mukkara P., Owens R. A., Baumstark T., Bruist M. F., Processing of potato spindle tuber viroid RNAs in yeast, a nonconventional host. J. Virol. 91, e01078-e17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latifi A., et al. , Replication of avocado sunblotch viroid in the cyanobacterium Nostoc sp. PCC 7120. J. Plant Pathol. Microbiol. 7, 341 (2016). [Google Scholar]
- 13.Di Serio F., et al. , Current status of viroid taxonomy. Arch. Virol. 159, 3467–3478 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Flores R., Randles J. W., Bar-Joseph M., Diener T. O., A proposed scheme for viroid classification and nomenclature. Arch. Virol. 143, 623–629 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Di Serio F., et al. ; Ictv Report Consortium , ICTV virus taxonomy profile: Avsunviroidae. J. Gen. Virol. 99, 611–612 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding B., The biology of viroid-host interactions. Annu. Rev. Phytopathol. 47, 105–131 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., et al. , A land plant-specific transcription factor directly enhances transcription of a pathogenic noncoding RNA template by DNA-dependent RNA polymerase II. Plant Cell 28, 1094–1107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang J., et al. , Potato spindle tuber viroid modulates its replication through a direct interaction with a splicing regulator. J. Virol. 92, e01004-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores R., et al. , Viroids, the simplest RNA replicons: How they manipulate their hosts for being propagated and how their hosts react for containing the infection. Virus Res. 209, 136–145 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Wang M. B., et al. , On the role of RNA silencing in the pathogenicity and evolution of viroids and viral satellites. Proc. Natl. Acad. Sci. U.S.A. 101, 3275–3280 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro B., et al. , Small RNAs containing the pathogenic determinant of a chloroplast-replicating viroid guide the degradation of a host mRNA as predicted by RNA silencing. Plant J. 70, 991–1003 (2012). [DOI] [PubMed] [Google Scholar]
- 22.van Kan J. A. L., Licensed to kill: The lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 11, 247–253 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Szabo L. J., Bushnell W. R., Hidden robbers: The role of fungal haustoria in parasitism of plants. Proc. Natl. Acad. Sci. U.S.A. 98, 7654–7655 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo Presti L., et al. , Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 66, 513–545 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Giraldo M. C., Valent B., Filamentous plant pathogen effectors in action. Nat. Rev. Microbiol. 11, 800–814 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Weiberg A., Wang M., Bellinger M., Jin H., Small RNAs: A new paradigm in plant-microbe interactions. Annu. Rev. Phytopathol. 52, 495–516 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Weiberg A., et al. , Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 342, 118–123 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowara D., et al. , HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 22, 3130–3141 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koch A., et al. , Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase-encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. U.S.A. 110, 19324–19329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hua C., Zhao J. H., Guo H. S., Trans-kingdom RNA silencing in plant-fungal pathogen interactions. Mol. Plant 11, 235–244 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Adams P. B., The potential of mycoparasites for biological control of plant diseases. Annu. Rev. Phytopathol. 28, 59–72 (1990). [DOI] [PubMed] [Google Scholar]
- 32.Bonfante P., Desirò A., Who lives in a fungus? The diversity, origins and functions of fungal endobacteria living in Mucoromycota. ISME J. 11, 1727–1735 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi D. Y., Crouch J. A., Bacterial/fungal interactions: From pathogens to mutualistic endosymbionts. Annu. Rev. Phytopathol. 47, 63–82 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Ghabrial S. A., Suzuki N., Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 47, 353–384 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Kondo H., Kanematsu S., Suzuki N., Viruses of the white root rot fungus, Rosellinia necatrix. Adv. Virus Res. 86, 177–214 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Xie J., Jiang D., New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 52, 45–68 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Anagnostakis S. L., Biological control of chestnut blight. Science 215, 466–471 (1982). [DOI] [PubMed] [Google Scholar]
- 38.Yu X., et al. , Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc. Natl. Acad. Sci. U.S.A. 110, 1452–1457 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiba S., et al. , A novel bipartite double-stranded RNA mycovirus from the white root rot Fungus Rosellinia necatrix: Molecular and biological characterization, taxonomic considerations, and potential for biological control. J. Virol. 83, 12801–12812 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mascia T., et al. , Infection of Colletotrichum acutatum and Phytophthora infestans by taxonomically different plant viruses. Eur. J. Plant Pathol. 10.1007/s10658-018-01615-9 (2019). [DOI] [Google Scholar]
- 41.Mascia T., et al. , Gene silencing and gene expression in phytopathogenic fungi using a plant virus vector. Proc. Natl. Acad. Sci. U.S.A. 111, 4291–4296 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andika I. B., et al. , Phytopathogenic fungus hosts a plant virus: A naturally occurring cross-kingdom viral infection. Proc. Natl. Acad. Sci. U.S.A. 114, 12267–12272 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao R. Y., Yeast for virus research. Microb. Cell 4, 311–330 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagy P. D., Yeast as a model host to explore plant virus-host interactions. Annu. Rev. Phytopathol. 46, 217–242 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Nerva L., Varese G. C., Falk B. W., Turina M., Mycoviruses of an endophytic fungus can replicate in plant cells: Evolutionary implications. Sci. Rep. 7, 1908 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andika I. B., Jamal A., Kondo H., Suzuki N., SAGA complex mediates the transcriptional up-regulation of antiviral RNA silencing. Proc. Natl. Acad. Sci. U.S.A. 114, E3499–E3506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rigling D., Van Alfen N. K., Regulation of laccase biosynthesis in the plant-pathogenic fungus Cryphonectria parasitica by double-stranded RNA. J. Bacteriol. 173, 8000–8003 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trail F., Common R., Perithecial development by Gibberella zeae: A light microscopy study. Mycologia 92, 130–138 (2000). [Google Scholar]
- 49.Eusebio-Cope A., et al. , The chestnut blight fungus for studies on virus/host and virus/virus interactions: From a natural to a model host. Virology 477, 164–175 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Jacob-Wilk D., Turina M., Van Alfen N. K., Mycovirus cryphonectria hypovirus 1 elements cofractionate with trans-Golgi network membranes of the fungal host Cryphonectria parasitica. J. Virol. 80, 6588–6596 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuss D. L., Mycoviruses, RNA silencing, and viral RNA recombination. Adv. Virus Res. 80, 25–48 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torres-Martínez S., Ruiz-Vázquez R. M., The RNAi universe in fungi: A varied landscape of small RNAs and biological functions. Annu. Rev. Microbiol. 71, 371–391 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Vance V., Vaucheret H., RNA silencing in plants–defense and counterdefense. Science 292, 2277–2280 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Chen Y., et al. , Characterization of RNA silencing components in the plant pathogenic fungus Fusarium graminearum. Sci. Rep. 5, 12500 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li L., Chang S. S., Liu Y., RNA interference pathways in filamentous fungi. Cell. Mol. Life Sci. 67, 3849–3863 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Segers G. C., Zhang X., Deng F., Sun Q., Nuss D. L., Evidence that RNA silencing functions as an antiviral defense mechanism in fungi. Proc. Natl. Acad. Sci. U.S.A. 104, 12902–12906 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiba S., Suzuki N., Highly activated RNA silencing via strong induction of dicer by one virus can interfere with the replication of an unrelated virus. Proc. Natl. Acad. Sci. U.S.A. 112, E4911–E4918 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun L., Nuss D. L., Suzuki N., Synergism between a mycoreovirus and a hypovirus mediated by the papain-like protease p29 of the prototypic hypovirus CHV1-EP713. J. Gen. Virol. 87, 3703–3714 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Koch A., et al. , An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 12, e1005901 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang M., Thomas N., Jin H., Cross-kingdom RNA trafficking and environmental RNAi for powerful innovative pre- and post-harvest plant protection. Curr. Opin. Plant Biol. 38, 133–141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang D., Wang M., Li S., Functional analysis of a viroid RNA motif mediating cell-to-cell movement in Nicotiana benthamiana. J. Gen. Virol. 98, 121–125 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Zhong X., Archual A. J., Amin A. A., Ding B., A genomic map of viroid RNA motifs critical for replication and systemic trafficking. Plant Cell 20, 35–47 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diener T. O., Origin and evolution of viroids and viroid-like satellite RNAs. Virus Genes 11, 119–131 (1995). [DOI] [PubMed] [Google Scholar]
- 64.Nuss D. L., Hypovirulence: Mycoviruses at the fungal-plant interface. Nat. Rev. Microbiol. 3, 632–642 (2005). [DOI] [PubMed] [Google Scholar]
- 65.García J. A., Pallás V., Viral factors involved in plant pathogenesis. Curr. Opin. Virol. 11, 21–30 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Rizza S., et al. , Microarray analysis of Etrog citron (Citrus medica L.) reveals changes in chloroplast, cell wall, peroxidase and symporter activities in response to viroid infection. Mol. Plant Pathol. 13, 852–864 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Itaya A., Matsuda Y., Gonzales R. A., Nelson R. S., Ding B., Potato spindle tuber viroid strains of different pathogenicity induces and suppresses expression of common and unique genes in infected tomato. Mol. Plant Microbe Interact. 15, 990–999 (2002). [DOI] [PubMed] [Google Scholar]
- 68.Xia C., et al. , Global transcriptomic changes induced by infection of cucumber (Cucumis sativus L.) with mild and severe variants of hop stunt viroid. Front. Microbiol. 8, 2427 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Serio F., et al. , Deep sequencing of the small RNAs derived from two symptomatic variants of a chloroplastic viroid: Implications for their genesis and for pathogenesis. PLoS One 4, e7539 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Navarro B., et al. , Deep sequencing of viroid-derived small RNAs from grapevine provides new insights on the role of RNA silencing in plant-viroid interaction. PLoS One 4, e7686 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adkar-Purushothama C. R., Sano T., Perreault J. P., Viroid-derived small RNA induces early flowering in tomato plants by RNA silencing. Mol. Plant Pathol. 19, 2446–2458 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adkar-Purushothama C. R., et al. , Small RNA derived from the virulence modulating region of the Potato spindle tuber viroid silences callose synthase genes of tomato plants. Plant Cell 27, 2178–2194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y., Lu J., Han Y., Fan X., Ding S. W., RNA interference functions as an antiviral immunity mechanism in mammals. Science 342, 231–234 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ding S. W., Voinnet O., Antiviral immunity directed by small RNAs. Cell 130, 413–426 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bologna N. G., Voinnet O., The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu. Rev. Plant Biol. 65, 473–503 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Margis R., et al. , The evolution and diversification of Dicers in plants. FEBS Lett. 580, 2442–2450 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Liu Q., Feng Y., Zhu Z., Dicer-like (DCL) proteins in plants. Funct. Integr. Genomics 9, 277–286 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Nakasugi K., et al. , De novo transcriptome sequence assembly and analysis of RNA silencing genes of Nicotiana benthamiana. PLoS One 8, e59534 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kryovrysanaki N., Alexiadis A., Grigoriadou A. M., Katsarou K., Kalantidis K., SERRATE, a miRNA biogenesis factor, affects viroid infection in Nicotiana benthamiana and Nicotiana tabacum. Virology 528, 164–175 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Katsarou K., Mavrothalassiti E., Dermauw W., Van Leeuwen T., Kalantidis K., Combined activity of DCL2 and DCL3 is crucial in the defense against Potato spindle tuber viroid. PLoS Pathog. 12, e1005936 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dadami E., et al. , DICER-LIKE 4 but not DICER-LIKE 2 may have a positive effect on potato spindle tuber viroid accumulation in Nicotiana benthamiana. Mol. Plant 6, 232–234 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Campo S., Gilbert K. B., Carrington J. C., Small RNA-based antiviral defense in the phytopathogenic fungus Colletotrichum higginsianum. PLoS Pathog. 12, e1005640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu J., Lee K. M., Cho W. K., Park J. Y., Kim K. H., Differential contribution of RNA interference components in response to distinct Fusarium graminearum virus infections. J. Virol. 92, e01756-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Minoia S., et al. , Specific argonautes selectively bind small RNAs derived from potato spindle tuber viroid and attenuate viroid accumulation in vivo. J. Virol. 88, 11933–11945 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Serio F., Martínez de Alba A. E., Navarro B., Gisel A., Flores R., RNA-dependent RNA polymerase 6 delays accumulation and precludes meristem invasion of a viroid that replicates in the nucleus. J. Virol. 84, 2477–2489 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Di Serio F., Daròs J. A., Ragozzino A., Flores R., Close structural relationship between two hammerhead viroid-like RNAs associated with cherry chlorotic rusty spot disease. Arch. Virol. 151, 1539–1549 (2006). [DOI] [PubMed] [Google Scholar]
- 87.Minoia S., et al. , Viroid-like RNAs from cherry trees affected by leaf scorch disease: Further data supporting their association with mycoviral double-stranded RNAs. Arch. Virol. 159, 589–593 (2014). [PubMed] [Google Scholar]
- 88.Biella S., Smith M. L., Aist J. R., Cortesi P., Milgroom M. G., Programmed cell death correlates with virus transmission in a filamentous fungus. Proc. Biol. Sci. 269, 2269–2276 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pearson M. N., Beever R. E., Boine B., Arthur K., Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 10, 115–128 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang M., Jin H., Spray-induced gene silencing: A powerful innovative strategy for crop protection. Trends Microbiol. 25, 4–6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang M., et al. , Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2, 16151 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shapira R., Choi G. H., Nuss D. L., Virus-like genetic organization and expression strategy for a double-stranded RNA genetic element associated with biological control of chestnut blight. EMBO J. 10, 731–739 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zeng W., et al. , Dicer-like proteins regulate sexual development via the biogenesis of perithecium-specific microRNAs in a plant pathogenic fungus Fusarium graminearum. Front. Microbiol. 9, 818 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Churchill A., Ciuffetti L., Hansen D., Van Etten H., Van Alfen N., Transformation of the fungal pathogen Cryphonectria parasitica with a variety of heterologous plasmids. Curr. Genet. 17, 25–31 (1990). [Google Scholar]
- 95.Hillman B. I., Supyani S., Kondo H., Suzuki N., A reovirus of the fungus Cryphonectria parasitica that is infectious as particles and related to the coltivirus genus of animal pathogens. J. Virol. 78, 892–898 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun L., Suzuki N., Intragenic rearrangements of a mycoreovirus induced by the multifunctional protein p29 encoded by the prototypic hypovirus CHV1-EP713. RNA 14, 2557–2571 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andika I. B., Kondo H., Tamada T., Evidence that RNA silencing-mediated resistance to beet necrotic yellow vein virus is less effective in roots than in leaves. Mol. Plant Microbe Interact. 18, 194–204 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Cao S., et al. , FgSsn3 kinase, a component of the mediator complex, is important for sexual reproduction and pathogenesis in Fusarium graminearum. Sci. Rep. 6, 22333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.